ABSTRACT
Myeloid cell leukemia-1 (MCL-1), closely related to B-cell lymphoma 2 (BCL-2), has a well-established role in cell survival and has emerged as an important target for cancer therapeutics. We have demonstrated that inhibiting MCL-1 is efficacious in suppressing tumour progression in pre-clinical models of breast cancer and revealed that in addition to its role in cell survival, MCL-1 modulated cellular invasion. Utilizing a MCL-1-specific genetic antagonist, we found two possible mechanisms; firstly MCL-1 directly binds to and alters the phosphorylation of the cytoskeletal remodeling protein, Cofilin, a protein important for cytoskeletal remodeling during invasion, and secondly MCL-1 modulates the levels SRC family kinases (SFKs) and their targets. These data provide evidence that MCL-1 activities are not limited to endpoints of extracellular and intracellular signaling culminating in cell survival as previously thought, but can directly modulate the output of SRC family kinases signaling during cellular invasion. Here we review the pleotropic roles of MCL-1 and discuss the implications of this newly discovered effect on protein kinase signaling for the development of cancer therapeutics.
KEYWORDS: Myeloid cell leukemia 1, SRC family kinases, invasion, protein kinase signaling, cancer, metastasis
Myeloid cell leukemia-1 (MCL-1), a unique pro-survival member or the BCL-2 family of proteins
Recently, work performed in our laboratory demonstrated that Myeloid cell leukemia-1 (MCL-1) antagonism suppressed tumour progression in pre-clinical models of breast cancer and revealed that in addition to its role in cell survival, MCL-1 modulated cellular invasion .1 MCL-1 was first described as an immediate-early response gene in human myeloid leukaemia cells induced to differentiate with phorbol ester.2 MCL-1 is best known as a pro-survival member of the BCL-2 family of proteins that regulates the intrinsic (mitochondrial) apoptotic cascade.3 MCL-1 is important for the survival of most normal and malignant tissues (reviewed in.4) The C-terminal region of MCL-1 shares homology with the BCL-2 family of proteins, which contain four BCL-2-homology (BH1–4)-domains that form a binding pocket for interaction with the pro-apoptotic BH3 only proteins and by doing so protect normal and malignant cells from cell death. The BCL-2 family members include two pro-apoptotic subgroups: the BH3-only sensor proteins (e.g. BIM, PUMA, NOXA, tBID etc), which trigger the intrinsic apoptotic cascade in response to cytotoxic insults or cellular stresses and BAX and BAK, the apoptotic effectors (reviewed elsewhere3). The BH domain hydrophobic pocket dictates MCL-1 binding specificity for BIM, tBID, PUMA, NOXA and BAK thereby restraining cellular apoptotic activity.
MCL-1 is important for cancer cell survival and therapeutic resistance
MCL1 is one of the most common somatic copy number amplifications, observed in 11% of cancers across multiple tissue types, with the highest rates observed in breast (36% of cases) and lung (54% of cases).5 MCL-1 protein levels correlate with outcome, tumor grade and therapeutic resistance in many cancers including those of the hematopoietic system, breast, lung and pancreas.6–12 MCL-1 has been validated to participate in neoplastic progression of B-cell lymphomas13 as well as haematopoietic progenitor/stem cell tumours14 and accelerate Myc induced myeloid leukaemia.15 Several studies have also shown that MCL-1 is a barrier to therapeutic sensitivity, including those that target BCL-2 (reviewed in16). MCL-1 is responsible for therapeutic resistance in a range of cancers including oral cancers,17 lung cancer,18 pancreatic cancer,19 ovarian cancer, colon cancer20 and triple negative breast cancer.21 MCL-1 also confers the survival of breast cancer cells in vitro22 and protects HER-2 positive breast cancers from hypoxia-induced death.23 It is for the above reasons that there have been significant advances in the development of small molecule inhibitors that bind to and inhibit MCL-1, including the most recent and promising compounds A1210477 and S63845,24,25 with the later inhibitor having high affinity, efficacy at low doses, and low toxicity. Recent work has shown that the MCL-1 inhibitor S63845 could increase the sensitivity of patient derived xenografts to docetaxel and traztuzumab.26 More work is to determine the genetic or proteomic biomarkers that would stratify patients to this type of therapy as a single agent or in combination with other therapies. Thus MCL-1 is a potent survival factor in hematopoietic and solid tumors and can be targeted with small molecule inhibitors to treat a wide range of cancers.
Protein kinase signalling control of MCL-1 activity
Unlike other members of the BCL-2 family, MCL-1 contains a unique 150 amino acid N-terminus consisting of PEST-like sequences (rich in proline (P), glutamic acid (E), serine (S), and threonine (T)) implicated in the control of protein stability and activity. Degradation of MCL-1 is thought to be primarily controlled primarily by ubiquitylation via 13 different lysine residues, and modulated by phosphorylation, targeting it to the proteasome.27 E3 ubiquitin-protein ligases known to regulate MCL-1 stability include HUWE1/MULE,28,29 SCF F-box containing proteins F-box/WD repeat-containing protein 7 (Fbw7,20,30) and F-box/WD repeat-containing protein 1A (βTrCP) and the deubuitinase Ubiquitin Specific Peptidase 9 (USPX,31). As a result MCL-1 has a short half-life of approximately 3 hours.32 MCL-1 expression and activity is also controlled by a variety of stress, growth factor, hormone, cytokine and signals culminating in receptor tyrosine kinase signalling involved in the stimulation of differentiation of myeloid lineage cells or in response to stress to enhance cell survival.7,33 A summary of the various signalling pathways and kinases responsible for the regulation of MCL-1 is provided in Table 1 and illustrated in Fig. 1. Best known in the regulation of MCL-1, is output of the PI3K/AKT, MAPK/ERK and JAK/STAT pathways that can be activated by a variety of receptor tyrosine kinases that including EGFR. The interleukin (IL) family of cytokines, which have roles in differentiation, growth and survival,34 also induce MCL-1 transcription. These include IL3,35,36 IL5,37 IL6,38,39 IL740 and IL15.41 Signalling downstream of the interleukin family predominantly occurs via the JAK/STAT pathway,37,39,41 with a STAT binding site present in the MCL-1 promoter.38 Consequently STAT5 has been shown to induce MCL-1 transcription.42 MCL-1 transcription is also activated after endoplasmic reticulum stress43 and hypoxia44 resulting in the death of diseased cells. Intracellular kinases (eg GSK3β, CDK1 and CDK2) downstream of these signalling pathways also control MCL-1 activity resulting via phosphorylation on nine potential phosphosites influencing the stability, activity, degradation and even localisation (summarised in Table 2). Thus MCL-1 is a critical cell survival factor in normal and malignant tissues that is induced and activated in response to a variety of extracellular and intracellular cues via protein kinase signalling.
Table 1.
Extracellular and intracellular regulation of MCL-1 function. The effects of extracellular and intracellular signalling (signalling pathways) on MCL-1 transcription in various cellular contexts.
| Signal | Pathway | Transcription Factors | Cell context | Outcome | References | ||
|---|---|---|---|---|---|---|---|
| Activation of Transcription | Cytokines | IL3 | PI3K/AKT | CREB Complex | Haematopoietic progenitor cell line | Increased survival when challenged with PD98059, LY294002, wortmannin and DMSO. | [35] |
| p38MAPK | PU.1 Complex | Haematopoietic progenitor cell line | Survival outcome unknown. | [36] | |||
| IL7 | Mouse derived RAG-2 deficient thymocytes | Increased survival was observed in unchallenged cells treated with IL7. | [40] | ||||
| IL6 | JAK | STAT3 | Choloangiocarcinoma cell lines | Addition of STAT3 inhibitor, AG490, or IL-6 antisera led to a downstream loss of MCL-1 expression and sensitized cells to apoptosis induced by tumor necrosis factor related apoptosis inducing ligand (TRAIL). | [38] | ||
| Isolated human macrophages | Sodium salicylate treatment resulted in loss of STAT3 signaling, and downstream loss of MCL-1 expression reduced survival. Overexpression of constitutively active STAT3 rescued this loss of viability. | [39] | |||||
| IL15 | JAK | STAT5 | Isolated mouse T-cells | Survival outcome unknown. | [41] | ||
| PI3K/AKT | |||||||
| IL5 | JAK | STAT5 | Haematopoietic progenitor cell lines | Increased survival was observed in unchallenged cells treated with IL5. | [37] | ||
| SCF | AKT/PKB | Haematopoietic progenitor cell lines | Increased survival was observed in unchallenged cells treated with SCF. | [37] | |||
| MAPK | TCF-SRF | HeLa ovarian cell lines | Expression of a repressive mutant of the TCF Elk-1, Elk-en, resulted in loss of MCL-1 expression and a reduction of viability in unchallenged cells. | [87] | |||
| ML-1 Myeloblastic cell line | Activated in response to TPA (12-O-tetradecanoylphorbol 13-acetate), colchine and vinblastine resulting in reduced apoptosis. | [88] | |||||
| Fusion Proteins | BCR-ABL | STAT5 | Chronic myeloid leukaemia cell lines | Reduced apoptosis in response to imatinib treatment. | [42] | ||
| Stress Signalling | ER Stress | MAPK | Humans melanoma cell lines | Reduced loss of survival in response to ER stress stimulators thapsigargin and tunicamycin. | [43] | ||
| IRE1α /ATF6 | |||||||
| Hypoxia | HIF1α | Heptoma cell lines | Reduced apoptosis induced by tert-Butyl Hydroperoxide. | [44] | |||
| Inhibition of Transcription | Integrated stress response | EIF2α | Choloangiocarcinoma cell lines, Pancreatic Cancer cell lines and Ovarian Cancer cell lines | Resulted in loss of survival in response to a range of stress stimuli to UV radiation, osmotic stress, arsenite, thapsigargin, tunicamycin and dithioreitol. | [89] | ||
Figure 1.
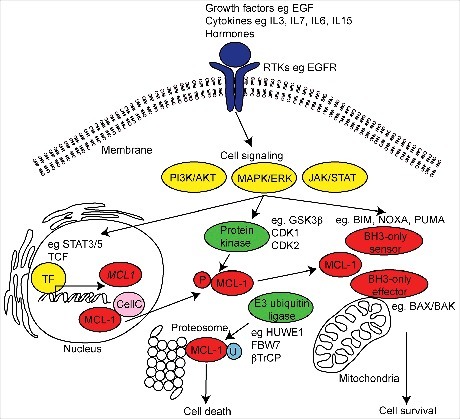
Schematic diagram of MCL-1 regulation and function. MCL-1 is induced by a variety of extracellular receptor tyrosine kinases (RTKs) (purple), growth factors, stress induced cytokines and hormones resulting in the activation of intracellular signals (yellow) and inducing the transcription of MCL-1 mRNA by target transcription factors (TF). MCL-1 activity and stability is regulated by E3 ubiquitin-ligase and protein kinase induced phosphorylation (P) during post-translational processing. The best recognized function of MCL-1 is its role in maintaining cell survival via interaction with the intrinsic apoptotic machinery at the mitochondria. MCL-1 can also participate in the regulation of mitochondrial structure and function, cell cycle (CellC) and DNA damage mechanisms. In diseased and damaged cells, MCL-1 can be ubiquitinated (U) and targeted for degradation at the proteasome resulting in cell death.
Table 2.
Protein kinase regulation of MCL-1. The effects of protein kinase phosphorylation on target residues of MCL-1 by protein kinases in various cellular contexts.
| MCL-1 target residue | Kinase | Cell context | Function | References | |
|---|---|---|---|---|---|
| Stability and Activity | Serine 64 | JNK, CDK1, CDK2, CDK1/Cyclin 1B, CDK2/Cyclin E, CDK2/Cyclin A | Cholangiocarcinoma, cervical and various cancer cell lines | Phospho-negative mutants had no effect on MCL-1 protein turnover, but increased interactions with BIM, NOXA and BAK have been observed. Phosphorylation is thought to occur during G2/M cell cycle progression and NOXA up-regulation enhanced phosphorylation. | [90-93] |
| Serine 121-Threonine 163 | JNK | Epithelial cell line HEK293 | Phosphorylation occurs downstream of stress. Phosphomimetic mutants increased MCL-1 half-life and anti-apoptotic activity. | [94,95] | |
| in vivo liver injury mouse model | |||||
| Threonine 92 and 163 | ERK | Breast Cancer cell lines | Phosphorylation increased the half-life of the MCL-1 protein, and enhanced interaction with PIN-1. | [90,96] | |
| CDK2/Cyclin E | Lymphocytic Leukaemia cell line | ||||
| Threonine 163 | GSK3β | Burkitt Lymphoma cell line | Phosphomimetics increased MCL-1 protein stability and led to chemoresistance. | ||
| ERK | [97,98] | ||||
| Degradation | Serine 155 and 159Threonine 163 | GSK3β | Breast Cancer cell lines | Phospho-negative mutants doubled the half-life of wild type MCL-1 and resulted in chemoresistance in MCF7 cells. | [31] |
| Serine-64-Threonine 92 | CDK1/Cyclin 1B | Cervical Cancer cell line | Phosphorylation occured during mitosis and targeted MCL-1 for degradation. | [91,99] | |
| Oral Cancer cell line | |||||
| Cervical Cancer cell line | [100] | ||||
| Serine 159 | GSK3β | IL-3 dependent mouse cell lines | Phosphorylation occured as a result of cellular stress and has been demonstrated to be regulated by SGK1, and targeted MCL-1 for degradation. | [101-103] | |
| JNK | Fibroblast cell lines | ||||
| Neuronal cell lines | |||||
| Localisation | Serine 162 | Cervical Cancer and epithelial cell lines | Located in the mitochondrial targeting motif of MCL-1, phospho-negative mutants are localised at the nucleus. | [100] | |
| Unknown | Threonine 70, 86, 156 | CDK1/Cyclin 1B | Cervical Cancer cell lines | Unknown | [100] |
MCL-1 is not just a mediator of cell survival
In addition to its role in cell survival, MCL-1 has been shown to possess multiple functions in different cellular compartments. Deletion of MCL-1 in mice resulted in peri-implantation lethality an effect that was independent of apoptosis.45 Additionally, MCL-1 and STAT3 have been shown to interact during embryonic implantation, which resulted in the expression of epithelial to mesenchymal markers, increased apoptosis and decreased invasion.46 MCL-1 is highly expressed in both human and mouse embryonic stem cells (ESCs), with the loss of MCL-1 through siRNA or up-regulation of NOXA by CDK1 inhibitor treatment leading to significant induction of cell death, pointing to MCL-1 playing an active role in ESC homeostasis.47
The divergent roles of MCL-1 are dependent on its post-translational modification and protein-protein interactions. Proteolytically cleaved amino-truncated MCL-1 can localise to the inner mitochondrial membrane and is important for mitochondrial structure and physiology.48,49 Furthermore, cell cycle progression by MCL-1 is also mediated through the direct recruitment and inhibition of CDK1, due a reduced capacity of CDK1 to bind to Cyclin 1B.50 In this manner MCL-1 binding may subvert the interaction of other target proteins thereby restricting kinase activity eg by binding to CDK1 and preventing cell cycle progression. MCL-1 has also been shown to directly interact with other proteins such as CDK1, PCNA and CHK1 in the nucleus, where it similarly regulates cell cycle progression and DNA damage.51 In the nervous system, MCL-1 is important for neural precursor cell survival52 but also for cell cycle progression in embryonic neural precursor cells. Furthermore, MCL-1 expression correlates with the levels of VEGF (vascular endothelial growth factor),53 and although this expression pattern is important for the survival of endothelial cells, it is also important for vessel sprouting and invasion.54,55
MCL-1 has also been implicated in the migration and invasion of normal and malignant tissues, for example MCL-1 has shown to be important for neuronal progenitor cell migration from the ventricular zone into the cortical plate during cortical neurogenesis.56 MCL-1 has further been demonstrated to play a role in the migration and invasion of colorectal57 and gastric58 cancer cell lines, whereby siRNA knockdown led to a loss of motility in wound healing and trans-well assays. Furthermore forced expression of MIR26a, which targets MCL-1 in breast cancer cell lines led to the loss of migration in wound healing assays.59 MIR26a does have other targets such as MTDH and EZH2 therefore it is still unclear whether this effect is solely dependent on the activities of MCL-1. These data suggest that MCL-1 possesses functions beyond merely its role in cell survival, including roles in mitochondrial physiology, cell cycle progression, DNA damage and possibly invasion but it has been difficult to discriminate these cellular functions from apoptosis as they are intrinsically linked to cellular viability.
MCL-1 is a new regulator of protein kinase signalling during invasion
We investigated the consequences of inhibiting MCL-1 in triple negative breast cancer cells,1 a subtype of breast cancer with limited treatment options and some of the poorest outcomes.60 We inhibited MCL-1 using a genetic approach via inducible expression of a modified form (L62A/F69A double mutant) of the short isoform of BIM (BIMs2A).61 This approach mimics that of small molecules (BH3-mimetics) targeting MCL-1 and was chosen as it was highly specific for MCL-1 and previously validated permitting an investigation of MCL-1 in a tumor cell autonomous manner.62 Expression of BIMs2A increased cell death in basal-like MDA-MB-468 cells but did not induce apoptosis in highly invasive claudin-low MDA-MB-231 cells when cultured on plastic. When seeded on contracted collagen I matrices that more accurately recapitulated key aspects of the in vivo microenvironment,63 MCL-1 antagonism suppressed invasion, an effect that was independent on its effect on apoptosis.1 Furthermore inhibition of MCL-1 significantly suppressed both the size and number of lung metastases in the lungs of mice bearing both MDA-MB-468 and MDA-MB-231 mammary intraductal xenografts, indicating that MCL-1 was essential for metastatic progression in both models. This specific model of antagonism provided definitive proof that MCL-1 controls invasive capacity of cancer cells, as what had been suggested previously using wound healing assays.59
To invade, cancer cells form specialised membrane protrusions termed invadopodia64 rich in filamentous (F)-actin filaments initiated by a kinase signalling cascade (often involving the SRC family kinases cSRC, FYN and YES and others).65 This signalling cascade results in the phosphorylation and activation of cytoskeletal remodelling proteins that include Dynamin, Cortactin, Cofilin, Talin, N-Wasp and ARP2/3 complex, augmented by the activity of GTPases CDC42 and RAC66 and the co-ordinated assembly of adhesion proteins (eg FAK) and those that promote F-Actin stabilisation (eg Paxillin, Vimentin).67
Additional work in our laboratory provided a potential mechanism for the effects of MCL-1 during cancer cell invasion and suggested that MCL-1 may modulate cytoskeletal remodelling during invasion. Kinomic profiling data revealed that MCL-1 inhibition altered a large number of proteins important for invasion and regulated by SRC family kinases.1 These included increased CSK levels (cSRC tyrosine kinase), a negative regulator of SRC family kinases,68 decreased total levels of the SRC family kinase, FYN, and the cSRC target, ABL. The cSRC target and adhesion protein FAK was also decreased as was the phosphorylation of Paxillin and Vimentin. Serine 3 phosphorylation of Cofilin 1 and 2 was increased, an effect that suppresses actin remodelling during invasion. All of these targets are regulated by SRC family kinase activity.69,70 Western blotting confirmed the observed decrease in total FAK and an increase in E-Cadherin (data not shown), suggestive of a more epithelial and less invasive state.71 MCL-1 antagonism also decreased the auto-phosphorylation site Y1148 in EGFR indicating suppression of invasion activity, perhaps due to loss of cSRC activation.72 Mass spectrometry and proximity ligation assays showed for the first time that MCL-1 was in direct contact with Cofilin, which perhaps is similar to what has been observed for MCL-1 and CDK1,51 may be important for restricting activity,1 via preventing the binding of its inhibitory partner Cortactin.73,74 Our data possibly places MCL-1 in complexes in direct contact with F-Actin signalling apparatus important for dynamic cytoskeletal changes during invasion (Fig. 2A). Although additional experimentation is required to confirm this hypothesis, these results suggest that MCL-1 may play a critical role in invasion via the modulation of SRC family kinase signalling.
Figure 2.
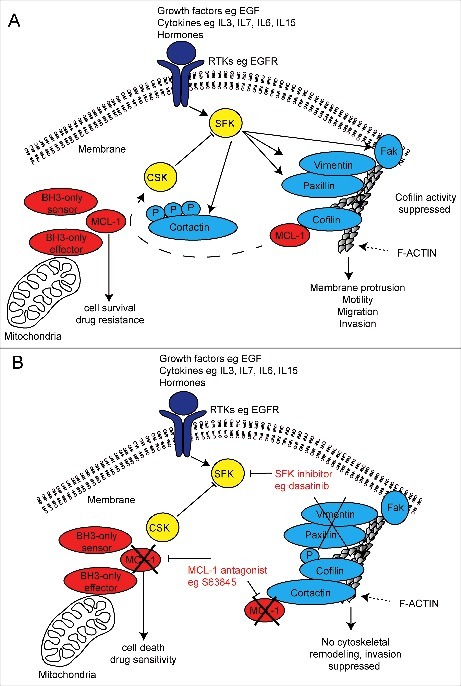
Schematic diagram of a putative mechanism for MCL-1 regulation of SRC family kinase signalling in invasive cancer cells and dual therapeutic strategy. (A) Receptor tyrosine kinase (RTK) activation induces SRC family kinase (SFK) signalling and its targets important for cytoskeletal invasion by cancer cells. MCL-1 binds and prevents serine 3 phosphorylation of Cofilin, which may prevent Cortactin inhibition of Cofilin, permitting cytoskeletal (F-actin) remodelling and cellular invasion. MCL-1 modulates the output of the SRC family kinases (eg Vimentin, Paxillin, FAK and CSK) via an unknown mechanism promotes cellular invasion. (B) MCL-1 antagonism using pharmaceutical inhibition (eg S63845) may allow Cortactin inhibition of Cofilin activity thereby preventing its cytoskeletal remodelling function and also alters the output of the SRC family kinases. When combined with SRC family kinase inhibitors (eg dasatinib, saracatinib and bosutinib), MCL-1 inhibition suppresses invasion while simultaneously induce cell death and increasing drug sensitivity.
MCL-1 regulation of SRC family kinase signalling; implications for cancer therapeutics
Our findings have significant implications for the treatment of cancers that rely on MCL-1 and SRC family kinase signalling for survival and metastatic progression. SRC family kinases are important in the development, maintenance, progression and the metastatic spread of several malignancies leading to extensive research into the development of agents that target this family. Examples of these agents include dasatinib (targeting ABL, cSRC and c-KIT), saracatinib (targeting cYES, FYN, LYN, BLK, FGR and LCK) and bosutinib (targeting SRC and ABR).65 Dasatinib has shown profound improvements in tumor and metastatic outcomes in pancreatic xenograft pre-clinical models in mice75,76 but despite this Phase II clinical trials of SFK inhibitors alone and/or in combination with gemcitabine have failed to show any improvement in progression free or overall survival in patients with advanced pancreatic adenocarcinoma.77–79 In breast cancer, FYN activation plays an important role in breast cancer cell motility and drug resistance in vitro,80 but so far trials of saracatinib targeting FYN have not succeeded.81
Conversely, Phase II clinical trials of single agent dasatinib have shown durable and objective clinical responses in a small proportion (5%) of patients with locally advanced and metastatic triple negative breast cancer.82 Combination trials have shown improved outcomes in patients with breast cancer, for example Phase I clinical trials of dasatinib with Capecitabine show clinical response rates of 56% in unselected patients.83 Other trials combining dasatinib with paclitaxel84 and bosutinib with exemestane85 are currently underway in patients with advanced metastatic breast cancer and are showing improved responses compared to single agents alone. These preliminary results suggest that the efficacy of SFK inhibitors will likely be improved by combining these drugs with others that increase potency or have parallel cytotoxic activity.
As MCL-1 modulated the output of SFK signalling, we then examined whether MCL-1 antagonism could be one way to increase the efficacy of these SFK inhibitors. Encouragingly, MCL-1 inhibition greatly enhanced the anti-invasive potential of dasatinib in 3D organotypic assays in vitro and suppressed tumour progression in pre-clinical models of breast cancer.1 The next logical step is to investigate whether this effect extends to other inhibitors of SRC family and their targets (eg saracatinib, bosutinib and others), as recently achieved for other highly metastatic cancers,86 and examine efficacy of this new dual targeting therapeutic strategy in clinical trials for metastatic disease (Fig. 2B). Importantly the advantage of using an MCL-1 antagonist to improve potency of anti-metastatic agents is the simultaneous suppression of cell survival and increased therapeutic sensitivity that may result in a substantial improvement in patient survival.
Concluding remarks
MCL-1 is an important regulator of normal and cancer cell viability but there is increasing evidence that MCL-1 has additional roles in mitochondrial structure and function, cell cycle regulation, DNA damage response and cellular invasion. Receptor tyrosine kinase signalling upstream of MCL-1 is important for its effects but our new evidence suggests that MCL-1 can also feed back to directly modulate protein kinase invasion signalling during metastasis (Fig. 2). Although more work is needed to understand to fully understand the mechanisms underlying these effects, the regulation of the cytoskeletal machinery by MCL-1 regulation via modulation of protein kinase signalling provides a valuable opportunity to increase the potency of drugs that antagonise these networks. For those SRC family kinase inhibitors that have largely disappointed in clinical trials, it may now be prudent to consider pharmaceutical inhibitors of MCL-1 (eg S6384524,25) in combination with these to improve clinical response.
Funding Statement
This work was supported by grants from the Cure Cancer Australia Foundation, NHMRC Australia, Cancer Council NSW (SRO and CJO RG17-02), Banque Nationale de Paris-Paribas Australia and New Zealand and RT Hall Trust, Mostyn Family Foundation, Cue Clothing Co., Estee Lauder Australia and by fellowships from the Cancer Institute NSW (DG-O) NHMRC Australia (CJO 1043400), National Breast Cancer Foundation fellowship (SRO ECF-13-08) and Len Ainsworth (PT).
Abbreviations
- ABL
Abelson murine leukaemia viral oncogene homolog 1
- AKT/PKB
Protein kinase B
- BAX/BCL2L4
Bcl-2 associated X protein/Bcl-2-like protein 4
- BAK/BCL2L7
Bcl-2 homologous antagonist killer/Bcl-2-like protein 7
- BCL-2
B-cell lymphoma 2
- BIM/BCL2L11
Bcl-2-like protein 11
- BH
BCL-2 homology
- BLK
Tyrosine-protein kinase Blk
- βTrCP
F-box/WD repeat-containing protein 1A
- CDC42
Cell division control protein 42 homolog
- CDK1
Cyclin dependent kinase 1
- CDK2
Cyclin dependent kinase 2
- CHK1/CHEK1
Checkpoint kinase 1
- CSK
cSRC tyrosine kinase
- cKIT/CD117
Tyrosine-protein kinase Kit
- cSRC
Tyrosine kinase Src
- cYES
Tyrosine-protein kinase Yes
- EGFR/HER1
Epidermal growth factor receptor
- ERK
Extracellular signal-related kinases
- ESC
Embryonic stem cell
- EZH2
Enhancer of zeste homolog 2
- FAK/PTK2
Focal adhesion kinase/protein tyrosine kinase 2
- FBW7
SCF F-box containing proteins F-box/WD repeat-containing protein 7
- FGR/SRC2
Gardner-Rasheed feline sarcoma viral
- FYN
Tyrosine-protein kinase Fyn
- GSK3β
Glycogen synthase kinase-3 beta
- HUWE1/MULE
E3 ubiquitin ligase HUWE1/MCL-1 ubiquitin ligase E3
- JAK
Janus kinase
- JNK
c-Jun N-terminal kinases
- LCK
Tyrosine-protein kinase Lck
- LYN
Tyrosine protein kinaseLyn
- MAPK
Mitogen-activated protein kinases
- MCL-1
Myeloid cell leukemia-1
- MIR26a
microRNA 26a
- MTDH/AEG1
Metadherin/Astrocytevelevated gene-1
- MYC
Avian myelocytomatosis viral oncogene homolog
- NOXA/PMAIP1
Phorbol-12-myristate-13-acetate-induced protein 1
- N-Wasp
Neural Wiskott-Aldrich syndrome protein
- PCNA
Proliferating cell nuclear antigen
- PI3K
Phosphoinositide 3-kinase
- PUMA/BBC3
p53 upregulated modulator of apoptosis/BCL-2 binding component 3
- RAC
Ras-related C3 botulinum toxin substrate 1
- SFK
SRC family kinase
- STAT
Signal transducer and activator of transcription
- STAT3
Signal transducer and activator of transcription 3
- STAT5
Signal transducer and activator of transcription 5
- tBID
truncated BH3- interacting-domain death agonist
- USPX
Ubiquitin Specific Peptidase 9
- YES
Tyrosine-protein kinase Yes
Disclosure statement
The authors declare no competing or financial interests.
References
- 1.Young AI, Law AM, Castillo L, et al.. MCL-1 inhibition provides a new way to suppress breast cancer metastasis and increase sensitivity to dasatinib. Breast cancer research: BCR. 2016;18(1):125. doi: 10.1186/s13058-016-0781-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kozopas KM, Yang T, Buchan HL, et al.. MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL2. Proceedings of the National Academy of Sciences of the United States of America 1993;90(8):3516–3520. doi: 10.1073/pnas.90.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strasser A, Cory S, Adams JM. Deciphering the rules of programmed cell death to improve therapy of cancer and other diseases. Embo J. 2011;30(18):3667–3683. doi: 10.1038/emboj.2011.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perciavalle RM, Opferman JT. Delving deeper: MCL-1's contributions to normal and cancer biology. Trends Cell Biol. 2013;23(1):22–29. doi: 10.1016/j.tcb.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beroukhim R, Mermel CH, Porter D, et al.. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463(7283):899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abulwerdi F, Liao C, Liu M, et al.. A novel small-molecule inhibitor of mcl-1 blocks pancreatic cancer growth in vitro and in vivo. Mol Cancer Ther. 2014;13(3):565–575. doi: 10.1158/1535-7163.MCT-12-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akgul C. Mcl-1 is a potential therapeutic target in multiple types of cancer. Cellular and molecular life sciences: CMLS. 2009;66(8):1326–1336. doi: 10.1007/s00018-008-8637-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chowdry RP, Sica GL, Kim S, et al.. Phosphorylated Bcl-2 and Mcl-1 as prognostic markers in small cell lung cancer. Oncotarget. 2016; February 18. doi: 10.18632/oncotarget.7485. [DOI] [Google Scholar]
- 9.Goodwin CM, Rossanese OW, Olejniczak ET, et al.. Myeloid cell leukemia-1 is an important apoptotic survival factor in triple-negative breast cancer. Cell Death Differ. 2015;22(12):2098–2106. doi: 10.1038/cdd.2015.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leverson JD, Zhang H, Chen J, et al.. Potent and selective small-molecule MCL-1 inhibitors demonstrate on-target cancer cell killing activity as single agents and in combination with ABT-263 (navitoclax). Cell Death Dis. 2015;6:e1590. doi: 10.1038/cddis.2014.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Modugno M, Banfi P, Gasparri F, et al.. Mcl-1 antagonism is a potential therapeutic strategy in a subset of solid cancers. Experimental cell research. 2015;332(2):267–277. doi: 10.1016/j.yexcr.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 12.Quinn BA, Dash R, Azab B, et al.. Targeting Mcl-1 for the therapy of cancer. Expert opinion on investigational drugs. 2011;20(10):1397–1411. doi: 10.1517/13543784.2011.609167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou P, Levy NB, Xie H, et al.. MCL1 transgenic mice exhibit a high incidence of B-cell lymphoma manifested as a spectrum of histologic subtypes. Blood. 2001;97(12):3902–3909. doi: 10.1182/blood.V97.12.3902. [DOI] [PubMed] [Google Scholar]
- 14.Campbell KJ, Bath ML, Turner ML, et al.. Elevated Mcl-1 perturbs lymphopoiesis, promotes transformation of hematopoietic stem/progenitor cells, and enhances drug resistance. Blood. 2010;116(17):3197–3207. doi: 10.1182/blood-2010-04-281071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beverly LJ, Varmus HE. MYC-induced myeloid leukemogenesis is accelerated by all six members of the antiapoptotic BCL family. Oncogene. 2009;28(9):1274–1279. doi: 10.1038/onc.2008.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams MM, Cook RS. Bcl-2 family proteins in breast development and cancer: could Mcl-1 targeting overcome therapeutic resistance? Oncotarget. 2015;6(6):3519–3530. doi: 10.18632/oncotarget.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palve V, Mallick S, Ghaisas G, et al.. Overexpression of Mcl-1L splice variant is associated with poor prognosis and chemoresistance in oral cancers. PLoS One. 2014;9(11):e111927. doi: 10.1371/journal.pone.0111927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toge M, Yokoyama S, Kato S, et al.. Critical contribution of MCL-1 in EMT-associated chemo-resistance in A549 non-small cell lung cancer. Int J Oncol. 2015;46(4):1844–1848. doi: 10.3892/ijo.2015.2861. [DOI] [PubMed] [Google Scholar]
- 19.Wei D, Zhang Q, Schreiber JS, et al.. Targeting mcl-1 for radiosensitization of pancreatic cancers. Translational oncology. 2015;8(1):47–54. doi: 10.1016/j.tranon.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wertz IE, Kusam S, Lam C, et al.. Sensitivity to antitubulin chemotherapeutics is regulated by MCL1 and FBW7. Nature. 2011;471(7336):110–114. doi: 10.1038/nature09779. [DOI] [PubMed] [Google Scholar]
- 21.Balko JM, Giltnane JM, Wang K, et al.. Molecular Profiling of the Residual Disease of Triple-Negative Breast Cancers after Neoadjuvant Chemotherapy Identifies Actionable Therapeutic Targets. Cancer discovery. 2014;4(2):232–45. doi: 10.1158/2159-8290.CD-13-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao Y, Nimmer P, Sheppard GS, et al.. MCL-1 Is a Key Determinant of Breast Cancer Cell Survival: Validation of MCL-1 Dependency Utilizing a Highly Selective Small Molecule Inhibitor. Molecular cancer therapeutics. 2015;14(8):1837–1847. doi: 10.1158/1535-7163.MCT-14-0928. [DOI] [PubMed] [Google Scholar]
- 23.Bashari MH, Fan F, Vallet S, et al.. Mcl-1 confers protection of Her2-positive breast cancer cells to hypoxia: therapeutic implications. Breast cancer research: BCR. 2016;18(1):26. doi: 10.1186/s13058-016-0686-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruncko M, Wang L, Sheppard GS, et al.. Structure-guided design of a series of MCL-1 inhibitors with high affinity and selectivity. J Med Chem. 2015;58(5):2180–2194. doi: 10.1021/jm501258m. [DOI] [PubMed] [Google Scholar]
- 25.Kotschy A, Szlavik Z, Murray J, et al.. The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature. 2016;538(7626):477–482. doi: 10.1038/nature19830. [DOI] [PubMed] [Google Scholar]
- 26.Merino D, Whittle JR, Vaillant F, et al.. Synergistic action of the MCL-1 inhibitor S63845 with current therapies in preclinical models of triple-negative and HER2-amplified breast cancer. Sci Transl Med. 2017;9(401).(pii):eaam7049. doi: 10.1126/scitranslmed.aam7049. [DOI] [PubMed] [Google Scholar]
- 27.Zhong Q, Gao W, Du F, Wang X. Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell. 2005;121(7):1085–1095. doi: 10.1016/j.cell.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 28.Czabotar PE, Lee EF, van Delft MF, et al.. Structural insights into the degradation of Mcl-1 induced by BH3 domains. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(15):6217–6222. doi: 10.1073/pnas.0701297104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gomez-Bougie P, Menoret E, Juin P, et al.. Noxa controls Mule-dependent Mcl-1 ubiquitination through the regulation of the Mcl-1/USP9X interaction. Biochem Biophys Res Commun. 2011;413(3):460–464. doi: 10.1016/j.bbrc.2011.1008.1118. Epub 2011 Sep 1011. doi:. [DOI] [PubMed] [Google Scholar]
- 30.Inuzuka H, Shaik S, Onoyama I, et al.. SCF(FBW7) regulates cellular apoptosis by targeting MCL1 for ubiquitylation and destruction. Nature. 2011;471(7336):104–109. doi: 10.1038/nature09732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding Q, He X, Xia W, et al.. Myeloid cell leukemia-1 inversely correlates with glycogen synthase kinase-3beta activity and associates with poor prognosis in human breast cancer. Cancer Res. 2007;67(10):4564–4571. doi: 10.1158/0008-5472.CAN-06-1788. [DOI] [PubMed] [Google Scholar]
- 32.De Biasio A, Vrana JA, Zhou P, et al.. N-terminal truncation of antiapoptotic MCL1, but not G2/M-induced phosphorylation, is associated with stabilization and abundant expression in tumor cells. J Biol Chem. 2007;282(33):23919–23936. doi: 10.1074/jbc.M700938200. [DOI] [PubMed] [Google Scholar]
- 33.Warr MR, Shore GC. Unique biology of Mcl-1: therapeutic opportunities in cancer. Curr Mol Med. 2008;8(2):138–147. doi: 10.2174/156652408783769580. [DOI] [PubMed] [Google Scholar]
- 34.Arai KI, Lee F, Miyajima A, Miyatake S, et al.. Cytokines: coordinators of immune and inflammatory responses. Annu Rev Biochem. 1990;59:783–836. doi: 10.1146/annurev.bi.59.070190.004031. [DOI] [PubMed] [Google Scholar]
- 35.Wang JM, Chao JR, Chen W, et al.. The antiapoptotic gene mcl-1 is up-regulated by the phosphatidylinositol 3-kinase/Akt signaling pathway through a transcription factor complex containing CREB. Mol Cell Biol. 1999;19(9):6195–6206. doi: 10.1128/MCB.19.9.6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang JM, Lai MZ, Yang-Yen HF. Interleukin-3 stimulation of mcl-1 gene transcription involves activation of the PU.1 transcription factor through a p38 mitogen-activated protein kinase-dependent pathway. Mol Cell Biol. 2003;23(6):1896–1909. doi: 10.1128/MCB.23.6.1896-1909.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang HM, Huang CJ, Yen JJ. Mcl-1 is a common target of stem cell factor and interleukin-5 for apoptosis prevention activity via MEK/MAPK and PI-3K/Akt pathways. Blood. 2000;96(5):1764–1771. [PubMed] [Google Scholar]
- 38.Isomoto H, Kobayashi S, Werneburg NW, et al.. Interleukin 6 upregulates myeloid cell leukemia-1 expression through a STAT3 pathway in cholangiocarcinoma cells. Hepatology. 2005;42(6):1329–1338. doi: 10.1002/hep.20966. [DOI] [PubMed] [Google Scholar]
- 39.Liu H, Ma Y, Cole SM, et al.. Serine phosphorylation of STAT3 is essential for Mcl-1 expression and macrophage survival. Blood. 2003;102(1):344–352. doi: 10.1182/blood-2002-11-3396. [DOI] [PubMed] [Google Scholar]
- 40.Opferman JT, Letai A, Beard C, et al.. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426(6967):671–676. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]
- 41.Shenoy AR, Kirschnek S, Hacker G. IL-15 regulates Bcl-2 family members Bim and Mcl-1 through JAK/STAT and PI3K/AKT pathways in T cells. Eur J Immunol. 2014;44(8):2500–2507. doi: 10.1002/eji.201344238. [DOI] [PubMed] [Google Scholar]
- 42.Aichberger KJ, Mayerhofer M, Krauth MT, et al.. Identification of mcl-1 as a BCR/ABL-dependent target in chronic myeloid leukemia (CML): evidence for cooperative antileukemic effects of imatinib and mcl-1 antisense oligonucleotides. Blood. 2005;105(8):3303–3311. doi: 10.1182/blood-2004-02-0749. [DOI] [PubMed] [Google Scholar]
- 43.Jiang CC, Lucas K, Avery-Kiejda KA, et al.. Up-regulation of Mcl-1 is critical for survival of human melanoma cells upon endoplasmic reticulum stress. Cancer Res. 2008;68(16):6708–6717. doi: 10.1158/0008-5472.CAN-08-0349. [DOI] [PubMed] [Google Scholar]
- 44.Piret JP, Minet E, Cosse JP, Ninane N, et al.. Hypoxia-inducible factor-1-dependent overexpression of myeloid cell factor-1 protects hypoxic cells against tert-butyl hydroperoxide-induced apoptosis. J Biol Chem. 2005;280(10):9336–9344. doi: 10.1074/jbc.M411858200. [DOI] [PubMed] [Google Scholar]
- 45.Rinkenberger JL, Horning S, Klocke B, et al.. Mcl-1 deficiency results in peri-implantation embryonic lethality. Genes Dev. 2000;14(1):23–27. [PMC free article] [PubMed] [Google Scholar]
- 46.Renjini AP, Titus S, Narayan N, Murali M, et al.. STAT3 and Mcl-1 unite to cause mesenchymal epithelial transition. J Cell Sci. 2014;127(Pt 8):1738–50. doi: 10.1242/jcs.138214. [DOI] [PubMed] [Google Scholar]
- 47.Huskey NE, Guo T, Evason KJ, et al.. CDK1 inhibition targets the p53-NOXA-MCL1 axis, selectively kills embryonic stem cells, and prevents teratoma formation. Stem Cell Reports. 2015;4(3):374–389. doi: 10.1016/j.stemcr.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morciano G, Giorgi C, Balestra D, et al.. Mcl-1 involvement in mitochondrial dynamics is associated with apoptotic cell death. Mol Biol Cell. 2016;27(1):20–34. doi: 10.1091/mbc.E15-01-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perciavalle RM, Stewart DP, Koss B, et al.. Anti-apoptotic MCL-1 localizes to the mitochondrial matrix and couples mitochondrial fusion to respiration. Nat Cell Biol. 2012;14(6):575–583. doi: 10.1038/ncb2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jamil S, Sobouti R, Hojabrpour P, et al.. A proteolytic fragment of Mcl-1 exhibits nuclear localization and regulates cell growth by interaction with Cdk1. The Biochemical journal. 2005;387(Pt 3):659–667. doi: 10.1042/BJ20041596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jamil S, Stoica C, Hackett TL, et al.. MCL-1 localizes to sites of DNA damage and regulates DNA damage response. Cell cycle (Georgetown, Tex). 2010;9(14):2843–2855. doi: 10.4161/cc.9.14.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Malone CD, Hasan SM, Roome RB, et al.. Mcl-1 regulates the survival of adult neural precursor cells. Mol Cell Neurosci. 2012;49(4):439–447. doi: 10.1016/j.mcn.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 53.Kuramoto K, Sakai A, Shigemasa K, et al.. High expression of MCL1 gene related to vascular endothelial growth factor is associated with poor outcome in non-Hodgkin's lymphoma. Br J Haematol. 2002;116(1):158–161. doi: 10.1046/j.1365-2141.2002.03253.x. [DOI] [PubMed] [Google Scholar]
- 54.Watson EC, Whitehead L, Adams RH, et al.. Endothelial cell survival during angiogenesis requires the pro-survival protein MCL1. Cell Death Differ. 2016;23(8):1371–1379. doi: 10.1038/cdd.2016.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee WS, Park YL, Kim N, et al.. Myeloid cell leukemia-1 is associated with tumor progression by inhibiting apoptosis and enhancing angiogenesis in colorectal cancer. Am J Cancer Res. 2015;5(1):101–113. [PMC free article] [PubMed] [Google Scholar]
- 56.Hasan SM, Sheen AD, Power AM, et al.. Mcl1 regulates the terminal mitosis of neural precursor cells in the mammalian brain through p27Kip1. Development. 2013;140(15):3118–3127. doi: 10.1242/dev.090910. [DOI] [PubMed] [Google Scholar]
- 57.Koehler BC, Scherr AL, Lorenz S, et al.. Beyond cell death – antiapoptotic Bcl-2 proteins regulate migration and invasion of colorectal cancer cells in vitro. PLoS One. 2013;8(10):e76446. doi: 10.1371/journal.pone.0076446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee WS, Kim N, Park YR, et al.. Myeloid cell leukemia-1 promotes epithelial-mesenchymal transition of human gastric cancer cells. Oncol Rep. 2015;34(2):1011–1016. doi: 10.3892/or.2015.4040. [DOI] [PubMed] [Google Scholar]
- 59.Gao J, Li L, Wu M, Liu M, et al.. MiR-26a Inhibits Proliferation and Migration of Breast Cancer through Repression of MCL-1. PLoS One. 2013;8(6):e65138. doi: 10.1371/journal.pone.0065138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O'Toole SA, Beith JM, Millar EKA, et al.. Therapeutic targets in triple negative breast cancer. J Clin Pathol. 2013;66(6):530–542. doi: 10.1136/jclinpath-2012-201361. [DOI] [PubMed] [Google Scholar]
- 61.Lee EF, Czabotar PE, van Delft MF, et al.. A novel BH3 ligand that selectively targets Mcl-1 reveals that apoptosis can proceed without Mcl-1 degradation. J Cell Biol. 2008;180(2):341–355. doi: 10.1083/jcb.200708096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Glaser SP, Lee EF, Trounson E, et al.. Anti-apoptotic Mcl-1 is essential for the development and sustained growth of acute myeloid leukemia. Genes Dev. 2012;26(2):120–125. doi: 10.1101/gad.182980.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Timpson P, McGhee EJ, Erami Z, et al.. Organotypic collagen I assay: a malleable platform to assess cell behaviour in a 3-dimensional context. Journal of visualized experiments: JoVE. 2011;(56):e3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamaguchi H, Pixley F, Condeelis J. Invadopodia and podosomes in tumor invasion. European journal of cell biology. 2006;85(3–4):213–218. doi: 10.1016/j.ejcb.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 65.Wheeler DL, Iida M, Dunn EF. The role of Src in solid tumors. The oncologist. 2009;14(7):667–678. doi: 10.1634/theoncologist.2009-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buccione R, Orth JD, McNiven MA. Foot and mouth: podosomes, invadopodia and circular dorsal ruffles. Nat Rev Mol Cell Biol. 2004;5(8):647–657. doi: 10.1038/nrm1436. [DOI] [PubMed] [Google Scholar]
- 67.Yamaguchi H, Wyckoff J, Condeelis J. Cell migration in tumors. Curr Opin Cell Biol. 2005;17(5):559–564. doi: 10.1016/j.ceb.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 68.Okada M. Regulation of the SRC family kinases by Csk. International journal of biological sciences. 2012;8(10):1385–1397. doi: 10.7150/ijbs.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reynolds AB, Kanner SB, Bouton AH, et al.. SRChing for the substrates of Src. Oncogene. 2014;33(37):4537–4547. doi: 10.1038/onc.2013.416. [DOI] [PubMed] [Google Scholar]
- 70.Roy S, Ruest PJ, Hanks SK. FAK regulates tyrosine phosphorylation of CAS, paxillin, and PYK2 in cells expressing v-Src, but is not a critical determinant of v-Src transformation. J Cell Biochem. 2002;84(2):377–388. doi: 10.1002/jcb.10025. [DOI] [PubMed] [Google Scholar]
- 71.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 72.Jorissen RN, Walker F, Pouliot N, et al.. Epidermal growth factor receptor: mechanisms of activation and signalling. Experimental cell research. 2003;284(1):31–53. doi: 10.1016/S0014-4827(02)00098-8. [DOI] [PubMed] [Google Scholar]
- 73.Oser M, Condeelis J. The cofilin activity cycle in lamellipodia and invadopodia. J Cell Biochem. 2009;108(6):1252–1262. doi: 10.1002/jcb.22372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oser M, Yamaguchi H, Mader CC, et al.. Cortactin regulates cofilin and N-WASp activities to control the stages of invadopodium assembly and maturation. J Cell Biol. 2009;186(4):571–587. doi: 10.1083/jcb.200812176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Morton JP, Karim SA, Graham K, et al.. Dasatinib inhibits the development of metastases in a mouse model of pancreatic ductal adenocarcinoma. Gastroenterology. 2010;139(1):292–303. doi: 10.1053/j.gastro.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 76.Nobis M, McGhee EJ, Morton JP, et al.. Intravital FLIM-FRET imaging reveals dasatinib-induced spatial control of src in pancreatic cancer. Cancer Res. 2013;73(15):4674–4686. doi: 10.1158/0008-5472.CAN-12-4545. [DOI] [PubMed] [Google Scholar]
- 77.Chee CE, Krishnamurthi S, Nock CJ, et al.. Phase II study of dasatinib (BMS-354825) in patients with metastatic adenocarcinoma of the pancreas. The oncologist. 2013;18(10):1091–1092. doi: 10.1634/theoncologist.2013-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Evans TR, Van Cutsem E, Moore MJ, et al.. Phase 2 Placebo-Controlled, Double-Blind Trial of Dasatinib Added to Gemcitabine for Patients with Locally-Advanced Pancreatic Cancer. Ann Oncol. 2017;28(2):354–361. doi: 10.1093/annonc/mdw607. [DOI] [PubMed] [Google Scholar]
- 79.Renouf DJ, Moore MJ, Hedley D, et al.. A phase I/II study of the Src inhibitor saracatinib (AZD0530) in combination with gemcitabine in advanced pancreatic cancer. Invest New Drugs. 2012;30(2):779–786. doi: 10.1007/s10637-010-9611-3. [DOI] [PubMed] [Google Scholar]
- 80.Elias D, Ditzel HJ. Fyn is an important molecule in cancer pathogenesis and drug resistance. Pharmacol Res. 2015;100:250–254. doi: 10.1016/j.phrs.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 81.Gucalp A, Sparano JA, Caravelli J, et al.. Phase II trial of saracatinib (AZD0530), an oral SRC-inhibitor for the treatment of patients with hormone receptor-negative metastatic breast cancer. Clin Breast Cancer. 2011;11(5):306–311. doi: 10.1016/j.clbc.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Finn RS, Bengala C, Ibrahim N, et al.. Dasatinib as a single agent in triple-negative breast cancer: results of an open-label phase 2 study. Clinical cancer research: an official journal of the American Association for Cancer Research. 2011;17(21):6905–6913. doi: 10.1158/1078-0432.CCR-11-0288. [DOI] [PubMed] [Google Scholar]
- 83.Somlo G, Atzori F, Strauss LC, et al.. Dasatinib plus capecitabine for advanced breast cancer: safety and efficacy in phase I study CA180004. Clinical cancer research: an official journal of the American Association for Cancer Research. 2013;19(7):1884–1893. doi: 10.1158/1078-0432.CCR-12-0652. [DOI] [PubMed] [Google Scholar]
- 84.Fornier MN, Morris PG, Abbruzzi A, et al.. A phase I study of dasatinib and weekly paclitaxel for metastatic breast cancer. Ann Oncol. 2011;22(12):2575–2581. doi: 10.1093/annonc/mdr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moy B, Neven P, Lebrun F, et al.. Bosutinib in combination with the aromatase inhibitor exemestane: a phase II trial in postmenopausal women with previously treated locally advanced or metastatic hormone receptor-positive/HER2-negative breast cancer. The Oncologist. 2014;19(4):346–347. doi: 10.1634/theoncologist.2014-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Erami Z, Herrmann D, Warren SC, et al.. Intravital FRAP Imaging using an E-cadherin-GFP Mouse Reveals Disease- and Drug-Dependent Dynamic Regulation of Cell-Cell Junctions in Live Tissue. Cell Rep. 2016;14(1):152–167. doi: 10.1016/j.celrep.2015.1012.1020 Epub 2015 Dec 1024. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vickers ER, Kasza A, Kurnaz IA, et al.. Ternary complex factor-serum response factor complex-regulated gene activity is required for cellular proliferation and inhibition of apoptotic cell death. Mol Cell Biol. 2004;24(23):10340–10351. doi: 10.1128/MCB.24.23.10340-10351.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Townsend KJ, Trusty JL, Traupman MA, et al.. Expression of the antiapoptotic MCL1 gene product is regulated by a mitogen activated protein kinase-mediated pathway triggered through microtubule disruption and protein kinase C. Oncogene 1998;17(10):1223–1234. doi: 10.1038/sj.onc.1202035. [DOI] [PubMed] [Google Scholar]
- 89.Fritsch RM, Schneider G, Saur D, et al.. Translational repression of MCL-1 couples stress-induced eIF2 alpha phosphorylation to mitochondrial apoptosis initiation. J Biol Chem. 2007;282(31):22551–22562. doi: 10.1074/jbc.M702673200. [DOI] [PubMed] [Google Scholar]
- 90.Choudhary C, Mann M. Decoding signalling networks by mass spectrometry-based proteomics. Nat Rev Mol Cell Biol. 2010;11(6):427–439. doi: 10.1038/nrm2900. [DOI] [PubMed] [Google Scholar]
- 91.Harley ME, Allan LA, Sanderson HS, Clarke PR. Phosphorylation of Mcl-1 by CDK1-cyclin B1 initiates its Cdc20-dependent destruction during mitotic arrest. Embo J. 2010;29(14):2407–2420. doi: 10.1038/emboj.2010.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kobayashi S, Lee SH, Meng XW, Mott JL, Bronk SF, Werneburg NW, Craig RW, Kaufmann SH, Gores GJ. Serine 64 phosphorylation enhances the antiapoptotic function of Mcl-1. J Biol Chem. 2007;282(25):18407–18417. doi: 10.1074/jbc.M610010200. [DOI] [PubMed] [Google Scholar]
- 93.Nakajima W, Hicks MA, Tanaka N, Krystal GW, Harada H. Noxa determines localization and stability of MCL-1 and consequently ABT-737 sensitivity in small cell lung cancer. Cell Death Dis. 2014;5:e1052. doi: 10.1038/cddis.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Inoshita S, Takeda K, Hatai T, et al.. Phosphorylation and inactivation of myeloid cell leukemia 1 by JNK in response to oxidative stress. J Biol Chem. 2002;277(46):43730–43734. Epub 42002 Sep 43739. doi: 10.1074/jbc.M207951200. [DOI] [PubMed] [Google Scholar]
- 95.Kodama T, Hikita H, Kawaguchi T, et al.. Mcl-1 and Bcl-xL regulate Bak/Bax-dependent apoptosis of the megakaryocytic lineage at multistages. Cell Death Differ. 2012;19(11):1856–1869. doi: 10.1038/cdd.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ding Q, Huo L, Yang JY, et al. et al.. Down-regulation of myeloid cell leukemia-1 through inhibiting Erk/Pin 1 pathway by sorafenib facilitates chemosensitization in breast cancer. Cancer Res. 2008;68(15):6109–6117. doi: 10.1158/0008-5472.CAN-08-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Domina AM, Vrana JA, Gregory MA, et al.. MCL1 is phosphorylated in the PEST region and stabilized upon ERK activation in viable cells, and at additional sites with cytotoxic okadaic acid or taxol. Oncogene. 2004;23(31):5301–5315. doi: 10.1038/sj.onc.1207692. [DOI] [PubMed] [Google Scholar]
- 98.Nifoussi SK, Vrana JA, Domina AM, et al.. Thr 163 phosphorylation causes Mcl-1 stabilization when degradation is independent of the adjacent GSK3-targeted phosphodegron, promoting drug resistance in cancer. PLoS One. 2012;7(10):e47060. doi: 10.41371/journal.pone.0047060 Epub 0042012 Oct 0047069. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chu R, Terrano DT, Chambers TC. Cdk1/cyclin B plays a key role in mitotic arrest-induced apoptosis by phosphorylation of Mcl-1, promoting its degradation and freeing Bak from sequestration. Biochem Pharmacol. 2012;83(2):199–206. doi: 10.1016/j.bcp.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Thomas E, Lee-Pullen T, Rigby P, et al.. Receptor activator of NF-kappaB ligand promotes proliferation of a putative mammary stem cell unique to the lactating epithelium. Stem Cells (Dayton, Ohio). 2012;30(6):1255–1264. doi: 10.1002/stem.1092. [DOI] [PubMed] [Google Scholar]
- 101.Iqbal S, Howard S, LoGrasso PV. Serum- and Glucocorticoid-Inducible Kinase 1 Confers Protection in Cell-Based and in In Vivo Neurotoxin Models via the c-Jun N-Terminal Kinase Signaling Pathway. Mol Cell Biol. 2015;35(11):1992–2006. doi: 10.1128/MCB.01510-01514 Epub 02015 Mar 01530. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Maurer U, Charvet C, Wagman AS, et al.. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21(6):749–760. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 103.Morel C, Carlson SM, White FM, et al.. Mcl-1 integrates the opposing actions of signaling pathways that mediate survival and apoptosis. Mol Cell Biol. 2009;29(14):3845–3852. doi: 10.1128/MCB.00279-00209 Epub 02009 May 00211. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]


