This article describes the syntheses, crystal structures and theoretical calculations of six group IIB coordination compounds containing a 2-{[(2-methoxyphenyl)imino]methyl}phenol ligand to provide further insight into the role of π-stacking and hydrogen bonding in metallosupramolecular assembly.
Keywords: π-stacking, hydrogen bonding, noncolvalent interactions, metallosupramolecular assembly, crystal structure, coordination compound, group IIB
Abstract
The supramolecular chemistry of coordination compounds has become an important research domain of modern inorganic chemistry. Herein, six isostructural group IIB coordination compounds containing a 2-{[(2-methoxyphenyl)imino]methyl}phenol ligand, namely dichloridobis(2-{(E)-[(2-methoxyphenyl)azaniumylidene]methyl}phenolato-κO)zinc(II), [ZnCl2(C28H26N2O4)], 1, diiodidobis(2-{(E)-[(2-methoxyphenyl)azaniumylidene]methyl}phenolato-κO)zinc(II), [ZnI2(C28H26N2O4)], 2, dibromidobis(2-{(E)-[(2-methoxyphenyl)azaniumylidene]methyl}phenolato-κO)cadmium(II), [CdBr2(C28H26N2O4)], 3, diiodidobis(2-{(E)-[(2-methoxyphenyl)azaniumylidene]methyl}phenolato-κO)cadmium(II), [CdI2(C28H26N2O4)], 4, dichloridobis(2-{(E)-[(2-methoxyphenyl)azaniumylidene]methyl}phenolato-κO)mercury(II), [HgCl2(C28H26N2O4)], 5, and diiodidobis(2-{(E)-[(2-methoxyphenyl)azaniumylidene]methyl}phenolato-κO)mercury(II), [HgI2(C28H26N2O4)], 6, were synthesized and characterized by X-ray crystallography and spectroscopic techniques. All six compounds exhibit an infinite one-dimensional ladder in the solid state governed by the formation of hydrogen-bonding and π–π stacking interactions. The crystal structures of these compounds were studied using geometrical and Hirshfeld surface analyses. They have also been studied using M06-2X/def2-TZVP calculations and Bader’s theory of ‘atoms in molecules’. The energies associated with the interactions, including the contribution of the different forces, have been evaluated. In general, the π–π stacking interactions are stronger than those reported for conventional π–π complexes, which is attributed to the influence of the metal coordination, which is stronger for Zn than either Cd or Hg. The results reported herein might be useful for understanding the solid-state architecture of metal-containing materials that contain M II X 2 subunits and aromatic organic ligands.
Introduction
Over the last two decades, the supramolecular chemistry of metal-containing compounds has attracted intense attention, due not only to their fascinating structures (Holliday & Mirkin, 2001 ▸; Brammer, 2004 ▸), but also their potential applications in diverse fields such as medicine (McKinlay et al., 2010 ▸; Reedijk, 2009 ▸), ion and molecular recognition (Custelcean et al., 2012 ▸; Busschaert et al., 2015 ▸) and catalysis (Wang et al., 2013 ▸; Wiester et al., 2011 ▸).
The ultimate goal of supramolecular chemistry is to understand the inherent complexities of the association mechanisms of molecular and ionic building blocks organized through noncovalent intermolecular interactions with prescribed properties and functions (Lehn, 1995 ▸; Steed & Atwood, 2013 ▸). In the context of metallosupramolecular chemistry (Braga & Grepioni, 2000 ▸; Braga et al., 1998 ▸), hydrogen bonding (Reedijk, 2013 ▸; Azhdari Tehrani et al., 2016 ▸) and halogen bonding (Khavasi et al., 2015 ▸; Khavasi & Azhdari Tehrani, 2013 ▸; Li et al., 2016 ▸) have been widely used so far to drive the self-assembly of coordination compounds, because of their directionality and versatility (Politzer et al., 2010 ▸; Desiraju, 1998 ▸). However, there are some reports that provide evidence suggesting the crucial role of nondirectional intermolecular interactions, such as π–π stacking (Khavasi & Azizpoor Fard, 2010 ▸; Janiak, 2000 ▸; Khavasi & Sadegh, 2014 ▸; Semeniuc et al., 2010 ▸), for designing the supramolecular architecture of metal-containing species in the solid state. In this regard, supramolecular chemists and crystal engineers have explored and studied the use of noncovalent interactions as a key tool for constructing supramolecular architectures of metal-containing building units in the solid state in which X-ray crystallography could provide a detailed picture of the supramolecular structure (Desiraju, 2014 ▸; Blake et al., 1999 ▸; Đaković et al., 2018 ▸). These studies reveal an undeniable contribution of such noncovalent interactions to the organization and stabilization of the ultimate crystal structures. These studies also revealed that the ultimate supramolecular architecture of self-assembled metal-containing compounds could be affected by various factors, such as ligand and metal geometries (Khavasi et al., 2012 ▸; Hajiashrafi et al., 2013 ▸), counter-ions (Schottel et al., 2006 ▸; Zeng et al., 2010 ▸) and reaction conditions (Khavasi & Mohammad Sadegh, 2010 ▸; Mahata et al., 2009 ▸).
In continuation of our research aimed at understanding the role of noncovalent interactions in the fabrication and self-assembly of metal-containing building blocks (Hajiashrafi et al., 2013 ▸, 2016 ▸; Kielmann & Senge, 2018 ▸), a series of coordination compounds, namely [ZnL 2Cl2] (1), [ZnL 2I2] (2), [CdL 2Br2] (3), [CdL 2I2] (4), [HgL 2Cl2] (5) and [HgL 2I2] (6), where L is 2-{[(2-methoxyphenyl)azaniumylidene]methyl}phenolate, have been synthesized and characterized using X-ray crystallography and different spectroscopic techniques (see Scheme 1). Geometrical, Hirshfeld surface analysis and theoretical calculations reveal the importance of π–π stacking interactions, as well as hydrogen bonding, in governing the crystal packing of this series of isostructural metal-containing compounds.
Experimental
Materials and apparatus
Chemicals and reagents were purchased from commercial sources. 2-Hydroxybenzaldehyde, 2-methoxyaniline and anhydrous M II halides, where M is Zn, Cd and Hg, were purchased from Sigma–Aldrich and Merck, and used as received. The Schiff base ligand 2-{[(2-methoxyphenyl)imino]methyl}phenol (L) was prepared according to a previously reported method (Song et al., 2013 ▸). The IR spectra were recorded on a Nicolet FT–IR 100 spectrometer in the range 500–4000 cm−1 using the KBr disk technique. Elemental analyses (C, H and N) were performed using an ECS 4010 CHN-O made in Costech, Italy. Melting points were measured by an Electrothermal 9100 melting-point apparatus and corrected. The measurements were carried out using 10 mg of a powdered sample sealed in aluminium pans with a mechanical crimp.
Computational methods
The geometries of the complexes included in this study were computed at the M06-2X/def2-TZVP level of theory using the crystallographic coordinates within TURBOMOLE 7.0 (Ahlrichs et al., 1989 ▸). We have used the crystallographic coordinates instead of the optimized complexes because we are interested in estimating the binding energies of several assemblies as they stand in the crystal structure, instead of investigating the most favourable geometry for a given complex. The interaction energies were calculated with correction for the basis set superposition error (BSSE) by using the Boys–Bernardi counterpoise technique (Boys & Bernardi, 1970 ▸). The ‘atoms-in-molecules’ (AIM) analysis of the electron density was performed at the same level of theory using the AIMAll program (Keith, 2013 ▸).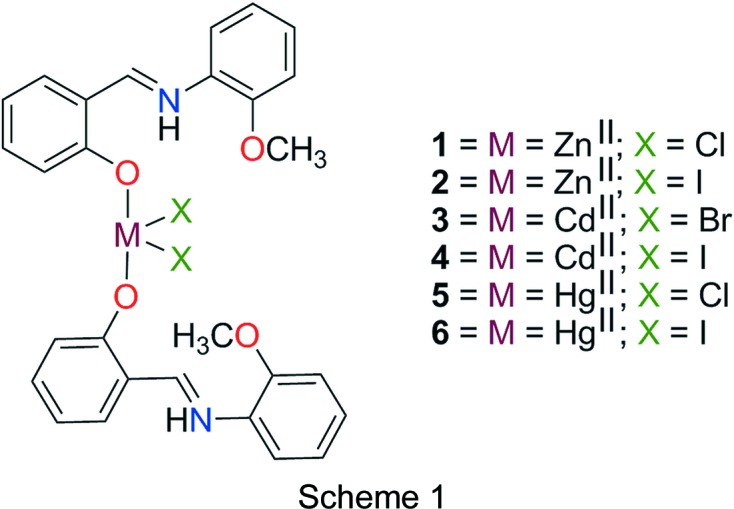
Synthesis and crystallization
The ligand 2-{[(2-methoxyphenyl)imino]methyl}phenol (L) was utilized previously for the preparation of a number of coordination compounds (Song et al., 2013 ▸; Gong et al., 2014 ▸; Reddy et al., 2003a ▸,b ▸; Li & Yuan, 2012 ▸). L was synthesized by reacting 2-hydroxybenzaldehyde (0.53 ml, 5 mmol) with 2-methoxyaniline (0.56 ml, 5 mmol) in ethanol. After stirring for 30 min at 323 K, the ligand precipitated from the reaction mixture as an orange powder which was filtered off, washed several times with cold ethanol and normal hexane, and then dried under vacuum.
The six coordination compounds [ZnL 2Cl2] (1), [ZnL 2I2] (2), [CdL 2Br2] (3), [CdL 2I2] (4), [HgL 2Cl2] (5) and [HgL 2I2] (6) were synthesized by combining a solution of MX 2 (0.1 mmol; M = Zn, Cd or Hg and X = Cl, Br or I) in methanol (5 ml) and a solution of L (0.2 mmol) in methanol (5 ml) with stirring. Each mixture was heated at 333 K for about 30 min. Reduction of the solvent volume resulted in the formation of a yellow-to-orange precipitate. The precipitate was filtered off, washed with methanol (3 × 2 ml) and then dried in vacuo. The solid was subsequently dissolved in boiling methanol, ethanol or acetonitrile (10 ml) and filtered. Upon slow evaporation of the filtrate at room temperature, crystals of complexes 1–6 suitable for X-ray crystallography were obtained (Hope, 1994 ▸; Senge, 2000 ▸). The coordination compounds were characterized using X-ray crystallography, FT–IR spectroscopy and elemental analysis.
Analytical data for L
M.p. 330 K. FT–IR (KBr, ν/cm−1, selected bands): 3445 (w, broad), 1246 (s), 3061 (w), 1615 (s), 792 (s), 849 (m).
Analytical data for 1
Yield 52%. M.p. 505–507 K. FT–IR (KBr, ν/cm−1, selected bands): 3679 (w), 3447 (w), 1637 (s), 1525 (m), 1382 (s), 1025 (m), 800 (m), 750 (m). Analysis calculated for C28H26Cl2N2O4Zn (%): C 56.92, H 4.44, N 4.74; found: C 56.86, H 4.42, N 4.70.
Analytical data for 2
Yield 70%. M.p. 520–522 K. FT–IR (KBr, ν/cm−1, selected bands): 3676 (w), 3447 (w), 1614 (s), 1542 (m), 1385 (s), 1019 (m), 805 (m), 754 (m). Analysis calculated for C28H26I2N2O4Zn (%): C 43.47, H 3.39, N 3.62; found: C 43.36, H 3.42, N 3.58.
Analytical data for 3
Yield 54%. M.p. 543–545 K. FT–IR (KBr, ν/cm−1, selected bands): 3674 (w), 3445 (m), 1620 (s), 1530 (m), 1484 (m), 1383 (s), 1020 (m), 794 (m), 753 (m). Analysis calculated for C28H26Br2CdN2O4 (%): C 46.28, H 3.61, N 3.85; found: C 46.24, H 3.40, N 3.82.
Analytical data for 4
Yield 46%. M.p. 461–463 K. FT–IR (KBr, ν/cm−1, selected bands): 3679 (w), 3447 (m), 1637 (s), 1520 (m), 1380 (m), 1025 (m), 796 (m), 741 (m). Analysis calculated for C28H26CdI2N2O4 (%): C 40.98, H 3.19, N 3.41; found: C 40.88, H 3.12, N 3.44.
Analytical data for 5
Yield 65%. M.p. 438–440 K. FT–IR (KBr, ν/cm−1, selected bands): 3675 (w), 3435 (m), 1622 (s), 1534 (m), 1378 (m), 1028 (m), 794 (m), 750 (m). Analysis calculated for C28H26Cl2HgN2O4 (%): C 46.32, H 3.61, N 3.86; found: C 46.28, H 3.62, N 3.92.
Analytical data for 6
Yield 70%. M.p. 383–385 K. FT–IR (KBr, ν/cm−1, selected bands): 3675 (w), 3446 (m), 1630 (s), 1523 (m), 1382 (m), 1018 (m), 796 (m), 738 (w). Analysis calculated for C28H26HgI2N2O4 (%): C 37.00, H 2.88, N 3.08; found: C 36.94, H 2.80, N 3.10.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 1 ▸. C- and N-bound H atoms were placed in their expected calculated positions and refined as riding, with N—H = 0.88 Å and C—H = 0.95–0.99 Å, and with U iso(H) = 1.5U eq(C) for methyl H atoms and 1.2U eq(N,C) otherwise. In the structure of 2, the C and N atoms were restrained to have similar isotropic displacement parameters. Atoms N1A, N1B and C14B were restrained to have close to isotropic displacement parameters. The structure was solved as a rotational twin rotated from the first domain by 179.8° about the reciprocal axis 0.002 1.000 0.001 and the real axis 0.434 1.000 0.197. The twin law to convert hkl from the first to this domain (SHELXL TWIN matrix) was −0.999 0.004 −0.001, 0.866 0.998 0.395, 0.007 0.003 −0.999. The structure of 3 was solved as a rotational twin rotated from the first domain by 179.7° about the reciprocal axis −0.003 −0.997 1.000 and the real axis 0.311 1.000 −0.257. The twin law to convert hkl from the first to this domain (SHELXL TWIN matrix) was −1.001 0.001 −0.004, 0.498 0.590 −0.407, −0.487 −1.593 −0.589. The structure of 5 was solved as a rotational twin rotated from the first domain by 179.9° about the reciprocal axis −0.001 1.000 −0.999 and the real axis 0.345 1.000 −0.274. The twin law to convert hkl from the first to this domain (SHELXL TWIN matrix) was −1.000 −0.001 0.001, 0.541 0.570 −0.431, −0.543 −1.570 −0.570. The structure of 6 was solved as a rotational twin rotated from the first domain by 149.8° about the reciprocal axis 1.000 0.235 0.787 and the real axis 1.000 0.533 0.319. The twin law to convert hkl from the first to this domain (SHELXL TWIN matrix) was 0.534 0.949 0.308, 0.116 −0.693 0.359, 1.269 −0.145 −0.569.
Table 1. Experimental details.
| 1 | 2 | 3 | |
|---|---|---|---|
| Crystal data | |||
| Chemical formula | [ZnCl2(C28H26N2O4)] | [ZnI2(C28H26N2O4)] | [CdBr2(C28H26N2O4)] |
| M r | 590.78 | 773.68 | 726.73 |
| Crystal system, space group | Triclinic, P

|
Triclinic, P

|
Triclinic, P

|
| Temperature (K) | 100 | 100 | 100 |
| a, b, c (Å) | 9.1926 (2), 10.6101 (2), 14.8057 (3) | 9.2709 (19), 10.020 (2), 16.248 (4) | 9.2772 (3), 10.0935 (3), 16.1021 (5) |
| α, β, γ (°) | 94.188 (1), 97.716 (1), 114.409 (1) | 98.56 (4), 100.50 (4), 110.09 (3) | 97.699 (2), 100.586 (2), 111.149 (2) |
| V (Å3) | 1289.80 (5) | 1356.7 (6) | 1348.92 (8) |
| Z | 2 | 2 | 2 |
| Radiation type | Mo Kα | Mo Kα | Mo Kα |
| μ (mm−1) | 1.20 | 3.22 | 3.81 |
| Crystal size (mm) | 0.26 × 0.12 × 0.11 | 0.14 × 0.07 × 0.03 | 0.27 × 0.13 × 0.10 |
| Data collection | |||
| Diffractometer | Bruker SMART APEXII area detector | Bruker APEXII area detector | Bruker APEXII area detector |
| Absorption correction | Multi-scan (SADABS; Bruker, 2016 ▸) | Multi-scan (TWINABS; Bruker, 2012 ▸) | Multi-scan (TWINABS; Bruker, 2012 ▸) |
| T min, T max | 0.691, 0.747 | 0.612, 0.746 | 0.538, 0.745 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 93163, 11965, 9331 | 7281, 7281, 4608 | 10130, 10130, 8342 |
| R int | 0.053 | 0.116 | 0.020 |
| (sin θ/λ)max (Å−1) | 0.822 | 0.606 | 0.634 |
| Refinement | |||
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.035, 0.084, 1.02 | 0.067, 0.195, 0.99 | 0.040, 0.126, 1.03 |
| No. of reflections | 11965 | 7281 | 10130 |
| No. of parameters | 336 | 337 | 337 |
| No. of restraints | 0 | 174 | 0 |
| H-atom treatment | H-atom parameters constrained | H-atom parameters constrained | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.77, −0.71 | 2.19, −1.18 | 1.04, −0.70 |
| 4 | 5 | 6 | |
|---|---|---|---|
| Crystal data | |||
| Chemical formula | [CdI2(C28H26N2O4)] | [HgCl2(C28H26N2O4)] | [HgI2(C28H26N2O4)] |
| M r | 820.71 | 726.00 | 908.90 |
| Crystal system, space group | Triclinic, P

|
Triclinic, P

|
Triclinic, P

|
| Temperature (K) | 100 | 100 | 100 |
| a, b, c (Å) | 9.3200 (3), 10.0498 (3), 16.6239 (5) | 9.2456 (4), 10.1510 (4), 15.8499 (6) | 9.2783 (14), 10.0060 (15), 16.695 (3) |
| α, β, γ (°) | 99.140 (1), 100.528 (1), 109.332 (1) | 96.5447 (15), 99.7441 (15), 112.6735 (14) | 98.777 (1), 100.296 (1), 109.396 (1) |
| V (Å3) | 1403.58 (8) | 1326.25 (9) | 1400.4 (4) |
| Z | 2 | 2 | 2 |
| Radiation type | Mo Kα | Mo Kα | Mo Kα |
| μ (mm−1) | 3.01 | 6.04 | 7.74 |
| Crystal size (mm) | 0.40 × 0.26 × 0.14 | 0.17 × 0.14 × 0.08 | 0.38 × 0.19 × 0.13 |
| Data collection | |||
| Diffractometer | Bruker SMART APEXII area detector | Bruker APEXII area detector | Bruker APEXII area detector |
| Absorption correction | Numerical (SADABS; Bruker, 2016 ▸) | Multi-scan (TWINABS; Bruker, 2012 ▸) | Multi-scan (TWINABS; Bruker, 2012 ▸) |
| T min, T max | 0.416, 0.667 | 0.630, 0.746 | 0.441, 0.746 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 110757, 13807, 10917 | 22779, 22779, 21098 | 11185, 11185, 10132 |
| R int | 0.055 | 0.012 | 0.071 |
| (sin θ/λ)max (Å−1) | 0.838 | 0.668 | 0.650 |
| Refinement | |||
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.033, 0.067, 1.03 | 0.022, 0.052, 1.03 | 0.032, 0.112, 1.08 |
| No. of reflections | 13807 | 22779 | 11185 |
| No. of parameters | 336 | 337 | 337 |
| No. of restraints | 0 | 0 | 0 |
| H-atom treatment | H-atom parameters constrained | H-atom parameters constrained | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 1.04, −1.33 | 1.29, −0.60 | 1.48, −1.78 |
Results and discussion
Crystal structure analysis
X-ray crystallography revealed that compounds 1–6 are isostructural and crystallize in the triclinic space group P
 (Fig. 1 ▸ and Table 1 ▸). The asymmetric units of these structures contain two L ligands, two halide ions and a metal ion of group IIB. Crystal structure analysis reveals that in compounds 1–6, the M
II ion is in a distorted trigonal pyramidal geometry, with four-coordinate geometry indices, τ4 (Yang et al., 2007 ▸), of 0.83, 0.85, 0.83, 0.84, 0.75, and 0.77, respectively. Selected bond lengths and angles are listed in Table 2 ▸ and are in agreement with the values reported for similar compounds (Shkol’nikova et al., 1970 ▸; Gong et al., 2014 ▸). The trigonal pyramidal geometry around M
II is made up of two halide ions and two phenolate O atoms from two different L ligands. It should be noted that N-salicylideneanilines may exist in different tautomeric forms and the tautomeric isomerization reaction between the enol and keto forms is accompanied by intra- and intermolecular proton transfer (Dürr & Bouas-Laurent, 2003 ▸; Cohen & Schmidt, 1962 ▸; Cohen et al., 1964 ▸; Tsuchimoto et al., 2016 ▸). The Schiff base ligand L shows a self-isomerization induced by an intramolecular proton transfer from the hydroxy O to the imine N atom through an O—H⋯N hydrogen bond (Hoshino et al., 1988 ▸; Alarcón et al., 1999 ▸). Thus, the ligand is a zwitterion with the negative and positive charges located at atoms O1B and N1B, respectively (Charland et al., 1989 ▸; Redshaw et al., 2013 ▸; Tsuchimoto et al., 2016 ▸; Kargili et al., 2014 ▸). This is supported by the geometry of the ligand and the unambiguous location of the H atom attached to atom N1B. The ligand almost keeps it coplanarity upon coordination; the dihedral angles between the planes of the two aromatic rings of ligand L lie in the range 4.10–10.68° for compounds 1–6 (see supporting information), which is a consequence of intramolecular N—H⋯O hydrogen bonding. In this form, L can act as a monodentate ligand, where it is coordinated to the metal ion via the phenolate O atom. It should be noted that at basic pH, the L ligand may act as a tridentate ligand through the imine N, phenolate O and methoxy O atoms (Gong et al., 2014 ▸; Song et al., 2013 ▸; Reddy et al., 2003a
▸).
(Fig. 1 ▸ and Table 1 ▸). The asymmetric units of these structures contain two L ligands, two halide ions and a metal ion of group IIB. Crystal structure analysis reveals that in compounds 1–6, the M
II ion is in a distorted trigonal pyramidal geometry, with four-coordinate geometry indices, τ4 (Yang et al., 2007 ▸), of 0.83, 0.85, 0.83, 0.84, 0.75, and 0.77, respectively. Selected bond lengths and angles are listed in Table 2 ▸ and are in agreement with the values reported for similar compounds (Shkol’nikova et al., 1970 ▸; Gong et al., 2014 ▸). The trigonal pyramidal geometry around M
II is made up of two halide ions and two phenolate O atoms from two different L ligands. It should be noted that N-salicylideneanilines may exist in different tautomeric forms and the tautomeric isomerization reaction between the enol and keto forms is accompanied by intra- and intermolecular proton transfer (Dürr & Bouas-Laurent, 2003 ▸; Cohen & Schmidt, 1962 ▸; Cohen et al., 1964 ▸; Tsuchimoto et al., 2016 ▸). The Schiff base ligand L shows a self-isomerization induced by an intramolecular proton transfer from the hydroxy O to the imine N atom through an O—H⋯N hydrogen bond (Hoshino et al., 1988 ▸; Alarcón et al., 1999 ▸). Thus, the ligand is a zwitterion with the negative and positive charges located at atoms O1B and N1B, respectively (Charland et al., 1989 ▸; Redshaw et al., 2013 ▸; Tsuchimoto et al., 2016 ▸; Kargili et al., 2014 ▸). This is supported by the geometry of the ligand and the unambiguous location of the H atom attached to atom N1B. The ligand almost keeps it coplanarity upon coordination; the dihedral angles between the planes of the two aromatic rings of ligand L lie in the range 4.10–10.68° for compounds 1–6 (see supporting information), which is a consequence of intramolecular N—H⋯O hydrogen bonding. In this form, L can act as a monodentate ligand, where it is coordinated to the metal ion via the phenolate O atom. It should be noted that at basic pH, the L ligand may act as a tridentate ligand through the imine N, phenolate O and methoxy O atoms (Gong et al., 2014 ▸; Song et al., 2013 ▸; Reddy et al., 2003a
▸).
Figure 1.
(a)–(f) The molecular structures of compounds 1–6, respectively, showing the atom labelling. Displacement ellipsoids are drawn at the 50% probability level.
Table 2. Selected bond lengths (Å) and angles (°) for compounds 1–6 .
| Compound | 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|---|
| Bond lengths | M1—X1 | 2.240 (4) | 2.588 (3) | 2.537 (1) | 2.720 (1) | 2.371 (1) | 2.658 (1) |
| M1—X2 | 2.243 (4) | 2.568 (3) | 2.545 (1) | 2.710 (1) | 2.369 (1) | 2.654 (1) | |
| M1—O2A | 1.985 (1) | 1.990 (10) | 2.216 (4) | 2.231 (2) | 2.259 (2) | 2.387 (5) | |
| M1—O2B | 1.983 (1) | 1.990 (10) | 2.225 (4) | 2.216 (2) | 2.356 (2) | 2.378 (5) | |
| Bond angles | X1—M1—X2 | 124.8 (2) | 123.7 (1) | 130.0 (1) | 130.1 (1) | 148.2 (1) | 145.1 (1) |
| O1A—M1—X1 | 103.9 (3) | 104.0 (4) | 104.4 (1) | 103.7 (1) | 98.3 (1) | 102.0 (1) | |
| O1A—M1—X2 | 104.0 (3) | 103.6 (4) | 103.6 (1) | 104.8 (1) | 100.4 (1) | 98.7 (1) | |
| O1B—M1—X1 | 105.5 (3) | 106.2 (4) | 104.8 (1) | 102.9 (1) | 98.8 (1) | 98.6 (1) | |
| O1B—M1—X2 | 102.8 (3) | 104.2 (4) | 101.9 (1) | 103.5 (1) | 100.7 (1) | 102.3 (1) | |
| O1A—M1—O1B | 116.8 (4) | 115.8 (5) | 112.0 (2) | 111.6 (1) | 105.6 (1) | 105.8 (2) |
As shown in Fig. 2 ▸, the crystal packing of compounds 1–6 consists of mononuclear units which are connected in the crystallographic a direction through a combination of π–π stacking interactions involving the C=N group of the ligand and C—H⋯π interactions. These units are then linked to other units via C—H⋯X (X = Cl, Br and I) hydrogen-bonding interactions in the bc plane. The intermolecular contacts involved in the crystal packing of compounds 1–6 can be quantified via Hirshfeld surface analysis (Spackman & Jayatilaka, 2009 ▸; Mackenzie et al., 2017 ▸). The analysis shows that in compounds 1–6, the H⋯H interactions have the highest priority (the highest contribution to the Hirshfeld surface) and the C—H⋯π, M—X⋯H and π–π interactions have the next highest priorities, respectively. Also, it has been found that the probability of hydrogen-bonding M—X⋯H interactions involving metal-bound halogen increases for a given metal on going from a lighter to a heavier halogen atom. Selected contribution percentages are shown as a histogram in Fig. 3 ▸.
Figure 2.
Representation of the self-assembly of compounds 1–6, showing the association of discrete units through π–π stacking interactions in the crystallographic a direction and C—H⋯X (X = Cl, Br and I) hydrogen bonding in the bc plane.
Figure 3.
Relative contributions of the various noncovalent contacts to the Hirshfeld surface area in complexes 1–6.
Theoretical study
Six ML 2 X 2 (M = Zn, Cd or Hg and X = Cl, Br or I) complexes have been synthesized and characterized by X-ray diffraction analysis (see Fig. 1 ▸). The ligand is monocoordinated to the metal centre and presents an extended π-system that comprises two phenyl rings and an imino group that connects both aromatic moieties.
The solid-state architecture of all six structures is governed by the formation of π-stacking interactions between the aromatic ligands. In particular, each ligand forms infinite one-dimensional (1D) ladders in the crystal packing, as detailed for compounds 1, 3 and 5 in Fig. 4 ▸ as representative systems.
Figure 4.
Partial view of the X-ray crystal structures in compounds (a) 1 (Zn), (b) 3 (Cd) and (c) 5 (Hg).
We have focused the theoretical study on a comparison of the energetic features shown by the π-stacking and hydrogen-bonding interactions (depending on the type of metal) observed in the crystal packing of compounds 1–6 described above. In particular, we have analyzed the π–π and C—H⋯X noncovalent interactions that are crucial to understanding their solid-state architectures. First of all, in order to study the donor–acceptor ability of the ML 2 X 2 complexes, we have computed the molecular electrostatic potential (MEP) surface of a model system (compound 1), which is shown in Fig. 5 ▸. As expected, the most negative electrostatic potential corresponds to the region of the Cl ligands (−75 kcal mol−1). The MEP surface also reveals that the N—H group is totally inaccessible since it is involved in an intramolecular hydrogen bond with the O atom of the ligand. Consequently, the most positive part is located in the region of the exocyclic C—H group at the molecular plane, also influenced by the aromatic C—H groups (40 kcal mol−1). Therefore, hydrogen-bonding interactions between these groups (C—H⋯X) should be electrostatically favoured. Furthermore, perpendicular to the molecular plane, we found that each aromatic ring presents negative MEP values (−17 and −8 kcal mol−1); therefore, face-to-face π–π stacking interactions are not electrostatically favoured (electrostatic repulsion). Remarkably, the electrostatic potential over the π-system of the linker (C=N) is positive, thus explaining the large displacement observed in the antiparallel π-stacking interactions highlighted in Fig. 4 ▸ and further discussed below.
Figure 5.
MEP surface of compound 1. The MEP values at selected points are given in kcal mol−1.
In isostructural Zn compounds 1–3, we have computed the interaction energies of the self-assembled π-stacked dimers (shown in Fig. 6 ▸ a) that are responsible for the formation of the 1D ladders shown in Fig. 3 ▸. The self-assembled dimers are stabilized by a combination of hydrogen bonds and π–π stacking interactions involving the C=N group of the ligand. The dimerization energies in 1 and 2 (ΔE1 = −33.0 kcal mol−1 and ΔE2 = −31.4 kcal mol−1, respectively) are very large due to the contribution of both hydrogen-bonding (red dashed lines in Fig. 6 ▸) and π–π interactions (blue dashed lines in Fig. 6 ▸), where the former involves the most positive (C—H groups, see Fig. 5 ▸) and the most negative (belts of the halide ligands) potential regions of the metal compound. In an effort to calculate the contribution of the different forces that govern the formation of the self-assembled dimers, we have computed additional theoretical models where the halide ligands that establish the hydrogen bonds have been replaced by hydride ligands (see Fig. 6 ▸ b) and consequently the hydrogen-bonding interactions between the halide ligands and the C—H groups are not formed. As a result, the interaction energies are reduced to ΔE3 = −24.8 kcal mol−1 and ΔE4 = −22.5 kcal mol−1 in 1 and 2, respectively. Therefore, the contribution of both symmetrically equivalent hydrogen-bonding interactions can be roughly estimated by the difference (they are −8.2 and 8.9 kcal mol−1 for 1 and 2, respectively) and it is similar in both compounds. Furthermore, we have used additional dimers where the ZnCl2 group in 1 or the ZnI2 group in 2 has been removed (see Fig. 6 ▸ c) in order to evaluate the influence of the metal coordination on the interaction energy. The resulting interaction energies are almost identical for both complexes (ΔE5 = −14.0 kcal mol−1 and ΔE6 = −14.1 kcal mol−1 for 1 and 2, respectively) and reveal the strong influence of the metal coordination on the π–π stacking interaction. This is likely due to the stronger dipole–dipole interaction in the antiparallel arrangement of the assembly. It is also worthy to mention that the π–π interaction energy computed for these compounds is large compared to other π-stacking interactions (i.e. benzene dimer). This is due to the special arrangement of the two π-systems where the C=N bond is located over the aromatic ring (see the on-top representation in Fig. 6 ▸). This fact is in very good agreement with the MEP surface represented in Fig. 5 ▸ and explains the large interaction energy since two electrostatically enhanced π(CN)⋯π interactions are established.
Figure 6.
(a) Interaction energies of the self-assembled π-stacked dimers observed in the solid state of compounds 1 and 2. (b)/(c) Interaction energies in several theoretical models of 1 and 2. (d) On-top representation of the π-stacking interaction. All distances are in Å.
In Cd compounds 3 and 4, the π-stacking binding mode is very similar to that described before for 1 and 2. As mentioned above, hydrogen-bonding and π–π interactions control the dimer formation (see Fig. 7 ▸ a). The computed interaction energies of the self-assembled dimers are almost identical (ΔE7 = −30.9 kcal mol−1 and ΔE8 = −29.9 kcal mol−1 for 3 and 4, respectively), indicating that the halide (Br or I) has a minimal influence on the binding energy. Compared to 1 and 2, the interaction energies are less favourable, thus revealing a larger influence of the Zn ion on the binding energy of the assembly compared to Cd. Also, in both compounds, we have computed theoretical models where the Br or I ligands have been replaced by H atoms and consequently the hydrogen bonds are not formed (see Fig. 7 ▸ b). As a result, the interaction energies are reduced to ΔE9 = −22.7 kcal mol−1 and ΔE10 = −21.6 kcal mol−1 in 3 and 4, respectively. Therefore, this contribution (both hydrogen bonds) can be roughly estimated by the difference (−8.2 and −8.3 kcal mol−1 for 3 and 4, respectively). These values are very close to those found for compounds 1 and 2, thus indicating that the contribution of the hydrogen bonds is not influenced by the type of transition metal (Zn or Cd). Furthermore, we have used an additional dimer, where the CdBr2 and CdI2 groups have been removed. The interaction energies are further reduced to ΔE9 = −13.3 kcal mol−1 and ΔE6 = −13.6 kcal mol−1 for 3 and 4, respectively, which is in agreement with the Zn complexes, revealing a strong influence of the metal coordination on the strength of the π-stacking interaction.
Figure 7.
(a) Interaction energies of the self-assembled π-stacked dimers observed in the solid state of compounds 3 and 4. (b)/(c) Interaction energies in several theoretical models of 3 and 4. All distances are in Å.
For Hg compounds 5 and 6, we have performed an equivalent study (see Fig. 8 ▸). The computed interaction energies of the self-assembled dimers are almost identical (ΔE13 = −28.3 kcal mol−1 and ΔE14 = −25.1 kcal mol−1 for 5 and 6, respectively), indicating that Hg has a smaller effect on the interaction energy than Cd and Zn. Also, in both Hg compounds, we have computed theoretical models where the Cl− or I− ligands have been replaced by H− ligands and consequently the hydrogen bonds are not formed (see Fig. 8 ▸ b). As a result, the interaction energies are reduced to ΔE15 = −19.9 kcal mol−1 and ΔE16 = −17.5 kcal mol−1 in 5 and 6, respectively. Therefore, this contribution (both hydrogen bonds) can be roughly estimated as −8.4 and −7.6 kcal mol−1 for 5 and 6, respectively. These values are in agreement with those found for compounds 1–4, thus confirming that the interaction energy of the hydrogen bonds is not influenced by the type of transition metal (Zn/Cd/Hg). Furthermore, we have used an additional dimer, where the HgCl2 and HgI2 groups have been eliminated. Consequently, the interaction energies are further reduced to ΔE17 = −13.0 kcal mol−1 and ΔE18 = −12.7 kcal mol−1 for 5 and 6, respectively; which is in agreement to the rest of complexes commented on above and confirms the strong influence of the metal coordination on the strength of the π-stacking interaction.
Figure 8.
(a) Interaction energies of the self-assembled π-stacked dimers observed in the solid state of compounds 5 and 6. (b)/(c) Interaction energies in several theoretical models of 5 and 6. All distances are in Å.
In order to provide additional evidence for the existence of the C—H⋯X hydrogen-bond and π–π stacking interactions, we have analyzed the self-assembled π-stacked dimer of compound 3 (as an exemplifying model) using Bader’s theory of ‘atoms in molecules’ (AIM) (Bader, 1991 ▸), which provides an unambiguous definition of chemical bonding. The AIM theory has been successfully used to characterize and understand a great variety of interactions, including those described herein. In Fig. 9 ▸ we show the AIM analysis of compound 3. It can be observed that the π–π interaction is characterized by the presence of three bond critical points that interconnect three C atoms of each aromatic ligand. The interaction is further characterized by several ring and cage critical points. Furthermore, the distribution of critical points reveals the existence of two symmetrically disposed sets of C—H⋯Br hydrogen-bonding interactions. Each one is characterized by a bond critical point and a bond path connecting one H atom of the C—H groups with the Br− ligand, thus confirming the formation of the trifurcated hydrogen bonds. The value of the Laplacian at the bond critical points is positive, as is common in closed-shell interactions.
Figure 9.
AIM analysis of the self-assembled dimers retrieved from the X-ray structure of compound 3. Bond, ring and cage critical points are represented by red, yellow and green spheres, respectively. The bond paths connecting bond critical points are also represented by dashed lines.
Conclusion
We herein reported the syntheses and structural characterization of six new metal complexes based on the 2-{[(2-methoxyphenyl)imino]methyl}phenol ligand. All compounds exhibited an infinite 1D ladder in the solid state governed by the formation of hydrogen-bonding and π–π stacking interactions in the solid state. The crystal structure of these compounds was studied using geometrical and Hirshfeld surface analyses. They have also been studied using M06-2X/def2-TZVP calculations and Bader’s theory of ‘atoms in molecules’. The energies associated with the interactions, including the contribution of the different forces, have been evaluated. In general, the π–π stacking interactions are stronger than those reported for conventional π–π complexes, that is attributed to the influence of the metal coordination, which is stronger for Zn than for either Cd or Hg. The results reported herein might be useful for understanding the solid-state architecture of metal-containing materials that contain M II X 2 subunits and organic aromatic ligands.
Supplementary Material
Crystal structure: contains datablock(s) Compound_1, Compound_2, Compound_3, Compound_4, Compound_5, Compound_6, global. DOI: 10.1107/S2053229618018314/sk3704sup1.cif
Structure factors: contains datablock(s) Compound_1. DOI: 10.1107/S2053229618018314/sk3704Compound_1sup2.hkl
Structure factors: contains datablock(s) Compound_2. DOI: 10.1107/S2053229618018314/sk3704Compound_2sup3.hkl
Structure factors: contains datablock(s) Compound_3. DOI: 10.1107/S2053229618018314/sk3704Compound_3sup4.hkl
Structure factors: contains datablock(s) Compound_4. DOI: 10.1107/S2053229618018314/sk3704Compound_4sup5.hkl
Structure factors: contains datablock(s) Compound_5. DOI: 10.1107/S2053229618018314/sk3704Compound_5sup6.hkl
Structure factors: contains datablock(s) Compound_6. DOI: 10.1107/S2053229618018314/sk3704Compound_6sup7.hkl
Acknowledgments
The authors gratefully acknowledge financial support from the Research Council of Alzahra University, and the Centre de Tecnologies de la Informació (CTI) at the UIB for computational facilities.
Funding Statement
This work was funded by Science Foundation Ireland grant IvP 13/IA/1894. MINECO/AEI of Spain grant CTQ2017-85821-R to A. Frontera and A. Bauzá.
References
- Ahlrichs, R., Bär, M., Häser, M., Horn, H. & Kölmel, C. (1989). Chem. Phys. Lett. 162, 165–169.
- Alarcón, S., Pagani, D., Bacigalupo, J. & Olivieri, A. (1999). J. Mol. Struct. 475, 233–240.
- Azhdari Tehrani, A., Abedi, S. & Morsali, A. (2016). Cryst. Growth Des. 17, 255–261.
- Bader, R. F. (1991). Chem. Rev. 91, 893–928.
- Blake, A. J., Champness, N. R., Hubberstey, P., Li, W.-S., Withersby, M. A. & Schröder, M. (1999). Coord. Chem. Rev. 183, 117–138.
- Boys, S. F. & Bernardi, F. D. (1970). Mol. Phys. 19, 553–566.
- Braga, D. & Grepioni, F. (2000). Acc. Chem. Res. 33, 601–608. [DOI] [PubMed]
- Braga, D., Grepioni, F. & Desiraju, G. R. (1998). Chem. Rev. 98, 1375–1406. [DOI] [PubMed]
- Brammer, L. (2004). Chem. Soc. Rev. 33, 476–489.
- Bruker (2012). TWINABS. Bruker AXS Inc., Madison, Wisconsin, USA.
- Bruker (2015). SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
- Bruker (2016). APEX3 and SADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
- Busschaert, N., Caltagirone, C., Van Rossom, W. & Gale, P. A. (2015). Chem. Rev. 115, 8038–8155. [DOI] [PubMed]
- Charland, J., Gabe, E., Khoo, L. & Smith, F. (1989). Polyhedron, 8, 1897–1901.
- Cohen, M. & Schmidt, G. (1962). J. Phys. Chem. 66, 2442–2446.
- Cohen, M., Schmidt, G. & Flavian, S. (1964). J. Chem. Soc. pp. 2041–2051.
- Custelcean, R., Bonnesen, P. V., Duncan, N. C., Zhang, X., Watson, L. A., Van Berkel, G., Parson, W. B. & Hay, B. P. (2012). J. Am. Chem. Soc. 134, 8525–8534. [DOI] [PubMed]
- Đaković, M., Soldin, Ž., Kukovec, B.-M., Kodrin, I., Aakeröy, C. B., Baus, N. & Rinkovec, T. (2018). IUCrJ, 5, 13–21. [DOI] [PMC free article] [PubMed]
- Desiraju, G. (1998). Chem. Commun. pp. 891–892.
- Desiraju, G. R. (2014). Angew. Chem. Int. Ed. 53, 604–605.
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
- Dürr, H. & Bouas-Laurent, H. (2003). In Photochromism: Molecules and Systems. Amsterdam: Elsevier.
- Gong, D., Wang, B., Jia, X. & Zhang, X. (2014). Dalton Trans. 43, 4169–4178. [DOI] [PubMed]
- Hajiashrafi, T., Kharat, A. N., Love, J. A. & Patrick, B. O. (2013). Polyhedron, 60, 30–38.
- Hajiashrafi, T., Ziarani, G. M., Kubicki, M., Fadaei, F. T. & Schenk, K. J. (2016). Polyhedron, 119, 260–266.
- Holliday, B. J. & Mirkin, C. A. (2001). Angew. Chem. Int. Ed. 40, 2022–2043. [PubMed]
- Hope, H. (1994). Prog. Inorg. Chem. 41, 1–19.
- Hoshino, N., Inabe, T., Mitani, T. & Maruyama, Y. (1988). Bull. Chem. Soc. Jpn, 61, 4207–4214.
- Janiak, C. (2000). J. Chem. Soc. Dalton Trans. pp. 3885–3896.
- Kargili, H., Alpaslan, G., Macit, M., Erdönmez, A. & Büyükgüngör, O. (2014). Opt. Spectrosc. 116, 179–186.
- Keith, T. A. (2013). AIMAll. Version 13.05.06. TK Gristmill Software, Overland Park, KS, USA.
- Khavasi, H. R. & Azhdari Tehrani, A. (2013). Inorg. Chem. 52, 2891–2905. [DOI] [PubMed]
- Khavasi, H. R. & Azizpoor Fard, M. (2010). Cryst. Growth Des. 10, 1892–1896.
- Khavasi, H. R., Barforoush, M. M. & Fard, M. A. (2012). CrystEngComm, 14, 7236–7244.
- Khavasi, H. R. & Mohammad Sadegh, B. M. (2010). Inorg. Chem. 49, 5356–5358. [DOI] [PubMed]
- Khavasi, H. R., Norouzi, F. & Azhdari Tehrani, A. (2015). Cryst. Growth Des. 15, 2579–2583.
- Khavasi, H. R. & Sadegh, B. M. M. (2014). Dalton Trans. 43, 5564–5573. [DOI] [PubMed]
- Kielmann, M. & Senge, M. O. (2018). Angew. Chem. Int. Ed. 58, 418–441. [DOI] [PMC free article] [PubMed]
- Lehn, J.-M. (1995). In Supramolecular Chemistry. Weinheim: VCH.
- Li, B., Zang, S.-Q., Wang, L.-Y. & Mak, T. C. (2016). Coord. Chem. Rev. 308, 1–21.
- Li, L. & Yuan, F. (2012). Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 42, 994–998.
- Mackenzie, C. F., Spackman, P. R., Jayatilaka, D. & Spackman, M. A. (2017). IUCrJ, 4, 575–587. [DOI] [PMC free article] [PubMed]
- Mahata, P., Prabu, M. & Natarajan, S. (2009). Cryst. Growth Des. 9, 3683–3691.
- McKinlay, A. C., Morris, R. E., Horcajada, P., Férey, G., Gref, R., Couvreur, P. & Serre, C. (2010). Angew. Chem. Int. Ed. 49, 6260–6266. [DOI] [PubMed]
- Politzer, P., Murray, J. S. & Clark, T. (2010). Phys. Chem. Chem. Phys. 12, 7748–7757. [DOI] [PubMed]
- Reddy, P. A., Nethaji, M. & Chakravarty, A. R. (2003a). Eur. J. Inorg. Chem. pp. 2318–2324.
- Reddy, P. A., Nethaji, M. & Chakravarty, A. R. (2003b). Inorg. Chem. Commun. 6, 698–701.
- Redshaw, C., Walton, M., Clowes, L., Hughes, D. L., Fuller, A. M., Chao, Y., Walton, A., Sumerin, V., Elo, P. & Soshnikov, I. (2013). Chem. Eur. J. 19, 8884–8899. [DOI] [PubMed]
- Reedijk, J. (2009). Eur. J. Inorg. Chem. pp. 1303–1312.
- Reedijk, J. (2013). Chem. Soc. Rev. 42, 1776–1783. [DOI] [PubMed]
- Schottel, B. L., Chifotides, H. T., Shatruk, M., Chouai, A., Pérez, L. M., Bacsa, J. & Dunbar, K. R. (2006). J. Am. Chem. Soc. 128, 5895–5912. [DOI] [PubMed]
- Semeniuc, R. F., Reamer, T. J. & Smith, M. D. (2010). New J. Chem. 34, 439–452.
- Senge, M. O. (2000). Z. Naturforsch. Teil B, 55, 336–344.
- Sheldrick, G. M. (2015a). Acta Cryst. C71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. A71, 3–8.
- Shkol’nikova, L., Obodovskaya, A. & Shugam, E. (1970). J. Struct. Chem. 11, 47–53.
- Song, X., Wang, Z., Zhao, J. & Hor, T. A. (2013). Chem. Commun. 49, 4992–4994. [DOI] [PubMed]
- Spackman, M. A. & Jayatilaka, D. (2009). CrystEngComm, 11, 19–32.
- Steed, J. W. & Atwood, J. L. (2013). In Supramolecular Chemistry. London: John Wiley & Sons.
- Tsuchimoto, M., Yoshida, N., Sugimoto, A., Teramoto, N. & Nakajima, K. (2016). J. Mol. Struct. 1105, 152–158.
- Wang, Z. J., Clary, K. N., Bergman, R. G., Raymond, K. N. & Toste, F. D. (2013). Nat. Chem. 5, 100–103. [DOI] [PubMed]
- Wiester, M. J., Ulmann, P. A. & Mirkin, C. A. (2011). Angew. Chem. Int. Ed. 50, 114–137. [DOI] [PubMed]
- Yang, L., Powell, D. R. & Houser, R. P. (2007). Dalton Trans. pp. 955–964. [DOI] [PubMed]
- Zeng, F., Ni, J., Wang, Q., Ding, Y., Ng, S. W., Zhu, W. & Xie, Y. (2010). Cryst. Growth Des. 10, 1611–1622.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) Compound_1, Compound_2, Compound_3, Compound_4, Compound_5, Compound_6, global. DOI: 10.1107/S2053229618018314/sk3704sup1.cif
Structure factors: contains datablock(s) Compound_1. DOI: 10.1107/S2053229618018314/sk3704Compound_1sup2.hkl
Structure factors: contains datablock(s) Compound_2. DOI: 10.1107/S2053229618018314/sk3704Compound_2sup3.hkl
Structure factors: contains datablock(s) Compound_3. DOI: 10.1107/S2053229618018314/sk3704Compound_3sup4.hkl
Structure factors: contains datablock(s) Compound_4. DOI: 10.1107/S2053229618018314/sk3704Compound_4sup5.hkl
Structure factors: contains datablock(s) Compound_5. DOI: 10.1107/S2053229618018314/sk3704Compound_5sup6.hkl
Structure factors: contains datablock(s) Compound_6. DOI: 10.1107/S2053229618018314/sk3704Compound_6sup7.hkl











