ABSTRACT
DDR1 and DDR2 are expressed in skin but their expression differs according to the skin compartment, epidermis, dermis, hypodermis and to the embryonic origin of the cells. In skin, it seems that during physiological processes such as wound healing or pathological processes such as tumorigenesis or systemic sclerosis development only one of the DDR is dysregulated. Furthermore, the altered DDR in pathological process is not necessarily the DDR implicated in basal homeostasis. Indeed, in epidermis, while DDR1 is the main DDR involved in melanocyte homeostasis, DDR2 seems to be the main DDR implicated in melanoma. On the contrary, in dermis, while DDR2 is necessary for normal wound healing, dysregulation of DDR1 is associated with abnormal wound healing leading to keloid. In conclusion, targeting DDR could be a therapeutic solution, however side effects have to be managed carefully.
KEYWORDS: Melanocyte, CCN3, keratinocyte, fibroblast, integrins, cadherin, cell adhesive proteins
Human skin is one of the most important organs in the human body since its main role is to protect the body against deleterious external factors such as ultraviolet (UV) light, trauma, microorganisms and chemicals. It is composed of three layers: the hypodermis, dermis and epidermis (Figure 1). The epidermis, which is the external layer, is a keratinized pluristratified epithelium composed of keratinocytes (90%), melanocytes (5%) in the basal layer, Merkel cells and Langerhans cells. The dermis is a connective tissue that supports and nourishes the epidermis since the latter is not vascularized. It is mainly composed of elastin and fibrillar collagen I and III, whose levels vary with age. The dermo-epidermal junction secreted by keratinocytes and by fibroblasts contains collagen IV and VII and ensures the cohesion of the epidermis and dermis. The hypodermis is a loose connective tissue mainly composed of adipocytes and connected to the dermis by collagen fibers and elastin.
Figure 1.
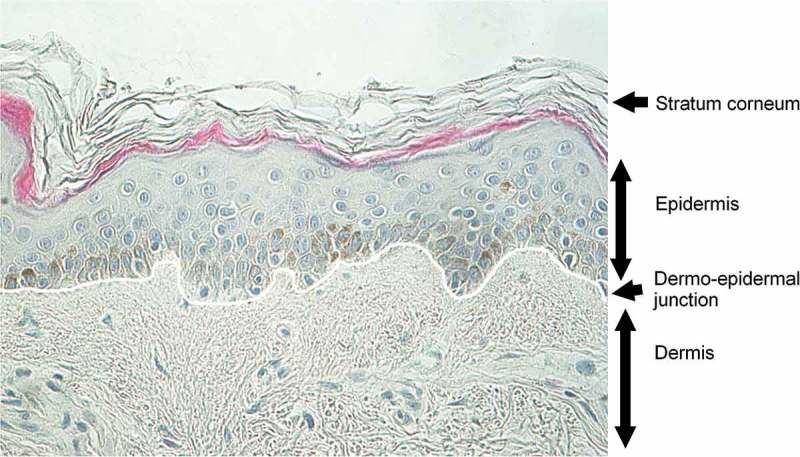
Morphology of adult human skin. Staining on human mammary skin from 45-year-old phototype III female. Fontana-Masson staining revealing melanin in dark and hematoxylin counterstaining revealing nucleus in blue.
Physiological expression of Discoidin Domain receptors in human skin
In the literature, Discoidin Domain Receptors (DDRs) have been widely described in epithelia and connective tissues but few studies have concerned DDRs in skin. In extracts from whole normal skin, mRNA of both DDR1 and DDR2 were detected [1]. However, expression of DDR1 and DDR2 varies according to cell type, DDR1 being mostly expressed in epithelial cells (Figure 2(a)) whereas DDR2 is mostly expressed by cells present in connective tissues originating from the mesoderm [1,2]. As skin layers have two embryonic origins, i.e. the epidermis originating from the ectoderm, dermis and the hypodermis originating from the mesoderm, keratinocytes and melanocytes should mainly express DDR1 whereas fibroblasts and adipocytes should mainly express DDR2.
Figure 2.
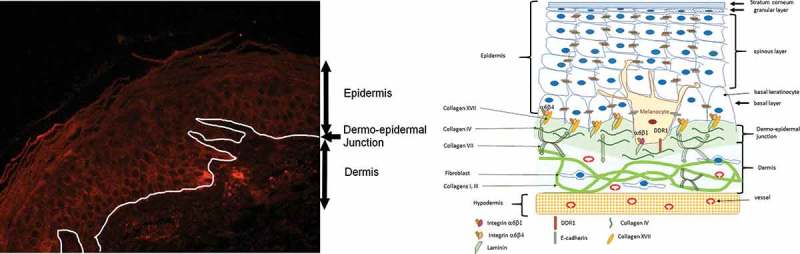
(a) Expression of DDR1 in human adult skin. Immunohistochemistry performed on mammary skin from 35-year-old phototype II female. Anti-DDR1 (abcam ab37838) detected using HRP-Envision+ system (Dako) followed by incubation with VIP (vector laboratory). DDR1 appears in violet-brown. Hematoxylin counterstaining reveals nucleus in blue. (b) Schematic representation of DDR1 localization in melanocyte.
DDRs in epidermis and epidermal cells
DDR1 and DDR2 expression in the epidermis has received little attention and immunostaining detects only DDR1 in keratinocytes (Figure 2(a)) [3,4]. DDR1 expression is maintained in keratinocyte monolayers and 3D models [4–6]. In culture, DDR1 is also expressed by melanocytes [4–6].
DDR1 is expressed by keratinocytes but its role has not yet been explored [3,4]. Di Marco et al. hypothesized that DDR1 represents the nerve growth factor (NGF) receptor and acts solely as a growth-mediating tyrosine kinase receptor and not as a potential adhesion molecule [5]. On the contrary, in melanocytes, DDR1 acts as an adhesion molecule by interacting with collagen IV on the basal membrane, thus controlling the basal localization of melanocytes [6]. In melanocytes, DDR1 is under the positive control of CCN3 (third member of the CCN family, the acronym representing the first three members of the family: Cysteine-rich angiogenic protein 61, Connective growth factor, Nephroblastoma overexpressed) [4,6]. However, no direct interaction between DDR1 and CCN3 has been described to date. DDR1 maintains melanocyte homeostasis both in basal conditions (Figure 2(b)) and under stress since upregulation of CCN3 by UV increases the number of DDR1 at the basal pole of melanocytes, thereby preventing detachment and apoptosis of melanocytes [7]. This mechanism might pass through p53 since DDR1 is a target of p53 [8] and p21, a downstream target of p53, is regulated by CCN3 [6]. Another hypothesis is that integrins (α5β1͵α6β1 and αvβ5)͵ the main receptors of CCN3, are involved in the regulation of DDR1 expression induced by CCN3 [9].
DDRs in dermis and dermal fibroblasts
The dermis is composed of two layers, the papillary dermis and reticular dermis which contain papillary or reticular fibroblasts. Their structures differ especially with regard to the direction in which the collagen is orientated and their fibrillar organization. Furthermore, according to where they are located, fibroblasts secrete different factors at various levels such as matrix metalloproteinase (MMP) and collagens, mostly type I and III [10–13]. Owing to this diversity, the expression and role of DDR1 and DDR2 in fibroblasts from other locations are not the same as those in dermal fibroblasts. Nevertheless, immunostaining revealed the expression of DDR1 in normal adult skin dermal cells, probably fibroblasts and vessels (Figure 2(a)) [3], and that of DDR2 in fibroblasts [1]. Transcriptomic analysis confirmed the expression of both DDRs in cultured fibroblasts [1]. However, the differential expression of DDRs in papillary and reticular fibroblasts remains elusive.
Using DDR2 null mice, Olaso et al. demonstrated that DDR2 promotes proliferation, collagen I secretion and MMP2 production and activation in skin fibroblasts [14]. DDR2 knock-out (DDR2 KO) does not affect DDR1 mRNA [15]. In dermal fibroblasts, overexpression of DDR2 leads to type I collagen induction via the downregulation of microRNA-196a (miR-196a), whereas the knock-down of DDR2 leads to the downregulation of collagen expression via the upregulation of miR-196a [1]. In dermal fibroblasts, DDR2 can be downregulated at mRNA and protein levels by exogenous TGF-β1 (Transforming growth factor), a skin pro-inflammatory cytokine, leading to the migration of cells such as fibroblasts [1,16]. On the contrary, no skin phenotype was described in DDR1 knock-out mice [17].
DDRs in hypodermis and adipocytes
DDR2 expression has been investigated using 3T3-L1 murine embryonic cells, which can be differentiated in adipocytes [18]. Zurakowski et al. suggested that DDR2 may play a regulatory role in adipogenesis, altering insulin responsiveness in preadipocytes and adipocytes [18]. DDR expression in hypodermis or skin adipocytes has not yet received attention.
DDR expression in cutaneous wound healing
Dermal wound healing is a dynamic process that includes cell migration, cell proliferation, cell differentiation, matrix synthesis and matrix remodeling. Matrix synthesis and remodeling are mainly due to production by fibroblasts and myofibroblasts of collagens and MMPs, especially MMP-2 [19].
DDR expression during normal wound healing
In the early gestational stage, the mammalian fetus heals cutaneous wounds rapidly with little acute inflammatory response and without scarring [20]. Human and rat DDR1 and DDR2 genes are highly homologous, suggesting that the involvement of DDRs in wound healing is common to all mammals [21]. Fibroblasts from early-gestational fetal rat skin express a high level of DDR1, synthesize more total collagen and have greater prolyl hydroxylase activity than adult fibroblasts [22].
Wound healing has been studied in both DDR1 and DDR2 knock-out mice. Wound healing occurs normally in DDR1 null mice [17] whereas it is delayed in DDR2 null mice [14]. The delay is due to a defective recruitment of myofibroblasts, a reduced ability of fibroblasts to contract the wound, a decrease in collagen I production and a decrease in collagen crosslinking. These findings were found to be due respectively to a decrease in lysyl oxidase (LOX), lysyl hydroxylase (LH1), secreted protein acid and rich in cystein (SPARC) at mRNA and protein levels and a decrease in Src phosphorylation [23]. A recent study by Coelho et al. [24] in a rat model of splinted (see 2.2) and unsplinted wounding process showed that physiological wound healing is associated with a transient expression of DDR1 and its phosphorylated form pDDR1 (activated DDR1). Its expression preceded the appearance of myofibroblasts (α-sma positive cells), so it could be primarily expressed in proto-myofibroblasts [25].
DDR expression in pathological wound healing
In their rat model, Coelho et al. showed that mechanical inhibition of wound contraction induces maintenance of both pDDR1 and myofibroblasts in wounds together with an increase in collagen alignment [24]. Dysregulation of normal cutaneous wound healing may lead to the formation of keloids. Keloids are due to the accumulation of extracellular matrix components, especially thick collagen bundles. Fibroblasts from keloids express more epidermal growth factor receptor (EGFR), DDR1 and adaptor protein Shc than normal fibroblasts and are constitutively highly phosphorylated [26]. Transactivation of the EGFR (phosphorylation) is required for Akt activation and collagen I production [27]. Thus, increased phosphorylation of EGFR may induce excessive production of collagen which stimulates DDR1 and its activation, thereby inducing the formation of docking sites for modular downstream signaling molecules such as Shc [28]. Shc in turn initiates numerous downstream cascades such as cell cycle progression and cell survival [29]. Thus, Chin et al. postulated that by regulating collagen-fibroblast signaling, DDR1 plays an important role in modulating overexpression of collagen in keloids [26]. While DDR2 KO in DDR2 null mice delays wound healing, it does not alter the normal wounding process [14] and leads to the formation of typical scar tissue.
DDR expression in skin diseases
DDR expression in vitiligo
Since non-segmental vitiligo (NSV) is a skin disorder characterized by the progressive loss of melanocytes leading to depigmentation, some authors have investigated the involvement of DDR1 and its upstream regulator CCN3 [3,4,30]. Genetic variants of DDR1 have been associated with NSV in two independent populations in Brazil [30], in a Korean population [31] and in a Chinese population [32]. In vitiligo patients, DDR1 was found to be homogeneously expressed in the epidermis and presented a chicken-wire pattern [3]. However, a decreased expression of DDR1 in non-lesional and lesional skin of NSV patients was observed in the whole epidermis [3,4,33]. Collagen IV is also decreased in vitiligo patients [4], which may explain the weak attachment of melanocytes via DDR1 to the basal membrane and their high susceptibility to detachment under stress. A recent study in mesangial cells by Borza et al. [34] demonstrated that DDR1 is a positive regulator of collagen IV and that this ability requires efficient collagen I binding and kinase activity. Since keratinocytes and melanocytes are in contact with the dermo-epidermal junction and not the dermis where collagen I is located, the mechanism of regulation may be different. Fukunaga-Kalabis et al. demonstrated that melanocytes interact only with collagen IV and not with collagen I [6]. Nevertheless, even if the mechanism of regulation is different, a decrease in collagen IV in vitiligo skin may result from a decrease in DDR1. Studies have shown that E-cadherin is stabilized by DDR1 and that DDR1 inhibition by RNA interference decreases E-cadherin at the cell surface [35–37]. This is consistent with the decrease in E-cadherin observed in melanocytes and keratinocytes from NSV skin [38,39]. Membranous E-cadherin is decreased in pigmented reconstructed epidermis under stress such as H2O2 (oxidative stress observed in vitiligo patients) or sera from NSV patients, leading to the detachment of melanocytes [39,40]. DDR1 expression should be analyzed in this model of stressed epidermal reconstructs used to reproduce vitiligo in order to verify whether the observed decrease in E-cadherin is associated with a decrease in DDR1. If DDR1 was to play two distinct roles in vitiligo, i.e. an intrinsic one due to the attachment of melanocytes to the basal membrane via an interaction between DDR1 and collagen IV and an inducible one due to its effect on melanocyte-keratinocyte interaction via E-cadherin degradation [36], this could explain the diversity of NSV expression in patients.
DDR expression in systemic sclerosis
Systemic sclerosis (SSc) is a complex systemic autoimmune disease that is characterized by microvascular dysfunction, immune activation, and interstitial and perivascular fibrosis affecting the skin and internal organs [41]. The interrelationships between these processes are unknown, and the elucidation of the interplay between these three pathologic hallmarks would enhance knowledge of the pathogenesis of this disease and, consequently, improve treatment options [42]. Around half of SSc patients have pigmentary disturbances (hypo or hyperpigmentation), which can occur prematurely even before fibrosis [43,44]. One of these pigmentary disorders is vitiligo-like depigmentation [45–47] but DDR1 expression in melanocytes from these patients has not yet been investigated. However, while there is no difference in DDR1 mRNA expression in whole human skin, DDR2 is greatly decreased in both limited and diffuse SSc [1]. Detection of DDR2 by immunohistochemistry showed that DDR2 is decreased in spindle-shaped fibroblasts in SSc skin as compared to normal skin and that this decrease is associated with an increase of the number of thickened collagen bundles [1]. In vitro, DDR2 is decreased both at the mRNA and protein level in fibroblasts from SSc patients and this downregulation persists until passage 15 [1]. The down-regulation of DDR2 in SSc fibroblasts results from stimulation by autocrine TGF-β signaling, which also downregulates miR-196a. Down-regulation of DDR2 in normal fibroblasts is thought to induce upregulation of miR-196a and thus a decrease in collagen I. However, since miR-196a is downregulated in SSc fibroblasts by TGF-β1 overproduction, DDR2 cannot counteract its effect and excessive collagen I is produced [1]. Since collagen I is the most abundant extracellular protein in SSc skin [48], and because DDR2 has a greater affinity for collagen I than DDR1 [15], it is logical that DDR2 and not DDR1 is altered in SSc skin.
DDR expression in skin cancers
Mutations and the altered expression of DDRs are found in many types of cancers, suggesting their implication in the development and progression of cancer. However, according to the cell type and cancer stage, DDRs can act as pro-tumorigenic or as anti-tumorigenic receptors [49].
DDR expression in human skin carcinoma
While the implication of DDRs in lung, breast, liver, pancreas and head-and-neck (group of cancers that starts within the mouth, nose, throat, larynx, sinuses, or salivary glands) carcinoma is indubitable [Valiat50], their role in skin carcinoma still remains to be demonstrated. As in normal epithelial cells, DDR1 forms complexes with E-cadherin in collectively invading A431, the human squamous cell carcinoma cell line. Wang et al. hypothesized that this complex could negatively regulate DDR1 activity and collagen-mediated cell spreading [37]. However, Hidalgo-Carcedo et al. hypothesized that DDR1 plays another role not linked to collagen binding. They found that endogenous DDR1 was localized in the apical zone of cell-cell contacts and that this localization required the E-cadherin function and helped to recruit Par3 and Par6 [51].
The complex DDR1/Par3/Par6 controls the localization of RhoE, which can antagonize Rho-ROCK-mediated regulation of actomyosin. Thus, DDR1 retains actomyosin activity at low levels, which allows the maintenance of cell cohesion during collective cell migration [51]. In hepatocellular carcinoma, decreased expression of miR-199a-5p contributes to an increase in cell invasion by deregulation of DDR1 activity [52,53]. In mouse cutaneous cell carcinoma keratinocytes (PAM212 cells), overexpression of miR-199a-5p has no effect on their proliferation but inhibits their migration. Among the mRNAs deregulated by overexpression of miR-199a-5p, DDR1 mRNA is the most down-regulated [54]. In in situ human cutaneous squamous cell carcinoma (cSSC), DDR1 is upregulated as compared to unaffected skin [54]. This upregulation of DDR1 is concomitant to downregulation of miR-199a-5p [54]. Overexpression of miR-199a-5p downregulates DDR1 in HaCat cells (human immortalized keratinocytes) and PAM212 [54], E-cadherin in A431 and increases MMP2 and MMP9 activity in A431 [55]. While E-cadherin is high in in situ cSSC tumors, it is downregulated in invasive SCC [56]. Since miR-199a-5p regulates both DDR1 and E-cadherin, one may hypothesize that DDR1 is also downregulated in invasive SCC. Thus, according to the hypothesis of Hidalgo-Carcedo, actomyosin activity would increase and spreading would be initiated. Indeed, in SCC and melanoma Rho, ROKC and actomyosin have been implicated in the metastatic cascade [57].
While there is some data on DDR1 and skin carcinoma, DDR2 expression has not been described in skin carcinoma until now. However, in 2015 a genomic analysis of metastatic cutaneous squamous cell carcinoma, which represents only 5% of cSSC, revealed a silent mutation in DDR2 [58]. A pure DDR2 mutation was found in 13.8% of cSSC [59].
DDR expression in melanoma
Melanoma cells escape physiological control by various mechanisms. These include the downregulation of receptors implicated in their interactions with keratinocytes (communication and adhesion), mainly E-cadherin and the loss of anchorage to the basement membrane due to altered expression of extracellular-matrix (ECM) binding proteins [60]. Owing to the involvement of DDR1 in the adhesion of melanocytes to the basement membrane (collagen IV) and to the fact that DDR1/ E-cadherin complexes sequester DDR1 at cell junctions, thereby preventing DDR1 activation (phosphorylation) and consequently the spreading of cells [37], it seems interesting to analyze DDR1 in melanoma. Indeed, the expression of CCN3, which regulates DDR1 in normal melanocytes [4], is downregulated in advanced melanoma and CCN3 may be a MMP2/9 repressor [61]. On the other hand, Vallachi et al. suggested that CCN3 affects the expression and function of integrin receptors modulating the interaction of melanoma cells with ECM, leading to melanoma progression [62]. Thus, CCN3 seems to affect melanoma progression through integrins and not DDR1.
In M24met cells (amelanotic melanoma cells), both DDR1 and DDR2 are expressed but only phosphorylated DDR2 induces a cell cycle arrest at the G1/S checkpoint when cells are cultured on fibrillar collagen (FC) (2D, 3D) [63]. This arrest, also observed in A2058 (melanoma cell lines from metastatic lymph node), is independent of cell spreading and cytoskeletal organization and was not observed when cells were cultured on monomeric fibrillar collagen [63]. However, the arrest is transient since these cells secrete MMP2 which, once activated, alters collagen and allows the cells to escape FC-induced cell cycle arrest [63]. DDR2 is probably not the sole regulator of MMP-2 expression and activation in the presence of FC. Among the integrins which are the main established receptor for collagen, integrins α2β1 are involved in M24met adhesion to fibrillary collagen [64] but DDR2 is not involved in this signaling [63].
In slie mice, i.e. DDR2 mutant mice which look like null mice, it has been shown after injection of B16 melanoma cells in the tail vein that loss of stromal DDR2 alleviates B16 melanoma hypoxia and restrains melanoma metastasis. This decrease in tumor metastasis ability is probably due to the increased number of pericytes, which are able to synthesize basement matrices such as collagen and thus prevent vascular leakage [65]. This finding is in accordance with Badiola et al. who showed that A375 human melanoma cells silenced for DDR2 are less prone to inducing liver metastasis than controlled A375 cells after intrasplenic injection of melanoma cells in mice [66]. This decrease in metastasis ability is due to downregulation of MMP2 and MMP9 activities via decreased phosphorylation of JNK [66]. More recently, an in vitro study using B16 BL6 murine melanoma cells silenced for DDR2 showed that phosphorylated DDR2 regulates both ERK (extracellular signal-regulated kinases) and NF-KB (nuclear factor kappa-light-chain-enhancer of activated B cells) pathways, which in turn modulate MMP2 and MMP9 and thus the migratory and invasive phenotype of melanoma cells [67].
DDR expression in fibrosarcoma
Fibrosarcomas are rare malignant mesenchymal tumors originating from fibroblasts that produce variable amounts of collagen. They are composed of immature proliferating fibroblasts or undifferentiated anaplastic spindle cells [68] and usually occur in soft tissues such as connective tissue like the dermis. In HT1080, a fibrosarcoma cell line, DDR1 was significantly less expressed than DDR2 [69]. In this cell line, phosphorylated DDR2 (pDDR2) is implicated in cell cycle arrest in G0/G1 when they are cultured on fibrillar collagen [63], whereas activated DDR2 induced proliferation in normal fibroblasts [14]. However, in HT080, DDR2 activation differs according to the quality of collagen (adult vs old), suggesting the involvement of DDR2 in the regulation of cell proliferation by collagen aging [69]. The latter result may partly explain why tumors are more proliferative in elderly patients.
Briefly, DDRs may be pro- or anti-tumorigenic and pro- or anti-metastatic in skin according to the origin of the tumor, as is the case in other tissue. Nevertheless, it seems that only one DDR drives the tumorigenic or metastatic effect. In cutaneous carcinoma, DDR1 seems to have an anti-metastatic effect by inhibiting spreading. In cutaneous melanoma, DDR2 seems to be pro-metastatic by increasing spreading, while pDDR2 seems to be anti- tumorigenic in fibrosarcoma by inhibiting growth.
Conclusion
In the epidermis, DDR1 is implicated in melanocyte homeostasis whereas DDR2 is involved in melanoma and metastatic spreading. On the other hand, DDR1 plays a role in cutaneous squamous cell carcinoma spreading. In the dermis, DDR1 is involved in abnormal wound healing by the formation of keloid tissue, while DDR2 is involved in normal wound healing, systemic sclerosis and likely fibrosarcoma. Further studies are needed to decipher the role of each DDR in each compartment. It will be interesting to investigate DDR1 expression in keratinocytes in the epidermis, especially in all phototypes and at various ages, since CCN3 expression varies according to these parameters [70]. Otherwise, since skin cancers occur more frequently with age, appear mainly in photoexposed areas and are more frequent in low phototype individuals [71], experiments addressing the expression of both DDR after chronic UV or solar irradiation of skin cells, epidermal reconstructs, skin explants or mice might unravel their role in the initiation and progression of non-melanoma skin cancers and melanoma. Furthermore, a decrease in DDR1 is frequently observed when patients with vitiligo are treated for melanoma. If DDR1 levels vary in keratinocytes at various stages of melanoma, this could be a marker of response to treatment. Such future perspectives pave the way for targeting DDR as a therapeutic solution or use as a diagnostic marker, since the expression of DDRs differs according to the cell type and compartment.
Disclosure statement
No potential conflict of interest was reported by the author.
References
- [1].Makino K, Jinnin M, Aoi J, et al. Discoidin domain receptor 2–microRNA 196a–mediated negative feedback against excess Type I collagen expression is impaired in scleroderma dermal fibroblasts. J Invest Dermatol. 2013;133:110–119. [DOI] [PubMed] [Google Scholar]
- [2].Alves F, Vogel W, Mossie K, et al. Distinct structural characteristics of discoidin I subfamily receptor tyrosine kinases and complementary expression in human cancer. Oncogene. 1995;10:609–618. [PubMed] [Google Scholar]
- [3].Reichert-Faria A, Jung JE, Moreschi Neto V, et al. Reduced immunohistochemical expression of Discoidin Domain Receptor 1 (DDR1) in vitiligo skin. J Eur Acad Dermatol Venereol. 2013;27:1057–1059. [DOI] [PubMed] [Google Scholar]
- [4].Ricard AS, Pain C, Daubos A, et al. Study of CCN3 (NOV) and DDR1 in normal melanocytes and vitiligo skin. Exp Dermatol. 2012;21:411–416. [DOI] [PubMed] [Google Scholar]
- [5].Di Marco E, Cutuli N, Guerra L, et al. Molecular cloning of trkE, a novel trk-related putative tyrosine kinase receptor isolated from normal human keratinocytes and widely expressed by normal human tissues. J Biol Chem. 1993;268:24290–24295. [PubMed] [Google Scholar]
- [6].Fukunaga-Kalabis M, Martinez G, Liu Z-J, et al. CCN3 controls 3D spatial localization of melanocytes in the human skin through DDR1. J Cell Biol. 2006;175:563–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Santiago-Walker A, Li L, Haass NK, et al. Melanocytes: from morphology to application. Skin Pharmacol Physiol. 2009;22:114–121. [DOI] [PubMed] [Google Scholar]
- [8].Ongusaha PP, Kim J, Fang L, et al. p53 induction and activation of DDR1 kinase counteract p53-mediated apoptosis and influence p53 regulation through a positive feedback loop. EMBO J. 2003;22:1289–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yeh Y-C, Lin -H-H, Tang M-J.. A tale of two collagen receptors, integrin β1 and discoidin domain receptor 1, in epithelial cell differentiation. Am J Physiol Cell Physiol. 2012;303:C1207–C1217. [DOI] [PubMed] [Google Scholar]
- [10].Tracy LE, Minasian RA, Caterson EJ. Extracellular matrix and dermal fibroblast function in the healing wound. Adv Wound Care. 2016;5:119–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sorrell JM, Baber MA, Caplan AI. Site-matched papillary and reticular human dermal fibroblasts differ in their release of specific growth factors/cytokines and in their interaction with keratinocytes. J Cell Physiol. 2004;200:134–145. [DOI] [PubMed] [Google Scholar]
- [12].Ali Bahar M, Bauer B, Tredget EE, et al. Dermal fibroblasts from different layers of human skin are heterogeneous in expression of collagenase and types I and III procollagen mRNA. Wound Repair Regen. 2004;12:175–182. [DOI] [PubMed] [Google Scholar]
- [13].Sorrell JM, Caplan AI. Fibroblast heterogeneity: more than skin deep. J Cell Sci. 2004;117:667–675. [DOI] [PubMed] [Google Scholar]
- [14].Olaso E, Labrador J-P, Wang L, et al. Discoidin domain receptor 2 regulates fibroblast proliferation and migration through the extracellular matrix in association with transcriptional activation of matrix metalloproteinase-2. J Biol Chem. 2002;277:3606–3613. [DOI] [PubMed] [Google Scholar]
- [15].Olaso E, Lin H-C, Wang L-H, et al. Impaired dermal wound healing in discoidin domain receptor 2-deficient mice associated with defective extracellular matrix remodeling. Fibrogenesis Tissue Repair. 2011;4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Han G, Li F, Singh TP, et al. The pro-inflammatory role of TGFβ1: a paradox? Int J Biol Sci. 2012;8:228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Vogel WF, Abdulhussein R, Ford CE. Sensing extracellular matrix: an update on discoidin domain receptor function. Cell Signal. 2006;18:1108–1116. [DOI] [PubMed] [Google Scholar]
- [18].Zurakowski H, Gagnon A, Landry A, et al. Discoidin domain receptor 2 impairs insulin-stimulated insulin receptor substrate-1 tyrosine phosphorylation and glucose uptake in 3T3-L1 adipocytes. Horm Metab Res Horm Stoffwechselforschung Horm Metab. 2007;39:575–581. [DOI] [PubMed] [Google Scholar]
- [19].Okada A, Tomasetto C, Lutz Y, et al. Expression of matrix metalloproteinases during rat skin wound healing: evidence that membrane type-1 matrix metalloproteinase is a stromal activator of pro-gelatinase A. J Cell Biol. 1997;137:67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Longaker MT, Adzick NS. The biology of fetal wound healing: a review. Plast Reconstr Surg. 1991;87:788–798. [DOI] [PubMed] [Google Scholar]
- [21].Chin GS, Lee S, Hsu M, et al. Discoidin domain receptors and their ligand, collagen, are temporally regulated in fetal rat fibroblasts in vitro. Plast Reconstr Surg. 2001;107:769–776. [DOI] [PubMed] [Google Scholar]
- [22].Chin GS, Kim WJ, Lee TY, et al. Differential expression of receptor tyrosine kinases and Shc in fetal and adult rat fibroblasts: toward defining scarless versus scarring fibroblast phenotypes. Plast Reconstr Surg. 2000a;105:972–979. [DOI] [PubMed] [Google Scholar]
- [23].Olaso E. Downregulation of discoidin domain receptor 2 in A375 human melanoma cells reduces its experimental liver metastasis ability. Oncol Rep. 2011;26:971–978. [DOI] [PubMed] [Google Scholar]
- [24].Coelho NM, Arora PD, van Putten S, et al. Discoidin domain receptor 1 mediates myosin-dependent collagen contraction. Cell Rep. 2017;18:1774–1790. [DOI] [PubMed] [Google Scholar]
- [25].Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526–537. [DOI] [PubMed] [Google Scholar]
- [26].Chin GS, Liu W, Steinbrech D, et al. Cellular signaling by tyrosine phosphorylation in keloid and normal human dermal fibroblasts. Plast Reconstr Surg. 2000b;106:1532–1540. [DOI] [PubMed] [Google Scholar]
- [27].Wu D, Peng F, Zhang B, et al. Collagen I induction by high glucose levels is mediated by epidermal growth factor receptor and phosphoinositide 3-kinase/Akt signalling in mesangial cells. Diabetologia. 2007;50:2008–2018. [DOI] [PubMed] [Google Scholar]
- [28].Vogel W, Gish GD, Alves F, et al. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell. 1997;1:13–23. [DOI] [PubMed] [Google Scholar]
- [29].Wary KK, Mainiero F, Isakoff SJ, et al. The adaptor protein Shc couples a class of integrins to the control of cell cycle progression. Cell. 1996;87:733–743. [DOI] [PubMed] [Google Scholar]
- [30].Silva de Castro CC, do Nascimento LM, Walker G, et al. Genetic variants of the DDR1 gene are associated with vitiligo in two independent Brazilian population samples. J Invest Dermatol. 2010;130:1813–1818. [DOI] [PubMed] [Google Scholar]
- [31].Kim H-J, Uhm YK, Yun JY, et al. Association between polymorphisms of discoidin domain receptor tyrosine kinase 1 (DDR1) and non-segmental vitiligo in the Korean population. Eur J Dermatol EJD. 2010;20:231–232. [DOI] [PubMed] [Google Scholar]
- [32].Liang Y, Yang S, Zhou Y, et al. Evidence for two susceptibility loci on chromosomes 22q12 and 6p21-p22 in Chinese generalized vitiligo families. J Invest Dermatol. 2007;127:2552–2557. [DOI] [PubMed] [Google Scholar]
- [33].Elgarhy LH, Abdullatif A, Abdelazim R, et al. Discoidin domain receptor-1 as a player in impairment of melanocytes adhesion process in vitiligo. G Ital Dermatol E Venereol Organo Uff Soc Ital Dermatol E Sifilogr. 2016;151:473–479. [PubMed] [Google Scholar]
- [34].Borza CM, Su Y, Tran T-L, et al. Discoidin domain receptor 1 kinase activity is required for regulating collagen IV synthesis. Matrix Biol J Int Soc Matrix Biol. 2017;57–58:258–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Eswaramoorthy R, Wang C-K, Chen W-C, et al. DDR1 regulates the stabilization of cell surface E-cadherin and E-cadherin-mediated cell aggregation. J Cell Physiol. 2010;224:387–397. [DOI] [PubMed] [Google Scholar]
- [36].Yeh Y-C, Wu -C-C, Wang Y-K, et al. DDR1 triggers epithelial cell differentiation by promoting cell adhesion through stabilization of E-cadherin. Mol Biol Cell. 2011;22:940–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wang C-Z, Yeh Y-C, Tang M-J. DDR1/E-cadherin complex regulates the activation of DDR1 and cell spreading. Am J Physiol Cell Physiol. 2009;297:C419–C429. [DOI] [PubMed] [Google Scholar]
- [38].Benzekri L, Hmamouchi I, Gauthier Y. Possible patterns of epidermal melanocyte disappearance in nonsegmental vitiligo: a clinicopathological study. Br J Dermatol. 2015;172:331–336. [DOI] [PubMed] [Google Scholar]
- [39].Wagner RY, Luciani F, Cario-André M, et al. Altered E-cadherin levels and distribution in melanocytes precedes clinical manifestations of vitiligo. J Invest Dermatol. 2015;135:1810–1819. [DOI] [PubMed] [Google Scholar]
- [40].Cario-André M, Pain C, Gauthier Y, et al. The melanocytorrhagic hypothesis of vitiligo tested on pigmented, stressed, reconstructed epidermis. Pigment Cell Res Spons Eur Soc Pigment Cell Res Int Pigment Cell Soc. 2007;20:385–393. [DOI] [PubMed] [Google Scholar]
- [41].Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med. 2009;360:1989–2003. [DOI] [PubMed] [Google Scholar]
- [42].Truchetet M-E, Demoures B, Eduardo Guimaraes J, et al. Platelets induce thymic stromal lymphopoietin production by endothelial cells: contribution to fibrosis in human systemic sclerosis. Arthritis Rheumatol. 2016;68:2784–2794. Hoboken NJ. [DOI] [PubMed] [Google Scholar]
- [43].Jewett LR, Hudson M, Malcarne VL, et al. Sociodemographic and disease correlates of body image distress among patients with systemic sclerosis. PLoS One. 2012;7:e33281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Darrigade AS, Vedie AL, Gauthier C, et al. Pigmented skin patches without scleroderma as a predominant clinical symptom revealing systemic sclerosis. Clin Exp Dermatol. 2015;41:379–382. [DOI] [PubMed] [Google Scholar]
- [45].De Villiers WJ, Jordaan HF, Bates W. Systemic sclerosis sine scleroderma presenting with vitiligo-like depigmentation and interstitial pulmonary fibrosis. Clin Exp Dermatol. 1992;17:127–131. [DOI] [PubMed] [Google Scholar]
- [46].Rai VM, Balachandran C. Pseudovitiligo in systemic sclerosis. Dermatol Online J. 2005;11:41. [PubMed] [Google Scholar]
- [47].Fujimoto N, Hamaguchi Y, Tanaka T. Vitiligo-like depigmentation with perifollicular pigment retention in systemic sclerosis treated successfully with suplatast tosilate. Eur J Dermatol. 2200;1:110–112. [DOI] [PubMed] [Google Scholar]
- [48].Mauch C, Kreig T. Fibroblast-matrix interactions and their role in the pathogenesis of fibrosis. Rheum Dis Clin North Am. 1990;16:93–107. [PubMed] [Google Scholar]
- [49].Borza CM, Pozzi A. Discoidin domain receptors in disease. Matrix Biol J Int Soc Matrix Biol. 2014;34:185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Valiathan RR, Marco M, Leitinger B, et al. Discoidin domain receptor tyrosine kinases: new players in cancer progression. Cancer Metastasis Rev. 2012;31:295–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Hidalgo-Carcedo C, Hooper S, Chaudhry SI, et al. Collective cell migration requires suppression of actomyosin at cell-cell contacts mediated by DDR1 and the cell polarity regulators Par3 and Par6. Nat Cell Biol. 2011;13:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Cheung -H-H, Davis AJ, Lee T-L, et al. Methylation of an intronic region regulates miR-199a in testicular tumor malignancy. Oncogene. 2011;30:3404–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Shen Q, Cicinnati VR, Zhang X, et al. Role of microRNA-199a-5p and discoidin domain receptor 1 in human hepatocellular carcinoma invasion. Mol Cancer. 2010;9:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kim B-K, Kim I, Yoon SK. Identification of miR-199a-5p target genes in the skin keratinocyte and their expression in cutaneous squamous cell carcinoma. J Dermatol Sci. 2015;79:137–147. [DOI] [PubMed] [Google Scholar]
- [55].Wang S, Cao KE, He Q, et al. miR-199a-5p induces cell invasion by suppressing E-cadherin expression in cutaneous squamous cell carcinoma. Oncol Lett. 2016;12:97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Barrette K, Van Kelst S, Wouters J, et al. Epithelial-mesenchymal transition during invasion of cutaneous squamous cell carcinoma is paralleled by AKT activation. Br J Dermatol. 2014;171:1014–1021. [DOI] [PubMed] [Google Scholar]
- [57].Rodriguez-Hernandez I, Cantelli G, Bruce F, et al. Rho, ROCK and actomyosin contractility in metastasis as drug targets. F1000Research. 2016;5:783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Li YY, Hanna GJ, Laga AC, et al. Genomic analysis of metastatic cutaneous squamous cell carcinoma. Clin Cancer Res Off J Am Assoc Cancer Res. 2015;21:1447–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Tsai M-C, Li W-M, Huang C-N, et al. DDR2 overexpression in urothelial carcinoma indicates an unfavorable prognosis: a large cohort study. Oncotarget. 2016;7:78918–78931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Haass NK, Smalley KSM, Li L, et al. Adhesion, migration and communication in melanocytes and melanoma. Pigment Cell Res. 2005;18:150–159. [DOI] [PubMed] [Google Scholar]
- [61].Fukunaga-Kalabis M, Martinez G, Telson SM, et al. Downregulation of CCN3 expression as a potential mechanism for melanoma progression. Oncogene. 2008;27:2552–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Vallacchi V, Daniotti M, Ratti F, et al. CCN3/nephroblastoma overexpressed matricellular protein regulates integrin expression, adhesion, and dissemination in melanoma. Cancer Res. 2008;68:715–723. [DOI] [PubMed] [Google Scholar]
- [63].Wall SJ, Werner E, Werb Z, et al. Discoidin domain receptor 2 mediates tumor cell cycle arrest induced by fibrillar collagen. J Biol Chem. 2005;280:40187–40194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Henriet P, Zhong ZD, Brooks PC, et al. Contact with fibrillar collagen inhibits melanoma cell proliferation by up-regulating p27KIP1. Proc Natl Acad Sci U S A. 2000;97:10026–10031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Zhang S, Bu X, Zhao H, et al. A host deficiency of discoidin domain receptor 2 (DDR2) inhibits both tumour angiogenesis and metastasis. J Pathol. 2014;232:436–448. [DOI] [PubMed] [Google Scholar]
- [66].Badiola I, Villacé P, Basaldua I, et al. Downregulation of discoidin domain receptor 2 in A375 human melanoma cells reduces its experimental liver metastasis ability. Oncol Rep. 2011;26:971–978. [DOI] [PubMed] [Google Scholar]
- [67].Poudel B, Lee Y-M, Kim D-K. DDR2 inhibition reduces migration and invasion of murine metastatic melanoma cells by suppressing MMP2/9 expression through ERK/NF-κB pathway. Acta Biochim Biophys Sin. 2015;47:292–298. [DOI] [PubMed] [Google Scholar]
- [68].Meral O, Uysal H. Comparative proteomic analysis of fibrosarcoma and skin fibroblast cell lines. Tumour Biol J Int Soc Oncodevelopmental Biol Med. 2015;36:561–567. [DOI] [PubMed] [Google Scholar]
- [69].Saby C, Buache E, Brassart-Pasco S, et al. Type I collagen aging impairs discoidin domain receptor 2-mediated tumor cell growth suppression. Oncotarget. 2016;7:24908–24927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Brassie M, Pain C, Ezzedine K, et al. CCN3 and CCN5, new factors associated with skin pigmentation. J Pigment Disord. 2016;3:239. [Google Scholar]
- [71].D’Orazio J, Jarrett S, Amaro-Ortiz A, et al. UV radiation and the skin. Int J Mol Sci. 2013;14:12222–12248. [DOI] [PMC free article] [PubMed] [Google Scholar]


