ABSTRACT
Neutrophils are highly motile innate immune cells; they actively migrate in response to inflammatory signals. Using two-photon intravital microscopy, we discovered that neutrophils form stable clusters upon phototoxicity at a certain threshold. Without significant damage to the collagen structure of mouse dermis, neutrophils aggregated together with nearby neutrophils. Surprisingly, this in situ neutrophil clustering resulted in rigorous changes of migratory direction. The density of residing neutrophils was also a critical factor affecting clustering. Additionally, we found that the triggering point of neutrophil aggregation was correlated with the structure of the extracellular matrix in the ear dermis, where autofluorescence was strongly observed. This swarming behavior of neutrophils may reflect an unknown communication mechanism of neutrophils during migration under sterile injury.
KEYWORDS: Neutrophil, migration, two-photon microscopy, intravital imaging, phototoxicity
Introduction
Neutrophil aggregation is considered a hallmark of inflammation. Neutrophils are mainly recruited through pathogen-associated molecular pattern (PAMP) and/or damage-associated molecular pattern (DAMP) signaling [1]. DAMP signaling causes sterile inflammation occurred in the absence of any microorganism, which results in the production of pro-inflammatory cytokines and the recruitment of leukocytes similar to microbially induced inflammation through PAMP [2]. Recently, intravital imaging of ear dermis or organs of mice successfully visualized neutrophil aggregation process in sterile damage conditions in a real-time manner [3–5]. Indeed, functional studies of neutrophil migration have been conducted from the view point of chemotactic strategies under inflammation [6,7].
“Neutrophil swarming” can be classified in two ways based on migration direction: (1) neutrophil forward migration and (2) neutrophil reverse migration[8]. Forward migration of neutrophils refers to the general migration pattern of neutrophils, cells moving from the bloodstream to damaged interstitial areas, through transendothelial migration (TEM) [9-13]. DAMPs such as ATP, HMGB1 protein, N-formyl peptides (fMLP), and uric acid act as early cues for neutrophil aggregation [14–17]. After initiation, neutrophil aggregation is amplified, and neutrophil clusters are created along damaged/infected areas. Thus, neutrophil migration is regarded as a consequence of sterile- or infection-oriented inflammatory condition. Although neutrophils are typically known to migrate toward injured sites, neutrophils have recently been shown to move back to the bloodstream during inflammation; this phenomenon has been called reverse migration of neutrophils (rTEM)[8,18].
Mitigation of neutrophil aggregation is critical for the prevention of serious tissue damage or vessel rupture of the damaged site [19,20]. For instance, the failure of various organ is induced by intensive leukocyte accumulation during sepsis and septic shock [21]. Therefore, since the communication mechanism behind neutrophil migration, accumulation, and dissociation is important to maintaining balance of the immune system, better understanding thereof would be helpful in numerous areas of medicine and clinical research. In this study, we performed two-photon intravital imaging of mouse ear dermis to delicately trace neutrophil migration under laser-induced damage. Neutrophil aggregation due to phototoxicity emerged upon surpassing a particular threshold, after which a change in the direction of migrating neutrophils was observed. Beyond this threshold, gathering of neutrophils was observed. However, below this threshold, neutrophils transiently contacted each other without aggregation, and then neutrophils randomly migrated away from the injury site. Interestingly, we found that neutrophils tended to aggregate on collagen fibers or distinct autofluorescent structures of the extracellular matrix, which may be cluster-triggering sites. Although neutrophils are known to actively communicate with other leukocytes by producing various chemokines and cytokines [22–26], details thereon remain elusive. The results from our study on neutrophil swarming may open a new venue by which to elucidate mechanisms related with neutrophil communication.
Results
Two-photon intravital imaging of the mouse ear dermis was performed to detect cell motility of intradermally transferred GFP+ neutrophils. When these neutrophils (5 × 104 cells in 5 μl PBS) were transferred to the ear, the neutrophil-containing PBS volume inflated the mouse ear dermis. However, after about 30 min, the inflated ear deflated back to the initial shape, as illustrated in Figure 1A. GFP+ neutrophils were motile and generally migrated away from the initially located site to all directions (Fig. 1B and Video 1). This motility of extracted neutrophils indicated that neutrophils could become active as a consequence of isolation process from the bone marrow. In addition, in situ injection of neutrophils has caused needle puncture on the ear skin, resulting in the dermis damage, even though care was taken to minimize damage by needle puncture. Thus, both the tissue damage by needle puncture and neutrophil stimulation in the isolation procedure from the bone marrow may induce neutrophil migration in the intradermally transferred ear derma. It may be possible that intradermally transferred neutrophils show different motility compared to the neutrophils those originally existing in wild type mouse, because the density of intradermally transferred neutrophils could be much higher than intact physiological conditions.
Figure 1.
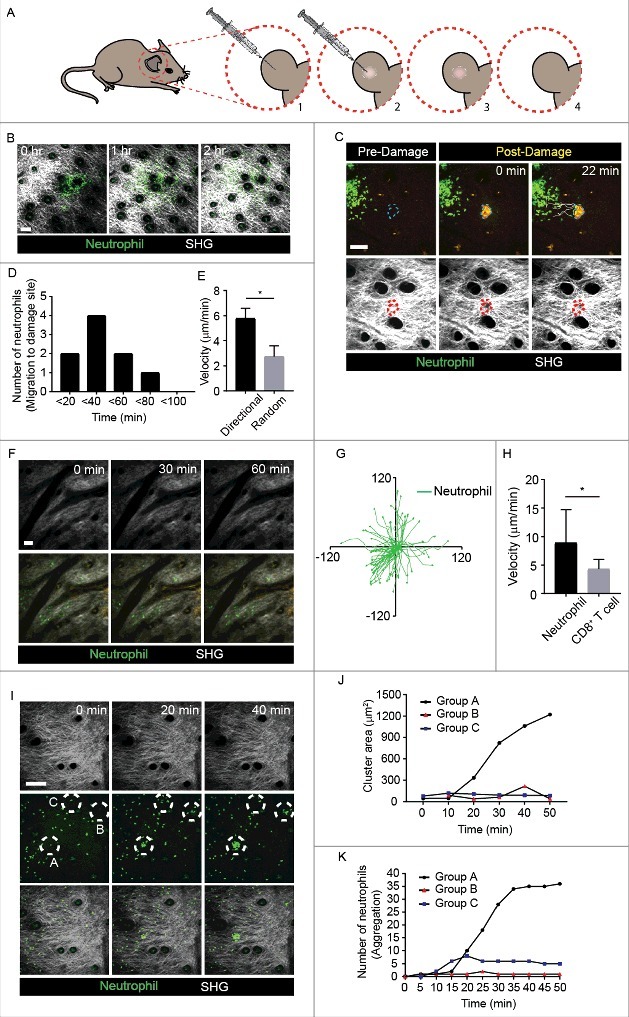
Neutrophil formed clusters during random migration in non-inflammatory mouse ear dermis (A) Schematic representation of intradermal injection of GFP+ neutrophils into the mouse ear. [1] Neutrophils extracted from bone morrow of LysM-GFP+ mice were transferred into the mouse ear using a syringe. [2-3] Intradermal injection of neutrophils induced the formation of a swelling due to the injection volume. [4] The swelling disappeared after about 30 min, as the neutrophil solution was absorbed. (B) Neutrophils were motile in mouse ear dermis, when intradermally transferred. Scale bar, 100 μm. The images were representative of at least five independent videos. (C) Comparison of mouse ear before and after laser exposure. Collagen structure (SHG) was remodeled its shape after laser burn. Laser burn is shown in a yellow circular form, since autofluorescence of green and red were induced. Scale bar, 100 μm. The images were representative of at least five independent videos. (D-E) High motility of neutrophils and their directionality toward sterile damage were analyzed. *P = 0.00079. (F) With low density level, neutrophil neither contacted each other nor formed clusters along time. Scale bar, 50 μm. The images were representative of at least five independent videos. (G) Neutrophils non-directionally migrated out from the initially located site. (H) When intradermally transferred, migration velocity of Neutrophils was faster than that of CD8+ T cells. *P < 0.05. (I) Two different patterns of cell clustering were observed and compared to non-directional migration, as follows: Group A, inflammation-free neutrophil aggregation; Group B, temporal cluster formation and dissociation; Group C, random migration spot. Scale bar, 100 μm. Circles represent the three groups. SHG image confirmed that there was no damage to the ear dermis. The images were representative of at least five independent videos. (J) Cluster size must increase above the threshold to achieve stable aggregation; once firm aggregation was established, cluster size increased rapidly. Group A, B, and C in (I) were drawn in the graph. Images and data are representative of at least 3 independent experiments. (K) Neutrophil numbers were counted in the aggregation of group A, B and C in (I).
When ear dermis was locally injured by laser burn, neutrophils obviously showed directional migration to the injured site (Fig. 1C) and Video 2). The number of directionally migrating neutrophils to the damaged site showed a peak at 40 min post laser burn and then gradually decreased (Fig. 1D). The velocity of neutrophils migrating towards the damage was higher than that of the randomly migrating neutrophils (Fig. 1E). This phenomenon means that, although neutrophil migration out from the initially injected site occurred by somewhat activation from needle puncture and isolation procedure, laser burn-induced stimulation is much stronger signal for neutrophil migration.
Then, to investigate the characteristics of neutrophil migration based on their density, the migration patterns of neutrophils were compared based on comparative distances among neutrophils. These sparsely located neutrophils showed random but much less motility (Fig. 1F and Video 3). This phenomenon indicates that there was no external cue to induce neutrophil migration when neutrophil concentration was low. Also, to compare the characteristics of neutrophil migration with those for migration of other leukocytes, we investigated the motility of intradermally transferred T cells under the same experimental conditions. When intradermally transferred CD8+ T cells were compared with neutrophils, T cells barely migrated along the injection site; neutrophils showed more active migration at longer distances and faster velocity (Fig. 1G and H). Hence, as an innate immune cell, neutrophil was autonomously motile, while T cell, an adaptive immune cell, showed opposite characteristics in this experimental condition.
If neutrophil density was increased, compared to low density of neutrophils described in Figure 1F, it may cause migrating neutrophils to frequently contact each other. We hypothesized that such contact may eventually affect motility of adjacent neutrophils. Motile neutrophils were observed to smoothly migrate along the dermis from the initial site; they then started to form small clusters by contacting adjacent neutrophils. This neutrophil clustering was repeatedly observed (Video 4). The formed neutrophil clusters were observed to either increase in size or rapidly dissociate (Fig. 1I and Video 4). When a sufficient number of neutrophils were recruited to a certain point, they suddenly aggregated with each other, increasing the stability and size of the cluster for more than 30 min (Group A in Fig. 1I-K and Video 4). However, when these neutrophils did not reach the certain level of threshold, the rough neutrophil group rapidly dissociated (Group B in Fig. 1I-K and Video 4). Neutrophil cluster formation was obvious, compared to randomly migrating neutrophils (Group C in Fig. 1I-K and Video 4). These distinct patterns of aggregation could be observed in the differences in cluster diameter (Fig. 1J-K and Video 4). This phenomenon implies the existence of a neutrophil-induced threshold to determine the increment of clustering. Thus, the continuous early-phase neutrophil clustering threshold, which could be more than 10 neutrophils’ accumulation within 20 min, was critical for further stable aggregation of neutrophils.
Video 5 shows this “out-migration pattern” of neutrophils from the initial site in the first hour, which was similar to the pattern described above (Fig. 1B and Video 1). Interestingly, we observed that randomly migrating neutrophils frequently formed clusters through continuous contact with adjacent neutrophils. Remarkably, several neutrophil clusters firmly retained their intact structure for more than 30 min, after which the out-migrating neutrophils suddenly changed their direction and moved back to the initial site. This unique phenomenon resulted in massive and intense accumulation of neutrophils (Fig. 2A and Video 5). This pattern of neutrophil aggregation by the retraction of out-migration eventually became irreversible due to the rigidity. Once the early-phase neutrophils formed a constellation, the cluster area rapidly expanded with noticeably increased retracting velocity (Fig. 2B-C). Thus, neutrophil cluster formation reinforced the regression of out-migrating neutrophils to their originally located site. The mechanism behind the initiation of this neutrophil clustering and the cause of the regression of neutrophil migration in non-inflammatory conditions are still unknown, as illustrated in the cartoon (Fig. 2D). Paracrine effects of neutrophils during physical contact between them might play an important role in this phenomenon [27]. Positive feedback by LTB4, which allows different phases of neutrophil swarming, is also a strong candidate [5]. Various chemokines produced by neutrophils may also be responsible for this phenomenon, as the pattern of chemokines released by neutrophils is known to be strictly dependent on the type of stimulus and/or associated with specific inflammatory/immunological contexts [28].
Figure 2.
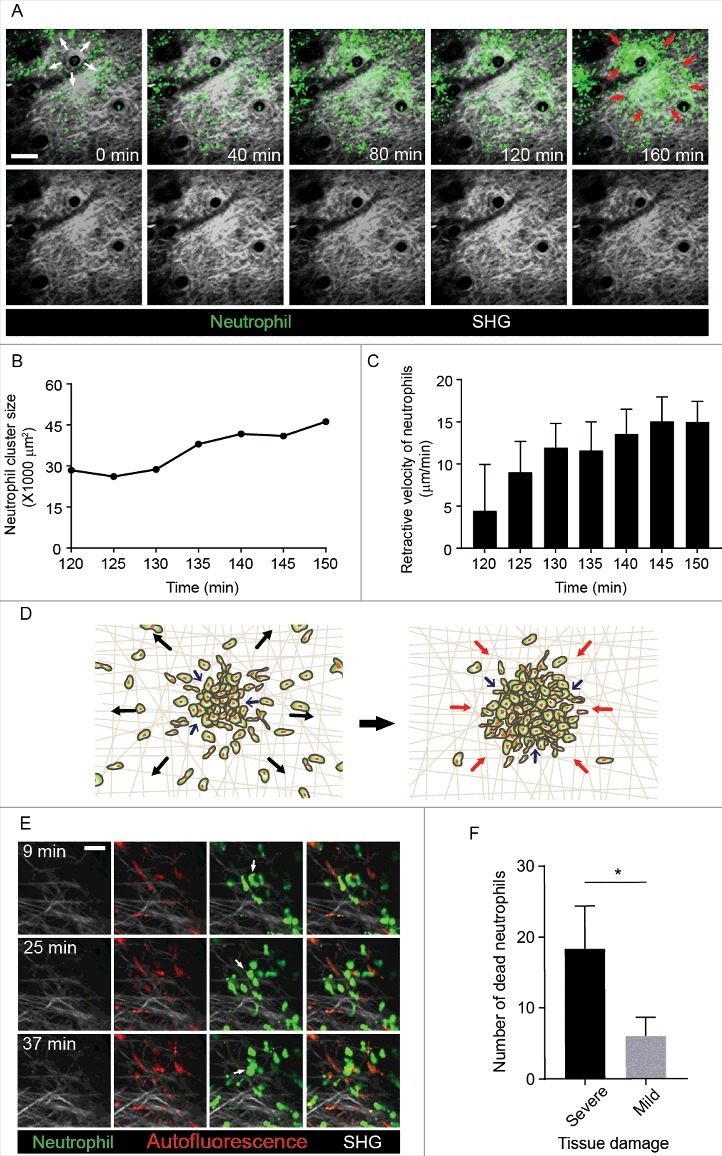
Neutrophil cluster enforced out-migrating neutrophils to draw back to the original residence (A) Neutrophils formed clusters in non-inflammatory conditions. Two phases of neutrophil migration were observed. In the first 120 min, circulating neutrophils migrated out along the tissue from the initial site, as indicated by the white arrows; after 120 min, neutrophils quickly moved back and formed massive cluster units. Red arrows represent the regressive neutrophils migrating towards the initial site. Scale bar, 100 μm. The images were representative of three independent videos. (B) Graph showing the correlation between cluster area size and time during retraction of neutrophils to the initial site in each time point. (C) Graph showing retraction velocity of neutrophils in each time point. (D) Illustration of neutrophil migratory pattern based on Video 5. (E) Images of neutrophils (green), autofluorescence structure (red), and SHG (grey) are represented. Arrows symbolize neutrophil death. Scale bar, 30 μm. The images were representative of five independent videos. (F) Comparison of cell death rates on autofluorescence and collagen structures. *P = 0.035. Images and data are representative of at least 3 independent experiments.
When the dermis is damaged by sterile inflammation, the extracellular matrix is remodeled, and swarm aggregation develops along the damaged site [29]. We confirmed that laser burn on the mouse ear could induce directed migration of neutrophils to the damaged site (Fig. 1C). Second harmonic generation (SHG) have been used to detect fibrillar collagen structures within connective tissues in vivo using two-photon microscopy[30]. Tissue damage by laser burn was visualized as a blank hole in the SHG and an autofluorescence in both green and red channels, as an evidence of the damage to the extracellular matrix structure (Fig. 1C and Video 2).
Although collagen fibers look intact along the interstitial neutrophil attraction pathway, exposure of tissue to laser during two-photon microscopy imaging may induce an omen of minor tissue burn. We speculated that the neutrophil clustering in the ear dermis might be associated with a specific structural pattern of the extracellular matrix in ear dermis. Under two-photon microscopy, some parts of the extracellular matrix of ear dermis had bright red autofluorescence (Fig 2E). A few hours after intradermal transfer, some neutrophils became slowly immotile and then died (Fig 2E and Video 6). In video 6, death of neutrophil coincided with its body spreading onto the collagen fiber (SHG) or another red autofluorescent structure. However, Spreading and death of neutrophils occurred more frequently onto red autofluorescent site than the SHG site (Fig. 2F). We also observed that additional neutrophils aggregated onto the stopped and dead neutrophils in this red autofluorescent site. Therefore, we suggest that additional neutrophil clusters might develop in this area, which could be a starting point for neutrophil clustering.
Discussion
In this study, we found the existence of a threshold for forward neutrophil swarming. The driving forces of neutrophil aggregation did not exist, when the distances among neutrophils were too large and when neutrophil numbers were insufficient. However, once a certain quantity of neutrophils became close to one another, cluster formation was initiated at not only with high speed, but also with obvious directionality. As shown in Figures 2E and 2F, we were able to detect that neutrophil aggregation starts from an immobilized neutrophil on collagen structures in the dermis. One hypothesis of this phenomenon was that the death of intradermally transferred neutrophils may result in exposure of cytosolic contents of this leukocyte subset. This exposure of cytosolic contents like neutrophil extracellular traps (NET) and chemokines of neutrophils may enhance the migration cue of massive neutrophil call-backs [31-33]. Further investigation of relationship among the structure of the extracellular matrix, random migration, cluster formation of neutrophils, and their signaling patterns, therefore, is crucial for gaining a better understanding of neutrophil migration patterns.
Forward neutrophil migration comprises four sub-steps: (1) early signal detection, (2) early phase recruitment of neutrophils, (3) amplification of recruitment, and (4) additional and prolonged signaling with consistent directionality [34,35]. In Figure 2A, however, intradermally transferred neutrophils moved away from initially injected site for about an hour, and then, rapidly formed tight and large clusters. This phenomenon might have occurred due to the cumulative pressure of PBS from the very beginning of neutrophil injection. Therefore, to exclude this possibility of neutrophil movement induced by cumulative pressure of PBS volume, we waited until the swollen ear dermis was fully subsided (Fig. 1A).
Also, there is no evidence that “overwhelming” of a threshold occurs at which step, among the four sub-steps of forward neutrophil migration. Discovering where the threshold occurs is essential, since many drugs are being developed to target the inhibition of the four sub-steps. For example, to alleviate chronic obstructive pulmonary disease (COPD), researchers have targeted CXCR2, which manages phase 1 and 2 of the forward neutrophil migration [8,36,37]. Conducting further in vitro experiment to clarify in which step the threshold overwhelming occurs could help provide clues to prevent excessive neutrophils cluster formation.
Materials and Methods
Experimental Design
To visualize neutrophil motility and morphology, LysM-GFP-expressing neutrophils were intradermally transferred into the ear of albino B6 mice using a syringe. Albino B6 mice were used to prevent the interference of autofluorescence of the dermal pigment that is usually observed on normal black 6 mice [35]. When these neutrophils (5 × 104 cells in 5 μl PBS) were transferred to the ear, neutrophil-containing PBS \ inflated the mouse ear dermis. However, after about 30 min, the inflated ear deflated back to its initial shape, as illustrated in Figure 1A. Therefore, after the intradermal injection, most of the LysM-GFP-expressing neutrophils were absorbed into the ear dermis at the injection site, and the inflated site was undetectable.
Mice
C57BL/6 (Orient bio) and Tyrc-2J/c-2J (albino B6) (The Jackson Laboratory, MN) mice were purchased. LysM-GFP mice [38] were maintained in a specific pathogen-free environment at the animal facilities of Yonsei University College of Medicine. For experiments, heterogenetic mice (GFP/+) were used. The institutional review board of Yonsei University College of Medicine approved all the protocols for all animal experiments.
Neutrophil preparation
Neutrophils were isolated from the femur and tibia bone marrow of LysM-GFP mouse. These bone marrows were washed out with 30 ml mixture of Dulbecco Modified Eagle Medium (Hyclone) with 10% Fetal Bovine Serum (Atlas). Erythrocytes were removed using ACK lysis buffer (Gibco). Then, neutrophils were extracted by negative selection using EasySep a mouse neutrophil enrichment kit (Stemcell technologies).
CD8+ T cell preparation
CD8+ T cells were preparation based on a previous study[39]. Briefly, CD8+ T cells were prepared from negative selection using DynaBeads (Invitrogen). Cells were activated by culturing them on a CD3 (10 μg/ml) Ab-coated dish in the presence of CD28 (2 μg/ml) and 10 unit/ml IL-2 for 5 days. Cells were stained with CMFDA (Thermo) before intradermal injection.
Two-photon intravital microscopy of mouse ear dermis
Mice were anesthetized with an intraperitoneal injection of Zoletil at a dose of 30 mg/kg. Neutrophils (5 × 104 cells in 5 μl PBS) were intradermally transferred into ear dermis of albino B6 mouse using an insulin syringe 1 hour before imaging. For imaging the cell motility and morphology in the mouse ear dermis, the following procedures were performed [40]. Briefly, the anesthetized mice were laid in a lateral recumbent position on a custom-designed platform, while maintaining the temperature at 37°C. Two-photon intravital imaging was performed using a multiphoton microscope, LSM 7 MP (Zeiss). For two-photon excitation, light of 880–900 nm wavelength was used for imaging green, red, and second harmonic generation. Images were acquired at a resolution of 512 × 512 pixels using step sizes of 1 μm to a depth of 30–50 μm every 30–60 sec.
Laser burn to induce phototoxicity
Laser burn was induced to cause tissue injury in the ear dermis with the laser of two-photon microscopy, using a modified version of a protocol described previously[5,35]. 800 nm of laser was emitted to the confined portion of the ear dermis with high power, around 80 mW. When performed properly, burned dermis was shown in yellow and sometimes even with blister.
Data analysis
All the image data were analyzed using Volocity (Perkin-Elmer), ImageJ (U.S. National Institutes of Health). Measurement of the patterns and velocity of migrating neutrophils and CD8+ T cells was conducted using Volocity 6.3.1. To quantify the single cell migration pattern or velocity of leukocytes consecutively, color threshold was pre-adjusted to certain points. Then, the velocity of selected objects could be measured. Counting the number of neutrophils and measuring velocity for a small number of cells migrating to the damage site were conducted manually. Determination of the areas of massive cell clusters was measured by ImageJ. Calculated pixels of cluster areas were converted to square micrometers. The number of dead neutrophils on severely and mildly damaged tissue was quantified in total running time. For each data set, at least three-five independent images were analyzed.
Statistical analysis
Statistical significance (P < 0.05) was computed using the Student's t-test or Mann-Whitney test with the Prism 7 (GraphPad Software).
Authorship Contributions
S.P. performed the experiments and wrote the manuscript. Y.C. analyzed the data. E. P. helped editing the manuscript. Y-M.H. designed the study, performed the experiments, and wrote the manuscript.
Funding Statement
This study was supported by the faculty research grant of Yonsei University College of Medicine [6-2016-0132] and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) [2016R1A2B4008199] (Y-M. H.).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Williams MR, Azcutia V, Newton G, Alcaide P, Luscinskas FW. Emerging mechanisms of neutrophil recruitment across endothelium. Trends Immunol 2011; 32:461–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol 2010; 10:826–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].McDonald B, Kubes P. Innate Immune Cell Trafficking and Function During Sterile Inflammation of the Liver. Gastroenterology 2016; 151:1087–95. [DOI] [PubMed] [Google Scholar]
- [4].McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, et al.. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 2010; 330:362–6. [DOI] [PubMed] [Google Scholar]
- [5].Lammermann T, Afonso PV, Angermann BR, Wang JM, Kastenmuller W, Parent CA, et al.. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature 2013; 498:371–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Czepielewski RS, Jaeger N, Marques PE, Antunes MM, Rigo MM, Alvarenga DM, et al.. GRPR antagonist protects from drug-induced liver injury by impairing neutrophil chemotaxis and motility. Eur J Immunol 2017; 47:646–57. [DOI] [PubMed] [Google Scholar]
- [7].Kamp ME, Shim R, Nicholls AJ, Oliveira AC, Mason LJ, Binge L, et al.. G Protein-Coupled Receptor 43 Modulates Neutrophil Recruitment during Acute Inflammation. PLoS One 2016; 11:e0163750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].de Oliveira S Rosowski EE, Huttenlocher A. Neutrophil migration in infection and wound repair: going forward in reverse. Nat Rev Immunol 2016; 16:378–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ley K. Integration of inflammatory signals by rolling neutrophils. Immunol Rev 2002; 186:8–18. [DOI] [PubMed] [Google Scholar]
- [10].Gambardella L, Vermeren S. Molecular players in neutrophil chemotaxis–focus on PI3K and small GTPases. J Leukoc Biol 2013; 94:603–12. [DOI] [PubMed] [Google Scholar]
- [11].Weninger W, Biro M, Jain R. Leukocyte migration in the interstitial space of non-lymphoid organs. Nat Rev Immunol 2014; 14:232–46. [DOI] [PubMed] [Google Scholar]
- [12].Lammermann T. In the eye of the neutrophil swarm-navigation signals that bring neutrophils together in inflamed and infected tissues. J Leukoc Biol 2016; 100:55–63. [DOI] [PubMed] [Google Scholar]
- [13].Nourshargh S, Alon R. Leukocyte migration into inflamed tissues. Immunity 2014; 41:694–707. [DOI] [PubMed] [Google Scholar]
- [14].Cordeiro JV, Jacinto A. The role of transcription-independent damage signals in the initiation of epithelial wound healing. Nat Rev Mol Cell Biol 2013; 14:249–62. [DOI] [PubMed] [Google Scholar]
- [15].Pittman K, Kubes P. Damage-associated molecular patterns control neutrophil recruitment. J Innate Immun 2013; 5:315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Broggi A, Granucci F. Microbe- and danger-induced inflammation. Mol Immunol 2015; 63:127–33. [DOI] [PubMed] [Google Scholar]
- [17].Venereau E, Ceriotti C, Bianchi ME. DAMPs from Cell Death to New Life. Front Immunol 2015; 6:422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nourshargh S, Renshaw SA, Imhof BA. Reverse Migration of Neutrophils: Where, When, How, and Why? Trends Immunol 2016; 37: 273–86. [Google Scholar]
- [19].Hirano Y, Aziz M, Wang P. Role of reverse transendothelial migration of neutrophils in inflammation. Biol Chem 2016; 397:497–506. [DOI] [PubMed] [Google Scholar]
- [20].Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol 2010; 10:427–39. [DOI] [PubMed] [Google Scholar]
- [21].Drifte G, Dunn-Siegrist I, Tissieres P, Pugin J. Innate immune functions of immature neutrophils in patients with sepsis and severe systemic inflammatory response syndrome. Crit Care Med 2013; 41:820–32. [DOI] [PubMed] [Google Scholar]
- [22].Kobayashi M, Tsuda Y, Yoshida T, Takeuchi D, Utsunomiya T, Takahashi H, et al.. Bacterial sepsis and chemokines. Curr Drug Targets 2006; 7:119–34. [DOI] [PubMed] [Google Scholar]
- [23].Orman MA, Ierapetritou MG, Berthiaume F, Androulakis IP. The dynamics of the early inflammatory response in double-hit burn and sepsis animal models. Cytokine 2011; 56:494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jones CN, Moore M, Dimisko L, Alexander A, Ibrahim A, Hassell BA, et al.. Spontaneous neutrophil migration patterns during sepsis after major burns. PLoS One 2014; 9:e114509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kasten KR, Muenzer JT, Caldwell CC. Neutrophils are significant producers of IL-10 during sepsis. Biochem Biophys Res Commun 2010; 393:28–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tavares-Murta BM, Zaparoli M, Ferreira RB, Silva-Vergara ML, Oliveira CH, Murta EF, et al.. Failure of neutrophil chemotactic function in septic patients. Crit Care Med 2002; 30:1056–61. [DOI] [PubMed] [Google Scholar]
- [27].Nemeth T, Mocsai A. Feedback Amplification of Neutrophil Function. Trends Immunol 2016; 37:412–24. [DOI] [PubMed] [Google Scholar]
- [28].Tecchio C, Cassatella MA. Neutrophil-derived chemokines on the road to immunity. Semin Immunol 2016; 28:119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kienle K, Lammermann T. Neutrophil swarming: an essential process of the neutrophil tissue response. Immunol Rev 2016; 273:76–93. [DOI] [PubMed] [Google Scholar]
- [30].Wolf K, Alexander S, Schacht V, Coussens LM, von Andrian UH, van Rheenen J, et al.. Collagen-based cell migration models in vitro and in vivo. Semin Cell Dev Biol 2009; 20:931–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol 2006; 6:173–82. [DOI] [PubMed] [Google Scholar]
- [32].Borregaard N. Neutrophils, from marrow to microbes. Immunity 2010; 33:657–70. [DOI] [PubMed] [Google Scholar]
- [33].Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 2013; 13:159–75. [DOI] [PubMed] [Google Scholar]
- [34].Soehnlein O, Steffens S, Hidalgo A, Weber C. Neutrophils as protagonists and targets in chronic inflammation. Nat Rev Immunol 2017; 17:248–61. [DOI] [PubMed] [Google Scholar]
- [35].Ng LG, Qin JS, Roediger B, Wang Y, Jain R, Cavanagh LL, et al.. Visualizing the neutrophil response to sterile tissue injury in mouse dermis reveals a three-phase cascade of events. J Invest Dermatol 2011; 131:2058–68. [DOI] [PubMed] [Google Scholar]
- [36].Lazaar AL, Sweeney LE, MacDonald AJ, Alexis NE, Chen C, Tal-Singer R. SB-656933, a novel CXCR2 selective antagonist, inhibits ex vivo neutrophil activation and ozone-induced airway inflammation in humans. Br J Clin Pharmacol 2011; 72:282–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Moss RB, Mistry SJ, Konstan MW, Pilewski JM, Kerem E, Tal-Singer R, et al.. Safety and early treatment effects of the CXCR2 antagonist SB-656933 in patients with cystic fibrosis. J Cyst Fibros 2013; 12:241–8. [DOI] [PubMed] [Google Scholar]
- [38].Faust N, Varas F, Kelly LM, Heck S, Graf T. Insertion of enhanced green fluorescent protein into the lysozyme gene creates mice with green fluorescent granulocytes and macrophages. Blood 2000; 96:719–26. [PubMed] [Google Scholar]
- [39].Lim K, Hyun YM, Lambert-Emo K, Capece T, Bae S, Miller R, et al.. Neutrophil trails guide influenza-specific CD8(+) T cells in the airways. Science 2015; 349:aaa4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hyun YM, Sumagin R, Sarangi PP, Lomakina E, Overstreet MG, Baker CM, et al.. Uropod elongation is a common final step in leukocyte extravasation through inflamed vessels. J Exp Med 2012; 209:1349–62. [DOI] [PMC free article] [PubMed] [Google Scholar]


