ABSTRACT
Hepatitis B virus (HBV) infection remains an important public health problem in China, and adults need to be vaccinated. This systematic review and meta-analysis assessed the appropriate immunization of adults in China. Only randomized controlled trials (RCTs) were eligible, and seroprotection was defined as anti-HBs≥ 10 mIU/ml; 18,308 participants in 27 studies were included. Relative risk (RR) and random effects models were used. Twenty micrograms of HBV vaccine resulted in a better response than 10 μg (RR: 1.05, 95% confidence interval (CI): 1.02 to 1.08), and the 0-, 1-, and 6-month schedule was more effective than the 0-, 1-, and 2 – or 3-month schedule (RR: 0.98, 95% CI: 0.96 to 1.00). No significant differences were observed between 10 μg and 5 μg (RR: 1.05, 95% CI: 0.88 to 1.01); (yeast-derived hepatitis B vaccines) YDV and recombinant Chinese hamster ovary cell (CHO) hepatitis B vaccine (RR: 1.01, 95% CI: 0.98 to 1.04); domestic and imported (RR: 1.02, 95% CI: 0.99 to 1.05); or 0-, 1-, and 6-month and 0-, 1-, and 12-month schedules (RR: 1.02, 95% CI: 0.89 to 1.08). In conclusion, 20 μg of vaccine is recommended for adults in China, and the 0-, 1-, and 12-month immunization program schedule is also worth choosing when it is not possible to complete the 0-, 1-, and 6-month schedule.
KEYWORDS: Hepatitis B virus, hepatitis B vaccine, anti-HBs, adult, meta-analysis
Introduction
Hepatitis B virus (HBV) is still an important worldwide public health problem. It is estimated that 257 million persons, or 3.5% of the population, are living with chronic HBV infection worldwide.1 The prevalence of HBV infection varies significantly in different areas; China is a highly endemic area for HBV infection.2
Today, after decades of HBV mass vaccination, the HBsAg prevalence in children has decreased significantly, but there remains a large proportion of adults who are as yet unvaccinated.3 In addition, Chen WG et al. analyzed 7119 newly discovered patients with chronic HBV infection and found that those aged 30–50 had the highest incidence; another report from the USA also showed that the highest proportion of new HBV infections occurs in the population aged 25 to 44.4,5 The Advisory Committee on Immunization Practices (ACIP) recommends vaccination for all unvaccinated adults at risk for HBV infection, and Britain and Italy also have adopted vaccination programs for adults at high risk for HBV infection.6–8 However, in China, adult hepatitis B vaccination has not been systematically performed, and the recommendation for adults from the national Centers for Disease Control follows the conventional immunization programs available for infants.9 Therefore, the need for vaccination among adults in China should receive wide attention.
At present, the factors that influence the immune response can be divided into two types: personal factors, such as overweight, smoking, age, gender, and region, which are difficult to change in vaccination; and immunization program factors, such as dosage and immunization schedule, which can be adjusted for better immune effect.9–42 In this study, we focused on immunization program factors. In the last decades, numerous emerging studies9,14–40,43 in China have been conducted to explore the factors that influence immunologic response to hepatitis B vaccine in adults. However, it is still inconclusive which immunization programs are the most appropriate. Therefore, we conducted a systematic review and meta-analysis to assess a more appropriate immunization program for adults in China.
Results
Characteristics of eligible studies
As shown in the flow diagram (Figure 1), a total of 3180 potentially eligible articles were identified by searching the relevant databases and the references of eligible studies, and 3008 records were excluded after screening the titles and abstracts. After reviewing the full texts, 27 studies that included 75 cohorts were included in this study.9,14–40 Of these 27 studies, 22 were published in Chinese, and 5 were published in English.
Figure 1.
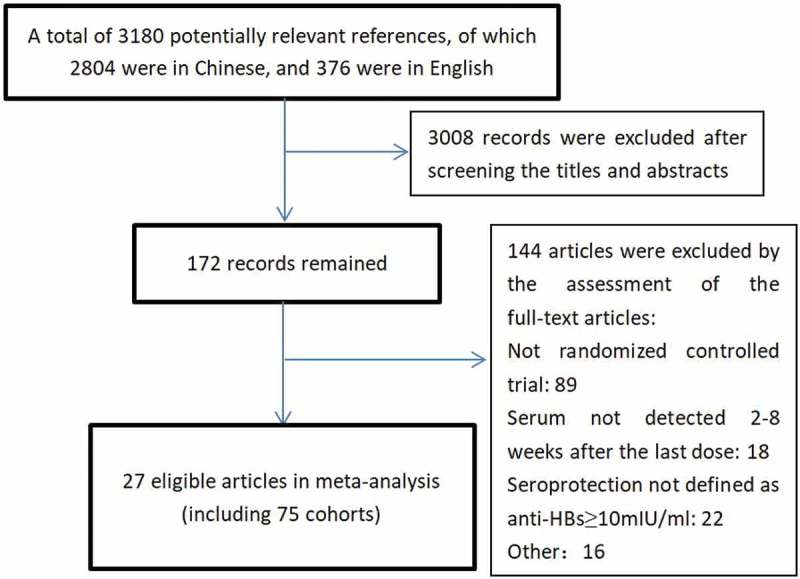
Flow diagram of the study selection process.
The characteristics of the included studies are shown in Table 1. All included studies were RCTs. The publication years of the included studies were concentrated between 2001 and 2017. All participants in these studies were older than 15 years, and most of them were aged between 16 and 50. Among these 75 cohorts, 21 used the CHO vaccine, 48 used YDVs made in China, and the rest used Engerix-B (an HBV vaccine made by GlaxoSmithKline). The standard 0-, 1-, and 6-month schedule was used in 53 cohorts; the 0-, 1-, and 2-month or the 0-, 1-, and 3-month schedules were used in 19 cohorts; and the other 3 cohorts used the 0-, 1-, and 12-month schedule. The positive rates of all cohorts included in the study ranged from 63.59% to 100%.
Table 1.
Characteristics of the 27 studies included in the meta-analysis.
| NO | Author | Design | Year | Age | Gender | No | vaccine | dosage | schedule | population characteristics | Geometric mean titer (IU/L) |
seroprotective rate anti-HBs ≥ 10 IU/L |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Chen W. G. et al. | RCT | 2001 | 18–20 | NA | 138 | CHO | 10 µg | 0.1.6 | seronegative students | NA | 100.00% |
| 149 | CHO | 10 µg | 0.1.2 | NA | 97.99% | |||||||
| 2 | Wang C. X. et al. | RCT | 2002 | 18–55 | 38/55 | 93 | YDV | 5 µg | 0.1.2 | seronegative adults | 18.88 | 68.82% |
| 35/71 | 106 | YDV | 10 µg | 0.1.2 | 54.47 | 84.91% | ||||||
| 3 | Yuan Y. B. et al. | RCT | 2003 | 20–60 | NA | 48 | YDV | 5 µg | 0.1.6 | seronegative teachers |
193.37 | 75.00% |
| 49 | YDV | 10 µg | 0.1.6 | 315.58 | 91.84% | |||||||
| 47 | YDV | 20 µg | 0.1.6 | 477.81 | 97.87% | |||||||
| 4 | Chen Y. Z. et al. | RCT | 2005 | 15–60 | NA | 92 | YDV | 5 µg | 0.1.2 | seronegative adults | 34.45 | 72.83% |
| 85 | YDV | 10 µg | 0.1.2 | 41.16 | 77.65% | |||||||
| 91 | YDV | 5 µg | 0.1.6 | 48.15 | 83.52% | |||||||
| 85 | YDV | 10 µg | 0.1.6 | 67.91 | 89.41% | |||||||
| 159 | CHO | 10 µg | 0.1.2 | 42.59 | 84.28% | |||||||
| 190 | CHO | 20 µg | 0.1.2 | 77.90 | 90.53% | |||||||
| 170 | CHO | 10 µg | 0.1.6 | 76.98 | 88.24% | |||||||
| 192 | CHO | 20 µg | 0.1.6 | 123.82 | 97.40% | |||||||
| 5 | Li W. Q. et al. | RCT | 2008 | 17–21 | 29/31 | 60 | YDV | 10 µg | 0.1.6 | seronegative students | NA | 98.30% |
| 42/18 | 60 | CHO | 10 µg | 0.1.6 | NA | 95.00% | ||||||
| 25/35 | 60 | CHO | 20 µg | 0.1.6 | NA | 96.70% | ||||||
| 37/21 | 58 | Engerix-B | 20 µg | 0.1.6 | NA | 96.50% | ||||||
| 6 | Dong M. H. et al. | RCT | 2009 | 20–55 | 200/101 | 301 | YDV | 5 µg | 0.1.6 | seronegative adults | NA | 94.40% |
| 231/101 | 332 | YDV | 10 µg | 0.1.6 | NA | 94.60% | ||||||
| 7 | Ji X. L. et al. | RCT | 2009 | >20 | NA | 238 | CHO | 10 µg | 0.1.6 | seronegative adults | NA | 96.20% |
| 267 | YDV | 10 µg | 0.1.6 | NA | 95.90% | |||||||
| 8 | Zhang W. et al. | RCT | 2011 | 18–45 | 141/180 | 321 | CHO | 10 µg | 0.1.6 | seronegative adults | NA | 88.80% |
| 129/192 | 321 | CHO | 20 µg | 0.1.6 | NA | 95.30% | ||||||
| 9 | Liu C. C. et al. | RCT | 2012 | 18–45 | NA | 114 | CHO | 10 µg | 0.1.6 | seronegative adults | 134.57 | 89.47% |
| 108 | CHO | 20 µg | 0.1.6 | 921.11 | 99.07% | |||||||
| 10 | Yu S. F. et al. | RCT | 2012 | 16–49 | NA | 241 | YDV | 10 µg | 0.1.3 | seronegative adults | 107.97 | 76.76% |
| 290 | YDV | 10 µg | 0.1.6 | 306.90 | 86.21% | |||||||
| 240 | YDV | 10 µg | 0.1.12 | 587.49 | 89.17% | |||||||
| 11 | Guo Y. H. et al. | RCT | 2013 | 18–74 | NA | 140 | CHO | 20 µg | 0.1.6 | seronegative adults | 1230.3 | 99.40% |
| 140 | Engerix-B | 20 µg | 0.1.6 | 602.6 | 97.00% | |||||||
| 12 | Chen S. Y. et al. | RCT | 2013 | 16–49 | NA | 190 | YDV | 10 µg | 0.1.3 | seronegative adults | 94.96 | 88.95% |
| 191 | YDV | 10 µg | 0.1.6 | 145.12 | 90.05% | |||||||
| 13 | Liu J. Y. et al. | RCT | 2013 | 18–49 | NA | 2011 | YDV | 20 µg | 0.1.6 | seronegative adults | NA | 85.78% |
| 2290 | CHO | 20 µg | 0.1.6 | NA | 90.65% | |||||||
| 14 | Xu M. Q. et al. | RCT | 2013 | 18–35 | NA | 60 | YDV | 10 µg | 0.1.6 | seronegative adults | NA | 75.00% |
| 60 | YDV | 20 µg | 0.1.6 | NA | 93.30% | |||||||
| 60 | Engerix-B | 20 µg | 0.1.6 | NA | 95.00% | |||||||
| 15 | Xu F. et al. | RCT | 2013 | 16–49 | 99/267 | 366 | YDV | 10 µg | 0.1.3 | seronegative adults | 1863.60 | 98.36% |
| 88/174 | 262 | YDV | 10 µg | 0.1.6 | 883.85 | 96.18% | ||||||
| 52/88 | 140 | Engerix-B | 20 µg | 0.1.3 | 629.59 | 97.86% | ||||||
| 72/100 | 172 | Engerix-B | 20 µg | 0.1.6 | 993.09 | 95.35% | ||||||
| 16 | Huang X. Y. et al. | RCT | 2014 | 17–59 | NA | 65 | YDV | 10 µg | 0.1.6 | seronegative adults | 202.99 | 89.04% |
| 72 | CHO | 10 µg | 0.1.6 | 201.98 | 91.14% | |||||||
| 17 | Song J. P. et al. | RCT | 2014 | ≥ 16 | NA | 591 | YDV | 20 µg | 0.1.6 | seronegative adults | 575.40 | 99.15% |
| 254 | YDV | 10 µg | 0.1.6 | 422.30 | 96.46% | |||||||
| 18 | Fu Q. P. et al. | RCT | 2015 | ≥ 16 | NA | 479 | YDV | 10 µg | 0.1.6 | seronegative adults | 718.86 | 98.96% |
| 523 | YDV | 20 µg | 0.1.6 | 1112.34 | 98.66% | |||||||
| 19 | Zhou Y. et al. | RCT | 2015 | 16–49 | NA | 217 | CHO | 20 µg | 0.1.3 | seronegative adults | 31.99 | 63.59% |
| 218 | CHO | 20 µg | 0.1.12 | 893.53 | 95.87% | |||||||
| 20 | Li J. et al. | RCT | 2015 | 20–46 | 66/93 | 159 | YDV | 10 µg | 0.1.3 | seronegative adults | 91.69 | 88.05% |
| 30/71 | 101 | YDV | 20 µg | 0.1.3 | 290.23 | 94.06% | ||||||
| 21 | Zhang L. et al. | RCT | 2015 | 18–49 | 59/59 | 118 | YDV | 20 µg | 0.1.6 | seronegative adults | 88.14 | 88.14% |
| 65/68 | 133 | CHO | 20 µg | 0.1.6 | 90.23 | 90.22% | ||||||
| 22 | Zhou B. Q. et al. | RCT | 2015 | 16–49 | NA | 151 | YDV | 10 µg | 0.1.3 | seronegative adults | 807.98 | 98.01% |
| 174 | YDV | 10 µg | 0.1.6 | 930.68 | 99.43% | |||||||
| 189 | YDV | 10 µg | 0.1.12 | 720.28 | 93.65% | |||||||
| 23 | Guo M. J. et al. | RCT | 2015 | 16–49 | NA | 254 | YDV | 10 µg | 0.1.3 | seronegative adults | 128.75 | 99.61% |
| 212 | YDV | 10 µg | 0.1.6 | 381.27 | 100.00% | |||||||
| 200 | Engerix-B | 20 µg | 0.1.3 | 249.70 | 99.50% | |||||||
| 182 | Engerix-B | 20 µg | 0.1.6 | 498.09 | 100.00% | |||||||
| 24 | Cao Y. et al. | RCT | 2016 | 18–21 | 361/289 | 650 | YDV | 20 µg | 0.1.3 | seronegative students | 486.96 | 89.70% |
| 361/289 | 650 | YDV | 20 µg | 0.1.6 | 407.91 | 95.70% | ||||||
| 25 | Wang H. et al. | RCT | 2016 | 25–55 | 125/168 | 293 | YDV | 20 µg | 0.1.6 | seronegative adults | 1033.38 | 93.17% |
| 126/163 | 289 | YDV | 20 µg | 0.1.6 | 600.75 | 97.23% | ||||||
| 126/167 | 293 | CHO | 20 µg | 0.1.6 | 1627.05 | 98.98% | ||||||
| 26 | Yang L. N. et al. | RCT | 2016 | 16–50 | 99/144 | 243 | YDV | 10 µg | 0.1.6 | seronegative adults | 304.11 | 100.00% |
| 50/101 | 151 | YDV | 10 µg | 0.1.6 | 906.07 | 100.00% | ||||||
| 107/143 | 250 | CHO | 10 µg | 0.1.6 | 330.33 | 99.60% | ||||||
| 65/86 | 151 | Engerix-B | 10 µg | 0.1.6 | 453.25 | 100.00% | ||||||
| 51/59 | 110 | CHO | 20 µg | 0.1.6 | 142.98 | 99.10% | ||||||
| 48/83 | 131 | Engerix-B | 20 µg | 0.1.6 | 1335.45 | 96.90% | ||||||
| 27 | Wen Q. et al. | RCT | 2017 | 18–55 | NA | 160 | YDV | 20 µg | 0.1.3 | seronegative adults | NA | 98.13% |
| 160 | YDV | 20 µg | 0.1.6 | NA | 97.50% |
RCT: randomized controlled trial; NA: not available; YDV: yeast-derived recombinant vaccines; CHO: recombinant hepatitis vaccine made by Chinese hamster ovary cells; Engerix-B: a hepatitis B vaccine made by Glaxo Smith Klin.
Meta-analysis results
A total of 18,307 participants from 27 studies were included, of whom 16,909 achieved an adequate immune response (anti-HBs≥ 10 mIU/ml).
Eleven studies9,14–17,19,22,27,33,35,39 involving 4855 participants were included in the evaluation of the differences in response to vaccination with 20 μg and 10 μg; a total of 13 studies are listed in the forest plot because some studies had more than two cohorts. Compared with the 10-μg dose, the 20-μg dose resulted in a significantly more positive response to vaccination (relative risk [RR]: 1.05, 95% confidence interval [CI]: 1.02 to 1.08) (Figure 2A). A meta-analysis of 4 studies14,22,26,28 involving 633 participants revealed no significant difference in the rates of response to vaccination between 5 μg and 10 μg of vaccine (RR: 1.05, 95% CI: 0.88 to 1.01) (Figure 2B).
Figure 2.
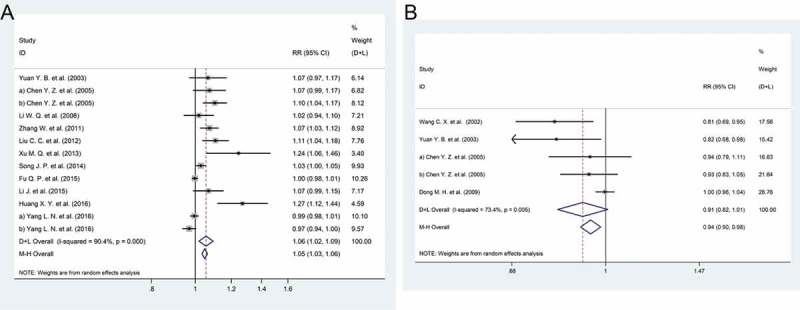
Forest plot. (a) The relative risks of the response to the HBV vaccine comparing the 20 μg and 10 μg doses. (b) The relative risks of the response to the HBV vaccine comparing the 5 μg and 10 μg doses.
Eight studies14,20,21,27,29,32,34,39 involving 7289 participants used different vaccine production methods to define the experimental and control groups. The group receiving the CHO vaccine was defined as the experimental group, and the group receiving YDV was defined as the control group. There was no significant difference in the positive rate of response to vaccination found between the experimental and control groups (RR: 1.01, 95% CI: 0.98 to 1.04) (Figure 3A). In addition, the source of the vaccine as a factor affecting its immune effect was discussed in 5 studies.17,21,27,30,39 The meta-analysis revealed that no significant difference was observed in immune efficacy between homemade or imported vaccines (RR: 1.02, 95% CI: 0.99 to 1.05) (Figure 3B).
Figure 3.
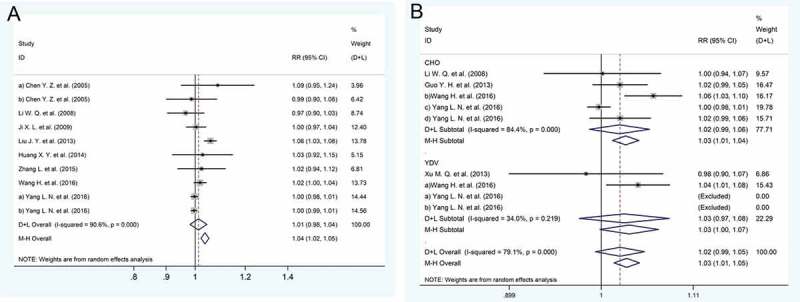
Forest plot. (a) The relative risks of the response to the HBV vaccine comparing CHO and YDV. (b) The relative risks of the response to the HBV vaccine comparing the domestic and imported vaccines.
A total of 5996 participants from 9 studies14,23–25,31,36,37,40 received two different immune schedules; 3016 of those participants received a 0-, 1-, and 2-month or a 0-, 1-, and 3-month immune schedule, and the other 2980 participants received the normal 0-, 1-, and 6-month schedule. The meta-analysis revealed that the 0-, 1-, and 2-month or the 0-, 1-, and 3-month schedules had significantly lower positive immune response rates than the normal schedule (RR: 0.98, 95% CI: 0.96 to 1.00) (Figure 4A). In addition, we compared the differences in immune effects between the 0-, 1-, and 6-month and the 0-, 1-, and 12-month schedules,23,37 and there was no significant difference in immune response observed between these two immune schedules (RR: 1.02, 95% CI: 0.89 to 1.08) (Figure 4B).
Figure 4.
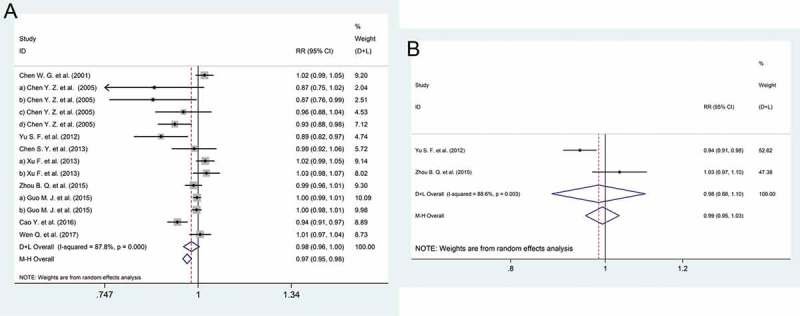
Forest plot. (a) The relative risks of the response to the HBV vaccine comparing the 0, 1, and 2 or 3 month schedule and the 0, 1, and 6 month schedule. (b) The relative risks of the response to the HBV vaccine comparing the 0, 1, and 12 month schedule and the 0, 1, and 6 month schedule.
Sensitivity analysis and publication bias
A sensitivity analysis was conducted to estimate the reliability of the results. Generally, the results showed no significant change if any single study was excluded (Figs S1-S4), but significant sensitivity was found in the results between different immune programs (Figs S5-S6). In this study, we used funnel plots to observe publication bias, but studies with fewer than eight articles were excluded. Funnel plot asymmetry was assessed by Begg’s test and revealed no significant publication bias was in the following groups (10 vs. 20 μg: z = 1.28, p = 0.200; YDV vs. CHO: z = 0.89, p = 0.371; domestic vs. imported: z = 0.30, p = 0.764; 0–1-2 or 3vs. 0–1-6 months: z = 1.42, p = 0.155, Figs S7-S10).
Discussion
The aim of this meta-analysis was to help develop a better immunization strategy for adults in China. This meta-analysis showed that adults in China will achieve a higher response with a 20-µg dose, and a 0–1-6 or a 0–1-12 schedule after completion of vaccination against hepatitis B.
Currently, the WHO recommends a standard pediatric dose of 5–10 µg HBsAg and a standard adult dose of 10–20 µg,44 and in America, ACIP recommends that adults above 20 years of age be vaccinated with a 20-µg dose of hepatitis B vaccine.45 In China, due to early yeast derived recombinant HB vaccine(YDV) was transferred from Merck of America in 1989 and other recombinant HB vaccines were later self-developed, doses of 5–10 µg and 10–20 µg HBsAg were commonly used by children and adults, respectively. But now, 10 and 20 µg doses of hepatitis B vaccines are widely used in different populations. Our study showed that the immunization effect of the 20-μg dose was significantly better than that of the 10-μg dose (RR: 1.05, 95% CI: 1.02 to 1.08). This result is consistent with the results of previous studies in other countries. An RCT in India showed that a 20-μg dose of vaccine had a better immune effect than the 10-μg dose,46 and another study in Italy showed that the 20-μg dose of hepatitis B vaccine had no significant higher positive rate but had a significant higher GMT compared with a 10-μg dose.47 In addition, we compared the immunological effects of the 5- and 10-μg doses, and the RR was 0.94 (95% CI: 0.88 to 1.01), indicating that there was no significant difference in the positive response rate. However, it was noteworthy that the RR was 0.96 (95% CI: 0.92 to 0.99) in the fixed model, and the sensitivity analysis showed that the result was significantly different when the study conducted by Dong M et al.28 was excluded, meaning that it is likely that the 10-μg dose hepatitis B vaccine had a better effect than the 5-μg dose; however, more studies are needed to confirm this result. Therefore, we can conclude that the 20-μg dose of hepatitis B vaccine may be more suitable for adults in China.
The first recombinant subviral particle vaccine, recombinant hepatitis B vaccine was licensed by FDA in 1986. Subsequently, inactive hepatitis B vaccine from the plasma of chronically infected patients was gradually replaced in the world, and China stopped producing this kind of vaccine in 1998.48,49 At present, there are two main types of recombinant hepatitis B vaccines derived from yeast and Chinese Hamster Ovary cells (CHO) expressing S or preS1/preS2/S gene. Yeast derived recombinant HB vaccine(YDV) is widely used around the world, and CHO derived recombinant HB vaccine is licensed in Israel and in some countries in East Asia.44. In China, the hepatitis B vaccines made by Saccharomyces cerevisiae, Hansenula polymorpha, and Chinese hamster ovary cells (CHO) are widely used. Many studies have compared the immune effects of the CHO and YDV in China, but the results were inconsistent.14,19–21,27,29,34,39 Our study showed there was no significant difference in the positive rate between CHO and YDV (RR: 1.01, 95% CI: 0.98 to 1.04), meaning that the CHO and YDV vaccines are both suitable for adult vaccination in China. In addition, in China, some people believe that the imported hepatitis B vaccine had a better immune effect, but the meta-analysis showed that the domestic hepatitis B vaccine had the same positive rate as the imported hepatitis B vaccine (RR: 1.02, 95% CI: 0.99 to 1.05). There are also no significant differences in side effects between domestic and imported vaccines.50,51 Therefore, adults in China can choose the CHO or YDV and domestic or imported as they please.
Three doses of HBV vaccine are recommended by the WHO for children, adolescents, and adults, with the second dose administered at least 1 month after and the third dose and 6 months after the first dose.44 However, in China, there are more than 230 million migrant workers who regularly move between cities and are at high risk for HBV infection.52 For these migrant workers, the 0-, 1-, and 6-month schedule may not be appropriate because they sometimes stay in one place for a few months and then move on to their next location. Therefore, many researchers have suggested administering the third dose 2 or 3 months after the first dose and have compared the immune effect between this schedule and the normal schedule.14,23–25,31,36,40,43 This meta-analysis showed that compared with the normal schedule, the 0-, 1-, and 2- or the 3-month schedule may had a lower positive response rate. However, the sensitivity analysis showed that result changed when some studies were excluded, meaning that more studies should be conducted to explore this problem. Another 0-, 1-, and 12-month immune schedule has been suggested because nearly all migrant workers return home during the Spring Festival. This meta-analysis showed that there was no significant difference between the 0-, 1-, and 12-month schedule and the normal schedule. However, only two studies were included in this meta-analysis. Therefore, we suggest that the 0-, 1-, and 6-month schedule should still be the first choice, but for those who cannot complete this schedule, the 0-, 1-, and 12-month schedule is also worth considering.
This study had some limitations. First, the number of studies included was too small. Publication bias may exist in some meta-analyses because of the small number of eligible studies. Second, quality assessment is lacking in this study. When we used various methods53-57 to evaluate the quality of the research, we found that almost all articles were similar in quality because the Chinese literature is simple in the description of the methods. Third, significant heterogeneity was present in this study, perhaps due to differences characteristics in different study populations. In the included studies, all participants were aged over than 15 years; some studies only included college students, while others included participants aged over than 40 years. Age is an important factor that affects the immune response, and the combination of studies with different age groups may result in significant heterogeneity. In addition, the prevalence of HBsAg varies from region to region, although the studies included were all from China. Furthermore, some personal factors, such as BMI, smoking status, alcohol status, and concomitant disease, were poorly reported in some included studies, with limited inclusion in subgroup analyses. Despite these limitations, in this work, several measures had been taken to avoid clinical and methodological heterogeneity. For example, we only selected RCTs in our study, we excluded studies with small samples, we defined the ending variables, and all participants in the studies were Chinese adults. Therefore, we believe that our study will make a great contribution to hepatitis B vaccination in adults in China.
Conclusion
This meta-analysis showed that the 20-μg dose of HBV vaccine is recommended for adult immunization in China. The immune effect between CHO and YDV was not significantly different, and the use of imported or domestic vaccines did not affect the immune effect. The standard schedule is the most appropriate in adult immunization, but the 0-, 1-, and 12-month schedule is also worth choosing when it is not possible to complete the standard schedule.
Material and methods
Search strategy
The search was performed in December 2017 with no restrictions regarding publication dates. Studies were identified through searches of the following 5 databases: the China Knowledge Resource Integrated Database (CNKI), Wanfang Med Online, the VIP database, PubMed, and the Cochrane Library. The search terms were ‘hepatitis B vaccine’ OR ‘hepatitis B vaccination’ OR ‘HBV vaccine’ OR ‘HBV vaccination’ OR ‘hepatitis B immunity’ OR ‘HBV immunity’ and ‘adult’ OR ‘adolescent’ and ‘China’. In addition, the reference lists of potentially relevant manuscripts were reviewed to obtain other eligible studies.
Inclusion criteria
The included studies met the following criteria: (1) The design of the study was a randomized controlled trial (RCT), and the sample size was ≥ 20; (2) the subjects were from the general population aged ≥ 15 years and had never been vaccinated for hepatitis B, and they were negative for anti-HBs, HBsAg, and HBeAg before vaccination; (3) the vaccine was the monovalent recombinant type, and the schedule consisted of 3 doses that were not given with accelerated timing, irrespective of type, dosage, route, or site of injection; and (4) seroprotection was defined as anti-HBs≥ 10 mIU/ml, and the serum should be tested 2–8 weeks after the last dose.
Data collection
Two authors (W.Z.K and B.H.D) independently assessed the studies to determine whether they met the inclusion criteria and fulfilled the objective of this meta-analysis, and disagreements were resolved through discussion with a third author. The authors were not blinded to the names of the studies, authors, journals, or results. We extracted the following data from the eligible studies: author, publication year, study design, age of participants, numbers of male and female participants, vaccination schedule, type and dosage of the vaccine, and the seroprotection rate after the last dose.
Statistical analysis
In this study, we calculated the relative risks (RR) and 95% confidence intervals (CIs) by comparing the seroconversion rates in the experimental and control groups of the included studies. Statistical heterogeneity among studies was examined by the Q and I2 statistics; an I2 value > 50% indicated significant heterogeneity. In addition, a random effects model was used to analyze the data when there was significant heterogeneity; otherwise, a fixed-effect model was selected.
Subgroup analyses were defined according to the reported data, and studies or results were grouped according to the type of vaccine (Chinese hamster ovary (CHO) or yeast-derived recombinant vaccine (YDV)), the origin of vaccine (domestic or imported), vaccination schedule (0.1.2–3, 0.1.6 or 0.1.12), and the dose of vaccine (5, 10 or 20 μg). A sensitivity analysis was performed to estimate the stability of the model by removing each study in turn, and publication bias was assessed through Begg’s Test. All statistical analyses in this study were conducted with Stata 12.0 software (Stata Corp., College Station, TX, USA).
Funding Statement
This study was financially supported by a Project supported by the National Scientific and Technological Major Project of China (2017ZX10105001-002). This funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplementary Material
Supplemental data for this article can be accessed here.
References
- 1.Organization WH Global hepatitis report, 2017.
- 2.Liaw YF, Chu CM.. Hepatitis B virus infection. Lancet (London, England). 2009. February 14;373(9663):582–592. doi: 10.1016/S0140-6736(09)60207-5. [DOI] [PubMed] [Google Scholar]
- 3.Cui F, Shen L, Li L, Wang H, Bi S, Liu J, Zhang G, Wang F, Zhang H, Sun X, et al. Prevention of chronic hepatitis B after 3 decades of escalating vaccination policy, China. Emerg Infect Dis. 2017;23(5):765–772. doi: 10.3201/eid2305.161477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen W. Epidemiological survey of 7119 patients with chronic hepatitis B. Chin J Nosocomiology. 2010;20(5):655 (In Chinese). [Google Scholar]
- 5.Daniels D, Grytdal S, Wasley A. Surveillance for acute viral hepatitis - United States, 2007. Morbidity Mortality Weekly Rep Surveill Summ. 2009;58(3):1. [PubMed] [Google Scholar]
- 6.Bridges CB, Woods L, Coyne-Beasley T. Advisory Committee on Immunization Practices (ACIP) recommended immunization schedule for adults aged 19 years and older—United States, 2013. Morbidity Mortality Weekly Rep Surveill Summ. 2013;62 Suppl 1(5):9. [PubMed] [Google Scholar]
- 7.Bonanni P. 1995. Implementation in Italy of a universal vaccination programme against hepatitis B. Vaccine. 13:S68–S71. doi: 10.1016/0264-410X(95)80058-L. [DOI] [PubMed] [Google Scholar]
- 8.Zuckerman J, Langer B. Hepatitis B vaccination in a school age population: a feasibility study. J Med Virol. 2005;76(1):47–54. doi: 10.1002/jmv.20372. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Yao J, Shan H, Chen Y, Jiang ZG, Ren JJ, Xu K, Ruan B, Yang S, Wang B, et al. Comparison of the effect of two different doses of recombinant hepatitis B vaccine on immunogenicity in healthy adults. Hum Vaccin Immunother. 2015;11(5):1108–1113. doi: 10.4161/21645515.2014.988547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banatvala J, Damme PV, Oehen S. Lifelong protection against hepatitis B: the role of vaccine immunogenicity in immune memory. Vaccine. 2000;19(7–8):877. [DOI] [PubMed] [Google Scholar]
- 11.Hammitt L, Hennessy T, Fiore A, Zanis C, Hummel K, Dunaway E, et al. Hepatitis B immunity in children vaccinated with recombinant hepatitis B vaccine beginning at birth: a follow-up study at 15 years. Vaccine. 2007;25(39–40):6958–6964. doi: 10.1016/j.vaccine.2007.06.059. [DOI] [PubMed] [Google Scholar]
- 12.Kang G, Ma F, Chen H, Yang Y, Guo S, Wang Z, et al. Efficacy of antigen dosage on the hepatitis B vaccine response in infants born to hepatitis B-uninfected and hepatitis B-infected mothers. Vaccine. 2015;33(33):4093. doi: 10.1016/j.vaccine.2015.06.081. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Zhang W, Lyu JJ, Zhang JJ, Liu JY, Yan BY, et al. [Comparison of antibody persistence after primary immunization with 5 μg and 10 μg recombinant hepatitis B vaccine among newborns with normal and high response: a five-year following-up]. Chin J Epidemiol. 2017;38(9):1156 (In Chinese). [DOI] [PubMed] [Google Scholar]
- 14.Chen YZ, Jiang RJ, Shen JJ, Chen CB, Zhou CL, Tang Q, et al. Study on the immunization schedule and dose of genetic recombinant hepatitis B vaccine wed for adults. Chin J Vaccines Immunization. 2005;11(02):100–105. (In Chinese). [Google Scholar]
- 15.Zhang W, Han L, Lin C, Wang H, Pang X, Li L, et al. Surface antibody and cytokine response to recombinant Chinese hamster ovary cell (CHO) hepatitis B vaccine. Vaccine. 2011;29(37):6276–6282. doi: 10.1016/j.vaccine.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 16.Liu C, Tang Y, Wang R, Sui J, Cai X, Zhang W. Evaluation of the immune effect of Chinese hamster ovary cell derived hepatitis B vaccine among adults. Chin J Prev Med. 2012;(09):701–703. (In Chinese). [Google Scholar]
- 17.Xu M, Niu Z, Yu J, Gao S, He L, Ye Y. Immunogenicity of different doses or areas of recombinant yeast-derived hepatitis B vaccine on the youth. Mod Prev Med. 2013;(15):2905–2906. (In Chinese). [Google Scholar]
- 18.Xing W, Liu C, Tang Y, Wang R, Xu Z, Zhang W. Evaluation on effect of different doses of domestic recombinant yeast-derived hepatitis B vaccine on adult immunization. Occup Health. 2014;(01):88–89+93 (In Chinese). [Google Scholar]
- 19.Huang X. The immune effect of different doses of hepatitis b vaccine in adult immunization. Chin J Clin Rational Drug Use. 2016;(35):59–60. (In Chinese). [Google Scholar]
- 20.Liu J, Yan B, Zhang L, Lv J, Feng Y, Ji F. Comparison on the antibody response and influenced factors of hepatitis B vaccine made by recombinant deoxyribonucleic acid techniques among adults. Chin J Vaccines Immunization. 2013;(02):142–146+153 (In Chinese). [Google Scholar]
- 21.Wang H, Cai B, Rao D, Liu M, Li Y, Liang X. Rapid immunization effects of a new type of 60 mug hepatitis B vaccine compared with traditional 20 mug hepatitis B vaccines in adults. Hum Vaccines and Immunotherapeutics. 2016;12(11):2921–2926. doi: 10.1080/21645515.2016.1206676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan Y, Zheng X, Wang Z, Yu S. The effect and safety of different doses of recombinant yeast hepatitis b vaccine on adult immunity. Chin J Infect Dis. 2003;(06):46–47. (In Chinese). [Google Scholar]
- 23.Yu S, Ling J, Qian X, Hu Z, Liu T. Three adult immunization schedules of hepatitis B vaccine. Paper presented at: Disease Surveillance 2012. (In Chinese) [Google Scholar]
- 24.Cao Y. Analysis of the immune response in adults vaccinated with 20 micrograms of recombinant hepatitis B. J Pathog Biol. 2016;11(05):444–447. (In Chinese). [Google Scholar]
- 25.Chen WG, Ye SD, Jia GF, Tian CS, Luo X. The immune effect of genetic recombinant hepatitis B vaccine made by Chinese hamster ovary cells (CHO). Dis Surveill. 2001;(06):214–216. (In Chinese). [Google Scholar]
- 26.Wang C, Feng Z, Diao L, Zhang Y, Dong C, Li M. The immune effect of adult vaccine with recombinant yeast-derived hepatitis B vaccine made in China. Chin J Health Lab Technol. 2002;(06):702 (In Chinese). [Google Scholar]
- 27.Li W, Li B, Liang X, Wang H, Zhou H, Bao H. Evaluation of immune effect of recombinant hepatitis B vaccine with different doses. J Jilin University (Medicine Edition). 2008;34(06):1050–1053. (In Chinese). [Google Scholar]
- 28.Dong MH, Lin YT, Qian YH, Jiang Y, Deng Y, Ding S, et al. Immunity effect of homemade recombinant yeast-derived hepatitis B vaccine in adults. Mod Med J China. 2009;11(2):24–26. (In Chinese). [Google Scholar]
- 29.Ji X, Liu J, Liu Z, Liu S, Zhnag Y. Observation of immunity effectiveness of different recombinant hepatitis B in adult population. Chin J Public Health Manag. 2009;(06):653–654. (In Chinese). [Google Scholar]
- 30.Guo Y, Liu Q, Xu J, Fan J, Ye Y, Feng X, et al. Study on the immune effects of CHO 20 micrograms Chinese hamster ovary (CHO) cell hepatitis B vaccine among adults. Chin J Dis Control Prev. 2013;17(11):967–970. (In Chinese). [Google Scholar]
- 31.Xu F, He F, Zhou B, Zhao Q, Yan R. Observation on immunization effect of hepatitis B vaccine in adults by different dosages and schedules. Dis Surveill. 2013;(01):38–41. (In Chinese). [Google Scholar]
- 32.Huang X, Fang S, Song X, Wu W, Li R, Guo S. Evaluation of the immunization effect and safety of different domestic hepatitis B vaccines. Mod Prev Med. 2014;(20):3781–3783. (In Chinese). [Google Scholar]
- 33.Song J, Kang G, Wang Y, Dong C, Ma F, Guo S, et al. Immunogenicity and safety of 20 microgram dosages of hepatitis B vaccine by recombinant deoxyribonucleic acid techniques in saccharomyces cerevisiae yeast among low- and non- response populations aged 16 and over. Chin J Vaccines Immunization. 2014;b(04):299–304. (In Chinese). [Google Scholar]
- 34.Zhang L, Liu J, Lu J, Yan B, Song L, Li L, et al. Antibody response to revaccination among adult non-responders to primary Hepatitis B vaccination in China. Hum Vaccin Immunother. 2015;11(11):2716–2722. doi: 10.1080/21645515.2015.1045172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fu Q, Chen H, Zhou Q, Chen X, et al. Safety and immunogenicity of hepatitis B vaccine made by recombinant deoxyribonucleic acid techniques in saccharomyces cerevisiae yeast (20ug/ml) in healthy people aged ≥ 16. Chin J Vaccines Immunization. 2015;21(01):72–76+116 (In Chinese). [Google Scholar]
- 36.Guo M, Yao J, Li J. An observation on the immune effect of hepatitis B vaccine by different immunization schedules among adults. Zhejiang J Prev Med. 2015;(08):757–760. (In Chinese). [Google Scholar]
- 37.Zhou B, Xu F, Zhang Y, Yao J. Comparison of effect on immunization schedules of recombinant hepatitis B Vaccine (Hansenula polymorpha) in adults. Chin J Vaccines Immunization. 2015;(05):495–498. (In Chinese). [Google Scholar]
- 38.Zhou Y, Yang L, Li L. Immunization effect of Chinese hamster ovary (CHO) cell hepatitis B vaccine with different schedules in adults. Dis Surveill. 2015;(3):180–183. (In Chinese). [Google Scholar]
- 39.Yang L, Yao J, Li J, Chen Y, Jiang ZG, Ren JJ, et al. Suitable hepatitis B vaccine for adult immunization in China. Immunol Res. 2016. February;64(1):242–250. doi: 10.1007/s12026-015-8742-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wen Q. Analysis of immune effect and safety of different procedures of adult hepatitis B vaccinations. China Health Ind. 2017;(02):40–41. (In Chinese). [Google Scholar]
- 41.Yang S, Tian G, Cui Y, Ding C, Deng M, Yu C, et al. Factors influencing immunologic response to hepatitis B vaccine in adults. Sci Rep. 2016;6:27251. doi: 10.1038/srep27251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ott J, Stevens G, Wiersma S. 2012. The risk of perinatal hepatitis B virus transmission: hepatitis B e antigen (HBeAg) prevalence estimates for all world regions. BMC Infect Dis. 12:131. doi: 10.1186/1471-2334-12-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen SY, Wang XC, Dong XL, Xu HT, Wang FD, Tang ZF, et al. Effects of two immunization schedules of recombinant yeast-derived hepatitis B vaccine for adults. Chin Prev Med. 2013;14(02):96–98. (In Chinese). [Google Scholar]
- 44.Organization WH Hepatitis B vaccines: WHO position paper – july 2017. Releve Epidemiologique Hebdomadaire. 2017;92(27):369. [PubMed] [Google Scholar]
- 45.Mast EE, Weinbaum CM, Fiore AE, Alter MJ, Bell BP, Finelli L, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR. Recommendations and Reports: Morbidity and Mortality Weekly Report. Recommendations and Reports. 2006;55(RR–16):1–33. [PubMed] [Google Scholar]
- 46.Velu V, Nandakumar S, Shanmugam S, Shankar EM, Thangavel S, Kulkarni PS, et al. Comparative efficacy of two dosages of recombinant hepatitis B vaccine in healthy adolescents in India. Pediatr Infect Dis J. 2007;26(11):1038–1041. doi: 10.1097/INF.0b013e3181342887. [DOI] [PubMed] [Google Scholar]
- 47.Chiaramonte M, Majori S, Ngatchu T, Moschen ME, Baldo V, Renzulli G, et al. Two different dosages of yeast derived recombinant hepatitis B vaccines: a comparison of immunogenicity. Vaccine. 1996;14(2):135–137. [DOI] [PubMed] [Google Scholar]
- 48.Gerlich WH. Prophylactic vaccination against hepatitis B: achievements, challenges and perspectives. Med Microbiol Immunol. 2015;204(1):39–55. doi: 10.1007/s00430-014-0373-y. [DOI] [PubMed] [Google Scholar]
- 49.JiangBo W. A brief history of hepatitis b vaccine development. Chin J Microbiol Immunol. 2013;33(1). (In Chinese). [Google Scholar]
- 50.Yang H, Chen L, Huang J, Liang X, Chen X. Study of domestic and imported recombinant Hepatitis B vaccine safety. J Med Theory Pract. 2015;(2):141–142. (In Chinese). [Google Scholar]
- 51.Fang J, Sun L, Liu J, Rong J, Hu B, Fu C. A study on the immunological effect of recombinant hepatitis B vaccine domestic and import. Chin J Prev Med. 2012;(7). (In Chinese). [Google Scholar]
- 52.Yao J, Li J, Chen Y, Shan H, Dai XW, Yang LN, et al. The response of hepatitis B vaccination on seronegative adults with different vaccination schedules. Hum Vaccin Immunother. 2015;11(5):1102–1107. doi: 10.4161/21645515.2014.985500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Casp UK Critical appraisal skills programme (CASP): checklists. 2013. https://casp-uk.net/casp-tools-checklists/ [Google Scholar]
- 54.Chalmers TC, Smith H, Blackburn B, et al. A method for assessing the quality of a randomized control trial. Control Clin Trials. 1981;2(1):31–49. [DOI] [PubMed] [Google Scholar]
- 55.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0. The Cochrane Collaboration. 2013.
- 56.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. [DOI] [PubMed] [Google Scholar]
- 57.Moseley AM, Herbert RD, Sherrington C, Maher CG. Evidence for physiotherapy practice: a survey of the Physiotherapy Evidence Database (PEDro). Aust J Physiother. 2002;48(1):43–49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


