On the structure of testis seminiferous tubules and the development of [spermatids]
By E. Sertoli
(Table III and IV)
Our knowledge of the structure of many organs is comprehensive and detailed, thanks to the abundant and meticulous studies carried out by capable histologists. However, the analysis of other important organs remains absolutely incomplete, insofar as no one or only a few researchers have studied them, or they have done so either in a partial or limited fashion.
The list of long-neglected organs includes the testes. On the one hand, the deepest details of the bone structure, muscles, nervous organs, salivary glands, gastroenteric mucosa, livers, and kidneys have been revealed. Pflueger, Schrön, His, Waldeyer, and many others, whose names I will not mention for the sake of brevity, splendidly described the structure of the female genital gland. On the other hand, our histologic knowledge of the tubules of the male genital gland has been limited to the scant research work carried out, in not so recent times, by Kölliker and Henle. It is true that histologists investigated how [spermatids]1 proliferated from [spermatocytes].2 However, they did not consider looking at the precise shape, arrangement, development, and changes that all the cells in the seminiferous tubules of the testes undergo.
In recent years, though, testes have been saved from a long oblivion. Following my discovery of special branched cells [(called Sertoli cells, starting around 1900)],3 and once it was understood that the structure of seminiferous tubules is far more complex than thought until recently, testicles have been the subject of a number of more or less successful investigations by Merkel, Ebner, La Valette St-George, Mihalkowics, Neumann, Blumberg, and myself.
Nevertheless, in spite of the increasing number of studies, the detailed structure of seminiferous tubules is still unknown and the function of their cells has not been determined yet. In fact, two opposing opinions emerged on the relation between these cells and the growth of [the flagellum].4 Some support the traditional view first promoted by Kölliker and Henle, which was later shared by Schweigger-Seidel and La Valette St-George, and favored in more recent times by Merkel and myself. According to this view, [spermatids] generate from [germ cells]5 in seminiferous tubules. A new hypothesis suggested by Ebner and shared by Mihalkowics, Neumann, Blumberg, and others is that [flagella] grow from the central end of the cells I discovered in the year 1865, subsequently called [Sertoli cells].6
In an attempt to reconcile these discordant opinions, I put my efforts to gain a clear understanding of the seminiferous tubule's structure and how it relates to the growth of [flagella] and the production of seminal fluids. I therefore undertook a number of studies on the testicles of man and several mammals; those findings were previously published in the years 1871 and 1875.
The new theory promoted by Ebner on the growth of [spermatids] is mainly based on observations made on rat testes. Therefore, I will devote the first part of my work on the structure of seminiferous tubules of the rat, leaving my findings on other animal species and man to be published at a later time.
Seminiferous tubules structure and development of [spermatids] in rats
Rats (Mus decumanus) lend themselves better than any other animal species to the study of testis structure. Their seminiferous tubules are very large and connected to one another through a thin and loose interstitial tissue. This makes it extremely easy to isolate relatively long tubule tracts by using needles. The high production of spermatozoa in these animals occurs on a regular basis, which allows for quick location of these components at their various stages of development.
The tubule structure is very complicated and differs at various points along a single tubule, insofar as the development of [spermatids] doesn’t occur at the same time throughout its entire length, but to a different extent in each tract of the tubule.7
This feature, observed for the first time by Ebner (1)8 and which I can absolutely confirm, is paramount and should be taken into account for the study of the tubule's structure. As this structure varies according to the developmental stage of [spermatids], what we see if we observe a scrape preparation of random tubule sections in which [spermatids] are at different stages of development is a number of components very dissimilar in shape, so that any relationship among them is hardly established. I believe it is precisely because we kept ignoring this circumstance that we have not yet been able to identify the real shape, arrangement, and genetic link of the abundant cells contained in seminiferous tubules.
Prior to illustrating the tubules’ content, it is worth a brief digression to describe how [flagella] develop in order to facilitate the understanding of concepts that will be outlined further on.
As I will demonstrate later, I am firmly convinced that [spermatids] develop from the [germ cells] contained in the seminiferous tubules.
On this basis, I divide the time in which a [flagellum] develops and reaches maturity into three main stages. This subdivision is not completely arbitrary. It is true that the spermatozoon goes through a continual process of metamorphosis from the beginning to the end of its development, and it only undergoes slight, gradual changes. It is therefore impossible to precisely determine the beginning and ending of each phase. However, in each of these three stages, the development of one of the three parts that constitute the [spermatid] takes place, coinciding with the main structural changes of seminiferous tubules.
In the first, [(or round spermatid)] stage, the component from which the spermatozoon originates still looks very much like a cell and it undergoes little change. This stage begins when the [germ cell] shows its first signs of development with the formation of the sperm tail. It concludes when, following the cell elongation, the nucleus reaches one of its ends and the alterations in shape that will lead to the formation of the head are about to start.
The second [(, or elongating spermatid)] stage begins at this point and it concludes when the nucleus evolves into the head. However, by the end of this period, the head is still attached to the protoplasm9 of the elongated, flattened cell [(now termed an elongated spermatid)]; the enlarged tail emanates from its largest end.
During the third [(, or condensing spermatid)] stage, the [spermatids] become fully developed. The filament for the formation of the midpiece [(of the flagellum, or tail)] connects to the head. The cellular protoplasm gradually disappears, generating the well-known appendage joined to the midpiece. Initially, the [spermatids] form sheaf-like structures arranged in a radiating pattern, with the heads toward the tubule periphery. At the end of this stage, mature [spermatids] move through the center of the tubule and orient themselves parallel to the direction of the tubule.
This last stage occurs simultaneously with the first developmental stage of a new generation of [spermatids], which grow downward in the following tract of the tubule.
When one examines a relatively long tract of fresh tubule, which is easily found in rat testes, slightly magnified and soaked in aqueous humor or serum, this appears different at various stages of [spermatid] development.
While between the end of the first stage and the beginning of the second a given tract of the tubule contains only cells, during the second stage it is possible to identify [spermatid] heads in the following portion ever more precisely. Finally, in the third stage, one sees the filaments [(of condensing spermatids)] connected in a sheaf-like shape, which initially move from the periphery toward the center and gradually place themselves along the center of the tubule during the third stage.
As Ebner correctly observed, the development of [spermatids] occurs downward, so that the youngest are situated toward the end of the tubule, whereas the oldest are found in the opposite direction. Therefore, they develop in a direction opposite to the outward movement of [spermatids] from the tubules.
The three stages of development [(of spermatids)] I highlighted occur in the same tubule tract, whose length is not constant; similarly, the segment where each stage takes place varies as well.
By isolating a very long portion of a tubule and observing it under the microscope, one can see that a single tubule contains several generations of [spermatids]. At the point where one generation approaches its end, a new one begins to form as described above, occupying another portion of the tubule. This generation is followed by a third one further ahead, and so on.
Having provided a brief overview of the development of [spermatids] for the sake of a better understanding of my work, I now consider the tubule structure.
Seminiferous tubules of the rat are composed of a very thin sheath10 and some content. This is a thick layer of cells of different shape and nature surrounding a lumen in the middle of the tubule, where there are seminal fluids with spermatozoa and cellular residues. The average diameter of the seminiferous tubules in rats is 0.33 mm and the cellular layer is approximately 0.1 mm thick [(on either side of the lumen)]. Thus, the diameter of the lumen is about 0.13 mm.
The sheath of the seminiferous tubule of the rat is composed of a single transparent, thin membrane; when soaked in carmine, it is possible to see a few pale, flat oval nuclei that are evenly arranged [(these are now termed peritubular myoid cells)]. Ebner (1)11 and Mihalkowics (2)12 showed that this membrane is not anhistous [(without definite structure)], as it is composed of many flat polyhedral cells13 [(peritubular myoid cells)] connected to one another by their edges. This is easily observed when one treats fresh tubules with a solution of silver nitrate.
It is not just by treating the tubule with silver salt that the cellular structure of the membrane can be identified. I was also able to observe it in isolated portions of tubules hardened in Müller fluid. Figure 17 shows one of the sheath portions of the tubule. The borders of [(peritubular myoid cells)] are clearly visible and marked by well-defined transparent lines. Their diameter varies between 0.03 and 0.05 mm. Approximately in the middle of each cell a flat oval nucleus with a maximum diameter of 0.015 mm is defined by light contours. From the side view, the nucleus looks like a thickening of the sheath proper.
Figure 17.
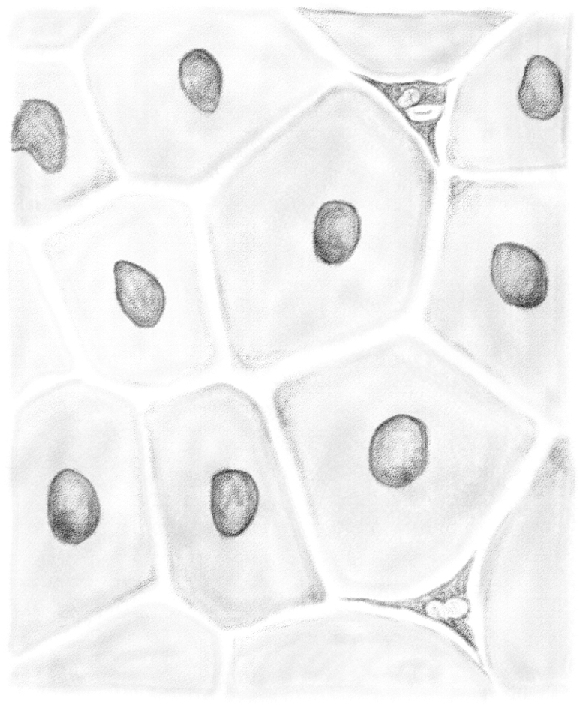
(Table IV.) Sheath of the seminiferous tubule, hardened in Müller's solution. 480/1. N.B. All the drawings, except those of fresh preparations, have been made using Oberhäuser's microscope equipped with a camera lucida. Archivio per le Scienze Mediche, year II, no. 1 and 2, 1877.
Between the [peritubular myoid cells] described above, very peculiar components are often found, which I will turn to later.
Two categories of cells of different shape and function are present in the seminiferous tubules.
Cells of the first category stay in the tubules for their entire span life, until cell turnover. We can therefore call them fixed cells [(Sertoli cells)].
The other category of cells, instead, continuously changes and renews; this constant movement is the product of the secretory activity of the testis. These cells are therefore motile [germ cells].
Fixed cells [(Sertoli cells)]
The first category consists of those cells I described and named branched cells [Sertoli cells] (1),14 which compose the epithelium of the tubule.
These cells have the shape of a cylinder or tapered cylinder and they are located within the tubule in such a way that its largest and more distant end is in contact with the internal face of the tubule membrane, whereas the narrower, central one delimits the tubule cavity or lumen. Therefore, a cylindrical epithelium covers the internal wall of a tube.
Unlike a simple cylindrical epithelium, though, these cells are in contact only at their peripheral end, because they are separated by the second group of cells [(germ cells)].
The cells composing the epithelium of seminiferous tubules can also be isolated and examined in a fresh state in isotonic solution. The use of any special hardening solutions to make them visible is therefore unnecessary, although such solutions fix the shape these cells assume in the tubule when they are surrounded by the other components. I state this for those who intended to refute some of the morphological characteristics of the cells I described [Sertoli cells] and even to deny the very existence of these cells, considering them artificial products derived from the action of the preserving solutions I used.
Figure 1 (table IV) shows a [Sertoli cell] found in the seminiferous tubule of a recently killed rat, isolated in its own aqueous humor. [Sertoli] cells treated in this way show a transparent, slightly granular protoplasm in which—just as in the cell shown in—coarser granules defined by well-marked borders, with the appearance of lipid droplets, are situated at the periphery of the cell. These lipid droplets, however, are not present in all the [Sertoli cells], and not in the same amount.15 I will describe in which cells the lipid droplets are visible later. The protoplasm [of the Sertoli cell] has a neat border in the peripheral region of the cell, at the point where this contacts the tubule membrane. The contours of the remaining part, if the cell is examined in a fresh state, appear delicate and sometimes blurry. The side view of the uneven cell shows a slightly protruding protoplasm with equally indistinct borders.
Figure 1.
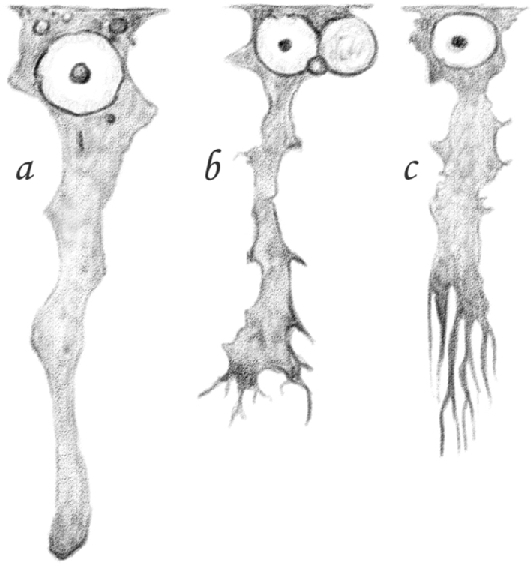
(Table IV.) Three [Sertoli cells] of a seminiferous tubule: a fresh. 600/1; b [(and likely c as well)] hardened in Müller's solution. 480/1.
The protoplasm of the examined cells is also very soft and can be easily torn, damaged, or destroyed. This feature, together with the extremely delicate appearance of its border, shows that [Sertoli cells] of the tubule, unlike the ones composing other cylindrical epithelia, are not delimited by the layer of membrane or thickened protoplasm that contains the softest part of the protoplasm and give it a specific texture.
Another characteristic of this protoplasm that, I think, derives precisely from the lack of a delimiting layer is its stickiness. Hence, under the microscope, these cells often appear surrounded by other components of the tubule from which they cannot or are unlikely to detach. Their detachment is more likely to occur when the cells have been soaked in a preserving solution, e.g. in Müller solution. As this hardens and exerts pressure on the protoplasm of adjacent, adhering cells, it causes their surface to detach. It should also be noted that, if we examine the same cells sometime after the animal's death, it is even harder to isolate them in an isotonic solution, such as serum, than it is immediately after the animal has been killed. Borders are also less clear cut, because hardened substances deposit on their surface. I am of the opinion that, sometime after death, a spontaneous coagulation of some albuminoids in the intercellular fluid causes the cells to become entangled, thus helping to further bond them together in the tubule.
[Sertoli cells] of seminiferous tubules contain a nucleus that is always found in the peripheral region of the cell. This is in accordance with the rule that the nucleus of cells composing any glandular cylindrical epithelium is typically situated in the cellular end in contact with the membrane acinus or the glandular tube. The nucleus appears as a spherical or slightly oval vesicle composed of a membrane with a well-marked double border and a clear, transparent, almost homogeneous content. Within this nucleus, there is only one relatively large nucleolus, which is roundish, well defined, and opaque.
In the many observations I carried out, not just in rats but also in other animals and man, I never found more than one nucleus in the [Sertoli cells] of seminiferous tubules. According to my measurements of the cells in a fresh state, their average length is 0.053 mm, and the peripheral width is 0.011 mm; cells become gradually and irregularly narrower, and their size diminishes to variable degrees toward the central region, where they are approximately 0.004 mm. The average diameter of the nucleus is 0.010 mm; the nucleolus is typically large enough to be at least approximately measurable, with a diameter of 0.002 mm.
As to the response of fresh cells to the main reagents used to determine their chemical composition, in particular the nature of the protoplasm, these change in distilled water. The protoplasm swells and partially dissolves, and what is left is only a granular, shapeless cluster surrounding a stretched nucleus with better-defined borders. Both dilute and concentrated acetic acid makes the cell extremely pale, so that the protoplasm is only visible because a number of insoluble granules remain in the reactive agent. The protoplasm, however, only partially dissolves in acetic acid; although swollen and pale, the remainder becomes more visible again after rinsing the cover slip with distilled water. The nucleus appears extremely faded as well, and its membrane and nucleolus are almost invisible. All these parts, however, do not disappear when they dissolve. As I demonstrated elsewhere (1),16 concentrated solutions of sodium chloride, which greatly affect the rounded components of the tubule, barely alter [Sertoli cells] by making the nucleus less visible.
Dilute alkalis, potash, and soda dissolve the protoplasm and the nucleus, and only lipid droplets are left. The examined cells look different after they have been conveniently hardened in situ by means of a preserving solution such as Müller's. They appear darker, more opaque, notably narrower and slender; this is explained by the fact that the solution in which they were placed lead to coagulation and exerted pressure on the protoplasm of these cells.
The middle part or body of the cells, which assumed a more markedly prismatic shape, also has more pronounced, longer side ridges with a crest shape. These occupy the space between the rounded components in contact with the cells. When hardened on site under the same pressure as in the tubule, the entire cell maintains the shape it had to assume when the surrounding roundish components exerted their pressure on it.
I must therefore state that Merkel's conclusion regarding the shape of the extensions [(of Sertoli cells)] is correct, as I previously acknowledged.
These protoplasmic extensions would not be pre-formed, as I already stated in 1871 (1),17 but they would rather be the result of the pressure exerted by [germ cells] on the protoplasm, which is forced to occupy the space they left empty. [Germ cells] therefore find themselves within a sort of imperfect receptacle composed of [Sertoli cells] and their extensions.
This is further proven by the fact that the amount of crest-shaped extensions and the width of the receptacles they constitute varies in the cells depending on the number and size of the components surrounding them. While they are rare and distant in the cells surrounded by the large third stage [pachytene spermatocytes], they are abundant and narrower in the cells enclosed by those small components from which [spermatids] originate. The difference is easily perceived when we look at [Sertoli cells], in Figure 1b,c (table IV), and cells in Figure 11 are compared with those in Figure 13.
Figure 11.
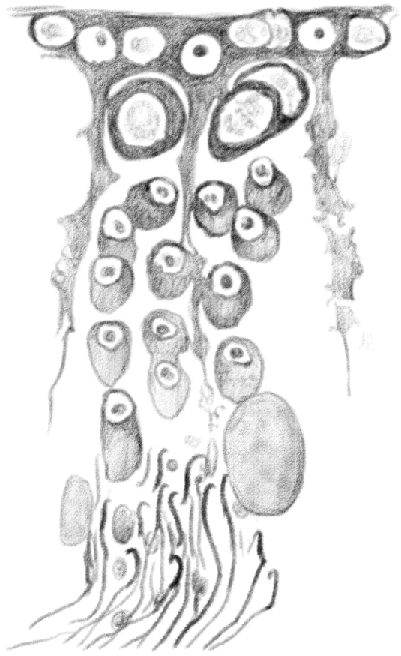
(Table IV.) Idem [(the same as above)], in which [spermatids] of the old generation detached from [Sertoli cells]. In the following generation, the nuclei traveled to the periphery of [the round spermatids]. [Spermatogonia] assumed a roundish shape and penetrated in the tubule, and second stage [leptotene spermatocytes] grew larger. 480/1. (This represents a stage IX tubule [Perey, Clermont, and Leblond Amer J Anat (1961)]. Germ cells included would be: rare type A spermatogonial stem cells, type A differentiating spermatogonia, leptotene spermatocytes, pachytene spermatocytes, and elongating spermatids.)
Figure 13.
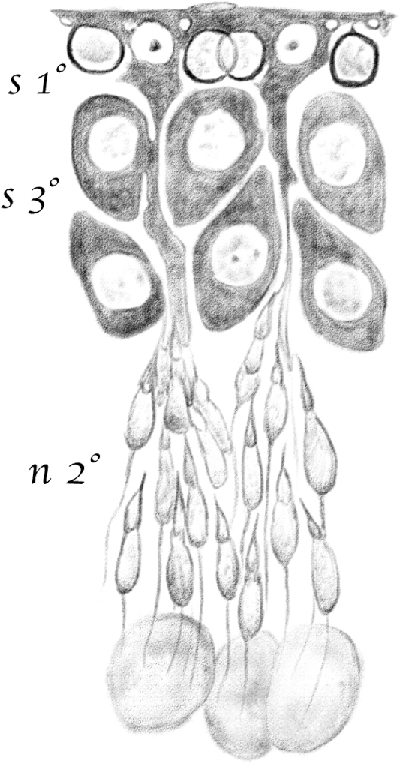
(Table IV.) Idem [(the same as above)], in which [spermatids] of the young generation are in their second [(elongating)] stage of development (n2°). [Spermatocytes] are in their third stage [(late pachytene)] (s3°), and [spermatogonia] completely transformed into first stage [leptotene spermatocytes] (s1°), which are found inside the basal surface of [Sertoli cells]. 480/1. (This represents a stage IX–XI tubule [Perey, Clermont, and Leblond Amer J Anat (1961)]. Germ cells included would be: rare type A spermatogonial stem cells, type A differentiating spermatogonia, leptotene spermatocytes, pachytene spermatocytes, and elongating spermatids.)
I also noticed that these extensions are hardly visible or only slightly protruding in fresh cells examined in isotonic solution. I believe this occurs because, once the cause that leads to their formation has been removed, the protoplasm assumes the original shape of a cylindrical [Sertoli cell]. When [germ cells] are absent, these cells have a distinctly cylindrical shape with regular straight edges, and there is no side extension of any shape, as one can see by examining these cells in the testis tubules of a fetus, or near the rete testis, as I observed in the testicles of a pseudohermaphrodite goat (1).18
It is also important to understand how the two ends of [Sertoli cells] in seminiferous tubules appear.
As I mentioned earlier, the peripheral [(basal)] end is the largest one. A large portion of it is in contact with the tubule membrane and constitutes the basal surface of the [Sertoli cell].
The width and shape of this basal surface varies. In some tracts of the same tubule, between the peripheral end of [Sertoli cells] and the sheath, where [spermatids] are in their second [(elongating)] developmental stage, there is no other component, or there are only a few distant cells where the ones which previously occupied this space moved more inward. In these tracts of the tubule, a large surface of the [Sertoli cells] is in contact with the sheath. The edges of this surface immediately couple to the homologous edges of neighboring cells, so that at first sight one might think that the internal wall of the tubule is covered with adjacent [Sertoli cells].
Figure 2 (table IV) shows the peripheral layer of a tubule tract from its outside to its inside, where it is possible to observe the mentioned characteristics of [Sertoli cells]. Their basal surfaces form an array of hexagonal plates marked by well-defined borders, in the middle of which is a clear nucleus with a nucleolus in the peripheries, as described earlier. Behind the peripheral borders of [Sertoli cells], it is possible to see [germ cells] situated slightly deeper between these peripheral regions.
Figure 2.
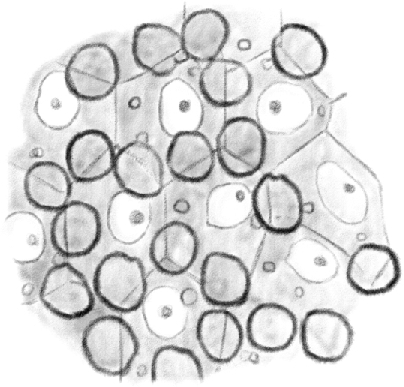
(Table IV.) Cross-cut section of the content periphery of a seminiferous tubule in which [spermatids] are in their second [(elongating)]developmental stage, seen from the outside to the inside. Clear nuclei belong to [Sertoli cells]; under the mosaic lines formed by the basal surface of [Sertoli cells], it is possible to see first stage [spermatocytes]. 480/1. (This represents a stage IX–XI tubule [Perey, Clermont, and Leblond Amer J Anat (1961)], based on the presence of elongating spermatids; at these stages, the first stage spermatocytes would be leptotene.)
In other tracts of the tubule, where [spermatids] are in their initial [(round)] or final [(condensing/condensed)] developmental stage, the peripheral surface of the cells in contact with the tubule wall is narrower and of a different shape. This occurs because components of a different nature, which I will describe later, come to lie between the edges of this surface and the sheath. Figure 6 shows the change in shape and size of the surface in contact with the periphery of [Sertoli cells] better than any words.
Figure 6.
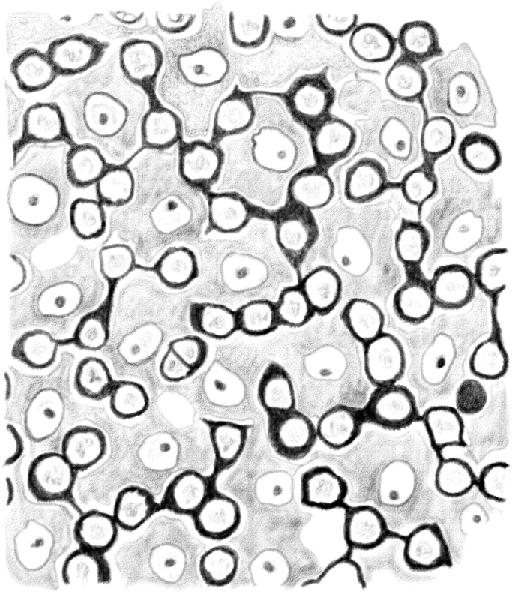
(Table IV.) Cross-cut section of the content periphery of a tubule in which [spermatids] are approaching the end of their third [(condensing)] developmental stage. Second stage [intermediate (In) and type B spermatogonia] dividing and penetrating between the basal surface of [Sertoli cells] are shown. 480/1.
The new components, however, do not cause the cells to detach from one another and separate, as they connect through equally well-defined edges delimiting all the polygonal areas occupied by [Sertoli cells]. In this case, though, they connect more inward, beneath the [germ cells] I described earlier.
Figure 3 shows a tubule tract seen from its outside to its inside, as in Figure 2. Here it is possible to identify the mosaic formed by the peripheral ends of [Sertoli cells] connected together, whose borders are partially covered and hidden by [germ cells] lying between them and the tubule membrane.
Figure 3.
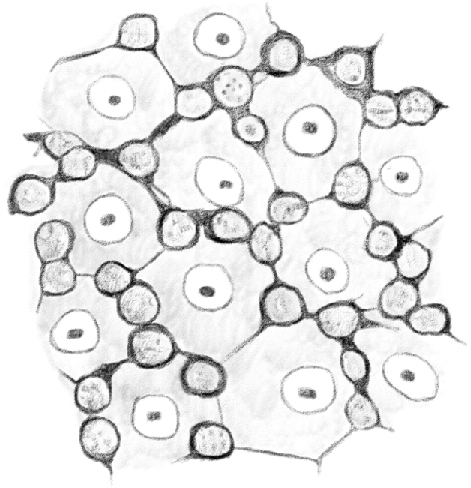
(Table IV.) Idem [(the same as above)] of a tubule in which [spermatids] are in their third [(condensing)] stage of development. The basal surface of [Sertoli cells] form a mosaic here as well, above which second stage [intermediate (In) and type B spermatogonia] are visible. 480/1. (This represents a stage I–VIII tubule [Perey, Clermont, and Leblond Amer J Anat (1961)], based on the presence of elongating spermatids; at these stages, the first stage spermatocytes would be leptotene.)
Figures 10 and 13 are side views that clearly illustrate how the peripheral ends of [Sertoli cells] connect to one another. Figure 13, which corresponds to Figure 2, shows a connection above the [germ cells], whereas in Figure 10, which refers to Figures 3 and 6, they connect underneath.
Figure 10.
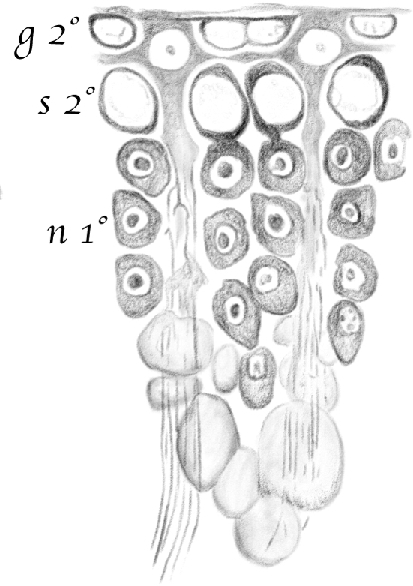
(Table IV.) Portion of a cross-cut section of a seminiferous tubule where [spermatids] of the older generation are in the third [(condensing)] stage of development and the younger is at the beginning of the first [(round)] stage. 2nd g (g2°)—second stage [intermediate (In) spermatogonia]; 2nd s (s2°)—second stage [early pachytene spermatocytes]; 1st n (n1°)—[spermatids] in their first [(round)] developmental stage.[Spermatids] in their third [(condensing)] stage are arranged in a sheaf-like pattern around [Sertoli cells]. 480/1. (This represents a stage I tubule [Perey, Clermont, and Leblond Amer J Anat (1961)]. Germ cells included would be: rare type A spermatogonial stem cells, type A undifferentiated and intermediate (In) spermatogonia, pachytene spermatocytes, and round and condensing spermatids.)
It follows that the connected peripheries of [Sertoli cells] do not form a mesh in which other components are located, as suggested by Ebner (1),19 but rather they are adjacent along their entire perimeter. However, they leave niches inside or outside the contact line, where the [germ cells] lie.
It should be recalled that Neumann (2)20 expressed the same opinion when he detected the polygonal shape of the peripheries of [Sertoli cells], which he called Fussplatte. Nonetheless, Mr Neumann only noticed the connections occurring outside the [germ cells] which, in his words, “von innen her gevissermassen in das Protoplasma der Epithelien eingedrückt sind, so dass sie und durch eine sehr dünne Schicht des letzteren von der Tunica propria der Kanälchen geschieden sind” (3).21
This is perfectly in line with what I stated regarding the arrangement of peripheries in the tubule tracts, where [spermatids] enter their second [(elongating)] developmental stage. It is also consistent with Figure 2 (table IV) and Figure 7, the latter of which shows the tubule epithelium seen from its inside to its outside, with peripheral [germ cells] placed in the niches.
Figure 7.
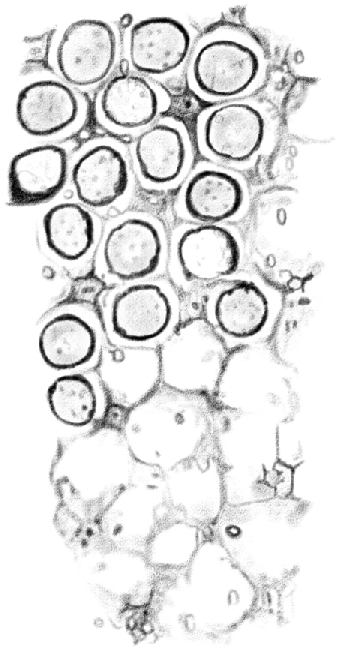
(Table IV.) Cross-cut section of the tubule, as in Figure 2, seen from the center to the periphery. The upper half shows [spermatocytes] positioned in niches beneath the basal surface of [Sertoli cells]; in the lower half, the niches are empty. [Sertoli cells] appear on the corners like cross-cut sections of prisms with three, four, or five concave sides. 480/1.
Firmly convinced, like many others, that [germ cells] only had a marginal role in [spermatid] formation, Neumann did not carefully study their development and changes, so he failed to notice the stage in which they are in contact with the tubule wall, which I draw in Figures 2, 6, and 10.
A peculiar feature of the periphery or bases of [Sertoli cells] is the presence of lipid droplets of different sizes, which are absent in the body and middle part of the same cells. One point should be highlighted with regard to these droplets. A fact that I have currently only distinctly observed in the rat should be remembered, as it contributes to demonstrate that [Sertoli cells] do not play a mere mechanical role within the tubules. When one examines the cells of the tubule tracts in which [spermatids] enter their second developmental [(elongating)] stage, it is possible to discern an abundance of lipid droplets in their periphery, some of which are rather large (Figures 2, 5, 7, 12, and 13). Quite to the contrary, if we look at the same cells at the point where [spermatids] are approaching the end of their third [(condensing)] stage, we see that there is no droplet at all in their peripheral region (Figures 3, 6, 10). This is easily seen not just in the vertical and tangent sections, but also by examining a long tract of an entire tubule under a microscope. Where [spermatids] are in their second [(elongating)] stage, one can observe many droplets scattered within the tubule membrane, which gradually rarify until they disappear when [spermatids] are in their third [(condensing)]stage. Such a peculiar behavior of the peripheries of [Sertoli cells] also allows for a determination of the developmental stage of [spermatids] and its content structure in a given tract by means of a cursory tubule examination.
Figure 5.
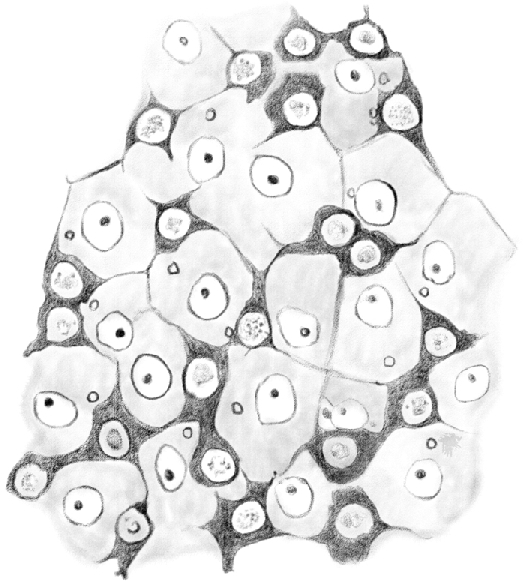
(Table IV.) First stage [type A spermatogonia] dividing and forming an irregular net. The basal surfaces of [Sertoli cells] are visible in the net meshes. 480/1.
Figure 12.
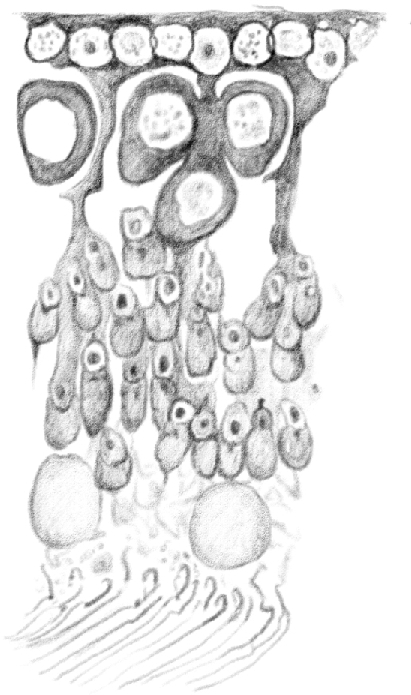
(Table IV.) Idem [(the same as above)], in which [condensed spermatids] of the old generation are positioned along the tubule axis. [Round spermatids] of the young generation move from the first [(round)] to the second [(elongating)] developmental stage. Second stage [type B differentiated spermatogonia] gradually become first stage [preleptotene spermatocytes], and second stage [early pachytene spermatocytes] enter their third stage [(late pachytene)]. 480/1. (This represents a stage VII–VIII tubule [Perey, Clermont, and Leblond Amer J Anat (1961)]. Germ cells included would be: rare type A spermatogonial stem cells, type A undifferentiated spermatogonia, preleptotene spermatocytes, pachytene spermatocytes, round and early elongating spermatids, and condensed spermatids.)
It should be noted that this temporary presence of lipids in [Sertoli cells] is due to a mere infiltration, not to fatty degeneration.
Before concluding the description of the periphery of [Sertoli cells], I will add that the thin lines marking their edges are not always easily identified. A rather strong hardening effect of Müller solution is needed, so the testicle should be soaked in it for several weeks.
The study of the central region of [Sertoli cells] is even more important, insofar as the main controversies surrounding the structure of testicles arise from its behavior and relationship with developing [spermatids]. I mentioned earlier that Ebner and his disciples believe [spermatids] originate from this central region [(from Sertoli cells)].
It is not easy, nor always possible, to see this central region. For the reasons outlined above, this is either torn and detached from the cell or it is surrounded and hidden by developing [spermatids], which adhere to it in the same way as [germ cells] enclose the middle portion. Only [spermatid] adherence in the periphery of [Sertoli cells] becomes gradually greater as [(spermatids)] enter more advanced developmental stages. Therefore, this depends not only on the stickiness of cellular protoplasm or the coagulation of the surrounding solution, but also on the shape of the components that adhere to it. This is especially true in the case of the rat because of the hook-shaped head of its [spermatids]. Nonetheless, it is possible to isolate and identify cells having a perfectly clear central region, which appears roundish and sometimes even slightly enlarged when observed in the fresh state and in aqueous humor, as shown in Figure 1a (table IV).
This region has a much different look when observed in cells conveniently hardened to maintain the shape they had to assume in the tubule because of the pressure of surrounding components. In this case, the central region of the examined cells is irregular, with few cavities or niches of different shapes circumscribed by abundant protrusions, just as larger cavities in the middle portion are delimited by projecting crests.
These cavities or niches take the shape of the components from which they originate, namely the [spermatids] in their various developmental stages.
Indeed, when one looks at the central region of [Sertoli cells] in the tubule tracts where [spermatids] are entering their second [(elongating)] stage, that is when the [spermatid] nucleus has not yet significantly changed its rounded shape, one sees relatively distant protrusions delimiting and forming wide rounded niches, as shown in Figure 1b (table IV). If, on the contrary, we examine [Sertoli cells] in a tubule tract where [spermatids] have reached an advanced stage of development and the head has already assumed its characteristic shape, what we see are long, narrow, parallel furrows longitudinally extended in the central region, as shown in Figure 1c.
In my opinion, the fact that the niches change shape according to the configuration of the components surrounding the [spermatids] is crucial, as it demonstrates that these are only impressions caused by the [spermatid] head. Due to the internal pressure of the tubule, the head becomes sunken into the soft protoplasm of [Sertoli cells], the same way as [germ cells] in contact with the body and periphery of the same [Sertoli cells] produce niches or receptacles that are variable in terms of width, depth, and completeness. If the cells were previously hardened in situ, the niches remain visible, whereas they mostly disappear if they were isolated in the fresh state and were no longer subject to any pressure within the tubule.
Motile cells [(germ cells)]
The second category of cells undergoes a continuous process of change and renewal. For this reason, I referred to them at the beginning of this work as motile cells [(germ cells)].22 From the periphery of the tubule, where they are formed, they travel between the [Sertoli cells] toward its center. As they move, they are subject to a number of alterations, which eventually lead to the formation of [spermatids]. Just as with [spermatid] development, changes in [germ cells] occur sequentially in seminiferous tubules. Therefore, [germ cells] appear different depending on the tract in which they are found.
Very little information has been published by histologists on these fundamental cells contained in the tubule. [Germ cells] were referred to interchangeably as seminiferous, seminal, or rounded testes cells, and were only subdivided into cells with a granular nucleus and cells with a smooth nucleus. The former were small in the periphery [(spermatogonia and preleptotene, leptotene, and zygotene spermatocytes)] and large in the center [(pachytene spermatocytes)]. From these, they inferred, without knowing how, the existence of the latter [(spermatids)], which were believed to be responsible for the formation of [spermatozoa].
It was only Ebner who noticed the centripetal movement of these cells and some of the main changes they undergo as [spermatids] develop. Nevertheless, convinced as he was that they did not contribute to the development of [spermatozoa] and that they only played a secondary role, he neglected to scrupulously examine them. Partly because of this preconceived idea, and partly because of his preparation method, he missed a great deal, neglected a great deal, and he misunderstood a great deal.23
Based on the changes [germ cells] undergo, I divide them into three main groups, which I call germ cells [(spermatogonia)],24seminiferous cells [(spermatocytes)], and nematoblasts25 [(round spermatids)]. Cells of the first group generate the second; these in turn serve as the vehicle through which [flagella] and third group cells, or [round spermatids], travel. [Later spermatids (elongating and condensing)] develop directly from this third group.
[(Spermatogonia)]
These cells are found in the periphery, between the membrane and the epithelium, where they form a simple layer, the germ stratum.
It seemed appropriate to me to call them germ cells [(spermatogonia)], as they are responsible for the formation of all the cells contained in the tubule except [Sertoli cells].
These cells should not be confused with Ebner's so-called Keimnetz, which are quite different. Keimnetz cells undoubtedly correspond to my description of the peripheries of [Sertoli cells] (1).26 [Spermatogonia] would correspond, instead, and only when they have fully developed, to what Ebner described as small rounded cells with a large granular nucleus positioned in the meshes of its Keimnetz, or to granular heaps (granulirte sich stark imbibirende Klümpchen27). These cells, together with the Keimnetz, form what Ebner called Wandschicht.
[Spermatogonia] can be found along the entire seminiferous tubule, scarce in some tracts, abundant elsewhere. Their features, shape, and size vary according to their age.
They undergo two stages of development. In animals, in which the process of spermatozoa formation is active, the production and transformations of these cells occur at the same pace. The difference between the two stages resides in the shape and position of the cells.
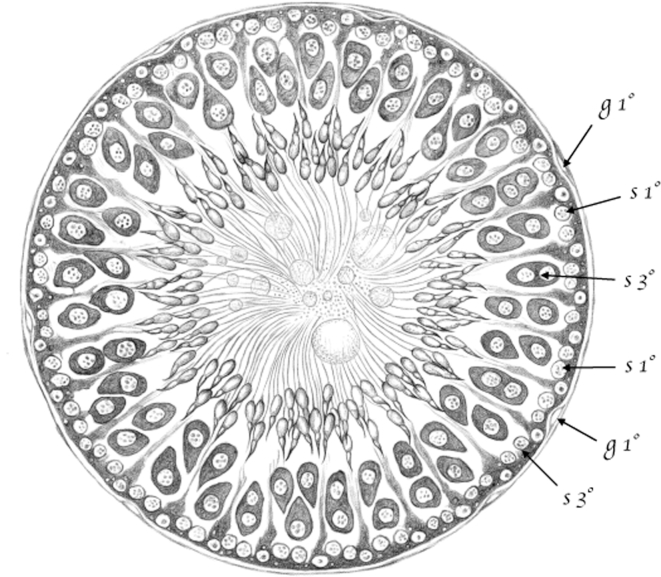
First stage [spermatogonia (type A)]—first stage [spermatogonia (type A)] are found in the seminiferous tubule tracts where [spermatids] are in their second [(elongating)] developmental stage, namely when the [spermatid] nucleus extends and forms a hook, which is the head of the [spermatozoon].
Here, [spermatogonia] appear as beautiful star-shaped cells in contact with the inner face of the sheath proper. Figure 4 shows these cells as pictured from the front. In spite of all my efforts, I have not been able to find any prior description of these cells.28 As shown in the figure mentioned above, they are composed of an opaque protoplasm, with no granulation, which can become intensely stained by carmine. In the midst of it, there is a large, rounded, clear protoplasm with granulations in its inner part. The nucleolus is not visible, not because it is missing, but because it can be hidden by the granulations in the center of the nucleus.
Figure 4.
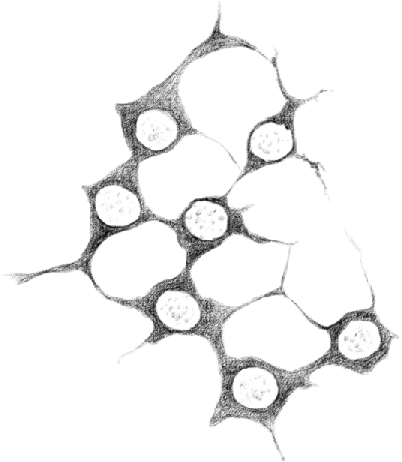
(Table IV.) First stage [type A spermatogonia] (hardened in Müller solution). 480/1.
These cells look flat, longer than thicker. As they move from the center toward the periphery, they become less and less thick. Therefore, the protoplasm looks more opaque and obscure in the largest area around the nucleus than in the thin periphery, where it is demarcated by a delicate, simple contour. This clearly demonstrates that [spermatogonia] do not lack a membrane at all.
Interestingly, these cells have four to five extensions that give them their peculiar star-shaped design. These extensions detach from the large-based cell and become gradually thinner as they move in opposite directions, although always in parallel with the tubule wall and never toward the center. As shown in Figures 4 and 5, they are mostly straight and they join the extensions of neighboring cells, so they often appear slightly swollen in the nodal points. In some cases, they are large and short, so as to form a sort of protoplasmic bridge connecting two neighboring cells.29
The fusion of a certain number of [spermatogonia] with short or long extensions forms a mesh adjacent to the tubule membrane, to which the mesh can sometimes adhere when hardened in Müller solution. The mesh is not always homogenous, as some spaces are devoid of cells.
First stage [spermatogonia] are situated between the tubule membrane and the epithelium. More specifically, they are found in correspondence with the peripheral edges of [Sertoli cells] and not within their center, which stays in contact with the sheath proper.
Peripheral edges of [Sertoli cells] connect to one another beneath [spermatogonia], when these are present. In this way, they form shallow niches where [spermatogonia] position themselves (see Figures 5, 14, table IV, and Figure 11st g, table III).
Figure 14.
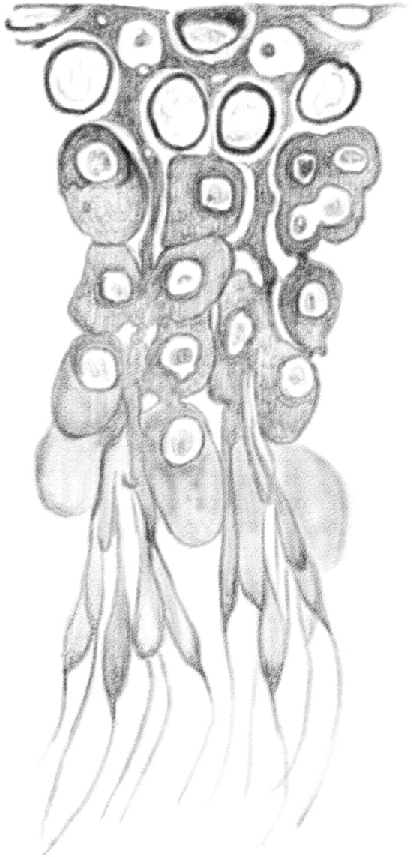
(Table IV.) Idem [(the same as above)], in which [spermatids] enter their third [(condensing)] stage of development. [Secondary spermatocytes and nascent round spermatids] originate from third stage [late pachytene spermatocytes]. First stage [zygotene spermatocytes] grow larger before entering the second stage [(early pachytene spermatocytes)]. It is possible to see [spermatogonia] in contact with the sheath, having the appearance of cells moving from the first [(type A spermatogonia)] to the second [(intermediate, or In spermatogonia)] stage. 480/1. (This represents a stage XIV–I tubule [Perey, Clermont, and Leblond Amer J Anat (1961)]. Germ cells included would be: rare type A spermatogonial stem cells, type A differentiating spermatogonia and intermediate spermatogonia, zygotene and pachytene spermatocytes, and round and condensing spermatids. Multiple transitions are taking place at this point in the epithelium.)
Figure 1.
(Table III.) Cross-cut section of a seminiferous tubule in rats, in a point in which [spermatids] are in their second [(elongating)]developmental stage. 200/1. (Appears to be a stage XI–XIV tubule [Perey, Clermont, and Leblond Amer J Anat (1961)]; at these stages, the seminiferous epithelium would contain the following germ cells: rare type A spermatogonial stem cells, type A differentiating spermatogonia, leptotene (stage XI) and zygotene (stages XII–XIV) spermatocytes, pachytene (stages XI–XIII) and diplotene (XIV) spermatocytes, and condensing spermatids.) 1st g (g1°) – first stage [spermatogonia]; 1st s (s1°) – first stage [spermatocytes (leptotene & zygotene)]; 3rd s (s3°) – Third stage [spermatocytes (pachytene)].
It must be noted that the [spermatogonial] extensions do not take an arbitrary direction, but follow the borders of [Sertoli cells]; therefore, in each mesh these extensions form with the respective cells from which they originate, one can see the periphery of a [Sertoli cell] (Figure 5, table IV).
So far, I have illustrated how [spermatogonia] appear when they are isolated by macerating and slightly hardening the tubules in Müller solution. I will now add that it is also possible to prepare and see them fresh in serum or aqueous humor. Even in this case, their shape is the same as I described, with the only difference that the protoplasm is more homogeneous and transparent, the nucleus is smooth, without any granulations in the middle, and there are between one and three small nucleoli. If treated with dilute acetic acid, the protoplasm appears even more transparent, while the nucleus looks more opaque and darker-colored, although still smooth.
According to the measurements I made, the average body of a first stage [spermatogonium (type A)] has a width (diameter) of about 0.015 mm; the diameter of the nucleus is 0.011 mm; the cell maximum thickness is 0.007 mm.
The number of [spermatogonia] varies along the tubule tract, and they are not present across its entire length. They are scarce in those sections of the tubule where [spermatids] are at the beginning of their second [(elongating)] developmental stage. Here, [spermatogonia] are scattered, individually or in clusters, within the sheath proper, leaving large areas unoccupied. Toward the end of the second [(elongating spermatid)] stage, however, their number grows significantly, until they form a thick mesh at the beginning of the third [(condensing spermatid)] stage, as shown in Figure 5.
This increase in [spermatogonia] occurs because existing ones divide. Several observations I made confirm this process of cell reproduction, which is nothing extraordinary. Often, I found two nuclei instead of one in some [spermatogonia]. I also saw binucleated cells clearly narrowing down in the middle, indicating their division. Similarly, the protoplasmic bridges [(intercellular bridges)] I mentioned earlier, which connect two cells together, are undoubtedly a product of these narrowing and subsequent distancing of two cells caused by the division of a single cell. Indeed, I am firmly convinced that many, if not all the extensions of first stage [spermatogonia (type A)] originate in this way.30
Therefore, [spermatogonia] form and multiply when a generation of [spermatids] is in its second [(elongating)] developmental stage. This cellular multiplication becomes increasingly intense during the third [(condensing)] developmental stage of [spermatids], although at this point [spermatogonia] are changing and are entering their second stage.
Second stage [spermatogonia (intermediate and type B)]—As first stage [spermatogonia (type A)] continue to multiply, they increase in number and become smaller in those tubule tracts where [spermatids] are in their third [(condensing)] developmental stage. Their shape changes as well, and they come to form a layer of cells that I considered appropriate to call second stage [spermatogonia (intermediate and type B)].
These are small, roundish cells with a small amount of protoplasm surrounding a large, clear, and very granular nucleus. They have a diameter of only 0.010 mm, which shows that [spermatogonia] become gradually smaller due to the process of cell division and multiplication.
Their shape changed as well, and they are no longer star-shaped as first stage [spermatogonia (type A)]. Because they lost their extensions, they now appear roundish. Before the large star-shaped cells of the first stage [spermatogonia (type A)] turn into the small rounded ones of the second stage [spermatogonia (intermediate and type B)], they go through some intermediate stages. Indeed, on the corners where several [Sertoli cells] join one another, some [spermatogonia] are still triangle-shaped, and their extensions are small and short. Other [spermatogonia] have two or just one extension connecting two or more cells in a row on the same line.31 I will now illustrate a peculiar feature of second stage [spermatogonia]. The propagation by division of the nucleus and the protoplasm continues in this stage until a certain number of cells have been produced. These cells often have two nuclei with the shape of two hemispheres, divided by a thin layer of protoplasm. In turn, these hemispheres contain two recently formed, more distant nuclei; their protoplasm, narrow in the middle, becomes increasingly narrower, until it causes the division of two new cells. Before they detach, the same division process occurs in each of them, so that one often sees three, four or, even more cells connect to one another as in a rosary.
All these cell rows form a net. Each mesh of the net contains the periphery of a tubule [Sertoli cell], as shown in Figures 3 and 6. The edges of these peripheral regions subside and form, together with their neighboring peripheries, a number of niches that become occupied by second stage [spermatogonia (intermediate and type B)].
Because [spermatogonia] are in contact with the tubule sheath proper, the peripheries of [Sertoli cells] can only connect to one another on their lower side, namely within [spermatogonia]. Consequently, the mosaic tiles formed by the epithelium have to move beneath [spermatogonia] when these are present, as I mentioned earlier (Figure 3). The surface of [Sertoli cells] in contact with the tubule membrane, therefore, will not have the polygonal shape drawn in Figure 2 (table IV), but the star shape of the meshes formed by [spermatogonia], as in Figure 6.
Figure 10 in table IV and Figure 2 in table III show a side view of the second stage [spermatogonial (intermediate and type B)] position and the relationship with the tubule sheath and the peripheries of [Sertoli cells].
Figure 2.
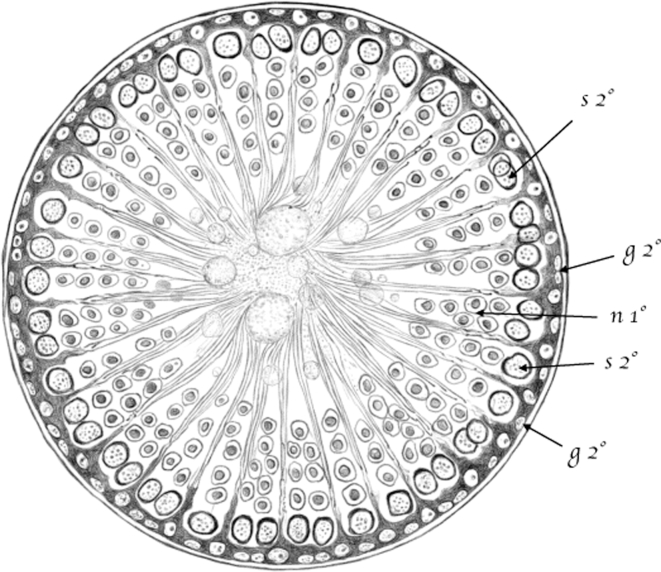
(Table III.) Cross-cut section of a seminiferous tubule in which [spermatids] are approaching the end of their third [(condensing)] developmental stage. 200/1. (Appears to be a stage I–IV tubule [Perey, Clermont, and Leblond Amer J Anat (1961)]; at these stages, the seminiferous epithelium would contain the following germ cells: rare type A spermatogonial stem cells, type A undifferentiated spermatogonia, intermediate (In) spermatogonia, pachytene spermatocytes, round and condensing spermatids.) 2nd g (g2°) – Second stage [spermatogonia]; 2nd s (s2°) – Second stage [spermatocytes (early pachytene stage)]; 1st n (n1°)—[spermatids] at the beginning of their first [(round)] stage of development (new generation). Third stage [spermatids], belonging to an older generation, are arranged in a sheaf-like pattern around [Sertoli cells]. Figures I and II (Table III) show [Sertoli cells] arranged in a sheaf-like pattern, with a clear nucleolated nucleus at the periphery. In the tubule lumen, glass-like spheres [(residual bodies)] and [spermatid] tails are visible from both the side view and the cross-cut section. In order to make the figures clearer, an empty space has been left between the sheath and the tubule content.
[Spermatogonia], however, do not stay in this position. As they move downward within the tubule and reach the tract where the second half of the first [spermatid] developmental stage occurs, a huge number of cramped, perfectly spherical [spermatogonia] slowly make their ways into the tubule between [Sertoli cells]. Toward the beginning of the second stage [(as intermediate and type B spermatogonia)], they become [spermatocytes] and place themselves within the peripheries of [Sertoli cells]. At the same time, new [spermatogonia] start to develop and multiply in the sheath proper, forming yet another layer of cells that replaces the transformed one.
The question that arises is: From where do the first [spermatogonia] come?
I do not think anyone has purposely studied the cells contained in the tubule, with the exception of Ebner who, having found that the cells in the meshes of his Keimnetz were very similar in appearance to white blood cells, considered the possibility that they might proceed directly from these cells migrating to the tubule through the membrane.
While acknowledging that this appears to be the most likely hypothesis, the way [germ cells] in the tubule originate from white blood cells is far more complex than Ebner thought. [Germ cells] found in the Keimnetz meshes and observed by Ebner are undoubtedly [spermatogonia] at the end of their second stage [(intermediate and type B spermatogonia)], or [spermatocytes] in their first stage [(preleptotene, leptotene, and zygotene)]. The former [type B spermatogonia] do not directly derive from white cells but rather, as I mentioned earlier, from first stage [type A spermatogonia], which do not look similar to white cells in the slightest. Therefore, it remains to be determined from where those star-shaped [type A spermatogonia] generating rounded [spermatocytes] originate, and how they enter the tubules.
I was never able to observe white blood cells undergoing any transformation in the star-shaped [type A spermatogonia] described, nor have I ever hit white cells passing through the tubule membrane in the solutions I prepared with utmost care for that specific purpose. Furthermore, I never noticed real white cells inside the membrane, as one should easily do if they were continuously moving within the tubules.
Instead, I observed peculiar cells in some membrane portions, scattered on the corners formed when three or more thin cell layers of the membrane connect to one another.
These [spermatogonia] have the appearance of a very dark, finely grained mass with blurred edges. Their shape is the same as the space left after the cell layers joined together. In a certain segment every two layers, it is also possible to see extremely delicate extensions with similarly blurred, indistinct borders. In the middle, they have a granular, often narrowing nucleus, which indicates cell division. Figure 17 accurately shows what I observed.
Now the question is: Are these cells, scattered along the entire tubule, responsible for generating the [spermatogonia] they partially resemble? Or are they in charge of forming new membrane layers, thus contributing to the growth and renovation of the membrane? Further research will yield answers to this question, which I am still unable to find.
[Spermatocytes]
The second category of [germ cells are] [spermatocytes, and] their function is to produce both the cells from which [spermatids] generate and the seminal fluid in which these are suspended. They correspond to those [germ cells] in which Henle found a coarsely grained nucleus after they were treated with acetic and chromic acid.
So far, however, the description of these [spermatocytes] has been vague and imprecise. They have been confused either with the cells from which they originate [(spermatogonia)] or the ones they generate themselves [(spermatids)]. Their birth, death, and gradual morphological transformations have not been illustrated, nor have their real position within the tubules, or their relationship with other cells, ever been clarified.
Therefore, Henle, who for the first time noticed the difference between this category of cells [(spermatocytes)] and the one with smooth nuclei [(spermatids)], which I will describe later, did not add much information. He was barely able to observe that they can have one, two, or more nuclei which, under the action of the acids mentioned above, resemble a cluster of dark granulates, that they are located in the middle of the cell, and that their size ranges from 0.012 to 0.015 mm. He also stated that he was not able to say whether these cells were “Jugendzustände der Zellen mit glattem Kern oder in einer rückschreitenden Metamorphose begriffen sind.”32 With regard to their position, he made it explicitly clear that “die Vertheilung der beiderlei Zellen innerhalb der Samenkanälchen lässt keine Regel erkennen” (1).33
Kölliker, who carried out the first important studies on the development of [spermatids] in mammals, only distinguished rounded [spermatocytes] by size and number of nuclei. Besides the epithelium, which he thought was made of flat polygonal cells, he only noticed in the tubule “rundliche Zellen in mehrfachen Reihen, unter denen haufig welche in Vermehrungzuständen, d. h. mit 2 Nucleolis in Einem Kerne, 2 Nucleis in einer Zelle und eingeschnurte mit zwei Kernen, vorkommen, zu innerst endlich liegen kleine Zellen mit einem Kerne und in geringerer Zahl grössere Zellen mit 2–5 ja selbst 10 und 20 hellen Kernen von 5–8 μ Grösse”(2).34 The latter cells would generate [spermatozoa].
According to La Valette St-George, the name Hodenzellen would include cells with a large, clear nucleus and a distinct nucleolus, cells with large or small granular nuclei, and cells with one or more smooth nuclei (3).35 The last two would be a variant of the same typology: the second would derive from the first and, in both, one may be able to observe the amoeboid movements first observed by La Valette in the Hodenzellen.
With the exception of the first group of cells which, judging by his description, “Sehr haufig sieht man solche Kerne frei liegen oder in einem Protoplasmarest von feinkörniger Beschaffenheit eingebettet,”36 as well as his figures, especially 1b, I believe to be mere portions of [Sertoli cells], the last two categories are insufficiently illustrated. The first of these two categories would correspond to the [spermatocytes] I intend to describe.
Neither Merkel nor Neumann provided any further or more precise details on these cells. The former neglected them completely and studied the cells that directly produce [spermatozoa]; the latter mentioned them only to agree with Ebner that they are not in the least related with the production of [spermatids].
Ebner (1),37 instead, studied them more accurately and observed that their size gradually changed depending on the [spermatid] developmental stage, as he himself subdivided the development of each generation of [spermatids] into several stages. He also noticed that these cells travel from the periphery (where he believed they are generated by blood white cells) toward the center, where they would be destroyed to form seminal fluid.
Not even Ebner, however, convinced as he was that the role of [spermatocytes] was marginal, did dwell upon the evidence he found in many of his preparations, and often misinterpreted it. To him, [spermatogonia], as well as [spermatocytes] and those from which spermatozoa originate [(spermatids)], all fall under the same category of runde Hodenzellen, in spite of the marked differences in shape, position, and function.
Therefore, it is necessary to study this category of [germ cells] contained in the tubules, which I call seminiferous [(spermatocytes)], more accurately.
The life span of [spermatocytes] should be subdivided into three separate stages. This division is not arbitrary, but natural and fundamental, insofar as they change their position within the tubules and interact with other cells in a different way in each of the three stages.
First stage [spermatocytes (preleptotene, leptotene, and zygotene)]38—These cells are small rounded components with a little protoplasm surrounding a large spherical nucleus. If observed in their fresh state in serum, one can see a finely grained protoplasm that surrounds like a thin peel a granular cluster with a blurred edge, the nucleus. After treating these cells with dilute acetic acid, the layer of protoplasm swells, becomes more transparent and clearer, while nuclear borders appear more marked and have more evident granules. If the same cells are hardened in Müller fluid, the coagulated protoplasm forms a thin, dark layer with a neat, marked contour, whereas the nucleus appears clearer, with scattered granules (see table IV, Figures 7 and 13).
The many measurements carried out reveal that these cells have an average diameter of 0.012 mm. The diameter can be slightly smaller or larger during the transition from [spermatogonia] to [spermatocytes], or from first stage [(preleptotene, leptotene, and zygotene)] to second stage [(early pachytene)] [spermatocytes].
First stage [spermatocytes (preleptotene, leptotene, and zygotene)] are located between [Sertoli cells], in the tubule periphery and within their basal surface. Therefore, unlike [spermatogonia], situated outside the lines connecting [Sertoli cells] to one another, [spermatocytes] are located inside, and they are separated from the tubule membrane by the basal surface of the cells forming the epithelium (table IV, Figures 2 and 13).39
These cells, however, are not always found in the tubules, nor are they found everywhere in such tubules, but only when or where [spermatids] are in their second [(elongating)] stage of development. There, the cells form a simple layer partially situated within the level of the nuclei of [Sertoli cells], in which third stage [spermatocytes (late pachytene)] are found (see Figure 11st s, table III, and Figure 13, table IV).
First stage [spermatocytes (preleptotene, leptotene, and zygotene)] originate directly from second stage [spermatogonia (intermediate and type B)]. A look at Figures 10–13 of table IV will suffice to immediately note the veracity of this statement. Second stage [spermatogonia (intermediate and type B)], after they have divided to form a real layer of cells, like the one shown in Figures 3, 6, and 10, have now become roundish, and they slowly enter the tubule, moving away from the sheath proper, as in Figures 11 and 12. Therefore, [spermatocytes] travel toward the center between the peripheral regions of [Sertoli cells]. Whereas earlier, when they were still [spermatogonia], they remained within the [Sertoli cells], now they stay outside, so that they are completely separated from the tubule membrane (Figures 2 and 13, table IV).
During this inward movement, the cells grow steadily: first stage [spermatocytes (preleptotene, leptotene, and zygotene)] have a diameter of 0.012 mm or bigger, while the diameter of [spermatogonia] in their last stage is only 0.010 mm.
Nuclear granulations grow in number as well, and the small protoplasm surrounding the nucleus has a more marked border. This, together with their more pronounced spherical shape and their bigger size, makes first stage [spermatocytes (preleptotene, leptotene, and zygotene)] more easily identifiable in their vertical and tangential sections than [spermatogonia].
It seems to me that first stage [spermatocytes (preleptotene, leptotene, and zygotene)] do not undergo any division process; at least, I never observed any evidence of it. Once [spermatocytes] originate from [spermatogonia], they steadily grow until they reach their second stage [(early pachytene)].
Second stage [spermatocytes (early pachytene)]—These cells differ only slightly from first stage [spermatocytes (preleptotene, leptotene, and zygotene)]. The main difference lies in their bigger size, as their diameter is approximately 0.015 mm. The nucleus is much larger, just as the protoplasm, which forms a thicker layer around the nucleus (table IV, Figure 10).
Like first stage [spermatocytes (preleptotene, leptotene, and zygotene)], they form a simple layer, slightly more inward. In it, the enlarged cells press further against one another and are compressed to the side so that, from a frontal view, one can see polygonal cells composing a mosaic. Scattered openings at regular distances allow the body of [Sertoli cells], analogously compressed by enlarged [spermatocytes], to pass through.
As second stage [spermatocytes (early pachytene)] derive from first stage [spermatocytes (preleptotene, leptotene, and zygotene)], they cannot be found in the same tubule tract at the same time. Second stage [spermatocytes (early pachytene)] arise later, which is to say they move downward in the tubule tract next to the tract where first stage [spermatocytes (preleptotene, leptotene, and zygotene)] are found.
Figure 2 (table III) shows the section of a tubule below the one shown in Figure 1. Here, one can see second stage seminiferous [spermatocytes (early pachytene)], which are absent where first stage [spermatocytes (preleptotene, leptotene, and zygotene)] are found. The same occurs in Figure 10 (table IV), where only second stage [spermatocytes (early pachytene)] are found in the peripheral region.
In this second period, or second tract of the tubule, first stage [spermatogonia (type A)] moved to the second stage [spermatogonia (intermediate and type B)], and third stage [spermatocytes (late pachytene)] formed [nascent round spermatids], while the generation of [spermatids] that was in its second [(elongating)] developmental stage is now in its third [(condensing)] one.
These cells will have second stage [spermatogonia (intermediate and type B)] toward the periphery, outside the [Sertoli cells’] basal surface and beyond; inside, there will be the [nascent round spermatids] or, when they are more developed, [spermatids] of a generation in its first [(round)] stage, and then [spermatids] of the previous generation in their third [(condensing)] developmental stage, leaning against [Sertoli cells].
Third stage [spermatocytes (late pachytene)]—[Spermatocytes] grow bigger and, after going through a second stage of development, they eventually enter their third stage [spermatocytes (late pachytene)].
If observed fresh and free in scrape preparations of aqueous humor, third stage [spermatocytes (late pachytene)] appear as large spheres (Figure 8), with a diameter ranging from 0.20 to 0.025 mm. Their protoplasm is finely grained, soft, and elastic, so that at the slightest pressure they easily become elongated and polyhedral, and they return to their previous shape as soon as the pressure is released. The protoplasm is not surrounded by a membrane; I, at least, was not able to find evidence of its existence in my research.
Figure 8.
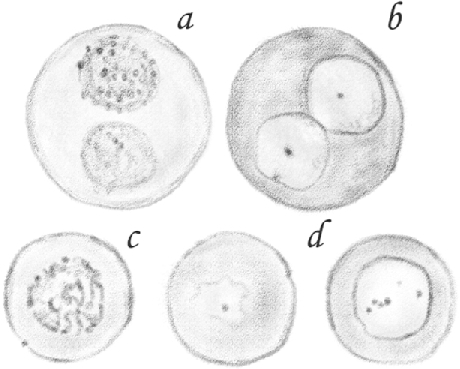
(Table IV.) Third stage [late pachytene spermatocytes]; b, d, observed in a fresh state: a, c, treated with dilute acetic acid. 600/1.
These [pachytene spermatocytes] contain one or two spherical nuclei having a diameter of 0.010–0.012 mm. These nuclei have a very delicate border and their content must be fresh or homogeneous, or finely grained like protoplasm, as they are hardly visible and it is possible to see them only because they have a pale, small nucleolus (Figure 8b, d). In fact, in some cases, the nucleus is not visible at all, or only its nucleolus appears, as shown in Figure 8d on the left. This is the appearance of cells with a granular nucleus. However, if one treats them with dilute acid, as Henle did, it can be immediately observed that the protoplasm becomes homogeneous and transparent, and the nucleus appears in the center in the form of a spherical cluster of big and dark granulates, as shown in Figure 8 a, c. I cannot tell whether these granulates are inside or only on the surface of the nucleus because they are so close together and dark that they block the view inside. The nucleus appears equally, but not so distinctly, granulated if the cells are treated with chromic acid or dichromate. These also make the protoplasm look more granular, dark-colored, hard, and stiff, so that if the cells were exposed to their action for a long time, in situ, they maintain the shape they took as a result of the pressure exerted within the tubule, thus appearing elongated, multifaceted, and mostly round tapered. The nucleus looks like a lighter-colored cavity with large granulations here and there (Figure 13, 3rds).
Unlike [spermatocytes] in their previous stages, third stage [spermatocytes (late pachytene)] form a double layer because they are located in pairs between the bodies of [Sertoli cells], as in Figure 13 (table IV) and Figure 1 (table III). This different arrangement is not due to an increase in the number of [spermatocytes] following the multiplication of second stage [spermatocytes (early pachytene)]—in fact, there is no multiplication at all—but only because the cells grow bigger as they enter the third stage [as late pachytene spermatocytes]. It is easy to understand how this occurs when one looks at Figures 10–13, which show the transition of [spermatocytes] from their second [(early pachytene)] to their third stage [(late pachytene)]. As second stage [spermatocytes (early pachytene)], already compressed against one another, grow bigger, they cannot stay on the same level any longer, so one of them has to gradually slide inwards, where it forms a second layer, as shown in Figure 13.
Third stage [spermatocytes (late pachytene)] originate regularly when first stage [round spermatids], previously found together with second stage [spermatocytes (in early pachytene)], enter their second stage. Therefore, third stage [spermatocytes (in late pachytene)] will be situated in the tubule segment next to the one where second stage [spermatocytes (early pachytene)], from which they originated, are placed. Cells of an earlier generation will occupy the previous tract. Thus, second stage [spermatocytes (early pachytene)] are found together with first stage [round spermatids] of the next generation.
Hence, the periphery of third stage [spermatocytes (late pachytene)] relates to the layer of first stage [spermatocytes (preleptotene, leptotene, and zygotene)], while their center relates to [spermatids] in their second [(elongating)] developmental stage, and their side to the body of [Sertoli cells], as third stage [spermatocytes (late pachytene)] are located between them.
I mentioned earlier that, in some cases, third stage [spermatocytes (late pachytene-diplotene)] have two nuclei. Since I never found more than one nucleus in second stage cells [spermatocytes (early pachytene)] or cells entering their third stage [spermatocytes (late pachytene)], it is natural to think that nuclear division begins in those [spermatocytes] that are in their last stage [(diplotene)] and reached their maximum size. This is indeed what occurs, as can be easily proven by observing a tubule tract where these cells are in their last stage and undergo the changes leading to the development of [nascent round spermatids]. Here, cells may have two, three, and even more nuclei.40
I cannot say with any degree of certainty how nuclear division occurs. In tubules soaked for 24 h in Müller solution, I was sometimes able to discern large [spermatocytes] having two, three, or four larger granules, as if they were nucleoli, far from one another. Slightly visible, granular, blurred lines in correspondence with the mentioned nucleoli divided the nucleus in two, three, or four parts. In other words, I noticed a faint sign of nuclear segmentation. Moreover, I often observed a small nucleus attached to a large one having the same aspect, as if it were a bud. Nonetheless, I was not able to track this process of multiplication, so it still remains to be understood whether it occurs by segmentation, gemmation [(budding)] of the primitive nucleus, or both.
After they divide, nuclei [(of resulting spermatids)] become smaller, clearer, and smooth. Also, the cellular protoplasm undergoes an important change remained unobserved so far: it splits itself into two different substances. The first, finely granular, arranges itself in small clusters around the nuclei, which in turn attract a portion of this substance. The other, homogeneous, transparent, glass-like, encloses the first together with the nuclei. This transparent substance constitutes the glass-like spheres Eiweisskugeln41 mainly located parallel to the direction of the tubule and whose origin is still unclear to current researchers of the seminiferous tubule structure.
Figure 9a shows a fresh cell soaked in aqueous humor, where it is possible to see how these glass-like spheres originate. Around each of the three nuclei resulting from the multiplication of a single, large nucleus, one can see the accumulation of a finely granular mass, which can only be the product of the partial separation of the protoplasm from the primitive cell. This forms three different cells enclosed in a large sphere of homogeneous substance.
Figure 9.
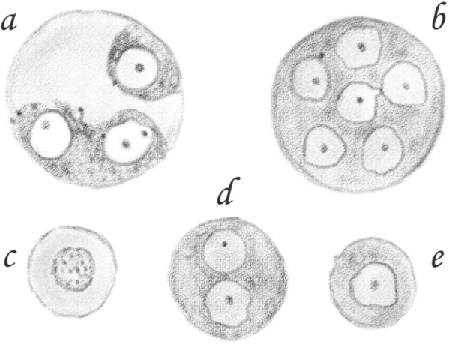
(Table IV.) [Nascent round spermatids]: b, d, [clusters]; e, [single]; c, [single,] treated with dilute acetic acid; a, [clusters of round spermatids] in which the division into [single round spermatids] and the formation of glass-like spheres is taking place. 600/1.
I observed many cells similar to the one shown in Figure 9. Some of them have a number of small cells with a granular protoplasm, and others only have one or two cells.
It is not plausible to think that such large spheres only contain one small cell; furthermore, many small cells are free in the tubule, and many spheres have no cell at all. It is therefore natural to think that the small cells formed inside the large [spermatocytes] in the manner described earlier gradually leave, so that only the homogenous portion of the protoplasm composing the glass-like spheres remains.
I was able to discern the cavities from which small cells had left in hardened glass-like spheres,42 but I never saw these cavities in fresh spheres.
If the substance contained in the large spheres is hardened in Müller solution when they are ready to separate from small cells, one will see that, when the spheres detach due to the preparation, a cavity or niche is still visible in the glass-like mass in place of the cells. Under natural conditions, instead, when cells detach from the glass-like substance, the latter forms clusters of spheres that fill the cavity left by the cells.
What happens when the spheres are hardened also demonstrates that the substance composing them is indeed a solid residue of the [spermatocyte]. It surrounds and holds together, for some time, the [spermatids,] originated from that very [spermatocyte].
Therefore, the so-called cysts in seminiferous tubules are not exactly so. I never noticed any sign of a membrane surrounding the glass-like mass with the small cells described above. The glass-like spheres move toward the center of the tubule; as they travel, they disintegrate, producing seminal fluid. This is the reason they decrease in number along the seminiferous tubule instead of growing as a result of the steady production, and only a few small spheres, if any, are found in the epididymis.
On the contrary, small cells remain in place and form the [nascent round spermatids].
The life cycle of [spermatocytes] ends, leading to the production of the seminal fluid and cells from which [spermatids] originate. Figure 14 shows the point where such transformation of [spermatocytes] occurs.
[Spermatids]
I include in the group of [spermatids] those cells originating from third stage [spermatocytes (late pachytene)] in the manner described above, which produce spermatozoa. I prefer this name to the term spermatoblasts used by Ebner, although with a different meaning, as I believe it more accurately indicates the cells’ function, which is precisely to produce [spermatozoa]. In fact, they contribute to little or no extent to the formation of semen which, as just mentioned, originates from a portion of protoplasm of [spermatocytes].
[Spermatids] were already described by Henle as cells with a smooth nucleus. Subsequently, Kölliker, Henle, Schweiger, Seidel, La Valette St-George, Merkel, and I highlighted their role in the production of spermatozoa.
[Round spermatids] are found in the form of [single] and [clustered round spermatids]. [Single round spermatids] are small spherical cells having a finely granular protoplasm and a smooth, clear, spherical nucleus with a small distinct nucleolus (Figure 9e). If treated with dilute acetic acid, their protoplasm swells, appears more transparent, and their nucleus becomes granular, although not as much as the nuclei of large [pachytene spermatocytes] (Figure 9c). The diameter of [single round spermatids] ranges from 0.012 to 0.014 mm; the nucleus is 0.008 mm.
[(A cluster of round spermatids is formed from those)] that did not separate after originating from [spermatocytes] and remained connected to one another, thus forming large cells with a diameter that can vary from 0.016 to 0.025 and 0.050 mm, and up to 10 or more nuclei (Figure 9b, d).
Both the protoplasm and the nuclei of these cells have the same appearance and microchemical features found in [single round spermatids].
A question may arise as to why no separation occurred, even when one assumes that these large [clusters of round spermatids] derive from third stage [late pachytene spermatocytes] and that glass-like spheres result from the division of protoplasmic substances and later detach from newly formed [round spermatids]. One might also ask whether the division of the two protoplasmic substances is due to decomposition, alteration, or if it is just part of the transformation process of [spermatocytes].
Firstly, I state that there are far fewer large [clusters of round spermatids] with many nuclei than small ones with a single nucleus. Moreover, the separation of a glass-like substance from the protoplasm of third stage [late pachytene spermatocytes] can be observed in preparations of extremely fresh testes with isotonic solution like the aqueous humor of the animal. Therefore, the detachment can neither be the result of decomposition nor alteration.
On the other hand, [clusters of round spermatids] can and do separate in a number of [single round spermatids], although a bit later, I was able to observe [clusters of round spermatids] in which the division process of their individual cells started when [later spermatids] were showing their first signs of development.
In some cases, though, they separate much later, so that the first stages of [spermatid] development take place within them, as I demonstrate later.
Because [round spermatids] are mostly [single], I believe that large cells with smooth nuclei are, in reality, a cluster of small cells, namely [single round spermatids], in which, for reasons unknown to me, the detachment of individual cells occurred later than in others.43
It should also be remembered that La Valette St-George states that large cells with smooth nuclei are a cluster of small cells, which means that each nucleus together with a small amount of protoplasm virtually forms a cell (1).44
As explained earlier, [single and clustered round spermatids] are found later than third stage [spermatocytes (late pachytene)], when first stage [spermatocytes (preleptotene, leptotene, and zygotene] of a more advanced generation entered their second stage [as early pachytene spermatocytes], and existing developing [spermatids] began their third [(condensing)] stage. Therefore, [round spermatids] populate the tubule tract next to the one in which third stage [spermatocytes (late pachytene)] are situated, which space they occupy. Here they form three to four layers of cells between the body and the central region of [Sertoli cells], surrounded by [spermatids] in their third [(condensing)] stage of development.
The external layer is in contact with the layer of second stage [spermatocytes (early pachytene)], while the inner part delimits the tubule lumen. Figures 2 (table III) and 10 (table IV) show the position and contact points of [round spermatids], although here the first signs of [later spermatid] development can be observed.
Development of [round spermatids]
[Elongating and condensing spermatids] originate from [round spermatids] as soon as these are produced by [spermatocytes]. Therefore, preparations of [round spermatids] also reveal the presence of [spermatids] showing the first signs of [flagella formation].
I studied the gradual changes [round spermatids] undergo until they become spermatozoa [spermatids] only in testis preparations of newly killed rats, as I already mentioned in my second prior communication (1).45 The solution I added to rarefy and more easily identify the cells was the aqueous humor of the same animal.
Having worked in the way I explained above, I believe no one might think that the phenomena I described as natural and normal were the product of decomposition or alteration.
I explained at the beginning of this work that the development of [spermatids] can be divided into three stages. The tail forms during the first stage [in round spermatids], the head during the second [stage in elongating spermatids], and the midpiece develops during the third stage [in condensing spermatids]. We now examine the phenomena occurring during these three stages closely.
First Stage [(round spermatid)]—The first observable fact in a cell growing into a spermatozoon is the development of a corpuscle [(chromatoid body)]46 in the protoplasm, in proximity to the nucleus, and a very thin and delicate thread, which is the tail of the future spermatozoon.
The [chromatoid body] is opaque, smooth, and roundish, and it resembles a nucleolus. It often has a spherical shape, in which case it looks darker. In other cases, it is oval and of a lighter color. It is likely to be mostly oval in shape, as one can see from the side view. If observed from one of its ends, instead, it looks spherical, darker, and thicker. Sometimes, because of the cell position, it seems located inside the nucleus, in which case it resembles a nucleolus. The [chromatoid body] is in contact with the external surface of the nucleus but it does not adhere to it, nor is it composed of a partial, circumscribed enlargement of its membrane. When the cell is observed in a given position under the microscope, a very small space in between them, which is wider at a much more advanced stage of [spermatid] development, might be seen.
If a [round spermatid] is treated with distilled water early in the first developmental stage, it slowly swells and the protoplasm becomes lighter and transparent, while its contours become barely visible. The [chromatoid body] is not affected at all. By using dilute acetic acid, the [spermatid] becomes initially smaller, granular and dark; the nucleus undergoes pretty much the same changes, and its borders appear darker and larger. The prolonged action of acetic acid causes the cell to swell, while the granules disappear. The protoplasm becomes clearer and barely visible; the nucleus looks lighter as well, and its contours appear thin again; the [chromatoid body] is no longer visible. If the preparation is rinsed again with water, the cell becomes once again smaller, dark, and granular, just as under the initial action of acetic acid. The [chromatoid body] is again visible; it swells, becomes lighter and invisible under the action of acetic acid, just like the thin granules in the protoplasm. However, the [chromatoid body] does not disintegrate and, unlike the granules, it resists the action of distilled water.
I never noticed two of these [chromatoid bodies] in the same cell. Therefore, until proven otherwise, I will be convinced that La Valette's observations in relation to the development of [spermatid] tails (1)47 and Merkel's conclusions regarding the first signs of the tip of a future [spermatid] head (2)48 refer in reality to the same fact.
With regard to the function of the [chromatoid body], just as in Merkel's case, no observation clearly demonstrated that they are responsible for the production of the tail. I often found the [chromatoid body] opposite to the cell end where the [flagellum] protrudes, too. On the other hand, contrary to what Merkel states, I still do not believe it contributes to form the tip of the future spermatozoon head, which he named Spitzenknopf. I will describe later my studies and conclusions regarding the Spitzenknopf and the formation of the free end of the head, and I will illustrate my observations of the [chromatoid body] on the side of the nucleus where no membrane thickening nor any formation of the mentioned Spitzenknopf occurs.
Finally, no evidence leads me to confirm Brunn's conclusions (3)49 that the [chromatoid body] would contribute to form a membrane around the [spermatid's] head (Kopfkappe).
Although unexplained, the presence of the [chromatoid body] in the [round spermatids] is an important and peculiar fact, insofar as it signals the beginning of the [later spermatid] development while its shape has not yet changed.
As mentioned, in the early developmental stage of the [spermatid], it is possible to see a [developing flagellum]50 attached to one of its peripheral ends, as La Valette St-George and then Merkel observed. I should like to dwell on this point more than they did, as I believe it is crucial.
The [developing flagellum] is extremely thin and barely visible under a powerful magnifying glass, so it easily goes unnoticed. It can be two or three times longer than the [spermatid] diameter, as one can see in the second cell shown in Figure 15. Its diameter is fairly stable along its entire length. It abruptly protrudes from a point of the cell surface, as if it came out of the very cell. However, I have to add that I never noticed any [developing flagellum] in a [spermatid] which connected to the nucleus or the [chromatoid body] mentioned above.
Figure 15.
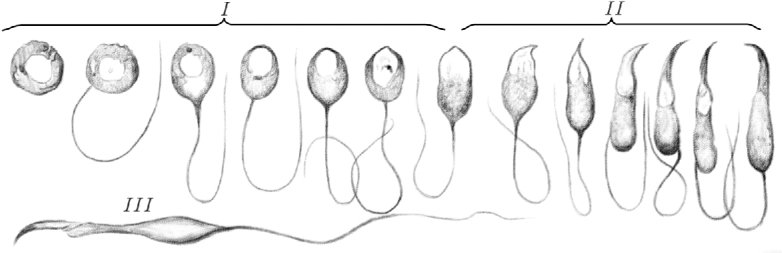
(Table IV.) Developing [spermatids]. I. First stage [(round)];—II. Second stage [(elongating)];—III. Beginning of the third stage [(condensing)].—Fresh cells in aqueous humor. 600/1.
If observed in a very fresh state, the [developing flagellum] shows the same, though extremely slow, vibratory movements as mature spermatozoa. In fact, these are so wide and vigorous that they cause the cell to which it is attached to move whenever the [developing flagellum] free end is prevented from oscillating.
Someone might think that this [developing flagellum] is only a tail piece accidentally attached to the cell. Such a supposition, though, is unsubstantiated because (i) the constant presence of the [developing flagellum] in first stage [round spermatids] cannot be an accidental circumstance; (ii) if the cell is shaken or rolled, the [developing flagellum] does not separate, as it should if it were only randomly attached; (iii) although insignificantly, the diameter of the [developing flagellum] decreases from the end attached to the cell to its free one, which is extremely thin; (iv) semimoving [developing flagella] are also attached to [clusters of round spermatids], in which the early stages of [spermatid] development take place and, most importantly, their number is the same as the nuclei contained in the [spermatid] (Figure 16).
Figure 16.
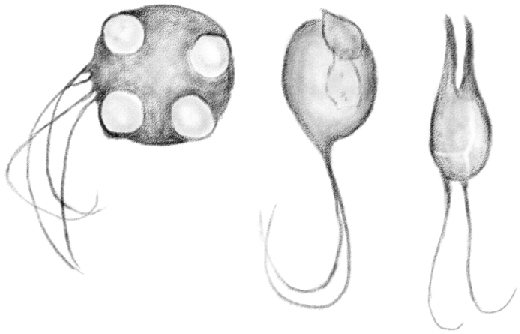
(Table IV.) Development of [spermatids] from [secondary spermatocytes].—Fresh cells in aqueous humor. 600/1.
The [developing flagellum] attached to the [round spermatids] is undoubtedly the tail of the future spermatozoon, which is therefore the first part to develop.51
The importance of such a statement is well understood as it demonstrates Pflueger's considerations (1)52 that the [spermatid] is a vibratile cell with a single [flagellum], as well as it irrefutably proves that, contrary to what Ebner stated, cells with a smooth nucleus do not disintegrate but lead to the formation of [later spermatids, and eventually spermatozoa]. In fact, while they still have the appearance of a cell, they already contain an essential part of the spermatozoon.
As the [flagellum] continues to grow, the nucleus, until now in its position, travels to a point of the cell surface opposed to the one where the [developing flagellum] originates. At the same time, the membrane of the nuclear hemisphere facing the cell surface becomes thicker, so that the border of this part of the nucleus appears well marked. This phenomenon, which is a peculiarity of the [spermatid] developmental process, was already observed by La Valette St-George and analyzed in detail by Merkel in his last work (2).53 Based on my observations, I share Merkel's conclusion that the thickening of the nuclear membrane occurs to the detriment of the nuclear content, which becomes less dense.
At some point at the very beginning of the first developmental stage [(of spermatids)], it is possible to observe both the [chromatoid body] in the protoplasm and the nucleolus in the nucleus of the [round spermatids]. The nucleolus is smaller than the [chromatoid body], which is now smaller than later on. However, when the border of a nuclear hemisphere begins to thicken, the nucleolus disappears and is no longer visible, while the [chromatoid body] appears more clearly. I could not tell whether the [developing flagellum] is already present when both the [chromatoid body] and the nucleolus are in the [round spermatids]. I never noticed it, but I might have missed it due to its extreme thinness, or because it accidentally detached from the examined cells.
While these transformations take place in the nucleus, the cell, once spherical, assumes an oval shape. At one of its ends, the nucleus with an enlarged border protrudes from the cell surface, while at the other it is possible to see the mentioned [developing flagellum]. As the development continues, the nucleus moves beyond the cell surface, so that the hemisphere with the thick membrane protrudes from the protoplasm. This, as a result, is positioned beneath the nucleus, like a bag wrapping the hemisphere with the thin border.
I was never able to observe a membrane in the [round spermatids], so I cannot confirm whether, as some maintain, the protruding nucleus is delimited by a membrane of the same cell, as if it were a cap.
At the same time as the [round spermatid] undergoes the changes described above, the initially thin [developing flagellum] becomes gradually longer and larger to the detriment of the protoplasm. The [developing flagellum] gradually connects to the protoplasm, so one might think that the protoplasm of the [spermatid] becomes longer here to form the [flagellum of the spermatozoon].
The [round spermatid] shape has only changed insignificantly so far and it still has the appearance of a cell. With the exception of the nucleus, which has undergone only minor changes compared to the ones occurring later on, the most salient feature of the [spermatid] first developmental stage is the tail formation.
Second Stage [(elongating spermatid)]—As the [spermatid] grows, the nucleus, once rounded, undergoes major changes leading to the formation of the [elongating spermatid] head.
Its spherical shape becomes oval, and its largest diameter aligns with the largest diameter of the cell. At its free edge, a slight protrusion of the thicker side of the membrane is visible, which I believe corresponds to what Merkel called Spitzenknopf. Later, in place of this protrusion, one sees a small aculeus [(acrosome)]54 often inclined sideward.
With regard to Merkel's Spitzenknopf, I am of the opinion that a slight and circumscribed protrusion is present at the thickest point of the nuclear membrane. Diagonally, this may look like a button firmly attached to the nuclear membrane. As the protrusion becomes more pronounced, the [(acrosome)] described and shown in Figure 15 originates and becomes longer as the [elongating spermatid] develops, while the body of the nucleus becomes smaller. This is how, in the second [(elongating)] developmental stage, the head of the future spermatozoon assumes the nail shape described by Ebner.
As it becomes longer, the [(acrosome)] gradually inclines sideward at its free edge, forming a hook, which is the typical shape of the spermatozoon head in rats. Meanwhile, the lower side of the nucleus becomes narrower in the cross direction, so that it loses its spherical shape, and it constitutes the basal surface of the hook. This is cut diagonally so that it faces the tipped side, and has a rhomboid shape with rounded angles, except for the lowest one.
The nuclear content contributes to form the [(acrosome)] and therefore the hook as well. It is delimited by a membrane that contains a thick double border at the top and becomes increasingly narrower, until it appears extremely thin at the base. It is precisely because of the membrane thickness and the [(acrosome)] thinness that the upper part of the nucleus looks opaque compared to the large lower part, where the nuclear membrane is thinner and the content more abundant.
The content of the nucleus is initially light colored, with rare and pale granules still present in its spherical side. Subsequently, these disappear; the light becomes more reflective while the content is darker and homogeneous, and it slowly assumes the appearance of a mature spermatozoon head.
At the same time that these profound changes affect the nucleus of the [round spermatid], the cellular protoplasm becomes longer, looking like a bag attached from its upper side to the diagonal basis of the spermatozoon head.
While the nucleus protrudes from a [round spermatid] edge and grows longer, the [chromatoid body] I described earlier, at a distance, becomes surrounded by shiny granules, which begin to appear in the protoplasm around this time. Once the nucleus formed the [(acrosome)], the [chromatoid body] becomes indistinguishable. In some cases, it disappears at an earlier time.
During the second developmental stage of the [elongating spermatid], the most prominent feature is, therefore, the head formation.
Third stage [(condensing spermatid)]—Once the head and tail of the future spermatozoon have been formed, the only missing part is the [round spermatid] protoplasm. As I mentioned, this assumes the shape of a bag connecting the head to the tail.
The area closer to the head grows longer and narrow, resembling a sort of neck attached to the head base, and forms a small swelling (Figure 15, table IV). Under the neck, the swollen body of the [condensing spermatid] gradually narrows toward its lower part and continues into the [flagellum], as shown in Figure 15, table IV. The protoplasmic mass is now ampule-shaped and finely granular, with shiny granules in its largest part.
The total length of the [condensing spermatid] is now 0.15 mm. The head is 0.015 mm long, the neck is 0.012, the body approximately 0.023, and the [flagellum] 0.10 mm.
As the [spermatid] keeps growing, the neck becomes increasingly thinner and longer. It remains attached to the hook base only from its obtuse angle, so that the low, acute one is free, thus reflecting the appearance of a completely formed spermatozoon. The [piece] connecting the tail to the head along the neck appears in this moment. The entire neck is now a thread and the body, now smaller, constitutes a granular, elongated mass, which remains attached to the thread midpiece for a while, forming the well-known Dujardin's appendage.
Finally, the [condensing spermatid] body or midpiece (Schweigger-Seidel's Mittelstück) is formed. This would be the product of the cellular protoplasm from which the [spermatid] develops, as accepted by the scientific community with the only exception of Kölliker. Like my colleagues, I cannot describe precisely how the midpiece is formed. I observed developing [spermatids] like the one shown in Figure 15 (table IV), in which it was impossible to see any [flagellum] continuing into the cellular protoplasm toward the head. Some other times, I observed them in a much more advanced stage and I noticed that the head was completely connected to the [developing flagellum]. How the connecting [piece] of the protoplasm originates is what still remains to be determined.
What is certain is that, during the third [(condensing)] stage, the midpiece formation takes place.
It seems to me of considerable importance that the first and second developmental stages of [flagellum development] can be observed in [clusters of nascent round spermatids] as well. Incidentally, this also demonstrates that spermatozoa originate from the [round spermatids] in the tubule.
Figure 16 shows a [cluster of round spermatids] with four nuclei on the cell surface having the typical thick membrane, and also four extremely thin tails, which moved in the solution exactly as the [developing flagellum of single round spermatids]. The other cell shown in Figure 16 represents a more advanced stage of development. Here, the two nuclei are elongated and protrude from the cell forming the [(acrosome)] described earlier. On its opposite side, one can see the tails of the future spermatozoa in the form of two long threads. In the third cell, the head of two [spermatids] with the same protoplasm as the [cluster of round spermatids] is almost completely formed. My observations did not provide enough evidence to determine whether [spermatids] reach their maturity when they remain so close together.
The above leads to the conclusion that [spermatids] develop from [germ cells] in the testis, as Kölliker first demonstrated. It also indicates that, without going into detail, they develop in the manner described by Henle, La Valette St-George, Schweigger-Seidel, and Merkel: the nucleus leads to the formation of the head, while the protoplasm contributes to form the [flagellum].
I believe it is especially important to note that first the tail, then the head, and finally the body or midpiece [of the flagellum] develop to form the spermatozoon. My division of the [spermatid] development into three main stages is therefore entirely justified.
[Spermatids] form and fully develop in situ, namely in the same place where the [round spermatids are] located. Only when they reach maturity do they pass though the center of the tubule to reach the rete testis. As Ebner rightly observed, they place themselves along the tubule lumen in such a way that they move ahead using their tails, which converge in a spiral motion parallel to the direction of the tubule.55
During the first developmental stage, [, round spermatids] are inside the layer of second stage [spermatocytes (early pachytene),] and occupy the room left by the body and central region of cylindrical [Sertoli cells] (Figure 2, table III; Figure 10, table IV). A more advanced generation of [spermatids] in their third [(condensing)] stage of development leans against [Sertoli cells]. This proves that a generation of [spermatids] in its last stage of development coexists in the same tubule tract with a new generation at the beginning of its development. It also clearly confirms Merkel's considerations that “Dass die ersten Entwickelungstadien der folgenden Spermatozoidengeneration schon zu einer Zeit durchlaufen werden, wo die vorhergehende noch in den Stutzzellen sitzt” [Sertoli cells] (1).56
Subsequently, while mature [condensed spermatids] enter the tubule lumen, developing ones take their place as they are pushed against [Sertoli cells] by [spermatocytes] moving from the second [(early pachytene)] to the third [(late pachytene)] stage (table IV, Figures 11 and 12).
In this regard, I do not agree with Merkel's opinion regarding the niches. He believes that the pockets of [Sertoli cells], as he calls them, are previously formed, and that [spermatids] in their first developmental stages place themselves in there once the old ones leave, as if they were somehow aware that those niches are made to host them. I already stated, when I described the central region of [Sertoli cells], that the niches are passively formed by the cells, nuclei, or [spermatids] that are pushed and compressed against it.
The detachment of mature [condensed spermatids] from [Sertoli cells] and their transition into the tubule lumen does not occur, as Ebner stated, because a new [round spermatid] develops, pushing ahead [mature spermatids].
The central extension of Ebner's so-called Keimnetz, which is the body and central region of [Sertoli cells], never detaches from its periphery, so there is not a new extension for each generation of [spermatids]. I always noticed that, immediately after [spermatids] detach, [Sertoli cells] are just as long as they were before and they reach the tubule lumen. Figure 11 shows precisely that [condensed spermatids] approached the center without affecting the [Sertoli cells].
It is true that, especially in scrape preparations, it is often possible to observe [spermatids] in a sheaf-like pattern around detached fragments of [Sertoli cells], as well as [Sertoli cells] devoid of their central region. However, by examining the point where the detachment occurred, one sees that it was caused by accidental damage of the same cells.
I could not explain in detail why mature [condensed spermatids] move toward the tubule lumen, but I tend to believe that the reason might be of a purely mechanical nature, as new [germ cells] grow and reproduce from the tubule periphery. I also think it should not cause surprise that the tails of mature [condensed spermatids] move, contrary to Ebner's conviction that they are completely motionless.57
Mature [condensed spermatids] in the rats I examined are longer than reported by Neumann, being approximately 0.19 mm long, with a 0.0099 mm long head, a 0.066 mm midpiece, and a 0.115 mm tail. This discrepancy might be due to a difference in the animals examined. The midpiece is often easy to identify because it is thick and bright. In most cases, at the point where it connects to the tail, I was able to observe a small brilliant point with the appearance of a vacuole.58
A comprehensive view of the entire developmental process of several [spermatid] generations will prove helpful. They originate in a given tubule tract and continuously follow one another. Each generation forms ab ovo [(from the beginning)], namely from first stage [type A spermatogonia], and goes through eight stages to fully develop. A generation of [spermatids] is present in the tubule in the following order: I. First stage [spermatogonia (type A)]. II. Second stage [spermatogonia (intermediate and type B)]. III. First stage [spermatocytes (preleptotene, leptotene, and zygotene]. IV. Second stage [spermatocytes (early pachytene)]. V. Third stage [spermatocytes (late pachytene)]. VI. First stage [round spermatids]. VII. Second stage [elongating spermatids]. VIII. Third stage [condensing spermatids].
In order to clarify the developmental sequence of [spermatid] generations in a given tubule tract and to illustrate how they relate to one another, I produced the following diagram based on the preparations I made, which faithfully represents and summarizes all of the above.
A half of a tubule segment is shown here [(see figure, above)]. The upper straight line represents its membrane, while the arrows indicate the lumen. The vertical dashed lines divide the tubule in as many developmental stages as a generation goes through (1,2,3, etc.), so that each section represents the length of a stage. The horizontal dashed lines demarcate areas of the same size indicating the stage of each generation in a given tubule tract (I, II, III, etc.). The solid lines represent individual generations; more specifically, the horizontal lines labeled with the letters on the left represent each generation's stage, while the diagonal lines connecting the horizontal ones indicate the transition of a given generation from one stage to the next. The diagonal lines followed by a horizontal line and ending in an arrow represent the [spermatids] entering the tubule lumen and their outward direction.
By examining a short tubule tract where first stage [spermatogonia (type A)]—namely, a generation of [round spermatids] in its first stage—are found, it is obviously not possible to see the following stage of the same generation. As I mentioned earlier, development does not occur simultaneously but in a stepwise process, downward within the tubule. It is therefore when one looks at a close, lower tract that the second stage of a recently formed generation, after going through the transition from the first to the second stage, becomes visible. Subsequently, in the following tract, the third stage of a more advanced generation will be found, and so on until the last generation.
Let us now suppose that tracts 1, 2, 3, etc. of the tubule represent the timeframe in which the stages of the same generation take place, instead of each individual developmental stage of several generations. In that case, the diagram would show all the eight stages that a given generation goes through from the moment it originates in the form of first stage [spermatogonia (type A)] to its end, when mature [condensed spermatids] enter the tubule lumen.
The figures in table III show two cross-cut sections of a seminiferous tubule in two different points. Apart from [Sertoli cells], Figure 1 shows, mainly at the periphery, first stage [spermatogonia (type A)] (1st g.), then first stage [spermatocytes (preleptotene, leptotene, and zygotene)] (1st s.), third stage [spermatocytes (late pachytene)] (3rd s.) and, finally, second stage [(elongating)] spermatids [elongating spermatids] (2nd sp.).59 Therefore, four generations are found here: the first in stage I, the second in III, the third in V, and the last one in VII.
Figure 2, instead, shows at the periphery the layer of second stage [spermatogonia (intermediate and type B)] (2nd g.), then second stage [spermatocytes (early pachytene)] (2nd s.), first stage [round spermatids] (1st n.), and lastly, third stage [condensing spermatids] (3rd sp.).60 Four generations are found in this tract as well, but this time they are in different stages: the first generation in stage II, the second in IV, the third in VI, and the last one in VIII.
The diagram helps to understand this concept, which appears fairly complicated in the two sections.
Let us suppose that an odd-numbered tubule tract, e.g. no. 3, and an even-numbered one, e.g. no. 4, are cut in half precisely in correspondence with the marker indicating Figures 1 and 2. It will be possible to see two sections representing the same structure as the ones in the figures mentioned above. The same holds for Figures 10–14 in table IV, which are indicated in the relevant diagram points. The diagram also shows the respective age of the various generations that, in some of their stages, are visible in the preparations shown in the mentioned figures of tables III and IV.
Both the figures and the diagram show that each tubule tract is populated all the time by four generations of [spermatids], each of them in a different stage. Each tract differs from the other as it contains generations in a different developmental stage.
Therefore, the odd-numbered tracts 1, 3, 5, 7 contain the most recent, that is the youngest generation in its stage I, the third generation in III, the second in V, and the oldest in VII. The following even-numbered tracts 2, 4, 6, 8, instead, contain the four generations that are in a more advanced stage: the youngest in its II, the third in IV, the second in VI, and the oldest generation in stage VIII. Thus, by superimposing an even-numbered tract on an odd-numbered one, or Figure 2 on Figure 1 of table III, one can see at the same point or tract all the developmental stages a generation undergoes, from the beginning to the end.
As the diagram shows, in the last portion of the even-numbered tracts, which contain older generations, the oldest one, now at its end, enters the tubule lumen. At the same time, a new generation originates from the periphery of the odd-numbered tracts, while each of the three existing generations enters a more advanced stage. Thus, a new generation is formed every two tracts, and every two tracts another generation becomes fully mature and enters the tubule lumen.
The length of the tracts corresponding to a given stage can vary, so that no. 1 can be shorter or longer than no. 2, which in turn can be shorter or longer than no. 3, and so on (1).61 For the sake of simplicity, I arbitrarily decided to draw segments of the same length in the diagram. However, the diagram clearly shows what the preparations reveal: in the same tract, be it longer or shorter than the others, the stages of the four generations it contains are of the same length. If this were not the case, it would not be possible to see in the sections of the tubules the continuous interrelation that exists between one category of cells and another, or between these and the various [spermatid] developmental stages.
Let us now turn to Ebner's theory, according to which [spermatids] would not originate from the [germ cells] of the tubule. Contrary to what I and other authoritative scientists state, Ebner maintains that [spermatids] originate from the central region of the so-called Keimnetz [(round spermatid)] extensions or, in other words, the central region of [Sertoli cells]. [Germ cells], instead, would dissolve, and their only function would be to produce seminal fluid.
The description of the tubule structure and [spermatid] development in the various animals I decided to study serves to disprove this theory. However, as it is based mainly on the research Ebner carried out on rats, I believe that it is appropriate to briefly demonstrate its incorrectness here, especially since there might not be another opportunity in the future.
My own observations, as well as Ebner's missing ones and, interestingly, even other observations made by Ebner himself, serve to demonstrate the inaccuracy of his theory.
The main reasons Ebner's theory cannot be accepted are as follows:
a) The changes that [round spermatids] undergo during their first developmental stage, when they still look like free roundish cells. The presence of the [chromatoid body] described, the thickening of the nuclear membrane, the peculiar movement of the nucleus, and, above all, the formation of a vibratile [developing flagellum], constitute sufficient evidence that [germ cells] with a smooth nucleus [(round spermatids)], rather than dissolving, become prepared to assume another shape.
b) The development of [spermatids] from [clusters of round spermatids]. It has never been questioned, not even by Ebner himself, that the large tubule cells or cysts with a number of smooth, small nuclei are not independent and autonomous. As I illustrated above, these cells, which [are clusters of round spermatids], undergo the same changes that characterize the first and second developmental stages of [spermatids].
c) The fact that the first developmental stage of [spermatids] takes place entirely while the older generation of [condensing spermatids] in its third stage, arranged in a sheaf-like pattern, is still attached to the [Sertoli cells] (the extensions of Ebner's Keimnetz). This point, previously highlighted by Merkel, is crucial. It disproves Ebner's and Neumann's conclusions that first stage [single round spermatids] are just cells detached from their spermatoblasts, insofar as, based on the same theory proposed by Ebner, the so-called Lappen of a new [round spermatid] cannot form until the older one has detached.
d) The continuous relation between the formation of [germ cells], specifically the [round spermatids], and the development of [spermatids]. This relation could not be understood or explained if the former did not lead to the latter and they did not have the same aim.
e) The constant length of [Sertoli cells] and the different shape of their central region depending on the [spermatid] developmental stage. Lastly, the presence of [Sertoli cells] and their cylindrical shape in the tubules where developing [spermatids] are absent.
I never observed any evidence of the fact that the central region of [Sertoli cells] detach from the body and the basal surface as a result of a natural process, nor that these cells become shorter, as Merkel admits. I myself observed, in scrape preparations, sheaves of [spermatids] connected to the central region of [Sertoli cells] that had detached from the remainder of the cell, as well as cells devoid of their central region. However, this detachment was not due to natural causes; the preparation had split the cells in two, as one could clearly see by examining the detachment point. It is not hard to understand how easily a cell can break when one considers that at one end it is attached to other cells, while at the other it carries a heavy sheaf of [spermatids], and in the middle it is thinner and narrow due to the pressure surrounding [germ cells] exert on it.
I previously mentioned that niches of different shapes are found in the central region of hardened [Sertoli cells]. These niches assume the shape of the [spermatid] head in contact with the [Sertoli cells]. Therefore, they are wide and roundish where the head still looks like a roundish nucleus, or narrow and hollow where it is elongated and hook-shaped. This means that the niches originate from the [spermatid] heads: the internal tubule pressure, which nobody ever took into due consideration, caused them to sink into the soft protoplasm of the [Sertoli cells’] central region, just like the [germ cells] on their side or periphery did, thus generating larger and rounded niches or cavities.
The borders of these niches are lamellate, so it is not possible to determine to which pedicles the developing [spermatids] are attached. In order to do so, they would have to be filiform [(threadlike)], and the niches should not be roundish.
What Ebner failed to observe in the microscopic examinations he carried out contradicts his theory as well.
He failed to notice the entire first developmental stage of [spermatids], which led him to conclude that [spermatids] are in their first [(round)] stage when in reality they already entered their second [(elongating)] one. What is even more serious, he observed the presence of the tail only when the [spermatids] had reached their fourth stage [(in his classification)] and their head was already hook-shaped, namely, in the third [condensing] developmental stage of my division. Leaving aside the formation of the [chromatoid body] and the initial nuclear changes, the fact that the tail develops when the [spermatids] are still rounded is beyond question. This was also observed by La Vallette St-George and Merkel, who noticed the tail movements as well. Although extremely thin at the beginning, the tail is already so pronounced when Ebner's first stage starts that, had he examined it under appropriate conditions, he would not have missed it.
It is therefore clear that the preparation method Ebner relied upon was flawed and lead him to erroneous conclusions, so he cannot convince us of the validity of his theory.
Either he did not examine fresh tubules or he did not use isotonic solutions, as proved when he states that [spermatids] in the tubule are motionless (l.c., p. 218). This is obviously incorrect, as I was able to repeatedly notice their movement. If Ebner had not failed to see the first developmental stage of [spermatids], he would not have conceived a further eighth stage, which might as well be identified as a resting stage.
Even what Ebner himself observed demonstrates that his theory is fallacious, insofar as:
He himself admits, on page 208 of his work, that the so-called Lappen are in most cases detached and isolated in scrape preparations, and that they appear like cells with a nucleus leaning against their walls (1).62 Now, isn’t it more natural and logical to admit that these components are in fact free cells, rather than the Lappen of another cell? In truth, it is. So much so that in his dissertation supervised by Neumann, Blumberg (2)63 had to clarify, in order not to deny the obvious, that [spermatids] develop in two ways, that is, both from Keimnetz [(Sertoli cells)] and from [germ cells]. What I described so far, as well as Merkel's most recent work (3),64 provide evidence of how mistaken Blumberg was.
According to Ebner, during stage VIII, many cells in the tubule appear elongated, with nuclei which become easily stained by hematoxylin, positioned in one of the cell ends. How could he not realize that these are the same cells as the ones described in (a), only in a slightly less advanced stage, corresponding to [spermatids] between their first [(round)] and second [(elongating)] stage? It should be remembered that stage VIII is followed by stage I, which, to Ebner, would correspond to a new generation. In reality, though, it is just a more advanced stage of the same generation found in VIII.
Ebner observed that during the third [(condensing spermatid)] stage the heads, already hook-shaped, are in the tubule periphery. This is absolutely true. Nonetheless, it is not clear how [spermatids] can climb along the [Sertoli cells] toward its periphery if they are attached to the central region, which is a fixed point. If they derive from the Keimnetz [(Sertoli cell)] extensions of the central region, then they should rather move toward the center. Instead, the fact that they travel toward the periphery is easily understood when one acknowledges that [spermatids] develop from free cells. Their head is free to move in the direction of the tubule radius, so they can reach the periphery through the elongated cell and, in particular, the protoplasmic neck of developing [spermatids].
Finally, in order to explain the detachment of [spermatid] sheaves and their transition into the tubule center, Ebner states that new extension originating from the Keimnetz push them toward the center. At the same time, he observes that some of these new extensions are 40 to 50 μm long, and that they are confused in the center with the granular mass surrounding the [spermatid] sheaves. However, based on what Ebner himself observed, there are reasons to believe that no detachment occurs at all, and that old and new extensions are in reality the same component, namely the body and central region of [Sertoli cells]. The only difference would be that the former are surrounded by [spermatids], while the latter are free, having traveled toward the center of the tubule. In fact, how can one suppose that newly born extensions are already so mature as to reach the length indicated by Ebner, which is in fact the length of [Sertoli cells]?
In this respect, it should be noted that even Neumann admits he was not able to observe the events preceding the formation of the so-called Lappen in the extensions’ (Keimnetz) development (l. c., p.315). I am convinced that, had Neumann been able to observe and understand these events, he would have not supported Ebner's theory.
The only argument in favor of this controversial theory is that [spermatids] are attached to the central region and body of [Sertoli cells], around which they are arranged in a sheaf-like pattern. However, if one supposes that this unnecessary relation implies the existence of a genetic link between [spermatids] and epithelium, then it should also be acknowledged that [germ cells] derive from [Sertoli cells], or the other way round, because isolation preparations reveal that they are often connected together.
Neumann relied on the research he carried out on [spermatid] development in the frog to support Ebner's theory. Nevertheless, even the soundness of his work has been questioned following La Valette St-George's accurate research (1).65
Therefore, the theory proposed by Ebner cannot be considered valid at all, and the total absence of supporting evidence does not allow to prove such a complex phenomenon. Indeed, the supposition that a spontaneous formation of nuclei (Kernartige Gebilde) may take place in a protoplasmic extension, and that this extension might be able to generate cells with the crucial physiological role of creating a new individual from egg cells—as [spermatids] are—goes against the theory, universally acknowledged, of cell formation.
I believe it is appropriate to spend a few words on the method I used, for other researchers may want to replicate my study on rat testes. It is a very simple one. I made scrape preparations with extremely fresh samples, or samples hardened in Müller solution. I found this solution very helpful, especially when I needed to observe the shape and position of individual cells. In fresh preparations from newly killed animals, I used aqueous humor from the eye, as it does not alter the composition of the cells.
My study is not based on randomly taken tubules. I isolated a given tubule a few inches long, which is easily done in rats. Then, I placed it on a microscope slide rinsed with a small amount of solution. Starting from one end, I cut several pieces, no longer than two or three millimeters, with a scalpel, and I examined them one by one, scraping them appropriately. By working in this way, the preparations are not a combination of disparate cells in different stages of [spermatid] development, which only confuse the observer. Instead, the gradual changes in the structure of the tubules, the transition from one component to the other, and every single transformation [single round spermatids] undergo before they become [elongating and condensing spermatids] are thus clearly visible.
In order to observe the layer of [spermatogonia] and first stage [spermatocytes (preleptotene, leptotene, and zygotene)], as well as the sheath proper, it is advisable not to use any needles. I took a small portion of a tubule, one or two mm long, from a sample soaked in Müller solution for 5 to 6 days, then I cut it along its length on the microscope slide. The solution should not be added before cutting the tubule, but only immediately after. The two halves can be stretched using needles, taking care to detach the central mass gently. At this point, the tubule can be observed under the slide. I initially used a small drop of a concentrated neutral solution of carmine, then distilled water and, finally, after having positioned the slide, I added glycerin or Farrants’ solution.
The tubules soaked for a few weeks in Müllers solution certainly can be cut on a microscope slide with a thin, sharp scalpel. Although complete segments can hardly ever be cut, they permit good preparations in which the mutual relations among the cells are only slightly altered. In order to have thinner and more complete segments, I put several portions of tubules of a hardened testis together in a not-too concentrated solution of gum arabic, so that it could easily penetrate inside. Then I let them soak for a few days, protected from the dust, until the gum arabic solution was evaporated to the consistency of a thick syrup. After arranging the portions in order in a paper case with gum arabic, I treated them with alcohol so that I could cut the portions appropriately. In order to observe the lamellae of the sheath proper, I let extremely fresh tubules soak in dilute solution of silver nitrate (1:400). By keeping them soaked in the salt a few minutes longer, the basal surface of [Sertoli cells] becomes colored due to the action of the light. This clearly demonstrates that the basal surface assumes a different shape in different tubule tracts, as I mentioned when I described [Sertoli cells]. Indeed, by examining the surface of a tubule treated with silver salt where second stage [spermatogonia (intermediate and type B)] are present, one can see, under the mosaic formed by the lamellae of the sheath proper, a number of dark or black plates with an irregular star shape, separated by light-colored roundish spaces. They represent the basal surface of [Sertoli cells], which are found here (similar to the ones shown in Figure 6). On the contrary, where second stage [spermatogonia (intermediate and type B)] are not present, namely when [spermatids)] are in their second [(elongating)] stage, the same plates are polygonal in shape and separated by small clear lines.
Footnotes
“Spermatozoa” replaced with “spermatids” throughout manuscript when these cells are still in the seminiferous epithelium.
“Seminiferous cells” replaced with “spermatocytes” throughout manuscript.
“Branched cells” and “epithelial cells” replaced with “Sertoli cells” throughout manuscript.
“Sperm filaments” replaced with “flagellum” or “flagella” throughout manuscript; here, although it appears that Sertoli specifically referred to the flagellum, the remainder of this section outlines the differing views on the production of the gamete (which we call spermatids until after spermiation, when they become testicular sperm).
“Round cells,” “roundish cells,” and “rounded cells” inside the epithelium replaced with “germ cells” throughout the manuscript.
This is the main concept addressed in this report, and it appears to have been the subject of quite a contentious debate between these two groups of scientists. In particular, note the language used throughout that Sertoli uses to passionately challenge the views and work of his contemporary, Victor von Ebner (1842–1925), an Austrian professor of histology and anatomy.
Here, Sertoli is alluding to the stages of the seminiferous epithelium, which were not codified until almost 100 years later [Perey, Clermont, and Leblond Amer J Anat (1961)].
Studies on the structure of the seminiferous canals, ecc. From the Institute for Physiol. U. Histol. In Graz, brsgb. By A. Rollet, 2 Hft.
Contents of a cell including the cytoplasm, nucleus, and organelles.
The “sheath” refers to the layer(s) of peritubular myoid cells that surround the seminiferous tubules.
Studies on the structure of the seminiferous canals, ecc. From the Institute for Physiol. U. Histol. In Graz, brsgb. By A. Rollet, 2 Hft., page 206.
Contribution to the anatomy and histology of the testis. Works from the Physiol. Institution for Leipzig, Jahrgang 1873.
“Polyhedral” or “polygonal” cells replaced with “peritubular myoid cells” throughout manuscript.
On the existence of special branched cells in the seminiferous tubules of the human testis, Il Morgani. 1865, page 31. – Observations on the structure of seminiferous tubules. First prior communication, Gazzetta Medica Italiana. 1871.
It was shown much later that there is indeed stage-dependency to the presence of lipid droplets in Sertoli cells [Kerr and Krester, J Reprod Fertil (1975)].
Research on the chemical composition of the testes. Gazzetta Medica Veterinaria. Year II. Milan, 1872.
Research on the chemical composition of the testes. Gazzetta Medica Veterinaria. Year II. Milan, 1872, p. 5.
Generali and Sertoli. On the Pseudohermaphroditism in a goat, Arch. Medicina Veterinaria, Milan, 1876.
Generali and Sertoli. On the Pseudohermaphroditism in a goat, Arch. Medicina Veterinaria, Milan, 1876, p. 207.
Studies on the development of spermatozoids. Arch. F. Mikr. 11, p. 292.
Translation: “They are effectively pressed, from the inside, into the protoplasm of the epithelium, so that they are separated from the tunica propria of the tubules by a very thin layer of the latter.” Studies on the development of spermatozoids. Arch. F. Mikr. 11, p. 308.
“Motile cells” will be replaced with “germ cells” throughout the remainder of the manuscript.
First of many strong opinions that Sertoli shares regarding von Ebner's scientific work and theories.
“Germ cells” will be replaced with “spermatogonia” throughout the remainder of the manuscript.
“Nematoblasts” will be replaced with “round spermatids” throughout the remainder of the manuscript; also, Sertoli referred to elongating and condensing spermatids as “spermatozoa.” Here, I am following current convention by calling all postmeiotic germ cells within the epithelium “spermatids,” and specifying their identity as either elongating or condensing. They will be called spermatozoa once they leave the epithelium at spermiation.
It was first described by Sertoli in man, where it is greatly developed. The knotty anastomosing cells, which, according to him, form the outermost cell of the seminal canals, are clearly what I call Keimentz.
Loose translation: “Granulated clumps.”
Perhaps the first mention of mammalian spermatogonia.
In this section, Sertoli describes the two types of cytoplasmic processes in spermatogonia: intercellular bridges (“large and short”), and multiple (4-5) thin extensions that appear to be distinct from bridges.
Another reference to intercellular bridges, which first form in type A spermatogonia.
These would likely be intercellular bridges.
Translation: youthful states of cells with smooth nucleus or in a backward transformation.
Translation: The distribution of the two cells within the seminiferous tubules does not reveal any rule. Eingeweidelehre, p. 356.
Translation: Basal cells in several rows, among which several are frequently found in reproductive states—that is with two nucleoli in one nucleus, two nuclei in one cell, and two nuclei in the nucleus. Finally, in the most inner part, are small cells with one nucleus, and in a small number larger cells with 2-5 and even 10 and 20 bright nuclei of 5-8 μm. Hand. Der Gewebelehre, p. 526.
Arch. f. Mikr. Anat 1 Bd.
Translation: Very often, such nuclei are exposed or embedded in a protoplasmic gravel of fine grained texture.
L.c.
These are likely preleptotene, leptotene, and zygotene spermatocytes.
This section mentions a structure that is the blood–testis barrier, although at that point in time there was no additional information regarding their structure or function.
Sertoli is describing the end of meiosis, which results in the formation of two secondary spermatocytes that will then each divide to form n=4 round spermatids.
This is a term that was used at the time to describe coagulated particles near the tubular lumina.
These may represent residual bodies, which is discarded spermatid cytoplasm left behind in the seminiferous epithelium following spermiation that is phagocytozed by Sertoli cells.
Prescient prediction!
Archiv f. Mikrosk. Anat. 3 Bd.
On the structure of seminiferous tubules, etc. Second prior communication. – Gazzetta medica italiana, Lombardia 51. 1875.
“Corpuscle” will be replaced by “chromatoid body” throughout the remainder of the manuscript. The chromatoid body is a male germ cell-specific cytoplasmic ribonucleoprotein structure present in spermatocytes and spermatids that may function in posttranscriptional gene regulation [an excellent review: Yokota, Acta Histochem Cytochem 41(4) (2008)]. Its discovery was credited to von Brunn in 1876, although the detailed account provided here by Sertoli clearly reveals he was characterizing it at the same time.
Arch. f. Mikr. Anat 3 Bd.
Unters. Aus dem Anat. Institut zu Rostock Hrsgb. Von Prof Merkel. 1874.
Arch. f. Mikr. Anat. 12 Bd.
Replaced “thread” with “developing flagellum,” and “sperm filament” with “flagellum” throughout the remainder of the manuscript.
This may be the first observation that flagella form in the earliest round spermatids, and then grows in subsequent spermatids.
About the ovum of mammals and man. 1863, p. 99.
About the ovum of mammals and man. 1863, p. 99.
Sertoli describes a structure he calls the “aculueus” which means a short pointed process (Latin for ‘small needle’), but I could not find this term used in this manner elsewhere in the literature of that era. This is apparently the acrosome, and therefore, will replace “aculeus” with “acrosome” throughout the remainder of the manuscript.
Not aware that spermatids use their flagella to move within the seminiferous epithelium.
Translation: the first developmental stages of the following generation of spermatids are already underway at a time when the previous one still sits in the Sertoli cells.
Not aware of evidence of motion exhibited by the flagella of developing spermatids.
Sertoli might be describing the cytoplasmic droplet, a small bit of residual cytoplasm that often resides on the flagellum after spermiation, and can be maintained in the epididymis, vas deferens, and after ejaculation.
There was no designation on the figure or mention in the legend for elongating spermatids, although they were incorporated into the figure title.
There was no designation on the figure or mention in the legend for condensing spermatids, although they were incorporated into the figure title.
It is not possible to indicate precisely how long each of these tracts is, because it is hard to determine exactly when they start and end. However, I was able to establish the length of the tubule tracts between two points where the [spermatids]] are oriented parallel to the direction of the tubule, that is, in the diagram, the length of 1 plus 2, 3 plus 4, etc. This length varies greatly: based on the 10 measurements I made, their average length is 37 mm, the minimum length is 27, and the maximum 52 mm.
“In the majority of cases, only the debris of these structures and the individual lobes appear to be isolated, like cells with small nuclei.”
On the development, the human body and the human body. The animals. Inaug. Diss., Konigsberg, 1873.
On the development, the human body and the human body. The animals. Inaug. Diss., Konigsberg, 1873.
The spermatogenesis in the amphibian. Arch. F. Mikr. Anat Vol. XII.
Grant Support
This project was funded by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (2R15HD072552 and 1R01HD090083 to CBG).