Abstract
The Xp22.31 segment of the short arm of the human X chromosome is a region of high instability with frequent rearrangement. The duplication of this region has been found in healthy people as well as in individuals with varying degrees of neurological impairment. The incidence has been reported in a range of 0.4-0.44% of the patients with neurological impairment. Moreover, there is evidence that Xp22.31 duplication may cause a common phenotype including developmental delay, intellectual disability, feeding difficulty, autistic spectrum disorders, hypotonia, seizures, and talipes. We report on a patient with microcephaly and trigonocephaly, moderate intellectual disability, speech and language delay, and poor social interaction in addition to minor but atypical dysmorphic features. This report provides further insight into the pathogenicity of the Xp22.31 duplication by extending knowledge of its clinical features. This case, in association with those reported in the literature, indicates that the Xp22.31 duplication may contribute to cause pathological phenotypes with minor facial dysmorphisms, microcephaly, and intellectual disability as main features.
Keywords: Developmental delay, Microcephaly, Tooth anomalies, Trigonocephaly, Xp22.31 duplication
Established Facts
• The Xp22.31 duplication has been reported in healthy people and in individuals with neurological impairment, including autistic spectrum disorder and intellectual disability, dysmorphic features, and foot anomalies.
Novel Insights
• Reported here is the case of a patient presenting with trigonocephaly and dysmorphic anomalies in addition to the classical features of Xp22.31 duplication - unreported thus far.
The Xp22.31 region of the short arm of the human X chromosome has been noted as being highly unstable and frequently subject to genomic rearrangement [Liu et al., 2011]. The Xp22.31 deletion has been reported in association with X-linked ichthyosis, a dermatologic disorder presenting with dry, scaly skin due to a deficiency of the enzyme steroid sulfatase (STS), usually arising from a mutation in the STS gene [Ballabio et al., 1987; Sitek et al., 2018]. Deletions of this region can also involve the KALIG1 gene, which is associated with a disorder manifesting with anosmia and hypogonadotropic hypogonadism called Kallman syndrome [Bick et al., 1992; Niu et al., 2018].
Duplication of the Xp22.31 has been reported in the healthy general population but also been described in individuals with pathological conditions. Therefore, it remains unclear whether this mutation is unrelated to a clinical disorder [Furrow et al., 2011] or potentially has some pathological involvement [Shaw-Smith et al., 2004].
In support of this mutation being a benign variant, there are reports of unaffected carriers with no apparent anomalies, for example in the parents of affected siblings who also manifest the same mutation. Again, variable expressivity has been shown in some cases but without clinical evidence of specific diagnostic markers [Horn et al., 2007; Scharer et al., 2008]. The possible pathological role of the Xp22.31 duplication is supported by studies performed using genetic surveys. In a large cohort of 7,793 samples carried out using array CGH and SNP array, Li et al. [2010] found this mutation in the healthy control population with a frequency of 0.15% compared to a frequency of 0.37% reported in individuals with abnormal phenotypes. The incidence of Xp22.31 duplication was seen in 0.44% of patients referred due to neurobehavioral phenotypes by Liu et al. [2011] in comparison with 0.41% reported in healthy controls. Faletra et al. [2012] report an incidence of 0.4% in patients with neurological disturbances in contrast to no cases observed among the 2,055 healthy individuals included in that study. A definite answer on the causative effect of Xp22.31 remains elusive, yet there is clinical evidence that the Xp22.31 mutation might have a pathogenic effect.
Here, we report on a 7.5-year-old boy with microcephaly and trigonocephaly, with moderate intellectual disability (ID), autism spectrum disorder (ASD), behavioral disturbances, minor facial dysmorphisms, and anomalies of the teeth and toes, features that are in line with and also expand the clinical expression of the patients with Xp22.31 microduplication.
Case Report
A 5.5-year-old boy was first admitted to the University Hospital “Policlinico Vittorio - Emanuele,” Catania, Italy, for diagnostic evaluation for his poor scholastic performance. He is the third child of healthy, unrelated Italian parents. His 14-year-old sister and 12-year-old brother are healthy and of weight, height and OFC within normal limits. At the time of gestation, the mother was 32 and the father was 36 years old: their OFCs are within the normal range and measure 55 cm and 57 cm, respectively. During the first 5 months, the mother was unaware of the pregnancy and was treated with oral contraceptives in response to a previous surgical operation for ovarian cysts. The mother reported having had a spontaneous miscarriage during the 5th month of a previous pregnancy. No infectious diseases were referred, nor were alcohol and illegal drugs consumed by the mother during the pregnancy. She also reported feeling fetal movements as expected. Fetal ultrasound revealed no anomalies with the exception of a small biparietal diameter. The child was born at 36 weeks of gestation by cesarean section. His birth weight was 2,750 g (10th percentile), height 50 cm (50th percentile), and OFC 32 cm (>3rd percentile). The Apgar scores were 7/9 at 1 and 5 min, respectively. Due to mild respiratory distress, the child was subjected to minor resuscitation and was ventilated by artificial manual breathing. The motor development milestones were reached as expected, whereas the speech development was delayed, starting at 16 months and using complete phrases at the age of 4 years. His scholastic performance was quite poor with teachers complaining about his immature behavior. A school teacher's aide was requested.
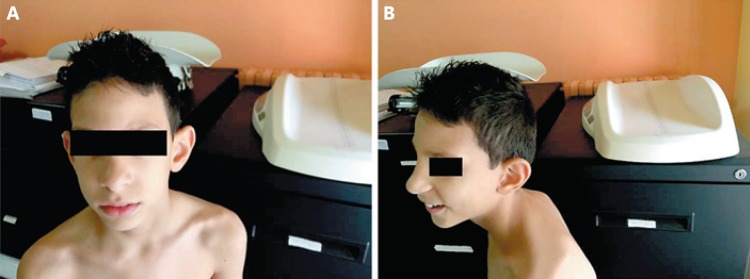
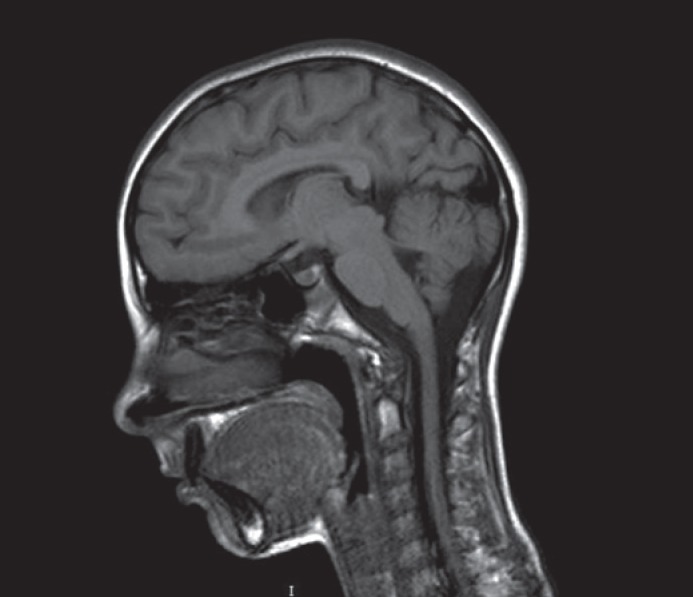
At the time of his first admission, the child presented with pleasant behavior, a nasal voice, and poor language. He seemed friendly but aloof and suffered from encopresis. At the physical examination, his weight was 20 kg (50th percentile), height 118 cm (90th percentile), and OFC 45 cm (>3 SD). Microcephaly and trigonocephaly were noticed in addition to facial dysmorphisms including a narrow, rectangular face shape with a protruding metopic suture. The forehead was high and narrow, the medial eyebrows sparse, palpebral fissures horizontally disposed, nasal tip rounded, philtrum flat, columnella protruding, upper lips thin, and retrognathia (Fig. 1A). To underline, the maxillary central incisors were long and wide, and the 3rd and 4th toes were shortened. Ears were protruding and low set (Fig. 1B). The Wechsler Intelligence Scale for Children - Third Edition (WISC-III) revealed an IQ of 48 (n.v. 80-120). Heart, thorax, and genital organs were normal. Routine laboratory analyses, including hemogram, electrolyte levels, plasma and urine amino acids, thyroid hormones, organic acids, plasma purines, and total cholesterol were within the normal ranges. Brain MRI indicated a small brain with a smooth cortex and increased surrounding cerebrospinal fluid (Fig. 2). EEG, ophthalmologic examination, and chest X-ray were normal. Color Doppler echocardiography displayed a middle-grade insufficiency of the tricuspid valve.
Fig. 1.
A, B The child at 5.5 years old showing a high and narrow forehead, rounded nasal tip, low-set ears, and retrognathia.
Fig. 2.
Brain MRI at the age of 5.5 years. Note the small brain with a smooth cortex.
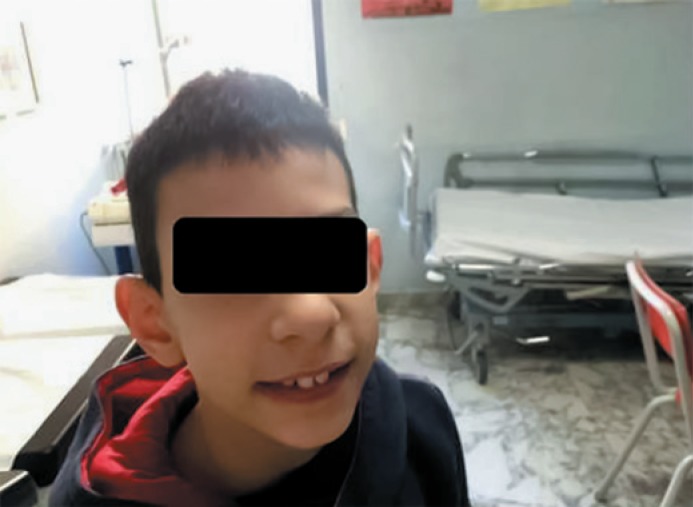
At the 2-year follow-up, the physical examination was unchanged (Fig. 3). At the age of 6.5 years, his weight was 22 kg (50th percentile), height 127 cm (90th percentile), and OFC 45 cm (>3rd percentile). At 7.5 years of age, his weight was 26 kg (75th percentile), height 130 cm (90th percentile), and OFC 45 cm (>3rd percentile). His scholastic performance remains insufficient, the child needs scholastic support, complains of encopresis, and his language is extremely limited.
Fig. 3.
The child at the age of 7.5 years of age with long and wide maxillary central incisors.
Materials and Methods
Genetic Testing
Proband DNA was labeled with Cy5 and control (human DNA-Promega) Cy3 was employed. A Human Genome CGH Microarray Kit 8 × 60K (Agilent), and CytoGenomics analysis software (genome assembly UCSC hg19), quality score (DLRS) <0.25 were used. The following analysis parameters were applied: 5 consecutive probes, algorithm threshold of 6.0., resolution 100-200 kb: ADM-2.
Results
Array-CGH analysis revealed the following rearrangement: arr[hg 19] Xp22.31(6,552,712-8,097,511)×2. This area includes 4 genes (HDHD1, STS, VCX, and PNPLA4). The rearrangement is a partial duplication of the short arm of chromosome X and extends for 1.5 Mb. Maternal array-CGH analysis displayed no CNVs. Paternal array CGH was not available.
Discussion
The child reported here presented with a variety of clinical anomalies including microcephaly and trigonocephaly, moderate ID with an IQ of 48, severe language impairment, poor social interaction, and minor facial dysmorphisms with long and wide maxillary central incisors. The child showed insufficient scholastic performance, behavioral disturbances, encopresis, and difficulties eating alone. He was aloof but friendly and endearing. Array CGH revealed a partial duplication of the short arm of the X chromosome extending 1.5 Mb (6,552,712-8,097,511). No CNVs were reported by the array-CGH analysis carried out in his mother.
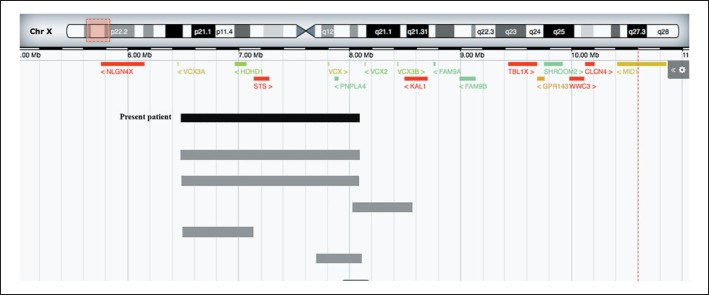
In Figure 4, the breakpoints and the genomic location in Xp22.31 from distal (Xpter) to proximal (X cen) of the X chromosome and the OMIM genes are shown. The region duplicated in Xp22.31 includes the following genes: HDHDIA, STS, VCX, PNPLA4, and KAL1 or ANOS1. HDHDIA encodes a pseudouridine 5′ phosphatase with unknown biological function. The STS gene encodes steroid sulfatase, an enzyme that hydrolyzes metabolic precursors for steroid acting on neurophysiological function. Duplication of the STS region in males has been reported as a benign finding [Furrow et al., 2011] and not causative of phenotype anomalies [Shaw-Smith et al., 2004], in contrast to what has been reported by Li et al. [2010] in a sample population study of 7,793 patients referred for neurological problems and multiple congenital anomalies in whom STS duplication was found in 29 patients. PNPLA4 encodes a member of the putative-like family of phospholipases. The encoded enzyme has triacylglycerol lipase and transacylase activities and has been involved in adipocyte triply-acid homeostasis and linked to human obesity. VCX2 encodes small and highly charged protein with unknown function and has been involved in human obesity [Steinberg et al., 2007]. KAL1 or ANOS1 encodes for a protein anosmin 1 involved in the migration of the neuroendocrine GnRH cells during embryogenesis [Shaw-Smith et al., 2004; Steinberg et al., 2007; Sitek et al., 2018].
Fig. 4.
The breakpoints and the genomic location in Xp22.31 from distal (Xpter) to proximal (X cen) are shown, and the OMIM genes are listed. Gray bars represent patients from the literature.
Patients with Xp22.31 have been reported in the literature both as healthy individuals, as children and adults with neurological impairment, and less frequently, as having variable dysmorphic features (Table 1). The most frequently reported neurological involvement consists of ASD, developmental delay (DD) or ID, which is present in about 50% of the reported cases. Three large cohorts of patients with Xp22.31 duplication have been reported in this decade. Li et al. [2010] described 35 individuals including 12 patients from the literature: DD/ID was reported in 24/35 (69%), hypotonia in 7/35 (20%), and seizures in 4/35 (11%). Moreover, abnormal behavior was reported in 13 (37%) and ASD in 9 out of 13 patients. Microcephaly was also reported in 4 out of 11 individuals. Variable dysmorphic features were reported in a few patients. Liu et al. [2011] reported on 14 individuals with this mutation: 9 (64%) manifested with DD/ID, 7 (50%) with ASD, 4 (28%) with hypotonia, and 2 (14%) with seizures. Other features from that study included 5 patients presenting with an abnormal head circumference, 4 with macrocephaly, and 1 with microcephaly. Esplin et al. [2014] reported on 9 patients: DD/ID was observed in all the patients, seizures in 4 (44%), talipes in 3 (33%), and feeding difficulties, ASD, and hypotonia in 2 patients, respectively. A single report from Faletra et al. [2011] described a patient with dysmorphic features and neurological involvement including DD/ID, talipes, and hypotonia.
Table 1.
Xp22.31 clinical features
| Features | Li et al., 2010 | Liu et al., 2011 | Faletra et al., 2012 | Esplin et al., 2014 | Present case |
|---|---|---|---|---|---|
| Hypotonia | 7/35 | 4/14 | 1/1 | 2/9 | 1/1 |
| DD/ID | 24/35 | 9/14 | 1/1 | 9/9 | 1/1 |
| Seizures | 4/35 | 2/14 | – | 4/9 | – |
| ASD | 9/13 | 7/14 | – | 2/9 | 1/1 |
| Microcephaly | 4/11 | 1/14 | – | – | 1/1 |
| Macrocephaly | – | 4/14 | – | – | – |
| Special features | – | – | Talipes, 1/1 | Talipes, 3/9 | Trigonocephaly, wide and high incisors, shortened 3rd and 4th toes |
ASD, autism spectrum disorder; DD/ID, developmental delay/intellectual disability.
The pathogenic role of this duplication is not clear and the clinical expression of the reported cases not always homogeneous [van Bon et al., 2009]. The modalities of presentation in individuals with Xp22.31 duplication might be explained by variable expressivity, decreased penetrance, and skewed X-inactivation [Scharer et al., 2008; Preumont et al., 2010; Niu et al., 2018]. All of these hypotheses need to be further confirmed. Additionally, the duplication might not act directly, but rather predispose the patients to the action of other factors that cause the clinical features [Seminara et al., 2000; Davies et al., 2009; Jiao et al., 2009]. However, the observation of talipes anomalies reported in 3 patients in association with the patient with talipes collected by Faletra et al. [2011] together with common neurological impairment have led Esplin et al. [2014] to recognize a classical, common phenotype for patients affected by the Xp22.31 duplication, including DD/ID, feeding difficulties, ASD, hypotonia, seizures, and talipes. The patient described here extends the spectrum of the anomalies reported by Esplin et al. [2014] with further notable signs such as microcephaly/trigonocephaly and 2 unreported anomalies, long and wide maxillary central incisors and shortened 3rd and 4th toes.
In this study, our patient provides further knowledge on the potentially pathogenic role of the Xp22.31 duplication and adds new impressive clinical signs. The role of the CNVs to directly cause these anomalies remains unclear, but the effect of genomic modifiers as determining factors seems highly probable.
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
The authors have no conflicts of interest to declare.
Acknowledgment
We wish to thank the American Manuscript Editors (USA) for editing the manuscript.
References
- 1.Ballabio A, Parenti G, Carrozzo R, Sebastio G, Andria G, et al. Isolation and characterization of a steroid sulfatase cDNA clone: genomic deletions in patients with X-chromosome-linked ichthyosis. Proc Natl Acad Sci USA. 1987;84:4519–4523. doi: 10.1073/pnas.84.13.4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bick D, Franco B, Sherins RJ, Heye B, Pike L, et al. Brief report: intragenic deletion of the KALIG-1 gene in Kallmann's syndrome. N Engl J Med. 1992;326:1752–1755. doi: 10.1056/NEJM199206253262606. [DOI] [PubMed] [Google Scholar]
- 3.Davies W, Humby T, Kong W, Otter T, Burgoyne PS, Wilkinson LS. Converging pharmacological and genetic evidence indicates a role for steroid sulfatase in attention. Biol Psychiatry. 2009;66:360–367. doi: 10.1016/j.biopsych.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esplin ED, Li B, Slavotinek A, Novelli A, Battaglia A, et al. Nine patients with Xp22.31 microduplication, cognitive deficits, seizures, and talipes anomalies. Am J Med Genet A. 2014;164A:2097–2103. doi: 10.1002/ajmg.a.36598. [DOI] [PubMed] [Google Scholar]
- 5.Faletra F, D'Adamo AP, Santa Rocca M, Carrozzi M, Perrone MD, et al. Does the 1.5 Mb microduplication in chromosome band Xp22.31 have a pathogenetic role? New contribution and a review of the literature. Am. 2012;J Med Genet A 158A:461–464. doi: 10.1002/ajmg.a.34398. [DOI] [PubMed] [Google Scholar]
- 6.Furrow A, Theisen A, Velsher L, Bawle EV, Sastry S, et al. Duplication of the STS region in males is a benign copy-number variant. Am. 2011;J Med Genet A 155A:1972–1975. doi: 10.1002/ajmg.a.33985. [DOI] [PubMed] [Google Scholar]
- 7.Horn D, Spranger S, Kruger G, Wagenstaller J, Weschke B, et al. Microdeletions and microduplications affecting the STS gene at Xp22.31 are associated with a distinct phenotypic spectrum. Medizinische Genetik. 2007;19:62. [Google Scholar]
- 8.Jiao X, Chen H, Chen J, Herrup K, Firestein BL, Kiledjian M. Modulation of neuritogenesis by a protein implicated in X-linked mental retardation. J Neurosci. 2009;29:12419–12427. doi: 10.1523/JNEUROSCI.5954-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li F, Shen Y, Kohler U, Sharkey FH, Menon D, et al. Interstitial microduplication of Kp22.31: causative of intellectual disability or benign copy number variant? Eur J Med Genet. 2010;53:93–99. doi: 10.1016/j.ejmg.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Liu P, Erez A, Nagamani SC, Bi W, Carvalho CM, et al. Copy number gain at Xp22.31 includes complex duplication rearrangements and recurrent triplications. Hum Mol Genet. 2011;20:1975–1988. doi: 10.1093/hmg/ddr078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niu Y, Zhou C, Xu H, Wang D, Chen Y, et al. Novel interstitial deletion in Xp22.3 in a typical X-linked recessive family with Kallmann syndrome. Andrologia. 2018 doi: 10.1111/and.12961. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 12.Preumont A, Rzem R, Vertommen D, van Schaftingen E. HDHD1, which is often deleted in X-linked ichthyosis, encodes a pseudouridine-5′-phosphatase. Biochem J. 2010;431:237–244. doi: 10.1042/BJ20100174. [DOI] [PubMed] [Google Scholar]
- 13.Scharer G, Manchester D, Bellus G, Pickler L, Saenz M, et al. Copy number variations (CNVs) in well-defined region of Xp22.31 are associated with syndromic phenotypes and intellectual disability. Further delineation of a new microdeletion/duplication syndrome. Am S Hum Genet. 2008;58:PI36. [Google Scholar]
- 14.Seminara SB, Oliveira LM, Beranova M, Hayes FJ, Crowley WF., Jr Genetics of hypogonadotropic hypogonadism. J Endocrinol Invest. 2000;23:560–565. doi: 10.1007/BF03343776. [DOI] [PubMed] [Google Scholar]
- 15.Shaw-Smith C, Redon R, Rickman L, Rio M, Willatt L, et al. Microarray based comparative genomic hybridisation (array-CGH) detects submicroscopic chromosomal deletions and duplications in patients with learning disability/mental retardation and dysmorphic features. J Med Genet. 2004;41:241–248. doi: 10.1136/jmg.2003.017731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sitek JC, Kulseth MA, Rypdal KB, Skodje T, Sheng Y, Retterstøl L. Whole-exome sequencing for diagnosis of hereditary ichthyosis. J Eur Acad Dermatol Venereol. 2018;32:1022–1027. doi: 10.1111/jdv.14870. [DOI] [PubMed] [Google Scholar]
- 17.Steinberg GR, Kemp BE, Watt MJ. Adipocyte triglyceride lipase expression in human obesity. Am J Physiol Endocrinol Metab. 2007;293:E958–E964. doi: 10.1152/ajpendo.00235.2007. [DOI] [PubMed] [Google Scholar]
- 18.van Bon BW, Mefford HC, Menten B, Koolen DA, Sharp AJ. Further delineation of the 15q13 microdeletion and duplication syndromes: a clinical spectrum varying from non-pathogenic to a severe outcome. J Med Genet. 2009;46:511–523. doi: 10.1136/jmg.2008.063412. [DOI] [PMC free article] [PubMed] [Google Scholar]