Abstract
Objectives
We compared the effectiveness of bisphosphonates combined with activated vitamin D administered for therapy of aromatase inhibitor-induced osteoporosis after a breast cancer operation and primary postmenopausal osteoporosis through propensity score matching.
Methods
Forty-eight postmenopausal patients with estrogen receptor-positive early breast cancer, who had postoperative adjuvant treatment with aromatase inhibitors and whose T-score of bone mineral density (BMD) decreased below −2.5 (AI group), and 48 patients of primary postmenopausal osteoporosis (PO group) enrolled in this retrospective observational study. They were administered monthly risedronate or minodronate, and daily alfacalcitol or eldecalcitol were combined. Their BMD (L2–4, L-BMD), serum-corrected calcium, serum phosphate, tartrate-resistant acid phosphatase 5b (TRACP-5b), bone alkaline phosphatase (BAP), estimated glomerular filtration rate, urine calcium/creatinine ratio, intact-parathyroid hormone, and 25-hydroxy vitamin D were measured before treatment and until 24 months.
Results
L-BMD values increased with time compared with the baseline values in each group, and there was no significant difference in the groups. Percentage value of TRACP-5b decreased rapidly after 6 months and maintained low level until 24 months in both groups. Percentage value of BAP in the AI group decreased continuously until 24 months. In contrast, the percentage change in the PO group plateaued after 6 months.
Conclusions
It is suggested that monthly oral bisphosphonate combined with activated Vitamin D is an effective therapy to increase BMD in the aromatase inhibitor-induced osteoporosis after breast cancer operation. Monitoring of kidney function and concentration of Ca in blood and urine may be necessary.
Keywords: Breast cancer, Aromatase inhibitor-induced osteoporosis, Primary postmenopausal osteoporosis, Monthly oral bisphosphonate, Activated vitamin D
1. Introduction
About two-thirds of all breast cancer patients are hormone-dependent, either estrogen receptor or progesterone receptor which are expressed by tumor cells [1]. Therefore, endocrine therapy is an important option in the adjuvant treatment by 2 mechanisms: to prevent cancer cells to interact with estrogen receptors by use of selective estrogen receptor modulators (SERMs) and to inhibit tissue conversion of androgen into estrogen with aromatase inhibitors. Tamoxifen, one of the SERMs, has been the standard care for the adjuvant treatment of breast cancer, however, aromatase inhibitors have shown better overall responses than tamoxifen by decreased estrogen production, that results in reduced risk of recurrence in postmenopausal women with breast cancer [[2], [3], [4]]. The third generation aromatase inhibitors, anastrozole, letrozole and exemestane, are used recently for first-line hormonal therapy in these women. However, these aromatase inhibitors cause significant enhancement of bone turnover markers and are responsible for accelerated bone loss, resulting in increased fracture incidence [5,6]. Because advances in breast cancer treatment of postmenopausal women have led to long-term survival improvement, increased bone loss and risk of fracture should be prevented promptly. The American Association of Clinical Oncology, as well as the European Society for Clinical and Economical aspects of Osteoporosis and Osteoarthritis, proposed orientations regarding this issue to start bisphosphonate therapy for prevention of fragile fractures in all patients with a T-score below −2.5 [7,8]. However, there are very few reports that compared effectiveness of bisphosphonates administered for therapy of aromatase inhibitor-induced osteoporosis after breast cancer operation and primary postmenopausal osteoporosis. In this retrospective observational study, the effect of oral bisphosphonates combined with activated vitamin D in these 2 groups was compared. A propensity score-matched analysis was used to assess and compare the outcomes between the groups after eliminating confounders such as age, bone mineral density (BMD) of lumbar spine and biochemical markers of bone turnover.
2. Methods
Fifty-eight postmenopausal patients with estrogen receptor-positive early breast cancer, who had postoperative adjuvant treatment with aromatase inhibitors in our institutions between April 2014 and March 2017, enrolled in this case-control study conducted in 2 centers. Study design of this research is shown as Fig. 1. During aromatase inhibitor therapy BMD was measured every 6 months, and in the case of those whose T-score decreased below −2.5 administrations monthly oral bisphosphonates combined with activated vitamin Ds was indicated (AI group). As a control, the same number of cases as AI group whose BMDs of baseline were included in the range of mean ± standard deviation were selected randomly from the patients of primary postmenopausal osteoporosis who had combination therapy of monthly oral bisphosphonates and activated vitamin D between April 2014 and March 2017 (PO group). Risedronate 75 mg or minodronate 50 mg per month, and alfacalcitol 0.5 μg or eldecalcitol 0.75 μg per day were combined. Patients were excluded when they had a history of poorly controlled hyper tension (higher than 180 over 110 mmHg) or diabetes mellitus (hemoglobin A1c level greater than 7.0%), chronic kidney disease in the stage of 3 and 4 or, hyper- or hypothyroidism, or other complications that influence bone metabolism, as well as any previously prescribed osteoporosis treatment including hormone replacement therapy.
Fig. 1.
Study design of this research. AI group, therapy of aromatase inhibitor-induced osteoporosis; PO group, patients of primary postmenopausal osteoporosis; RIS, risedronate; ALF, alfacalcidol; ELD, eldecalcitol; MIN, minodronate.
This study was approved by the Ethics Review Committee at Kawakita General Hospital institutional ethics review boards prior to its commencement (H2016-0019) and was conducted in accordance with the ethical tenets outlined in the 1964 Declaration of Helsinki for research involving human subjects. Written informed consent was obtained from all patients after detailed explanation of therapeutic agents.
2.1. BMD assessment
For each group, we measured lumber spine (L2–4) BMD (L-BMD) at the beginning of treatment and at 6, 12, 18, and 24 months after the start of therapy. Primary end point of this study was assessment of effectiveness of oral bisphosphonates combined with activated vitamin D for the aromatase inhibitor-induced osteoporosis after breast cancer operation by increase of BMD. BMD was measured using dual-energy X-ray absorptiometry (HOLOGIC Co., QDR4500, Tokyo, Japan). When existing fracture or ectopic ossification in lumber spine was found, this region was excluded from evaluation. The cases of advanced osteoarthritis and severe scoliosis of spine were also omitted.
2.2. Biochemical markers of bone turnover
We investigated tartrate-resistant acid phosphatase 5b (TRACP-5b), bone alkaline phosphatase (BAP), serum-corrected calcium (corrected Ca), serum phosphate, and estimated glomerular filtration rate (eGFR) before treatment and at 6, 12, 18, and 24 months after the commencement of therapy. For measurement of the bone turnover markers, TRACP-5b (Enzyme Immunoassay, DS Pharma Biomedical Co., Ltd., Osaka, Japan) and BAP (chemiluminescence enzyme immunoassay, Beckman Coulter, Inc., CA, USA) were used, respectively. Intact-parathyroid hormone (PTH) and, Ca and creatinine (Cr) in urine, which were used to calculate Ca/Cr ratio, were obtained before treatment and after 12 and 24 months. 25-Hydroxy vitamin D (25(OH)D) was also measured before treatment.
2.3. Statistical analysis
In order to eliminate the confounding effects of the nonrandom assignment of patients when assessing the patient outcomes, we used a propensity score-matched analysis with the software R ver. 3.0.2 (The R Foundation for Statistical Computing, Vienna, Austria). The matching variables of age, body mass index, L-BMD, TRACP-5b, BAP, corrected Ca, serum iP, eGFR, PTH, Ca/Cr ratio, and 25(OH)D were inserted for a propensity score matching algorithm between the AI and PO groups. However, we could not match the propensity scores successfully when age was included in the examined factor. Therefore, it was excluded from the matching variables. Overall, 48 patients in each AI and PO group were enrolled before statistical comparison. The actual measurement values over time of the examined factors were compared with the baseline values in each group using a Wilcoxon signed-rank test. The percentage changes of these examined factors from the baseline values were compared between the groups using a Mann-Whitney U-test. The statistical significance between the 25(OH)D and percentage change in bone turnover markers, TRACP-5b and BAP, was detected using the Pearson product-moment correlation coefficient (P < 0.05). A P-value of <0.05 was considered to be statistically significant.
3. Results
Forty-eight patients in each AI and PO group were included in the final statistical analyses, whose baseline data are summarized on Table 1, Table 2. Because a peak age of onset of breast cancer is forties, age of patients in AI group was significantly younger than that in PO group. No patient complained of any major acute phase response complication, such as fever or joint pain, nor were any fresh fragile fractures encountered in the study period. Hypercalcemia over 10.5 mg/dL of corrected Ca occurred in one and 2 cases in the AI and PO groups, respectively. These cases were administered eldecalcitol concurrently.
Table 1.
Baseline clinical characteristics.
| Characteristic | AI group (n = 48) | PO group (n = 48) | P-value |
|---|---|---|---|
| Age, yr | 63.5 ± 8.2 (51–78) | 76.4 ± 6.6 (63–85) | <0.05 |
| BMI, kg/cm2 | 20.0 ± 7.4 | 20.8 ± 3.7 | NS |
| L-BMD, g/cm2 | 0.7 ± 0.1 | 0.7 ± 0.1 | NS |
| TRACP-5b, mU/dL | 376.2 ± 121.7 | 473.3 ± 199.5 | NS |
| BAP, μg/L | 22.5 ± 10.5 | 15.2 ± 7.7 | NS |
| Corrected Ca, mg/dL | 9.6 ± 0.3 | 9.3 ± 0.4 | NS |
| Serum iP, mg/dL | 3.9 ± 0.4 | 3.6 ± 0.5 | NS |
| eGFR, mL/min/1.73m2 | 81.8 ± 15.2 | 72.6 ± 21.0 | NS |
| 25(OH)D, ng/mL | 21.0 ± 4.7 | 16.3 ± 5.9 | NS |
| Intact-PTH, pg/mL | 56.0 ± 23.9 | 35.8 ± 14.6 | NS |
Values are presented as mean ± standard deviation (range).
AI group, therapy of aromatase inhibitor-induced osteoporosis; PO group, patients of primary postmenopausal osteoporosis.
BMI, body mass index; L-BMD, L2–4 bone mineral density; TRACP-5b, tartrate-resistant acid phosphatase 5b; BAP, bone alkaline phosphatase; corrected Ca, serum-corrected calcium; serum iP, serum phosphate; GFR, estimated glomerular filtration rate; 25(OH)D, 25-hydroxy vitamin D; PTH, parathyroid hormone; NS, not significant.
Propensity score-matched analysis.
Table 2.
Baseline clinical characteristics.
| Variable | AI group (n = 48) | PO group (n = 48) |
|---|---|---|
| Previous fracture | Vertebral fracture (n = 2) Radius fracture (n = 2) |
Vertebral fracture (n = 38) Femoral neck fracture (n = 6) Pelvic fracture (n = 2) |
| Complications | Endometriosis (n = 2) Myoma uteri (n = 2) Hyperlipidaemia (n = 2) Hypertension (n = 2) Diabetes mellitus (n = 2) Thyroid cancer (n = 2) |
Hyperlipidaemia (n = 6) Hypertension (n = 4) Asthma (n = 2) Osteoarthrosis (n = 2) Diabetes mellitus (n = 4) Behcet disease (n = 2) |
AI group, therapy of aromatase inhibitor-induced osteoporosis; PO group, patients of primary postmenopausal osteoporosis.
3.1. Change in BMD
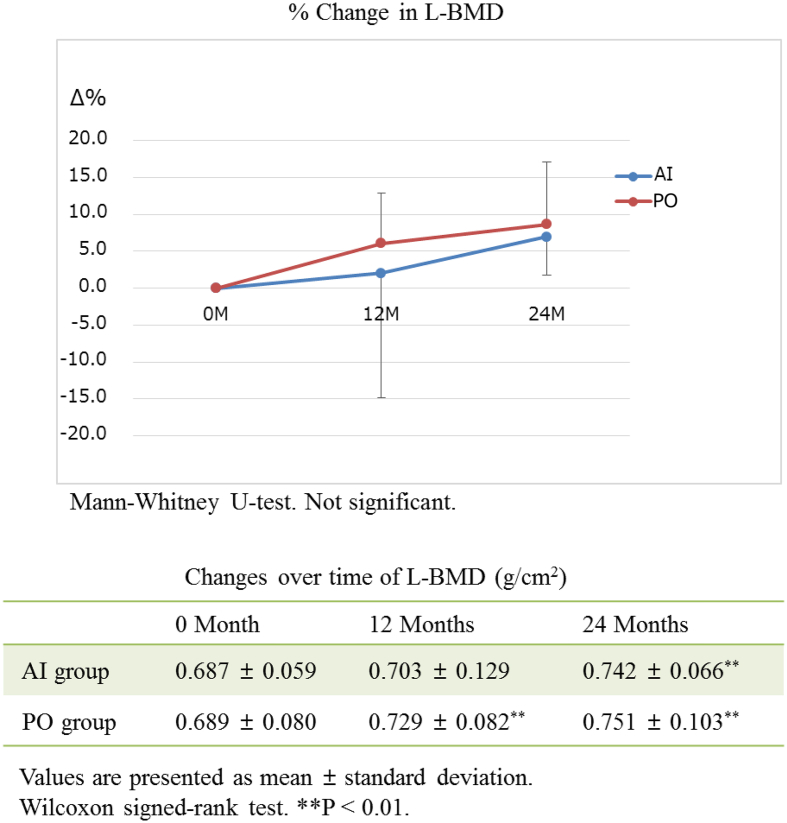
The actual measured L-BMD values increased with time compared with the baseline values in each group (P < 0.01) (Fig. 2). There was no significant difference in the actual measured L-BMD value nor percentage change in L-BMD in the groups.
Fig. 2.
The actual measured L-BMD values increase with time compared with the baseline values in each group (P < 0.01). There is no significant difference in the actual measured L-BMD value nor percentage change in L-BMD in the groups. L-BMD, L2–4 bone mineral density; AI group, therapy of aromatase inhibitor-induced osteoporosis; PO group, patients of primary postmenopausal osteoporosis.
3.2. Biochemical markers of bone turnover
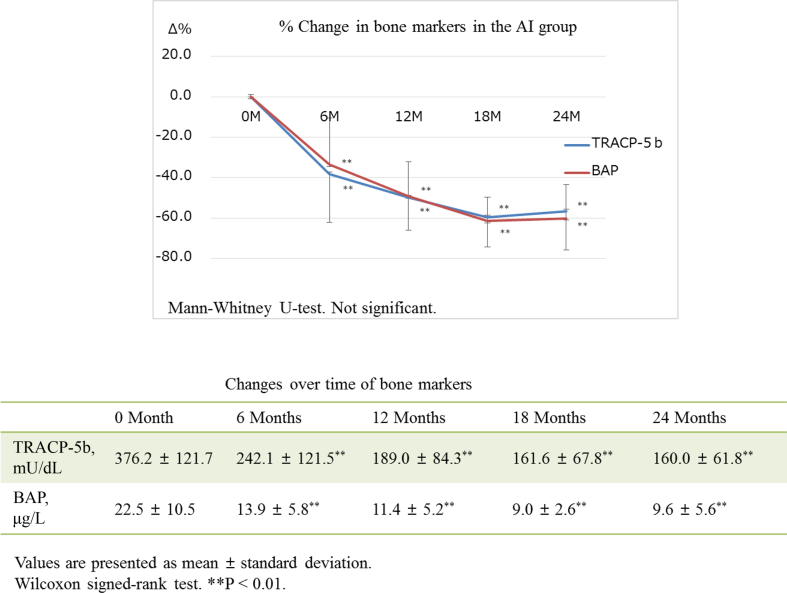
The actual measured value in TRACP-5b decreased significantly after 6 months compared with baseline value and continued to slowly decrease until 24 months after the commencement of therapy in each group (Fig. 3, Fig. 4). There was no significant difference in the actual measured value in TRACP-5b in the groups. The actual measured values in BAP decreased significantly after 6 months compared with baseline value and continued to decrease in each group down to the same level after 24 months (Fig. 3, Fig. 4). There was no significant difference in the actual measured value in BAP in the groups. Percentage value of TRACP-5b decreased rapidly after 6 months and maintained low level until 24 months in both groups (Fig. 3, Fig. 4). There was no significant difference in the percentage value of TRACP-5b in the groups. Percentage value of BAP in the AI group decreased continuously until 24 months (Fig. 3). In contrast, the percentage change of BAP in the PO group plateaued after 6 months (Fig. 4). The 25(OH)D value positively correlated with percentage change in TRACP-5b through the observation period (each P < 0.01) and in BAP at 6 months (P < 0.01) in the AI group. There was no correlation between the 25(OH)D value and percentage change in bone turnover markers in the PO group through the observation period. The corrected Ca, Serum iP, eGFR, and intact-PTH value, as well as Urine Ca/Cr rate, maintained within normal ranges through the treatment period in both AI and PO groups (Table 3). However, the eGFR value decreased significantly compared with the baseline value in each group, after 18 months in the AI group and after 12 months in the PO group. The intact-PTH value also decreased significantly compared with the baseline value in each group after 24 months.
Fig. 3.
Both percentage value of TRACP-5b and BAP decrease continuously until 24 months in the AI group. The actual measured value in TRACP-5b and BAP decrease significantly after 6 months compared with baseline value and continue to slowly decrease until 24 months (P < 0.01). TRACP-5b, tartrate-resistant acid phosphatase 5b; BAP, bone alkaline phosphatase; AI group, therapy of aromatase inhibitor-induced osteoporosis; PO group, patients of primary postmenopausal osteoporosis.
Fig. 4.
Both percentage value and actual measured value of TRACP-5b and BAP decrease rapidly after 6 months and maintain low level until 24 months in the PO group. The actual measured value in TRACP-5b and BAP decrease significantly after 6 months compared with baseline value until 24 months (P < 0.01). TRACP-5b, tartrate-resistant acid phosphatase 5b; BAP, bone alkaline phosphatase; AI group, therapy of aromatase inhibitor-induced osteoporosis; PO group, patients of primary postmenopausal osteoporosis.
Table 3.
Changes over time of the variables.
| Variable | 0 Month | 6 Months | 12 Months | 18 Months | 24 Months |
|---|---|---|---|---|---|
| AI group | |||||
| Corrected Ca, mg/dL | 9.6 ± 0.3 | 9.6 ± 0.3 | 9.5 ± 0.3 | 9.6 ± 0.4 | 9.5 ± 0.4 |
| Serum iP, mg/dL | 3.9 ± 0.4 | 3.6 ± 0.4* | 3.5 ± 0.3** | 3.6 ± 0.5** | 3.4 ± 0.4** |
| eGFR, mL/min/1.73 m2 | 81.8 ± 15.2 | 82.5 ± 15.6 | 79.9 ± 16.2 | 78.3 ± 15.4** | 76.1 ± 11.6** |
| Urine Ca/Cr ratio | 0.24 ± 0.19 | – | 0.18 ± 0.10 | – | 0.18 ± 0.11 |
| Intact-PTH, pg/mL | 56.0 ± 23.9 | – | 42.9 ± 17.1 | – | 49.7 ± 19.1** |
| PO group | |||||
| Corrected Ca, mg/dL | 9.3 ± 0.4 | 9.3 ± 0.4 | 9.4 ± 0.4 | 9.6 ± 0.4 | 9.5 ± 0.4 |
| Serum iP, mg/dL | 3.6 ± 0.5 | 3.8 ± 0.5 | 3.5 ± 0.4 | 3.5 ± 0.4 | 3.5 ± 0.4 |
| eGFR, mL/min/1.73 m2 | 72.6 ± 21.0 | 70.0 ± 16.4 | 68.2 ± 13.9* | 64.9 ± 12.1** | 63.4 ± 12.5** |
| Urine Ca/Cr ratio | 0.23 ± 0.15 | – | 0.29 ± 0.15* | – | 0.27 ± 0.14 |
| Intact-PTH, pg/mL | 35.8 ± 14.6 | – | 33.8 ± 18.5 | – | 31.2 ± 15.9** |
Values are presented as mean ± standard deviation.
AI group, therapy of aromatase inhibitor-induced osteoporosis; PO group, patients of primary postmenopausal osteoporosis.
Corrected Ca, serum-corrected calcium; serum iP, serum phosphate; eGFR, estimated glomerular filtration rate; Cr, creatinine; PTH, parathyroid hormone.
Wilcoxon signed-rank test.
*P < 0.05. **P < 0.01.
4. Discussion
Several studies that compared aromatase inhibitors with tamoxifen in postmenopausal breast cancer patients revealed a better overall response, disease-free survival and reduction of recurrence risk for aromatase inhibitors [2,3]. With the increasing use of aromatase inhibitors as an alternative to tamoxifen, their serious side effect, bone loss increasing risk of fracture, has to be addressed in postmenopausal women under adjuvant-aromatase inhibitor treatment of breast cancer for their bone health and quality of life. For this purpose, increased physical activity, and vitamin D (a dose of up to 10,000 IU/wk or at least 800 IU/day) and calcium (at least 1000 mg/day) supplementation are recommended [7,8]. In the patients with several risk factors such as age, smoking, alcohol intake, previous fragility fracture, corticosteroid therapy, diseases or anticancer therapies that may contribute to impaired bone health, BMD is to be measured regularly. When T-score decreases below −2.5, antiosteoporosis therapy using bisphosphonates [[9], [10], [11], [12], [13]] or denosumab [14] should be started. It is reported that reduction rate of BMD in aromatase inhibitor-induced osteoporosis is significantly rapid compared to postmenopausal osteoporosis [15,16], however, there are no previous clinical reports that compared the efficacy of bisphosphonate therapy in combination with activated Vitamin D on these pathological conditions, as far as we know. In our study, the effect of monthly oral bisphosphonates combined with activated vitamin Ds, which are used extensively for treatment of osteoporosis, in the AI and PO groups was compared through propensity score matching. L-BMD values increased with time compared with the baseline values in each group, and there was no significant difference in the actual measured L-BMD value nor percentage change in L-BMD in the groups. These results suggest that bisphosphonate therapy combined with activated Vitamin D is effective in the aromatase inhibitor-induced osteoporosis as in postmenopausal osteoporosis. Concentration of 25(OH)D provides a useful index of sufficiency level of vitamin D in the body and there is a report that 25(OH)D of postmenopausal women with an existing vertebral fracture was less than 20 ng/mL [17]. In this study, average serum 25(OH)D was 21.0 ng/mL in the AI group and 16.3 ng/mL in the PO group, that suggests that postmenopausal women in this group were deficient in vitamin D and at high risk of low BMD and fragile fracture. Therefore, they were all administered activated vitamin D through the observation period and reached normal levels of vitamin D.
Percentage value of TRACP-5b decreased rapidly after 6 months and maintained low level until 24 months in both groups. Percentage value of BAP in the AI group decreased continuously until 24 months. In contrast, the percentage changes in the PO group plateaued after 6 months. The mechanism of different decreasing patterns of these markers in the groups is unknown. However, the fact that the BAP/TRACP-5b ratio in the AI group tended to be higher than in the PO group may suggest because the age of patients in the AI group was younger than in the PO group, compensatory activation of bone formation for enhanced bone absorption caused by deficiency in estrogen, has been maintained relatively. Aromatase inhibitors enhance bone turnover significantly and cause accelerated bone loss, which results in increased fracture risk. Meanwhile, the L-BMD values increased with time as in the PO group even in the AI group in which decrease rate of bone mass is large. Considering that the increasing rate of BMD of lumbar vertebra is low when concentration of serum 25(OH)D is low [18,19], the fact that the 25(OH)D value in the baseline tended to be larger in the AI group than in the PO group may affect the result. Furthermore, the 25(OH)D value positively correlated with percentage change in TRACP-5b through the observation period in the AI group, but not in the PO group. It is suggested that the higher the 25(OH)D value in the base line is, the more rapidly the bone metabolism maker decreases, that can result in increase of bone density.
Hypercalcemia occurred in 1 and 2 patients in the AI and PO groups, respectively, and all 3 cases were coadministered eldecalcitol. Although both eldecalcitol and alfacalcitol are activated vitamin D, the former is superior to the latter in the increasing effect of BMD and preventive effect of vertebral fracture [20]. On the contrary, the risk of hypercalcemia and hypercalciuria accompanied by administration of eldecalcitol is reported as in alfacalcitol [20]. Therefore, monitoring of kidney function and concentration of Ca in blood and urine may be necessary when activated vitamin D is administered for a long time in the aromatase inhibitor therapy.
Fifty-eight patients with breast cancer, who had postoperative adjuvant treatment with aromatase inhibitors, enrolled in this case-control study. The information on the stage, histologic type, estrogen-receptor or progesterone-receptor positivity, and other molecular markers of breast cancer group should be provided in order to show the homogeneity of the AI group. More specifically, the AI group is divided into the subgroups according to these clinical data and the efficacy of combined therapy of monthly oral bisphosphonate and activated vitamin D should be compared with each subgroup. We would like to make an attempt on such detailed analysis in the next study.
5. Conclusion
It is suggested that monthly oral bisphosphonate combined with activated vitamin D is an effective therapy to increase BMD in the aromatase inhibitor-induced osteoporosis after breast cancer operation. Monitoring of kidney function and concentration of Ca in blood and urine may be necessary because the eGFR and intact-PTH value decreased over time.
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Footnotes
Peer review under responsibility of The Korean Society of Osteoporosis.
References
- 1.Rugo H.S. The breast cancer continuum in hormone-receptor-positive breast cancer in postmenopausal women: evolving management options focusing on aromatase inhibitors. Ann Oncol. 2008;19:16–27. doi: 10.1093/annonc/mdm282. [DOI] [PubMed] [Google Scholar]
- 2.Oyan B. Potential role of bone-directed therapy on the superiority of aromatase inhibitors over tamoxifen. Med Hypotheses. 2011;77:1028–1030. doi: 10.1016/j.mehy.2011.08.040. [DOI] [PubMed] [Google Scholar]
- 3.Goss P.E., Ingle J.N., Pritchard K.I., Ellis M.J., Sledge G.W., Budd G.T. Exemestane versus anastrozole in postmenopausal women with early breast cancer: NCIC CTG MA.27--a randomized controlled phase III trial. J Clin Oncol. 2013;31:1398–1404. doi: 10.1200/JCO.2012.44.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howell A., Cuzick J., Baum M., Buzdar A., Dowsett M., Forbes J.F. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet. 2005;365:60–62. doi: 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 5.Hadji P. Aromatase inhibitor-associated bone loss in breast cancer patients is distinct from postmenopausal osteoporosis. Crit Rev Oncol Hematol. 2009;69:73–82. doi: 10.1016/j.critrevonc.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 6.Cuzick J., Sestak I., Baum M., Buzdar A., Howell A., Dowsett M. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010;11:1135–1141. doi: 10.1016/S1470-2045(10)70257-6. [DOI] [PubMed] [Google Scholar]
- 7.izzoli R., Body J.J., DeCensi A., Reginster J.Y., Piscitelli P., Brandi M.L. Guidance for the prevention of bone loss and fractures in postmenopausal women treated with aromatase inhibitors for breast cancer: an ESCEO position paper. Osteoporos Int. 2012;23:2567–2576. doi: 10.1007/s00198-011-1870-0. [DOI] [PubMed] [Google Scholar]
- 8.Hillner B.E., Ingle J.N., Chlebowski R.T., Gralow J., Yee G.C., Janjan N.A. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol. 2003;21:4042–4057. doi: 10.1200/JCO.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Van Poznak C., Hannon R.A., Mackey J.R., Campone M., Apffelstaedt J.P., Clack G. Prevention of aromatase inhibitor-induced bone loss using risedronate: the SABRE trial. J Clin Oncol. 2010;28:967–975. doi: 10.1200/JCO.2009.24.5902. [DOI] [PubMed] [Google Scholar]
- 10.Lester J.E., Dodwell D., Purohit O.P., Gutcher S.A., Ellis S.P., Thorpe R. Prevention of anastrozole-induced bone loss with monthly oral ibandronate during adjuvant aromatase inhibitor therapy for breast cancer. Clin Canc Res. 2008;14:6336–6342. doi: 10.1158/1078-0432.CCR-07-5101. [DOI] [PubMed] [Google Scholar]
- 11.Brufsky A.M., Bosserman L.D., Caradonna R.R., Haley B.B., Jones C.M., Moore H.C. Zoledronic acid effectively prevents aromatase inhibitor-associated bone loss in postmenopausal women with early breast cancer receiving adjuvant letrozole: Z-FAST study 36-month follow-up results. Clin Breast Canc. 2009;9:77–85. doi: 10.3816/CBC.2009.n.015. [DOI] [PubMed] [Google Scholar]
- 12.Hines S.L., Sloan J.A., Atherton P.J., Perez E.A., Dakhil S.R., Johnson D.B. Zoledronic acid for treatment of osteopenia and osteoporosis in women with primary breast cancer undergoing adjuvant aromatase inhibitor therapy. Breast. 2010;19:92–96. doi: 10.1016/j.breast.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brufsky A.M., Harker W.G., Beck J.T., Bosserman L., Vogel C., Seidler C. Final 5-year results of Z-FAST trial: adjuvant zoledronic acid maintains bone mass in postmenopausal breast cancer patients receiving letrozole. Cancer. 2012;118:1192–1201. doi: 10.1002/cncr.26313. [DOI] [PubMed] [Google Scholar]
- 14.Body J.J. New developments for treatment and prevention of bone metastases. Curr Opin Oncol. 2011;23:338–342. doi: 10.1097/CCO.0b013e328347918b. [DOI] [PubMed] [Google Scholar]
- 15.Hadji P. Aromatase inhibitor-associated bone loss in breast cancer patients is distinct from postmenopausal osteoporosis. Crit Rev Oncol Hematol. 2009;69:73–82. doi: 10.1016/j.critrevonc.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Coleman R.E., Body J.J., Gralow J.R., Lipton A. Bone loss in patients with breast cancer receiving aromatase inhibitors and associated treatment strategies. Canc Treat Rev. 2008;34(Suppl 1):S31–S42. doi: 10.1016/j.ctrv.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Ikegami S., Kamimura M., Uchiyama S., Kato H. Women with insufficient 25-hydroxyvitamin D without secondary hyperparathyroidism have altered bone turnover and greater incidence of vertebral fractures. J Orthop Sci. 2011;16:573–580. doi: 10.1007/s00776-011-0107-6. [DOI] [PubMed] [Google Scholar]
- 18.Ishijima M., Sakamoto Y., Yamanaka M., Tokita A., Kitahara K., Kaneko H. Minimum required vitamin D level for optimal increase in bone mineral density with alendronate treatment in osteoporotic women. Calcif Tissue Int. 2009;85:398–404. doi: 10.1007/s00223-009-9295-x. [DOI] [PubMed] [Google Scholar]
- 19.Roux C., Binkley N., Boonen S., Kiel D.P., Ralston S.H., Reginster J.Y. Vitamin D status and bone mineral density changes during alendronate treatment in postmenopausal osteoporosis. Calcif Tissue Int. 2014;94:153–157. doi: 10.1007/s00223-013-9763-1. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka S., Kuroda T., Yamazaki Y., Shiraki Y., Yoshimura N., Shiraki M. Serum 25-hydroxyvitamin D below 25 ng/mL is a risk factor for long bone fracture comparable to bone mineral density in Japanese postmenopausal women. J Bone Miner Metabol. 2014;32:514–523. doi: 10.1007/s00774-013-0520-3. [DOI] [PubMed] [Google Scholar]