Abstract
Background
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide. It has been widely established that the early detection of HCC enables more treatment options with improvements in prognosis and survival.
Objectives
The aim of this study was to assess the diagnostic accuracy of both circulating miR-215 and squamous cell carcinoma antigen-IgM (SCCA-IgM) as serum biomarkers for HCC by examining their diagnostic sensitivity, specificity, accuracy, and predictive values in hepatitis C virus (HCV)-induced HCC patients.
Subjects and methods
This study included 60 patients with HCV-related HCC. In addition, 60 patients with HCV-related liver cirrhosis (LC) and 60 apparently healthy subjects were involved, and served as diseased and healthy control groups, respectively. The relative expression levels of miR-215 were detected using quantitative real-time PCR. SCCA-IgM levels in serum were measured by enzyme immunoassay. We used receiver operating characteristic (ROC) curve to calculate the diagnostic accuracy against alpha-fetoprotein (AFP).
Results
Relative miR-215 expression levels increased the most in HCC patients compared to that in healthy or diseased controls (P<0.001). Serum concentration of SCCA-IgM was significantly higher in HCC group than that in the two control groups. We performed multivariate analysis using AFP level, focal lesion size, and portal vein thrombosis as independent variables. ROC curves showed that the optimum diagnostic miR-215 cutoff value for identifying HCC patients from cirrhotic ones was 417 (sensitivity, 97%; specificity, 91%) and for SCCA-IgM was 95 AU/mL (sensitivity, 92%; specificity, 98%). Moreover, the superiority of both miR-215 and SCCA-IgM to AFP is obvious in our study and this superiority is more evident in distinguishing HCC with AFP levels <200 ng/mL and HCC patients with small-sized focal lesions from cirrhotic patients.
Conclusion
Cell-free miR-215 and serum SCCA-IgM could be used for early diagnosis of HCC either each one as a single marker or with AFP complement measurement.
Keywords: miR-215, SCCA-IgM, AFP, LC, HCC
Introduction
Hepatocellular carcinoma (HCC) is the most common malignant tumor of the liver. It is the sixth most common cancer in men and women combined with respect to the incidence rate.1 Furthermore, HCC is the third most common cause of cancer-related deaths.2 A broad geographic variability has been observed for HCC, which is mainly attributable to a different distribution of the leading predisposing conditions, which are mainly represented by hepatitis B virus (HBV) and hepatitis C virus (HCV) infections.3 HCC represents an important public health problem in Egypt where up to 90% of HCC cases are attributable to HCV infection.4 The prognosis of HCC is poor due to late presentation with advanced disease as early detection is still difficult due to the lack of early symptoms. As well, the biomarkers that show high sensitivity and specificity are yet missed.
The diagnosis of HCC depends on both alpha-fetoprotein (AFP) as a screening marker and radiological imaging studies. Normally, the levels of AFP are below 10 ng/mL, but AFP greater than 200 ng/mL is suggestive of HCC. However, as the sensitivity of AFP for HCC is about 67%, normal AFP serum levels or levels <200 ng/mL do not exclude HCC.5 Thus, so far, there is no early reliable diagnostic marker for the prediction of malignant transformation in the liver. Hence, searching another tumor marker that together with AFP could improve the diagnosis of HCC is still crucial. miRNA expression signature had been suggested in many studies as cancer diagnostic and prognostic biomarkers. miRNAs as biomarkers have their advantages, such as stability and high sensitivity. They comprise a class of highly conserved noncoding RNAs of ~22 nucleotides in length. Mature miRNAs may interact with the 3′-untranslated regions (UTRs) of target mRNAs to form RNA-induced silencing complexes, resulting in inhibition of translation or mRNA cleavage.6 As well, previous studies have revealed that miRNAs may regulate gene expression at posttranscriptional level and perform a role in regulating epigenetic machinery, including DNA methylation and histone modification.7
Squamous cell carcinoma antigen (SCCA) is a member of the high molecular weight family of serine protease inhibitors named serpins. They are physiologically found in the granular layers of normal squamous epithelium but are also found to be typically expressed by neoplastic cells of epithelial origin in different cancers, for example, cancer cervix, lung cancer, and head and neck cancers. Hence, they can be used as biomarkers for these malignancies. Overexpression of SCCA variants (SCCA1 and SCCA2) has been reported in all surgically resected HCC specimens but in none of the normal control livers as detected by immunohistochemistry.8 The discovery of a novel class of tumor markers comprising circulating IgM antibodies forming immune complexes with specific cancer biomarkers has provided interesting opportunities for HCC patient management.9,10 IgM immune complexes can be detected in the serum of HCC patients as reported for SCCA; and the assessment of squamous cell carcinoma antigen-IgM (SCCA-IgM) immune complexes has allowed a higher diagnostic performance than the determination of the free, not complex one.11
Both miR-215 and SCCA-IgM were recently suggested to be predictive and/or prognostic biomarkers of HCC risk in patients with cirrhosis in whom the marker predicted histological progression.12 Therefore, the overall objective of this study was to measure the relative expression levels of cell-free miR-215 as well as the levels of SCCA-IgM in the serum of HCV-induced HCC patients. Also in this study, we aimed to assess the diagnostic accuracy of these two serum markers against the conventional marker, AFP for distinguishing HCC patients from liver cirrhosis (LC) patients.
Subjects and methods
Subjects
This study is a case–control, hospital-based study. It was carried out at the Clinical Pathology, Tropical Medicine and Internal Medicine Departments, Faculty of Medicine, Minia University. It was approved by the institutional review board of the Minia Faculty of Medicine, Minia University, Egypt. Moreover, this study was conducted in accordance with the ethical guidelines of both Declaration of Helsinki and International Conference on Harmonization – Good Clinical Practice.
One hundred and twenty patients with chronic HCV-related liver diseases were included in this study along with 60 apparently healthy subjects as a healthy control group (group I). We randomly selected healthy volunteers from the medical and paramedical staff working in the same hospital after their agreement to participate in this study as control subjects. Their ages ranged from 49 to 60 years. The patients were selected from the outpatient’s clinic and the inpatient’s of the Tropical Medicine and Internal Medicine Departments, Minia University Hospital and were divided into two groups (Group II and Group III). Written informed consent was obtained from every participant.
Group II composed of 60 patients with post HCV LC diagnosed by clinical, biochemical, and abdominal ultrasonographic findings. Group III composed of 60 patients with HCV-associated HCC on top of LC. HCC was defined on the basis of ultrasound, computed tomography (CT) or magnetic resonance imaging characteristics, serology (AFP), and liver function tests to evaluate the patients. Because AFP cutoff level of 200 ng/mL is the most suggestive of HCC,13 HCC group was subdivided into two subgroups according to their AFP serum levels: subgroup IIIa with AFP serum levels <200 ng/mL and subgroup IIIb with AFP serum levels ≥200 ng/mL.13 Furthermore, according to their portal vein status, HCC patients were categorized into HCC patients with patent portal vein or portal vein thrombosis (PVT). Also, HCC patients were grouped according to the size of their liver focal lesions (tumor size) into HCC patients with tumor size <3 cm and HCC patients with tumor size ≥3 cm. All patients were proved to be negative for HBV infection and positive for HCV infection by enzyme immunoassay (EIA) and quantitative real-time PCR (qRT-PCR). Patients with other coexisting liver disease (eg, schistosomiasis infection, autoimmune hepatitis), other malignancies, other infectious diseases (eg, HIV infection), or autoimmune diseases were excluded.
Clinical data collection and laboratory methods
Detailed history was collected from all subjects besides complete clinical examination. Venous blood was withdrawn from all subjects by sterile venipuncture. These blood samples were used for analyzing complete blood counts (CBCs), prothrombin time, liver function tests, serum levels of AFP, serum levels of SCCA-IgM, and relative expression levels of circulating miR-215. CBCs were detected via automated cell counter (Sysmex KX-21N; TAO Medical Incorporation, Japan). Prothrombin time was measured using STAGO COMPACT CT Coagulation Analyzer (Diamond Diagnostics, USA). Liver function tests (aspartate aminotransferase [AST], alanine aminotransferase [ALT], bilirubin, and total protein and albumin [ALB]) were determined by automated chemistry auto-analyzer system Konelab 20i (Thermo Electron Incorporation, Vantaa, Finland). Serum AFP levels were measured by electro-chemiluminescence immunoassay using Cobas e 411 Analyzer (Roche Diagnostics, Indianapolis, IN, USA). In addition, serum SCCA-IgM levels were analyzed via EIA using anti-SCCA-IgM antibodies (Hepa-IC, Xeptagen; Xeptagen SpA, Marghera, Venice, Italy) according to the manufacturer’s instructions.
Quantitative detection of the relative expression levels of circulating miR-215 was performed by qRT-PCR technique. First, total RNA including miRNAs was extracted using miR-Neasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Total RNA was further reverse transcribed to cDNA through miScript II RT Kit supplied by Qiagen followed by qRT-PCR using miScript SYBR Green PCR Kit supplied by Qiagen. SYBR Green PCR Kit contained Universal PCR Master Mix II. The thermal protocol was run on DTlite Real-Time PCR System (DNA technology, Moscow, Russia). The relative amount of miR-215 to SNORD68_11 (sno68) (reference gene) was calculated using cycle threshold (Ct) method. Ct was calculated by DT master software: ΔCt=Ct (target gene)–Ct (reference gene).
The miR-215 real-time PCR forward primers were designed as follows: miRNA-215-qPCRF, AUGACCUAUGAAUUGACAGAC.
Statistical analyses
Statistical analyses were performed using SPSS software-version 17 (SPSS Inc., Chicago, IL, USA). Descriptive statistical analyses were done for parametric quantitative data, and the data are presented as mean±SD ranges; nonparametric quantitative data were presented as median; and qualitative data were presented as n (%). Analyses were done for parametric quantitative data among three groups using one-way ANOVA followed by unpaired t-test to compare between two study groups. For comparison of not normally distributed data (nonparametric), statistical chi-squared test was used. Linear correlation was done between either miR-215 or SCCA-IgM and other study variables and between each other using Pearson’s correlation coefficient and Spearman’s rank correlation. P-value≤0.05 was considered statistically significant. miR-215 expression levels were presented as fold change relative to the healthy control group. Fold changes of >1.0 or <-1.0 and P-value ≤0.05 were considered significant. Receiver operating characteristic (ROC) curve was applied to identify the area under the curve (AUC) along with best cutoff point that yields the best sensitivity and specificity. In addition, we calculated positive predictive value (PPV), negative predictive value (NPV), and accuracy of each variable vs AFP for predicting HCC.
Results
Baseline and clinicopathologic characteristics of the enrolled subjects
Sixty patients with HCC were enrolled in this study. Sixty patients with LC and 60 apparently healthy subjects were served as control groups (diseased and healthy controls, respectively). All the patients were Egyptians and HCV positive. The baseline demographic and clinical characteristics of the enrolled subjects were presented in Table 1. The mean±SD of age in group I was 53.4±5.4 years with a male/ female ratio of 36/24, in group II was 57.8±7.4 years with a male/female ratio of 42/18, and in group III was 59±7.8 years with a male/female ratio of 40/20. There were no statistically significant differences in regard to age and sex among the three groups. Concerning clinical features, there were 31.7% of the HCC patients who had PVT and 56.7% with tumor size <3 cm. Percentages of cases with ascites were included in Table 1.
Table 1.
Demographic data and clinical features of studied groups
| Variables | Group I – control (n=60) | Group II – LC (n=60) | Group III – HCC (n=60) | P-values | ||
|---|---|---|---|---|---|---|
| Age (years) | 0.533 | |||||
| Range | 49–60 | 47–65 | 45–81 | I vs II | I vs III | II vs III |
| Mean±SD | 53.4±5.4 | 57.8±7.4 | 59±7.8 | 0.880 | 0.360 | 0.680 |
| Sex, n (%) | 0.842 | |||||
| Male | 36 (60) | 42 (70) | 40 (66.7) | I vs II | I vs III | II vs III |
| Female | 24 (40) | 18 (30) | 20 (33.3) | 0.690 | 0.734 | 0.794 |
| Ascites, n (%) | ||||||
| No | 60 (100) | 12 (20) | 5 (8.3) | – | ||
| Mild | – | 15 (25) | 10 (16.7) | |||
| Moderate | – | 20 (33.3) | 25 (41.7) | |||
| Massive | – | 13 (21.7) | 20 (33.3) | |||
| PVT, n (%) | 0 | 0 | 19 (31.7) | – | ||
| Tumor size, n (%) | ||||||
| <3 (cm) | 0 | 0 | 34 (56.7) | – | ||
| ≥3 (cm) | 0 | 0 | 26 (43.3) | |||
Abbreviations:HCC, hepatocellular carcinoma; LC, liver cirrhosis; PVT, portal vein thrombosis.
Routine laboratory results of patients and control groups
The CBC findings are presented in Table 2. There were no statistically significant differences of white blood cells (WBCs) count in patient with LC in comparison to healthy controls (P=0.958); however, the group of patients with HCC had a statistically higher WBCs count when compared to both healthy controls (P=0.025) and LC patients (P=0.007). There was a statistically significant decrease in the mean hemoglobin levels in patient’s groups when compared to the control group (P<0.001). Also, there was no statistically significant difference in hemoglobin levels in HCC group when compared to LC group (P=0.418). The platelet count showed no statistically significant difference in the group of patients with LC when compared to HCC group (P=0.288), but there was a statistically significant decrease in their counts in patient’s groups when compared to the control group (P<0.001).
Table 2.
Comparison between studied groups regarding routine laboratory data
| Variables | Group I – control (n=60) | Group II – LC (n=60) | Group III – HCC (n=60) | P-values | ||
|---|---|---|---|---|---|---|
| I vs II | I vs III | II vs III | ||||
| WBCs (¥103/μL) | ||||||
| Range | 4.5–7.9 | 3–8.5 | 3.6–15.6 | 0.958 | 0.025a | 0.007a |
| Mean±SD | 5.4±1.6 | 5.7±1.7 | 8±3.2 | |||
| Hb (g/dL) | ||||||
| Range | 13.1–16.6 | 4.9–14 | 4.9–13.5 | <0.001a | <0.001a | 0.418 |
| Mean±SD | 14.7±1.5 | 10.5±2.6 | 9.9±1.6 | |||
| Platelets (¥103/μL) | ||||||
| Range | 187–332 | 74–162 | 79–241 | <0.001a | <0.001a | 0.288 |
| Mean±SD | 266.6±54.3 | 122.9±25 | 138.8±43 | |||
| ALT (U/L) | ||||||
| Range | 18.1–23 | 40–99 | 67–228 | 0.002a | <0.001a | <0.001a |
| Mean±SD | 19.7±1.9 | 67.8±20.3 | 122.2±40.1 | |||
| AST (U/L) | ||||||
| Range | 12–25 | 47–115 | 91–299 | 0.004a | <0.001a | <0.001a |
| Mean±SD | 16±4.9 | 81.1±25.6 | 180.3±59.4 | |||
| Total bilirubin (mg/dL) | ||||||
| Range | 0.2–1 | 1.9–3.9 | 2.1–16.9 | <0.001a | <0.001a | <0.001a |
| Mean±SD | 0.5±0.3 | 2.7±0.8 | 5.3±3.6 | |||
| Direct bilirubin (mg/dL) | ||||||
| Range | 0.04–0.3 | 0.7–2.3 | 0.8–11.7 | <0.001a | <0.001a | <0.001a |
| Mean±SD | 0.2±0.1 | 1.2±0.4 | 3.3±2.6 | |||
| ALB (g/dL) | ||||||
| Range | 4.8–5.4 | 1.4–3.5 | 1.5–4.4 | <0.001a | <0.001a | 0.414 |
| Mean±SD | 5.1±0.2 | 2.4±0.6 | 2.6±0.6 | |||
| INR | ||||||
| Range | 1–1.1 | 1.3–2.7 | 1–3.4 | <0.001a | 0.002a | 0.256 |
| Mean±SD | 1±0 | 1.7±0.4 | 1.5±0.5 | |||
Notes:
Significant difference at P-value≤0.05.
Abbreviations: ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; Hb, hemoglobin; HCC, hepatocellular carcinoma; INR, international normalized ratio; LC, liver cirrhosis; WBC, white blood cell.
Likewise, liver function tests are presented in Table 2. When cirrhotic patients’ groups with and without HCC were compared with healthy control group, the formers had statistically significant higher levels of AST, ALT, total bilirubin, and international normalized ratio (INR) (P<0.05) and statistically significant lower serum ALB levels (P≤0.001). HCC patients had statistically significant higher levels of total bilirubin, ALT, and AST than cirrhotic patients without HCC (P≤0.001). Conversely, there were no statistically significant differences in relation to serum ALB and INR levels when HCC patients were compared to LC patients (P<0.414 and <0.256, respectively).
AFP serum levels, cell-free miR-215 relative expression levels, and SCCA-IgM serum levels within different studied groups
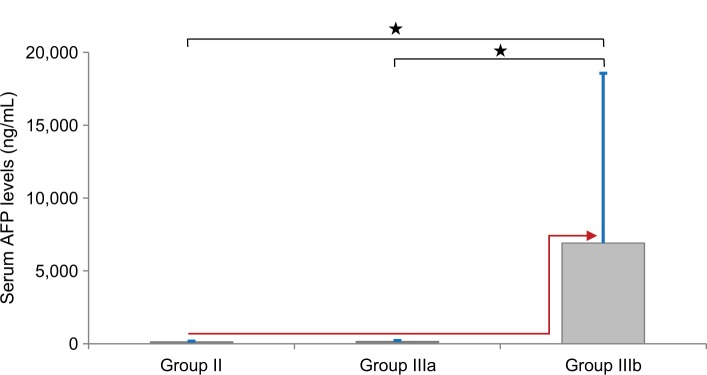
The serum levels of AFP, cell-free miR-215, and SCCA-IgM were detailed in Table 3. As the AFP values in the current study were not normally distributed in HCC group, we presented its results using the median value. The median AFP levels were 2.5, 29.8, and 300 ng/mL in control, LC, and HCC groups, respectively. These values showed statistically significant higher serum levels of AFP in the HCC group compared to the other two groups (P<0.001). Moreover, dividing the HCC group according to AFP levels revealed a statistically nonsignificant differences between group IIIa and group II vs a significant differences between group IIIa compared to both group I and group IIIb (P=0.06,<0.001, and <0.001, respectively; Figure 1).
Table 3.
Comparison between patients and control groups as regards AFP, cell-free miR-215 and SCCA-IgM
| Variables | Group I – control (n=60) | Group II – LC (n=60) | Group III – HCC (n=60) | P-values | |||
|---|---|---|---|---|---|---|---|
|
AFP (ng/mL) Range Mean±SD Median |
1.8–3.7 2.6±0.7 2.5 |
6–117 45.3±38.2 29.8 |
20–46,000 3,979.4±9,398.8 300 |
I vs II | I vs III | II vs III | |
| 0.007a | <0.001a | <0.001a | |||||
| Group IIIa HCC with AFP <200 (n=25) | Group IIIb HCC with AFP ≥200 (n=35) | I vs IIIa | II vs IIIa | IIIa vs IIIb | |||
| 20–197 71.9±56 63.9 |
287–46,000 6,910±11,650 1,109 |
<0.001a | 0.06 | <0.001a | |||
|
miR-215 Range Mean±SD |
11.9–25 18.4±5.3 |
104–538 205.4±134.3 |
417–3,100 1,018.0±600.6 |
I vs II | I vs III | II vs III | |
| <0.001a | <0.001a | <0.001a | |||||
| Group IIIa HCC with AFP <200 (n=25) | Group IIIb HCC with AFP ≥200 (n=35) | I vs IIIa | II vs IIIa | IIIa vs IIIb | |||
| 417–1,001 758±157 |
588–3,100 1,213±727 |
<0.001a | <0.001a | <0.001a | |||
|
SCCA-IgM (AU/mL) Range Mean±SD |
4–66 21±15 |
23–187 50±3 |
3.2–630 316±155 |
I vs II | I vs III | II vs III | |
| <0.001a | <0.001a | <0.001a | |||||
| Group IIIa HCC with AFP <200 (n=25) | Group IIIb HCC with AFP ≥200 (n=35) | I vs IIIa | II vs IIIa | IIIa vs IIIb | |||
| 3.2–460 250.2±151.6 |
8.6–630 386.63±126.1 |
<0.001a | 0.001a | 0.001a | |||
Notes:
Significant difference at P-value≤0.05.
Abbreviations: AFP, alpha-fetoprotein; HCC, hepatocellular carcinoma; LC, liver cirrhosis; SCCA-IgM, squamous cell carcinoma antigen-IgM.
Figure 1.
Comparison of serum AFP levels between LC group (Group II) and HCC subgroups (Group IIIa and IIIb).
Notes: The values are presented as mean±SD. *Significant difference at P-value≤0.05. Red arrow indicates how AFP serum levels had been jumped from both LC (Group II) and HCC with AFP levels <200 ng/mL (Group IIIa) groups to HCC with AFP levels ≥200 ng/mL (Group IIIb). Blue bar indicates SD.
Abbreviations: AFP, alpha-fetoprotein; HCC, hepatocellular carcinoma; LC, liver cirrhosis.
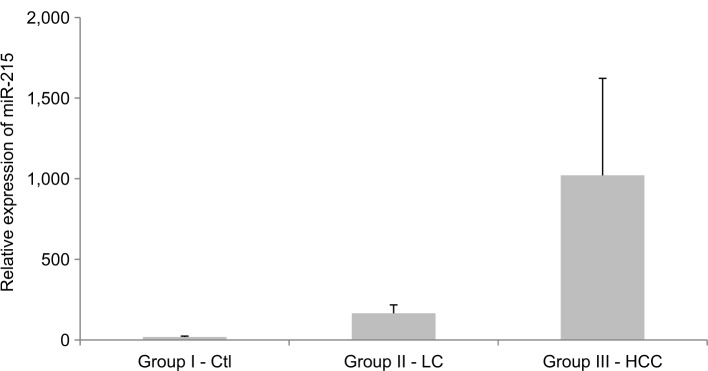
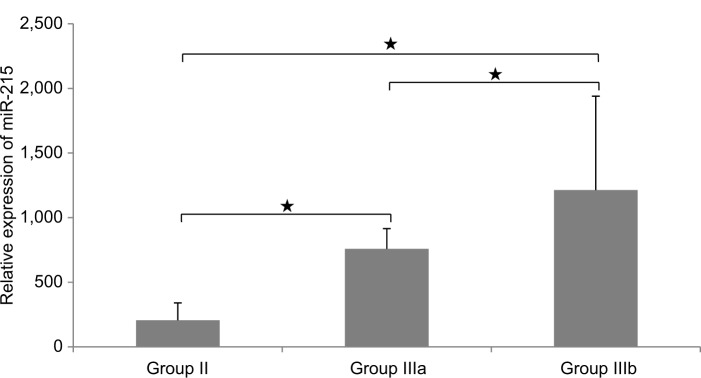
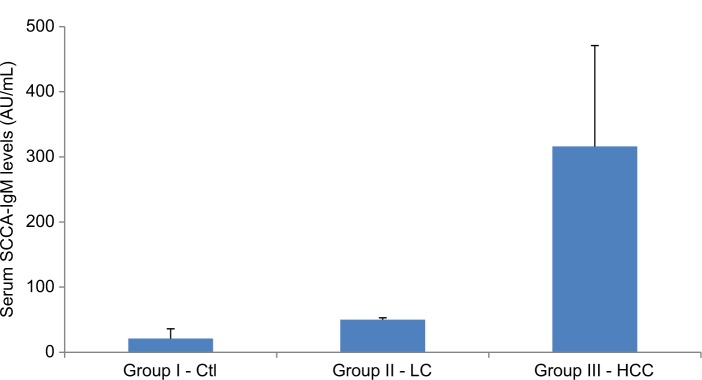
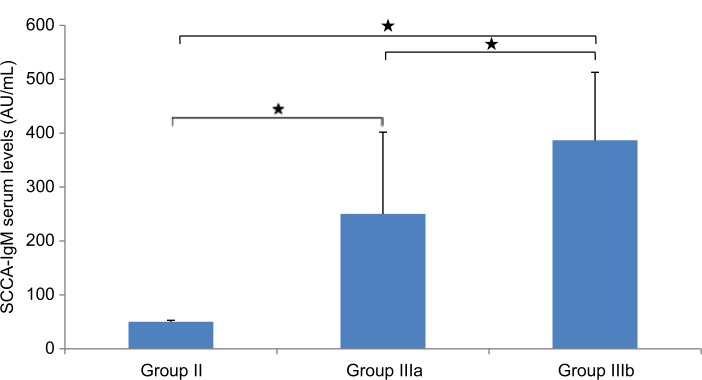
miR-215 relative expression levels were presented as mean±SD (Table 3 and Figures 2 –5): group I, 18.4±5.3; group II, 205.4±134.3; group III, 1018±600.6. In addition, miR-215 relative expression levels in subgroup IIIa were 758.0±157.03 and in subgroup IIIb were 1213.1±727.2. There was a statistically significant increase in miR-215 expression levels in group II when compared to that in group I (P<0.001) and in subgroup IIIa when compared to that in group I, group II, and subgroup IIIb (P<0.001). Also, mean±SD of SCCA-IgM serum levels was 21±15 in healthy control group, 50±3 in LC patients, and 316±155 in HCC group (Table 3 and Figures 4 and 6). Serum SCCA-IgM levels were statistically significantly higher in HCC patients than LC and healthy control groups (P<0.001). SCCA-IgM serum levels within HCC subgroups and their comparisons are listed Table 3 and shown in Figure 7.
Figure 2.
The relative miR-215 expression levels among the studied groups.
Notes: Expression levels were detected using qRT-PCR assay. Relative expression was analyzed by ∆Ct method using SNORD68_11 as reference gene. The values are presented as mean±SD.
Abbreviations: Ctl, control; HCC, hepatocellular carcinoma; LC, liver cirrhosis; qRT-PCR, quantitative real-time PCR.
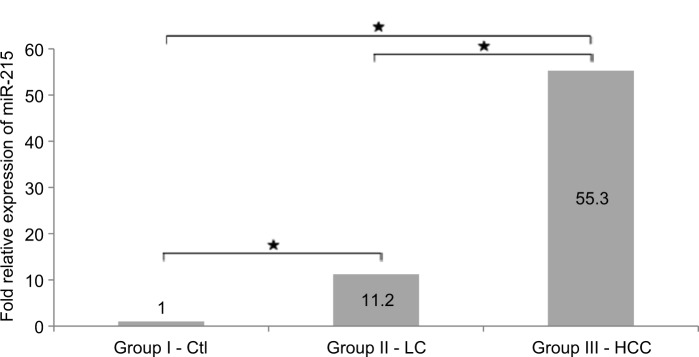
Figure 3.
Fold change of miR-215 relative expression levels among studied groups.
Notes: *Significant difference at P-value≤0.05. miR-215 expression levels were presented as fold change relative to healthy control group. Fold change of >1.0 or <-1.0 plus P-value≤0.05 were considered significant.
Abbreviations: Ctl, control; HCC, hepatocellular carcinoma; LC, liver cirrhosis.
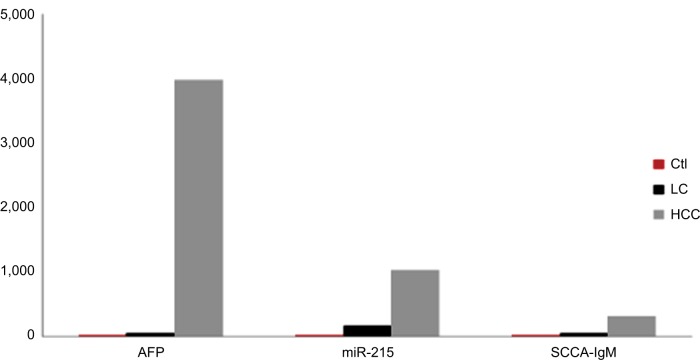
Figure 4.
Comparison between studied groups as regards AFP serum levels, miR-215 relative expression levels, and serum levels of SCCA-IgM.
Note: The values are presented as mean levels.
Abbreviations: AFP, alpha-fetoprotein; Ctl, control; HCC, hepatocellular carcinoma; LC, liver cirrhosis; SCCA-IgM, squamous cell carcinoma antigen-IgM.
Figure 5.
Comparison between LC group (Group II) and HCC subgroups (Groups IIIa and IIIb) as regards miR-215 relative expression levels.
Notes: The values are presented as mean±SD. *Significant difference at P-value ≤0.05. (Group IIIa) HCC with AFP levels <200 ng/mL and (Group IIIb) HCC with AFP levels ≥200 ng/mL.
Abbreviations: HCC, hepatocellular carcinoma; LC, liver cirrhosis.
Figure 6.
Comparison between patients and control groups as regards serum SCCA-IgM levels.
Note: The values are presented as mean±SD.
Abbreviations: Ctl, control; HCC, hepatocellular carcinoma; LC, liver cirrhosis; SCCA-IgM, squamous cell carcinoma antigen-IgM.
Figure 7.
Comparison between LC group (Group II)b and HCC subgroups (Groups IIIa and IIIb) regarding SCCA-IgM serum levels.
Notes: The values are presented as mean±SD. *Significant difference at P-value ≤0.05. (Group IIIa) HCC with AFP levels <200 ng/mL and (Group IIIb) HCC with AFP levels ≥200 ng/mL.
Abbreviations: HCC, hepatocellular carcinoma; LC, liver cirrhosis; SCCA-IgM, squamous cell carcinoma antigen-IgM.
Table 4 shows a comparison among the mean±SD of AFP levels, miR-215 expression levels, and SCCA-IgM levels in HCC patients who were categorized according to the tumor and the presence or absence of PVT. There was a statistically significant increase in AFP levels in HCC patients with tumor size >3 cm when compared to HCC patients with smaller tumor size and in HCC patients with PVT when compared to those with patent portal vein (P<0.001). The same trend was seen in miR-215 relative expression levels. Similarly, we compared serum levels of SCCA-IgM according to the tumor size and patency of portal vein. We found that the mean values of SCCA-IgM serum levels in patients with tumor size ≥3 cm were insignificantly higher than that in patients with tumor size <3 cm (P=0.094). The same was found in regard to PVT (P=0.207).
Table 4.
Comparison within HCC group regarding AFP serum levels, circulating miR-215 relative expression levels, and SCCA-IgM serum levels
| Variables | HCC with tumor size <3 cm (n=34) | HCC with tumor size ≥3 cm (n=26) | P-values | HCC with patent PV (n=41) | HCC with PVT (n=19) | P-values |
|---|---|---|---|---|---|---|
| AFP (ng/mL) | ||||||
| Mean±SD | 1,090±3,849 | 8,869±13,371 | <0.001a | 1,052±3,946 | 8,371±12,991 | <0.001a |
| Median | 143 | 2,300 | 112 | 19,400 | ||
| miR-215 | ||||||
| Mean±SD | 775±159 | 1,429±820 | <0.001a | 766±150 | 1,396±801 | <0.001a |
| SCCA-IgM (AU/mL) | ||||||
| Mean±SD | 210±133.5 | 297±118.7 | 0.094 | 300±152.1 | 365±159.1 | 0.207 |
Notes:
Significant difference at P-value≤0.05.
Abbreviations: AFP, alpha-fetoprotein; HCC, hepatocellular carcinoma; PV, portal vein; PVT, portal vein thrombosis; SCCA-IgM, squamous cell carcinoma antigen-IgM.
Correlation between circulating miR-215 relative expression levels along with SCCA-IgM serum levels and other laboratory data plus clinical findings within HCC group
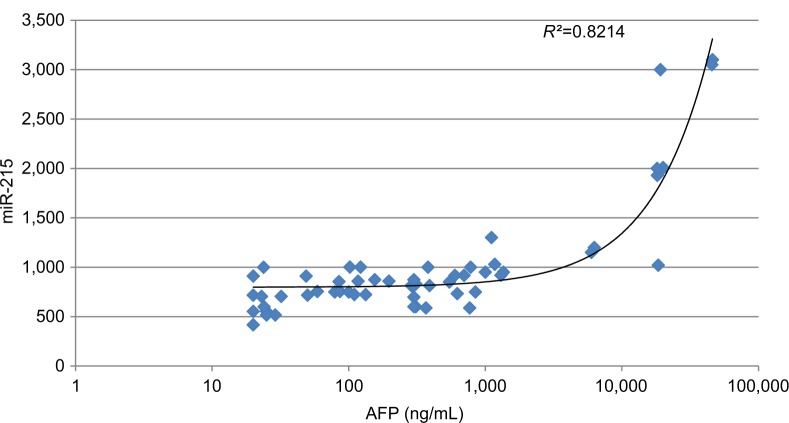
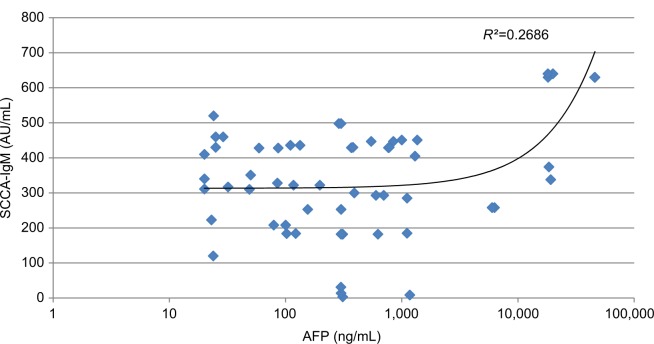
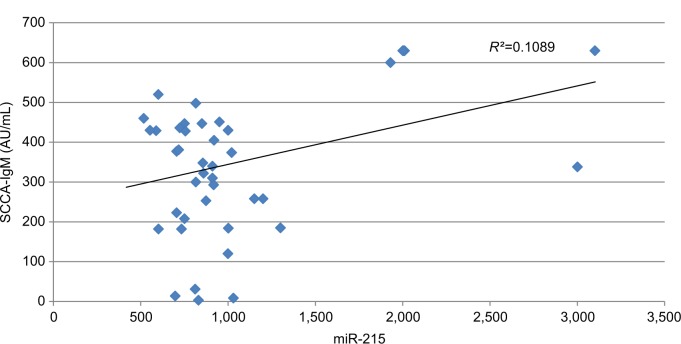
Our data show that there was a statistically significant moderate positive correlation between miR-215 expression levels in HCC group and both tumor size (r=0.527 and P=0.001) and PVT (r=0.554 and P=0.001). Also, there was a fair positive correlation between miR-215 expression levels and both total bilirubin (r=0.287 and P=0.016) and direct bilirubin (r=0.307 and P=0.010) within HCC group. However, there were no significant correlations among miR-215 and age, WBCs, Hb, platelet count, ALT, AST, ALB, total protein, and INR (Table 5). In addition, there were no significant correlations between SCCA-IgM and clinicopathologic characteristics of HCC patients besides their routine laboratory data (Table 6). There was statistically no significant correlation between miR-215 and AFP levels in HCC patients (r=0.821 and P=0.074; Figure 8). Contradictory, serum levels of SCCA-IgM in HCC group were directly correlated with AFP serum levels in a significant manner (r=0.269 and P=0.028; Figure 9). Figure 10 shows the correlation between miR-215 relative expression levels and serum levels of SCCA-IgM within HCC group (r=0.109 and P<0.001).
Table 5.
Correlation between miR-215 expression levels and age, some laboratory data in addition to some clinicopathologic findings within HCC group
| Group III | miR-215 | |
|---|---|---|
| r | P-values | |
| Age (years) | 0.129 | 0.285 |
| WBCs (×103/μL) | –0.018 | 0.885 |
| Hb (g/dL) | –0.141 | 0.244 |
| Platelets (×103/μL) | –0.138 | 0.256 |
| ALT (U/L) | 0.117 | 0.334 |
| AST (U/L) | 0.087 | 0.476 |
| Total bilirubin (mg/dL) | 0.287 | 0.016a |
| Direct bilirubin (mg/dL) | 0.307 | 0.010a |
| ALB (g/dL) | –0.025 | 0.836 |
| INR | 0.065 | 0.594 |
| Tumor sizeb | 0.527 | <0.001a |
| PVTb | 0.554 | <0.001a |
Notes:Pearson’s correlation coefficient.
Significant correlation at P-value ≤0.05.
Spearman’s correlation coefficient. r=0.75–1 (strong correlation), r=0.5–0.74 (moderate correlation), r=0.25–0.49 (fair correlation), and r=0.1–0.24 (weak correlation). Bold data indicates r with P-values Significant (P-value≤0.05).
Abbreviations: ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; Hb, hemoglobin; HCC, hepatocellular carcinoma; INR, international normalized ratio; WBC, white blood cell.
Table 6.
Correlation between SCCA-IgM serum levels and age, some laboratory data in addition to some clinicopathologic findings within HCC group
| Group III | SCCA-IgM | |
|---|---|---|
| r | P-values | |
| Age (years) | 0.135 | 0.351 |
| WBCs (×103/μL) | 0.047 | 0.748 |
| Hb (g/dL) | –0.450 | 0.755 |
| Platelets (×103/μL) | –0.222 | 0.121 |
| ALT (U/L) | 0.194 | 0.177 |
| AST (U/L) | 0.242 | 0.091 |
| Total bilirubin (mg/dL) | –0.009 | 0.951 |
| Direct bilirubin (mg/dL) | –0.031 | 0.831 |
| ALB (g/dL) | –0.023 | 0.370 |
| INR | 0.067 | 0.642 |
| Tumor sizea | 0.143 | 0.083 |
| PVTa | 0.017 | 0.111 |
Notes:
Pearson’s correlation coefficient
Spearman’s correlation coefficient. r=0.75–1 (strong correlation), r=0.5–0.74 (moderate correlation), r=0.25–0.49 (fair correlation), and r=0.1–0.24 (weak correlation).
Abbreviations: ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; Hb, hemoglobin; HCC, hepatocellular carcinoma; INR, international normalized ratio; PVT, portal vein thrombosis; SCCA-IgM, squamous cell carcinoma antigen-IgM; WBC, white blood cell.
Figure 8.
Correlation between AFP serum levels and miR-215 relative expression levels in HCC group.
Abbreviations: AFP, alpha-fetoprotein; HCC, hepatocellular carcinoma.
Figure 9.
Correlation between AFP and SCCA-IgM serum levels in HCC group.
Abbreviations: AFP, alpha-fetoprotein; HCC, hepatocellular carcinoma; SCCA-IgM, squamous cell carcinoma antigen-IgM.
Figure 10.
Correlation between miR-215 relative expression levels and SCCA-IgM serum levels in HCC group.
Abbreviations: HCC, hepatocellular carcinoma; SCCA-IgM, squamous cell carcinoma antigen-IgM.
Diagnostic accuracy of serum miR-215 and SCCA-IgM vs AFP for identifying HCC patients from LC patients
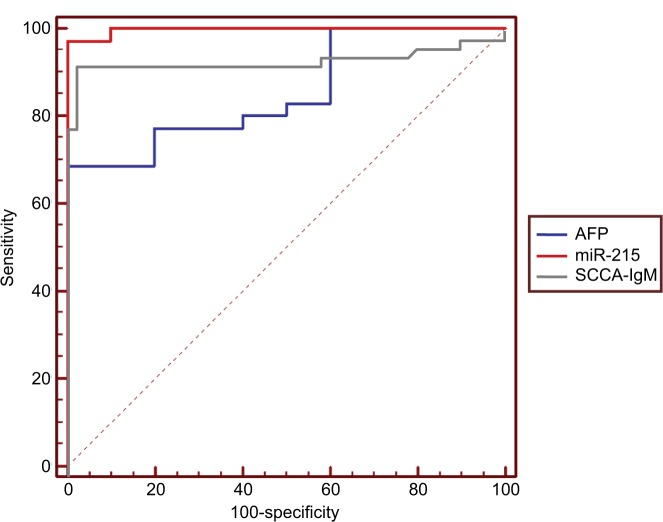
For differentiating HCC from LC, ROC curve was applied. The AUC value of AFP was 0.854 (95% CI, 0.764–0.920, P<0.001). miR-215 showed an AUC value of 0.997 (95% CI, 0.954–1.0, P<0.001; Figure 11). The sensitivity of AFP as a diagnostic marker for HCC was 77% with specificity of 81% when applying a cutoff level of 117 ng/mL, which was the cutoff with the maximal sum of sensitivity and specificity (Table 7). Similarly, the sensitivity of miR-215 as a marker for distinguishing these patients from LC patients was 97.14% and its specificity value was 91%. The cutoff level of 417 for miR-215 was the one that showed the maximum sensitivity and specificity (Table 7). Also, ROC curves showed that the optimum cutoff value for SCCA-IgM was 95 AU/mL (AUC, 0.932; sensitivity, 92%; specificity, 98%; 95% CI, 0.888–0.991, P<0.001; Table 7 and Figure 11).
Figure 11.
ROC curve analysis of AFP against both circulating miR-215 and SCCA-IgM for prediction of HCC from LC.
Abbreviations: AFP, alpha-fetoprotein; HCC, hepatocellular carcinoma; LC, liver cirrhosis; ROC, receiver operating characteristic; SCCA-IgM, squamous cell carcinoma antigen-IgM.
Table 7.
Diagnostic performances of both miR-215 and SCCA-IgM vs AFP for distinguishing HCC patients from LC patients
| Variables | Optimal cutoff | AUC | P-values | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) |
|---|---|---|---|---|---|---|---|---|
| AFP | >117 | 0.854 | <0.001* | 77.0 | 81 | 100 | 47.6 | 75.6 |
| miR-215 | >417 | 0.997 | <0.001* | 97.14 | 91 | 97.1 | 90 | 95.66 |
| SCCA-IgM | >95 | 0.932 | <0.001* | 92 | 98 | 97.9 | 92.5 | 95 |
Notes:
P-value is significant ≤0.05.
Abbreviations: AFP, alpha-fetoprotein; AUC, area under the curve; HCC, hepatocellular carcinoma; LC, liver cirrhosis; NPV, negative predictive value; PPV, positive predictive value; SCCA-IgM, squamous cell carcinoma antigen-IgM.
Discussion
Egypt was reported to have the highest prevalence of HCV infection in the world. About 14.7% of the Egyptian popu lation between 15 and 59 years of age is chronically HCV infected.14 Moreover, about 85% of these patients develop persistent infection with high risk for LC and HCC to the extent that HCC, mostly related to HCV infection, constitutes 13% of all cancers.15,16 Although the diagnostic role of serum AFP is well recognized in advanced HCC, in early stages of the disease or with small HCC focal lesions, only 2.4%–22% of patients presented with serum AFP levels ≥200 ng/mL.17 Also, radiofrequency ablation (RFA), which is among the more frequently therapeutics of choice for HCC, is more effective in treating early single HCC focal lesion (<3 cm). RFA has reduced efficiency for HCC ≥3 cm.18 Therefore, early diagnosis of small single HCC focal lesion would be valuable for starting RFA, thus being more effective with good prognosis. Accordingly, finding more reliable and early diagnostic markers for small single lesion HCC is vital either alone or in combination with AFP. Hence, multiple previous studies recommended the use of more than one marker for prediction, screening, or diagnosis of HCC.19–21
Zhang et al7 reported in their study that a panel of 3 miRNAs when combined with AFP had a higher diagnostic efficacy in the diagnosis of HCC than AFP alone. The current study revealed a significant difference in miR-215 expression levels among the three studied main groups. Besides, our results showed a stepwise increase in the relative expression levels of circulating miR-215 in apparently healthy control subjects (mean±SD=18.4±5.4), LC patients (mean±SD=205.4±143), and HCC patients (mean±SD=1018±600.6). Furthermore, the mean miR-215 values in the present study remain significantly higher in HCC patients subgroup IIIa with AFP levels <200 ng/mL than control and LC groups. This may indicate a stronger role for miR-215 in the diagnosis of HCC with lower AFP levels.22
These results were in concordance with the studies by Gui et al22 and Ali et al23 who reported statistically significant higher serum expression levels of miR-215 in HCC patients compared to both control and LC groups. Yang et al24 reported deregulation of miR-215 levels with micro-vascular invasion or growth advantage in HCC patients, and Mamdouh et al25 identified a panel of three miRNAs including miR-215 that had a high diagnostic accuracy of HCV-associated HCC. Zhang et al26 detected statistically significant upregulated expression of miR-215 in the serum of chronic HCV patients and HCC patients. They reported that serum miR-215 distinguished HCC from healthy individuals with 80% sensitivity and 91% specificity. Thus, miR-215 in Zhang et al study is tumorigenesis, and its overexpression indicates the increased HCC severity.26 Liu et al27 reported that the upregulation of miR-215 leads to an elevation of HBV X (HBx) protein levels and hence the development of HCC. Moreover, Wang et al28 found that cell-free miR-215 expression levels were upregulated in HCC patients and were associated with bad prognosis. In addition, miR-215 expression levels were significantly upregulated in para-formaldehyde-fixed, paraffin-embedded (FFPE) tissues in Egyptian HCC patients in the study by El-Halawany et al.29 In disagreement with our results, Ren et al30 reported that miR-215 inhibits migration and invasion of HCC cells and inhibits HCC growth in vivo. Likewise, many other studies stated that miR-215 has a tumor suppression activity but these studies were detecting miR-215 expression levels in HCC tissues not in patients’ serum.31–34
The abovementioned contradictory results may reflect a dual miRNA tumor suppressor/oncogenic role, even within the same tumor type. Moreover, this variability may refer to the adversative mechanisms that regulate or being regulated by miRNAs expression, which mayinvolve cell proliferation, differentiation, migration, invasion, and angiogenesis pathways.35 The tumor suppressor role of miR-215 was documented in renal cell carcinoma, glioma, and acute myeloid leukemia in addition to gastric, colon, and lung cancers.36–41 In HCC, this suppressor role operates via suppressing expression of the oncogene PCAT-1.30 Conversely, being one of the p53-inducible miRNAs, miR-215 may play its oncogenic role through targeting YY-1, KDM1B, ZEB2, XIAP, and RB1.30,42,43
In agreement with many authors,44,45 our results revealed statistically significant increased serum levels of SCCA-IgM in HCC patients compared to LC and control groups. However, they were studying the unbound SCCA levels (not IgM bound). Moreover, SCCA-IgM-reduced levels in response to treatment spur were shown in Pozzan et al11 study. The Pozzan et al study results are valuable in considering a role of SCCA-IgM in the prognosis of HCC. Also, the present study revealed a significant direct correlation between SCCA-IgM and AFP, which is in agreement with the study by Mossad et al.46 However, Hussein et al44 failed to find a significant correlation between free-SCCA and AFP. Similarly, Soyemi et al47 reported no statistically significant difference between HCC and control groups regarding free SCCA levels, but HCC cases in this study were Nigerian population, most of them had HBV infection as a base underlying HCC.
Our study revealed statistically significant increase in miR-215 expression levels in HCC patients who had bigger tumor size with significant positive correlation between them (r=0.53 and P<0.001). These findings were in agreement with the study by Demerdash et al,48 but they investigated different miRNAs. PVT had been found to be an important bad prognostic indicator associated with worsened hepatic function and high rate of recurrence after ablation.49 Our results demonstrated that both AFP and miR-215 levels were significantly increased in patients with HCC associated with PVT with a strong significant positive correlation between miR-215 expression levels and PVT (r=0.55 and P<0.001). Correlations between PVT and miRNAs were reported in previous studies.49,50 Conversely, the mean serum levels of SCCA-IgM were not correlated with the tumor burden or the presence of PVT. This may indicate that this marker reflects rather the intrinsic biologic activity of the tumor and/or the extent of the patient’s immune response than the extent of neoplastic involvement.
Trying to detect the potential applications of miR-215 and SCCA-IgM in distinguishing HCC apart from LC, we applied ROC curve analysis to identify their diagnostic performances against AFP. The ROC curve analysis showed that AFP level of 117 ng/mL is the best cutoff value that yields best sensitivity and specificity (77% and 81%, respectively) with 75.6% accuracy, which is higher than that of the previously established cutoff value of 200 ng/mL, which showed 30% sensitivity and 100% specificity (PPV, 100%; NPV, 58%; AUC, 0.663; 95% CI, 0.610–0.716) in the study by Jang et al.51 The miR-215 best cutoff value was 417 with 97.14% sensitivity, 91% specificity, and 95.66% accuracy. For HCC with small-sized solitary focal lesion (<3 cm), the ROC curve analysis for AFP showed 63% sensitivity, 87% specificity, and 68.8% accuracy, whereas the ROC curve analysis for miR-215 showed 96% sensitivity, 100% specificity, and 96.88% accuracy (data not shown). Furthermore, the performance of miR-215 has shown a greater AUC in differentiating either all HCC patients or those with only small focal lesions from LC (0.997 and 0.995, respectively) than AFP (0.854 and 0.777, respectively; data not shown). Samie El-Sherif et al52 reported that AFP showed 62% sensitivity, 171 ng/mL cutoff value, and AUC of 0.886 or 0.813, respectively, in differentiating both HCC patients and those with only small focal lesions (2–5 cm) from control subjects. Also, it was reviewed in the study by Loosen et al53 that a panel of seven miRNAs (miR-29a, miR-29c, miR-133a, miR-143, miR-145, miR-192/215, and miR-505) can detect HCC with AFP levels <20 ng/mL, this panel showed superior AUC than AFP in diagnosing small-sized HCC lesion (AUC=0.833 vs 0.727) and early stage ones (AUC=0.824 vs 0.754). In our study, measuring the serum levels of miR-215 either alone or with AFP could have a useful diagnostic tool for surveillance of HCV-related HCC or to differentiate it from HCV-related LC and to identify early hepatic carcinogenesis. Consequently, the increased proportion of patients with early diagnosed HCC may affect improved prognosis.
The data that were exported from previous studies carried on Egyptian population suggested that circulating miR-215 could well differentiate HCC from normal and diseased controls. Mamdouh et al25 reported that circulating miR-143, miR-215, and miR-224 were significantly upregulated in HCV-induced HCC patients and that miR-215 could tell apart between HCC-positive and HCC-negative subjects with cutoff value of 8.137 (AUC, 0.754; sensitivity, 78.66%; specificity, 8.137). Similarly, Ali et al analyzed the diagnostic efficacy of both miR-141 and miR-215 for differentiation among LC, chronic hepatitis C (CHC), and HCC cases. They showed that the AUC of miR-215 for diagnosing hepatic diseases was 0.872. They also found that miR-215 could distinguish between CHC and LC as well as between HCC and other liver diseases with AUC of 0.899 and 0.818, respectively.23 However, the present study had a larger sample size besides the concurrent comparison between the performances of miR-215 and the conventional marker AFP. Conversely, both El-Halawany et al29 and Ashmawy et al31 were concerned about measuring miR-215 expression levels inside hepatocytes not serum cell-free miR-215s.
It was reviewed in the studies by Mishra and Merlino and by Mishra that miRNAs could be detected in section slides. Although tissue-specific miRNAs represent unique identifiers for tumor origin and type, the most prominent advantage would be the high-throughput sequencing of miRNAs in serum as being noninvasive biomarkers.54,55 As a biomarker, circulating miRNAs were reported to be advantageous in stability and high sensitivity.56 Brase et al57 used a panel of miRNAs as an example for the relation of some specific miRNAs with prostate cancer tumor progression. Elemeery et al35 evaluated serum miRNA panel as biomarkers for early diagnosis of HCC in Egyptian patients. They considered this panel to be early biomarkers for tracking the progress of liver fibrosis to HCC. Similarly, in a large patient cohort analysis, Lu et al58 reported that miRNAs expression signature could be used as an HCC prognostic tool.
Furthermore, many studies reported closely related cutoff values for SCCA-IgM using ROC curve. The cutoff values for SCCA-IgM using ROC curve were 95 AU/mL in the current study, 104 AU/mL in the study by Giannelli et al,59 and 89 AU/ mL in the study by Pozzan et al.11 In concordance with other studies, the serum SCCA-IgM showed more diagnostic accuracy with greater AUC, sensitivity, and specificity values than did AFP in HCC patients, discriminating them from those with cirrhosis.11,46 In the study by Buccione et al,60 the best cutoff value for SCCA-IgM was 200 AU/mL with 57% sensitivity and 89% specificity. Mossad et al46 in their cross-sectional study reported a fair significant correlation between SCCA-IgM and AFP (P=0.007 and r=0.284) with higher diagnostic performance for SCCA-IgM (88% sensitivity and 66% specificity) than that for AFP (70% sensitivity and 53% specificity). On the contrary, Soyemi et al47 reported inability of the serum SCCA values to discriminate HCC patients from control subjects (AUC=0.525, sensitivity=75%, and specificity=27%). These conflicting results could be explained by population differences, varied tumor characteristics, diverse marker cutoff values, and dissimilarity in tumor stages. In addition, the diversity between enrolled patients regarding the etiology of their liver diseases or the different mechanisms underlying their carcinogenesis and the multiplicity in the detection methods even for the same biomarker in different studies could play roles.
To the best of our knowledge, there are no published researches that investigated the clinical impact of both cir culating miR-215 and SCCA-IgM together in a head-to-head comparison for the diagnoses of HCV-induced HCC via ROC curve and against AFP. Most of the previous studies focused on miR-215 expression in HCC tissues and cell lines (in vitro) or in animal models (in vivo).27,32 For SCCA, most of the previous studies concentrated on the total SCCA not its IgM bound form. In addition, biomarkers performances may vary depending on the etiology of liver disease. Consequently, the difference in the etiology of LC and HCC in our study from some previous studies (usually on top of HBV infection)27 plus targeting Egyptian populations with their specific genotyping and phenotyping characteristics for HCV-associated liver diseases may add a point of novelty as well as explaining the divergences in results.
The advantages of our study that may increase its clinical significance include the following: 1) comparing both cell-free miR-215 expression levels and SCCA-IgM serum levels with the conventional serum HCC biomarker, AFP; 2) the control subjects include both apparently healthy individuals and non-HCC liver-diseased patients (LC); and 3) the same etiological base (HCV induced) for Egyptian HCC patients and the diseased controls with their own specific viral genotyping and phenotyping will add to the knowledge about Egyptian patients per se and is assumed to avoid results bias. Despite the previous advantages, our study also had several limitations: 1) all the patients belong to one region, which necessitates external validation for the results. This weak point may be considered by others to be a powerful point avoiding results bias depending on the homogenous data for the enrolled subjects whom are subjected to the same circumstances; 2) the serum miR-215 and SCCA-IgM levels were not assessed as serum prognostic or treatment monitoring markers by follow-up; 3) larger sample size studies including more numbers of both apparently healthy and diseased controls are required in prospect research to further expose the predictive values of these markers; 4) the factors affecting the diagnostic performance of the studied biomarkers need specific consideration in large-scale prospective studies. These include tumor invasiveness, the number of focal lesions, tumor differentiation, the HCC histopathological grade, and the TNM stage; and 5) noninclusion of chronic HCV carriers as another subgroup of diseased controls deprived this study from a valuable aspect to start investigating the disease from an earlier point.
Thus, dynamic changes in both serum miR-215 expression levels, SCCA-IgM serum levels, and AFP serum levels during transformation from chronic hepatitis to LC then to HCC could be assessed in future studies. Similar studies regarding other miRNAs could facilitate the molecular subclassification of HCC patients aiming to design a “tailor-made therapy” model that fits each individualized HCC patient according to its corresponding miRNA-based molecular footprint instead of “one-size-fits-all” therapy models.
Conclusion
Our results indicated the applicability of miR-215 and SCCA-IgM as talented noninvasive serum markers for early diagnosis of HCC with enhanced diagnostic performances containing higher specificity and higher sensitivity commands. The role of these improved markers in the prognosis of HCC along with its therapy monitoring and its possible potentials in personalized medicine should be further investigated. Thus, this study could serve as a base for advanced investigations, preferably large-scale validation in clinical trials to validate the use of miR-215 and/or SCCA-IgM either as a single biomarker or in complement measurement with AFP for early diagnosis of HCC. The dynamic changes in both serum miR-215 expression levels and SCCA-IgM serum levels against AFP serum levels during transformation from chronic hepatitis to LC then to HCC could be assessed in prospective studies. Similar studies regarding other miRNAs could facilitate the tumor profiling in a form of molecular subclassification of HCC patients aiming to design their personalized therapy. Hopefully, cell-free miR-215 and serum SCCA-IgM would be one of the biomarkers for HCC “tailor-made therapy” after tumor profiling.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Faculty of Medicine, Minia University, and with the 1964 Declaration of Helsinki and its later amendments. Informed consent was obtained from all individual participants included in the study.
Acknowledgments
The authors would like to express their sincere appreciation to the participants involved in this study.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Bosetti C, Turati F, La Vecchia C. Hepatocellular carcinoma epidemiology. Best Pract Res Clin Gastroenterol. 2014;28(5):753–770. doi: 10.1016/j.bpg.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Center MM, Desantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19(8):1893–1907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 4.Goldman R, Ressom HW, Abdel-Hamid M, et al. Candidate markers for the detection of hepatocellular carcinoma in low-molecular weight fraction of serum. Carcinogenesis. 2007;28(10):2149–2153. doi: 10.1093/carcin/bgm177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134(6):1752–1763. doi: 10.1053/j.gastro.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 6.Molasy M, Walczak A, Szaflik J, Szaflik JP, Majsterek I. MicroR-NAs in glaucoma and neurodegenerative diseases. J Hum Genet. 2017;62(1):105–112. doi: 10.1038/jhg.2016.91. [DOI] [PubMed] [Google Scholar]
- 7.Zhang EL, Gu J, Zhang ZY, Dong KS, Liang BY, Huang ZY. MicroRNA expression profiling in patients with hepatocellular carcinoma of familial aggregation and hepatitis B virus infection. Oncol Lett. 2017;14(1):971–976. doi: 10.3892/ol.2017.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pontisso P, Calabrese F, Benvegnù L, et al. Overexpression of squamous cell carcinoma antigen variants in hepatocellular carcinoma. Br J Cancer. 2004;90(4):833–837. doi: 10.1038/sj.bjc.6601543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuen MF, Tanaka Y, Fong DY, et al. Independent risk factors and predictive score for the development of hepatocellular carcinoma in chronic hepatitis B. J Hepatol. 2009;50(1):80–88. doi: 10.1016/j.jhep.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 10.Trevisani F, De Notariis S, Rapaccini G, et al. Semiannual and annual surveillance of cirrhotic patients for hepatocellular carcinoma: effects on cancer stage and patient survival (Italian experience. Am J Gastroenterol. 2002;97(3):734–744. doi: 10.1111/j.1572-0241.2002.05557.x. [DOI] [PubMed] [Google Scholar]
- 11.Pozzan C, Cardin R, Piciocchi M, et al. Diagnostic and prognostic role of SCCA-IgM serum levels in hepatocellular carcinoma (HCC) J Gastroenterol Hepatol. 2014;29(8):1637–1644. doi: 10.1111/jgh.12576. [DOI] [PubMed] [Google Scholar]
- 12.Tsuchiya N, Sawada Y, Endo I, Saito K, Uemura Y, Nakatsura T. Biomarkers for the early diagnosis of hepatocellular carcinoma. World J Gastroenterol. 2015;21(37):10573–10583. doi: 10.3748/wjg.v21.i37.10573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng YC, Chan CS, Chen GH. The effectiveness of serum alpha-fetoprotein level in anti-HCV positive patients for screening hepatocellular carcinoma. Hepatogastroenterology. 1999;46(30):3208–3211. [PubMed] [Google Scholar]
- 14.Guerra J, Garenne M, Mohamed MK, Fontanet A. HCV burden of infection in Egypt: results from a nationwide survey. J Viral Hepat. 2012;19(8):560–567. doi: 10.1111/j.1365-2893.2011.01576.x. [DOI] [PubMed] [Google Scholar]
- 15.Berry V, Arora R, Paul P. Hepatitis C clinical outcome and diagnosis. J K Sci. 2005;7(3):129–131. [Google Scholar]
- 16.Motawi TK, Shaker OG, El-Maraghy SA, Senousy MA. Serum microRNAs as potential biomarkers for early diagnosis of hepatitis C virus-related hepatocellular carcinoma in Egyptian patients. PLoS One. 2015;10(9):e0137706. doi: 10.1371/journal.pone.0137706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsai JF, Jeng JE, Chuang LY, et al. Serum insulin-like growth factor-II as a serologic marker of small hepatocellular carcinoma. Scand J Gastroenterol. 2005;40(1):68–75. doi: 10.1080/00365520410009311. [DOI] [PubMed] [Google Scholar]
- 18.Imai K, Beppu T, Chikamoto A, et al. Comparison between hepatic resection and radiofrequency ablation as first-line treatment for solitary small-sized hepatocellular carcinoma of 3 cm or less. Hepatol Res. 2013;43(8):853–864. doi: 10.1111/hepr.12035. [DOI] [PubMed] [Google Scholar]
- 19.Zekri AN, Youssef AS, El-Desouky ED, et al. Serum microRNA panels as potential biomarkers for early detection of hepatocellular carcinoma on top of HCV infection. Tumour Biol. 2016;37(9):12273–12286. doi: 10.1007/s13277-016-5097-8. [DOI] [PubMed] [Google Scholar]
- 20.Yang L, Xu Q, Xie H, Gu G, Jiang J. Expression of serum miR-218 in hepatocellular carcinoma and its prognostic significance. Clin Transl Oncol. 2016;18(8):841–847. doi: 10.1007/s12094-015-1447-z. [DOI] [PubMed] [Google Scholar]
- 21.Zuo D, Chen L, Liu X, et al. Combination of miR-125b and miR-27a enhances sensitivity and specificity of AFP-based diagnosis of hepatocellular carcinoma. Tumour Biol. 2016;37(5):6539–6549. doi: 10.1007/s13277-015-4545-1. [DOI] [PubMed] [Google Scholar]
- 22.Gui J, Tian Y, Wen X, et al. Serum microRNA characterization identifies miR-885-5p as a potential marker for detecting liver pathologies. Clin Sci. 2011;120(5):183–193. doi: 10.1042/CS20100297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ali S, Alahmady ZZ, Yamany HA, Abul-Fotouh AM. Serum expression levels of miR-141 and miR-215 for differentiation between liver cirrhosis, chronic hepatitis C and hepatocellular carcinoma patients. Microbiol Res J Int. 2017;20(3) [Google Scholar]
- 24.Yang YM, Lee WH, Lee CG, et al. Gα12 gep oncogene deregulation of p53-responsive microRNAs promotes epithelial-mesenchymal transition of hepatocellular carcinoma. Oncogene. 2015;34(22):2910–2921. doi: 10.1038/onc.2014.218. [DOI] [PubMed] [Google Scholar]
- 25.Mamdouh S, Khorshed F, Aboushousha T, et al. Evaluation of mir-224, mir-215 and mir-143 as serum biomarkers for HCV associated hepatocellular carcinoma. Asian Pac J Cancer Prev. 2017;18(11):3167–3171. doi: 10.22034/APJCP.2017.18.11.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang ZQ, Meng H, Wang N, et al. Serum microRNA 143 and microRNA 215 as potential biomarkers for the diagnosis of chronic hepatitis and hepatocellular carcinoma. Diagn Pathol. 2014;9:135. doi: 10.1186/1746-1596-9-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu F, You X, Chi X, et al. Hepatitis B virus X protein mutant HBxΔ127 promotes proliferation of hepatoma cells through up-regulating miR-215 targeting PTPRT. Biochem Biophys Res Commun. 2014;444(2):128–134. doi: 10.1016/j.bbrc.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Wang L, Wang YM, Xu S, et al. MicroRNA-215 is upregulated by treatment with Adriamycin and leads to the chemoresistance of hepatocellular carcinoma cells and tissues. Mol Med Rep. 2015;12(4):5274–5280. doi: 10.3892/mmr.2015.4012. [DOI] [PubMed] [Google Scholar]
- 29.El-Halawany MS, Ismail HM, Zeeneldin AA, et al. Investigating the pretreatment miRNA expression patterns of advanced hepatocellular carcinoma patients in association with response to TACE treatment. Biomed Res Int. 2015;2015:1–12. doi: 10.1155/2015/649750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ren Y, Shang J, Li J, et al. The long noncoding RNA PCAT-1 links the microRNA miR-215 to oncogene CRKL-mediated signaling in hepatocellular carcinoma. J Biol Chem. 2017;292(43):17939–17949. doi: 10.1074/jbc.M116.773978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ashmawy AM, Elgeshy KM, Abdel Salam ET, et al. Crosstalk between liver-related microRNAs and Wnt/β-catenin pathway in hepatocellular carcinoma patients. Arab J Gastroenterol. 2017;18(3):144–150. doi: 10.1016/j.ajg.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 32.Braconi C, Valeri N, Gasparini P, et al. Hepatitis C virus proteins modulate microRNA expression and chemosensitivity in malignant hepatocytes. Clin Cancer Res. 2010;16(3):957–966. doi: 10.1158/1078-0432.CCR-09-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gong J, Zhang JP, Li B, et al. MicroRNA-125b promotes apoptosis by regulating the expression of Mcl-1, Bcl-w and IL-6R. Oncogene. 2013;32(25):3071–3079. doi: 10.1038/onc.2012.318. [DOI] [PubMed] [Google Scholar]
- 34.Shimizu S, Takehara T, Hikita H, et al. The let-7 family of microRNAs inhibits Bcl-xL expression and potentiates sorafenib-induced apoptosis in human hepatocellular carcinoma. J Hepatol. 2010;52(5):698–704. doi: 10.1016/j.jhep.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 35.Elemeery MN, Badr AN, Mohamed MA, Ghareeb DA. Validation of a serum microRNA panel as biomarkers for early diagnosis of hepatocellular carcinoma post-hepatitis C infection in Egyptian patients. World J Gastroenterol. 2017;23(21):3864–3875. doi: 10.3748/wjg.v23.i21.3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White NM, Khella HW, Grigull J, et al. miRNA profiling in metastatic renal cell carcinoma reveals a tumour-suppressor effect for miR-215. Br J Cancer. 2011;105(11):1741–1749. doi: 10.1038/bjc.2011.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deng Y, Huang Z, Xu Y, et al. MiR-215 modulates gastric cancer cell proliferation by targeting RB1. Cancer Lett. 2014;342(1):27–35. doi: 10.1016/j.canlet.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 38.Ye M, Zhang J, Zhang J, Miao Q, Yao L, Zhang J. Curcumin promotes apoptosis by activating the p53-miR-192-5p/215-XIAP pathway in non-small cell lung cancer. Cancer Lett. 2015;357(1):196–205. doi: 10.1016/j.canlet.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 39.Chen Z, Han S, Huang W, et al. MicroRNA-215 suppresses cell proliferation, migration and invasion of colon cancer by repressing Yin-Yang 1. Biochem Biophys Res Commun. 2016;479(3):482–488. doi: 10.1016/j.bbrc.2016.09.089. [DOI] [PubMed] [Google Scholar]
- 40.Hu J, Sun T, Wang H, et al. MiR-215 is induced post-transcriptionally via HIF-Drosha complex and mediates glioma-initiating cell adaptation to hypoxia by targeting KDM1B. Cancer Cell. 2016;29(1):49–60. doi: 10.1016/j.ccell.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang YX, Zhang TJ, Yang DQ, et al. Reduced miR-215 expression predicts poor prognosis in patients with acute myeloid leukemia. Jpn J Clin Oncol. 2016;46(4):350–356. doi: 10.1093/jjco/hyv204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Braun CJ, Zhang X, Savelyeva I, et al. p53-Responsive micrornas 192 and 215 are capable of inducing cell cycle arrest. Cancer Res. 2008;68(24):10094–10104. doi: 10.1158/0008-5472.CAN-08-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Georges SA, Biery MC, Kim SY, et al. Coordinated regulation of cell cycle transcripts by p53-Inducible microRNAs, miR-192 and miR-215. Cancer Res. 2008;68(24):10105–10112. doi: 10.1158/0008-5472.CAN-08-1846. [DOI] [PubMed] [Google Scholar]
- 44.Hussein MM, Ibrahim AA, Abdella HM, Montasser IF, Hassan MI. Evaluation of serum squamous cell carcinoma antigen as a novel biomarker for diagnosis of hepatocellular carcinoma in Egyptian patients. Indian J Cancer. 2008;45(4):167–172. doi: 10.4103/0019-509x.44666. [DOI] [PubMed] [Google Scholar]
- 45.El Ezawy H, Shebil N, Mounis A. Assessment of serum SCCA and KL-6 as tumor markers and their correlation with tumor size. J Am Sci. 2012:172–179. [Google Scholar]
- 46.Mossad NA, Mahmoud EH, Osman EA, Mahmoud SH, Shousha HI. Evaluation of squamous cell carcinoma antigen-immunoglobulin M complex (SCCA-IGM) and alpha-L-fucosidase (AFU) as novel diagnostic biomarkers for hepatocellular carcinoma. Tumour Biol. 2014;35(11):11559–11564. doi: 10.1007/s13277-014-2467-y. [DOI] [PubMed] [Google Scholar]
- 47.Soyemi OM, Otegbayo JA, Ola SO, Akere A, Soyemi T. Comparative diagnostic efficacy of serum squamous cell carcinoma antigen in hepatocellular carcinoma. BMC Res Notes. 2012;5(1):403. doi: 10.1186/1756-0500-5-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Demerdash HM, Hussien HM, Hassouna E, Arida EA. Detection of microRNA in hepatic cirrhosis and hepatocellular carcinoma in hepatitis C genotype-4 in Egyptian patients. Biomed Res Int. 2017;2017:1–10. doi: 10.1155/2017/1806069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harfoush RAH, Meheissen MA, Elwafa RAHA, Elwazzan DA. The role of circulating microRNAs as markers of disease progression in hepatitis C virus infected Egyptian patients. Adv Microbiol. 2016;6(4):320–331. [Google Scholar]
- 50.Sun JX, Shi J, Li N, et al. Portal vein tumor thrombus is a bottleneck in the treatment of hepatocellular carcinoma. Cancer Biol Med. 2016;13(4):452–458. doi: 10.20892/j.issn.2095-3941.2016.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jang ES, Jeong SH, Kim JW, Choi YS, Leissner P, Brechot C. Diagnostic performance of alpha-fetoprotein, protein induced by vitamin K absence, osteopontin, Dickkopf-1 and its combinations for hepatocellular carcinoma. PLoS One. 2016;11(3):e0151069. doi: 10.1371/journal.pone.0151069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Samie El-Sherif AA, Kamal Eldin AM, Higazi A, Keryakos H, Mohamed HI, Rahman Meshref DA. Diagnostic outcomes of soluble major histocompatibility complex class I related chain molecule A and Des-γ carboxy prothrombin versus alpha-fetoprotein for hepatitis C virus-induced hepatocellular carcinoma in Egyptian patients. Immunome Res. 2016;12(3):2–12. [Google Scholar]
- 53.Loosen SH, Schueller F, Trautwein C, Roy S, Roderburg C. Role of circulating microRNAs in liver diseases. World J Hepatol. 2017;9(12):586–594. doi: 10.4254/wjh.v9.i12.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mishra PJ, Merlino G. MicroRNA reexpression as differentiation therapy in cancer. J Clin Invest. 2009;119(8):2119–2123. doi: 10.1172/JCI40107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mishra PJ. MicroRNAs as promising biomarkers in cancer diagnostics. Biomark Res. 2014;2:19. doi: 10.1186/2050-7771-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sempere LF. Recent advances in miRNA-based diagnostic applications. Expert Rev Mol Diagn. 2012;12(6):557–559. doi: 10.1586/erm.12.47. [DOI] [PubMed] [Google Scholar]
- 57.Brase JC, Johannes M, Schlomm T, et al. Circulating miRNAs are correlated with tumor progression in prostate cancer. Int J Cancer. 2011;128(3):608–616. doi: 10.1002/ijc.25376. [DOI] [PubMed] [Google Scholar]
- 58.Lu M, Kong X, Wang H, Huang G, Ye C, He Z. A novel microRNAs expression signature for hepatocellular carcinoma diagnosis and prognosis. Oncotarget. 2017;8(5):8775–8784. doi: 10.18632/oncotarget.14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Giannelli G, Fransvea E, Trerotoli P, et al. Clinical validation of combined serological biomarkers for improved hepatocellular carcinoma diagnosis in 961 patients. Clin Chim Acta. 2007;383(1–2):147–152. doi: 10.1016/j.cca.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 60.Buccione D, Fatti G, Gallotta A, et al. Serum SCCA-IgM as a predictor of hepatocellular carcinoma in patients with liver cirrhosis. Open J Gastroenterol. 2012;02(02):56–61. [Google Scholar]