Abstract
Policies and practices have proliferated to optimize prescribers’ use of their states’ prescription drug monitoring programs, which are statewide databases of controlled substances dispensed at retail pharmacies. Our study assessed the effectiveness of three such policies: comprehensive legislative mandates to use the program, laws that allow prescribers to delegate its use to office staff, and state participation in interstate data sharing. Our analysis of information from a large commercial insurance database indicated that comprehensive use mandates implemented during 2011–15 were associated with a 6–9 percent reduction in opioid prescriptions with high risk for misuse and overdose. We also found delegate laws to be associated with reductions of a similar magnitude for selected outcomes. In general, the effects of all three policies strengthened over time, especially beginning in the second year after implementation. Our findings support comprehensive use mandates and delegate laws to optimize prescribers’ use of drug monitoring programs, but the results will need updates in the context of evolving state opioid policies—including the increasing integration of drug monitoring data with electronic health records.
Prescription drug monitoring programs (PDMPs) are statewide databases of controlled substances dispensed at retail pharmacies. By providing a nearly complete picture of controlled substance use by individual patients, the programs can assist prescribers in identifying unsafe use or misuse of prescription opioids without compromising safe and medically warranted use of pain medications. Thus, the programs are a prominent tool in states’ efforts to combat the opioid crisis. Their success depends on the actual use of PDMPs by prescribers at the point of care. However, existing data suggest that use of the programs overall is low and varied,1,2 which raises concerns about the effectiveness of this policy tool.
In recent years states have adopted a series of policies and practices aimed at improving prescribers’ use of PDMPs. Legislative mandates that prescribers use the programs at the point of care are prominent, but these use mandates vary substantially in their comprehensiveness and strength.3,4 For example, by the end of 2017 only twenty of the thirty-seven states that had some type of use mandate in effect had adopted mandates that applied to prescribers of controlled substances in all settings, required use of the program at initial prescription and at least annually thereafter, and did not rely on prescribers’ discretion on whether to use the program.5 Evidence is accumulating that use mandates that fall short of these criteria provide limited value in reducing overall opioid prescribing;4 have a much weaker effect in curbing excessive opioid use,6 compared to stronger and more comprehensive mandates; and have no effect in reducing deaths associated with opioid overdose.6,7
Policies and practices other than legislative mandates have also proliferated in recent years.3,8 These policies are designed to lower prescriber burden when using the PDMP (for example, by allowing office staff to check the PDMP on behalf of the authorized prescriber) or to enhance the program’s timeliness (by updating its information daily), comprehensiveness (by enabling interstate data sharing), or usability (by enhancing the user interface or providing alerts). National evidence is limited on the effectiveness of these policies independent from the effects of mandates.9–11 However, data from selected states suggest that there were increases in the use of PDMPs and user satisfaction after implementation of delegation laws, more timely reporting, or improved PDMP interfaces.3
We assessed the effects of PDMP policies and practices implemented in the period 2011–15 on patterns of prescriptions that put patients at high risk of misuse and overdose (“high-risk prescriptions”). We focused on three types of policies: comprehensive mandates to use a PDMP at the point of care, legislation allowing delegate access to PDMP, and participation in interstate data sharing. These policies were selected because of the promises they present for increasing prescribers’ use of PDMPs, their adoption by a sufficient number of states during the study period, the relative homogeneity of policies adopted across states, and the availability of reliable data on effective dates of the policies for all adopting states.
We used data from a large commercial insurance claims database and focused on the nonelderly adult population (ages 18–64) with private insurance. In 2016 an estimated 5.6 million such adults nationwide used prescription opioids nonmedically, compared to 2.1 million with Medicaid, 0.7 million with Medicare, and 0.3 million with both Medicare and Medicaid (authors’ analysis of data from the 2016 National Survey on Drug Use and Health).12 The implications of PDMP policies for privately insured adults remain understudied: Only a few studies have used population aggregate data covering people with all types of insurance.7,13 By focusing on the privately insured, we provide policy makers with much-needed evidence concerning the largest population that states’ drug control policies are designed to benefit.
Study Data And Methods
Data
Our data for 2011–15 came from the Health Care Cost Institute’s insurance claims database,14 which includes claims for about fifty million people per year enrolled in a health insurance plan offered or administered (that is, self-insured plans) by Aetna, Humana, and UnitedHealthcare. The institute’s data include beneficiary enrollment information and inpatient facility, outpatient facility, physician, and pharmacy claims. Pharmacy claims provide information on the National Drug Code, days of supply, quantity and unit, and strength/dosage for each prescription.
Population And Sample
Our study population consisted of privately insured adults ages 18–64 who had at least one opioid prescription in 2011–15. Only patients who were continuously enrolled in employment-based or individual-market plans in a calendar year were included in the sample. We excluded patient-years in which the patient had a cancer diagnosis to focus on people receiving opioids for noncancer pain. All forty-eight states with an operating PDMP by the end of 2015 were adequately represented in each year of the sample (online Appendix Exhibit A1).15
Measures
Our unit of analysis was the patient-quarter. We examined four measures of high-risk opioid prescriptions conditional on having any opioid prescription in a quarter: overlapping opioid prescriptions for seven days or more days;16,17 opioid prescriptions from three or more prescribers;18 overlapping opioid and benzodiazepine prescriptions for seven or more days;19–21 and a very high standardized dosage of opioids, indicated by a daily dose exceeding 120 morphine milligram equivalents (MMEs).21–23 These measures were selected because they either strongly suggest “doctor shopping” (patients’ obtaining multiple prescriptions from multiple doctors) or misuse (patients’ using opioids in a manner or dose other than prescribed) or are associated with substantially increased risks of overdose – behaviors that PDMPs are designed to curtail. Opioid-benzodiazepine overlap is a salient measure because of its high risk of overdose and because all PDMPs now monitor Schedule IV drugs, including benzodiazepines. When deriving these measures, we “assigned” days’ supply of a prescription to adjacent quarters if the prescription was filled in one quarter and extended into the next.
Implementation of PDMP policies was defined based on the effective date of legislation (for comprehensive use mandates and delegation laws) or the “go-live” date of interstate data sharing. The National Alliance for Model State Drug Laws24 provided us with effective dates of pertinent state legislation. During our study period, the most robust way of enabling interstate data sharing was through state participation in PMP InterConnect, provided by the National Association of Boards of Pharmacy.25 Participating states sign a memo of understanding and develop an interface to connect their PDMP with PMP InterConnect. Each state controls access to its data via a dashboard within PMP InterConnect, allowing selected states to share the data. By the end of our study period, ten states had implemented comprehensive use mandates, thirty-eight had allowed prescribers to delegate PDMP use to an office staff member, and thirty were participating in interstate data sharing via PMP InterConnect (Appendix Exhibit A2).15 We set each of the three policy indicators to 1 for each full quarter after the effective date of the policy in a given state, and 0 otherwise.
Analysis
The staggered implementation of PDMP policies across states created a natural experiment. We examined changes in study outcomes from before to after policy implementation in implementing states, using states that had not implemented the policy as controls. Our main analysis focused on the twenty-eight states with a fully operating PDMP (one that users could access) by the end of 2010. States that launched PDMPs later were more likely to have adopted mandates and other PDMP-enhancing policies at the same time or shortly after they launched PDMPs, which made it challenging to statistically isolate the effects of PDMP-enhancing policies from the effects of launching a PDMP. In a secondary analysis, we assessed the robustness of our findings by including all forty-eight states that had an operating PDMP by the end of 2015 (the twenty-eight states mentioned above and the twenty with newly implemented PDMPs during 2011–15; the excluded states were Missouri and Pennsylvania, as well as the District of Columbia).
We estimated a linear probability model and a logistic model for each outcome. The main independent variables were the three dichotomous PDMP policy variables indicating that a specific state-quarter was before or after implementation of a given policy. The models included a set of dichotomous state indicators (state fixed effects) to control for differences between states that did not change over time and a set of calendar-quarter fixed effects (from the second quarter of 2011 to the fourth quarter of 2015) to control for nationwide trends in the outcomes. Each model also controlled for patients’ sex, age (at the beginning of a quarter), pain-related diagnoses based on health care claims in the calendar year, any mental health condition, and any substance use condition (which was further broken down into alcohol use disorders, drug use disorders, and tobacco use). (The International Classification of Diseases, Ninth Revision (ICD-9), diagnostic codes used to define these conditions are in Appendix Exhibit A3.)15 We did not include patient ZIP code–level sociodemographic profiles in the final models because missing ZIP codes would have dropped 2.5 percent of the sample from analysis and because excluding these controls did not change the main findings. All analyses took into account clustering of quarters of the same patient in deriving robust standard errors.
Our main analysis estimated the average effect of the policies, regardless of how long they had been in effect. We conducted an additional analysis to allow the effect to differ by time since implementation. Specifically, in place of the dichotomous policy indicators, we included indicators that the index quarter was within 0–6 months, 7–12 months, or 13 or more months after implementation. We also included indicators of 0–6, 7–12, 13–18, and 19 or more months before implementation for implementing (versus nonimplementing) states, to determine whether trends in a given outcome leading to policy implementation differed by state implementation status. This analysis, known as an “event study” in the economics literature,26 treated 0–6 months before implementation as the reference period in which the difference between the implementing and nonimplementing states was held to zero.
In the secondary analysis that included all forty-eight states with an operating PDMP by the end of 2015, we included a dichotomous indicator of an operating PDMP in addition to all policy indicators considered in the main analysis.
Limitations
Our study had a number of limitations. First, our measures of high-risk prescriptions represent direct behavioral targets of PDMP policies. Our study did not provide data on implications for downstream outcomes of public health significance such as opioid-related overdose events and deaths.
Second, whether a state adopts a PDMP policy and the timing of such adoption may be correlated with the opioid epidemic in the state, leading to potentially biased estimates. Our event study analysis provided a way to check whether implementing and nonimplementing states followed parallel trends prior to the policy—a critical assumption of our statistical approach.
Third, co-occurrence of state drug control policies is the norm rather than the exception. In particular, comprehensive use mandates and delegate laws could have synergistic effects. Because six of the seven states with comprehensive mandates in our main analysis had adopted delegate laws before or at the same time as their comprehensive mandates took effect, it was challenging to assess the added effects of having both policies above and beyond having comprehensive mandates alone. The effects we estimated pertaining to comprehensive mandates thus more closely reflect the combined effects of comprehensive mandates and delegate laws. On the other hand, seventeen of the twenty-three states with delegate laws did not adopt comprehensive use mandates. The effect estimates pertaining to delegate laws thus captured their effects independent of the effects of the mandates.
Fourth, we did not include non-PDMP policies that could have direct implications for opioid prescribing. Previous studies have found that state laws governing pain clinics did not change effect estimates pertaining to PDMPs.6 Other state policies, such as day or dosage limits for first opioid prescriptions, were implemented more recently (that is, after our study years). While adequately accounting for all state policies is difficult and beyond the scope of this study, our estimates may reflect overestimation of true effects.
Finally, we were not able to control for any prescriber characteristics because prescriber identifiers were encrypted in our data and could not be linked to external sources.
Study Results
The sample for our main analysis contained 6,244,784 patient-quarters with at least one day of opioid prescriptions, contributed by 3,314,040 unique patients from the twenty-eight states with an operating PDMP by the end of 2010. (Characteristics pertaining to the sample and study outcomes are summarized in Appendix Exhibit A4.)15 Of the patient-quarters, 7.4 percent had overlapping opioid prescriptions for seven or more days, 2.8 percent had three or more prescribers of opioids, 10.4 percent had overlapping opioid and benzodiazepine prescriptions for seven or more days, and 5.8 percent had a daily morphine milligram equivalent of more than 120. Because these outcomes were of different scales, we report below changes associated with the policy relative to the sample means. Results reported are based on linear probability models. Results based on logistic models (in terms of marginal changes in outcomes) were similar and are reported in Appendix Exhibit A5.15
Policies’ Overall Effects
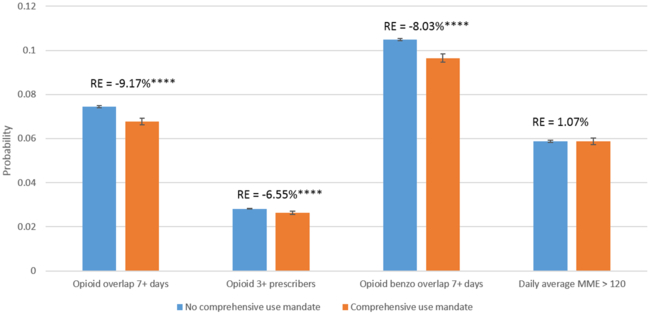
Based on our main analysis of the twenty-eight states with an operating PDMP by the end of 2010, a comprehensive use mandate was associated with a 9.2 percent reduction in the probability of overlapping opioid prescriptions, a 6.6 percent reduction in the probability of having three or more opioid prescribers, and an 8.0 percent reduction in the probability of having overlapping opioid and benzodiazepine prescriptions (Exhibit 1). A comprehensive use mandate was not associated with a significant difference in having a daily MME greater than 120.
Exhibit 1.
Changes in the probability of high-risk opioid prescriptions after the implementation of a comprehensive mandate to use a prescription drug monitoring program, 2011–15
Source/Notes: SOURCE Authors’ analysis of data for 2011–15 from the Health Care Cost Institute’s insurance claims database. NOTES The exhibit shows the predicted changes in the probabilities of outcomes associated with the implementation of a mandate among privately insured adults who were ages 18–64, had at least one opioid prescription in the study period, and lived in the twenty-eight states that had an operating program by the end of 2010. The whiskers indicate 95% confidence intervals. The percentages (relative effects) indicate the difference between probabilities with and without a mandate. High-risk opioid prescriptions are explained in the text. MME is morphine milligram equivalent. ****p < 0.001
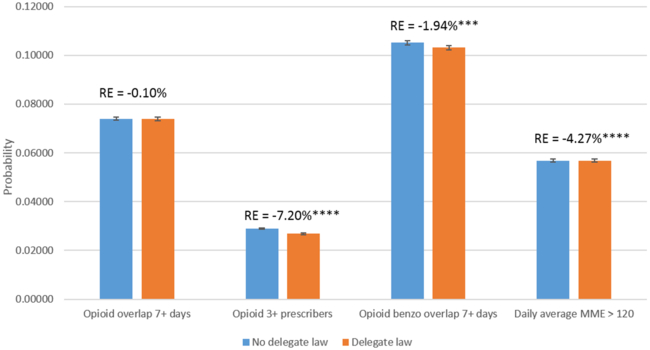
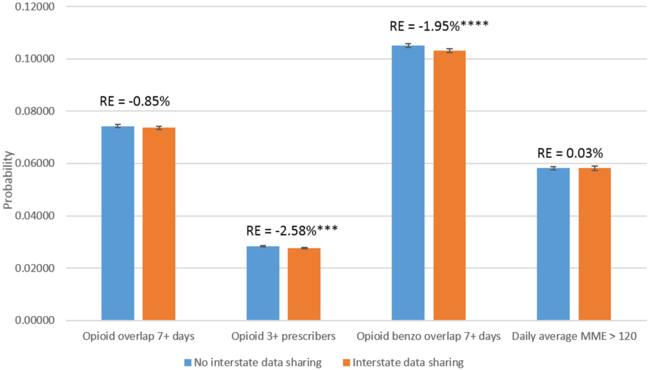
Delegate laws were associated with a 7.2 percent reduction in the probability of having three or more prescribers of opioids, a 1.9 percent reduction in the probability of having overlapping opioid and benzodiazepine prescriptions, and a 4.3 percent reduction in the probability of having a daily MME greater than 120 (Exhibit 2). Having interstate data sharing was associated with a 2.6 percent reduction in having three or more prescribers of opioids and a 2.0 percent reduction in having overlapping opioid and benzodiazepine prescriptions, but not with the other two outcomes (Exhibit 3).
Exhibit 2.
Changes in the probability of high-risk opioid prescriptions after the implementation of legislation allowing delegate access to prescription drug monitoring program databases, 2011–15
Source/Notes: SOURCE Authors’ analysis of data for 2011–15 from the Health Care Cost Institute’s insurance claims database. NOTES The exhibit shows the predicted changes in the probabilities of outcomes associated with the implementation of the legislation among the population described in the notes to Exhibit 1. The whiskers indicate 95% confidence intervals. The percentages (relative effects) indicate the difference between probabilities with and without the legislation. High-risk opioid prescriptions are explained in the text. MME is morphine milligram equivalent. ***p < 0.01 ****p < 0.001
Exhibit 3.
Changes in the probability of high-risk opioid prescriptions after states’ participation in interstate data sharing in prescription drug monitoring programs, 2011–15
Source/Notes: SOURCE Authors’ analysis of data for 2011–15 from the Health Care Cost Institute’s insurance claims database. NOTES The exhibit shows the predicted changes in the probabilities of outcomes associated with data sharing among the population described in the notes to Exhibit 1. The whiskers indicate 95% confidence intervals. The percentages (relative effects) indicate the difference between probabilities with and without data sharing. High-risk opioid prescriptions are explained in the text. MME is morphine milligram equivalent. ***p < 0.01 ****p < 0.001
Policies’ Effects Over Time
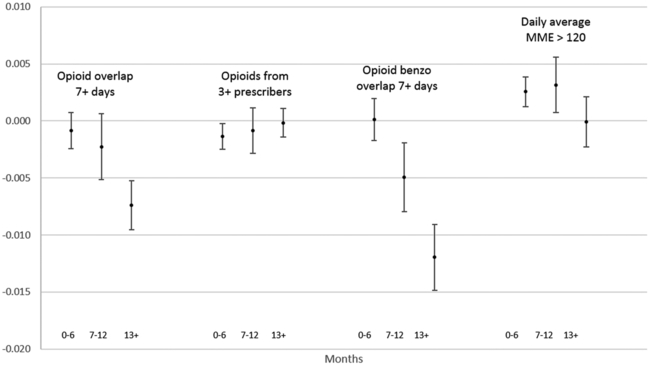
Our secondary analysis allowed the effects of the policies to differ over time. The results indicate that the reductions in the probabilities of high-risk prescriptions associated with a comprehensive use mandate strengthened over time (Exhibit 4). For example, while the estimated effect on the probability of overlapping opioid prescriptions was not significantly different from zero in the first year (0–6 and 7–12 months), the estimated effect increased to 0.74 percentage points (10 percent of the sample mean) after the mandate had been in effect for at least thirteen months. For delegate laws, reductions in the probability of overlapping opioid and benzodiazepine prescriptions strengthened from close to zero in the first six months to 0.4 percentage points (3.8 percent of the sample mean) after at least thirteen months (Appendix Exhibit A6).15 For interstate data sharing, substantial reductions were seen after at least thirteen months in overlapping opioid prescriptions (3.1 percent of the sample mean) and a daily MME greater than 120 (13.2 percent of the sample mean) – even though the policy was not associated with changes in either outcome in the overall analysis. Results of the event studies also indicate that for all three measures of high-risk opioid prescriptions, outcomes in implementing and nonimplementing states largely followed parallel trends leading to policy implementation (Appendix Exhibit A6).15
Exhibit 4.
Changes in the probability of high-risk opioid prescriptions after the implementation of a comprehensive mandate to use a prescription drug monitoring program, by time since mandate implementation, 2011–15
Source/Notes: SOURCE Authors’ analysis of data for 2011–15 from the Health Care Cost Institute’s insurance claims database. NOTES The exhibit shows the predicted changes in the probabilities of outcomes associated with the implementation of a mandate among the population described in the notes to Exhibit 1, relative to the period 0–6 months before implementation. The whiskers indicate 95% confidence intervals. MME is morphine milligram equivalent.
Policies’ Effects For All States With A Prescription Drug Monitoring Program By 2015
Our analysis that included forty-eight states with an operating PDMP by the end of 2015 generated findings that were largely comparable with those from the main analysis (Appendix Exhibit A7).15 For example, a comprehensive use mandate was associated with a 10 percent reduction in the probability of overlapping opioid prescriptions, a 5 percent reduction in the probability of having three or more opioid prescribers, a 7 percent reduction in the probability of overlapping opioid and benzodiazepine prescriptions (all p < 0.001), and a 3 percent reduction in the probability of having a daily MME exceeding 120 (p < 0.01).
Discussion
In recent years states have adopted various policies and practices to optimize prescribers’ use of prescription drug monitoring programs. We found states’ adoption of comprehensive use mandates to be associated with a 6–9 percent reduction in almost all measures of high-risk prescriptions. These estimates—if applied to all nonelderly, privately insured people in the US in 2016—suggest that the adoption of comprehensive use mandates by every state would be associated with over 36,000 fewer people having overlapping opioid prescriptions and over 44,000 fewer people having overlapping opioid and benzodiazepine prescriptions in any given quarter (Appendix Exhibit A8).15 (Total impacts of these policies are likely to be much greater when accounting for people with other insurance status.) We found delegate laws to be associated with reductions of a similar magnitude for selected measures of high-risk prescriptions. Our results suggest that, in general, the effects of all three policies strengthened over time, especially beginning in the second year after a policy took effect.
Our findings support comprehensive use mandates as an effective policy tool to curb high-risk opioid prescriptions. The relative effect sizes of comprehensive mandates estimated in this study (6–9 percent of the sample mean) are comparable to and, in some cases, greater than what has been reported in previous studies that focused on the Medicaid4 and Medicare populations.6 In our main analysis of the twenty-eight states with an operating PDMP by the end of 2010, four of the seven states with comprehensive use mandates did not implement the mandate until 2015 and therefore had limited exposure to the policy. Our estimates may have been driven by the much longer experience of the three early-adopting states, Kentucky (2012), West Virginia (2012), and Tennessee (2013). Given our finding that the effect of such mandates strengthened over time, it is plausible that the true effects of comprehensive mandates, if given enough time, could be greater than estimated. On the other hand, because the three states presented higher opioid prescribing rates than the rest of the country,27 our estimates pertaining to comprehensive use mandates might not be generalizable and should be updated when data for more recent years become available.
Our findings indicate that legislation that allows physician office staff to check the PDMP on behalf of the prescriber (independent from comprehensive use mandates) was associated with a reduction in the probability of having three or more prescribers of opioids in a quarter, a behavior that strongly suggests doctor shopping. The time required to check the PDMP at the point of care – usually by logging into an online portal completely separate from the electronic health record (EHR) – was perceived by physicians as a major barrier to using the PDMP.28,29 Being able to delegate the task to office staff may significantly reduce the burden on physicians and, in turn, make it more likely that they will use the PDMP to inform their prescribing decisions.
The implications of our findings regarding the mandate and delegation policies may change in future years, especially in the context of increasing integration of PDMPs with EHRs. Such integration should substantially lower the time burden of using a PDMP, especially if clinicians can access its information with a single click from within the EHR or if PDMP information is automatically integrated into the EHR. A recent study of a statewide initiative to automate PDMP queries from emergency department EHRs found a substantial increase in PDMP queries for patients with previous dispensing of controlled substances, albeit no difference in opioid prescribing or quantity.30 Increased integration should further strengthen prescriber use mandates by making it easier for prescribers to use a PDMP; meanwhile, delegate access might become less important.
We did not find states’ participation in interstate data sharing to be associated with meaningful changes in high-risk opioid prescriptions overall, although an additional analysis indicated substantial reductions in selected high-risk outcomes starting in the second year after the data sharing went live. Patients engaged in doctor shopping for opioids across state lines were likely a very small proportion of the entire population of prescription opioid users. The effect of interstate data sharing, if any, could have been diluted in a population-based analysis. Future research should reexamine the policy effects by focusing on subpopulations of patients who, because of either their previous high-risk behaviors or their geographic location (for example, proximity to state borders), are at a higher risk of engaging in doctor shopping in another state.
Conclusion
Our analysis of private insurance claims data provides evidence that supports comprehensive use mandates and delegate laws as policies to optimize prescribers’ use of prescription drug monitoring programs and curb high-risk opioid prescriptions. Our analysis also suggests that policy effects became stronger over time, especially starting in the second year after implementation. To further promote prescribers’ use of PDMPs, states should consider adopting comprehensive use mandates and laws allowing delegate access, if they have not already done so. Future research should continue to assess the utility of interstate data sharing, emerging PDMP-EHR integration, and other policy innovations in this arena.
Supplementary Material
Acknowledgments
Yuhua Bao, Phyllis Johnson, and Philip Jeng were supported by a pilot grant from the Center for Health Economics of Treatment Interventions for Substance Use Disorder, HCV, and HIV (CHERISH), a National Institute on Drug Abuse Center of Excellence (Grant No. P30DA040500), and by the National Institute of Mental Health (Grant No. R01MH104200). Zachary Meisel and Bruce Schackman are funded by the National Institute on Drug Abuse (Grant No. P30DA040500). Meisel is also supported by the Patient-Centered Outcomes Research Institute (Grant No. CDR-1511–33496). The authors thank Sherry Green, former CEO of the National Alliance for Model State Drug Laws (NAMSDL), and Chad Zadrazil, managing legislative attorney at NAMSDL, for providing prescription drug monitoring program policy data. The authors also thank the National Association of Boards of Pharmacy for providing data on states’ participation in PMP InterConnect.
Bios
Bio 1: Yuhua Bao (yub2003@med.cornell.edu) is an associate professor of healthcare policy and research at Weill Cornell Medical College, in New York City.
Bio 2: Katherine Wen is a PhD student in the Department of Policy Analysis and Management, Cornell University, in Ithaca, New York.
Bio 3: Phyllis Johnson is a programmer analyst in the Department of Healthcare Policy & Research, Weill Cornell Medical College.
Bio 4: Philip J. Jeng is a research coordinator in the Department of Healthcare Policy & Research, Weill Cornell Medical College.
Bio 5: Zachary F. Meisel is the director of the Center for Emergency Care Policy and Research and an associate professor in the Department of Emergency Medicine, both at the Perelman School of Medicine, and a senior fellow at the Leonard Davis Institute of Health Economics, all at the University of Pennsylvania, in Philadelphia.
Bio 6: Bruce R. Schackman is a professor of healthcare policy and research at Weill Cornell Medical College and director of the Center for Health Economics of Treatment Interventions for Substance Use Disorder, HCV, and HIV.
Notes
- 1.Rutkow L, Turner L, Lucas E, Hwang C, Alexander GC. Most primary care physicians are aware of prescription drug monitoring programs, but many find the data difficult to access. Health Aff (Millwood). 2015;34(3):484–92 PubMed. [DOI] [PubMed] [Google Scholar]
- 2.Irvine JM, Hallvik SE, Hildebran C, Marino M, Beran T, Deyo RA. Who uses a prescription drug monitoring program and how? Insights from a statewide survey of Oregon clinicians. J Pain. 2014;15(7):747–55 PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pew Charitable Trusts. Prescription drug monitoring programs: evidence-based practices to optimize prescriber use [Internet]. Philadelphia (PA): Pew Charitable Trusts; [updated 2016 Dec; cited 2018. August 27]. Available from: http://www.pewtrusts.org/~/media/assets/2016/12/prescription_drug_monitoring_programs.pdf [Google Scholar]
- 4.Wen H, Schackman BR, Aden B, Bao Y. States with prescription drug monitoring mandates saw a reduction in opioids prescribed to Medicaid enrollees. Health Aff (Millwood). 2017;36(4):733–41 PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Alliance for Model State Drug Laws. Mandated use of prescription drug monitoring programs (PMPs)—map [Internet]. Harrisburg (PA): NAMSDL; 2018 Jan 2 [cited 2018. August 27]. Available from: http://www.namsdl.org/library/Mandated%20Use%20%20of%20PMPs%20-%20State%20Map%20(1-2-18)/ [Google Scholar]
- 6.Buchmueller TC, Carey C. The effect of prescription drug monitoring programs on opioid utilization in Medicare. Am Econ J Econ Policy. 2018;10(1):77–112. [Google Scholar]
- 7.Patrick SW, Fry CE, Jones TF, Buntin MB. Implementation of prescription drug monitoring programs associated with reductions in opioid-related death rates. Health Aff (Millwood). 2016;35(7):1324–32 PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prescription Drug Monitoring Program Center of Excellence at Brandeis. COE briefing: PDMP prescriber use mandates: characteristics, current status, and outcomes in selected states [Internet]. Waltham (MA): The Center; [revised 2016 May; cited 2018. August 27]. Available from: http://www.pdmpassist.org/pdf/Resources/Briefing_on_mandates_3rd_revision_A.pdf [Google Scholar]
- 9.Fink DS, Schleimer JP, Sarvet A, Grover KK, Delcher C, Castillo-Carniglia A, et al. Association between prescription drug monitoring programs and nonfatal and fatal drug overdoses: a systematic review. Ann Intern Med. 2018;168(11):783–90 PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pauly NJ, Slavova S, Delcher C, Freeman PR, Talbert J. Features of prescription drug monitoring programs associated with reduced rates of prescription opioid-related poisonings. Drug Alcohol Depend. 2018;184:26–32 PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pardo B. Do more robust prescription drug monitoring programs reduce prescription opioid overdose? Addiction. 2017;112(10):1773–83 PubMed. [DOI] [PubMed] [Google Scholar]
- 12.Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. National Survey on Drug Use and Health 2016 (NSDUH-2016-DS0001) [Internet]. Rockville (MD): Substance Abuse and Mental Health Services Administration; 2017 Oct 27 [cited 2018. August 27]. Available from: https://www.datafiles.samhsa.gov/study-dataset/national-survey-drug-use-and-health-2016-nsduh-2016-ds0001-nid17185 [Google Scholar]
- 13.Dowell D, Zhang K, Noonan RK, Hockenberry JM. Mandatory provider review and pain clinic laws reduce the amounts of opioids prescribed and overdose death rates. Health Aff (Millwood). 2016;35(10):1876–83 PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Health Care Cost Institute [home page on the Internet]. Washington (DC): HCCI; c2018 [cited 2018. August 28]. Available from: http://www.healthcostinstitute.org/ [Google Scholar]
- 15.To access the appendix, click on the Details tab of the article online.
- 16.Prescription Drug Monitoring Program Training and Technical Assistance Center. Definitions of PBSS measures [Internet]. Waltham (MA): The Center; [cited 2018. August 28]. Available from: http://www.pdmpassist.org/pdf/COE_documents/Add_to_TTAC/Definitions%20of%20PBSS%20Measures.pdf [Google Scholar]
- 17.Seal KH, Shi Y, Cohen G, Cohen BE, Maguen S, Krebs EE, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307(9):940–7 PubMed. [DOI] [PubMed] [Google Scholar]
- 18.Wilsey BL, Fishman SM, Gilson AM, Casamalhuapa C, Baxi H, Zhang H, et al. Profiling multiple provider prescribing of opioids, benzodiazepines, stimulants, and anorectics. Drug Alcohol Depend. 2010;112(1–2):99–106 PubMed. [DOI] [PubMed] [Google Scholar]
- 19.Sun EC, Dixit A, Humphreys K, Darnall BD, Baker LC, Mackey S. Association between concurrent use of prescription opioids and benzodiazepines and overdose: retrospective analysis. BMJ. 2017;356:j760 PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hawkins EJ, Malte CA, Grossbard JR, Saxon AJ. Prevalence and trends of concurrent opioid analgesic and benzodiazepine use among Veterans Affairs patients with post-traumatic stress disorder, 2003–2011. Pain Med. 2015;16(10):1943–54 PubMed. [DOI] [PubMed] [Google Scholar]
- 21.Braden JB, Russo J, Fan MY, Edlund MJ, Martin BC, DeVries A, et al. Emergency department visits among recipients of chronic opioid therapy. Arch Intern Med. 2010;170(16):1425–32 PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meara E, Horwitz JR, Powell W, McClelland L, Zhou W, O’Malley AJ, et al. State legal restrictions and prescription-opioid use among disabled adults. N Engl J Med. 2016;375(1):44–53 PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gwira Baumblatt JA, Wiedeman C, Dunn JR, Schaffner W, Paulozzi LJ, Jones TF. High-risk use by patients prescribed opioids for pain and its role in overdose deaths. JAMA Intern Med. 2014;174(5):796–801 PubMed. [DOI] [PubMed] [Google Scholar]
- 24.National Alliance for Model State Drug Laws [home page on the Internet]. Harrisburg (PA): NAMSDL; c 2018 [cited 2018. August 28]. Available from: http://www.namsdl.org/ [Google Scholar]
- 25.National Association of Boards of Pharmacy. PMP InterConnect [Internet]. Mount Prospect (IL): NABP; c 2018 [cited 2018. August 28]. Available from: https://nabp.pharmacy/initiatives/pmp-interconnect [Google Scholar]
- 26.Heckman JJ, Smith JA. The pre-programme earnings dip and the determinants of participation in a social programme. Implications for simple programme evaluation strategies. Econ J (London). 1999;109(457):313–48. [Google Scholar]
- 27.Centers for Disease Control and Prevention. Opioid overdose: U.S. state prescribing rates, 2015 [Internet]. Atlanta (GA): CDC; [last updated 2017 Jul 31; cited 2018. August 28]. Available from: https://www.cdc.gov/drugoverdose/maps/rxstate2015.html [Google Scholar]
- 28.Smith RJ, Kilaru AS, Perrone J, Paciotti B, Barg FK, Gadsden SM, et al. How, why, and for whom do emergency medicine providers use prescription drug monitoring programs? Pain Med. 2015;16(6):1122–31 PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hildebran C, Cohen DJ, Irvine JM, Foley C, O’Kane N, Beran T, et al. How clinicians use prescription drug monitoring programs: a qualitative inquiry. Pain Med. 2014;15(7):1179–86 PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun BC, Charlesworth CJ, Lupulescu-Mann N, Young JI, Kim H, Hartung DM, et al. Effect of automated prescription drug monitoring program queries on emergency department opioid prescribing. Ann Emerg Med. 2018;71(3):337–347.e6 PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.