Abstract
Romosozumab, a specific inhibitor of sclerostin, is a unique approach to therapy for postmenopausal osteoporosis and related disorders. The elucidation of sclerostin deficiency as the molecular defect of syndromes of high bone mass with normal quality, and the pivotal role of sclerostin as a mediator of osteoblastic activity and bone formation, provided the platform for the evaluation of inhibitors of sclerostin to activate bone formation. An extensive preclinical program and 2 large fracture endpoint trials with romosozumab, a sclerostin-binding antibody, have been completed. This review will highlight the results of those studies and describe the current status of romosozumab as a potential therapy for osteoporosis.
Keywords: Romosozumab, Sclerostin, Osteoporosis, Fracture risk, Manuscript category
1. Introduction
Osteoporosis is a disorder of increased fracture risk characterized by low bone mass and microarchitectural deterioration of the trabecular and cortical skeletal envelopes [1]. To restore the damaged and disconnected trabecular architecture will require strategies to stimulate new bone formation. The most commonly used treatments for osteoporosis, however, are antiremodeling drugs that decrease bone formation as well as bone resorption, precluding their ability to restore skeletal architecture or to cure osteoporosis. Parathyroid hormone analogues do increase bone formation but also activate bone resorption, limiting the anabolic or bone forming response.
The discovery of sclerostin as a key inhibitor of bone formation was made by groups evaluating patients with 2 rare autosomal recessive syndromes associated with high bone mass [2]. Sclerostiosis is a disorder characterized by very high bone mass due to inactivating mutations of the SOST gene on chromosome 17q21, the gene that codes for sclerostin. Excess bone growth during childhood results in frontal bossing, cranial and basilar stenosis, cranial nerve entrapment, and mandibular hypertrophy. Patients with Van Buchem disease, a somewhat less severe disorder, have a separate noncoding deletion of a gene required for normal transcription of the SOST gene. Heterozygous cases of both disorders have moderately high bone mass without other phenotypic or clinical features. Sclerostin is most highly expressed in osteocytes. Binding of sclerostin to low-density lipoprotein receptor-related proteins 5 and 6 (LRP5 and LRP6) prevents activation of canonical Wnt signaling in bone, resulting in decreased bone formation. These findings stimulated interest in exploring the potential of antisclerostin therapy as a strategy to increase bone formation and to restore skeletal architecture in patients with osteoporosis.
2. Preclinical studies
Genetic deficiency of sclerostin in rodents is associated with high bone mass, increased bone formation in both trabecular and cortical bone, normal bone quality and increased bone strength, recapitulating the high bone mass syndrome of sclerostiosis [3]. The mineralization of the bone matrix in sclerostin-deficient animals is normal or reduced, accounting for the lack of bone brittleness seen in patients with osteopetrosis due to osteoclast deficiency or dysfunction.
Inhibition of sclerostin by monoclonal antibodies in rats and monkeys resulted in robust anabolic responses on trabecular, endocortical, intracortical and periosteal bone surfaces [4]. In aged, ovariectomized rats, antisclerostin therapy increased trabecular and cortical bone thickness and reduced cortical porosity. After 5 weeks of treatment, the skeletal abnormalities induced by ovariectomy were corrected, and bone mass and bone strength exceeded the sham-operated control animals. In gonad-intact female cynomolgus monkeys, treatment with a humanized antisclerostin antibody for 2 months transiently increased markers of bone formation and induced anabolic responses on all skeletal surfaces. Bone mineral density (BMD) in the lumbar spine (LS), femoral neck, proximal tibia, and distal radius increased significantly, correlated with a substantial increase in LS and femoral diaphyseal bone strength [5].
The skeletal response to antisclerostin therapy in old mice was similar to that observed in younger animals, important since osteoporosis is primarily a disorder of older men and women [4]. The anabolic response to antisclerostin therapy was restored upon retreatment following a short treatment free interval. Following antisclerostin therapy with an inhibitor of RANK ligand, a potent antiremodeling agent, preserved or amplified the gain in bone mass achieved with the antisclerostin therapy. The skeletal response to antisclerostin therapy was not blunted in animals pre-treated with bisphosphonates.
3. Clinical studies
Single and multiple dose phase 1 studies (ClinicalTrials.gov Identifiers: NCT01059435 and NCT01825785) with romosozumab (originally known as AMG 785/CDP7851) in healthy men and women demonstrated a brisk increase in biochemical indices of bone formation accompanied by a decrease in markers of bone resorption [6,7]. These divergent effects of romosozumab on bone formation and bone resorption are very distinct from the reductions in both resorption and formation by antiremodeling agents and the increases in both components of the remodeling cycle by teriparatide and abaloparatide [8]. BMD values, measured by dual-energy X-ray absorptiometry in the LS and total hip (TH), increased by 5.2% and 1.1%, respectively, when measured 85 days after the single-dose. Similar results were observed in the ascending multiple dose study [6]. Romosozumab was administered by subcutaneous (SQ) injections of 1 or 2 mg/kg every 2 weeks (Q2W) or 2 or 3 mg/kg every 4 weeks for 3 months. The biochemical marker responses to the injections were maintained during the first 2 months of dosing but were somewhat blunted following the final dose compared to the initial dose. Pharmacokinetics of romosozumab were similar in men and women.
In a placebo-controlled phase 1b study (ClinicalTrials.gov Identifier: NCT01825785), the effects of romosozumab on volumetric BMD (vBMD) and bone structure were assessed by high resolution quantitative computed tomography (HR-QCT) scans of the LS in 48 subjects (32 women, 16 men) with low bone mass who received active treatment with doses ranging from 1–3 mg/kg Q2W for 3 months, followed by no therapy for an additional 3 months [9]. At 3 months, HR-QCT assessments of trabecular BMD and stiffness increased by 9.5% and 26.9%, respectively, and were significantly greater than the changes in the placebo group (−3.0% and −2.7%, respectively). These improvements were maintained during the 3-month off-treatment follow-up period.
An international phase 2 dose-ranging study (ClinicalTrials.gov Identifier: NCT00896532) assessed responses to romosozumab treatment in 419 postmenopausal women with low bone mass [10]. The patients, ages 55–85, were randomly assigned to receive monthly SQ doses of romosozumab (70, 140, or 210 mg) or doses of 140 mg or 210 mg every 3 months, or placebo injections [10]. Other patients were randomly assigned to receive open label alendronate 70 mg once weekly (QW) or teriparatide 20 μg SQ daily. As seen in the phase 1 studies, romosozumab therapy resulted in rapid and substantial increases in serum bone formation markers (serum P1NP and alkaline phosphatase [AP]) but a decrease in the bone resorption marker serum β-CTX. Serum P1NP values peaked at 4 weeks, returned to baseline between 3 and 6 months and were below baseline for the remainder of the treatment interval. All doses of romosozumab increased BMD at both the spine and proximal femur. The largest increases at 12 months were observed with romosozumab 210 mg once monthly (QM), the dose chosen for phase 3 studies. BMD in the LS and TH had increased by 11.3% and 4.1%, respectively, and these gains were significantly greater than with teriparatide or alendronate. During the second year of the study, markers of bone formation and resorption remained below baseline in women who continued romosozumab (McClung MR et al. J Bone Miner Res, 2014;29[Supp. 1] oral presentation 1152). Consistent with the lack of anabolic effect demonstrated by bone markers during the second year of romosozumab therapy, smaller increases in BMD occurred than had been observed during the first year. After 2 years, romosozumab therapy was discontinued. In patients randomly switched to placebo for 12 months, BMD values in the spine and hip returned to or toward baseline values. Serum β-CTX values rose above baseline before returning toward pretreatment values while markers of bone formation gradually returned to baseline values. In patients who were switched to denosumab 60 mg SQ every 6 months (Q6M), BMD increased in a fashion similar to the increases during the second year of romosozumab therapy.
In a similar phase 2 study, 252 postmenopausal Japanese women with osteoporosis received romosozumab in doses of 70, 140, and 210 mg QM or placebo (ClinicalTrials.gov Identifier: NCT01101061) [11]. All doses resulted in significant gains in BMD at the LS and proximal femur compared to baseline and to placebo. At 12 months with the 210 mg QM dose, the average gains were 16.9% and 4.7% in the LS and TH, respectively. Changes in serum markers of bone turnover were similar to those observed in the international phase 2 study.
In a subset of patients from the international phase 2 study, areal and vBMD of the LS and TH was assessed by quantitative computed tomography (QCT) in patients who received placebo (n = 27), teriparatide 20 μg daily (n = 31) or romosozumab 210 mg QM (n = 24) for 12 months [12] (Table 1). DXA BMD increased 12.3% in the LS and 3.9% in TH with romosozumab compared to 6.9% and 0.8%, respectively, with teriparatide. Gains in both vBMD and estimated strength, assessed by finite element analysis, of the hip and spine were significantly greater with romosozumab than with teriparatide [13]. The skeletal effects of romosozumab have also been compared with teriparatide in a randomized but open label phase 3 study (ClinicalTrials.gov Identifier: NCT01796301) in 436 postmenopausal women with osteoporosis who had previously taken bisphosphonates for at least three years (mean duration 5.6 years) [14]. After 12 months of therapy, TH areal BMD by DXA increased by 2.6% (95% confidence interval [CI], 2.2–3.0) in the romosozumab group while a decrease by 0.6% (95% CI, −1.0 to −0.2) was observed in the women who received teriparatide. Integral vBMD of the hip, based on QCT analysis, increased more in the romosozumab group, while trabecular vBMD increased similarly in both treatment groups. The decrease in cortical vBMD of the hip observed with teriparatide (−3.6%) was not observed with romosozumab (+1.1%). QCT-derived estimates of hip bone strength increased significantly more with romosozumab at 12 months (2.5%) than with teriparatide (−0.7%).
Table 1.
Comparison of changes in areal and volumetric BMD and in estimated bone strength over 12 months of therapy with romosozumab and teriparatide.
| Reference | Romosozumab 210 mg QM | Teriparatide 20 μg/d | |
|---|---|---|---|
| Areal BMD (DXA) | |||
| Lumbar spine | [12] | 12.3%a | 6.9% |
| Total hip | 3.9%a | 0.8% | |
| Integral volumetric BMD (QCT) | |||
| Lumbar spine | [12] | 17.7%a | 12.9% |
| Total hip | 4.1%a | 1.2% | |
| Estimated bone strength (FEA by QCT) | |||
| Lumbar spine | [13] | 27.3%a | 18.5% |
| Total hip | 3.6%a | −0.7% | |
BMD, bone mineral density; QM, once monthly; DXA, dual-energy X-ray absorptiometry; QCT, quantitative computed tomography; FEA, finite element analysis.
p ≤ 0.05 vs. teriparatide.
Two phase 3 studies have evaluated the effects of romosozumab on fracture risk reduction. The Fracture Study in Postmenopausal Women with Osteoporosis (FRAME) (ClinicalTrials.gov Identifier: NCT01575834) is an international, randomized, double blind study of 7180 women, average age 71 years, who received either romosozumab 210 mg QM or placebo for 12 months followed then by an additional 12 months of open label therapy with denosumab 60 mg Q6M in both treatment groups [15]. During the first 12 months of therapy, romosozumab reduced the incidence of new vertebral fractures by 73% (0.5% vs. 1.8% with placebo). Among those women with spinal x-rays available for analysis, vertebral fractures occurred in 16 of 3321 patients in the romosozumab group and in 59 of 3322 patients in the placebo group. Romosozumab therapy significantly reduced clinical fracture risk by 36% at 12 months and nonvertebral fracture risk by 25% compared to placebo, but the reduction in non-vertebral fractures was not statistically significant (adjusted p = 0.10).
There was a difference in nonvertebral fracture response according to the location of the study sites. In a preplanned subgroup analysis, a significant interaction of nonvertebral fracture risk reduction with geography was observed [15]. In Latin American study sites, the risk of nonvertebral fracture was much lower than in other parts of the world, and there was no effect of romosozumab therapy on nonvertebral fracture risk. In a post hoc analysis, nonvertebral fracture risk was significantly reduced after 12 months of therapy by 42% in all study sites after excluding those from Latin America. During the year of open label denosumab therapy, 80% fewer women who had received romosozumab (5) during year 1 had vertebral fractures than in the group who had taken placebo (25) during the first year.
The Active-Controlled Fracture Study in Postmenopausal Women with Osteoporosis at High Risk (ARCH) (ClinicalTrials.gov Identifier: NCT01631214) compared the effects of SQ romosozumab 210 mg QM with oral alendronate 70 mg QW for 12 months, followed by open label alendronate therapy in both treatment groups for up to an additional 2 years [16]. A total of 4093 women were enrolled in the study. These women were selected to be at much higher risk of fracture than were the women in the placebo-controlled FRAME study. There were two sets of entry criteria for ARCH: (1) a BMD T-score of −2.5 or less at the TH or femoral neck and either one or more moderate or severe vertebral fractures or two or more mild vertebral fractures; or (2) a bone mineral density T-score of −2.0 or less at the TH or femoral neck and either two or more moderate or severe vertebral fractures or a fracture of the proximal femur that had occurred 3–24 months before randomization. The average age of the study participants was 74.3 years, and more than half of the women were age 75 or older. The average T-score values in the LS and TH were −2.96 and −2.80, respectively. Ninety-nine percent of the women had a history of a fragility fracture since age 45, including 96.1% of women with an adjudicated vertebral fracture at baseline. The primary endpoints of the study were the reduction in vertebral fracture incidence at 12 and 24 months and the cumulative incidence of clinical fractures (nonvertebral and symptomatic vertebral fracture) at the time of the primary analysis (when clinical fracture had been confirmed in at least 330 patients and all the patients had completed the month 24 visits). Secondary endpoints included nonvertebral and hip fracture risk reduction at the primary analysis.
After 12 months of therapy, new vertebral fractures occurred in 4.0% of the women who received romosozumab and in 6.1% of alendronate-treated women, resulting in a relative reduction in vertebral fracture risk, compared to alendronate, of 37% (p = 0.003). Clinical fracture risk was reduced by 28% (hazard ratio [HR], 0.72; 95% CI, 0.54–0.96) with romosozumab compared to alendronate. Similarly, nonvertebral fracture risk was 26% lower with romosozumab at 12 months, but this did not quite achieve statistical significance (p = 0.06). BMD at the LS increased by 13.7% after 12 months of romosozumab treatment compared to 5.0% with alendronate. Likewise, the increase in TH BMD was greater with romosozumab (6.2%) vs. alendronate (2.8%).
The benefit of romosozumab on vertebral fracture risk continued during the second year of the study during which all women received alendronate therapy. Compared to the group that received alendronate during year 1, vertebral fracture risk was 48% lower (6.2% vs. 11.9%) in the women who had taken romosozumab. During that second year, 45 women who had taken romosozumab had new vertebral fractures while receiving alendronate vs. 115 women who took alendronate in both years 1 and 2. BMD increased similarly in both treatment groups during year 2, with total increases over 2 years of 15.2% and 7.1% in the LS and TH, respectively, in women who received romosozumab followed by alendronate.
In the primary analysis, clinical fracture risk was reduced by 27% (9.7% vs. 13.0%; HR, 0.73; 95% CI, 0.61–0.88; p < 0.001) with romosozumab followed by alendronate (9.7%) compared to alendronate alone (13.0%). Additionally, in the romosozumab-alendronate group, nonvertebral fracture risk was significantly reduced by 19% (p = 0.04) as was hip fracture risk (38% lower, p = 0.02).
4. Safety
In the clinical trials, romosozumab has been well tolerated. Mild injection site reactions were reported in 4.4%–5.2% of participants receiving romosozumab 210 mg QM vs. 2.6%–2.9% of controls [15,16]. One patient in a phase 1 romosozumab study developed transient, symptomatic elevation of serum transaminase [7], but alterations in liver function have not been generally observed with romosozumab therapy. Mild, transient and asymptomatic reductions in serum calcium with the expected reciprocal increases in PTH occurred infrequently with the higher doses in the phase 2 study [10]. Patients with vitamin D deficiency were excluded from the FRAME and ARCH studies, and a loading dose of 50,000 to 60,000 IU of vitamin D was given at the beginning of the trial to participants whose baseline serum 25-hydroxyvitamin D level was 40 ng per mL or lower [15,16]. In addition, all women received daily supplements of calcium (500–1000 mg) and vitamin D (600–800 IU) during the studies. In FRAME, the average serum calcium decreased by 2% after 1 month of romosozumab therapy, and 1 subject who received romosozumab in both FRAME and ARCH developed asymptomatic hypocalcemia.
Anti-romosozumab antibodies have been detected in 15%–20% of patients during the first year of therapy, including about 3% of patients whose antibodies had neutralizing activity in vitro [10,15,16]. However, there was no evidence that these antibodies altered the efficacy or safety of therapy, and neither the presence nor titer of antibodies correlated with injection site reactions or other adverse events.
Because cellular proliferation in many tissues is governed by canonical Wnt signaling, theoretical concern existed about the possibility of inducing malignancy with antisclerostin therapy [17]. No treatment related effects on tumor incidence was observed in a lifetime study in rats treated with romosozumab [18]. These results differ from the dose-dependent induction of osteosarcoma in similar rat toxicity studies with other anabolic therapies teriparatide and abaloparatide [19,20].
No important vascular safety signals with romosozumab were noted in FRAME [15]. Adjudicated cardiovascular events occurred in 1.1% of women receiving placebo and in 1.2% of those who took romosozumab. Deaths occurred in 0.8% of the romosozumab groups and in 0.6% of women receiving placebo. In ARCH, however, after 12 months of therapy, adjudicated cardiovascular events and stroke were reported more often with romosozumab (0.8% for both) than with alendronate (0.3% for both adverse events) [16]. Thirty of the 2040 women (1.5%) in the romosozumab group died during the first year of ARCH compared to 21 of 2014 women (1.0%) taking alendronate. The 0.5% difference in mortality persisted throughout follow-up period while all patents were taking alendronate.
The mechanism of the possible adverse vascular effect of romosozumab is not known. Any explanation would have to account for the disparity of results between the placebo controlled study (FRAME) and the active comparator study (ARCH). AP activity in vascular smooth muscle cells is a marker of vascular calcification [21], and bone AP is transiently stimulated by romosozumab therapy. Sclerostin is up-regulated during vascular calcification in vitro, but whether this is a cause of or response to the calcification is not known [22,23]. Additional analyses of the results from FRAME and ARCH, including comparisons of the different patient populations, will hopefully shed light on the underlying mechanism and clinical relevance of the cardiovascular findings in ARCH.
5. Current status
The results of FRAME formed the basis of the recent filing with regulatory agencies for registration of romosozumab as a treatment for postmenopausal osteoporosis. That application is pending review in Europe. In the United States, the original application was withdrawn, and a new application that includes both the efficacy and safety data from ARCH is being submitted. Meanwhile, follow-up of women in the FRAME and ARCH studies is on-going. Additional information will be available from the international phase 2 study including the effects of retreatment with romosozumab. Results will be available soon from small studies evaluating the BMD and turnover responses to romosozumab in men with osteoporosis (ClinicalTrials.gov Identifier: NCT02186171) and in Korean women with postmenopausal osteoporosis (ClinicalTrials.gov Identifier: NCT02791516).
6. Commentary
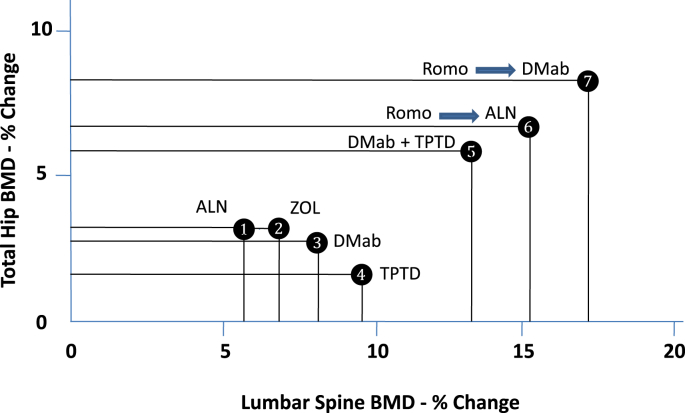
The discovery of sclerostin was quickly recognized as an opportunity to develop a unique new therapy for osteoporosis that, by blocking sclerostin action, would activate bone formation while reducing bone resorption. That possibility grew in likelihood as the clinical features of sclerostin deficiency syndromes were explored and by the ability of anti-sclerostin therapy to normalize bone mass and strength in animals. Most of the promise of that idea has been realized with romosozumab followed by an anti-remodeling drug. That strategy effectively reduces the risk of important fractures and produces faster and greater increases in BMD and bone strength than with any current osteoporosis treatment [15,16,24,25] (Fig. 1). However, in addition to concerns about possible vascular side effects, there are limitations to the promise of romosozumab as a possible “cure” for osteoporosis. Monthly dosing, administered by health professionals, will be inconvenient. More importantly, the robust anabolic effect of treatment is limited to the first few months of therapy, curtailing the progressive, large increases in bone mass. Perhaps a strategy can be identified whereby patients can experience the anabolic effects of romosozumab on multiple occasions with a limited interval or the use of alternate drugs between courses. Romosozumab is very effective in treatment-naïve patients, but, although not directly compared in head-to-head studies, the BMD response to therapy appears to be more modest in patients who have taken bisphosphonates [14].
Fig. 1.
Percent changes from baseline at 24 months (M) in bone mineral density (BMD) of the lumbar spine and total hip in postmenopausal women with osteoporosis with various treatment regimens. 1, alendronate (ALN) [16]; 2, zoledronic acid (ZOL) [24]; 3, denosumab (DMab) [25]; 4, teriparatide (TPTD) [25]; 5, denosumab plus teriparatide [25]; 6, romosozumab 12 M and alendronate 12 M [16]; 7, romosozumab 12 M and denosumab 12 M [15].
These limitations, though, do not detract from the impressive effectiveness of fracture risk reduction with romosozumab. While the relative reduction in vertebral fracture risk during the first year of therapy in FRAME was similar (61%) to that observed with other osteoporosis drugs, FRAME was the first study to demonstrate that beginning treatment with an anabolic agent, followed by a potent antiremodeling drug, was more effective than starting with antiremodeling therapy. An 80% greater reduction in vertebral fracture risk was observed during year 2 of FRAME in patients who received romosozumab followed by denosumab compared to the placebo-denosumab group [15].
In ARCH, the effects of romosozumab were clearly superior to alendronate therapy, the most extensively studied and most commonly used osteoporosis drug [16]. Furthermore, the 48% greater reduction in vertebral fractures during year 2 in the romosozumab-alendronate group confirmed the superiority of using an anabolic agent followed by an antiremodeling drug compared to starting with antiremodeling therapy.
The results of FRAME and ARCH should convince clinicians and payers that beginning treatment with an anabolic agent in patients at very high or imminent risk of fracture is the most effective and the appropriate treatment plan, especially since romosozumab-would be used for only 12 months.
Hopefully, the question of cardiovascular safety will not derail registration and availability of romosozumab for the treatment of women with postmenopausal osteoporosis at high-risk of fracture. If approved, there should be few cautions or contraindications other than pregnancy and hypersensitivity. Hypocalcemia is an expected response to the first dose of romosozumab because of the inhibition of bone resorption combined with the mineralization of the large amount of new bone matrix formed by treatment. It is my opinion that patients with hypocalcemia or vitamin D deficiency should not be treated with romosozumab, that adequate intakes of calcium and vitamin D should be ensured before treatment is begun, and that serum calcium should be monitored after initial dosing in patients at risk for hypocalcemia. It would also be wise to avoid therapy in patients with or at risk of skeletal metastases or with other high bone remodeling conditions such as Paget disease of bone.
Beyond the use of romosozumab to treat postmenopausal and age-related osteoporosis, it is appealing to consider the use of this the unique anabolic response to romosozumab therapy in several states of low bone turnover including idiopathic osteoporosis in young adults, anorexia nervosa, adynamic renal osteodystrophy and glucocorticoid-induced osteoporosis. Studying the effects of antisclerostin therapy in those conditions will be very interesting.
7. Conclusions
Romosozumab is an exciting potential therapy for osteoporosis, and, if approved, its availability will usher in a new era of osteoporosis therapy.
Conflicts of interest
Author receives consulting fees and honorarium from Amgen and Radius Health. Except for that, no potential conflict of interest relevant to this article was reported.
Footnotes
Peer review under responsibility of The Korean Society of Osteoporosis.
References
- 1.NIH consensus development panel on osteoporosis prevention, diagnosis, and therapy. Osteoporosis prevention, diagnosis, and therapy. J Am Med Assoc. 2001;285:785–795. [Google Scholar]
- 2.van Lierop A.H., Appelman-Dijkstra N.M., Papapoulos S.E. Sclerostin deficiency in humans. Bone. 2017;96:51–62. doi: 10.1016/j.bone.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Li X., Ominsky M.S., Niu Q.T., Sun N., Daugherty B., D'Agostin D. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res. 2008;23:860–869. doi: 10.1359/jbmr.080216. [DOI] [PubMed] [Google Scholar]
- 4.Ominsky M.S., Boyce R.W., Li X., Ke H.Z. Effects of sclerostin antibodies in animal models of osteoporosis. Bone. 2017;96:63–75. doi: 10.1016/j.bone.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 5.Ominsky M.S., Boyd S.K., Varela A., Jolette J., Felx M., Doyle N. Romosozumab improves bone mass and strength while maintaining bone quality in ovariectomized cynomolgus monkeys. J Bone Miner Res. 2017;32:788–801. doi: 10.1002/jbmr.3036. [DOI] [PubMed] [Google Scholar]
- 6.Padhi D., Allison M., Kivitz A.J., Gutierrez M.J., Stouch B., Wang C. Multiple doses of sclerostin antibody romosozumab in healthy men and postmenopausal women with low bone mass: a randomized, double-blind, placebo-controlled study. J Clin Pharmacol. 2014;54:168–178. doi: 10.1002/jcph.239. [DOI] [PubMed] [Google Scholar]
- 7.Padhi D., Jang G., Stouch B., Fang L., Posvar E. Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res. 2011;26:19–26. doi: 10.1002/jbmr.173. [DOI] [PubMed] [Google Scholar]
- 8.McClung M.R., San Martin J., Miller P.D., Civitelli R., Bandeira F., Omizo M. Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch Intern Med. 2005;165:1762–1768. doi: 10.1001/archinte.165.15.1762. [DOI] [PubMed] [Google Scholar]
- 9.Graeff C., Campbell G.M., Peña J., Borggrefe J., Padhi D., Kaufman A. Administration of romosozumab improves vertebral trabecular and cortical bone as assessed with quantitative computed tomography and finite element analysis. Bone. 2015;81:364–369. doi: 10.1016/j.bone.2015.07.036. [DOI] [PubMed] [Google Scholar]
- 10.McClung M.R., Grauer A., Boonen S., Bolognese M.A., Brown J.P., Diez-Perez A. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370:412–420. doi: 10.1056/NEJMoa1305224. [DOI] [PubMed] [Google Scholar]
- 11.Ishibashi H., Crittenden D.B., Miyauchi A., Libanati C., Maddox J., Fan M. Romosozumab increases bone mineral density in postmenopausal Japanese women with osteoporosis: a phase 2 study. Bone. 2017;103:209–215. doi: 10.1016/j.bone.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Genant H.K., Engelke K., Bolognese M.A., Mautalen C., Brown J.P., Recknor C. Effects of romosozumab compared with teriparatide on bone density and mass at the spine and hip in postmenopausal women with low bone mass. J Bone Miner Res. 2017;32:181–187. doi: 10.1002/jbmr.2932. [DOI] [PubMed] [Google Scholar]
- 13.Keaveny T.M., Crittenden D.B., Bolognese M.A., Genant H.K., Engelke K., Oliveri B. Greater gains in spine and hip strength for romosozumab compared with teriparatide in postmenopausal women with low bone mass. J Bone Miner Res. 2017;32:1956–1962. doi: 10.1002/jbmr.3176. [DOI] [PubMed] [Google Scholar]
- 14.Langdahl B.L., Libanati C., Crittenden D.B., Bolognese M.A., Brown J.P., Daizadeh N.S. Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: a randomised, open-label, phase 3 trial. Lancet. 2017;390:1585–1594. doi: 10.1016/S0140-6736(17)31613-6. [DOI] [PubMed] [Google Scholar]
- 15.Cosman F., Crittenden D.B., Adachi J.D., Binkley N., Czerwinski E., Ferrari S. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375:1532–1543. doi: 10.1056/NEJMoa1607948. [DOI] [PubMed] [Google Scholar]
- 16.Saag K.G., Petersen J., Brandi M.L., Karaplis A.C., Lorentzon M., Thomas T. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377:1417–1427. doi: 10.1056/NEJMoa1708322. [DOI] [PubMed] [Google Scholar]
- 17.Krishnamurthy N., Kurzrock R. Targeting the Wnt/beta-catenin pathway in cancer: update on effectors and inhibitors. Canc Treat Rev. 2018;62:50–60. doi: 10.1016/j.ctrv.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chouinard L., Felx M., Mellal N., Varela A., Mann P., Jolette J. Carcinogenicity risk assessment of romosozumab: a review of scientific weight-of-evidence and findings in a rat lifetime pharmacology study. Regul Toxicol Pharmacol. 2016;81:212–222. doi: 10.1016/j.yrtph.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Vahle J.L., Sato M., Long G.G., Young J.K., Francis P.C., Engelhardt J.A. Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1-34) for 2 years and relevance to human safety. Toxicol Pathol. 2002;30:312–321. doi: 10.1080/01926230252929882. [DOI] [PubMed] [Google Scholar]
- 20.Jolette J., Attalla B., Varela A., Long G.G., Mellal N., Trimm S. Comparing the incidence of bone tumors in rats chronically exposed to the selective PTH type 1 receptor agonist abaloparatide or PTH(1-34) Regul Toxicol Pharmacol. 2017;86:356–365. doi: 10.1016/j.yrtph.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Sheen C.R., Kuss P., Narisawa S., Yadav M.C., Nigro J., Wang W. Pathophysiological role of vascular smooth muscle alkaline phosphatase in medial artery calcification. J Bone Miner Res. 2015;30:824–836. doi: 10.1002/jbmr.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu D., Mackenzie N.C., Millán J.L., Farquharson C., MacRae V.E. The appearance and modulation of osteocyte marker expression during calcification of vascular smooth muscle cells. PLoS One. 2011;6 doi: 10.1371/journal.pone.0019595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gay A., Towler D.A. Wnt signaling in cardiovascular disease: opportunities and challenges. Curr Opin Lipidol. 2017;28:387–396. doi: 10.1097/MOL.0000000000000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Black D.M., Delmas P.D., Eastell R., Reid I.R., Boonen S., Cauley J.A. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–1822. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 25.Leder B.Z., Tsai J.N., Uihlein A.V., Burnett-Bowie S.A., Zhu Y., Foley K. Two years of Denosumab and teriparatide administration in postmenopausal women with osteoporosis (The DATA Extension Study): a randomized controlled trial. J Clin Endocrinol Metab. 2014;99:1694–1700. doi: 10.1210/jc.2013-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]