Abstract
Osteoporosis and fragility fractures have become major global public health concerns, and they can be prevented by maximizing peak bone mass during childhood and adolescence with weight-bearing physical activity, which can result in stronger and healthier bones that significantly decrease the risk of osteoporosis and fragility fractures in adulthood and the elderly years. From a public health perspective, implementing weight-bearing physical activity for children and adolescents is best achieved with school-based exercise interventions, and a review of school-based exercise interventions was conducted to determine their effectiveness in increasing bone mineral density (BMD) and/or bone mineral content (BMC). Seventeen studies were reviewed, all school-based exercise interventions utilized jumping exercises, and 15 of the 17 studies found at least one significant increase in measures of BMD and/or BMC for the total body, and/or at the hip, vertebrae, and/or wrist. One study that found no significant differences did report significant increases in bone structural strength, and the other study with no significant differences had exercises that measured and reported the lowest ground reaction forces (GRFs) of only 2–3 times body weight (BW), whereas the other studies that showed significant increase(s) in BMD and/or BMC had exercise with measured and reported GRFs ranging from 3.5 × to 8.8 × BW. School-based exercise interventions are time- and cost-efficient and effective in increasing BMD and/or BMC in children and adolescents, but must incorporate high-intensity exercise, such as high-impact jumping of sufficient GRFs, in order to significantly increase bone mineralization for osteoporosis and fragility fracture prevention later in life.
Keywords: Bone, Exercise, Fracture, Osteoporosis, School
1. Introduction
The World Health Organization (WHO) declares that osteoporosis is a major global public health problem that is highly prevalent in populations throughout the world [1]. Osteoporosis is a severe skeletal disease of reduced bone mineral density (BMD) that is clinically diagnosed as being 2.5 standard deviations below the adult peak mean [2], and this reduction of BMD decreases bone strength and increases its risk for fragility fractures. Fragility fractures are most prevalent at the hip, particularly the bone at the femoral neck; the vertebrae, particularly the bones of the lumbar spine; and at the wrist, such as the bone of the distal radius. Decreased BMD and diagnosed osteoporosis increase the risk of fragility fractures in populations [3]. Osteoporosis affects hundreds of millions of individuals from all over the world [4], and the amount of people who are susceptible to fragility fractures is underestimated, as many fragility fractures occur in individuals who do not even meet the clinical definition of osteoporosis [5], and the majority of individuals who suffer from low-trauma fractures do not have osteoporosis based on its clinical definition [6]. Also considered to be a “silent” disease, as most individuals with osteoporosis or low BMD do not know that they are at risk of bone and fragility fractures until they actually occur, it is also currently incurable, as there are no present treatments that are capable of fully replenishing the reduced BMD.
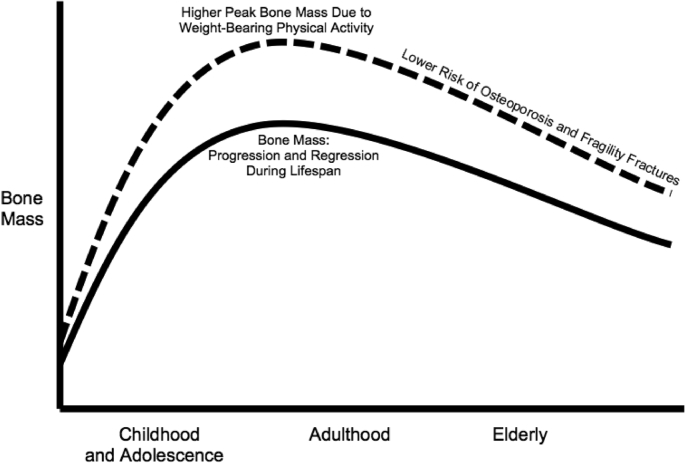
The WHO reports that there are numerous ways to prevent osteoporosis and fragility fractures, though the most optimal approach is to have adequate amounts of calcium intake and weight-bearing physical activity all throughout the lifespan, but especially during youth such as childhood and adolescence when BMD can be maximized, and this enhances bone strength to prevent osteoporosis and fragility fractures later in life into adulthood [1]. During the lifespan, most bone acquisition happens during youth at childhood and adolescence, and it can continue to minimally increase in early adulthood, but then continuously decreases in later adulthood and into the elderly years. Thus, childhood and adolescence is when maximal peak bone mass should be attained, in which it can be maintained throughout the rest of the lifespan [7]. Furthermore, of the two main osteoporosis preventive behaviors of calcium intake and weight-bearing physical activity, weight-bearing physical activity is a more important factor than calcium intake in achieving maximal peak bone mass during youth [8], particularly if it is high-impact jumping or high-intensity running or resistance training, as that may have higher impact on bone mass [9]. And higher peak bone mass at youth with weight-bearing physical activity during youth is sustained into older adulthood [10]. Therefore, children and adolescents who engage in weight-bearing physical activity have the ability to attain higher maximal peak bone mass in order to most optimally prevent osteoporosis and fragility fractures later in life (Fig. 1).
Fig. 1.
Bone health throughout the lifespan. The solid line represents the natural progression of bone mass increasing during childhood and adolescence into early adulthood, and then its natural regression of continuously decreasing into older adulthood and the elderly years, which increases the risk of osteoporosis and fragility fractures. The dashed line represents how weight-bearing physical activity creates larger increases in peak bone mass during childhood and adolescence, which leads to continuously higher bone mass into adulthood and the elderly years to reduce the risk of osteoporosis and fragility fractures.
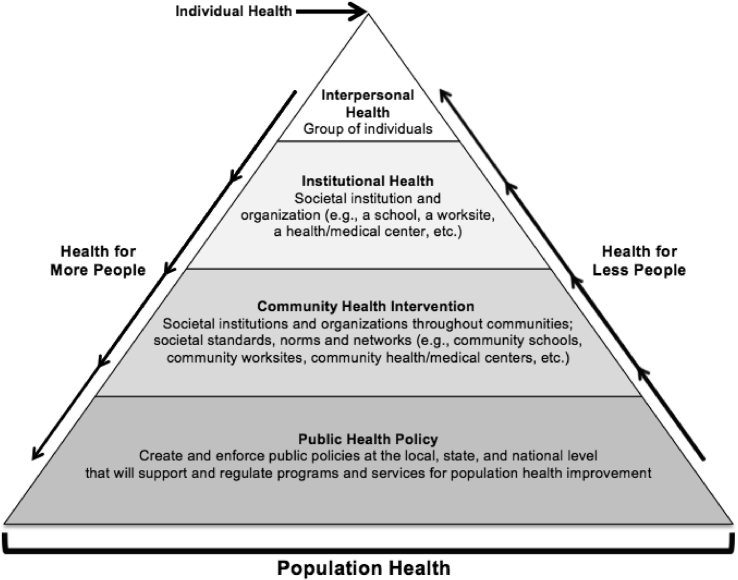
As a global public health problem, the social ecological model can be used in public health practice to affect different amounts of populations through various levels of health [11]. Implementing community health interventions will have more of an impact on population health improvement than traditionally used individual health interventions, as they impact more people and directly connect to public health policy, which can implement, enforce and support wide-spread community health interventions to have a greater impact on population health (Fig. 2). With children and adolescents being optimal populations for osteoporosis and fragility fracture prevention due to their ability to most efficiently increase BMD, schools are an ideal location to implement community health interventions incorporating weight-bearing physical activity for this population in public health practice.
Fig. 2.
Social ecological model to be applied for children and adolescents in osteoporosis and fragility fracture prevention.
School-based exercise intervention studies have investigated what specific types of weight-bearing physical activities can improve bone health, particularly in the assessment of bone mineralization measured in BMD and/or bone mineral content (BMC). The purpose of this review is to determine if and how school-based exercise interventions may increase bone mineralization in children and adolescents, in order to find what types of exercises should be implemented in school settings to effectively increase BMD and/or BMC.
2. Review of the literature
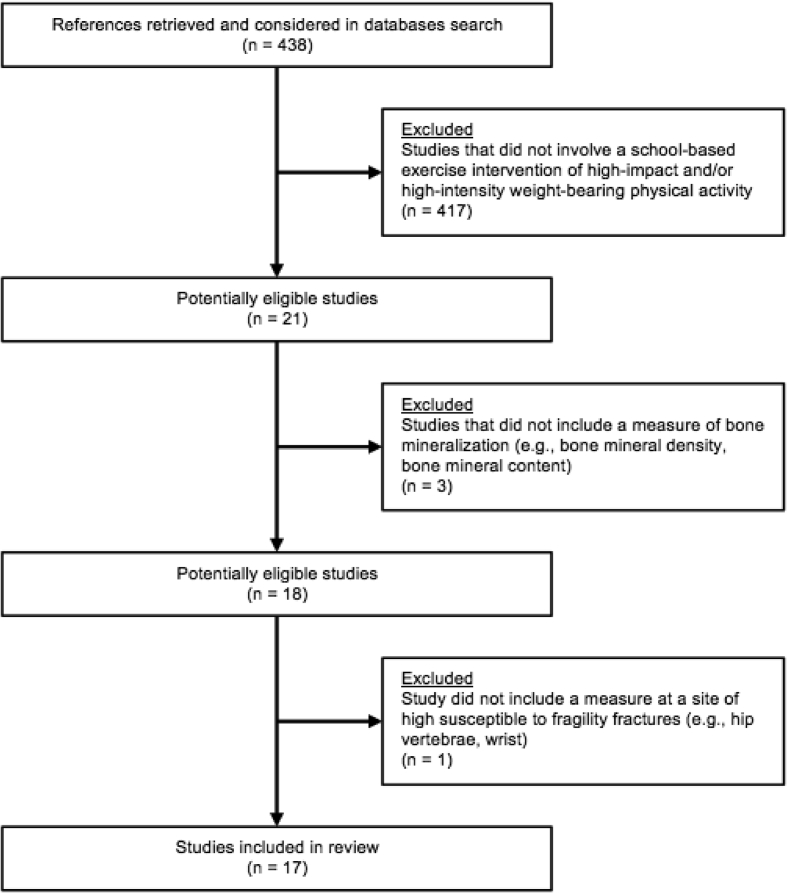
A review of the literature using PubMed and Google Scholar was conducted, and search terms entered included “school,” “bone,” “exercise,” and/or “physical” for “physical activity” or “physical education.” After a thorough examination of all search results, there were numerous studies of school-based exercise interventions, and due to various deciding factors, 21 studies were considered to be most suitable for this review of school-based controlled exercise interventions as they utilized high-impact and/or high-intensity weight-bearing physical activity specifically designed to improve bone health. Of those 21 studies, 3 did not include a measure of bone mineralization, such as BMD or BMC, and were excluded from the review. In addition, 1 study was excluded that did not include a measure at a skeletal site of high susceptibility to fragility fractures, which resulted in 17 articles found to be studies of school-based exercise interventions of high-impact and/or high-intensity weight-bearing physical activity that measured bone mineralization in either BMD and/or BMC of children and/or adolescents in comparison to control participants (Fig. 3, Table 1). As all 17 studies investigated at least one school-based exercise intervention [[12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28]], 1 study compared 2 different school-based exercise interventions [12], and 1 study included school-based exercise and/or nutrition interventions [18]. Of the 17 school-based exercise interventions, 15 studies found at least one significant increase in measures of BMD and/or BMC [12,13,[15], [16], [17],[19], [20], [21], [22], [23], [24], [25], [26], [27], [28]], and of the 2 studies that did not find significant increases [14,18], 1 of those studies did report increases in other measures of bone strength [14], and the other study found no significant differences [18]. School-based exercise interventions varied in the types and amounts of exercises performed, though all interventions included sessions that were from 10 to 40 minutes ranging from 2 to 5 times per week, and the duration of these studies ranged from 7 months up to 43 months, with one study reaching 91 months due to inclusion of follow-up measures. Exercises were integrated into physical education classes and/or into activities at school [[13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28]], or as an after school activity [12]. All 17 studies measured school-based exercise intervention effects on BMD and/or BMC at various locations of the skeleton, such as the total body and skeletal sites that are common areas of fragility fractures, including the femoral neck, proximal femur and trochanter for the hip; the lumbar spine for the vertebrae; and the distal radius for the wrist.
Fig. 3.
Flowchart of studies included in review.
Table 1.
Aspects of school-based exercise intervention on bone mineralization outcomes.
| Study (n = 17) | Participants description | School-based exercise intervention details | Bone mineralization outcomes |
|---|---|---|---|
| Larsen et al., 2018 [12] | Intervention groups: n = 95 boys and girls for Small-Sided Ball Game Group (SSG), mean age: 9.3 ± 0.4 years at pretest and 10.1 ± 0.4 years at posttest, n = 83 boys and girls for Circuit Strength Training (CST), mean age: 9.3 ± 0.3 years at pretest and 10.0 ± 0.3 at posttest. Control group: n = 116 boys and girls, mean age: 9.3 ± 0.3 years at pretest and 10.0 ± 0.4 years at posttest. | SSG: 40 minutes of 3-on-3 football, basketball and unihockey 3 days per week for 10 months. CST: 40 minutes of exercise periods (30s of exercise with 45s of rest) consisting of plyometric and strength training exercises such as jumps, sit ups, push ups, etc. 3 days per week for 10 months. (Frederikssund and Copenhagen, Denmark) | Significant increase in bone mineral density (BMD) for total body in SSG (P = 0.001) and CST (P = 0.005) and at leg in SSG (P < 0.001) and CST (P = 0.017), and in bone mineral content (BMC) for total body in CST (P < 0.01) and at leg in SSG (P = 0.002) and CST (P = 0.004) compared to control group. |
| Nogueira et al., 2015 [13] | Intervention group: n = 104 boys, mean age: 10.5 ± 0.5 years at baseline and 11.3 ± 0.5 years at follow-up (BMD/BMC measured: n = 12). Control group: n = 68 boys, mean age: 10.7 ± 0.6 years at baseline and 11.4 ± 0.6 years at follow-up (BMD/BMC measured: n = 6). | 10 Minutes of high-intensity movements based on capoeira consisting of approximately 20 jumps, hops, tuck jumps, and jump squats each, 60–70 star jumps, 10 jump lunges per leg, 50–60 gingea kicks, 15 handstands and cartwheels each, and 8 bencao with jumps per leg, 3 days per week for 9 months. (Gold Coast, Queensland, Australia) | Significant increase in BMC at the lumbar spine (P = 0.039) and distal radius (P = 0.03) in the intervention group compared to control group, but no significant differences for total body or at femoral neck. (Significant increase in calcaneal broadband ultrasound attenuation [BUA] in intervention group (P = 0.001) compared to control group.) |
| Nogueira et al., 2014 [14] | Intervention group: n = 71 girls, mean age: 10.5 ± 0.6 years at baseline and 11.3 ± 0.6 years at follow-up (BMD/BMC measured: n = 30). Control group: n = 67 girls, mean age: 10.7 ± 0.6 years at baseline and 11.4 ± 0.6 years at follow-up (BMD/BMC measured: n = 6). | 10 Minutes of high-intensity movements based on capoeira consisting of approximately 20 jumps, hops, tuck jumps, and jump squats each, 60–70 star jumps, 10 jump lunges per leg, 50–60 gingea kicks, 15 handstands and cartwheels each, and 8 bencao with jumps per leg, 3 days per week for 9 months. (Gold Coast, Queensland, Australia) | No significant differences in BMD or BMC for total body or at femoral neck, lumbar spine, trochanter or distal radius between intervention group and control group. (Significant increase in BUA in intervention group (P = 0.046) compared to control group. Significant increase in bone structural strength at lumbar spine (P = 0.006) in participants with BMD/BMC measures in intervention group compared to control group.) |
| Meyer et al., 2011 [15] | Intervention groups: n = 129 Prepubertal boys and girls, mean age: 8.3 ± 2.0 years, and n = 95 Early-pubertal boys and girls, mean age: 10.9 ± 1.0 years. Control groups: n = 29 Prepubertal boys and girls, mean age: 7.8 ± 1.8 years, n = 38 Early-pubertal boys and girls, mean age: 11.2 ± 0.9 years. | 10 Minutes of jumping activities consisting of hopping, jumping up and down stairs, and jump roping, 5 days per week for 1 school year. (Aargau and Baselland, Switzerland) | Significant increase in BMC for total body (P = 0.001) and at femoral neck (P = 0.037) and lumbar spine (P = 0.01) and in BMD for total body (P = 0.001) and lumbar spine (P ≤ 0.001) in intervention group compared to control group, but no significant difference in BMD at femoral neck between groups. No significant gender group interaction, but pubertal stage group interactions were significant for BMC for total body (P = 0.02) and at femoral neck (P = 0.001) and in BMD at lumbar spine (P = 0.001) for larger effect in prepubertal children than pubertal children. |
| Gunter et al., 2008 [16] | Intervention group: n = 33, 19 boys and 14 girls, mean age: 7.4 ± 1.0 years at baseline and 15.0 ± 1.3 years at follow-up for boys (n = 18), 7.9 ± 0.8 years at baseline and 15.5 ± 1.4 years at follow-up for girls (n = 11). Control group: n = 24, 16 boys and 8 girls, mean age: 7.9 ± 1.1 years at baseline and 15.3 ± 1.4 years at follow-up for boys (n = 13), 8.1 ± 0.8 years at baseline and 15.8 ± 0.9 years at follow-up for girls (n = 7). | 20 Minutes of jumping off a 61-cm box progressing from 50 up to 100 jumps per session, 3 times per week for 7 months, with annual reassessments up to 91 months. (Corvallis, OR, USA) | Significant increase in BMC at total hip, femoral neck and trochanter (P < 0.05) in intervention group compared to control group after 7-month intervention, and significantly greater BMC at the total hip (P < 0.05) in the intervention group compared to control group at 91-month follow-up, but no significant differences in femoral neck and trochanter. |
| Gunter et al., 2008 [17] | Intervention group: n = 101, 47 boys and 54 girls, mean age: 8.7 ± 0.9 years at baseline and 12.5 ± 1.0 years at follow-up for boys (n = 24), 8.7 ± 0.8 years at baseline and 12.2 ± 0.7 years at follow-up for girls (n = 32). Control group: n = 104, 51 boys and 53 girls, mean age: 8.7 ± 0.8 years at baseline and 12.3 ± 0.9 years at follow-up for boys (n = 27), 8.5 ± 0.9 years at baseline and 12.0 ± 0.9 years at follow-up for girls (n = 29). | Jumping sessions averaging 90–100 jumps per session, for 72 jumping sessions throughout 43 months. (Corvalis, OR, USA) | Significant increase in BMC for total body and at lumbar spine, femoral neck and trochanter (P < 0.05) in intervention group compared to control group. |
| Nichols et al., 2008 [18] | Intervention groups: n = 61 boys and girls for Only Exercise, mean age: 9.7 ± 0.3 years, n = 9 boys and girls for Only Nutrition, mean age: 9.6 ± 0.3 years, and n = 14 boys and girls for both exercise and nutrition, mean age: 9.7 ± 0.4. Control group: n = 28 boys and girls, mean age: 9.7 ± 0.5 years. | Exercise: 15 minutes of jumping consisting of tuck jumps, side-to-side jumps, forward/backward jumps, and over items jumps. Nutrition: Education on dairy products and other calcium-rich foods. 2 times per week for 20 months. (Denton, TX, USA) | No significant differences in BMC of total body or at femoral neck or lumbar spine, and no significant differences in BMD at the femoral neck or lumbar spine among groups. |
| Weeks et al., 2008 [19] | Intervention group: n = 52, 22 boys and 30 girls, mean age: 13.8 ± 0.4 years for boys and 13.7 ± 0.4 years for girls. Control group: n = 47, 24 boys and 23 girls, mean age: 13.8 ± 0.4 years for boys and 13.7 ± 0.5 years for girls. | "Preventing Osteoporosis With Exercise Regimes in Physical Education": 10 minutes of directed jumping activities at the beginning of physical education (PE) classes consisting of jump hops, tuck-jumps, jump-squats, stride jumps, star jumps, lunges, side lunges, and skipping, totaling approximately 300 jumps of 0.2–0.4 m in height, 2 times per week for 8 months. (Gold Coast, Queensland, Australia) | Significant increase in BMC at the femoral neck (P = 0.04) for girls but not boys in intervention group compared to control group, but no other significant differences in lumbar spine or whole body among groups. |
| Macdonald et al., 2008 [20] | Intervention group: n = 293, 151 boys and 142 girls, mean age: 10.2 ± 0.5 years at baseline and 11.4 ± 0.6 years at follow-up for boys, 10.2 ± 0.6 years at baseline and 11.4 ± 0.6 years at follow-up for girls. Control group: n = 117, 62 boys and 55 girls, mean age: 10.3 ± 0.7 years at baseline and 11.4 ± 0.6 years at follow-up for boys, 10.2 ± 0.5 years at baseline and 11.4 ± 0.5 years at follow-up for girls. | In addition to (2) 40-min PE classes, along with 15 minutes of physical activity for 5 days per week that included skipping, dancing, and resistance exercises with resistance bands. Also utilized "Bounce at the Bell": 10 counter movement jumps, 3 times per day (morning bell, noon bell, and dismissal bell), 2–3 times per week for 16 months. (Vancouver and Richmond, British Columbia, Canada) | Significant increase in BMC at total body (P = 0.03) for boys and femoral neck (P = 0.04) for girls in intervention group compared to control group, but no other significant differences between groups. |
| Linden et al., 2006 [21] | Intervention group: n = 49 girls, mean age: 7.6 ± 0.6 years at baseline, 9.7 ± 0.6 years at follow-up. Control group: n = 50 girls, mean age: 7.9 ± 0.6 years at baseline, 9.8 ± 0.6 years at follow-up. | 200 Minutes per week of PE of various activities including games, running, jumping, and climbing, compared to the standard 60 minutes per week for 2 years. (Malmo, Sweden) | Significant increase in BMC at the lumbar spine (L2–4: P = 0.007, L3: P < 0.001) but none at the femoral neck in the intervention group compared to control group, and significant increase in BMD in total body (P = 0.006), at part of lumbar spine (L3: P = 0.006) and femoral neck (P = 0.007) in the intervention group compared to control group. |
| McKay et al., 2005 [22] | Intervention group: n = 51, 23 boys and 28 girls, mean age: 10.1 ± 0.5 years. Control group: n = 73, 34 boys and 39 girls, mean age: 10.2 ± 0.43 years. | "Bounce at the Bell": 10 counter movement jumps, 3 times per day: morning bell, noon bell, and dismissal bell, 2–3 times per week for 8 months. (Vancouver, British Columbia, Canada) | Significant increase in BMC at femoral neck (P = 0.019) and trochanter (P = 0.017) in intervention group compared to control group, with no significant gender group interaction. |
| MacKelvie et al., 2003 [23] | Intervention group: n = 32 girls, mean age: 9.9 ± 0.6 years at baseline. Control group: n = 43 girls, mean age: 10.4 ± 0.4 years at baseline. | 10- to 12-Minute session of high-impact jumps, consisting of a number of jumps that progressively increased from 50 to 100 and jump height from 10 cm to 50 cm through the school year, 3 times per week for 2 years. (Richmond, British Columbia, Canada) | Significant increase in BMC at the lumbar spine (P < 0.05) and femoral neck (P < 0.05) in intervention group compared to control group. |
| MacKelvie et al., 2002 [24] | Intervention group: n = 61 boys, mean age: 10.2 ± 0.6 years. Control group: n = 60 boys, mean age: 10.3 ± 0.7 years. | 10- to 12-Minute session of high-impact jumps, consisting of jumping jacks, lunge jumps, and drop jumps that progressively increased from 50 to 100 and jump height from 10 cm to 50 cm through the school year, 3 times per week for 7 months. (Richmond, British Columbia, Canada) | Significant increase in BMC for total body (P < 0.01) and in BMD at the proximal femur (P < 0.05) in the intervention group compared to control group, but no significant differences at the lumbar spine, femoral neck or trochanter. |
| Petit et al., 2002 [25] | Intervention groups: n = 44 Prepubertal girls, mean age: 10.0 ± 0.6 years, and n = 43 Early-pubertal girls, mean age: 10.4 ± 0.7 years. Control groups: n = 26 Prepubertal girls, mean age: 10.1 ± 0.5 years, n = 64 Early-pubertal girls, mean age: 10.5 ± 0.6 years. | 10- to 12-Minute session of high-impact jumps, consisting of a number of jumps that progressively increased from 50 to 100 and jump height from 10 cm to 50 cm through the school year, 3 times per week for 2 years. (Richmond, British Columbia, Canada) | Significant increase in BMD at femoral neck (P = 0.027) and trochanter (P = 0.016) in intervention group compared to control group for early-pubertal girls, but no significant differences for intervention group and control group for prepubescent girls. |
| Fuchs et al., 2001 [26] | Intervention group: n = 45, 25 boys and 20 girls, mean age: 7.5 ± 0.16 years. Control group: n = 44, 26 boys and 18 girls, mean age: 7.6 ± 0.17 years. | 20 Minutes of jumping off a 61-cm box progressing from 50 up to 100 jumps per session, 3 times per week for 7 months. (Corvallis, OR, USA) | Significant increase in BMC at femoral neck (P < 0.001) and lumbar spine (P < 0.05) and in BMD at the lumbar spine (P < 0.01) in intervention group compared to control group, but no significant difference in BMD at the femoral neck between groups. |
| MacKelvie et al., 2001 [27] | Intervention groups: n = 44 Prepubertal, mean age: 10.0 ± 0.6 years, and n = 43 Early-pubertal girls, mean age: 10.4 ± 0.7 years. Control groups: n = 26 Prepubertal girls, mean age: 10.1 ± 0.5 years, n = 64 Early-pubertal girls, mean age: 10.5 ± 0.6 years. | 10- to 12-Minute session of high-impact jumps, 3 times per week, consisting of a number of jumps that progressively increased from 50 to 100 and jump height from 10 cm to 50 cm through the school year, 3 times per week for 7 months. (Richmond, British Columbia, Canada) | Significant increase in BMD at the lumbar spine (P = 0.005) and femoral neck (areal BMD P = 0.038, volumetric BMD P = 0.019) in intervention group compared to control group for early-pubertal girls, but no significant differences for intervention group and control group for prepubescent girls. |
| McKay et al., 2000 [28] | Intervention group: n = 63 boys and girls. Control group: n = 81 boys and girls. Age range: 6.9–10.2 years. | 10- to 30-Minute session of "loading" activities consisting of 10 tuck jumps at the beginning, with games, circuit training, and dance that integrated jumps, 3 times per week for 8 months. (Richmond, British Columbia, Canada) | Significant increase in BMD at the trochanter (P = 0.03) in intervention group compared to control group, but no significant differences in the femoral neck or lumbar spine between groups. |
For weight-bearing physical activity, every school-based exercise intervention used various forms of jumping activities, most of which consisted of high-impact jumps with many sessions as short as just 10 min [[13], [14], [15],19,[22], [23], [24], [25],27,28]. High-impact jumping was shown to significantly increase BMD and/or BMC for the total body [12,15,17,20,21,24], at the hip [12,[15], [16], [17],[19], [20], [21], [22], [23], [24], [25], [26], [27], [28]] and the vertebrae [13,15,17,21,23,26,27]. High-impact loading at the hands also showed a significant increase at the wrist [13]. Of the 15 studies that found a significant increase in BMD and/or BMC, 14 of those 15 studies found a significant increase at the hip, which is the skeletal site where fragility fractures are most common and the consequences are most severe. One study also investigated the long-term effects of school-based exercise interventions with annual follow-up measures after the intervention had concluded, and showed lasting significant increases at the hip approximately 7 years after the intervention [16].
Of the 2 studies that did not find significant increases in BMD and/or BMC, one of those studies did measure and assess bone structural strength and found significant increases [14]. The other study did not find significant increases in BMD and/or BMC [18], and reported that the school-based exercise intervention produced ground reaction forces (GRFs) measures of only 2–3 times body weight (BW). Several of the studies that found significant increases in BMD and/or BMC also measured and reported GRFs, which were 3.5–5 × BW [23,24], 4–5 × BW [22], 8 × BW [16], 8.5 × BW [17], and 8.8 × BW [26]. The study by Nichols et al. [18] shows that exercises producing GRFs of only 2–3 × BW are insufficient to affect bone mineralization in children and adolescents by failing to significantly increase BMD and/or BMC; however, GRFs of at least 3.5 × BW are needed to significantly increase BMD and/or BMC (Table 2).
Table 2.
Ground reaction forces and bone mineral outcomes.
| Study | Ground reaction forces | Significant increase(s) in BMD and/or BMC |
|---|---|---|
| Gunter et al., 2008 [16] | 8 Times body weight (8 × BW) | Yes |
| Gunter et al., 2008 [17] | 8.5 Times body weight (8.5 × BW) | Yes |
| Nichols et al., 2008 [18] | 2–3 Times body weight (2–3 × BW) | No |
| McKay et al., 2005 [22] | 4–5 Times body weight (4–5 × BW) | Yes |
| MacKelvie et al., 2003 [23] | 3.5–5 Times body weight (3.5–5 × BW) | Yes |
| MacKelvie et al., 2002 [24] | 3.5–5 Times body weight (3.5–5 × BW) | Yes |
| Fuchs et al., 2001 [26] | 8.8 Times body weight (8.8 × BW) | Yes |
BW, body weight; BMD, bone mineral density; BMC, bone mineral content.
3. Discussion
School-based exercise interventions that provide high-impact jumping activities with GRFs of at least 3.5 × BW are effective in increasing BMD and/or BMC in children and adolescents, and GRFs as high as 8.8 × BW are also effective and appear to be safe for children and adolescents. These programs take up minimal time within school days, and these exercises can easily be integrated into already established physical education classes, in classroom activities, as well as after school programs, with minimal time and financial resources. School administrators and teachers can use numerous types of exercises to provide variety, and they can be creative in implementing and integrating them into the school day, such as with “Bounce at the Bell” that incorporated high-impact jumping in very short bouts 3 times a day at the morning bell, noon bell, and dismissal bell [20,22].
It is important to note that these exercise sessions are not time-consuming, as sessions as short as 10 minutes for 2 to 3 times per week were sufficient in increasing BMD and/or BMC. The duration of exercise can be minimal, and the most important factor is the intensity of the exercise. High-impact jumps, both off the ground and off of a 50 cm [[23], [24], [25],27] up to a 61-cm step [16,26], can provide adequate stimulation on bones to increase BMD and/or BMC. In another study on the effects of weight-bearing physical activity on bone mineralization in children and/or adolescents, Deere et al. [29] found that a stimulus of 4.2 or higher above gravitational force (g), such as high-impact jumping that provides over 5.1 g and/or running at speeds over 10 km/hr, is needed to significantly increase BMD, as stimuli below those levels are insufficient in increasing bone mineralization. Similarly, Herrmann et al. [30] found that just 10 minutes of moderate-to vigorous-intensity weight-bearing physical activity can significantly increase bone strength in children, whereas low-intensity weight-bearing physical activity is insufficient.
The WHO recommends both weight-bearing physical activity and calcium intake to maximize peak bone mass in children and adolescents to prevent osteoporosis and fragility fractures later in life, and although weight-bearing physical activity is the more important factor, calcium intake should not be neglected. There are many school-based exercise intervention studies investigating the effects of weight-bearing physical activity on bone mineralization, but school-based nutrition intervention studies investigating the effects of calcium intake on bone mineralization are scarce. Although a meta-analysis has shown that children with increased consumption of high-calcium foods such as milk and dairy products, even though not through school-based nutrition interventions, lead to significantly higher total body BMC [31]. Thus, school-based nutrition interventions can simply provide high-calcium foods and drinks during school meals to increase calcium intake in children and adolescents.
Osteoporosis drastically diminishes both the quality and quantity of life, as it reduces independence and hinders physical, mental and social well-being [32], and fragility fractures lead to permanent physical disability, decreased self-sufficiency, hospitalization, and increased frailty, morbidity and mortality [33]. Osteoporosis, and particularly a fragility fracture, leads to major detriments in physical function, including muscle loss, avoidance of physical activity due to fear of injury, and reduced physical capacity, all of which lead to a higher risk of more fractures and further loss of physical function [34]. After a fragility fracture, even when receiving osteoporosis treatments, the quality of life is significantly reduced [35]. There are significant declines in physical function afterwards that last for years, and though they can improve after a couple of years, they are still significantly worse than before experiencing the fragility fracture [36]. Using health care metrics, people with osteoporosis and suffer from fragility fractures have worse physical health-related quality of life (HRQoL) than those who have not suffered from a fragility fracture [37], and fragility fractures lead to loss of disability-adjusted life-year, which is a measure of years lost due to premature mortality and a year of a perfect state of heath lost due to disability, causing increased disability and mortality [38].
Fragility fractures are associated with a high public health burden due to costs, loss in HRQoL, and mortality [39]. When compared to other common health conditions, fragility fractures are associated with the highest hospital charges and the second highest hospital length of stay [40], and the hospital costs associated with the acute treatment of fragility fractures are substantial, as they lead to increased intensive care unit use and higher rates of mortality [41]. Treating fragility fractures is an enormous cost and the burden on health care systems will only increase as the population continues to become more elderly [42], the health care costs for osteoporosis treatment is also expected to continuously and greatly increase far into the foreseeable future [43], and the health care resources needed to treat and manage osteoporosis will become scarcer as more people suffer from fragility fractures [44]. Furthermore, many individuals do not adhere to osteoporosis medications to manage the disease, and this leads to increased health care resources used and higher costs [45]. The low-to nonadherence to osteoporosis medications is due to a variety of reasons, mainly because of their side effects and simply forgetting to take them [46]. But even with high-adherence to osteoporosis medications, osteoporosis treatment drugs have side effects that can increase mortality and shorten the lifespan, showing the need to prevent osteoporosis and fragility fractures to reduce or eliminate the need for these treatment drugs [47].
Osteoporosis continues to be a growing public health problem with trends showing increased prevalence of the disease in population health, with significantly increased health care costs and resources to treat and manage it [48], but if fragility fracture rates can be reduced, not only will the quality and quantity of life improve for those affected by the disease and low BMD, but health care systems will be positively impacted by substantial amounts of costs saved in health care and medical expenses [49]. With low worldwide public health awareness of osteoporosis and fragility fractures, there is a major public health need to prioritize osteoporosis and fragility fracture prevention [50], and this can be done by implementing school-based exercise interventions, which this review has shown to be a cost-saving way to effectively increase bone mineralization in children and adolescents with minimal time and economic resources, in order to greatly reduce the risk of osteoporosis and fragility fractures and the severe public health and health care burdens that accompany them.
This literature review did have limitations. High-impact jumping that produced GRFs of at least 3.5 × BW and up to 8.8 × BW are safe and effective for increasing BMD and/or BMC, as participants did not report injuries, but it is unknown what amount of GRF would reach a threshold in BMD and/or BMC increase, and what impact levels are too high and can cause harm, such as increased risk of skeletal (stress) fractures or joint injuries. And while 1 study did show that a school-based exercise intervention can have lasting effects on improved bone mineralization 7 years after the intervention [16], whether the effects will last up to older adulthood and the elderly years is unknown. But as previously noted, weight-bearing physical activity during youth has been shown to have sustained higher bone mass into adulthood [10], and it is reasonable to believe that school-based exercise interventions can have the same effectiveness, especially if implemented throughout the school years in growing children and adolescents. Due to these limitations, ideas for future research may include investigating other types of high-impact jumping, even those producing GRFs above 8.8 × BW, to test for continuous increases or thresholds for improving BMD and/or BMC, as well as prospective cohort studies that investigate if higher obtained BMD and/or BMC during youth from school-based exercise interventions lead to reduced osteoporosis and fragility fractures into older adulthood.
4. Conclusion
Osteoporosis and fragility fractures are major global public health problems that affect populations all throughout the world, and their consequences are severe for both individuals and national health care systems. Osteoporosis is a pediatric disease with geriatric consequences [51], as neglecting preventive measures during youth will result in costs of osteoporosis and fragility fractures later in life. Fortunately, osteoporosis and fragility fractures can be prevented with effective public health policies of implementing evidence-based community health interventions, such as school-based exercise interventions, to most effectively and efficiently prevent this disease and its consequences in populations all throughout the world.
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Footnotes
Peer review under responsibility of The Korean Society of Osteoporosis.
References
- 1.Genant H.K., Cooper C., Poor G., Reid I., Ehrlich G., Kanis J. Interim report and recommendations of the world health organization task-force for osteoporosis. Osteoporos Int. 1999;10:259–264. doi: 10.1007/s001980050224. [DOI] [PubMed] [Google Scholar]
- 2.Kanis J.A., Melton L.J., 3rd, Christiansen C., Johnston C.C., Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9:1137–1141. doi: 10.1002/jbmr.5650090802. [DOI] [PubMed] [Google Scholar]
- 3.Marshall D., Johnell O., Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312:1254–1259. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper C., Campion G., Melton L.J., 3rd Hip fractures in the elderly: a world-wide projection. Osteoporos Int. 1992;2:285–289. doi: 10.1007/BF01623184. [DOI] [PubMed] [Google Scholar]
- 5.Curtis E.M., Moon R.J., Harvey N.C., Cooper C. The impact of fragility fracture and approaches to osteoporosis risk assessment worldwide. Bone. 2017;104:29–38. doi: 10.1016/j.bone.2017.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lespessailles E., Cortet B., Legrand E., Guggenbuhl P., Roux C. Low-trauma fractures without osteoporosis. Osteoporos Int. 2017;28:1771–1778. doi: 10.1007/s00198-017-3921-7. [DOI] [PubMed] [Google Scholar]
- 7.Beck B.R., Snow C.M. Bone health across the lifespan–exercising our options. Exerc Sport Sci Rev. 2003;31:117–122. doi: 10.1097/00003677-200307000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Welten D.C., Kemper H.C., Post G.B., Van Mechelen W., Twisk J., Lips P. Weight-bearing activity during youth is a more important factor for peak bone mass than calcium intake. J Bone Miner Res. 1994;9:1089–1096. doi: 10.1002/jbmr.5650090717. [DOI] [PubMed] [Google Scholar]
- 9.Kohrt W.M., Bloomfield S.A., Little K.D., Nelson M.E., Yingling V.R. American college of sports medicine position stand: physical activity and bone health. Med Sci Sports Exerc. 2004;36:1985–1996. doi: 10.1249/01.mss.0000142662.21767.58. [DOI] [PubMed] [Google Scholar]
- 10.Kriska A.M., Sandler R.B., Cauley J.A., LaPorte R.E., Hom D.L., Pambianco G. The assessment of historical physical activity and its relation to adult bone parameters. Am J Epidemiol. 1988;127:1053–1063. doi: 10.1093/oxfordjournals.aje.a114881. [DOI] [PubMed] [Google Scholar]
- 11.McLeroy K.R., Bibeau D., Steckler A., Glanz K. An ecological perspective on health promotion programs. Health Educ Q. 1988;15:351–377. doi: 10.1177/109019818801500401. [DOI] [PubMed] [Google Scholar]
- 12.Larsen M.N., Nielsen C.M., Helge E.W., Madsen M., Manniche V., Hansen L. Positive effects on bone mineralisation and muscular fitness after 10 months of intense school-based physical training for children aged 8-10 years: the FIT FIRST randomised controlled trial. Br J Sports Med. 2018;52:254–260. doi: 10.1136/bjsports-2016-096219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nogueira R.C., Weeks B.K., Beck B.R. Targeting bone and fat with novel exercise for peripubertal boys: the CAPO kids trial. Pediatr Exerc Sci. 2015;27:128–139. doi: 10.1123/pes.2014-0069. [DOI] [PubMed] [Google Scholar]
- 14.Nogueira R.C., Weeks B.K., Beck B.R. An in-school exercise intervention to enhance bone and reduce fat in girls: the CAPO Kids trial. Bone. 2014;68:92–99. doi: 10.1016/j.bone.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Meyer U., Romann M., Zahner L., Schindler C., Puder J.J., Kraenzlin M. Effect of a general school-based physical activity intervention on bone mineral content and density: a cluster-randomized controlled trial. Bone. 2011;48:792–797. doi: 10.1016/j.bone.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 16.Gunter K., Baxter-Jones A.D., Mirwald R.L., Almstedt H., Fuchs R.K., Durski S. Impact exercise increases BMC during growth: an 8-year longitudinal study. J Bone Miner Res. 2008;23:986–993. doi: 10.1359/JBMR.071201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gunter K., Baxter-Jones A.D., Mirwald R.L., Almstedt H., Fuller A., Durski S. Jump starting skeletal health: a 4-year longitudinal study assessing the effects of jumping on skeletal development in pre and circum pubertal children. Bone. 2008;42:710–718. doi: 10.1016/j.bone.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Nichols D.L., Sanborn C.F., Essery E.V., Clark R.A., Letendre J.D. Impact of curriculum-based bone loading and nutrition education program on bone accrual in children. Pediatr Exerc Sci. 2008;20:411–425. doi: 10.1123/pes.20.4.411. [DOI] [PubMed] [Google Scholar]
- 19.Weeks B.K., Young C.M., Beck B.R. Eight months of regular in-school jumping improves indices of bone strength in adolescent boys and Girls: the POWER PE study. J Bone Miner Res. 2008;23:1002–1011. doi: 10.1359/jbmr.080226. [DOI] [PubMed] [Google Scholar]
- 20.Macdonald H.M., Kontulainen S.A., Petit M.A., Beck T.J., Khan K.M., McKay H.A. Does a novel school-based physical activity model benefit femoral neck bone strength in pre- and early pubertal children? Osteoporos Int. 2008;19:1445–1456. doi: 10.1007/s00198-008-0589-z. [DOI] [PubMed] [Google Scholar]
- 21.Linden C., Ahlborg H.G., Besjakov J., Gardsell P., Karlsson M.K. A school curriculum-based exercise program increases bone mineral accrual and bone size in prepubertal girls: two-year data from the pediatric osteoporosis prevention (POP) study. J Bone Miner Res. 2006;21:829–835. doi: 10.1359/jbmr.060304. [DOI] [PubMed] [Google Scholar]
- 22.McKay H.A., MacLean L., Petit M., MacKelvie-O'Brien K., Janssen P., Beck T. "Bounce at the Bell": a novel program of short bouts of exercise improves proximal femur bone mass in early pubertal children. Br J Sports Med. 2005;39:521–526. doi: 10.1136/bjsm.2004.014266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacKelvie K.J., Khan K.M., Petit M.A., Janssen P.A., McKay H.A. A school-based exercise intervention elicits substantial bone health benefits: a 2-year randomized controlled trial in girls. Pediatrics. 2003;112(6 Pt 1):e447. doi: 10.1542/peds.112.6.e447. [DOI] [PubMed] [Google Scholar]
- 24.MacKelvie K.J., McKay H.A., Petit M.A., Moran O., Khan K.M. Bone mineral response to a 7-month randomized controlled, school-based jumping intervention in 121 prepubertal boys: associations with ethnicity and body mass index. J Bone Miner Res. 2002;17:834–844. doi: 10.1359/jbmr.2002.17.5.834. [DOI] [PubMed] [Google Scholar]
- 25.Petit M.A., McKay H.A., MacKelvie K.J., Heinonen A., Khan K.M., Beck T.J. A randomized school-based jumping intervention confers site and maturity-specific benefits on bone structural properties in girls: a hip structural analysis study. J Bone Miner Res. 2002;17:363–372. doi: 10.1359/jbmr.2002.17.3.363. [DOI] [PubMed] [Google Scholar]
- 26.Fuchs R.K., Bauer J.J., Snow C.M. Jumping improves hip and lumbar spine bone mass in prepubescent children: a randomized controlled trial. J Bone Miner Res. 2001;16:148–156. doi: 10.1359/jbmr.2001.16.1.148. [DOI] [PubMed] [Google Scholar]
- 27.Mackelvie K.J., McKay H.A., Khan K.M., Crocker P.R. A school-based exercise intervention augments bone mineral accrual in early pubertal girls. J Pediatr. 2001;139:501–508. doi: 10.1067/mpd.2001.118190. [DOI] [PubMed] [Google Scholar]
- 28.McKay H.A., Petit M.A., Schutz R.W., Prior J.C., Barr S.I., Khan K.M. Augmented trochanteric bone mineral density after modified physical education classes: a randomized school-based exercise intervention study in prepubescent and early pubescent children. J Pediatr. 2000;136:156–162. doi: 10.1016/s0022-3476(00)70095-3. [DOI] [PubMed] [Google Scholar]
- 29.Deere K., Sayers A., Rittweger J., Tobias J.H. Habitual levels of high, but not moderate or low, impact activity are positively related to hip BMD and geometry: results from a population-based study of adolescents. J Bone Miner Res. 2012;27:1887–1895. doi: 10.1002/jbmr.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herrmann D., Buck C., Sioen I., Kouride Y., Marild S., Molnár D. Impact of physical activity, sedentary behaviour and muscle strength on bone stiffness in 2-10-year-old children-cross-sectional results from the IDEFICS study. Int J Behav Nutr Phys Activ. 2015;12:112. doi: 10.1186/s12966-015-0273-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huncharek M., Muscat J., Kupelnick B. Impact of dairy products and dietary calcium on bone-mineral content in children: results of a meta-analysis. Bone. 2008;43:312–321. doi: 10.1016/j.bone.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 32.Lips P., van Schoor N.M. Quality of life in patients with osteoporosis. Osteoporos Int. 2005;16:447–455. doi: 10.1007/s00198-004-1762-7. [DOI] [PubMed] [Google Scholar]
- 33.Leboime A., Confavreux C.B., Mehsen N., Paccou J., David C., Roux C. Osteoporosis and mortality. Joint Bone Spine. 2010;77(Suppl 2):S107–S112. doi: 10.1016/S1297-319X(10)70004-X. [DOI] [PubMed] [Google Scholar]
- 34.Kerr C., Bottomley C., Shingler S., Giangregorio L., de Freitas H.M., Patel C. The importance of physical function to people with osteoporosis. Osteoporos Int. 2017;28:1597–1607. doi: 10.1007/s00198-017-3911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim K.J., Jun H.J., Jeong H.S., Jeon D.J., Ji S.H. The relationship between fracture and quality of life in Korean adults receiving treatment for osteoporosis based on the 2010 Korean Community Health Survey. J Phys Ther Sci. 2015;27:2083–2086. doi: 10.1589/jpts.27.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fischer S., Kapinos K.A., Mulcahy A., Pinto L., Hayden O., Barron R. Estimating the long-term functional burden of osteoporosis-related fractures. Osteoporos Int. 2017;28:2843–2851. doi: 10.1007/s00198-017-4110-4. [DOI] [PubMed] [Google Scholar]
- 37.Al-Sari U.A., Tobias J., Clark E. Health-related quality of life in older people with osteoporotic vertebral fractures: a systematic review and meta-analysis. Osteoporos Int. 2016;27:2891–2900. doi: 10.1007/s00198-016-3648-x. [DOI] [PubMed] [Google Scholar]
- 38.Papadimitriou N., Tsilidis K.K., Orfanos P., Benetou V., Ntzani E.E., Soerjomataram I. Burden of hip fracture using disability-adjusted life-years: a pooled analysis of prospective cohorts in the CHANCES consortium. Lancet Public Health. 2017;2:e239–e246. doi: 10.1016/S2468-2667(17)30046-4. [DOI] [PubMed] [Google Scholar]
- 39.Marques A. Lourenço Ó, da Silva JA; Portuguese Working Group for the Study of the Burden of Hip Fractures in Portugal. The burden of osteoporotic hip fractures in Portugal: costs, health related quality of life and mortality. Osteoporos Int. 2015;26:2623–2630. doi: 10.1007/s00198-015-3171-5. [DOI] [PubMed] [Google Scholar]
- 40.Cunningham T.D., Martin B.C., DeShields S.C., Romero C.C. The impact of osteoporotic fractures compared with other health conditions in older adults living in Virginia, United States. Osteoporos Int. 2016;27:2979–2988. doi: 10.1007/s00198-016-3620-9. [DOI] [PubMed] [Google Scholar]
- 41.Weycker D., Li X., Barron R., Bornheimer R., Chandler D. Hospitalizations for osteoporosis-related fractures: economic costs and clinical outcomes. BoneKEy Rep. 2016;5:186–191. doi: 10.1016/j.bonr.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harvey N., Dennison E., Cooper C. Osteoporosis: impact on health and economics. Nat Rev Rheumatol. 2010;6:99–105. doi: 10.1038/nrrheum.2009.260. [DOI] [PubMed] [Google Scholar]
- 43.Burge R., Dawson-Hughes B., Solomon D.H., Wong J.B., King A., Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 44.Melton L.J., 3rd, Johnell O., Lau E., Mautalen C.A., Seeman E. Osteoporosis and the global competition for health care resources. J Bone Miner Res. 2004;19:1055–1058. doi: 10.1359/JBMR.040316. [DOI] [PubMed] [Google Scholar]
- 45.Kjellberg J., Jorgensen A.D., Vestergaard P., Ibsen R., Gerstoft F., Modi A. Cost and health care resource use associated with noncompliance with oral bisphosphonate therapy: an analysis using Danish health registries. Osteoporos Int. 2016;27:3535–3541. doi: 10.1007/s00198-016-3683-7. [DOI] [PubMed] [Google Scholar]
- 46.Clark E.M., Gould V.C., Tobias J.H., Horne R. Natural history, reasons for, and impact of low/non-adherence to medications for osteoporosis in a cohort of community-dwelling older women already established on medication: a 2-year follow-up study. Osteoporos Int. 2016;27:579–590. doi: 10.1007/s00198-015-3271-2. [DOI] [PubMed] [Google Scholar]
- 47.Abrahamsen B., Osmond C., Cooper C. Life expectancy in patients treated for osteoporosis: observational cohort study using national Danish prescription data. J Bone Miner Res. 2015;30:1553–1559. doi: 10.1002/jbmr.2478. [DOI] [PubMed] [Google Scholar]
- 48.Cauley J.A. Public health impact of osteoporosis. J Gerontol Biol Med Sci. 2013;68:1243–1251. doi: 10.1093/gerona/glt093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ohta H., Mouri M., Kuroda T., Nakamura T., Shiraki M., Orimo H. Decreased rate of hip fracture and consequent reduction in estimated medical costs in Japan. J Bone Miner Metabol. 2017;35:351–353. doi: 10.1007/s00774-016-0760-0. [DOI] [PubMed] [Google Scholar]
- 50.Harvey N.C., McCloskey E.V., Mitchell P.J., Dawson-Hughes B., Pierroz D.D., Reginster J.Y. Mind the (treatment) gap: a global perspective on current and future strategies for prevention of fragility fractures. Osteoporos Int. 2017;28:1507–1529. doi: 10.1007/s00198-016-3894-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hightower L. Osteoporosis: pediatric disease with geriatric consequences. Orthop Nurs. 2000;19:59–62. doi: 10.1097/00006416-200019050-00010. [DOI] [PubMed] [Google Scholar]