ABSTRACT
Although techniques for isolating and culturing adult cardiomyocytes were developed four decades ago, it still remains a challenge to isolate high yields of healthy viable cardiomyocytes, to maintain them in culture, and to use them successfully in experiments. This is due to the difficulty in deciding which adhesive substrate and buffer composition to use. Therefore this study aimed to (1) identify a robust experimental buffer to sustain survival of cultured adult rat cardiomyocytes (ARCMs) during control normoxic conditions, and (2) to identify an adhesive substrate that provides optimal cell adherence, not only during normoxia, but especially during simulated ischemia-reperfusion (SIR) experiments.
Adhesion and viability of ARCMs were evaluated on laminin, laminin-entactin (LE), and extracellular matrix (ECM) at concentrations between 25–200 ug/ml, in three different normoxic experimental buffers and under SIR conditions. Differences in normoxic buffer composition had no effect on the adherence of ARCMs, but had a significant effect on mitochondrial function and thus cell viability. HEPES buffered PBS supplemented with 10 mM glucose was not sufficient to sustain cell viability unless 2 mM pyruvate was added, yet a cocktail of PBS and M199 provided an even greater viability.
Finally, laminin, LE, and ECM retained similar numbers of ARCMs per concentration, but only provided efficient adhesion at concentrations ≥ 100 ug/ml.
KEYWORDS: Adhesive substrate, cell adhesion, cell survival, mitochondrial membrane potential difference, simulated ischemia reperfusion, contracture, hypercontracture
Introduction
Adult cardiomyocytes (ACMs) have been isolated and cultured since the 1970s1 and still hold a promising future for cardiovascular research. Although the use of isolated ACMs has gained popularity in cardiophysiology research, it is still a challenging task to isolate high numbers of healthy viable cardiomyocytes and retain them in culture, even for a period of 24 hours. Obstacles in culture are presented in deciding which medium and cell adhesive substrates to use in order to promote efficient cell attachment, survival and retention of morphology. Medium 199 (M199) is the preferred medium for culturing ACMs and is commonly supplemented with creatine, carnitine, and taurine.2,3 However, the investigator is free to add other agents such as HEPES,4 pyruvate, insulin,2,4 and the myosin II ATPase inhibitor blebbistatin5 or N-benzyl-p-toluene sulphonamide.6 Thus, an efficient culture medium is mostly obtained through trial and error, which was also the case in our laboratory.
We optimised a medium that sustained adult rat cardiomyocyte (ARCM) viability during overnight culture on 10 ug/ml laminin in 96 well plates, but during SIR experimentation the cells consistently washed off the culture surfaces, leaving no cells attached for evaluation. This showed that the 10 ug/ml laminin mainly used in the literature might be too low, or that laminin is not an efficient ACM adhesive substrate, and therefore other adhesives should be investigated. A suitable substrate for cell attachment is critical to avoid unwanted changes in cell morphology and function. Various types of adhesive substrates are commercially available, including gelatins, dis-intergrins, poly-lysine, vitronectins and extracellular matrix (ECM) components (collagens, fibronectins, proteoglycans, entactins and laminins).
Studies on attachment and survival of ACMs on various ECM substrates (laminin, collagens, fibronectin, ECM gel) have previously been carried out.7–14 Data from these studies showed that ACMs attach efficiently to basement membrane components of laminin and collagen type 4, weakly to fibronectin and do not attach to interstitial collagens (Type 1 & 3).8 Since then, laminin has been the most preferred adhesive substrate to culture ACMs15–19 and is used at low concentration ranges (10–35 μg/ml). All studies that evaluated adhesive substrates for ACMs investigated its role in culture, but no studies evaluated optimal adhesion during experimentation, and no studies have reported severe cell detachment during SIR.
The primary aim of the present study was to improve the numbers of ARCMs retained to 96-well culture surfaces, after applying a SIR protocol with several necessary cell washes, so that enough cells are available for evaluation. The secondary aim was to identify an experimental buffer that would sustain ARCM viability under control normoxic conditions. This was addressed by comparing the efficiency with which ARCMs adhere to different concentrations of laminin, ECM and LE, after overnight culture and SIR.
Materials and Methods
All reagents were obtained from Sigma-Aldrich, unless specified as otherwise.
Animal Care
Adult male Wistar rats were used in this study. The animals were kept on a 12 hour day/night cycle at a constant temperature of 22 ˚С and 40% humidity. All animals had free access to food (standard lab chow) and water. This study conformed to the conditions described in the “Revised South African National Standard for the care and use of Animals for Scientific purposes” (South African Bureau of Standards, SANS 10386, 2008). Animal use was approved by the Ethical Committee of the Faculty of Health Sciences, Stellenbosch University. Project number 10GL_LOP1 was assigned to the study.
Isolation of ARCMs
The cardiomyocyte isolation technique was based on a protocol published by Fischer and colleagues in 199120. Briefly, adult male Wistar rats (∼300 g) were anesthetized by intra-peritoneal injection of 60 mg sodium pentobarbital (Bayer). Hearts were excised and arrested in ice cold (4 °C) buffer A (6 mM KCl; 1 mM Na2HPO4, 0.2 mM NaH2PO4, 1.4 mM MgSO4, 128 mM NaCl, 10 mM HEPES, 11 mM D-glucose, 2 mM pyruvate and 3 mIU insulin (Humulin R by Lilly), 1% penicillin/streptomycin, pH 7.4) that contained 0.5 mM CaCl2. All buffers thereafter were warmed to 37 °C and gassed with 95% O2 and 5% CO2. The arrested hearts were attached to a Langendorff apparatus and retrogradely perfused with calcium free buffer A to wash out blood from the coronary arteries. After 5 minutes, digestion was started by perfusion with 50 ml buffer B (buffer A containing 0.5% BSA fatty acid free (Roche), 1.8 mg/ml collagenase Type II (240 U/mg, Worthington), 0.2 mg/ml protease XIV and 18.0 mM butanedione monoxim (BDM)). The first 10 ml of the digestion buffer was discarded, while the rest was recirculated and perfusion continued for ∼ 25 minutes until the heart was soft and soapy. 0.1 mM CaCl2 was added at 10 minutes and 20 minutes of the digestion period.
When heart digestion was complete, the ventricles were cut off and placed in a petri dish with 15 ml buffer D (2 parts buffer C (1 × buffer A containing 0.5% BSA fatty acid free, 0.5% BSA fraction V, 9.0 mM BDM) and 1 part buffer B) containing 0.3 mM CaCl2. Ventricular cardiomyocytes were separated by gently moving the tissue back and forth in the buffer with a tweezers until it was completely dissociated. The cell suspension was then filtered through a 200 μm nylon filter into a 50 ml conical tube. The cells were allowed to sediment by gravity for 10 minutes at room temperature and then spun at 60 × g for 1 minute. The supernatant containing dead cells, other cells and debris was removed. Calcium was re-introduced to the cells in a stepwise manner to a final concentration of 1.2 mM. The pellet was resuspended in buffer E1 (buffer C with 0.6 mM CaCl2), followed by buffer E2 (buffer C with 0.9 mM CaCl2) and lastly buffer E3 (buffer C with 1.2 mM CaCl2), with each step interspersed by cell sedimentation (7 minutes, 6 minutes and 5 minutes respectively). After calcium re-introduction, the final pellet of calcium tolerant cardiomyocytes was resuspended in a modified M199 culture buffer (40% Hanks’ salt M199 supplemented with 10 mM HEPES, 5 mM creatine, 5 mM carnitine, 5 mM taurine mixed with 60% buffer C (with 1.2 mM CaCl2, but without BDM)) with 10 μM blebbistatin.
Counting of ARCMs on a haemocytometer and seeding in culture plates
The cardiomyocytes were counted on a haemocytometer and viability determined by rod shape morphology. Before counting the cells, the ARCMs were thoroughly mixed by inversion to form a homogeneous cell mixture, but not shaken to avoid froth. A cell aliquot was loaded onto a haemocytometer until the chamber was filled, but not overfilled, to avoid overestimation of cell numbers. Normal healthy ACMs are rod-shaped and therefore they do not disperse as easily in liquid as round or spindle shaped cells. Consequently ACMs do sometimes distribute unevenly on a haemocytometer, and therefore it was imperative to confirm equal distribution of ACMs across the haemocytometer before counting the cells. Whenever cells were unevenly distributed, the haemocytometer was cleaned and a new cell aliquot loaded for counting. The number of viable rod shaped cells were counted in each of the four quadrants (each containing 16 squares) of the haemocytometer, summed together and divided by four to get the average viable cells. The same was done for the dead round cells to get the average number of dead cells. Each quadrant holds a volume of 0.1 ul and therefore the cell numbers per quadrant were multiplied by 10000 to provide cell numbers per ml. The percentage cell viability was determined by dividing the average number of live cells by the total number of cells (live plus dead), and multiplied by 100. Only when cell viability was ≥ 70% were the cardiomyocytes seeded into black clear bottom 96-well plates (Corning) that were pre-coated with cell adhesives, followed by overnight culture at 5% CO2 and 37 ˚С. Given that more cells were to be lost with SIR than normoxia, seeding density was 2500 cells/100 µl/well for normoxic experimental conditions and 5000 cells/100 µl/well for SIR conditions.
Coating of culture plates with LE, laminin and ECM
Black, clear bottom 96-well tissue culture plates (Corning) were coated with either LE (BD Biosciences), laminin, or ECM gel at a concentration range of 25–200 µg/ml. Each adhesive substrate was diluted in PBS (6 mM KCl, 1 mM Na2HPO4, 0.2 mM, NaH2PO4.H20, 1.4 mM MgSO4.7H20, 128 mM NaCl, 1.2 mM Ca2+, 10 mM HEPES, pH 7.4) to obtain the required final concentrations (25, 50, 75, 100, 125, 150, 175 and 200 μg/ml), and 5 µl was placed in the middle of each well. The coated plates were incubated overnight at 5% CO2 and 37 ˚С, washed twice with PBS buffer and air dried in a laminar flow hood prior to seeding of the cardiomyocytes.
Effect of different experimental buffers on ARCM attachment and mitochondrial viability when adhered to laminin, LE and ECM
After overnight culture, the cardiomyocytes were washed and stained with one of three experimental buffers, (1) PBS with 11 mM D-glucose (PBS-G), (2) PBS with 11 mM D-glucose and 2 mM pyruvate (PBS-GP), or (3) modified M199 wash buffer (MMWB) (40% Hanks’ salt M199 supplemented with 10 mM HEPES, 5 mM creatine, 5 mM carnitine, 5 mM taurine mixed with 60% buffer A (with 1.2 mM CaCl2, without insulin). All the buffers used were warmed to 37 ˚C, all incubations were done at 37 ˚C in a 5% CO2 incubator, and the number of washes or buffer changes was equal between normoxic control and the SIR protocol used. Briefly, the cultured cardiomyocytes were washed three times with 100 µl/well buffer so that each wash was followed by a 10 minute incubation period. Thereafter two consecutive washes were applied without incubation, followed by 10 minutes staining with 10 µg/ml JC-1 (5, 5¢, 6, 6¢-tetrachloro-1, 1’, 3’, 3’- tetraethylbenzimidazolocarbocyanine iodide) at 37 ˚C. The stain was removed, the cells washed twice and then incubated for 30 minutes for the stain to reach equilibrium. The number of washes or buffer changes were similar to the SIR protocol, except that the simulated ischemia (60 minutes) and reperfusion (15 minutes) duration was not mimicked. Fluorescence images of cardiomyocytes were captured on a Nikon fluorescent microscope at a 10 x objective, using a filter that allows excitation at 490 nm and emissions of 590 nm (green JC-1 monomers) and 530 nm (red JC-1 aggregates). The average red fluorescence (R) and green fluorescence (G) intensity was measured per cell per image, and used to quantify the average cell viability according to the equation R ÷ G, which is abbreviated as R/G. The R/G fluorescence ratio is high per cell in a viable cell population, but low in a dead cell population. Indeed, viable cells accumulate many red JC-1 aggregates in mitochondria and therefore display a high intensity of red fluorescence, while emitting a poor green fluorescence intensity. In contrast, dead cells accumulate many green JC-1 aggregates in the cytosol and no or few red JC-1 aggregates in the mitochondria.
ARCMs attachment and mitochondrial viability after SIR
After overnight culture, the cells were washed three times as described above, but only with MMWB. Simulated ischemia was induced for 1 hour with modified M199 ischemic buffer (40% Hanks’ salt M199 mixed with 60% PBS (with 1.2 mM CaCl2, without HEPES), pH 6.4, supplemented with 5 mM 2-deoxy-D-glucose and 5 mM sodium dithionite). Thereafter the cells were gently washed twice, followed by 15 minutes incubation (reperfusion). The cells were then stained with 10 µg/ml JC-1 for 10 minutes, washed twice and incubated for 30 minutes before capturing fluorescence images as described above.
Parameters measured and statistical analysis
The cardiomyocyte length was determined from a single line drawn along the length of each cardiomyocyte found in a 100x magnified field view (1244 µm x 933 µm), using ImageJ. The same line was used to measure the average red (R) and green (G) fluorescence intensity per ARCM, which was used to calculated the ratio of R/G as a measure of mtMPD, and thus viability. The total number of cells attached per treatment was also recorded for each field view, as an indication of cardiomyocyte adherence. All values are expressed as the mean ± standard error of the mean (SEM). GraphPad Prism 6 was used to compare results, using either one-way or two-way analysis of variance (ANOVA) with Sidak post hoc test applied. Probability values of less than 0.05 (p < 0.05) were considered significantly different.
Results
Effects of experimental buffers on cardiomyocyte attachment and mitochondrial viability
Based on the literature, PBS supplemented with glucose is normally the buffer used to perform experiments on either freshly isolated or cultured ACMs. Since the buffer used to perform experiments plays a vital role in cell survival, we compared the effects of different buffers, PBS-G (PBS + glucose), PBS-GP (PBS +glucose + pyruvate) and MMWB (Modified M199 wash buffer) on cell attachment and mitochondrial viability. These buffers were evaluated on ARCMs cultured on a range of concentrations (25–200 ug/ml) of laminin, ECM and LE, followed by assessment of mitochondrial viability with JC-1 and cell attachment.
As shown in Fig. 1, mitochondrial viability (R/G) was higher with MMWB compared to PBS-G and PBS-GP on laminin (Fig. 1a), LE (Fig. 1b) and ECM (Fig. 1c). On laminin, mitochondrial viability was significant lower with PBS-G compared to MMWB at 25 ug/ml (0.53 ± 0.14 vs 1.67 ± 0.38, p < 0.01), 50 ug/ml (0.57 ± 0.16 vs 1.58 ± 0.11, p < 0.01), 75 ug/ml (0.37 ± 0.15 vs 1.56 ± 0.08, p < 0.001), 100 ug/ml (0.48 ± 0.20 vs 1.72 ± 0.16, p < 0.001), 125 ug/ml (0.55 ± 0.17 vs 1.68 ± 0.15, p < 0.01), 150 ug/ml (0.47 ± 0.17 vs 1.25 ± 0.17, p < 0.05) and 175 ug/ml (0.41 ± 0.15 vs 1.48 ± 0.28, p < 0.01). Significant differences were also observed between PBS-GP and MMWB, but only at 75 ug/ml (0.77 ± 0.24 vs 1.56 ± 0.08, p < 0.05), 100 ug/ml (0.72 ± 0.26 vs 1.72 ± 0.16, p < 0.01), 125 ug/ml (0.73 ± 0.26 vs 1.68 ± 0.15, p < 0.05) and 175 ug/ml (0.56 ± 0.20 vs 1.48 ± 0.28, p < 0.05). Fluorescent images of the ARCMs can be viewed in the supplementary document.
Figure 1.
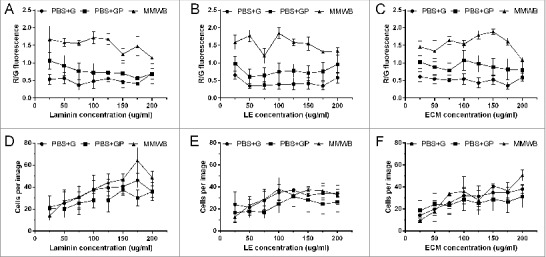
Effect of different experimental buffers (PBS-G, PBS-GP and MMWB) on the total number of cells attached per field view, as well as mitochondrial viability, under normoxic conditions. Mitochondrial membrane potential expressed as R/G fluorescence of ARCMs on (A) Laminin, (B) LE, and (C) ECM in. Total numbers of cells attached to laminin (D), LE (E) and ECM (F). Data are expressed as mean ± SEM (N = 6).
On ECM, similar trends were observed where R/G fluorescence was significantly higher; up to double or triple the R/G fluorescence for cells washed with MMWB compared to PBS-G at 25–175 ug/ml (25 ug/ml: 1.46 ± 0.17 vs 0.61 ± 0.15, p < 0.01, 175 ug/ml: 1.60 ± 0.10 vs 0.35 ± 0.10, p < 0.0001). Between MMWB and PBS-GP, mitochondrial viability of cells in MMWB was approximately double that of cells in PBS-GP at 75 ug/ml (1.64 ± 0.11 vs 0.78 ± 0.22, p < 0.05) and 100–150 ug/ml (125 ug/ml: 1.79 ± 0.12 vs 0.98 ± 0.24, p < 0.05). On LE, mitochondrial viability was also higher with MMWB compared to PBS-G at 25–175 ug/ml (25 ug/ml: 1.59 ± 0.21 vs 0.65 ± 0.12, p < 0.01 and at 175 ug/ml: 1.31 ± 0.03 vs 0.35 ± 0.11, p < 0.01), and for MMWB compared to PBS-GP at 75 ug/ml (1.20 ± 0.22 vs 0.64 ± 0.22), and 125–200 ug/ml (125 ug/ml: 1.58 ± 0.09 vs 0.78 ± 0.19, p < 0.05).
Interestingly, there were no significant differences in the numbers of cardiomyocytes attached to laminin (Fig. 1d), LE (Fig. 1e) and ECM (Fig. 1f) per field view, when washed with PBS-G, PBS-GP and MMWB.
ARCMs attachment at different concentration in normoxia versus SIR
In primary cultures of adult cardiomyocytes, the type of adhesive substrates used and their concentrations, are essential factors for efficient cell attachment, retention of morphology and promotion of cell survival. Laminin is commonly used in the literature at a concentration of 10 ug/ml, but at that concentration ARCMs washed off the plastic and glass culture surfaces used, during SIR in our laboratory. This made it impossible to accurately assess cell damage since very few cells remained attached for evaluation. Therefore laminin, ECM and LE were tested at concentrations of 25 ug/ml to 200 ug/ml under normoxia and SIR, for cardiomyocyte adhesion (Fig. 2) and cell injury (Fig. 3 & 4).
Figure 2.
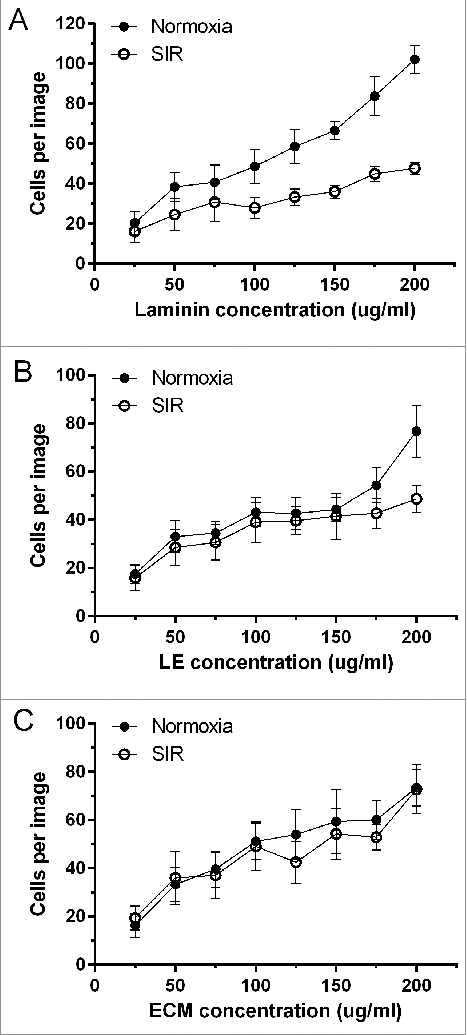
Total number of adherent ARCMs per field view at different concentrations of laminin, LE and ECM, during normoxia and after SIR. Data are expressed as mean ± SEM (N = 8).
Figure 3.
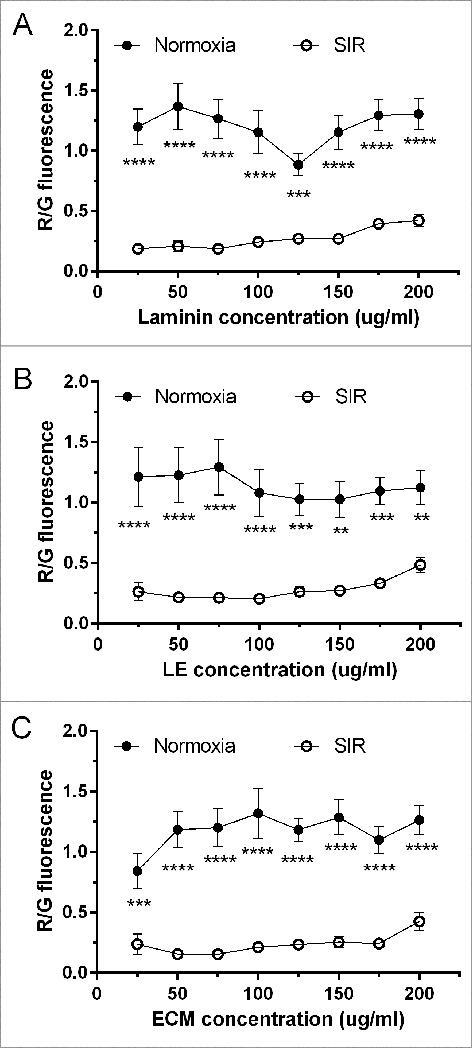
R/G fluorescence as an indicator of mitochondrial viability of ARCMs on laminin, LE and ECM, under normoxia versus SIR. Data are expressed as mean ± SEM (N = 8).
Figure 4.
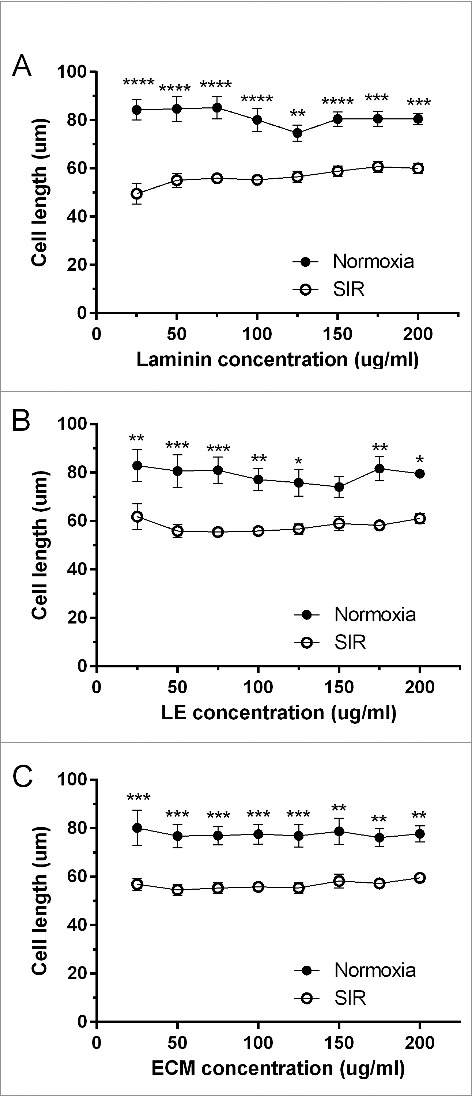
Average length in microns (um) of cardiomyocytes cultured on laminin, ECM and LE at 25–200ug/ml, under normoxia and after SIR. Data are expressed as mean ± SEM (N = 8).
As shown in Fig. 2, under normoxia, cell numbers attached per field view increased in a concentration dependent manner for all adhesives, with the lowest cell numbers at 25 ug/ml (laminin: 20 ± 6, LE: 17 ± 4, ECM: 16 ± 5), and the highest at 200 ug/ml (laminin: 102 ± 7, LE: 77 ± 11, ECM: 74 ± 8). There were no differences amongst the adhesives between 25–150 ug/ml when compared per concentration. However more cells adhered to laminin (84 ± 10) than to LE (54 ± 7, p < 0.05) at 175 ug/ml, and at 200 ug/ml to laminin (102 ±7) compared to ECM (74 ± 8, p < 0.05). Comparison of concentrations within the same adhesive showed there were no differences between lower concentrations of laminin (25–100 ug/ml), LE (25–150 ug/ml) and ECM (25–75 ug/ml), while cell adherence increased significantly at higher concentrations. Indeed, at 25 ug/ml laminin, total cells were significantly less (20 ± 6) than at 125 ug/ml (58 ± 9, p < 0.05), 150 ug/ml (67 ± 4, p < 0.001), 175 ug/ml (84 ± 10, p < 0.0001) and 200 ug/ml (102 ±7, p < 0.0001). When laminin concentration increased to 50 ug/ml (38 ± 7), 75 ug/ml (41 ± 9) and 100 ug/ml (49 ± 8), significant differences were only observed against 175 ug/ml (p < 0.01, and p < 0.05 versus 100 ug/ml) and 200 ug/ml (p < 0.0001). Differences became even smaller with higher laminin concentrations, where 200 ug/ml only showed a difference against 125 ug/ml (p < 0.01) and 150 ug/ml (p < 0.05). On LE, cells cultured at 25 ug/ml (18 ± 4) were significantly lower compared to cells on 175 ug/ml (54 ± 7, p < 0.05) and 200 ug/ml (77 ± 11, p < 0.0001). In contrast cells on LE at 50 ug/ml (33 ± 7, p < 0.01), 75 ug/ml (35 ± 5, p < 0.01), 100 ug/ml (43 ± 5, p< 0.05) and 125 ug/ml (43 ± 7, p < 0.05) were lower than 200 ug/ml (77 ± 11). When ECM was used, total cells from 100–200 ug/ml (51 ± 7 to 74 ± 8) were significantly higher than 25 ug/ml (16 ± 5), while at 50 ug/ml (33 ± 7) and 75 ug/ml (40 ± 8) significant differences were only observed against 200 ug/ml (74 ± 8).
When cardiomyocytes were subjected to SIR (Fig. 2), ECM retained significantly higher numbers of cells (73 ± 10, p < 0.05) compared to laminin (48 ± 3) at 200 ug/ml, but for all other concentrations all adhesives retained similar cell numbers. Differences were observed between high and low concentrations within the same adhesives only for LE and ECM, but not for laminin. On LE, significant differences were observed only between 25 ug/ml (16 ± 5) and 200 ug/ml (49 ± 6, p < 0.05), while on ECM significant differences were observed for 25 ug/ml (20 ± 5) versus 150 ug/ml (55 ± 11, p < 0.05), 175 ug/ml (53 ± 5, p < 0.05) and 200 ug/ml (73 ± 10, p < 0.0001). At 50 ug/ml (36 ± 11, p < 0.05) and 75 ug/ml (37 ± 10, p < 0.05) significant differences were only observed when these concentrations were compared with 200 ug/ml (73 ± 10).
The total number of cells were also compared for the same adhesive under normoxia and SIR conditions (Fig. 2). Significant differences were observed at higher concentrations from 125 ug/ml to 200 ug/ml and not at lower concentrations (25–100 ug/ml). As shown in Fig. 2a, on laminin, total cell numbers were significantly reduced with SIR compared to normoxia at 125 ug/ml (p < 0.05, 33 ± 4 vs. 59 ± 9), 150 ug/ml (p < 0.01, 36 ± 3 vs. 67 ± 4), 175 ug/ml (p < 0.001, 45 ± 4 vs. 84 ± 10) and 200 ug/ml (p < 0.0001, 48 ± 3 vs. 102 ± 7). When LE was used as an adhesive substrate (Fig. 2b), total cell numbers attached at 200 ug/ml were significantly reduced from normoxia (77 ± 11) to SIR (49 ± 6). There were no differences between the numbers of cells attached on ECM between concentrations under during SIR, or between conditions of normoxia and SIR for a given concentration (Fig. 2c).
mtMPD as an indicator of cell viability in cardiomyocytes subjected to normoxia and SIR
Adult cardiomyocytes were assessed for viability using JC-1 (Fig. 3), a dye that emits a green fluorescence in the cytosol and red fluorescence in the mitochondria of healthy cells, as dye is taken up by mitochondria due to the presence of a mtMPD. Therefore a loss in mtMPD will be reported by JC-1 as a loss in red fluorescence and a gain in green, and serves as an early indicator of apoptosis. Under normoxia, there was no significant difference observed in R/G ratio between laminin, LE or ECM at any of the concentrations used.
When cells were subjected to SIR, significant differences were only observed between low concentrations of laminin (25–75 ug/ml) and high concentrations (175–200 ug/ml). At 25 ug/ml laminin, R/G was significant less (0.19 ± 0.03) compared to 175 ug/ml (0.40 ± 0.04, p < 0.05) and 200 ug/ml (0.42 ± 0.05, p < 0.01). When the concentration was increased to 50–75 ug/ml, R/G was significantly lower at 50 ug/ml (0.21 ± 0.04) and 75 ug/ml (0.19 ± 0.03) compared to 175 ug/ml (0.40 ± 0.04, p < 0.05) and 200 ug/ml (0.42 ± 0.05, p < 0.01) respectively.
On L/E, cell viability was significantly lower at 25 ug/ml (0.26 ± 0.07, p < 0.01), 50 ug/ml (0.22 ± 0.03, p < 0.001), 75 ug/ml (0.21 ± 0.03, p < 0.001), 100 ug/ml (0.20 ± 0.03, p < 0.001), 125 ug/ml (0.26 ± 0.04, p < 0.01) and 150 ug/ml (0.27 ± 0.04, p < 0.05) compared to 200 ug/ml (0.49 ± 0.06). Similarly, R/G was significantly lower on ECM at 50 ug/ml (0.16 ± 0.02, p < 0.001), 75 ug/ml (0.15 ± 0.02, p < 0.001), 100 ug/ml (0.21 ± 0.03, p < 0.05) and 125 ug/ml (0.23± 0.04, p < 0.05) compared to 200 ug/ml (0.43 ± 0.08). When cell viability was compared per adhesive in normoxia and SIR, R/G fluorescence was significantly higher in normoxic conditions compared to SIR for all adhesives.
Cell length as indicator of cardiomyocyte contracture
Cell length is an important indicator of ACM injury, reporting on whether the cells are normal, contracted or hypercontracted. This is especially essential in studies of SIR where contracture/hypercontracture is indicative of a stiff heart wall that results in poor heart function. Fig. 4 shows the average cell length of cardiomyocytes on laminin, LE and ECM at 25–200 ug/ml under normoxia and after SIR. There was no significant difference between any concentrations of laminin, ECM and LE under normoxia), or with SIR. When cell length was compared within the same adhesive under normoxia and SIR (Fig. 4a-c), contracture was consistently induced with SIR, indicated by the significant reduction in cell length.
Discussion
This study aimed to establish conditions for optimal adherence of ARCMs to plastic 96 well microplates under conditions of normoxia and SIR. Adhesion and viability of ARCMs were evaluated on laminin, LE and ECM respectively, at concentrations that ranged between 25–200 ug/ml, and with increments of 25 ug/ml.
Effect of experimental buffer and adhesive substrate on ARCM adhesion
Adhesive substrates and buffer composition are considered essential for adherence and survival of cardiomyocytes in culture.3,4 Therefore comparisons between laminin, LE and ECM were made in three different experimental buffers to identify a robust buffer for normoxic conditions (Fig 1), before evaluation under SIR conditions. HEPES buffered PBS supplemented with metabolic substances are commonly used as control experimental buffers for cardiomyocytes.21–23 Hence the evaluated buffers consisted of a modified PBS supplemented with 10 mM HEPES, 1.2 mM Ca2+ and either: (1) 11 mM glucose (PBS-G), (2) 11 mM glucose and 2 mM pyruvate (PBS-GP), or (3) modified M199 (MMWB). The MMWB buffer contained a final concentration of 8.8 mM glucose, 3.2 mM Pyruvate, 3 mM creatine, 3 mM carnitine and 3 mM taurine, in addition to the amino acids and vitamins that are part of the M199 media composition.
All three experimental buffers resulted in similar numbers of adherent ARCMs for all adhesive substrates, per concentration tested (Fig. 1 d-f). The adherent cell numbers increased as adhesive substrate concentration increased, regardless of the type of adhesive substrate used. Thus adhesion of ARCMs was independent of buffer composition, but dependent on the concentration of adhesive substrate. In contrast, buffer composition had a significant effect on mitochondrial function and thus cell viability, while substrate concentration had no effect (Fig. 1 a-c). Indeed, mitochondrial function of ARCMs was by far superior with MMWB compared to PBS-G and PBS-GP, where PBS-G provided the poorest viability. PBS-G and PBS-GP each contained 11 mM glucose, which is commonly sufficient to sustain ex-vivo heart pump function, yet could not sustain survival of non-beating ARCMs here (Fig. 1 a-c). Only with the addition of 2 mM pyruvate, was it possible for PBS-GP to improve mitochondrial function above PBS-G. This finding suggests that insufficient amounts of pyruvate was possibly produced from glucose by glycolysis in PBS-G, which was compensated for by the 2 mM pyruvate in PBS-GP. It is possible that pyruvate concentrations higher than 2 mM are necessary, given that MMWB was the most robust buffer and contained 3.2 mM pyruvate. Yet, MMWB also contained various other factors such as vitamins, amino acids, creatine, carnitine and taurine, of which any compound/s could have caused the marked improvement in mitochondrial function. It is noteworthy that MMWB contained 8.8 mM glucose, which is less than that in PBS-G and PBS-GP, again indicating that a high glucose concentration was not as important for survival as pyruvate supplementation. The rate of glycolysis depends on the workload of the heart24,25 and thus it is reasonable to expect non-beating cardiomyocytes to have an extremely low glycolytic rate. This could explain why survival of ARCM was low with high glucose, but improved with pyruvate supplementation. MMWB proved to be the most robust experimental buffer and was therefore used for normoxic control conditions during SIR experiments in Figs. 2–4.
Effect of adhesive substrate on ARCM adhesion after SIR
The adhesion of ARCMs to laminin, LE and ECM were compared under SIR conditions to find a suitable adhesive substrate and concentration that would retain high enough cell numbers after SIR for analysis. The results indicate that laminin, ECM and LE retained similar numbers of ARCMs per adhesive substrate concentration, which increased as concentration increased, under normoxic and SIR conditions (Fig. 2). For all the adhesive substrates, concentrations lower than 100 µg/ml retained fewer cells than concentrations above 100 µg/ml. At least 30–40 ARCMs were retained per field view at 100x magnification, on 100 ug/ml of laminin, LE and ECM, which are enough cells for reliable analysis where test conditions are repeated at least in triplicate. These findings show that adhesive concentration is essential for cardiomyocyte adhesion, and that LE and ECM perform as well as laminin.
In contrast, the type of adhesive substrate and concentration had no effect on cell length and mitochondrial function during normoxia or SIR. Indeed, comparison of the adhesive substrates per treatment condition (normoxia or SIR) showed that all three adhesive substrates provided similar mitochondrial viability and cell length regardless of the concentrations used (Fig. 3). Furthermore, SIR injury was successfully induced on each adhesive substrate as seen by the severe reduction in mitochondrial function and cell length. Thus, in spite of SIR injury, sufficiently high numbers of ARCMs still remained attached to the 96 well microplates, when adhesion substrates were 100 ug/ml or above.
Limitations of the study
It is difficult to say which components in the MMWB were responsible for the dramatic improvement in mitochondrial function above that of PBS-G and PBS-GP. Pyruvate concentrations higher than 2 mM should also have been tested in the PBS-GP buffer to determine whether it could provide a similar or greater degree of viability than MMWB. However, this was beyond the scope of this study, since the aim was not to investigate which individual components are essential for ARCM survival during experimentation, but rather to find a robust experimental buffer for use as control in SIR experiments.
Conclusion
This is the first time that low (< 100 ug/ml) laminin concentrations are shown to provide insufficient adhesion to ARCMs during SIR conditions in 96 well microplates, yet laminin is routinely used in the literature at 5–20 ug/ml. Only high concentrations (≥ 100 ug/ml) of laminin, LE and ECM, respectively retained sufficient numbers of adherent ARCMs after SIR. LE and ECM performed as well as laminin with regards to adherence and survival of ARCMs, and can thus serve as cheaper alternatives. Furthermore, HEPES buffered PBS supplemented with 11 mM glucose is not sufficient to sustain cell viability of ARCMs during normoxia. Instead pyruvate in excess of 2 mM must be present in normoxic experimental buffer, although even greater viability can be attained by using a cocktail of PBS and M199.
Supplementary Material
References
- 1.Jacobson SL. Culture of Spontaneously Contracting Myocardial Cells from Adult Rats. Cell Struct Funct. 1977;2:1–9. doi: 10.1247/csf.2.1. [DOI] [Google Scholar]
- 2.Berger H-J, Prasad SK, Davidoff AJ, Pimental D, Ellingsen O, Marsh JD, Smith TW, Kelly RA. Continual Electric Field Stimulation Preserves Contractile Function of Adult Ventricular Myocytes in Primary Culture. Am J Physiol. 1994;266:H341–9. PMID:8304516. [DOI] [PubMed] [Google Scholar]
- 3.Volz A, Piper HM, Siegmund B, Schwartz P. Longetivity of adult ventricular rat heart muscle cells in serum-free primary culture. J Mol Cell Cardiol. 1991;23:161–73. doi: 10.1016/0022-2828(91)90103-S. PMID:2067025. [DOI] [PubMed] [Google Scholar]
- 4.Ellingsen O, Davidoff AJ, Prasad SK, Berger HJ, Springhorn JP, Marsh JD, Kelly RA, Smith TW. Adult rat ventricular myocytes cultured in defined medium: phenotype and electromechanical function. Am J Physiol – Heart Circ Physiol. 1993;265:H747–54. PMID:8368376. [DOI] [PubMed] [Google Scholar]
- 5.Kabaeva Z, Zhao M, Michele DE. Blebbistatin extends culture life of adult mouse cardiac myocytes and allows efficient and stable transgene expression. AJP Heart Circ Physiol. 2008;294:H1667–74. doi: 10.1152/ajpheart.01144.2007. PMID:18296569. [DOI] [PubMed] [Google Scholar]
- 6.Abi-Gerges N, Pointon A, Pullen GF, Morton MJ, Oldman KL, Armstrong D, Valentin JP, Pollard CE. Preservation of cardiomyocytes from the adult heart. J Mol Cell Cardiol. 2013;64:108–19. doi: 10.1016/j.yjmcc.2013.09.004. PMID:24051370. [DOI] [PubMed] [Google Scholar]
- 7.Bird SD, Doevendans PA, Van Rooijen MA Brutel De La Riviere A, Hassink RJ, Passier R, Mummery CL. The human adult cardiomyocyte phenotype. Cardiovasc Res. 2003;58:423–34. doi: 10.1016/S0008-6363(03)00253-0. PMID:12757876. [DOI] [PubMed] [Google Scholar]
- 8.Borg TK, Rubin K, Lundgren E, Borg K, Öbrink B. Recognition of extracellular matrix components by neonatal and adult cardiac myocytes. Dev Biol. 1984;104:86–96. doi: 10.1016/0012-1606(84)90038-1. PMID:6734942. [DOI] [PubMed] [Google Scholar]
- 9.Cooper G, Mercer WE, Hoober JK, Gordon PR, Kent RL, Lauva IK, Marino TA. Load regulation of the properties of adult feline cardiocytes. The role of substrate adhesion. Circ Res. 1986;58:692–705. doi: 10.1161/01.RES.58.5.692. PMID:3708766. [DOI] [PubMed] [Google Scholar]
- 10.Lundgren E, Borg T, Mårdh S. Isolation, characterization and adhesion of calcium-tolerant myocytes from the adult rat heart. J Mol Cell Cardiol. 1984;16:355–62. doi: 10.1016/S0022-2828(84)80606-9. PMID:6726824. [DOI] [PubMed] [Google Scholar]
- 11.Lundgren E, Terracio L, Mårdh S, Borg TK. Extracellular matrix components influence the survival of adult cardiac myocytes in vitro. Exp Cell Res1985 158:371–81. doi: 10.1016/0014-4827(85)90462-8. PMID:4007060. [DOI] [PubMed] [Google Scholar]
- 12.Lundgren E, Gullberg D, Rubin K, Borg TK, Terracio MJ, Terracio L. In vitro studies on adult cardiac myocytes: Attachment and biosynthesis of collagen type IV and laminin. J Cell Physiol. 1988;136:43–53. doi: 10.1002/jcp.1041360106. PMID:3294238. [DOI] [PubMed] [Google Scholar]
- 13.Lundgren E, Terracio L, Borg TK. Adhesion of cardiac myocytes to extracellular matrix components. Basic Res Cardiol. 1985;80 Suppl 1:69–74. doi: 10.1007/978-3-662-11041-6_13. PMID:3994641. [DOI] [PubMed] [Google Scholar]
- 14.Rubin K, Borg TK, Holmdahl R, Klareskog L, Obrink B. Interactions of mammalian cells with collagen. In Basement membrane and Cell Movement.Ciba Found Symp. 1984;108:93–116. doi: 10.1002/9780470720899.ch7. PMID:6569833. [DOI] [PubMed] [Google Scholar]
- 15.Banyasz T, Lozinskiy I, Payne CE, Edelmann S, Norton B, Chen B, Chen-Izu Y, Izu LT, Balke CW. Transformation of adult rat cardiac myocytes in primary culture. Exp Physiol. 2008;93:370–82. doi: 10.1113/expphysiol.2007.040659. PMID:18156167. [DOI] [PubMed] [Google Scholar]
- 16.Bistola V, Nikolopoulou M, Derventzi A, Kataki A, Sfyras N, Nikou N, Toutouza M, Toutouzas P, Stefanadis C, Konstadoulakis MM. Long-term primary cultures of human adult atrial cardiac myocytes: Cell viability, structural properties and BNP secretion in vitro. Int J Cardiol. 2008;131:113–22. doi: 10.1016/j.ijcard.2007.10.058. PMID:18255169. [DOI] [PubMed] [Google Scholar]
- 17.Heidkamp MC, Iyengar R, Szotek EL, Cribbs LL, Samarel AM. Protein kinase Cε-dependent MARCKS phosphorylation in neonatal and adult rat ventricular myocytes. J Mol Cell Cardiol. 2007;42:422–31. doi: 10.1016/j.yjmcc.2006.10.017. PMID:17157309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joshi-Mukherjee R, Dick IE, Liu T, O'Rourke B, Yue DT, Tung L. Structural and functional plasticity in long-term cultures of adult ventricular myocytes. J Mol Cell Cardiol. 2013;65:76–87. doi: 10.1016/j.yjmcc.2013.09.009. PMID:24076394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu X, Colecraft HM. Primary Culture of Adult Rat Heart Myocytes. J Vis Exp. 2009;9–11. doi: 10.3791/1308. PMID:19532115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer Y, Rose H, Kammermeier H. Highly insulin-responsive isolated rat heart muscle cells yielded by a modified isolation method. Life Sci. 1991;49:1679–88. doi: 10.1016/0024-3205(91)90310-8. PMID:1943473. [DOI] [PubMed] [Google Scholar]
- 21.Strijdom H, Genade S, Lochner A. Nitric Oxide Synthase (NOS) does not contribute to simulated ischaemic preconditioning in an isolated rat cardiomyocyte model. Cardiovasc Drugs Ther. 2004;18:99–112. doi: 10.1023/B:CARD.0000029027.50796.84. PMID:15162071. [DOI] [PubMed] [Google Scholar]
- 22.Ruiz-Meana M, Abellan A, Miro-Casas E, Agullo E, Garcia-Dorado D. Role of sarcoplasmic reticulum in mitochondrial permeability transition and cardiomyocyte death during reperfusion. AJP Hear Circ Physiol. 2009;297:H1281–9. doi: 10.1152/ajpheart.00435.2009. PMID:19684187. [DOI] [PubMed] [Google Scholar]
- 23.Abdallah Y, Kasseckert SA, Iraqi W, Said M, Shahzad T, Erdogan A, Neuhof C, Gündüz D, Schlüter K-D, Tillmanns H, et al.. Interplay between Ca2+ cycling and mitochondrial permeability transition pores promotes reperfusion-induced injury of cardiac myocytes. J Cell Mol Med. 2011;15:2478–85. doi: 10.1111/j.1582-4934.2010.01249.x. PMID:21199327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrison GJ, van Wijhe MH, de Groot B, Dijk FJ, Gustafson LA, van Beek JHGM. Glycolytic buffering affects cardiac bioenergetic signaling and contractile reserve similar to creatine kinase. Am J Physiol Heart Circ Physiol. 2003;285:H883–90. doi: 10.1152/ajpheart.00725.2002. PMID:12714331. [DOI] [PubMed] [Google Scholar]
- 25.Depré C, Rider MH, Hue L. Mechanisms of control of heart glycolysis. Eur J Biochem. 1998;258:277–90. doi: 10.1046/j.1432-1327.1998.2580277.x. PMID:9874192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


