Abstract
Fragility fracture is a serious clinical event, because it is associated with increased risk of mortality and reduced quality of life. The risk of fracture is determined by multiple risk factors, and their effects may be interactional. Over the past 10 years, a number of predictive models (e.g., FRAX, Garvan Fracture Risk Calculator, and Qfracture) have been developed for individualized assessment of fracture risk. These models use different risk profiles to estimate the probability of fracture over 5- and 10-year period. The ability of these models to discriminate between those individuals who will and will not have a fracture (i.e., area under the receiver operating characteristic curve [AUC]) is generally acceptable-to-good (AUC, 0.6 to 0.8), and is highly variable between populations. The calibration of existing models is poor, particularly in Asian populations. There is a strong need for the development and validation of new prediction models based on Asian data for Asian populations. We propose approaches to improve the accuracy of existing predictive models by incorporating new markers such as genetic factors, bone turnover markers, trabecular bone score, and time-variant factors. New and more refined models for individualized fracture risk assessment will help identify those most likely to sustain a fracture, those most likely to benefit from treatment, and encouraging them to modify their risk profile to decrease risk.
Keywords: Osteoporosis, Fracture, Fracture risk assessment, Genetic profiling, FRAX, Garvan
1. Why fracture risk assessment?
At the individual level, fragility fracture is a serious clinical problem, because it is associated with increased risk of recurrent fractures, reduced mobility and quality of life, and increased risk of mortality. An initial fracture, at any skeletal site, is a signal for further fractures, and the relative risk ranges between 1.4 and 4.9 [1], depending on the site of initial fracture. For instance, a woman with a hip fracture is associated with a 2.8- and 4.9-fold increase in subsequent fracture in women and men, respectively [1]. The time from an initial fracture to a subsequent fracture is also shorter than the time from no fracture to an initial fracture. The importance of fragility fracture also lies in the fact that individuals with a fracture tend to have reduced life expectancy, and the risk is greater in men than in women [2]. The relative risk of mortality in men with fracture (1.8 fold) is substantially greater than that in women (1.4 fold) [3]. The increased mortality risk was also observed in younger individuals with fracture [4]. Moreover, up to 24% women and 38% men will die within the first 3 months after experiencing a hip fracture [5]. Those who survive a fracture usually develop one or more of chronic pain, increased dependence, and reduce quality of life [6].
At the population level, fragility fracture remains a significant public health burden, because it is highly prevalent in the general population and can incur a substantial healthcare cost. The lifetime risk of fracture is approximately 50% in women and ∼30% in men aged 50 years [7]. It is little known that in women, the remaining lifetime risk of hip fracture is equivalent to or higher than the risk of invasive breast cancer [7], and in men, the risk of hip and clinical vertebral fractures (17%) is comparable to the risk prostate cancer [8]. Taken together, recent data clearly suggest that fragility fracture is a common and serious skeletal disorder that is expected to increase in magnitude over the next few decades as populations are rapidly aging.
There are high quality data suggesting that treating individuals at high risk of fracture or individuals with an initial fracture reduces the risk of subsequent fracture [9]. The magnitude of risk reduction typically ranges between 30% and 60% [10]. More importantly, there are high quality evidence that treatment of individuals with a fracture could reduce the risk of postfracture mortality. For instance, a large randomized controlled trial (RCT) showed that zoledronic acid treatment reduced the risk of post–hip-fracture mortality by 28%, when given within 3 months post hip surgery [11]. More recent studies have also suggested that individuals on oral bisphosphonates have lower risk of mortality [12]. Despite these evidence [12,13], less than 30% of women and less than 10% of men, who have already had an osteoporotic fracture, receive treatment to reduce their risk of subsequent fractures [14]. Thus, osteoporosis is an undertreated disease, and the undertreatment status could partly be responsible for excess mortality associated with fracture [2].
2. Risk factors for fracture: not just low BMD!
The risk of fracture is influenced by multiple risk factors, but the most robust risk factors are low bone mineral density (BMD) [15]. Each standard deviation lower in BMD is typically associated with a 2-fold increase in fracture risk [16]. The magnitude of association between BMD and fracture is equivalent to or greater than the association between serum cholesterol and cardiovascular disease [17]. Thus, measurement of BMD is considered the gold standard for the diagnosis of osteoporosis in elderly men and postmenopausal women. In 1994 the World Health Organization (WHO) expert panel proposed an operational definition of osteoporosis, by which a postmenopausal woman is considered to have osteoporosis if the woman's femoral neck BMD is decreased by at least 2.5 standard deviations as compared to mean value in young adults [18]. The operational criteria of osteoporosis for women were subsequently adopted for men [19]. Although the WHO criteria were criticized as a flawed approach [20], they have been widely used in clinical practice.
Apart from low BMD, a personal history of fracture is also an important risk factor for fracture [21]. The relative risk of fracture associated with a prior fracture ranged between 1.5 and 9.5 fold depending on age at assessment, number of prior fractures and the site of the incident fracture. Even a pre-existing asymptomatic vertebral fracture increases the risk of a second vertebral fracture and nonvertebral fracture by at least 4 fold [22]. On average, the risk of subsequent fracture among those with a prior fracture at any site is 2.2 times that of people without a prior fragility fracture [21].
It is, therefore, logical that the assessment of fracture risk has traditionally been based on the measurement BMD and a personal history of fracture. Furthermore, treatment initiation is indicated for individuals with low BMD (i.e., osteoporosis) and/or with a pre-existing low trauma fracture. This strategy appears to be logical and evidence-based because results from randomized clinical trials show that treating these patients (e.g., with osteoporosis and/or a prior fracture) did reduce their fracture risk.
Although low BMD is the most robust risk factor for fracture, it does not account for most fracture cases. Indeed, among those aged 50 years and older, more than 50% of women and up to 70% of men who sustained a fracture had not had osteoporosis [23] as defined by bone density criteria alone. Among individuals aged 60 years or older with low BMD (high risk group) 60% of women and 70% of men did not sustain an osteoporotic fracture within a 13-year follow-up. In other words, more than half of individuals with low BMD are “resistant to fracture.”
Further studies have shown that apart from low BMD and prior fracture, other factors such as advancing age, being woman, family history of fracture, excessive bone loss, low body weight, falls, and smoking behavior were also associated with fracture risk [24]. Indeed, at any given level of BMD, fracture risk varies widely in relation to the burden of other risk factors. Thus, for any one individual, the likelihood of fracture depends on a combination of these and other risk factors. This means that 2 individuals, both with “osteoporosis,” can have different risks of fracture because they have different non-BMD risk profile. Similarly, an osteoporotic individual can have the same risk of fracture as a nonosteoporotic individual due to the difference in constellation of risk factors between the 2 individuals. The multifactorial nature of fracture implies that the assessment of fracture risk should ideally take into account the full profile of risk factors of an individual.
A challenging issue is how to synthesize information from multiple risk factors for predicting fracture risk for an individual. It is commonly believed that clinical experience or clinical intuition could predict clinical outcome fairly accurately. Indeed, since the Hippocrates' time, doctors have been valued for their ability to predict their patients' outcome. However, in the presence of multiple risk factors, clinician's assessment can be problematic because they are unable to weigh information in a reproducible and objective manner. Statistical prognostic models have been shown to out-perform clinical judgment [25], because these models can objectively incorporate data from many risk factors and produce reproducible risk estimates.
3. Individualized assessment of fracture risk
In the past, the assessment of risk was based on a grouping approach [26]. In the risk grouping approach, a continuously distributed risk factor is usually categorized into distinct groups, and the estimate of risk is therefore applicable to a group of individuals rather than to an individual. For instance, the stratification of BMD measurement into osteoporosis vs. Nonosteoporosis based on T-score splits 2 men with T-scores of −2.45 and −2.50 into 2 distinct groups despite the trivial difference, and despite the possibility that the 2 men may have comparable risk of fracture if other risk factors are considered. Moreover, because of the broad categories, such a stratification approach classifies a 80-year-old man with T-score of −2.5 and a 70-year-old man with T-score of −3.0 into a single group, despite the 2 men have very different risk profiles! The risk grouping approach is conceptually simple and sometimes useful in clinical practice, its predictive value is poorer than the individualized approach due to the arbitrariness of any numerical cutoff value [27].
A better approach of risk assessment should recognize that each individual is unique. The uniqueness can be defined in terms of an individual's measured profile. For instance, instead of categorizing BMD into distinct groups, the individualized approach would consider BMD in its full measurement range. This is more logical since the relationship between BMD and fracture risk is continuous, there is no threshold value for BMD that accurately separates those who will from those who will not sustain a fracture. Thus, 2 individuals with a BMD T-score of −2.5 and −2.6 should have different risks of fracture, and of course, their risks are modified by other risk factors. This implies that by considering risk factors in their continuous scale the estimated risk can be better tailored to an individual.
A number of models for fracture risk assessment have been developed based on the idea of individualized approach (Table 1). The most common models include FRAX [28], Garvan Fracture Risk Calculator [29,30], and Qfracture [31]. FRAX uses 12 risk factors, including femoral neck BMD, anthropometric factors, lifestyle factors, and comorbidities. The Garvan Fracture Risk Calculator (Garvan) uses 5 risk factors, namely, age, sex, femoral neck BMD, prior fracture, and history of fall. The risk factors included in the Garvan model were identified by the Bayesian Model Averaging approach [32] which has been demonstrated to have superior performance than stepwise approach [33]. In the Garvan model, prior fracture and prior falls were quantified in terms of the number of events rather than simple binary classification (e.g., yes/no) variables. The Garvan provides 5- and 10-year risks of total fracture and hip fracture, FRAX provides 10-year risk of hip fracture and major osteoporotic fractures. We consider that 5-year estimate of risk is more manageable than 10-year risk for an individual, and this view is agreeable with a recent commentary that 5-year estimate of risk without mortality adjustment is more helpful than mortality-adjusted 10-year estimate [34].
Table 1.
FRAX and garvan fracture risk calculator.
| Characteristic | Garvan model | FRAX model |
|---|---|---|
| Risk factors considered | Sex Age No. of prior fractures No. of falls over the past 12 months Femoral neck BMD |
Sex Age Prior fracture (yes/no) Body weight Body height Femoral neck BMD Parental history of fracture Current smoking Alcohol (3 or more units/day) Chronic corticosteroid use Rheumatoid arthritis Secondary osteoporosis |
| Method for selecting variables | Bayesian Model Averaging method | NA |
| Adjustment for competing risk of mortality | No | Yes |
| Statistical model | Cox proportional hazards | NA |
| Output | 5- and 10-year risk of total fracture 5- and 10-year risk of hip fracture |
10-year risk of major fracture 10-year risk of hip fracture |
| Typical AUC, median (range) | ||
| Total fracture (in validation studies) | 0.74 (0.64–0.88) | 0.69 (0.54–0.83) |
| Hip fracture (in validation studies) | 0.79 (0.67–0.85) | 0.78 (0.70–0.88) |
| Website | Garvan.org.org/bone-fracture-risk | shef.ac.uk/FRAX |
BMD, bone mineral density; NA, not available; AUC, area under the receiver operating characteristic curve.
Data were derived from Nguyen and Eisman. J Clin Densitom 2017;20:368–78 [53].
FRAX (Centre for Metabolic Bone Diseases, University of Sheffield, Sheffield, UK), Garvan Fracture Risk Calculator (Garvan Institute of Medical Research, Sydney, Australia).
4. Performance of fracture risk assessment models
The performance of a predictive model is commonly assessed by 2 metrics: discrimination and calibration. Discrimination is the capability of a model to separate individuals who will sustain a fracture along a continuum from those who will not. The primary metric of discrimination is the area under the receiver operating characteristic curve (AUC) which evaluates the compromise between sensitivity and specificity, and is thus a global estimate of prognostic accuracy. Calibration assesses the agreement between observed and predicted risk of fracture over the range of predicted probabilities.
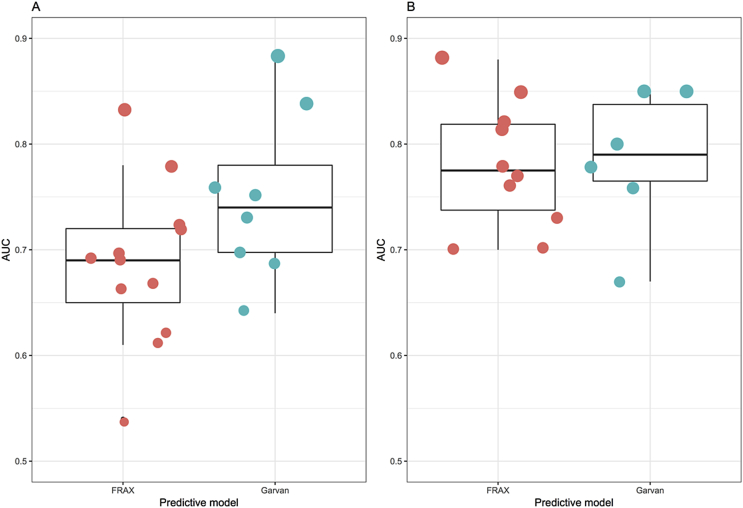
Over the past 10 years, there have been several independent studies examining the prognostic performance of the Garvan model [[35], [36], [37], [38]], FRAX [[39], [40], [41], [42], [43], [44]], or both Garvan and FRAX [35,[45], [46], [47], [48], [49], [50], [51], [52]]. In general, the discrimination for hip fracture was better than for total fractures (Fig. 1). In predicting hip fracture risk, the median AUC value for Garvan was 0.80, which was equivalent to that of FRAX (AUC, 0.78). In predicting major fracture risk, the median AUC value for Garvan and FRAX was 0.76 and 0.69, respectively [53]. However, it should be noted that as a norm, AUC value for outcome with low frequency (e.g., less than 100 events) such as hip fracture is often overoptimistic [54]. It appears that the discrimination of fracture in men was lower than women [55]. In certain populations [36,38,45], it appears that the Garvan model performed well in the discrimination of fracture, particularly in men [49]. For instance, in the Canadian Multicenter Osteoporosis Study, the Garvan model yielded good discrimination, particularly for hip fracture (AUC 0.80 for women and 0.85 for men) [36]. In a recent systematic review, the average AUC for total fracture by FRAX and Garvan was 0.67 (95% confidence interval, 0.64–0.71) and 0.70 (95% CI, 0.64–0.75) [56].
Fig. 1.
Area under the receiver operating characteristic curve of the FRAX and Garvan models for predicting total or major osteoporotic fractures (A) and hip fracture (B) from published validation studies. FRAX (Centre for Metabolic Bone Diseases, University of Sheffield, Sheffield, UK), Garvan Fracture Risk Calculator (Garvan Institute of Medical Research, Sydney, Australia). Data were derived from Nguyen and Eisman. J Clin Densitom 2017;20:368–78 [53].
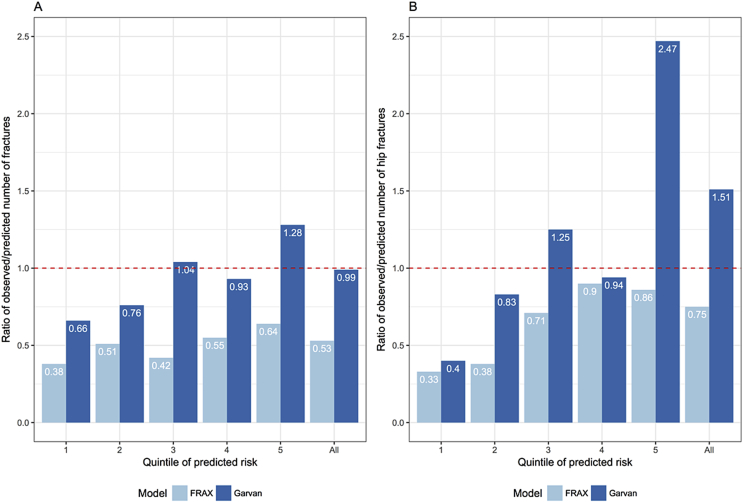
While the discriminatory ability of FRAX and Garvan was comparable, their calibration was very different. Most studies have consistently shown that FRAX tended to underestimate the risk of fracture [45,47,49,57], particularly in diabetic patients [58]. Several studies have indicated that the Garvan model had very good calibration. A validation study on 1422 postmenopausal women living in New Zealand found that the Garvan predicted fracture risk was 99% in agreement with the observed number of fractures; however the Garvan model tended to overestimate the risk of fracture among individuals in the top quartile of fracture risk [45] (Fig. 2) which was also noted in the initial development study [29,30]. In the CaMoS cohort, the Garvan model also shows a remarkable agreement between predicted 10-year probability of fracture and observed 10-year risk of fracture [36].
Fig. 2.
Ratio of observed over predicted number of fractures stratified by quintile of predicted probability of fracture, for total fracture (A) and hip fracture (B). FRAX (Centre for Metabolic Bone Diseases, University of Sheffield, Sheffield, UK), Garvan Fracture Risk Calculator (Garvan Institute of Medical Research, Sydney, Australia). Data were derived from Bolland et al. J Bone Miner Res 2011;26:420–7 [45].
The concordance in the predicted probabilities of fracture between Garvan and FRAX was modest, with the coefficient of correlation being 0.67 [59]. A reason for the discordance is that the Garvan model takes into account the prevalence of falls in the risk estimation, but the FRAX model did not [50]. A validation study in 2012 postmenopausal women of Polish background found that there was a considerable discrepancy in risk estimates between Garvan and FRAX models with the Garvan model predicting fracture more accurately than FRAX [46]. Despite the fact that there are differences in predicted risk of fracture between Garvan and FRAX, the majority of the differences do not seem to impact on treatment recommendation [60].
The discordance between Garvan and FRAX is expected, because the 2 models use different profiles of risk factors. In essence, the estimated risk is a conditional probability that is dependent on the risk factors and their statistical weights. The estimated weight associated with each risk factor is dependent on the statistical method that is used to model the relationship between the risk factor and fracture. The weights associated with 5 risk factors in the Garvan model were derived from on the multivariable Cox proportional hazards analysis [29], whereas the method of derivation of the FRAX model is not known [61]. Thus, an individual can have different predicted risks of fracture dependent on which factors are considered in the prediction [62]. It is also important to appreciate that the predicted risk is actually an average—a kind of “wisdom of the crowd” [63]—with “true” values fluctuating below or above the typical value. Therefore, an individual does not necessarily have a unique risk value. This subtle fact also explains why different valid predictive models can yield substantially different results for an individual.
Is the predicted fracture risk concordant with clinical guidelines? In a validation on 801 men who have been followed up for 10 years, Pluskiewicz et al. [49] found that the Garvan-predicted risk of fracture was more concordant with treatment indication than FRAX-predicted risk. For instance, among 218 men with a prior fracture (i.e., indicated for treatment), 82% of them had Garvan-predicted risk ≥20% compared with only 8% had FRAX-predicted risk ≥20%. Similarly, among men with osteoporosis (i.e., indicated for treatment), the proportion of men with ≥20% predicted risk by Garvan and FRAX was 72% and 10%, respectively [49]. Thus, it appears that the threshold of 20% predicted risk for defining “high-risk” is reasonably consistent with current clinical guidelines.
However, it remains unknown whether treating patients with high risk as defined by the current predictive models will reduce their risk of future fracture. Virtually all RCTs evaluating antifracture efficacy selected patients based on low BMD (i.e., osteoporosis) and/or the presence of a pre-existing fracture, and among these patients pharmacological interventions have shown good efficacy [10]. Because no clinical trials have been performed on individuals with high-risk of fracture based on either FRAX or Garvan, it is not known whether these patients can be benefited from pharmacological treatments. Nevertheless, post hoc analyses of RCTs appear to suggest that those with high risk of fracture at baseline (as assessed by FRAX) had a slightly greater relative risk reduction of fracture associated with denosumab [64] and bazedoxifene [65], but not with strontium ranelate [66] and raloxifene [67]. In another post hoc analysis [68] it was found that among women in the top 25th percentile of fracture probability (average probability of 24%), clodronate treatment reduced the risk of fracture by 23% over 3 years; among those in the top 10% percentile (average fracture probability of 30%), treatment reduced the fracture risk by 31% [68]. Taken together, these results seem to be consistent with the hypothesis that treatment of individuals at high risk or moderate risk identified by predictive models could reasonably be expected to reduce fractures.
5. Room for improvement
From the point of view of predictive accuracy, all current models for fracture risk assessment are suboptimal. Indeed, the average AUC value for total fracture prediction by FRAX and Garvan was only ∼0.7 [56] which may be considered “adequate.” The challenge is to find ways to improve the accuracy of fracture prediction. We postulate that the accuracy can be improved by incorporating new markers for fracture risk and by adopting new modeling strategies (Table 2).
Table 2.
Potential ways to improve predictive accuracy of fracture risk assessment.
| “New” markers | Modeling approaches |
|---|---|
| Genetic profiling | Fracture type specific prediction |
| Trabecular bone score | Machine learning |
| Bone turnover markers | Time-variant predictions |
| Ethnic-specific models |
5.1. Genetic profiling
It is well known that the risk of fragility fracture is partly influenced by genetic factors. Almost half of the variance in fracture susceptibility among individuals is due to hereditary factors [69]. Over the past 20 years or so, several large scale collaborative studies [70] have 62 loci that are associated with BMD; among the 62 single nucleotide polymorphisms (SNPs) identified, 8 SNPs were associated with fracture risk at the genome-wide significance level [70]. A common characteristic of these SNPs is that their effect sizes were modest, with odds ratios ranging between 1.1 and 1.4, suggesting that individually they have limited utility for fracture prediction.
Nevertheless, a genetic profiling may help improve the accuracy of fracture prediction. A simulation study showed that a genetic profile of up to 50 genetic variants, with each having a modest effect size (odds ratio, 1.01–1.35) could improve the accuracy of fracture prediction by 10% points of AUC [71]. In postmenopausal women of Korean background, a genetic profiling of 39 SNPs in 30 human genomic loci increased the precision of nonvertebral fracture prediction and help to define the risk threshold [72], while a profiling of 35 risk alleles was significantly associated the risk of vertebral fracture [72,73] in patients on bisphosphonate. Recently, we have shown that the incorporation of an “osteogenomic profile” of 62 BMD-associated SNPs into existing Garvan Fracture Risk Calculator could modestly improve the predictive accuracy of fracture [74], and this finding was consistent with a previous observation from MrOS study [75]. Taken together, these latest results studies suggest that genetic profiling could help improve the accuracy of fracture prediction over and above that of clinical risk factors.
5.2. Trabecular bone score
Trabecular bone score (TBS) is a measure of the distributional trabecular architecture [76]. TBS is derived as a texture parameter that reflects pixel grey level variation in dual-energy X-ray absorptiometry images. Previous studies have reported that TBS is significantly correlated with trabecular number, trabecular separation and structure model index [77]. Moreover, TBS was found to be associated with fracture risk in elderly women and diabetic patients [78] independently of BMD and classical clinical risk factors [79]. A recent meta-analysis found that TBS was a FRAX-independent predictor of fracture risk [80], suggesting that TBS could improve the discriminatory power of fracture risk assessment for an individual.
5.3. Bone turnover markers
Several cross-sectional and longitudinal studies have observed that fragility fractures occur not only because of low BMD but also as a result of rapid bone turnover that leads to adverse architectural changes. There is accumulating evidence that accelerated bone resorption is a risk factor for fracture, independent of BMD and other clinical risk factors [81]. For instance, increased urinary levels of the pyridinium crosslink, deoxypyridinoline (DPD), was associated with a 2- to 3-fold increase in the risk of hip fracture [82]. Increased urinary type I collagen C-telopeptide (CTX) and free DPD levels were associated with a 2-fold increase in hip fracture risk after adjusting for BMD and physical mobility [83]. In men, increased bone resorption was also associated with increased fracture risk [84]. A meta-analysis of longitudinal studies found that increased serum levels of serum aminoterminal propeptide of type I collagen and CTX were modestly associated with an increase in fracture risk in men and women [85]. These results strongly suggest that the incorporation of bone turnover markers into the existing prognostic models could improve the prediction of absolute fracture risk. However, the use of bone turnover markers for fracture risk assessment is faced with challenges in the standardization of measurements and treatment of intrasubject variability.
5.4. Fracture type-specific prediction
Existing individualized risk assessment models were developed for predicting the risk of total (or major) fractures and hip fracture. The implicit assumption behind the development of these models is that all fracture types share common risk factors. However, this assumption is unlikely true, as a risk factor for one fracture type may not be associated with another fracture type. For instance, fall is a major risk factor for hip fracture, but it is not a risk factor for vertebral fracture. Therefore, future models should move away from the “one size-fits-all” approach by focusing on specific fracture sites.
5.5. Machine learning approach
Most, if not all, existing models were developed under the assumption that there are no interactions between risk factors. However, this assumption may not be true, because complex interactions between risk factors are likely present but not detected by traditional statistical methods. In the presence of interactions or potential interactions, machine learning approach such as artificial neural network (ANN) can be useful in the prediction of fracture. By imitating human brain functions, ANN can model complex real-world relationships, including interacting variables. Recent studies have demonstrated that ANN performed better than traditional statistical models in terms of predicting vertebral fracture among postmenopausal women [86], and mortality following a hip fracture [87]. We and others [88,89] have also shown that for hip fracture prediction, ANN yielded a more accurate prediction than traditional statistical methods such as the logistic regression model.
From a conceptual viewpoint, it is important to distinguish between prediction and association [90]. Traditional statistical methods focus on association which is mainly concerned with the identification of statistically significant predictors to explain the relationship between the predictors and an outcome for a group of individuals. On the other hand, prediction is concerned with the derivation of rules based on observed data for forecasting specific outcomes for an individual. Although a strong association can translate into a good prediction, they are not synonymous. Indeed, a statistically significant association in a group of individuals does not necessarily translate into good prediction for an individual [91]. A risk factor may achieve statistical significance (e.g., P < 0.05) with large sample size even if it is a poor predictor of future outcome [91]. A risk factor or a set of risk factors may be statistically significantly associated with an outcome due to larger effect on a small number of events in the population, yet provide poor prediction for individuals in the population [92]. We propose that future fracture risk assessment models should move beyond association analysis and adopt more prediction analyses. Instead of finding factors that are associated with fracture, we should focus on the factors that have high predictive value of fracture risk. The factors that influence fracture risk are likely to be related and their effects on fracture risk are likely interactional. Association analysis striving for elegance and parsimony are unlikely inadequate to delineate their separate contributions or to capture their interactional effects. Prediction analysis using machine learning approach (e.g., ANN and deep learning) may be statistically less elegant but it could help identify potential highly predictive factors that are ignored by traditional association analysis [88,89].
5.6. Time-variant predictions
All risk factors change with time, and the rates of change are highly variable between individuals. For example, BMD in the elderly declines with advancing age, and the rates of decline vary substantially among individuals [93]. However, all existing predictive models assume that risk factors are constant with time. Of course, this assumption is not realistic, but it is a convenient starting point for building a predictive model. Therefore, one important aspect of future model development should take the time-varying nature of risk factors into account to achieve a better estimate of risk for an individual.
5.7. Ethnic-specific models
It is important to keep in mind that all existing predictive models (e.g., FRAX, Garvan, and Qfracture) were developed from data pertaining to North American and European populations, not Asian populations. These models have also been largely validated in Caucasian populations, and their performance in Asian populations are not well documented. Nevertheless, few studies have attempted to assess the utility of FRAX in the prediction of fracture in Asian individuals. In a validation analysis based on the Hong Kong Osteoporosis Study (n = 2266 postmenopausal women), the AUC of the FRAX model for predicting total fracture was ∼0.73, which is not substantially different from the model with BMD alone (AUC, 0.71) [94]. A study on 198 Chinese individuals with very recent fracture, Chen et al. [95] observed that the average FRAX-predicted fracture risk was 6.6%, with only 2 individuals (1%) had 10-year risk ≥20%, suggesting a poor calibration. In a Japanese population, FRAX model had a moderate discrimination for self-reported total fracture (AUC, 0.69), which is similar to the model with age and femoral neck BMD (AUC, 0.69) [42]. In an analysis of 405 postmenopausal women and 139 men with fracture Min et al. [96] observed a ∼2-fold difference in FRAX-predicted risk of fracture between the Korean FRAX model and Japanese FRAX model, despite the fact that the 2 populations have similar background risk. Taken together, these results suggest that the FRAX model has modest prognostic performance in Asian populations.
Thus, there is a strong need for the development of individualized fracture risk assessment models for Asian populations. This is true, because at the population level, the incidence of fracture in Asians is generally lower than that in Caucasian populations [97], and the distribution of behavioral risk factors for fracture is expected to be different between Asian and Caucasian populations. The prevalence of cigarette smoking in Asian women is lower than that in Caucasian women, but Asian men are more like to smoke than Caucasian men [98], and these ethnic-related differences need to be methodologically weighed in the estimation of fracture risk for an individual. It would be unrealistic to assume that Asian men and women share exactly the same risk factor profile as Caucasian populations; it is even more unrealistic to assume that the magnitude of association between smoking and fracture in Caucasian women is the same as in Asian women. Experience in the field of cardiovascular disease shows that the Caucasian based models (e.g., Framingham risk score and QRISK2) did not perform well in Asian populations [99]. International prospective population-based studies are urgently needed for the development and validation of new fracture risk assessment models for Asian populations.
Any statistical model is an imperfect representation of reality. Model development is a struggle between complexity and simplicity. Overly complex models with too many factors may yield better accuracy but they are of little practical use because it is hard to implement such models in practice. On the other hand, too simple models can miss high-risk individuals. Nevertheless, given the current modest calibration and discrimination of simple models, the addition of highly predictive factors to the existing models is likely to help improve the accuracy of prediction without increasing the burden complexity.
6. Conclusions
Fracture due to osteoporosis remains a major public health problem at the population level, and a serious event for an individual because it is associated with increased risk of mortality. Treatment of individuals with osteoporosis and/or a pre-existing fracture reduces the risk of future fracture [100]. More importantly, emerging evidence suggests that treatment of individuals with a fracture reduces the risk of mortality. Yet, the treatment uptake among high-risk individuals is disturbingly low. Providing an individualized estimate of short-term risk of fracture may improve appropriate treatment uptake and reduce the burden of fracture in the general population.
Over the past 10 years, a number of individualized risk assessment models have been developed and implemented in clinical setting [47]. The advance of these models represent a significant achievement of translational osteoporosis research. During the past decade, through the development, validation and use of these models, we have learned several lessons that may be highlighted as follows:
-
(1)
All existing risk assessment models, including FRAX, Garvan and QFact, have acceptable-to-good discrimination (AUC ranges between 0.6 and 0.8). The accuracy of prediction of these models is highly variable between populations. There is good evidence that incorporating new markers such as genetic profiling, TBS, and bone turnover markers into existing models may improve their prediction accuracy. Thus, the addition of these markers should be pursued.
-
(2)
Most existing fracture risk assessment models have poor calibration in external populations. It appears that the FRAX model underestimates while the Garvan model overestimates fracture risk. There is not consensus on the (predicted) fracture risk threshold above which treatment or intervention has a net worth.
-
(3)
The existing risk assessment models appear to work well for hip fracture risk than for total fracture. This seems to suggest future risk prediction models should be calibrated to individual fracture types rather than based on the “one-size-fits-all” approach.
-
(4)
The effectiveness of these individualized risk assessment models in real world clinical care remains uncertain. It is not known whether providing individuals with absolute risk of fracture will improve the treatment uptake. It is also not known whether treating individuals identified as “high-risk” by these models can reduce their risk of fracture and postfracture mortality.
-
(5)
The prognostic performance of existing fracture risk assessment models in Asian populations has not been well documented. However, few studies in Chinese, Japanese, and Korean populations have shown that these models have poor discrimination and calibration. As Asia is going to become an “epicenter” of osteoporosis, there is a strong need for the development of new risk fracture prediction models based on Asian data for local Asian populations.
-
(6)
Recent advances in statistical modeling and machine learning have opened new opportunities for improving fracture prediction. We envision that future prediction models should account for the interaction and time-variant nature of risk factors. Machine learning methods such as neural networks and deep learning are attractive options for improving the prognostic accuracy of existing risk assessment models.
The ultimate goal of risk assessment model is to provide clinicians and patients with accurate and reproducible risk estimate that helps guide clinical decisions. Current fracture risk assessment models have contributed substantially to the management of osteoporotic patients over the past decade. Still, much remains to be done to enhance the discrimination and calibration of existing models, as well as to develop new models for Asian populations using new statistical and machine learning technologies. New and more refined risk assessment models can help maximize benefits and preclude potential problems of overmedicalization and false assurance.
Conflicts of interest
The author is the developer of the Garvan Fracture Risk Calculator. Except for that, no potential conflict of interest relevant to this article was reported.
Footnotes
Peer review under responsibility of The Korean Society of Osteoporosis.
References
- 1.Center J.R., Bliuc D., Nguyen T.V., Eisman J.A. Risk of subsequent fracture after low-trauma fracture in men and women. J Am Med Assoc. 2007;297:387–394. doi: 10.1001/jama.297.4.387. [DOI] [PubMed] [Google Scholar]
- 2.Frost S.A., Nguyen N.D., Center J.R., Eisman J.A., Nguyen T.V. Excess mortality attributable to hip-fracture: a relative survival analysis. Bone. 2013;56:23–29. doi: 10.1016/j.bone.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Bliuc D., Nguyen N.D., Milch V.E., Nguyen T.V., Eisman J.A., Center J.R. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. J Am Med Assoc. 2009;301:513–521. doi: 10.1001/jama.2009.50. [DOI] [PubMed] [Google Scholar]
- 4.Shortt N.L., Robinson C.M. Mortality after low-energy fractures in patients aged at least 45 years old. J Orthop Trauma. 2005;19:396–400. doi: 10.1097/01.bot.0000155311.04886.7e. [DOI] [PubMed] [Google Scholar]
- 5.Haentjens P., Magaziner J., Colon-Emeric C.S., Vanderschueren D., Milisen K., Velkeniers B. Meta-analysis: excess mortality after hip fracture among older women and men. Ann Intern Med. 2010;152:380–390. doi: 10.1059/0003-4819-152-6-201003160-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adachi J.D., Adami S., Gehlbach S., Anderson F.A., Jr., Boonen S., Chapurlat R.D. Impact of prevalent fractures on quality of life: baseline results from the global longitudinal study of osteoporosis in women. Mayo Clin Proc. 2010;85:806–813. doi: 10.4065/mcp.2010.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen N.D., Ahlborg H.G., Center J.R., Eisman J.A., Nguyen T.V. Residual lifetime risk of fractures in women and men. J Bone Miner Res. 2007;22:781–788. doi: 10.1359/jbmr.070315. [DOI] [PubMed] [Google Scholar]
- 8.Cummings S.R., Black D.M., Rubin S.M. Lifetime risks of hip, Colles', or vertebral fracture and coronary heart disease among white postmenopausal women. Arch Intern Med. 1989;149:2445–2448. [PubMed] [Google Scholar]
- 9.Black D.M., Rosen C.J. Postmenopausal osteoporosis. N Engl J Med. 2016;374:2096–2097. doi: 10.1056/NEJMc1602599. [DOI] [PubMed] [Google Scholar]
- 10.Delmas P.D., Rizzoli R., Cooper C., Reginster J.Y. Treatment of patients with postmenopausal osteoporosis is worthwhile. The position of the International Osteoporosis Foundation. Osteoporos Int. 2005;16:1–5. doi: 10.1007/s00198-004-1813-0. [DOI] [PubMed] [Google Scholar]
- 11.Lyles K.W., Colon-Emeric C.S., Magaziner J.S., Adachi J.D., Pieper C.F., Mautalen C. Zoledronic acid in reducing clinical fracture and mortality after hip fracture. N Engl J Med. 2007;357:1799–1809. doi: 10.1056/NEJMoa074941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolland M.J., Grey A.B., Gamble G.D., Reid I.R. Effect of osteoporosis treatment on mortality: a meta-analysis. J Clin Endocrinol Metab. 2010;95:1174–1181. doi: 10.1210/jc.2009-0852. [DOI] [PubMed] [Google Scholar]
- 13.Center J.R., Bliuc D., Nguyen N.D., Nguyen T.V., Eisman J.A. Osteoporosis medication and reduced mortality risk in elderly women and men. J Clin Endocrinol Metab. 2011;96:1006–1014. doi: 10.1210/jc.2010-2730. [DOI] [PubMed] [Google Scholar]
- 14.Eisman J., Clapham S., Kehoe L. Osteoporosis prevalence and levels of treatment in primary care: the Australian BoneCare Study. J Bone Miner Res. 2004;19:1969–1975. doi: 10.1359/JBMR.040905. [DOI] [PubMed] [Google Scholar]
- 15.LaFleur J., McAdam-Marx C., Kirkness C., Brixner D.I. Clinical risk factors for fracture in postmenopausal osteoporotic women: a review of the recent literature. Ann Pharmacother. 2008;42:375–386. doi: 10.1345/aph.1K203. [DOI] [PubMed] [Google Scholar]
- 16.Marshall D., Johnell O., Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312:1254–1259. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen N.D., Pongchaiyakul C., Center J.R., Eisman J.A., Nguyen T.V. Identification of high-risk individuals for hip fracture: a 14-year prospective study. J Bone Miner Res. 2005;20:1921–1928. doi: 10.1359/JBMR.050520. [DOI] [PubMed] [Google Scholar]
- 18.Kanis J.A., Melton L.J., 3rd, Christiansen C., Johnston C.C., Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9:1137–1141. doi: 10.1002/jbmr.5650090802. [DOI] [PubMed] [Google Scholar]
- 19.Diagnosis of osteoporosis in men, premenopausal women, and children. J Clin Densitom. 2004;7:17–26. doi: 10.1385/jcd:7:1:17. [DOI] [PubMed] [Google Scholar]
- 20.Wasnich R.D. Consensus and the T-score fallacy. Clin Rheumatol. 1997;16:337–339. doi: 10.1007/BF02242447. [DOI] [PubMed] [Google Scholar]
- 21.Klotzbuecher C.M., Ross P.D., Landsman P.B., Abbott TAr, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res. 2000;15:721–739. doi: 10.1359/jbmr.2000.15.4.721. [DOI] [PubMed] [Google Scholar]
- 22.Pongchaiyakul C., Nguyen N.D., Jones G., Center J.R., Eisman J.A., Nguyen T.V. Asymptomatic vertebral deformity as a major risk factor for subsequent fractures and mortality: a long-term prospective study. J Bone Miner Res. 2005;20:1349–1355. doi: 10.1359/JBMR.050317. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen N.D., Eisman J.A., Center J.R., Nguyen T.V. Risk factors for fracture in nonosteoporotic men and women. J Clin Endocrinol Metab. 2007;92:955–962. doi: 10.1210/jc.2006-1476. [DOI] [PubMed] [Google Scholar]
- 24.Cummings S.R., Nevitt M.C., Browner W.S., Stone K., Fox K.M., Ensrud K.E. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332:767–773. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 25.Ross P.L., Gerigk C., Gonen M., Yossepowitch O., Cagiannos I., Sogani P.C. Comparisons of nomograms and urologists' predictions in prostate cancer. Semin Urol Oncol. 2002;20:82–88. doi: 10.1053/suro.2002.32490. [DOI] [PubMed] [Google Scholar]
- 26.van Staa T.P., Geusens P., Kanis J.A., Leufkens H.G., Gehlbach S., Cooper C. A simple clinical score for estimating the long-term risk of fracture in post-menopausal women. QJM. 2006;99:673–682. doi: 10.1093/qjmed/hcl094. [DOI] [PubMed] [Google Scholar]
- 27.Kattan M.W., Reuter V., Motzer R.J., Katz J., Russo P. A postoperative prognostic nomogram for renal cell carcinoma. J Urol. 2001;166:63–67. [PubMed] [Google Scholar]
- 28.Kanis J.A., Johnell O., Oden A., Johansson H., McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19:385–397. doi: 10.1007/s00198-007-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen N.D., Frost S.A., Center J.R., Eisman J.A., Nguyen T.V. Development of a nomogram for individualizing hip fracture risk in men and women. Osteoporos Int. 2007;18:1109–1117. doi: 10.1007/s00198-007-0362-8. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen N.D., Frost S.A., Center J.R., Eisman J.A., Nguyen T.V. Development of prognostic nomograms for individualizing 5-year and 10-year fracture risks. Osteoporos Int. 2008;19:1431–1444. doi: 10.1007/s00198-008-0588-0. [DOI] [PubMed] [Google Scholar]
- 31.Collins G.S., Mallett S., Altman D.G. Predicting risk of osteoporotic and hip fracture in the United Kingdom: prospective independent and external validation of QFractureScores. BMJ. 2011;342:d3651. doi: 10.1136/bmj.d3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoeting J. Bayesian model averaging: a tutorial. Stat Sci. 1999;14:382–401. [Google Scholar]
- 33.Wang D., Zhang W., Bakhai A. Comparison of Bayesian model averaging and stepwise methods for model selection in logistic regression. Stat Med. 2004;23:3451–3467. doi: 10.1002/sim.1930. [DOI] [PubMed] [Google Scholar]
- 34.Bolland M.J., Jackson R., Gamble G.D., Grey A. Discrepancies in predicted fracture risk in elderly people. BMJ. 2013;346 doi: 10.1136/bmj.e8669. e8669. [DOI] [PubMed] [Google Scholar]
- 35.Sandhu S.K., Nguyen N.D., Center J.R., Pocock N.A., Eisman J.A., Nguyen T.V. Prognosis of fracture: evaluation of predictive accuracy of the FRAX algorithm and Garvan nomogram. Osteoporos Int. 2010;21:863–871. doi: 10.1007/s00198-009-1026-7. [DOI] [PubMed] [Google Scholar]
- 36.Langsetmo L., Nguyen T.V., Nguyen N.D., Kovacs C.S., Prior J.C., Center J.R. Independent external validation of nomograms for predicting risk of low-trauma fracture and hip fracture. CMAJ. 2011;183:E107–E114. doi: 10.1503/cmaj.100458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmed L.A., Nguyen N.D., Bjornerem A., Joakimsen R.M., Jorgensen L., Stormer J. External validation of the Garvan nomograms for predicting absolute fracture risk: the Tromso study. PLoS One. 2014;9 doi: 10.1371/journal.pone.0107695. e107695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pluskiewicz W., Adamczyk P., Franek E., Leszczynski P., Sewerynek E., Wichrowska H. Ten-year probability of osteoporotic fracture in 2012 Polish women assessed by FRAX and nomogram by Nguyen, et al.-Conformity between methods and their clinical utility. Bone. 2010;46:1661–1667. doi: 10.1016/j.bone.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 39.Leslie W.D., Lix L.M., Johansson H., Oden A., McCloskey E., Kanis J.A. Independent clinical validation of a Canadian FRAX tool: fracture prediction and model calibration. J Bone Miner Res. 2010;25:2350–2358. doi: 10.1002/jbmr.123. [DOI] [PubMed] [Google Scholar]
- 40.Leslie W.D., Lix L.M., Langsetmo L., Berger C., Goltzman D., Hanley D.A. Construction of a FRAX(R) model for the assessment of fracture probability in Canada and implications for treatment. Osteoporos Int. 2011;22:817–827. doi: 10.1007/s00198-010-1464-2. [DOI] [PubMed] [Google Scholar]
- 41.Ensrud K.E., Lui L.Y., Taylor B.C., Schousboe J.T., Donaldson M.G., Fink H.A. A comparison of prediction models for fractures in older women: is more better? Arch Intern Med. 2009;169:2087–2094. doi: 10.1001/archinternmed.2009.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tamaki J., Iki M., Kadowaki E., Sato Y., Kajita E., Kagamimori S. Fracture risk prediction using FRAX(R): a 10-year follow-up survey of the Japanese Population-Based Osteoporosis (JPOS) Cohort Study. Osteoporos Int. 2011;22:3037–3045. doi: 10.1007/s00198-011-1537-x. [DOI] [PubMed] [Google Scholar]
- 43.Azagra R., Roca G., Encabo G., Aguye A., Zwart M., Guell S. FRAX(R) tool, the WHO algorithm to predict osteoporotic fractures: the first analysis of its discriminative and predictive ability in the Spanish FRIDEX cohort. BMC Musculoskelet Disord. 2012;13:204. doi: 10.1186/1471-2474-13-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ettinger B., Ensrud K.E., Blackwell T., Curtis J.R., Lapidus J.A., Orwoll E.S. Performance of FRAX in a cohort of community-dwelling, ambulatory older men: the Osteoporotic Fractures in Men (MrOS) study. Osteoporos Int. 2013;24:1185–1193. doi: 10.1007/s00198-012-2215-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bolland M.J., Siu A.T., Mason B.H., Horne A.M., Ames R.W., Grey A.B. Evaluation of the FRAX and Garvan fracture risk calculators in older women. J Bone Miner Res. 2011;26:420–427. doi: 10.1002/jbmr.215. [DOI] [PubMed] [Google Scholar]
- 46.Pluskiewicza W., Adamczykb P., Franekc E., Leszczynskid P., Seweryneke E., Wichrowskac H. Conformity between 10-year probability of any osteoporotic fracture assessed by FRAX and nomogram by Nguyen, et al. Bone. 2009;44(Suppl 2):S229–S230. doi: 10.1016/j.bone.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 47.Dagan N., Cohen-Stavi C., Leventer-Roberts M., Balicer R.D. External validation and comparison of three prediction tools for risk of osteoporotic fractures using data from population based electronic health records: retrospective cohort study. BMJ. 2017;356:i6755. doi: 10.1136/bmj.i6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gourlay M.L., Ritter V.S., Fine J.P., Overman R.A., Schousboe J.T., Cawthon P.M. Comparison of fracture risk assessment tools in older men without prior hip or spine fracture: the MrOS study. Arch Osteoporos. 2017;12:91. doi: 10.1007/s11657-017-0389-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pluskiewicz W., Adamczyk P., Franek E., Sewerynek E., Leszczynski P., Wichrowska H. FRAX calculator and Garvan nomogram in male osteoporotic population. Aging Male. 2014;17:174–182. doi: 10.3109/13685538.2013.875991. [DOI] [PubMed] [Google Scholar]
- 50.Billington E.O., Gamble G.D., Reid I.R. Reasons for discrepancies in hip fracture risk estimates using FRAX and Garvan calculators. Maturitas. 2016;85:11–18. doi: 10.1016/j.maturitas.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 51.Rubin K.H., Abrahamsen B., Friis-Holmberg T., Hjelmborg J.V., Bech M., Hermann A.P. Comparison of different screening tools (FRAX(R), OST, ORAI, OSIRIS, SCORE and age alone) to identify women with increased risk of fracture. A population-based prospective study. Bone. 2013;56:16–22. doi: 10.1016/j.bone.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 52.Chen S.J., Chen Y.J., Cheng C.H., Hwang H.F., Chen C.Y., Lin M.R. Comparisons of different screening tools for identifying fracture/osteoporosis risk among community-dwelling older people. Medicine (Baltim) 2016;95 doi: 10.1097/MD.0000000000003415. e3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nguyen T.V., Eisman J.A. Fracture risk assessment: from population to individual. J Clin Densitom. 2017;20:368–378. doi: 10.1016/j.jocd.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 54.Siontis G.C., Tzoulaki I., Ioannidis J.P. Predicting death: an empirical evaluation of predictive tools for mortality. Arch Intern Med. 2011;171:1721–1726. doi: 10.1001/archinternmed.2011.334. [DOI] [PubMed] [Google Scholar]
- 55.Nguyen T.V. Individualized assessment of fracture risk: contribution of "osteogenomic profile". J Clin Densitom. 2017;20:353–359. doi: 10.1016/j.jocd.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 56.Marques A., Ferreira R.J., Santos E., Loza E., Carmona L., da Silva J.A. The accuracy of osteoporotic fracture risk prediction tools: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74:1958–1967. doi: 10.1136/annrheumdis-2015-207907. [DOI] [PubMed] [Google Scholar]
- 57.van den Bergh J.P., van Geel T.A., Lems W.F., Geusens P.P. Assessment of individual fracture risk: FRAX and beyond. Curr Osteoporos Rep. 2010;8:131–137. doi: 10.1007/s11914-010-0022-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giangregorio L.M., Leslie W.D., Lix L.M., Johansson H., Oden A., McCloskey E. FRAX underestimates fracture risk in patients with diabetes. J Bone Miner Res. 2012;27:301–308. doi: 10.1002/jbmr.556. [DOI] [PubMed] [Google Scholar]
- 59.van Geel T.A., Nguyen N.D., Geusens P.P., Center J.R., Nguyen T.V., Dinant G.J. Development of a simple prognostic nomogram for individualising 5-year and 10-year absolute risks of fracture: a population-based prospective study among postmenopausal women. Ann Rheum Dis. 2011;70:92–97. doi: 10.1136/ard.2010.131813. [DOI] [PubMed] [Google Scholar]
- 60.Bolland M.J., Grey A., Gamble G., Reid I.R., Comment on Kanis Pitfalls in the external validation of FRAX. Osteoporos Int. 2013;24:389–390. doi: 10.1007/s00198-012-1977-y. [DOI] [PubMed] [Google Scholar]
- 61.Collins G.S., Michaelsson K. Fracture risk assessment: state of the art, methodologically unsound, or poorly reported? Curr Osteoporos Rep. 2012;10:199–207. doi: 10.1007/s11914-012-0108-1. [DOI] [PubMed] [Google Scholar]
- 62.Lemeshow S., Klar J., Teres D. Outcome prediction for individual intensive care patients: useful, misused, or abused? Intensive Care Med. 1995;21:770–776. doi: 10.1007/BF01704747. [DOI] [PubMed] [Google Scholar]
- 63.Galton F. Vox populi. Nature. 1907;75:450–451. [Google Scholar]
- 64.McCloskey E.V., Johansson H., Oden A., Austin M., Siris E., Wang A. Denosumab reduces the risk of osteoporotic fractures in postmenopausal women, particularly in those with moderate to high fracture risk as assessed with FRAX. J Bone Miner Res. 2012;27:1480–1486. doi: 10.1002/jbmr.1606. [DOI] [PubMed] [Google Scholar]
- 65.Kanis J.A., Johansson H., Oden A., McCloskey E.V. Bazedoxifene reduces vertebral and clinical fractures in postmenopausal women at high risk assessed with FRAX. Bone. 2009;44:1049–1054. doi: 10.1016/j.bone.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 66.Kanis J.A., Johansson H., Oden A., McCloskey E.V. A meta-analysis of the effect of strontium ranelate on the risk of vertebral and non-vertebral fracture in postmenopausal osteoporosis and the interaction with FRAX((R)) Osteoporos Int. 2011;22:2347–2355. doi: 10.1007/s00198-010-1474-0. [DOI] [PubMed] [Google Scholar]
- 67.Kanis J.A., Johansson H., Oden A., McCloskey E.V. A meta-analysis of the efficacy of raloxifene on all clinical and vertebral fractures and its dependency on FRAX. Bone. 2010;47:729–735. doi: 10.1016/j.bone.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 68.McCloskey E., Johansson H., Oden A., Aropuu A., Jalava T., Kanis J. Efficacy of clodronate on fracture risk in women selected by 10-year fracture probability. J Bone Miner Res. 2007;22:S131. [Google Scholar]
- 69.Michaelsson K., Melhus H., Ferm H., Ahlbom A., Pedersen N.L. Genetic liability to fractures in the elderly. Arch Intern Med. 2005;165:1825–1830. doi: 10.1001/archinte.165.16.1825. [DOI] [PubMed] [Google Scholar]
- 70.Richards J.B., Zheng H.F., Spector T.D. Genetics of osteoporosis from genome-wide association studies: advances and challenges. Nat Rev Genet. 2012;13:576–588. doi: 10.1038/nrg3228. [DOI] [PubMed] [Google Scholar]
- 71.Tran B.N.H., Nguyen N.D., Nguyen V.X., Center J.R., Eisman J.A., Nguyen T.V. Genetic profiling and individualized prognosis of fracture. J Bone Miner Res. 2011;26:414–419. doi: 10.1002/jbmr.219. [DOI] [PubMed] [Google Scholar]
- 72.Lee S.H., Lee S.W., Ahn S.H., Kim T., Lim K.H., Kim B.J. Multiple gene polymorphisms can improve prediction of nonvertebral fracture in postmenopausal women. J Bone Miner Res. 2013;28:2156–2164. doi: 10.1002/jbmr.1955. [DOI] [PubMed] [Google Scholar]
- 73.Lee S.H., Cho E.H., Ahn S.H., Kim H.M., Lim K.H., Kim B.J. Prediction of future osteoporotic fracture occurrence by genetic profiling: a 6-year follow-up observational study. J Clin Endocrinol Metab. 2016;101:1215–1224. doi: 10.1210/jc.2015-3972. [DOI] [PubMed] [Google Scholar]
- 74.Ho-Le T.P., Center J.R., Eisman J.A., Nguyen H.T., Nguyen T.V. Prediction of bone mineral density and fragility fracture by genetic profiling. J Bone Miner Res. 2017;32:285–293. doi: 10.1002/jbmr.2998. [DOI] [PubMed] [Google Scholar]
- 75.Eriksson J., Evans D.S., Nielson C.M., Shen J., Srikanth P., Hochberg M. Limited clinical utility of a genetic risk score for the prediction of fracture risk in elderly subjects. J Bone Miner Res. 2015;30:184–194. doi: 10.1002/jbmr.2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pothuaud L., Barthe N., Krieg M.A., Mehsen N., Carceller P., Hans D. Evaluation of the potential use of trabecular bone score to complement bone mineral density in the diagnosis of osteoporosis: a preliminary spine BMD-matched, case-control study. J Clin Densitom. 2009;12:170–176. doi: 10.1016/j.jocd.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 77.Hans D., Barthe N., Boutroy S., Pothuaud L., Winzenrieth R., Krieg M.A. Correlations between trabecular bone score, measured using anteroposterior dual-energy X-ray absorptiometry acquisition, and 3-dimensional parameters of bone microarchitecture: an experimental study on human cadaver vertebrae. J Clin Densitom. 2011;14:302–312. doi: 10.1016/j.jocd.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 78.Leslie W.D., Aubry-Rozier B., Lamy O., Hans D. Manitoba Bone Density P. TBS (trabecular bone score) and diabetes-related fracture risk. J Clin Endocrinol Metab. 2013;98:602–609. doi: 10.1210/jc.2012-3118. [DOI] [PubMed] [Google Scholar]
- 79.Silva B.C., Leslie W.D., Resch H., Lamy O., Lesnyak O., Binkley N. Trabecular bone score: a noninvasive analytical method based upon the DXA image. J Bone Miner Res. 2014;29:518–530. doi: 10.1002/jbmr.2176. [DOI] [PubMed] [Google Scholar]
- 80.McCloskey E.V., Oden A., Harvey N.C., Leslie W.D., Hans D., Johansson H. A meta-analysis of trabecular bone score in fracture risk prediction and its relationship to FRAX. J Bone Miner Res. 2016;31:940–948. doi: 10.1002/jbmr.2734. [DOI] [PubMed] [Google Scholar]
- 81.Akesson K., Ljunghall S., Jonsson B., Sernbo I., Johnell O., Gardsell P. Assessment of biochemical markers of bone metabolism in relation to the occurrence of fracture: a retrospective and prospective population-based study of women. J Bone Miner Res. 1995;10:1823–1829. doi: 10.1002/jbmr.5650101127. [DOI] [PubMed] [Google Scholar]
- 82.van Daele P.L., Seibel M.J., Burger H., Hofman A., Grobbee D.E., van Leeuwen J.P. Case-control analysis of bone resorption markers, disability, and hip fracture risk: the Rotterdam study. Bmj. 1996;312:482–483. doi: 10.1136/bmj.312.7029.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Garnero P., Hausherr E., Chapuy M.C., Marcelli C., Grandjean H., Muller C. Markers of bone resorption predict hip fracture in elderly women: the EPIDOS Prospective Study. J Bone Miner Res. 1996;11:1531–1538. doi: 10.1002/jbmr.5650111021. [DOI] [PubMed] [Google Scholar]
- 84.Meier C., Nguyen T.V., Center J.R., Seibel M.J., Eisman J.A. Bone resorption and osteoporotic fractures in elderly men: the dubbo osteoporosis epidemiology study. J Bone Miner Res. 2005;20:579–587. doi: 10.1359/JBMR.041207. [DOI] [PubMed] [Google Scholar]
- 85.Johansson H., Oden A., Kanis J.A., McCloskey E.V., Morris H.A., Cooper C. A meta-analysis of reference markers of bone turnover for prediction of fracture. Calcif Tissue Int. 2014;94:560–567. doi: 10.1007/s00223-014-9842-y. [DOI] [PubMed] [Google Scholar]
- 86.Eller-Vainicher C., Chiodini I., Santi I., Massarotti M., Pietrogrande L., Cairoli E. Recognition of morphometric vertebral fractures by artificial neural networks: analysis from GISMO Lombardia Database. PLoS One. 2011;6 doi: 10.1371/journal.pone.0027277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lin C.-C., Ou Y.-K., Chen S.-H., Liu Y.-C., Lin J. Comparison of artificial neural network and logistic regression models for predicting mortality in elderly patients with hip fracture. Injury. 2010;41:869–873. doi: 10.1016/j.injury.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 88.Kruse C., Eiken P., Vestergaard P. Machine learning principles can improve hip fracture prediction. Calcif Tissue Int. 2017;100:348–360. doi: 10.1007/s00223-017-0238-7. [DOI] [PubMed] [Google Scholar]
- 89.Ho-Le T.P., Center J.R., Eisman J.A., Nguyen T.V., Nguyen H.T. Prediction of hip fracture in post-menopausal women using artificial neural network approach. Conf Proc IEEE Eng Med Biol Soc. 2017;2017:4207–4210. doi: 10.1109/EMBC.2017.8037784. [DOI] [PubMed] [Google Scholar]
- 90.Shmueli G. To explain or to predict. Stat Sci. 2010;25:289–310. [Google Scholar]
- 91.Lo A., Chernoff H., Zheng T., Lo S.H. Why significant variables aren't automatically good predictors. Proc Natl Acad Sci U S A. 2015;112:13892–13897. doi: 10.1073/pnas.1518285112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pepe M.S., Janes H., Longton G., Leisenring W., Newcomb P. Limitations of the odds ratio in gauging the performance of a diagnostic, prognostic, or screening marker. Am J Epidemiol. 2004;159:882–890. doi: 10.1093/aje/kwh101. [DOI] [PubMed] [Google Scholar]
- 93.Nguyen T.V., Center J.R., Eisman J.A. Femoral neck bone loss predicts fracture risk independent of baseline BMD. J Bone Miner Res. 2005;20:1195–1201. doi: 10.1359/JBMR.050215. [DOI] [PubMed] [Google Scholar]
- 94.Cheung E., Cheung C.L., Kung A.W., Tan K.C. Possible FRAX-based intervention thresholds for a cohort of Chinese postmenopausal women. Osteoporos Int. 2014;25:1017–1023. doi: 10.1007/s00198-013-2553-9. [DOI] [PubMed] [Google Scholar]
- 95.Chen X.F., Li X.L., Zhang H., Liu G.J. Were you identified to be at high fracture risk by FRAX(R) before your osteoporotic fracture occurred? Clin Rheumatol. 2014;33:693–698. doi: 10.1007/s10067-014-2533-2. [DOI] [PubMed] [Google Scholar]
- 96.Min Y.K., Lee D.Y., Park Y.S., Moon Y.W., Lim S.J., Lee Y.K. A FRAX experience in Korea: fracture risk probabilities with a country-specific versus a surrogate model. J Bone Metab. 2015;22:113–118. doi: 10.11005/jbm.2015.22.3.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cheng S.Y., Levy A.R., Lefaivre K.A., Guy P., Kuramoto L., Sobolev B. Geographic trends in incidence of hip fractures: a comprehensive literature review. Osteoporos Int. 2011;22:2575–2586. doi: 10.1007/s00198-011-1596-z. [DOI] [PubMed] [Google Scholar]
- 98.Zhou W., Christiani D.C. East meets West: ethnic differences in epidemiology and clinical behaviors of lung cancer between East Asians and Caucasians. Chin J Canc. 2011;30:287–292. doi: 10.5732/cjc.011.10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tillin T., Hughes A.D., Whincup P., Mayet J., Sattar N., McKeigue P.M. Ethnicity and prediction of cardiovascular disease: performance of QRISK2 and Framingham scores in a U.K. tri-ethnic prospective cohort study (SABRE–Southall and Brent REvisited) Heart. 2014;100:60–67. doi: 10.1136/heartjnl-2013-304474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cosman F., de Beur S.J., LeBoff M.S., Lewiecki E.M., Tanner B., Randall S. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359–2381. doi: 10.1007/s00198-014-2794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]