Abstract
Objectives
Current treatments for osteoporosis were prevention of progression, yet it has been questionable in the stimulation of bone growth. The mesenchymal stem cells (MSCs) treatment for osteoporosis aims to induce differentiation of bone progenitor cells into bone-forming osteoblasts. We investigate whether human umbilical cord blood (hUCB)-MSCs transplantation may induce bone regeneration for osteoporotic rat model induced by ovariectomy.
Methods
The ovariectomized (OVX) group (n = 10) and OVX-MSCs group (n = 10) underwent bilateral ovariectomy to induce osteoporosis, while the Sham group (n = 10) underwent sham operation at aged 12 weeks. After a femoral defect was made at 9 months, Sham group and OVX group were injected with Hartmann solution, while the OVX-MSCs group was injected with Hartmann solution containing 1 × 107 hUCB-MSCs. The volume of regenerated bone was evaluated using micro-computed tomography at 4 and 8 weeks postoperation.
Results
At 4- and 8-week postoperation, the OVX group (5.0% ± 1.5%; 6.1% ± 0.7%) had a significantly lower regenerated bone volume than the Sham group (8.6% ± 1.3%; 12.0% ± 1.8%, P < 0.01), respectively. However, there was no significant difference between the OVX-MSCs and Sham groups. The OVX-MSCs group resulted in about 53% and 65% significantly higher new bone formation than the OVX group (7.7% ± 1.9%; 10.0% ± 2.9%, P < 0.05).
Conclusions
hUCB-MSCs in bone defects may enhance bone regeneration in osteoporotic rat model similar to nonosteoporotic bone regeneration. hUCB-MSCs may be a promising alternative stem cell therapy for osteoporosis.
Keywords: Mesenchymal stem cell transplantation, Osteoporosis
1. Introduction
Osteoporosis is one of the major systemic skeletal diseases worldwide today and is characterized by compromised bone strength resulting in bone fragility and heightened risk of bone fractures [1]. Osteoporotic patients experience low bone mass and density with microarchitectural deterioration of bone tissue in which osteoblast function and levels are reduced while osteoclast bone resorption is augmented [2]. Although no longer regarded as age- or sex-dependent, the presence of osteoporosis, specifically primary osteoporosis, is rapidly rising among the elderly and postmenopausal women experiencing estrogen deficiency [3].
A wide range of treatment for osteoporosis currently exists, including antiresorptive or bone regenerating pharmacological agents and lifestyle changes towards an altered diet and increased physical activity [4]. Bisphosphonates, the predominant drug-based therapy that inhibits bone resorption, is commonly prescribed; however, long-term safety concerns and serious adverse effects, such as osteonecrosis of the jaw and atypical femoral fractures, have limited its use [[5], [6], [7], [8]]. Moreover, most current drugs have shown to prevent the progression of osteoporosis yet are questionable in stimulating bone growth [9]. On the other hand, mesenchymal stem cells (MSCs) are a primary cell source for induction of bone regeneration and bone graft engineering [10]. These stem cells have the ability to self-renew and differentiate into multiple lineages, such as osteoblasts, chondrocytes, adipocytes and myocytes [11]. Physiologically, the occurrence of osteoporosis has been linked to a decrease in the osteogenic potential of MSCs in the bone marrow and periosteum [[12], [13], [14]], as shown by their diminished cell count and decreased ability to proliferate and differentiate into osteoblasts [13,[15], [16], [17]]. Thus, MSCs-based treatment of osteoporosis aims to induce differentiation and osteogenesis of bone progenitor cells into active, bone-forming osteoblasts.
Multiple tissues have been discovered to harbor MSCs, including bone marrow [18], adipose tissue [19], and umbilical cord blood [20]. Studies have reported encouraging results from MSCs transplantations performed in various osteoporotic models. Wang et al. [21] showed that bone-marrow derived MSCs transplantation enhanced trabecular thickness, bone apposition and osteoid formation, thus strengthening bone in ovariectomized (OVX) rabbit models. Moreover, Ye et al. [22] reported that adipose-derived stem cell transplantation promoted osteogenesis and inhibited adipogenesis of bone marrow mesenchymal stromal cells by activating bone morphogenic protein 2 and additionally increased local bone mineral density in OVX rabbit models. MSCs obtained from human umbilical cord blood (hUCB) were shown to improve trabecular and bone formation parameters, hence preventing OVX-induced bone loss in mouse models [23].
MSCs from bone marrow aspirates are deemed to have the greatest multilineage potential, thus is commonly used for tissue engineering and regenerative medicine [24]. However, MSCs from this origin are obtained through an invasive, painful procedure and may decrease in differentiation potency after cultivation or with the donor's age [25,26]. In contrast, hUCB provides a richer source of human MSCs [27] that displays low immunogenic potential [28] and may be harvested via simpler and noninvasive methods [29]. Moreover, hUCB derived MSCs are reported to exhibit higher proliferation rates [30] and greater capacity for osteogenic differentiation compared to other MSCs thus providing a strong rationale for the use of hUCB-derived MSCs (hUCB-MSCs) in stem cell regenerative therapy in bone-related diseases [[31], [32], [33]]. In this study, we aim to investigate whether hUCB-MSCs transplantation in femoral defects of OVX rats may help induce bone regeneration in osteoporotic rat models.
2. Methods
2.1. hUCB and mononuclear cell isolation
hUCB was obtained from a cord blood bank (LifeLine, Yongin, Korea). hUCB was drawn from the umbilical vein of newborns, collected into cord blood collection bags containing anticoagulant (Greencross LabCell Corp., Yongin, Korea), and stored at room temperature. Written informed consent was obtained from each parent. The mononuclear cells from hUCB were obtained by sterile a pyrogenic Ficoll-paque density gradient centrifugation (1.077 g/cm3, GE Healthcare, Chicago, IL, USA), according to the manufacturer's instructions, and washed with phosphate buffered saline, and plated at 1 × 106 cells/cm2 in low glucose Dulbecco Modified Eagle Medium (Gibco, Seoul, Korea) supplemented with 30% fetal bovine serum (Gibco), 1% antibiotics-antimycotics (Gibco). After 7 days, nonadherent cells were discarded, and adherent cells were cultured with three medium replacements per week. The cells were grown at 37 °C in a humidified atmosphere containing 5% CO2 for 14 days. MSC colonies were translated to a new T75 culture flask for expansion culture.
2.2. In vitro expansion culture of cord blood MSCs and hMSCs preparation
hUCB-MSCs were cultured in Mesenchymal Stem Cell Growth Medium (MSCsGM; Lonza, Basel, Switzerland), supplemented with MSCsGM SingleQuots (Lonza), and maintained at 37 °C in a humidified atmosphere containing 5% CO2. Approximately 70% of confluent cells were detached with 0.05% trypsin ethylenediaminetetraacetic acid (EDTA; Gibco) and replated at a density of 3 × 105 in a T175 culture flask. The hUCB-MSCs were also expanded in culture by repeated harvesting and replating of cells every seven days, up to the 10th passage.
For transplantation into animals, 1 × 107 cells were collected at the 6th passage and resuspended in 7.5 μL of Hartmann solution (JW Pharmaceutical, Seoul, Korea) with 1% albumin (Greencross LabCell Corp., Yongin, Korea).
2.3. Characterization of hUCB-MSCs
hUCB-MSCs were characterized by the cell surface protein profile and the differentiation capability. The harvested cells were analyzed for the expression of CD73, CD105, CD29, CD44, HLA-ABC, CD90, CD13, CD166, CD45, CD34, HLA-DR by flow cytometry with FACS laser cytometer (Beckman Coulter, Seoul, Korea). Approximately 0.5–1 × 105 cells per tube were used for cell surface antigen expression studies. To evaluate differentiation capability of hUCB-MSCs, multi lineage differentiation tests were performed in vitro. For induction of osteogenic or adipogenic differentiation, 70% confluent cells were incubated in respective differentiation medium (Lonza). For induction of chondrogenic differentiation, the hUCB-MSCs pellet was incubated in a polypropylene tube containing commercially available chondrogenic differentiation medium (Lonza).
After 3 weeks, the osteogenic differentiation ability was assessed by Von Kossa staining. After 4 weeks, adipogenic differentiation was assessed by oil red O staining. After 6 weeks, the harvested chondrogenic pellets were fixated with 10% formalin and paraffin embedded for histological processing. 5-μL-thin sections were stained with safranin O.
2.4. Osteoporotic rat model
All experimental procedures were approved by the Animal Experimentation Ethics Committee of Chungnam National University Hospital (CNUH-017-A0045). The female Sprague-Dawley rats aged 12 weeks were purchased from Damuel Science (Daejeon, Korea) and acclimated to conditions 1 week before and throughout the experimental period. The animals were housed in a room air-conditioned at 22 °C ± 2 °C with a 12-hour light/dark cycle and free access to commercial rodent diet.
OVX rats were used as osteoporotic models in this study [34]. The ovariectomy method utilized was the double dorso-lateral approach explained by Park et al. [35]. All animals at 12 weeks of age were randomly distributed into 3 groups: Sham group, OVX group, and OVX-MSCs group. The Sham group (n = 10) underwent Sham operation while the OVX (n = 10) and OVX-MSCs groups (n = 10) underwent bilateral ovariectomy to induce osteoporosis.
2.5. Femoral defect surgical methods
Six month following ovariectomy or sham operations (at 9 months of age), all rats underwent surgery creating a bone defect on right distal femurs of Sham rats and both left and right distal femurs on OVX rats. All rats were anaesthetized with isoflurane (Forane; Abbott Laboratories, North Chicago, IL, USA) at a 1:1 flow ratio of N2O and O2. Under sterile surgical conditions, linear incisions were made on the skin and subcutaneous tissue to expose the distal femoral bone. Using a micro motor 207A apparatus (HTY-M04 Saeshin Strong 207A + 107L; Henan Hongtaiyang Medical Apparatus and Instruments Co., Ltd., Henan, China), a defect cavity (1.2-mm diameter, 7-mm height) was created into the epiphysis using a rotary burr (1.2mm diameter, 7-mm height) under continuous saline irrigation to minimize overheating. Right femoral defects of Sham rats and OVX rats were injected with 7.5 μL of Hartmann solution. The right femoral defect of OVX-MSCs rats was injected with 7.5 μL of Hartmann solution containing 1 × 107 hUCB-MSCs. The gel-type adhesive (Greenplast Q, Greencross LabCell Corp., Yongin, Korea) was injected to fill the femoral defect and prevent leakage of solution. The subcutaneous tissue and muscle were closed using synthetic resorbable materials (4-0 Vicryl W9074; Ethicon, Somerville, NJ, USA). Outer skin was sutured with monofilament materials (2–0 Prolene W8623; Ethicon). Unrestricted movement and activity of the rat were observed postoperatively after recovery from anesthesia. At 4- and 8-week postoperation, 5 rats per group were euthanized and the left and right distal femur from each rat was isolated. Prior to micro-computed tomography (micro-CT) and histological analysis, samples were fixed in 4% paraformaldehyde solution for approximately 3–5 days.
2.6. Micro-CT analysis
The volume of regenerated bone was evaluated using micro-CT (Sky-Scan 1172 TM; Skyscan, Kontich, Belgium) scan under an X-ray voltage of 60 kV and a current of 167 μA using a 0.5-mm aluminum filter. The scanned data were reconstructed by image analysis software (CTAnalyzer; Skyscan, Kontich, Belgium). Measurements of radiopaque regions of interest relative to the entire defect volume were representative of the percent of bone volume formed.
2.7. Histological evaluation
The distal femoral specimens were decalcified in a 10% EDTA solution with a pH of 7.4 and dehydrated in a graded series of alcohol solutions (80%–100%). The specimens were then embedded in paraffin. Using a rotary microtome (HM 325; Microm, Walldorf, Germany), 5-μm sections of the samples were cut. Each specimen was stained with hematoxylin and eosin and then observed under a light microscope (DMR; Leica, Nussloch, Germany) equipped with a digital camera (DFC-480; Leica).
2.8. Statistical analysis
Quantitative measurements of newly formed was analyzed using IBM SPSS Statistics ver. 22.0 (IBM Co., Armonk, NY, USA). Because there is a small number of samples that are not normally distributed, Nonparametric analysis utilizing Kruskal-Wallis test and post hoc analysis by Mann-Whitney test was performed. P < 0.05 was considered statistically significant.
3. Results
3.1. Characterization of hUCB-MSCs
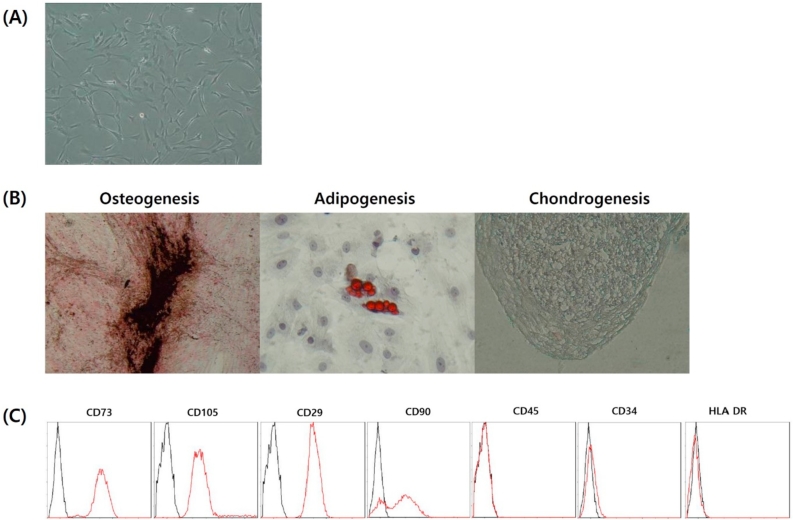
The in vitro expansion of hUCB-MSCs was observed under optical microscopy and confirmed the morphology of hUCB-MSCs as shown in Fig. 1A. The multilineage differentiation ability of hUCB-MSCs was demonstrated confirmed by positive staining for osteogenesis, adipogenesis and chondrogenesis after incubation in the respective differentiation mediums (Fig. 1B). Flow cytometry analyses showed that hUCB-MSCs were positive for MSCs-specific surface antigens CD73, CD105, CD29, CD90 and negative for hematopoietic cell-specific marker CD45, CD34, HLA-DR (Fig. 1C).
Fig. 1.
Stem cell properties of human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSCs). (A) hUCB-MSCs morphology at P5 (magnification, × 100), (B) differentiation capacity of hUCB-MSCs at P5 (magnification, × 100), and (C) characterization of CBMSCs at P5: representative FACS analyses shows that hUCB-MSCs were positive for MSCs specific surface marker CD73, CD105, CD29, CD90 and negative for hematopoietic cell specific marker CD45, CD34, HLA-DR. The black line is the isotypic control and the red line is the specific marker.
3.2. Animals
All animals underwent a successful recovery after operation. Moreover, histopathologic observations did not detect any necrosis in any specimens of this study.
3.3. Micro-CT analysis
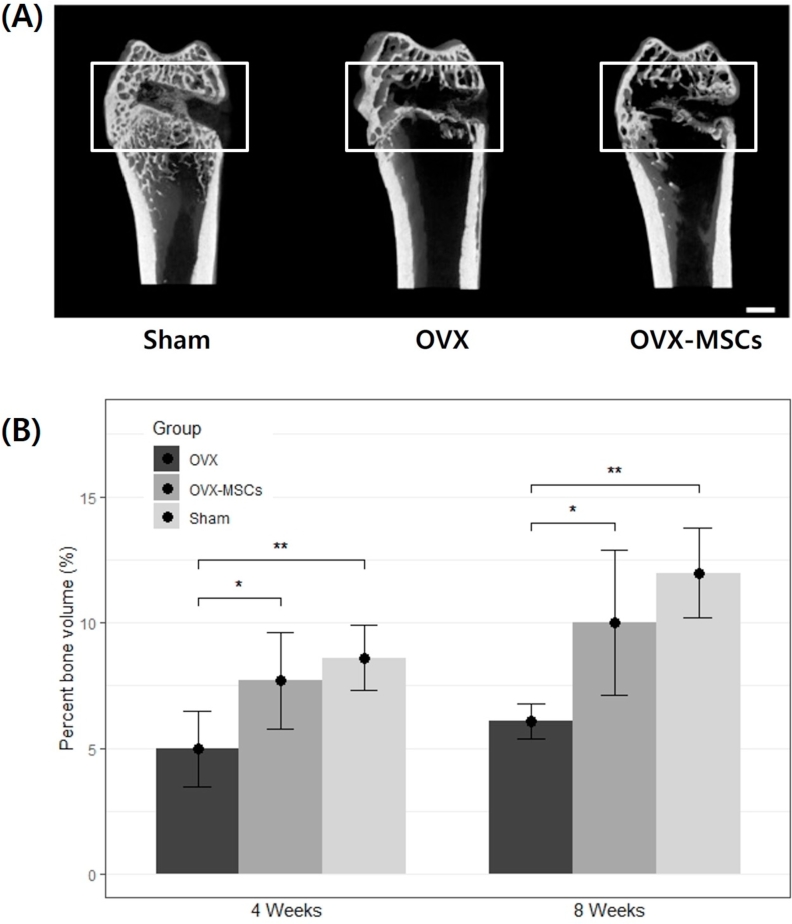
Fig. 2A shows the reconstructed 3-dimensional image of the distal femur at 8-week postoperation as analyzed by micro-CT. The images show that the Sham group had a denser trabecular bone density in the epiphyses and metaphysis bone microstructure of the distal femur in contrast to the OVX group. Bone formation at the margins of the defect was observed in all experimental and Sham groups, although with varying degrees. Within the defect, OVX-MSCs group showed a greater amount of new bone formation in the central and marginal area of the defect compared to the OVX group. In contrast, the Sham group resulted with the most active and dense bone regeneration throughout the defect cavity.
Fig. 2.
Micro-computed tomography (micro-CT) evaluation. (A) Three-dimensional reconstructed micro-CT image. Scale bar is 1 mm. (B) Quantitative analysis of the percent bone volume of the newly formed bone calculated from micro-CT data after surgery (n = 5). Each column represents the mean ± standard deviation (*P < 0.05, **P < 0.01). OVX, ovariectomized; OVX-MSCs, OVX-mesenchymal stem cells.
Quantitative measurements of newly formed bone was represented by percent bone volume calculations from micro-CT analyses as depicted in Fig. 2B. At both 4- and 8-week postoperation, the OVX group (5.0% ± 1.5%) (6.1% ± 0.7%) had a significantly lower percent bone volume than the Sham group, respectively (8.6% ± 1.3%) (12.0% ± 1.8%) (P < 0.01). However, there was no significant difference in percent bone volume between the OVX-MSCs and Sham group at all time periods. The OVX-MSCs (7.7% ± 1.9%) resulted with about 53% significantly higher new bone formation than the OVX (P < 0.05). Likewise, at 8-week postoperation, the percent bone volume was significantly higher in the OVX-MSCs group (10.0% ± 2.9%) compared with the OVX group by approximately 65%. These results suggest that the presence of hUCB-MSCs in bone defects may enhance bone regeneration in osteoporotic environments to levels similar to that of nonosteoporotic bone regeneration.
3.4. Histological evaluation
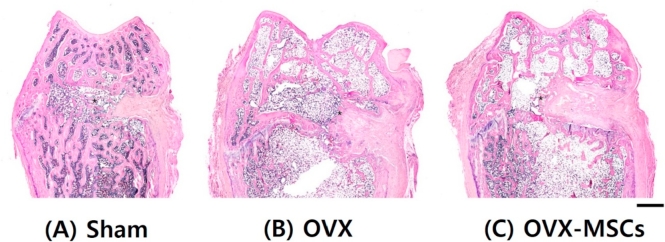
Histologic images with hematoxylin and eosin staining of the distal femur at 8-week postoperation are depicted in Fig. 3. The Sham group showed densely formed new trabecular bone within the defect area. The OVX-MSCs group showed thicker newly formed bone within the defect cavity as well as in the marginal regions.
Fig. 3.
Histological observations. Hematoxylin and eosin-stained histological morphology of the entire area of distal femurs 8 weeks after surgery. (A) Sham operation. (B) Ovariectomy. (C) Mesenchymal stem cells injected after ovariectomy. Asterisk, new bone. Scale bar is 1 mm. OVX, ovariectomized; OVX-MSCs, OVX-mesenchymal stem cells.
4. Discussion
In our study, we demonstrated that local injection of hUCB-MSCs enhanced the ability of new bone formation in osteoporotic rats. By establishing an estrogen-deficient OVX rat model, we compared bone regeneration in femoral defects under both healthy and osteoporotic conditions. Because local injection of hUCB-MSCs in OVX models increased bone formation to levels comparable to nonosteoporotic models, we concluded that the presence of hUCB-MSCs may be a valuable alternative in stem cell therapy for osteoporosis.
The pathogenesis of osteoporosis stems from the imbalance in bone remodeling due to defects in osteoid-secreting osteoblasts and augmented function of proteolytic osteoclasts. The disease is prevalent among estrogen-deficient postmenopausal women who experience dramatically increased bone turnover rates and corresponding loss of bone mass [36]. Estrogen is an important steroid in maintaining adequate bone mass and architecture thus withdrawal may lead to irreversible loss of thin trabecular elements and heightened incidence of fractures [37]. Therefore, deprivation of estrogen through ovariectomy procedures has been frequently used to mimic postmenopausal osteoporosis in experimental animals. In this study, the OVX group showed distinct osteoporotic features in the distal epiphyseal and metaphyseal femur in contrast to the Sham group (Fig. 2, Fig. 3). The micro-CT and histological results display significantly reduced trabecular density with greater porous features in the cancellous bone, thus demonstrate the establishment of osteoporosis in the rat models at 180 days postovariectomy. Likewise, previous reports confirm a time period of 180 days or longer after ovariectomy for cortical bone remodeling to simulate postmenopausal osteoporosis and result in a significant decrease in cancellous bone volume of up to 30%–35% in OVX rats [38,39].
MSCs extracted from various sources, including the bone marrow, adipose tissue, periosteum, and umbilical cord, have been previously utilized in bone regeneration and stem cell therapy for osteoporosis [29,40,41]. Diminished function and osteogenic potential of MSCs in the bone marrow and periosteum [[12], [13], [14]], demonstrated by reduced proliferation and osteodifferentiation, is normally coupled with osteoporosis [13,[15], [16], [17]]. Consequently, MSCs-based treatment aims to induce differentiation and osteogenesis of bone progenitor cells into active, bone-forming osteoblasts. Recently, MSCs-based methods have attracted much attention not only because of the cells’ self-renewal and pluripotent properties, but also due to their immunosuppressive capacity [42]. Bone marrow mesenchymal stromal cells were initially discovered to effectively differentiate into osteoblasts; however, the highly invasive harvesting procedure and the decline in proliferation and differentiation potential with increasing age have limited its clinical use [40,43]. On the other hand, hUCB-MSCs are harvested and stored via simpler methods [29] and have displayed higher osteogenicity and proliferation rates compared to other MSCs [[30], [31], [32],40]. In addition, hUCB-MSCs were reported to actively inhibit the allogeneic proliferation of responder lymphocytes [44] and retain immunosuppressive activity equal to bone marrow- and adipose-derived MSCs [30].
In this study, OVX rats that received xenograft hUCB-MSCs developed 53% and 65% more bone volume in the femoral defect compared to OVX rats treated with saline at 4- and 8-week postoperation, respectively (Fig. 2). Moreover, there were no significant differences in the percent bone volume between the OVX-MSCc and Sham groups. Despite being a xenograft stem cell transplantation, we found no evidence of immunogenic rejection. In fact, when Oh et al. [44] pretreated hUCB-MSCs with interferon-γ and pro-inflammatory cytokines interleukin (IL)-1β or tumor necrosis factor-α, there was no inflammatory responses provoked thus suggesting that hUCB-MSCs may be transplanted into inflammation regions without the necessary requirement of long-term immunosuppressive treatment. Furthermore, the secretion of immune inhibitory cytokines from hUCB-MSCs, including IL-10 and transforming growth factor-β, has also been previously discovered, hence concluding the mesenchymal content responsible for the tolerogenic characteristics of cord blood [45,46]. Analogous to these reports, our study suggests hUCB-MSCs xenotransplantations in bone defects of osteoporotic conditions may positively enhance bone regeneration abilities with minimal immunogenic responses.
Recently, there have been a few reports studying osteogenicity of hUCB-MSCs in vivo. Jo et al. [47] found that hUCB-MSCs attached on a scaffold in critical-sized femoral defects of rats showed significantly higher levels of osteogenesis than the scaffold-only group and the adipose tissue- and umibilical cord-derived MSCs group. Similar to our study, comparisons were based on total bone volume percentages, but additionally demonstrated significantly elevated trabecular numbers and radiologic scores in the hUCB-MSCs group [47]. Despite the poor engraftment of MSCs in bone tissue after systemic administration, this study discovered increased serum procollagen type-I N-telopeptide levels, elevated alkaline phosphatase activity, and heightened pro-osteogenic mRNA expression; thus, they proposed that the prevention of OVX-mediated bone loss may be attenuated by a paracrine mechanism [23]. This mechanism may be a possible explanation behind the increased bone formation observed in our study after the injection of hUCB-MSCs. In fact, a recent clinical study has demonstrated significant increases in insulin-like growth factor 1 levels and beneficial effects on bone mineral density following subcutaneous injection of allogenic hUCB mononuclear cells in osteoporotic patients [48].
Although hUCB-MSCs have the potential to be differentiated into multiple cell lineages, studies have reported the relatively low ability to differentiate towards the adipocyte lineage compared to bone marrow- and adipose-derived MSCs [40,49]. Moreover, the expression of BMP receptors was discovered predominantly on hUCB-MSCs and bone marrow MSCs [30]. In contrast to the paracrine effect, MSCs can also migrate and engraft into wounded areas and regulate repair via site-specific differentiation and provide a hospitable microenvironment [50,51]. Upon directed injection, MSCs can also attach to the surface of the trabecular bone [52]. Consequently, our study utilized a direct injection method into the femoral defect, in which the hUCB-MSCs may have homed to the trabecular surface thus providing local, direct osteogenesis.
One limitation to this study is the use of rodents as the animal model for osteoporosis hence the lack of Haversian systems and Haversian remodeling in the cortical bone. However, ovariectomy of all rat models generate cancellous and endocortical bone loss, which are the dominant causes of postmenopausal osteoporosis, and ultimately show the same pattern as the estrogen-deficient human [38]. On the contrary, bone loss induced by intracortical remodeling in the Haversian system plays a minor role [53]. Therefore, rodents are the most common animal model for osteoporosis studies [54].
Another limitation is the restricted area of analysis within the femoral defect (1.2-mm diameter, 7-mm height) for micro-CT quantitative measurements to maintain standardization among each specimen. Osteogenesis in areas outside the defect, including effects that would improve osteoporotic bone conditions, had to be excluded from the data. The number of specimens per experimental group was relatively small as well. Also, quantitative immunohistochemistry analysis would have provided more accurate results, since the osteoblast cells discovered at the margin of the femoral defect could not be distinguished as either injected human cells or cells originating from the femur of the rat.
In summary, our results demonstrate that xenograft transplantation of hUCB-MSCs into femoral defects of OVX rats enhance bone formation abilities. Considering the relatively immunosuppressive properties, the high osteogenic potential, and the simpler harvesting methods of hUCB-MSCs, our study demonstrates that hUCB-MSCs may be suitable for bone regenerative medicine and tissue engineering. Additional information regarding specific mechanism behind the bone healing process of UCB-MSCs is required. With the growing incidence of osteoporosis among female and elderly populations, hUCB-MSCs may be a promising alternative stem cell for future osteoporotic treatment.
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Acknowledgments
The authors thank student doctor Jaein Lee (Chungnam National University School of Medicine) for her invaluable contribution throughout this research. This study was partially supported by the National Research Foundation of Korea (2017R1A2B4005905).
Footnotes
Peer review under responsibility of The Korean Society of Osteoporosis.
References
- 1.Osteoporosis prevention, diagnosis, and therapy. NIH Consens Statement. 2000;17:1–45. [PubMed] [Google Scholar]
- 2.Raisz L.G. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest. 2005;115:3318–3325. doi: 10.1172/JCI27071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szulc P., Delmas P.D. Biochemical markers of bone turnover: potential use in the investigation and management of postmenopausal osteoporosis. Osteoporos Int. 2008;19:1683–1704. doi: 10.1007/s00198-008-0660-9. [DOI] [PubMed] [Google Scholar]
- 4.Das S., Crockett J.C. Osteoporosis - a current view of pharmacological prevention and treatment. Drug Des Dev Ther. 2013;7:435–448. doi: 10.2147/DDDT.S31504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan A.A., Sándor G.K., Dore E., Morrison A.D., Alsahli M., Amin F. Bisphosphonate associated osteonecrosis of the jaw. J Rheumatol. 2009;36:478–490. doi: 10.3899/jrheum.080759. [DOI] [PubMed] [Google Scholar]
- 6.Lo J.C., O'Ryan F.S., Gordon N.P., Yang J., Hui R.L., Martin D. Prevalence of osteonecrosis of the jaw in patients with oral bisphosphonate exposure. J Oral Maxillofac Surg. 2010;68:243–253. doi: 10.1016/j.joms.2009.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saleh A., Hegde V.V., Potty A.G., Lane J.M. Bisphosphonate therapy and atypical fractures. Orthop Clin N Am. 2013;44:137–151. doi: 10.1016/j.ocl.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Nieves J.W., Cosman F. Atypical subtrochanteric and femoral shaft fractures and possible association with bisphosphonates. Curr Osteoporos Rep. 2010;8:34–39. doi: 10.1007/s11914-010-0007-2. [DOI] [PubMed] [Google Scholar]
- 9.Valverde P. Pharmacotherapies to manage bone loss-associated diseases: a quest for the perfect benefit-to-risk ratio. Curr Med Chem. 2008;15:284–304. doi: 10.2174/092986708783497274. [DOI] [PubMed] [Google Scholar]
- 10.Fröhlich M., Grayson W.L., Wan L.Q., Marolt D., Drobnic M., Vunjak-Novakovic G. Tissue engineered bone grafts: biological requirements, tissue culture and clinical relevance. Curr Stem Cell Res Ther. 2008;3:254–264. doi: 10.2174/157488808786733962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Undale A.H., Westendorf J.J., Yaszemski M.J., Khosla S. Mesenchymal stem cells for bone repair and metabolic bone diseases. Mayo Clin Proc. 2009;84:893–902. doi: 10.4065/84.10.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katsara O., Mahaira L.G., Iliopoulou E.G., Moustaki A., Antsaklis A., Loutradis D. Effects of donor age, gender, and in vitro cellular aging on the phenotypic, functional, and molecular characteristics of mouse bone marrow-derived mesenchymal stem cells. Stem Cell Dev. 2011;20:1549–1561. doi: 10.1089/scd.2010.0280. [DOI] [PubMed] [Google Scholar]
- 13.Chen X.D., Shi S., Xu T., Robey P.G., Young M.F. Age-related osteoporosis in biglycan-deficient mice is related to defects in bone marrow stromal cells. J Bone Miner Res. 2002;17:331–340. doi: 10.1359/jbmr.2002.17.2.331. [DOI] [PubMed] [Google Scholar]
- 14.Verma S., Rajaratnam J.H., Denton J., Hoyland J.A., Byers R.J. Adipocytic proportion of bone marrow is inversely related to bone formation in osteoporosis. J Clin Pathol. 2002;55:693–698. doi: 10.1136/jcp.55.9.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mueller S.M., Glowacki J. Age-related decline in the osteogenic potential of human bone marrow cells cultured in three-dimensional collagen sponges. J Cell Biochem. 2001;82:583–590. doi: 10.1002/jcb.1174. [DOI] [PubMed] [Google Scholar]
- 16.Rodríguez J.P., Garat S., Gajardo H., Pino A.M., Seitz G. Abnormal osteogenesis in osteoporotic patients is reflected by altered mesenchymal stem cells dynamics. J Cell Biochem. 1999;75:414–423. doi: 10.1002/(sici)1097-4644(19991201)75:3<414::aid-jcb7>3.3.co;2-3. [DOI] [PubMed] [Google Scholar]
- 17.Bonyadi M., Waldman S.D., Liu D., Aubin J.E., Grynpas M.D., Stanford W.L. Mesenchymal progenitor self-renewal deficiency leads to age-dependent osteoporosis in Sca-1/Ly-6A null mice. Proc Natl Acad Sci U S A. 2003;100:5840–5845. doi: 10.1073/pnas.1036475100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bianco P. Stem cells and bone: a historical perspective. Bone. 2015;70:2–9. doi: 10.1016/j.bone.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Zuk P.A., Zhu M., Mizuno H., Huang J., Futrell J.W., Katz A.J. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 20.Erices A., Conget P., Minguell J.J. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235–242. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z., Goh J., Das De S., Ge Z., Ouyang H., Chong J.S. Efficacy of bone marrow-derived stem cells in strengthening osteoporotic bone in a rabbit model. Tissue Eng. 2006;12:1753–1761. doi: 10.1089/ten.2006.12.1753. [DOI] [PubMed] [Google Scholar]
- 22.Ye X., Zhang P., Xue S., Xu Y., Tan J., Liu G. Adipose-derived stem cells alleviate osteoporosis by enhancing osteogenesis and inhibiting adipogenesis in a rabbit model. Cytotherapy. 2014;16:1643–1655. doi: 10.1016/j.jcyt.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 23.An J.H., Park H., Song J.A., Ki K.H., Yang J.Y., Choi H.J. Transplantation of human umbilical cord blood-derived mesenchymal stem cells or their conditioned medium prevents bone loss in ovariectomized nude mice. Tissue Eng. 2013;19:685–696. doi: 10.1089/ten.tea.2012.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bashir J., Sherman A., Lee H., Kaplan L., Hare J.M. Mesenchymal stem cell therapies in the treatment of musculoskeletal diseases. Pharm Manag PM R. 2014;6:61–69. doi: 10.1016/j.pmrj.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Wagner W., Horn P., Castoldi M., Diehlmann A., Bork S., Saffrich R. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS One. 2008;3 doi: 10.1371/journal.pone.0002213. e2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stenderup K., Justesen J., Clausen C., Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33:919–926. doi: 10.1016/j.bone.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Weiss M.L., Medicetty S., Bledsoe A.R., Rachakatla R.S., Choi M., Merchav S. Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson's disease. Stem Cell. 2006;24:781–792. doi: 10.1634/stemcells.2005-0330. [DOI] [PubMed] [Google Scholar]
- 28.Majhail N.S., Brunstein C.G., Tomblyn M., Thomas A.J., Miller J.S., Arora M. Reduced-intensity allogeneic transplant in patients older than 55 years: unrelated umbilical cord blood is safe and effective for patients without a matched related donor. Biol Blood Marrow Transplant. 2008;14:282–289. doi: 10.1016/j.bbmt.2007.12.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Antebi B., Pelled G., Gazit D. Stem cell therapy for osteoporosis. Curr Osteoporos Rep. 2014;12:41–47. doi: 10.1007/s11914-013-0184-x. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X., Hirai M., Cantero S., Ciubotariu R., Dobrila L., Hirsh A. Isolation and characterization of mesenchymal stem cells from human umbilical cord blood: reevaluation of critical factors for successful isolation and high ability to proliferate and differentiate to chondrocytes as compared to mesenchymal stem cells from bone marrow and adipose tissue. J Cell Biochem. 2011;112:1206–1218. doi: 10.1002/jcb.23042. [DOI] [PubMed] [Google Scholar]
- 31.Schneider R.K., Puellen A., Kramann R., Raupach K., Bornemann J., Knuechel R. The osteogenic differentiation of adult bone marrow and perinatal umbilical mesenchymal stem cells and matrix remodelling in three-dimensional collagen scaffolds. Biomaterials. 2010;31:467–480. doi: 10.1016/j.biomaterials.2009.09.059. [DOI] [PubMed] [Google Scholar]
- 32.Shetty P., Cooper K., Viswanathan C. Comparison of proliferative and multilineage differentiation potentials of cord matrix, cord blood, and bone marrow mesenchymal stem cells. Asian J Transfus Sci. 2010;4:14–24. doi: 10.4103/0973-6247.59386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guillot P.V., De Bari C., Dell'Accio F., Kurata H., Polak J., Fisk N.M. Comparative osteogenic transcription profiling of various fetal and adult mesenchymal stem cell sources. Differentiation. 2008;76:946–957. doi: 10.1111/j.1432-0436.2008.00279.x. [DOI] [PubMed] [Google Scholar]
- 34.Tsuchida H., Hashimoto J., Crawford E., Manske P., Lou J. Engineered allogeneic mesenchymal stem cells repair femoral segmental defect in rats. J Orthop Res. 2003;21:44–53. doi: 10.1016/S0736-0266(02)00108-0. [DOI] [PubMed] [Google Scholar]
- 35.Park S.B., Lee Y.J., Chung C.K. Bone mineral density changes after ovariectomy in rats as an osteopenic model: stepwise description of double dorso-lateral approach. J Korean Neurosurg Soc. 2010;48:309–312. doi: 10.3340/jkns.2010.48.4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garnero P., Sornay-Rendu E., Chapuy M.C., Delmas P.D. Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J Bone Miner Res. 1996;11:337–349. doi: 10.1002/jbmr.5650110307. [DOI] [PubMed] [Google Scholar]
- 37.Klein-Nulend J., van Oers R.F., Bakker A.D., Bacabac R.G. Bone cell mechanosensitivity, estrogen deficiency, and osteoporosis. J Biomech. 2015;48:855–865. doi: 10.1016/j.jbiomech.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 38.Jee W.S., Yao W. Overview: animal models of osteopenia and osteoporosis. J Musculoskelet Neuronal Interact. 2001;1:193–207. [PubMed] [Google Scholar]
- 39.Wronski T.J., Dann L.M., Horner S.L. Time course of vertebral osteopenia in ovariectomized rats. Bone. 1989;10:295–301. doi: 10.1016/8756-3282(89)90067-7. [DOI] [PubMed] [Google Scholar]
- 40.Kern S., Eichler H., Stoeve J., Klüter H., Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cell. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 41.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 42.Ren G., Su J., Zhang L., Zhao X., Ling W., L'huillie A. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cell. 2009;27:1954–1962. doi: 10.1002/stem.118. [DOI] [PubMed] [Google Scholar]
- 43.Mendes S.C., Tibbe J.M., Veenhof M., Bakker K., Both S., Platenburg P.P. Bone tissue-engineered implants using human bone marrow stromal cells: effect of culture conditions and donor age. Tissue Eng. 2002;8:911–920. doi: 10.1089/107632702320934010. [DOI] [PubMed] [Google Scholar]
- 44.Oh W., Kim D.S., Yang Y.S., Lee J.K. Immunological properties of umbilical cord blood-derived mesenchymal stromal cells. Cell Immunol. 2008;251:116–123. doi: 10.1016/j.cellimm.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 45.Liu J., Lu X.F., Wan L., Li Y.P., Li S.F., Zeng L.Y. Suppression of human peripheral blood lymphocyte proliferation by immortalized mesenchymal stem cells derived from bone marrow of Banna Minipig inbred-line. Transplant Proc. 2004;36:3272–3275. doi: 10.1016/j.transproceed.2004.11.090. [DOI] [PubMed] [Google Scholar]
- 46.Tögel F., Hu Z., Weiss K., Isaac J., Lange C., Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Ren Physiol. 2005;289:F31–F42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 47.Jo C.H., Yoon P.W., Kim H., Kang K.S., Yoon K.S. Comparative evaluation of in vivo osteogenic differentiation of fetal and adult mesenchymal stem cell in rat critical-sized femoral defect model. Cell Tissue Res. 2013;353:41–52. doi: 10.1007/s00441-013-1619-5. [DOI] [PubMed] [Google Scholar]
- 48.Li J., Zhang L., Zhou L., Yu Z.P., Qi F., Liu B. Beneficial effects of non-matched allogeneic cord blood mononuclear cells upon patients with idiopathic osteoporosis. J Transl Med. 2012;10:102. doi: 10.1186/1479-5876-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morigi M., Rota C., Montemurro T., Montelatici E., Lo Cicero V., Imberti B. Life-sparing effect of human cord blood-mesenchymal stem cells in experimental acute kidney injury. Stem Cell. 2010;28:513–522. doi: 10.1002/stem.293. [DOI] [PubMed] [Google Scholar]
- 50.Shi Y., Hu G., Su J., Li W., Chen Q., Shou P. Mesenchymal stem cells: a new strategy for immunosuppression and tissue repair. Cell Res. 2010;20:510–518. doi: 10.1038/cr.2010.44. [DOI] [PubMed] [Google Scholar]
- 51.Liu Y., Wu J., Zhu Y., Han J. Therapeutic application of mesenchymal stem cells in bone and joint diseases. Clin Exp Med. 2014;14:13–24. doi: 10.1007/s10238-012-0218-1. [DOI] [PubMed] [Google Scholar]
- 52.Ocarino Nde M., Boeloni J.N., Jorgetti V., Gomes D.A., Goes A.M., Serakides R. Intra-bone marrow injection of mesenchymal stem cells improves the femur bone mass of osteoporotic female rats. Connect Tissue Res. 2010;51:426–433. doi: 10.3109/03008201003597049. [DOI] [PubMed] [Google Scholar]
- 53.Lelovas P.P., Xanthos T.T., Thoma S.E., Lyritis G.P., Dontas I.A. The laboratory rat as an animal model for osteoporosis research. Comp Med. 2008;58:424–430. [PMC free article] [PubMed] [Google Scholar]
- 54.Turner A.S. Animal models of osteoporosis–necessity and limitations. Eur Cell Mater. 2001;1:66–81. doi: 10.22203/ecm.v001a08. [DOI] [PubMed] [Google Scholar]