ABSTRACT
Extracellular matrix (ECM) provides cells scaffolding for cell migration and microenvironment for various cellular functions. Collagens are major ECM components in tissue and discoidin domain receptors (DDRs) are receptor tyrosine kinases (RTK) that recognise fibrillar collagens. Unlike other RTK, their ligands are solid ECM the that are abundantly present in the pericellular environment in various tissue, and thus its activation and regulations are unique amongst RTK family. It is emerging that DDRs may be the sensors that monitor and detects changes in ECM microenvironment and determines the cellular fates upon tissue injuries. In this mini-review, recent findings on the role of DDRs as microenvironment sensor and their roles in cell migration and invasion are discussed.
Keywords: adhesion signaling, adhesion, cell adhesion, cell migration, Cell signaling, collagen, DDR, extracellular matrix, ECM, invasion, integrins, MMP, microenvironment, migration, migration, MMP, RTK, tyrosine kinases
1. Introduction
In multicellular organisms, extracellular matrix (ECM) plays an essential role in maintaining functional tissue structures, as a scaffolding to support cell migration and as a reservoir for growth factors. Also, components of ECM can directly transmit signals, promoting cell survival and differentiation. Among various ECM proteins, collagen is the most abundant ECM molecules in our body and a major component of cellular microenvironment [1]. There are several classes of collagen binding receptors in mammals including Integrins, discoidin domain receptors (DDRs), glycoprotein IV, Leukocyte-associated Ig-like receptor-1, Mannose receptor family proteins including uPARAP (urokinase plasminogen activator receptor-associated protein)/Endo180 [2]. Among these molecules, there are two classes of receptors that are known to transmit collagen signals to the cells: collagen binding integrins [3] and discoidin domain receptors [4]. Integrins are heterodimers of non-covalently associated α and β subunits that is a major family of ECM receptors for cell adhesion [3]. Of the 24 distinct integrins in humans, four members including α1β1, α2β1, α10β1 and α11β1 are known to be collagen binding integrins [2]. They all have common β1 subunits and their ability to bind to collagen can be altered by their active- or inactive-conformation of the integrins that can be regulated by both outside-in and inside-out signals [3]. This allows cells to be able to control cell adhesion in a spatiotemporal manner. On the other hand, the ability of DDRs to bind to collagen is spontaneous and is not influenced by signals from outside or inside. Therefore, it requires additional steps to control binding of DDRs to collagen as well as its signaling. In this mini-review, recent findings of their unique regulatory mechanism and involvement in cell migration and microenvironment recognition are discussed.
2. Structure of ddrs and their expression
DDRs are type I transmembrane proteins. Their domain structure consists of a collagen binding discoidin (DS) domain, a DS-like domain, an extracellular juxtamembrane (JM) region, a transmembrane region, an intracellular JM region and a tyrosine kinase (TK) domain. N-terminal DS domain is the collagen binding domain in both DDR1 and 2. DDR1 has five different isoforms due to alternative splicing: DDR1a-e. Among these isoforms, DDR1d and DDR1e are kinase—deficient due to a frame shift and truncation. DDR1a (876 amino acids) and DDR1b (913 amino acids) are commonly expressed DDR1 isoforms, and DDR1b has an insertion of 37 amino acids at the intracellular JM region of DDR1a sequence. DDR1c has additional 6 amino acids insertion in TK domain in DDR1b sequence. DDR2 does not have such isoforms and consists of 588 amino acids [4, 5]. DDR1 is generally expressed in epithelial cells and immune cells including mononuclear cells, activated T-cells. DDR2 is generally expressed in mesenchymal cells including fibroblasts and chondrocytes, but it was also shown to be expressed in neutrophils [4]. In normal cells, there are no examples that both DDR1 and DDR2 are expressed, but several de-differentiated epithelial cancer cells and fibrosarcoma cells are shown to express both DDR1 and DDR2 while well-differentiated epithelial cancer cells seem to express the only DDR1, but not DDR2.
3. Ligand specificity
DDRs are unique among RTK family in being bound and activated by solid ECM components, collagens. Monomer collagens consist of three polypeptide α chains forming right-handed triple helical structure. Some collagens are composed of three identical α chains (homo-trimeric collagens), while others have two to three distinct α chains (heteromeric collagens) [1]. These monomeric collagens further assemble fibers or sheet-like networks. The most abundant collagens in our body are fibrillar collagens (type I, II and III), and also network-forming collagen in the basement membrane, type IV collagen, is also widely present in different organs [1]. While both DDRs recognize fibrillar collagens, type IV collagen is recognized only by DDR1 but not by DDR2 [4]. DDR-binding sites in homo-trimeric collagens have been identified by Leitinger's group using triple helical peptide library [6, 7]. The studies identified GVMGFO (O is hydroxyproline) as a high-affinity binding motif for both DDR1 and DDR2 that is present in collagen types I-III. Interestingly GVMGFO motif cannot be found in collagen IV, suggesting that DDR1 binds different motif in this collagen [6]. Although both DDRs recognize the same motif, surrounding sequences seem to influence DDR1 binding and DDR2 seems to have relaxed specificity. For instance, DDR1 has only one binding site in collagen III. While DDR2 binds at least four high-affinity binding sites within the collagen [6].
4. Regulation of ddr signaling: Ectodomain shedding
Most of RTK family proteins bind to soluble ligands for their activation. Their signal activation is determined by the availability of a specific ligand to each RTK, and once the ligand binds to the ectodomain of RTK, they can be endocytosed for signaling, followed by termination of the process. The level of signaling is dependent on the local concentration of these bioavailable ligands, and it can be regulated by their gene expression or liberation of ligands from the local ECM by proteolysis. On the other hand, ligands of DDRs, collagens, are present in the pericellular spaces as a solid matrix. Therefore, receptor activation processes found in other RTK cannot be applied to DDRs due to following problem: (i) ligand is abundantly present in extracellular space; and (ii) when DDRs bind to collagen in solid ECM, it cannot be dissociated or endocytosed to terminate DDR signaling. It was found that DDR1 employs a unique mechanism to regulate collagen signaling: ectodomain shedding. Significant shedding of DDR1 has been detected upon collagen stimulation of cells [8]. It was found that a disintegrin and metalloprotease 10 (ADAM10) is the responsible enzyme for this collagen-induced DDR1 shedding, and ADAM10-dependent DDR1 shedding plays important roles in termination of DDR1 signaling [9]. The shedding-resistant mutant has longer signaling retention time, suggesting that ADAM10-dependent DDR1 shedding is an important regulatory mechanism of DDR1-mediated collagen signaling. It was shown that DDR1 and ADAM10 form a stable complex even before collagen stimulation without cleavage, and collagen binding to DS domain of DDR1 somehow renders DDR1 available for ADAM10 to cleave [9]. As DDR1 binds to collagen matrix, it also acts as an adhesion molecule. However, for an adhesion molecule to support cell migration, dissociation is as important as adhesion to ECM molecules. ADAM10-dependent shedding seems to be a major mechanism to dissociate DDR1-dependent cell adhesion to promote cell migration since inhibition of the shedding or expression of a shedding-resistant DDR1 mutant significantly inhibited cell migration on the collagen matrix [9]. DDR1 is also spontaneously shed in low level, but responsible shedding enzyme and biological significance have not been identified yet. Although it was reported that ectopic expression of membrane-type 1 matrix metalloproteinase (MT1-MMP) could result in the constitutive shedding of DDR1, endogenous MT1-MMP was not shown to do this [10], and ADAM10 has been ruled out [9]. Shedding of DDR2, on the other hand, has not been described, and MT1-MMP did not shed even with ectopic expression [10].
5. Role of ddrs in cell migration
Both DDRs has been shown to modulate capability of cells to migrate. For DDR1, there are some conflicting reports showing that expression of DDR1 impacts cell migration/invasion negatively and positively. Wang et al. [11] reported that ectopic expression of DDR1a and DDR1b in either MDCK epithelial cells or NIH-3T3 fibroblasts inhibited collagen-stimulated α2β1 integrin-mediated cell migration by inhibiting Stat1/3 tyrosine phosphorylation. The same group subsequently reported that ectopic expression of either DDR1a or DDR1b in MDCK cells inhibited tubulogenesis in 3D-collagen matrix as well, while expression of the cytoplasmic domain-deleted dominant negative form of DDR1 did not. A proposed mechanism is through the interaction of DDR1 with SHP-2, that counteracts α2β1-integrin-mediated phosphorylation of STAT3, leading to inhibition of migration and tubulogenesis of MDCK cells [12]. In contrast, using smooth muscle cells derived from DDR1 null mice, it was shown that DDR1 plays a role in collagen-induced cell migration [13], and a similar finding was also reported for squamous cell carcinoma cells that ectopic expression of DDR1a stimulated cell migration on the collagen matrix [9]. T-cell migration through 3D collagen matrix was shown to be inhibited by addition of soluble DDR1-Fc fusion protein, suggesting that engagement of DDR1 to the collagen plays a role in T-cell migration [14]. Similar data were also reported in Th17 T-cells where either knockdown of DDR1 or addition of DDR1-Fc fusion protein inhibited cell migration in 3D collagen [15]. There are some reports showing that DDR1a and DDR1b have different effects on cell migration. In THP-1 human monocytic cells, ectopic expression of DDR1a significantly increased MCP-1 induced cell migration through collagen matrix, but the expression of DDR1b did not influence the cell migration [16]. Similar data was also reported on glioma cells where ectopic expression of DDR1a stimulated both cell migration and invasion while DDR1b expression did not influence [17]. In non-small lung cancer cells ectopic expression DDR1a but not DDR1b enhanced cell migration in the absence of collagen, however, both DDR1a and DDR1b promoted cell migration in a significant manner upon collagen stimulation [18]. A potential reason for the different effects and role of DDR1 and its variants may be cell-type specific or different experimental conditions, but difference upon ectopic expression of DDR1 may potentially be explained by the expression level of ADAM10 as a DDR1-sheddase in different cells, since DDR1 expression without efficient shedding of DDR1 ectodomain inhibits cell migration [9]. Nevertheless, endogenously expressed DDR1 seems to play a role in cell migration and invasion [13, 15].
DDR2 was also shown to support cell migration. It was reported that efficient migration of skin fibroblasts through Matrigel requires DDR2 as DDR2 null fibroblasts showed significantly reduced cell migration compared to cells from heterozygous mice, while expression of DDR2 in the knockout cells recovered their cell migration phenotype [19]. It was also reported that vascular smooth muscle cell migration induced by the hypoxic condition is due to increased DDR2 expression through the signal via p38 MAPK [20].
6. Role of ddrs as cell adhesion molecules
Cell migration requires repetition of cell adhesion to and cell detachment from their ECM substratum. DDRs mediates cell adhesion to collagen, and this may be one of the ways for DDRs to stimulate cell motility. It has been shown for DDR1 that detachment of DDR1-dependent cell adhesion can be carried out by ectodomain shedding by ADAM10 [9]. On the other hand, the well recongnised group of cell adhesion molecules is integrins, and collagen binding integrins including α1β1, α2β1, α10β1 and α11β1 are considered to play a role in cell migration on collagenous matrix [3]. Since DDRs and integrins are co-expressed in the cells, a cross-talk between DDRs and integrins is considered to be crucial for cell migration in collagenous environment. It was shown that DDR1 activation by collagen is independent from β1 integrin activation [21]. However, Xu et al. [22] have reported that activation of both DDR1 and DDR2 promotes α1β1- and α2β1-integrins mediated cell adhesion to collagen in 293 cells. This may partially explain proadhesive and promigratory effect of DDRs that has been decribed in different cell types [9, 13, 15–18]. Whether different promigratory effect in DDR1a and DDR1b can be explained by integrin cross talk still need to be addressed in future, and detailed molecular links between two collagen receptors are to be further investigated. On the contraly in MDCK epithelial cells and NIH3T3 cells DDR1 was found to inhibit cell migration by inhibiting α2β1 integrin-mediated Stat1/3 tyrosine phosphorylation [11]. It is not clear if this discrepancy is due to different cellular context or experimental procedures, but it needs to be further clarified in futurre.
Recently it was reported that binding of α1β1, α2β1, and α10β1 integrins to cartilage matrix depend on non-collagenous surface macromolecules rather than fibrillar collagens [23]. They confirmed that these β1integrins could bind to monomeric collagens in Mg2+-dependent manner, but failed to bind to fibrillar collagens. They also have shown that chondrocyte adhesion to fibrillar collagen was significantly inhibited by addition of DDR2 ectodomain, suggesting that role of DDRs as cell adhesion molecules in relative to integrins may be more significant within collagenous microenvironment. It is important to further investigate relative roles of DDRs and integrins as a cell adhesion molecules.
7. Modulation of matrix metalloproteinase expression and activity
Cell migration within 3D ECM environment can be classified into mesenchymal migration and amoeboid migration [24, 25]. In mesenchymal migration, cells degrade barrier ECM by proteinases to make a path, while in amoeboid migration cells migrate through ECM gaps by squeezing cell body without degrading ECM [24, 25]. The migration mode is dependent on the size of the gaps within ECM and ability of different cells to squeeze the cell body [25, 26]. It is considered that cancer, epithelial and stromal cells migrate through mesenchymal migration and leukocytes employ amoeboid migration [25], but this is likely to be determined by how squeezable the cell bodies are. Nevertheless, when migratory cells face to the barrier matrix that cells cannot migrate through without enlarging the gap, ECM degradation becomes an important step in migration, and role of DDRs in promoting cellular invasion is partially due to modulating expression and activity of matrix metalloproteinases (MMPs), a group of the enzymes that degrades ECM components (Table 1). Vogel et al. [27] first demonstrated that MMP-1 (interstitial collagenase) is upregulated with collagen stimulation through DDR2 in HT1080 human fibrosarcoma cell line. Ferri et al. [28] also reported that ectopic expression of DDR1 and DDR2 upregulated MMP-1 expression. Robert et al. [29] reported that DDR1 knock down downregulated MMP-7 production in human bronchial epithelial cells. It has also been reported that expression of DDR1 upregulates MMP-2 and MMP-9 secretion in different cells. Hou et al. [30] reported that DDR1 expression mediates MMP-2 and MMP-9 expression in mouse smooth muscle cell. Ram et al. [17] described that ectopic expression of DDR1a and DDR1b induced upregulated production of both proMMP-2 and proMMP-9, and collagen stimulation induced activation of proMMP-2 in the cells that overexpress DDR1a but not DDR1b or endogenous DDR1. A similar report was made by Park et al. [31], showing that ectopic expression of DDR1a or DDR1b upregulated proMMP-2 and proMMP-9 production in hepatocarcinoma cell line. Castro-Sanchez et al. [32] described that stimulation of DDR1 in breast cancer cells, MDA-MB-231 and ZR-75 cells, with type IV collagen have resulted expression of both proMMP-2 and proMMP-9, and this required protein kinase C, activation of epidermal growth factor receptor, production of arachidonic acid (AA) and AA metabolites.
Table 1.
A list of reports showing roles of DDRs in MMPs production. A list of references showing DDRs-mediated MMPs upregulation are shown. Upreguated MMPs, references, cell types and requirement of collagen stimulation for MMPs upregulation are shown.
| DDRs |
Upregulated MMPs |
References |
Cell types |
Requirement of collagen |
| DDR1 | MMP-1 | Ferri et al.28 | human smooth muscle cells | No |
| MMP-2 & MMP-9 | Hou et al.30 | mouse smooth muscle cells | No | |
| Ram et al.17 | human glioma cells | No | ||
| Park et al.31 | human hepatocellular carcinoma | No | ||
| Castro-Sanchez et al.32 | MDA-MB231, ZR-75 | Yes | ||
| MMP-7 | Roberts et al.29 | human smooth muscle cells | ND | |
| DDR2 | MMP-1 | Vogel et al.27 | HT1080 | Yes |
| Ferri et al.28 | human smooth muscle cells | No | ||
| MMP-13 | Xu et al.37 | human chondrocytes | No | |
| Sunk et al.38 | human chondrocytes | Yes | ||
| Vonk et al.39 | human chondrocytes | Yes | ||
| MMP-14 | Majkowska et al.45 | human skin fibroblasts | Yes | |
| human rheumatoid synovial cells |
not determined
There is a clear link of DDR2 with a human disease, osteoarthritis (OA), which is the most common arthritis displaying an age-dependent cartilage degradation accompanied by loss of joint function. Cartilage is made of small number of chondrocytes and large part of ECM components of type II collagen (45%), aggrecan proteoglycan (45%) and other minor components. During OA development both aggrecan and collagen are degraded by metalloproteinases belonging to ADAMTS (a disintegrin and metalloproteinase with thrombospondin motif) enzymes and MMPs produced from chondrocytes [33]. MMP-13 (collagenase 3) has been implicated in cartilage degradation in OA [34-36], and it has been reported that DDR2 is a receptor that mediates expression of MMP-13 in human chondrocytes [37-39]. Heterozygote DDR2 null mice were shown to be partially protected from experimental OA in mouse accompanied by a decreased level of MMP-13 expression [40], suggesting that DDR2-MMP-13 axis contributes to OA development. However, chondrocytes in cartilage are segregated from fibrillar collagen II in a territorial matrix in cartilage due to the pericellular matrix surrounding chondrocyte. Further investigation is needed to understand the events that allow chondrocyte DDR2 to recognize collagen II in territorial matrix during OA development.
DDR2 may also be related to another form of arthritis, rheumatoid arthritis (RA). RA is an autoimmune inflammatory disease characterized by degradation joint tissue by inflamed synovial pannus tissues. There are two collagenolytic enzymes implicated in cartilage degradation in RA, namely MMP-13 and MT1-MMP both expressed by RA synovial cells. Su et al. [41] reported that DDR2 mediates type II collagen-induced MMP-13 expression. Since MMP-13 was shown to highly expressed at pannus-cartilage junctions [42], it is possible that DDR2-mediated cartilage collagen signal is responsible for this. At the pannus-cartilage junction in RA joints, MT1-MMP was also shown to be highly expressed and responsible to degrade cartilage tissue [24, 43, 44]. Like MMP-13, DDR2 also mediates collagen-induced activation of MT1-MMP gene expression and the function in human fibroblasts and RA synovial cells [45]. Knocking down DDR2 or pharmacological inhibition of DDR2 kinase effectively inhibited collagen-induced MT1-MMP activity including proMMP-2 activation and cellular degradation of collagen, suggesting that cartilage collagen signaling through DDR2 may be an endogenous pathway for MT1-MMP upregulation in RA as well. Since MT1-MMP is an endogenous activator of proMMP-13 [46], upregulation of both MMP-13 and MT1-MMP at the pannus-cartilage junction may play an important role in the progression of RA.
It is known that collagen stimulation also induces MT1-MMP activation in some cancer cells, but neither DDR1 nor DDR2 signaling plays a role in HT1080 fibrosarcoma cells since DDR kinase inhibition did not suppress collagen-induced MT1-MMP activities. In MDA-MB-231 cells, type I collagen did not influence the expression of MT1-MMP, MMP-2, and MMP-9 [45]. These findings suggest that DDR2 signaling may be cell-type specific, and there is another pathway that mediates collagen-induced activation of MT1-MMP.
8. Role of ddr1 in the formation of invadosome membrane structure
When cells invade into ECM, they extend specialized membrane protrusions into ECM that contain a proteolytic enzyme to make a path for migration. These membrane structures are called invadosomes which is rich in actin, and MT1-MMP is the enzyme that is concentrated in these membrane structures [47]. Invadosomes include podosomes that normal cells display and invadopodia that transformed cells extend. They are both thin (0.4–5 μm) and relatively short (1–8 μm) extending into ECM attached basal side of the plasma membrane [47]. Recently it was found that endothelial cells can display peculiar linear actin-rich membrane structure upon culturing cells on collagen fibrils, which occurs along collagen fibrils, named linear invadosomes [48]. Like invadopodia and podosomes, it is rich in actin, cortactin, Tks5, and MT1-MMP [48]. It was then found that formation of the linear invadosomes in MDA-MB231 breast cancer cells is DDR1-dependent [49]. Interestingly DDR1 kinase activity is not required for this, but it is via Cdc42 small GTPase and its guanine nucleotide-exchange factor, TUBA [49]. Presumably binding of DDR1 to the collagen triggers local activation of Cdc42 to form this invadosome, which in turn contribute to cellular invasion into collagen-rich ECM. However, detailed molecular mechanism how DDR1 triggers this event and in vivo role of DDR1-mediated invadosome formation are still to be investigated.
9. DDRs as microenvironment sensors
As described above, binding sites of DDR1 and DDR2 in type II and III collagens have been identified [6, 7], and the precise atomic interaction has been elucidated by crystallographic studies [50]. However, based on the fibrillar structure of collagen solved by X-ray fiber diffraction analysis [51], the DDR binding sites may not be readily exposed on the surface of collagen fibrils. Flynn et al. [52] showed that in vitro collagen fibrillogenesis was inhibited in the presence of the soluble ectodomain of DDR1 or DDR2, supporting the notion that DDR binding sites in monomeric collagen are located inside the fibril structure and not readily accessible. It is, therefore, possible that collagen molecules are capable of stimulating DDR only when newly synthesizeed, but before forming a highly-ordered fibril structure, or when the collagen fibrils are proteolytically, chemically or mechanically damaged to expose DDR-binding sites. One of the high-affinity binding sites for DDR2 in type-II collagen is covered by telopeptide lesion of collagen. Thus proteinases that are capable of removing this part of collagen may be able to convert non-stimulus collagen fibrils into stimulator for DDRs. Indeed, Majkowska et al. [45] reported that while acid-extracted type I collagen fibril with intact telopeptide only moderately induced DDR2-mediated MT1-MMP activation in synovial fibroblasts, the pepsin-treated type I collagen fibrils, whose telopeptide was removed, effectively induced MT1-MMP activation. Furthermore, it was found that culturing rheumatoid synovial fibroblasts on intact cartilage stimulated MT1-MMP activation only moderately, while culturing on trypsin-treated partially damaged cartilage, whose aggrecan and other minor components are removed, stimulated MT1-MMP activation effectively [45]. These findings suggest that DDRs may not be activated by collagen within the intact and healthy tissue, and local proteolytic damages may need to occur to expose DDR binding sites. In other words, DDRs are receptors that detect changes in ECM microenvironment (Figure 1). DDR2 activation results in transcriptional activation of various MMPs as discussed above and detection of collagen by DDR2 in partially damaged tissue may play a role in triggering tissue remodeling and repair process by upregulating ECM degrading enzymes.
Figure 1.
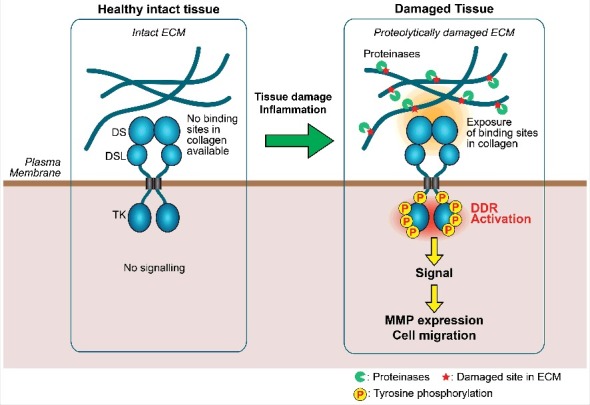
A model of microenvironment sensing by DDRs. When tissue is intact and healthy, DDR binding sites in collagen fibrils in tissue are not readily available for DDRs, thus DDRs cannot bind to collagens and the receptor activation does not occur. Upon tissue injuries causing proteolytic damage of ECM microenvironment, DDR binding sites within collagen become exposed, DDR activation tales place, and induce MMP expression and cell migration. This event may lead to tissue repair or contribute to further tissue damage and promote progression of diseases. DS, discoidin domain; DSL, discoidin-like domain; TK, tyrosine kinase domain.
10. Conclusions
DDRs are relatively under-investigated RTK, and unique among RTK super-family in its regulation, ligand property and being able to sense changes in ECM microenvironment as discussed above. DDRs are shown to involve in different cancers and arthritis, hence suggested that both DDRs are potential molecular targets for different diseases. There are several efforts being made to develop selective DDR kinase inhibitors, and there are some highly selective DDR1 inhibitors already developed [53-55]. However, pathophysiological roles of DDRs are still to be further revealed before applying DDR inhibitor drug to the clinic. For instance, an aspect of microenvironment sensor and their role in cell migration may be important in both physiological and pathological events, and benefits and detrimental effects upon inhibition need to be defined. Further detailed investigations of DDRs would contribute to potential novel means to full fill current unmet needs in future.
Funding Statement
The Kennedy Trust for Rheumatology Research ID: N/A. Arthritis Research UK programme grant (Ref: 19797).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Kadler KE, Baldock C, Bella J, et al.. Collagens at a glance. J Cell Sci. 2007;120(Pt 12):1955-8. [DOI] [PubMed] [Google Scholar]
- [2].Leitinger B. Transmembrane collagen receptors. Annu Rev Cell Dev Biol. 2011;27:265-90. [DOI] [PubMed] [Google Scholar]
- [3].Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673-87. [DOI] [PubMed] [Google Scholar]
- [4].Leitinger B. Discoidin domain receptor functions in physiological and pathological conditions. Int Rev Cell Mol Biol. 2014;310:39-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Valiathan RR, Marco M, Leitinger B, et al.. Discoidin domain receptor tyrosine kinases: new players in cancer progression. Cancer Metastasis Rev. 2012;31(1–2):295-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Xu H, Raynal N, Stathopoulos S, et al.. Collagen binding specificity of the discoidin domain receptors: binding sites on collagens II and III and molecular determinants for collagen IV recognition by DDR1. Matrix Biol. 2011;30(1):16-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Konitsiotis AD, Raynal N, Bihan D, et al.. Characterization of high affinity binding motifs for the discoidin domain receptor DDR2 in collagen. J Biol Chem. 2008;283(11):6861-8. [DOI] [PubMed] [Google Scholar]
- [8].Vogel WF. Ligand-induced shedding of discoidin domain receptor 1. FEBS Lett. 2002;514(2–3):175-80. [DOI] [PubMed] [Google Scholar]
- [9].Shitomi Y, Thogersen IB, Ito N, et al.. ADAM10 controls collagen signaling and cell migration on collagen by shedding the ectodomain of discoidin domain receptor 1 (DDR1). Mol Biol Cell. 2015;26(4):659-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fu HL, Sohail A, Valiathan RR, et al.. Shedding of Discoidin Domain Receptor 1 by Membrane-type Matrix Metalloproteinases. J Biol Chem. 2013;288(17):12114-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wang CZ, Hsu YM, Tang MJ. Function of discoidin domain receptor I in HGF-induced branching tubulogenesis of MDCK cells in collagen gel. J Cell Physiol. 2005;203(1):295-304. [DOI] [PubMed] [Google Scholar]
- [12].Wang CZ, Su HW, Hsu YC, et al.. A discoidin domain receptor 1/SHP-2 signaling complex inhibits alpha2beta1-integrin-mediated signal transducers and activators of transcription 1/3 activation and cell migration. Mole Biol Cell. 2006;17(6):2839-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lu KK, Trcka D, Bendeck MP. Collagen stimulates discoidin domain receptor 1-mediated migration of smooth muscle cells through Src. Cardiovasc Pathol. 2011;20(2):71-6. [DOI] [PubMed] [Google Scholar]
- [14].Hachehouche LN, Chetoui N, Aoudjit F. Implication of discoidin domain receptor 1 in T cell migration in three-dimensional collagen. Mol Immunol. 2010;47(9):1866-9. [DOI] [PubMed] [Google Scholar]
- [15].El Azreq MA, Kadiri M, Boisvert M, et al.. Discoidin domain receptor 1 promotes Th17 cell migration by activating the RhoA/ROCK/MAPK/ERK signaling pathway. Oncotarget. 2016;7(29):44975-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kamohara H, Yamashiro S, Galligan C, et al.. Discoidin domain receptor 1 isoform-a (DDR1alpha) promotes migration of leukocytes in three-dimensional collagen lattices. FASEB J. 2001;15(14):2724-6. [DOI] [PubMed] [Google Scholar]
- [17].Ram R, Lorente G, Nikolich K, et al.. Discoidin domain receptor-1a (DDR1a) promotes glioma cell invasion and adhesion in association with matrix metalloproteinase-2. J Neurooncol. 2006;76(3):239-48. [DOI] [PubMed] [Google Scholar]
- [18].Yang SH, Baek HA, Lee HJ, et al.. Discoidin domain receptor 1 is associated with poor prognosis of non-small cell lung carcinomas. Oncol Rep. 2010;24(2):311-9. [DOI] [PubMed] [Google Scholar]
- [19].Olaso E, Labrador JP, Wang L, et al.. Discoidin domain receptor 2 regulates fibroblast proliferation and migration through the extracellular matrix in association with transcriptional activation of matrix metalloproteinase-2. J Biol Chem. 2002;277(5):3606-13. [DOI] [PubMed] [Google Scholar]
- [20].Chen SC, Wang BW, Wang DL, et al.. Hypoxia induces discoidin domain receptor-2 expression via the p38 pathway in vascular smooth muscle cells to increase their migration. Biochem Biophys Res Commun. 2008;374(4):662-7. [DOI] [PubMed] [Google Scholar]
- [21].Vogel W, Brakebusch C, Fassler R, et al.. Discoidin domain receptor 1 is activated independently of beta(1) integrin. J Biol Chem. 2000;275(8):5779-84. [DOI] [PubMed] [Google Scholar]
- [22].Xu H, Bihan D, Chang F, et al.. Discoidin domain receptors promote alpha1beta1- and alpha2beta1-integrin mediated cell adhesion to collagen by enhancing integrin activation. PloS one. 2012;7(12):e52209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Woltersdorf C, Bonk M, Leitinger B, et al.. The binding capacity of alpha1beta1-, alpha2beta1- and alpha10beta1-integrins depends on non-collagenous surface macromolecules rather than the collagens in cartilage fibrils. Matrix Biol. 2017;63:91-105. [DOI] [PubMed] [Google Scholar]
- [24].Sabeh F, Shimizu-Hirota R, Weiss SJ. Protease-dependent versus -independent cancer cell invasion programs: three-dimensional amoeboid movement revisited. J Cell Biol. 2009;185(1):11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Willis AL, Sabeh F, Li XY, et al.. Extracellular matrix determinants and the regulation of cancer cell invasion stratagems. J Microsc. 2013;251(3):250-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wolf K, Te Lindert M, Krause M, et al.. Physical limits of cell migration: control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J Cell Biol. 2013;201(7):1069-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Vogel W, Gish GD, Alves F, et al.. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell. 1997;1(1):13-23. [DOI] [PubMed] [Google Scholar]
- [28].Ferri N, Carragher NO, Raines EW. Role of discoidin domain receptors 1 and 2 in human smooth muscle cell-mediated collagen remodeling: potential implications in atherosclerosis and lymphangioleiomyomatosis. Am J Pathol. 2004;164(5):1575-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Roberts ME, Magowan L, Hall IP, et al.. Discoidin domain receptor 1 regulates bronchial epithelial repair and matrix metalloproteinase production. Eur Respir J. 2011;37(6):1482-93. [DOI] [PubMed] [Google Scholar]
- [30].Hou G, Vogel WF, Bendeck MP. Tyrosine kinase activity of discoidin domain receptor 1 is necessary for smooth muscle cell migration and matrix metalloproteinase expression. Circ Res. 2002;90(11):1147-9. [DOI] [PubMed] [Google Scholar]
- [31].Park HS, Kim KR, Lee HJ, et al.. Overexpression of discoidin domain receptor 1 increases the migration and invasion of hepatocellular carcinoma cells in association with matrix metalloproteinase. Oncol Rep. 2007;18(6):1435-41. [PubMed] [Google Scholar]
- [32].Castro-Sanchez L, Soto-Guzman A, Guaderrama-Diaz M, et al.. Role of DDR1 in the gelatinases secretion induced by native type IV collagen in MDA-MB-231 breast cancer cells. Clin Exp Metastasis. 2011;28(5):463-77. [DOI] [PubMed] [Google Scholar]
- [33].Nagase H, Murphy G. Metalloproteinases in cartilage Matrix Breakdown: The Roles in Rheumatoid Arthritis and Osteoarthritis. In: Brix K, Stöcker W, editors. Proteases: Structure and Function. Vienna: Springer Vienna; 2013. p. 433-69. [Google Scholar]
- [34].Sato T, Konomi K, Yamasaki S, et al.. Comparative analysis of gene expression profiles in intact and damaged regions of human osteoarthritic cartilage. Arthritis Rheum. 2006;54(3):808-17. [DOI] [PubMed] [Google Scholar]
- [35].Little CB, Barai A, Burkhardt D, et al.. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 2009;60(12):3723-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Baragi VM, Becher G, Bendele AM, et al.. A new class of potent matrix metalloproteinase 13 inhibitors for potential treatment of osteoarthritis: Evidence of histologic and clinical efficacy without musculoskeletal toxicity in rat models. Arthritis Rheum. 2009;60(7):2008-18. [DOI] [PubMed] [Google Scholar]
- [37].Xu L, Peng H, Glasson S, et al.. Increased expression of the collagen receptor discoidin domain receptor 2 in articular cartilage as a key event in the pathogenesis of osteoarthritis. Arthritis Rheum. 2007;56(8):2663-73. [DOI] [PubMed] [Google Scholar]
- [38].Sunk IG, Bobacz K, Hofstaetter JG, et al.. Increased expression of discoidin domain receptor 2 is linked to the degree of cartilage damage in human knee joints: a potential role in osteoarthritis pathogenesis. Arthritis Rheum. 2007;56(11):3685-92. [DOI] [PubMed] [Google Scholar]
- [39].Vonk LA, Doulabi BZ, Huang C, et al.. Collagen-induced expression of collagenase-3 by primary chondrocytes is mediated by integrin {alpha}1 and discoidin domain receptor 2: a protein kinase C-dependent pathway. Rheumatology (Oxford). 2010; [DOI] [PubMed] [Google Scholar]
- [40].Xu L, Servais J, Polur I, et al.. Attenuation of osteoarthritis progression by reduction of discoidin domain receptor 2 in mice. Arthritis Rheum. 2010;62(9):2736-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Su J, Yu J, Ren T, et al.. Discoidin domain receptor 2 is associated with the increased expression of matrix metalloproteinase-13 in synovial fibroblasts of rheumatoid arthritis. Mol Cell Biochem. 2009;330(1–2):141-52. [DOI] [PubMed] [Google Scholar]
- [42].Konttinen YT, Salo T, Hanemaaijer R, et al.. Collagenase-3 (MMP-13) and its activators in rheumatoid arthritis: localization in the pannus-hard tissue junction and inhibition by alendronate. Matrix Biol. 1999;18(4):401-12. [DOI] [PubMed] [Google Scholar]
- [43].Miller MC, Manning HB, Jain A, et al.. Membrane type 1 matrix metalloproteinase is a crucial promoter of synovial invasion in human rheumatoid arthritis. Arthritis Rheum. 2009;60(3):686-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kaneko K, Williams RO, Dransfield DT, et al.. Selective Inhibition of Membrane Type 1 Matrix Metalloproteinase Abrogates Progression of Experimental Inflammatory Arthritis: Synergy With Tumor Necrosis Factor Blockade. Arthritis Rheumatol. 2016;68(2):521-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Majkowska I, Shitomi Y, Ito N, et al.. Discoidin domain receptor 2 mediates collagen-induced activation of membrane-type 1 matrix metalloproteinase in human fibroblasts. J Biol Chem. 2017;292(16):6633-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Knäuper V, Will H, López-Otín C, et al.. Cellular mechanisms for human procollagenase 3 (mmp 13) activation: evidence that mt1 mmp (mmp 14) and gelatinase a (mmp 2) are able to generate active enzyme. J Biol Chem. 1996;271(29):17124-31. [DOI] [PubMed] [Google Scholar]
- [47].Linder S. Invadosomes at a glance. J Cell Sci. 2009;122(Pt 17):3009-13. [DOI] [PubMed] [Google Scholar]
- [48].Juin A, Billottet C, Moreau V, et al.. Physiological type I collagen organization induces the formation of a novel class of linear invadosomes. Mol Biol Cell. 2012;23(2):297-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Juin A, Di Martino J, Leitinger B, et al.. Discoidin domain receptor 1 controls linear invadosome formation via a Cdc42-Tuba pathway. J Cell Biol. 2014;207(4):517-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Carafoli F, Bihan D, Stathopoulos S, et al.. Crystallographic insight into collagen recognition by discoidin domain receptor 2. Structure. 2009;17(12):1573-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Orgel JP, Irving TC, Miller A, et al.. Microfibrillar structure of type I collagen in situ. Proc Natl Acad Sci U S A. 2006;103(24):9001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Flynn LA, Blissett AR, Calomeni EP, et al.. Inhibition of collagen fibrillogenesis by cells expressing soluble extracellular domains of DDR1 and DDR2. J Mol Biol. 2010;395(3):533-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Yoda M, Kimura T, Tohmonda T, et al.. Systemic overexpression of TNFalpha-converting enzyme does not lead to enhanced shedding activity in vivo. PloS one. 2013;8(1):e54412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Wang Z, Bian H, Bartual SG, et al.. Structure-Based Design of Tetrahydroisoquinoline-7-carboxamides as Selective Discoidin Domain Receptor 1 (DDR1) Inhibitors. J Med Chem. 2016;59(12):5911-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kothiwale S, Borza CM, Lowe EW Jr., et al.. Discoidin domain receptor 1 (DDR1) kinase as target for structure-based drug discovery. Drug Discov Today. 2015;20(2):255-61. [DOI] [PMC free article] [PubMed] [Google Scholar]


