ABSTRACT
Estrogenic signals have been suggested to be important for the tumorigenesis and progression of endometrial cancer (EC) cells. Our present data showed that estrogen related receptor alpha (ERRα), while not ERRβ or ERRγ, was significantly elevated in EC cells and tissues when compared to their controls. Targeted inhibition of ERRα by siRNA or its inverse agonist XCT-790 can suppress the migration and invasion of EC cells. Both si-ERRα and XCT-790 decreased the expression of transforming growth factor-beta (TGF-β). ERRα can directly bind with the promoter of TGFB1 and then increase its transcription. Further, ERRα was involved in the positive self-feedback loop of TGF-β in EC cells. Targeted inhibition of ERRα/TGF-β can synergistically suppress the in vitro invasion of EC cells. Collectively, our data suggested that ERRα can trigger the cell migration and invasion via increasing the positive self-feedback regulation of TGF-β.
KEYWORDS: ERRα, endometrial cancer, TGF-β, migration, invasion
1. Introduction
As one of the most common gynecologic cancer, the incidence of endometrial cancer (EC) has increased worldwide [1]. There is 60,050 women diagnosed with EC, and 10,470 women died from this disease in 2016 [2]. Numerous factors such as exogenous hormone, obesity, late menopause can induce the turmorigenesis and progression of EC [3]. During the past decade, the primary treatment for EC is surgery followed by adjuvant radiation therapy and chemotherapy. However, metastasis is one of the major reasons responsible for treatment failure. It has been reported that 28% patients being diagnosed have regional or distant metastasis [4]. The five-year survival rate for EC patients with metastasis is only about 15–20% [5]. Therefor understanding the mechanisms for EC metastasis will be great helpful for the drug development and clinical treatment of EC patients.
As one of the most important hormone-responsive tumors, the metastasis of EC is frequently correlated with activity of estrogen signals and expression of estrogen receptors (ERα and ERβ) [6]. Targeted inhibition of ER has suggested a possible role in the development of novel therapy approach for EC treatment [7]. Estrogen receptor (ER)-related receptors (ERRs; ERRα, ERRβ, and ERRγ), which belong to the NR3B group of nuclear receptors, have high homologous DNA-binding domains with ERα [8]. Accumulating evidences indicate that orphan nuclear receptor ERR can regulate cell growth and tumorigenesis in various cancers [9]. For example, increased ERRα levels are correlated with a higher risk of recurrence and poor prognosis in breast cancer [10]. ERRα is one of the negative prognostic factors in human prostate cancer [11]. Recently, ERRα has been detected in human EC tissues and cell lines [12]. The upregulation of ERRα might be correlated with the tumor growth and advancement in uterine endometrial cancers [13]. Furthermore, expression of ERRα mRNA is positively correlated with FIGO stage (determined by the International Federation of Gynecology and Obstetrics with increasing numbers indicating the neoplastic progression, P = 0.019) and myometrial invasion (P = 0.043) of endometrial adenocarcinoma [14]. All these results suggest that ERRα is involve in the progression and metastasis of EC, while how ERRα contribute to the malignant state of EC remains unclear.
In order to elucidate the function and related mechanisms of ERRα in endometrial cancer, we checked the expression of ERRα in both cells and EC tissues in the present study. By performing gain and loss of function experiments, we demonstrated the promotion effects of ERRα on migration and invasion of EC cells via upregulation of TGF-β.
2. Materials and methods
2.1. Cells culture, transfection, and treatment
Human endometrial cell line endometrial stromal cell (ESC) and human endometrial cancer cell lines HEC1A, HEC1B, ECC1, AN3CA, KLE, and RL95-2 were purchased from the American Type Culture Collection (ATCC, Manassas, VA) and maintained with Dulbecco modified Eagle medium (DMEM)/F12 (HyClone, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS, Gibco, Carlsbad, CA, USA) at 37°C with 5% CO2. For cell transfection, siRNA for ERRα and siRNA negative control (siNC) obtained from Ambion (Austin, TX) were transfected with Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. The final working concentration of siRNA was 20 nM. To confirm the roles of TGF-β in ERRα regulated cell motility, recombinant TGF-β (10 ng/ml) or anti-TGF-β neutralization antibody (50 ng/ml) was added in to the culture medium of EC cells.
2.2. Human tissue collection
Total 32 paired of tumor tissues and adjacent normal endometrial samples (including 9 carcinoma tissues with lymphoma metastasis) were collected in our hospital during July 2012 to October 2016 according to the approval of Medical Ethical Committee of our hospital. All endometrial cancer samples were collected from patients did not receive any therapy before surgery. Informed consent was obtained from all patients. Total RNA in tissues were extracted by use of Trizol, then the mRNA expression of ERRα was measured by qRT-PCR. The protein expression of ERRα was measured by western blot analysis.
2.3. qRT-PCR
Total RNA was extracted from tissues or cells by use of Trizol Reagent (Invitrogen, CA, USA) according to the the manufacturer's instructions. Then, 1 μg of total RNA was converted to cDNA by use of the PrimeScript RT-PCR Kit (Takara Bio, Shiga, Japan). Samples for real-time PCR analysis were prepared using the Light Cycler 480 DNA SYBR Green I Master (Roche Diagnostic, Mannheim, Germany) in Light Cycler 480 system II (Roche Diagnostic, Mannheim, Germany) with the following primers: ERRα forward primer, 5′- CGA GAG GAG TAT GTT CTA CT-3′; and reverse primer, 5′- TGC AGA GCT TCT CGC AGC T-3′; ERRβ forward primer, 5′- CCG AGA GCT TGT GGT CAT CA-3′; and reverse primer, 5′- ACA CCA GCT TGT CGT CAT AG-3′; ERRγ forward primer, 5′- TAA TGC TAT CCT GCA GCT GG-3′; and reverse primer, 5′- CTG CAG CGC TTC ATG TAA GA-3′; IL-3 forward primer, 5′- CGG AGA ATC TGA CCT GCT GGA T-3′; and reverse primer, 5′- GAC ACT CGT ACT GTT GAC GCC T-3′; IL-4 forward primer, 5′- CCG TAA CAG ACA TCT TTG CTG CC-3′; and reverse primer, 5′- GAG TGT CCT TCT CAT GGT GGC T-3′; IL-6 forward primer, 5′- AGA CAG CCA CTC ACC TCT TCA G-3′; and reverse primer, 5′- TTC TGC CAG TGC CTC TTT GCT G-3′; IL-8 forward primer, 5′- GAG AGT GAT TGA GAG TGG ACC AC-3′; and reverse primer, 5′- CAC AAC CCT CTG CAC CCA GTT T-3′; IL-10 forward primer, 5′- TCT CCG AGA TGC CTT CAG CAG A-3′; and reverse primer, 5′- TCA GAC AAG GCT TGG CAA CCC A-3′; BMP1 forward primer, 5′- CCA ATG GCT ACT CTG CTC ACA TG-3′; and reverse primer, 5′- AAG CCA TCT CGG ACC TCC ACA T-3′; TGFB1 forward primer, 5′- TAC CTG AAC CCG TGT TGC TCT C-3′; and reverse primer, 5′- GTT GCT GAG GTA TCG CCA GGA A-3′; TNF-α forward primer, 5′- CTC TTC TGC CTG CTG CAC TTT G -3′; and reverse primer, 5′- ATG GGC TAC AGG CTT GTC ACT C -3′; VEGFA forward primer, 5′- TTG CCT TGC TGC TCT ACC TCC A -3′; and reverse primer, 5′- GAT GGC AGT AGC TGC GCT GAT A -3′; CSF forward primer, 5′- TGA GAC ACC TCT CCA GTT GCT G -3′; and reverse primer, 5′- GCA ATC AGG CTT GGT CAC CAC A -3′; GAPDH forward primer, 5′- ATG GTG AAG GTC GGT GTG AAC-3′; and reverse primer, 5′- TGT AGT TGA GGT CAA TGA AGG-3′. The level of gene expression in each sample was normalized to the respective GAPDH expression level.
2.4. Western blot analysis
Cells were lysed by use of the M-Per Mammalian Protein Extraction Reagent (Pierce Biotechnology, Woburn, MA). Total proteins (40 μg) were separated by 10% SDS-PAGE and transferred to PVDF membranes (Millipore, Billerica, MA, USA). The membrane was blocked by PBS containing 5% non-fat dry milk and incubated with primary monoclonal antibodies, against ERRα, ERRβ, ERRγ, MMP-2, MMP-9, TGF-β, or GAPDH at a dilution of 1:2000, at 4°C for overnight. After washed with PBS three times, membranes were further incubated with peroxidase-conjugated secondary antibodies provided by Cell Signaling Technology (Danvers, MA, USA). The immunoblot was visualized by use of ECL western blot detection reagent (Amersham, Piscataway, NJ, USA) and analyzed by ImageQuant LAS 4000 System (GE Healthcare, Little Chalfont, UK). GAPDH was blotted to show equal protein loading.
2.5. Wound healing and cell invasion assay
The effects of ERRα on the motility of EC cells were evaluated by use of wound healing and cell invasion assay according to the previous study [15]. For wound healing assay, cells were plated at 6-well plates with 90% confluence. After transfection or treatment, cells were wounded with a 1-mm-wide pipette tip and then incubated with serum-free medium for the indicated times. Distance migrated was quantitated by taking pictures at the indicated times.
The transwell plates (8 μm-pore size, 24-well plate, Corning Costar, Cambridge, MA) coated with Matrigel (BD Biosciences, Franklin Lakes, NJ) were used to measure the in vitro cell invasion ability. After transfection, 2 × 104 cells were seeded in the upper compartment of the transwell without FBS. The lower chamber was filled with culture media containing 10% FBS. At the end of the experiment, cells invaded to the lower surface of the filter were fixed with 10% formalin, stained with 0.1% crystal violet, and counted under a light microscope. Tests were repeated via three independent experiments.
2.6. Enzyme-linked immunosorbent assays (ELISA) for TGF-β
The effects of ERRα on the expression of TGF-β in medium of EC cells were evaluated by use of a TGF-β ELISA kit (Invitrogen, Basel, Switzerland). Briefly, supernatants of 2 × 106 cells treated with or without XCT-790, a potent and selective inverse agonist ligand of ERRα, for 24 h were collected and concentrated using an Amicon Ultra centrifugal filter (3K) (Millipore, Temecula, CA). Then levels of TGF-β in medium were measured by use of the kit according to the manufacturer's instructions.
2.7. Promoter activity of TGFB1
The effects of XCT-790 on the transcription activity of TGFB1 promoter were evaluated by use of a luciferase reporter assay on the basis of the previous study [16]. Briefly, the −436/+55 bp flanking the transcription start site of the human TGFB1promoter was cloned to generate the pTGF-β-luciferase plasmid. Then cells were transfected with pTGF-β-luciferase (250 ng) and the pSV-β-galactosidase vector (25 ng) (Promega, USA) and further treated with or without XCT-790. The luciferase activity (Promega, USA) and β-galactosidase activity (Applied Biosystems, Foster City, CA, USA) were analyzed by use of commercial kits according to the instructions.
2.8. Chromatin immunoprecipitation (ChIP) PCR
After treated with or without XCT-790, cells were crosslinked by use of formaldehyde to crosslink proteins and their interacting DNA. Then cells were collected, lysed, and sonicated to shear DNA into fragments of 500–1000 bp in length. The antibody against ERRα and human IgG were used for immunoprecipitation. The promoter of TGFB1 was amplified by use of the primer as follow: forward 5′-TCC TTG GCG TAG TAG TC-3′, and reverse 5′-CAA GAC TAT CGA CAT GGA G-3′.
2.9. Statistical analysis
All data were analyzed by use of the SPSS statistical software (Abbott Laboratories, USA). The data were presented as mean ± standard error. The comparison between two groups was analyzed by t-test (two-sided). In all samples, P < 0.05 was considered to be statistically significant.
3. Results
3.1. The expression of ERRα/β/γ in EC cells
Firstly, we used qRT-PCR to evaluate the mRNA expression of ERRα/β/γ in EC cells and compared the expression with that in human endometrial stromal cell (ESC). Our data showed that the expression of ERRα was significantly increased in human EC cells as compared to that in human ESC cells. The expression of ERRγ was also increased in some EC cell lines such as ECC1 and AN3CA as compared to that in ESC cells (Figure 1A). We then evaluated the protein expression of ERRα/β/γ in EC cells. In order to evaluate the relative expression of ERR family, we firstly evaluate the expression of GAPDH in equal amount of protein by western blot analysis. Then the adjusted amount of protein was loaded to obtain the comparable levels of GAPDH in each cell samples. Our data showed that the expression of ERRα, while not ERRβ or ERRγ, was significantly increased in EC cells as compared with that in human ESC cells (Figure 1B). These data showed that expression of ERRα was significantly increased in human EC cells.
Figure 1.
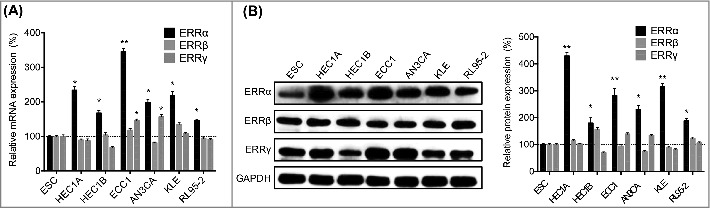
The expression of ERRα/β/γ in EC cells. (A) The mRNA expression of ERRα/β/γ in human EC and ESC cells were checked by qRT-PCR; (B) The protein expression of ERRα/β/γ in human EC and ESC cells were checked by western blotting (left) and quantitatively analyzed (right). Each experiment has been replicated for three times. *p < 0.05, **p < 0.01 as compared with the control group.
3.2. The expression of ERRα was up regulated in human EC tissues
We then checked the expression of ERRα in 32 human EC tissues paired with the adjacent non-neoplastic normal tissues (9 carcinoma tissues with lymphoma metastasis) (Table 1). Our data showed that the expression of ERRα in EC tissues was significantly greater than that in their matched adjacent non-neoplastic normal tissues (Figure 2A). In addition, the expression of ERRα was consistently increased even more in EC tissues with lymph node metastases (8/9, 88.9%, Figure 2B). The expression of ERRα in EC patients with lymph node metastases was significantly greater than that in patients without lymph node metastases (p < 0.05, Table 1). Collectively, clinical data revealed that expression of ERRα was increased in EC tissues and might be involved in cancer metastasis.
Table 1.
The detailed pathological features of clinical EC tissues (n = 32).
| Characteristics | N | Expression of ERRα | p value | |
|---|---|---|---|---|
| Tumor/Adjacent | Tumor | 32 | 0.94 ± 0.54 | <0.01 |
| Adjacent | 32 | 2.97 ± 1.41 | ||
| Age | ≤50 | 11 | 3.27 ± 1.01 | 0.802 |
| >50 | 21 | 3.02 ± 1.37 | ||
| FIGO stage | I∼II | 25 | 2.94 ± 0.95 | 0.168 |
| III∼IV | 7 | 3.11 ± 1.27 | ||
| Grade | 1∼2 | 19 | 2.83 ± 1.31 | 0.132 |
| 3 | 13 | 3.09 ± 1.54 | ||
| Lymph node metastasis | No | 23 | 2.67 ± 1.28 | 0.045 |
| Yes | 9 | 3.34 ± 1.23 | ||
Figure 2.
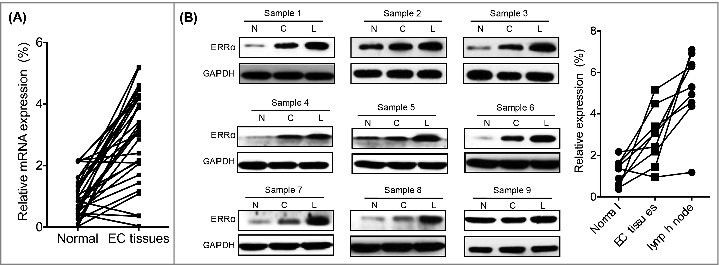
The expression of ERRα was increased in human EC tissues with lymph node metastases. (A) The mRNA expression of ERRα in 32 human EC tissues paired with the adjacent non-neoplastic normal tissues was measured by qRT-PCR and normalized to GAPDH; (B) The expression of ERRα in human EC tissues was detected by western blotting (left) and quantitatively analyzed (right). The expression of ERRα in cancer tissue (C) was higher than that in adjacent normal tissue (N). The higher expression of ERRα was observed in EC patients with lymph metastasis (L) in 88.9% (8/9) EC patients.
3.3. Targeted inhibition of ERRα suppressed migration and invasion of EC cells
We then evaluated the effects of ERRα on cell motility of EC cells. Firstly, we knocked down the expression of ERRα in both HEC1A and ECC cells by use of si-RNA (Figure 3A). Our data confirmed that si-NC had no effect on protein expression (Figure S1). The si-ERRα-1 was used for next study due to the better efficiency. Our data showed that si-ERRα-1 can decrease the expression of MMP-2 and MMP-9 in both HEC1A and ECC cells (Figure 3B). Wound healing assay showed that si-ERRα-1 can impair the wound healing of both HEC1A and ECC cells (Figure 3C). This was confirmed by the transwell analysis that knockdown of ERRα significantly inhibited the invasion of both HEC1A and ECC cells (Figure 3D), while si-NC had no effect on cell migration (Figure S2). In addition, cell viability assay showed that si-ERRα-1 had no significant effect on the proliferation of HEC1A or ECC1 cells (Figure S3). We further targeted inhibition of ERRα by its specific inverse agonist XCT-790. The results showed that XCT-790 can also inhibit the expression of MMP-2 and MMP-9 in HEC1A and ECC cells (Figure 3E) and suppress their invasion (Figure 3F), while vehicle control had no effect on protein expression (Figure S4). These results showed that targeted inhibition of ERRα can suppress the migration and invasion of EC cells.
Figure 3.
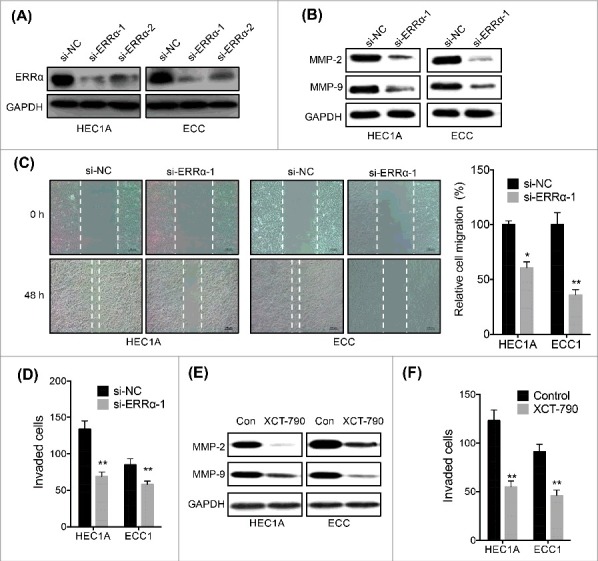
Targeted inhibition of ERRα suppressed migration and invasion of EC cells. (A) HEC1A and ECC cells were transfected with si-NC or si-ERRα for 24 h; (B) After transfected with si-NC or si-ERRα for 48 h, the expression of MMP-2/-9 in HEC1A and ECC cells were checked by western blotting; (C) The wound healing assay for HEC1A and ECC cells transfected with si-NC or si-ERRα for 48 h; (D) The in vitro invasion assay of HEC1A and ECC cells transfected with si-NC or si-ERRα for 48 h; (E) HEC1A and ECC cells were treated with or without XCT-790 (1 µM) for 48 h, the expression of MMP-2/-9 in HEC1A and ECC cells were checked by western blotting; (F) The in vitro invasion assay of HEC1A and ECC cells treated with or without XCT-790 (1 µM) for 48 h. Each experiment has been replicated for three times. * p<0.05, **p<0.01 as compared with the control group.
3.4. ERRα regulated the motility of EC cells via modulating TGFB1
We then investigated the mechanisms responsible for ERRα regulated motility of EC cells. We tested the mRNA expression of major cytokines in both si-ERRα and XCT-790 treated HEC1A cells. The IL-3, IL-4, IL-6, IL-8, IL-10, BMP1, TGFB1, TNF, VEGFA and CSF were measured due to their positive role in cell motility [17] and the progression of endometrial cancer [18, 19]. Our data showed that both si-ERRα (Figure 4A) and XCT-790 (Figure 4B) can significantly decrease the expression of TGFB1 in HEC1A cells. In addition, si-ERRα and XCT-790 also decreased TGFB1 in ECC cells (Figure 4C). ELISA confirmed that XCT-790 can decrease the protein secretion of TGF-β in both HEC1A and ECC cells (Figure 4D). To investigate whether TGF-β is involved in ERRα regulated the motility of cells, we treated HEC1A cells with XCT-790, TGF-β, or together. The in vitro invasion assay showed that TGF-β can attenuate the XCT-790 suppressed invasion of HEC1A (Figure 4E) and ECC (Figure 4F) cells. These data confirmed that ERRα regulated the motility of EC cells via modulating TGFB1.
Figure 4.
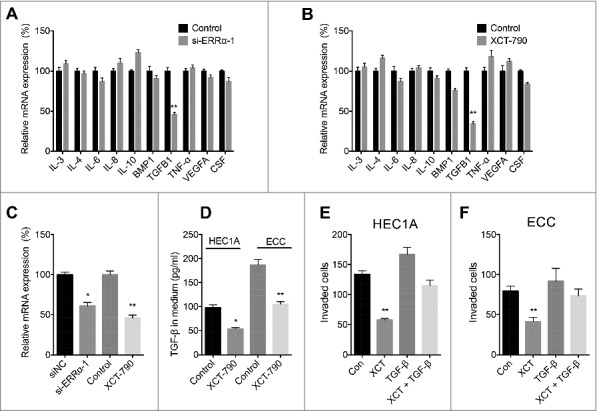
ERRα regulated the motility of EC cells via modulating TGFB1. HEC1A transfected with si-NC or si-ERRα (A) or treated with or without XCT-790 (1 µM) (B) for 24 h, then the mRNA expression of cytokines was measured by use of qRT-PCR; (C) ECC cells were transfected with si-NC or si-ERRα or treated with or without XCT-790 (1 µM) for 24 h, the mRNA of TGFB1 was measured by use of qRT-PCR; (D) HEC1A or ECC cells were treated with or without XCT-790 (1 µM) for 24 h, the expression of TGF-β in medium was measured by use of ELISA; HEC1A (E) or ECC (F) cells were treated with XCT-790 (1 µM), TGF-β (10 ng/ml) or together for 48 h, the in vitro invasion of cells was measured by tranwell assay. Each experiment has been replicated for three times. * p<0.05, **p<0.01 as compared with the control group.
3.5. ERRα regulated the transcription of TGFB1via directly binding to its promoter
We then investigated the mechanisms responsible for ERRα regulated expression of TGF-β. By use of XCT-790, we observed that the transcription of TGFB1 was rapidly decreased in HEC1A since the treatment of 1 h (Figure 5A). Similarly, XCT-790 also decreased the mRNA expression of TGFB1 in ECC cells after 1 h (Figure 5B). We then evaluated the promoter activity of TGFB1 in cells treated with or without XCT-790. The dual luciferase assay indicated that XCT-790 can suppress the promoter activity of TGFB1 in both HEC1A and ECC cells (Figure 5C). We then investigated whether ERRα can directly bind to the promoter of TGFB1 by ChIP assay. Our data showed that ERRα can bind with the promoter of TGFB1, while XCT-790 can decrease the binding between ERRα and promoter of TGFB1 in HEC1A cells (Figure 5D). Collectively, these data confirmed that ERRα regulated the transcription of TGFB1via directly binding to its promoter.
Figure 5.
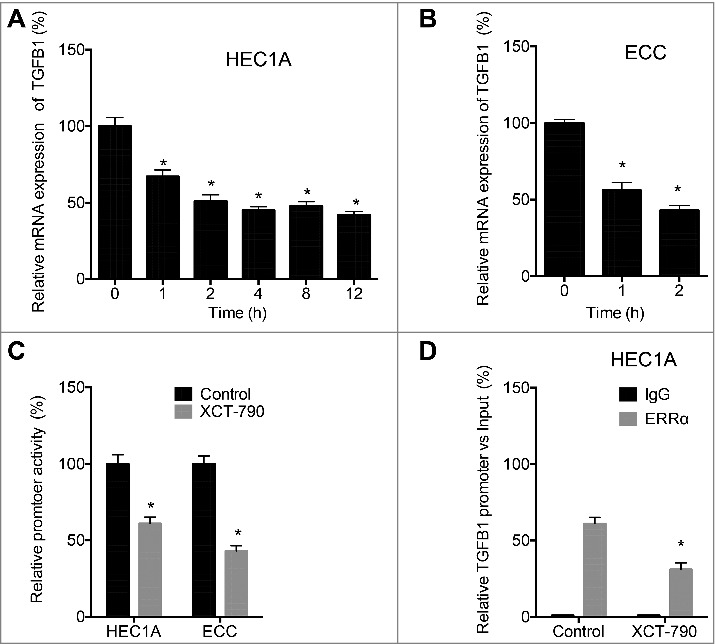
ERRα regulated the transcription of TGFB1 via directly binding to its promoter. HEC1A (A) or ECC (B) cells were treated with XCT-790 (1 µM) for the indicated times, the mRNA expression of TGFB1 was checked by qRT-PCR; (C) Cells were treated with or without 1 µM XCT-790, the luciferase activities of TGFB1 promoter were measured by use of the dual-luciferase assay; (D) HEC1A cells were treated with or without XCT-790 (1 µM) for 2 h, the binding between ERRα and promoter of TGFB1 was measured by ChIP assay. Each experiment has been replicated for three times. **p < 0.01 as compared with the control group.
3.6. ERRα was involved in the self-feedback loop of TGF-β in EC cells
Previous studies indicated that TGF-β can positively regulate its own expression in normal and transformed cells. Our data confirmed that exogenous TGF-β increased the mRNA (Figure 6A) and protein (Figure 6B) levels of TGF-β in both HEC1A and ECC cells. Intriguingly, TGF-β also increased the mRNA (Figure 6C) and protein (Figure 6B) expression of ERRα in both HEC1A and ECC cells. Considering that ERRα can regulate the transcription of TGF-β, we investigated the expression of TGF-β in HEC1A cells transfected with si-NC or si-ERRα. The results showed that si-ERRα can abolish TGF-β self-feedback loop in HEC1A cells (Figure 6D). We then checked the mRNA expression of TGFB1 in 32 human EC tissues paired with the adjacent non-neoplastic normal tissues. Our data showed that the expression of TGFB1 in EC tissues was significantly greater than that in the adjacent non-neoplastic normal tissues (Figure 6E). These data indicated that ERRα was involved in the self-feedback loop of TGF-β in EC cells.
Figure 6.
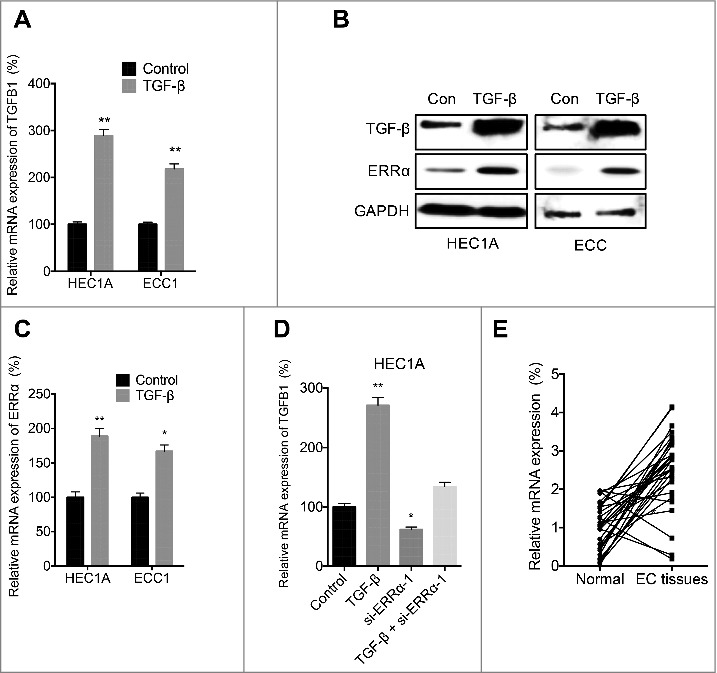
ERRα was involved in the self-feedback loop of TGF-β in EC cells. HEC1A or ECC cells were treated with or without 10 ng/ml TGF-β for 24 h, the mRNA of TGFB1 (A) and ERRα (C) was checked by qRT-PCR; the protein expression of TGF-β and ERRα was checked by western blotting (B); (D) After transfected with siNC or si- ERRα-1 for 12 h, the HEC1A cells were further treated with or without 10 ng/ml TGF-β for 24 h, the mRNA of TGFB1 was checked by qRT-PCR; (E) The mRNA expression of TGFB1 in 32 human EC tissues paired with the adjacent non-neoplastic normal tissues was measured by qRT-PCR and normalized to GAPDH. Each experiment has been replicated for three times. * p<0.05, **p<0.01 as compared with the control group.
3.7. Targeted inhibition of ERRα/TGF-β suppressed the motility of EC cells
Considering that ERRα can trigger the cell migration via upregulation the expression of TGF-β, we then evaluated the effects of targeted inhibition of ERRα/TGF-β on the motility of EC cells. Our data showed that both si-ERRα and TGF-β neutralization antibody can suppress the invasion of HEC1A cells, furthermore, they had synergistic effects to suppress the invasion of HEC1A cells (Figure 7A). This was confirmed by the results that si-ERRα and TGF-β neutralization antibody can synergistically inhibit the expression of MMP-2 in HEC1A and ECC cells (Figure 7B). Our data suggested that targeted inhibition of ERRα/TGF-β suppressed the motility of EC cells.
Figure 7.
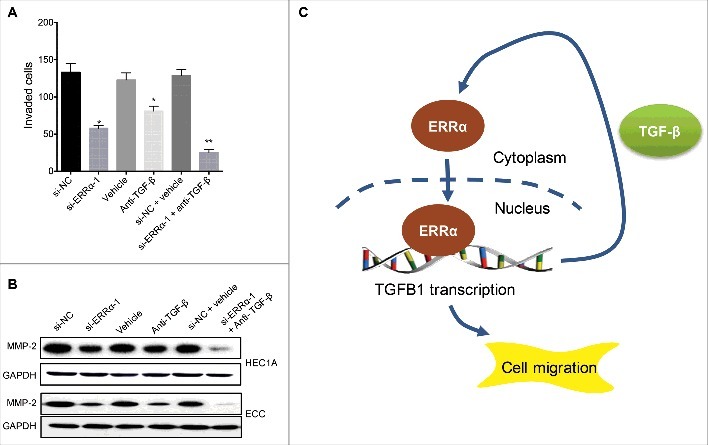
Targeted inhibition of ERRα/TGF-β suppressed the motility of EC cells. (A) HEC1A cells were treated with si-NC or si- ERRα-1 for 12 h before treated with or without anti-TGF-β neutralization antibody for another 48 h, the cell invasion was evaluated with transwell analysis; (B) Cells were treated as (A), the expression of MMP-2 was checked by western blotting (B). Each experiment has been replicated for three times. (C) The schematic of ERRα triggered cell migration and invasion via increasing the positive self-feedback regulation of TGF-β. * p<0.05, **p<0.01 as compared with the control group.
4. Discussion
Metastasis is one of the most important characteristics of EC cells, while the related under mechanisms are not well illustrated. Our present study showed that the expression of ERRα, while not ERRβ or ERRγ, was increased in the EC cells and tissues as compared with the normal controls. The expression of ERRα in EC patients with lymph node metastases was significantly greater than that in patients without lymph node metastases. Mechanically studies indicated that ERRα can bind to the promoter of TGF-β and then trigger the migration and invasion of EC cells. Silencing of ERRα can block the self-feedback loop of TGF-β and then synergistically increase the inhibition effects of anti-TGF-β neutralization antibody on the invasion of EC cells. Collectively, our study suggested that up regulation of ERRα in EC cells can trigger the cell migration and invasion via increasing the positive self-feedback regulation of TGF-β (Figure 7C).
Our present study confirmed that ERRα plays a positive role on tumorigenesis and development of various cancers including EC. It has been speculated that ERRα might be a candidate for prognostic factors in uterine endometrial cancer [13]. The expression of ERRα mRNA was positively correlated with FIGO stage (P = 0.019) and myometrial invasion (P = 0.043) of endometrial adenocarcinoma [14]. Similarly, our data showed that expression of ERRα in EC patients with lymph node metastases was significantly greater than that in patients without lymph node metastases (p<0.05, Table 1). Our data showed that ERRα can trigger the migration and invasion of EC cells. It was reported that knockdown of ERRα can suppress the angiogenesis and proliferation of uterine endometrial cancer cells [20]. The positive roles of ERRα on motility of cancer cells were also observed in colorectal [21] and triple negative breast [22, 23] cancer cells. Together with the published data, our present study suggested that ERRα might be a potential target to suppress the metastasis of EC cells.
Our results showed that ERRα triggers the migration and invasion of EC cells via strengthening the self-feedback loop of TGF-β in EC cells. This was evidenced by the results that ERRα can directly bind with the promoter of TGF-β, and TGF-β can increase the expression of ERRα. Knockdown of ERRα blocked the TGF-β induced its own expression. Recent study revealed that ERRα can mediate the TGF-β induced epithelial-mesenchymal transition (EMT) of osteosarcoma cells via upregulation of Snail [24]. As one of the most important cytokines, TGF-β can promote cancer metastasis by regulating the composition of extracellular matrix and induction of EMT [25]. High levels of TGF-β can promote cancer progression in an autocrine and/or paracrine manner that favours invasion and metastasis of EC cells [26]. TGF-β1 was also able to promote cell migration by up-regulating the activity of MMPs [27], which was observed in our present study that TGF-β mediated induced up regulation of MMP-2/-9. It was reported that TGF-β1 can trigger the cancer progression via activation of SMAD2/3 [28], whether SMAD2/3 mediates TGF-β1 induced ERRα in EC cell still needs further studies.
Taken together, our data showed that ERRα was over expressed in EC cells and tissues. It can positively regulate the migration and invasion of EC cells via up regulation of TGF-β1. Targeted inhibition of ERRα/ TGF-β can suppress the invasion of EC cells efficiently. Our study suggested that ERRα might be a potential target for EC treatment.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Bell DW. Novel genetic targets in endometrial cancer. Expert Opin Ther Tar. 2014;18:725–30. doi: 10.1517/14728222.2014.909414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- [3].Kurra V, Krajewski KM, Jagannathan J, et al.. Typical and atypical metastatic sites of recurrent endometrial carcinoma. Cancer Imaging. 2013;13:113–22. doi: 10.1102/1470-7330.2013.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].van der Steen MJ, de Waal YR, Westermann A, et al.. An impressive response to pazopanib in a patient with metastatic endometrial carcinoma. Neth J Med. 2016;74:410–3. [PubMed] [Google Scholar]
- [5].Thanapprapasr D, Thanapprapasr K. Molecular Therapy as a Future Strategy in Endometrial Cancer. Asian Pac J Cancer P. 2013;14:3419–23. doi: 10.7314/APJCP.2013.14.6.3419. [DOI] [PubMed] [Google Scholar]
- [6].Kent CN, Reed IKG. Regulation of epithelial-mesenchymal transition in endometrial cancer: connecting PI3K, estrogen signaling, and microRNAs. Clin Transl Oncol. 2016;18:1056–61. doi: 10.1007/s12094-016-1492-2. [DOI] [PubMed] [Google Scholar]
- [7].Pessoa JN, Freitas ACL, Guimaraes RA, et al.. Endometrial Assessment: When is it Necessary? J Clin Med Res. 2014;6:21–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Razzaque MA, Masuda N, Maeda Y, et al.. Estrogen receptor-related receptor gamma has an exceptionally broad specificity of DNA sequence recognition. Gene. 2004;340:275–82. doi: 10.1016/j.gene.2004.07.010. [DOI] [PubMed] [Google Scholar]
- [9].Giguere V. Transcriptional Control of Energy Homeostasis by the Estrogen-Related Receptors. Endocr Rev. 2008;29:677–96. doi: 10.1210/er.2008-0017. [DOI] [PubMed] [Google Scholar]
- [10].Suzuki T, Miki Y, Moriya T, et al.. Estrogen-related receptor alpha in human breast carcinoma as a potent prognostic factor. Cancer Res. 2004;64:4670–6. doi: 10.1158/0008-5472.CAN-04-0250. [DOI] [PubMed] [Google Scholar]
- [11].Fujimura T, Inoue S, Urano T, et al.. Increased Expression of Tripartite Motif (TRIM) 47 Is a Negative Prognostic Predictor in Human Prostate Cancer. Clin Genitourin Canc. 2016;14:298–303. doi: 10.1016/j.clgc.2016.01.011. [DOI] [PubMed] [Google Scholar]
- [12].Watanabe A, Kinoshita Y, Hosokawa K, et al.. Function of estrogen-related receptor alpha in human endometrial cancer. J Clin Endocrinol Metab. 2006;91:1573–7. doi: 10.1210/jc.2005-1990. [DOI] [PubMed] [Google Scholar]
- [13].Fujimoto J, Sato E. Clinical implication of estrogen-related receptor (ERR) expression in uterine endometrial cancers. J Steroid Biochem Mol Biol. 2009;116:71–5. doi: 10.1016/j.jsbmb.2009.04.012. [DOI] [PubMed] [Google Scholar]
- [14].Gao M, Sun P, Wang J, et al.. Expression of estrogen receptor-related receptor isoforms and clinical significance in endometrial adenocarcinoma. Int J Gynecol Cancer. 2006;16:827–33. doi: 10.1111/j.1525-1438.2006.00527.x. [DOI] [PubMed] [Google Scholar]
- [15].Seo E, Lim JS, Jun JB, et al.. Exendin-4 in combination with adipose-derived stem cells promotes angiogenesis and improves diabetic wound healing. J Transl Med. 2017;15:35. doi: 10.1186/s12967-017-1145-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fichtner-Feigl S, Strober W, Kawakami K, et al.. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat Med. 2006;12:99–106. doi: 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]
- [17].Setrerrahmane S, Xu H. Tumor-related interleukins: old validated targets for new anti-cancer drug development. Mol Cancer. 2017;16:153. doi: 10.1186/s12943-017-0721-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chopra V, Dinh TV, Hannigan EV. Serum levels of interleukins, growth factors and angiogenin in patients with endometrial cancer. J Cancer Res Clin Oncol. 1997;123:167–72. [DOI] [PubMed] [Google Scholar]
- [19].Daley-Brown D, Oprea-Ilies GM, Lee R, et al.. Molecular cues on obesity signals, tumor markers and endometrial cancer. Horm Mol Biol Clin Investig. 2015;21:89–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Matsushima H, Mori T, Ito F, et al.. Anti-tumor effect of estrogen-related receptor alpha knockdown on uterine endometrial cancer. Oncotarget. 2016;7:34131–48. doi: 10.18632/oncotarget.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ding SJ, Tang ZH, Jiang YJ, et al.. IL-8 Is Involved in Estrogen-Related Receptor alpha-Regulated Proliferation and Migration of Colorectal Cancer Cells. Digest Dis Sci. 2017;62:3438–46. doi: 10.1007/s10620-017-4779-4. [DOI] [PubMed] [Google Scholar]
- [22].Wu YM, Chen ZJ, Liu H, et al.. Inhibition of ERRalpha suppresses epithelial mesenchymal transition of triple negative breast cancer cells by directly targeting fibronectin. Oncotarget. 2015;6:25588–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Carnesecchi J, Forcet C, Zhang L, et al.. ERRalpha induces H3K9 demethylation by LSD1 to promote cell invasion. Proc Natl Acad Sci U S A. 2017;114:3909–14. doi: 10.1073/pnas.1614664114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chen Y, Zhang K, Li Y, et al.. Estrogen-related receptor alpha participates transforming growth factor-beta (TGF-beta) induced epithelial-mesenchymal transition of osteosarcoma cells. Cell Adh Migr. 2017;11:338–46. doi: 10.1080/19336918.2016.1221567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Katsuno Y, Lamouille S, Derynck R. TGF-beta signaling and epithelial-mesenchymal transition in cancer progression. Curr Opin Oncol. 2013;25:76–84. doi: 10.1097/CCO.0b013e32835b6371. [DOI] [PubMed] [Google Scholar]
- [26].Horiguchi M, Ota M, Rifkin DB. Matrix control of transforming growth factor-beta function. J Biochem. 2012;152:321–9. doi: 10.1093/jb/mvs089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Matsuzaki K, Seki T, Okazaki K. TGF-beta signal shifting between tumor suppression and fibro-carcinogenesis in human chronic liver diseases. Journal of Gastroenterology. 2014;49:971–81. doi: 10.1007/s00535-013-0910-2. [DOI] [PubMed] [Google Scholar]
- [28].Pathil A, Mueller J, Ludwig JM, et al.. Ursodeoxycholyl lysophosphatidylethanolamide attenuates hepatofibrogenesis by impairment of TGF-beta1/Smad2/3 signalling. Br J Pharmacol. 2014;171:5113–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


