ABSTRACT
Amebiasis, caused by intestinal infection with Entamoeba histolytica, is one of the leading causes of parasite infection-related mortality and morbidity globally. Although its pathogenesis, including determinant factors of infection outcome, remains unclear, recent clinical data indicate that the gut microbiome plays a role in determining the severity of amebiasis. Recently, we investigated the effects of the gut microbiome on neutrophil mediated protection from E. histolytica infection using a mouse model. We identified that surface expression of CXCR2 on neutrophils was diminished in mice with dysbiosis, which resulted in decreased neutrophil recruitment to the infection site, allowing more aggressive intestinal tissue damage by E. histolytica. Our results indicated that oxidase activity during E. histolytica infection was also diminished after dysbiosis, consistent with the results from prior research. Thus, the gut microbiome plays an important role in regulating neutrophil phenotype when fighting against external pathogens.
KEYWORDS: gut microbiomes, Entamoeba histolytica, neutrophils, chemokine receptor, CXCR2, amebiasis
Severity of entamoeba histolytica infection and the gut microbiome in clinical studies
Amebiasis, caused by intestinal infection with Entamoeba histolytica, is one of the leading causes of parasite infection-related mortality and morbidity around the world.1 Although disease severity ranges from self-limiting mild abdominal symptoms to life-threatening systemic disease, determinant factors of infection outcomes are still undefined.2 Even in the same patient, invasive symptomatic disease can develop after long-term asymptomatic colonization,3–5 and conversely, patients with amebic liver abscesses after medical treatment can be asymptomatic cyst passers.6 These results suggest that not only are the genetics of both host and pathogen important for determining clinical symptoms of infected individuals, but also the gut environment surrounding E. histolytica. A group from India previously reported that the gut microbiome could be different between individuals who show different clinical forms of E. histolytica infection (e.g. asymptomatic infection vs colitis, colitis vs liver abscess).7,8 Later, our group identified that the presence of one particular human commensal bacteria, Prevotella copri, in gut flora is associated with susceptibility of children to E. histolytica induced diarrheal disease in different geographic areas.9,10 Old papers reported that gut bacteria, such as O55 Escherichia coli and Shigella dysenteriae, directly affect on the virulence of E. histolytica in vitro experiments.11–13 Also, it was known that disease severity of autoimmune diseases, such as inflammatory bowel diseases and rheumatoid, are highly influenced by the gut microbiome.14–16 However, effect of gut microbiome on host immune response to external pathogen are rarely investigated in previous studies.
Roles of neutrophils in the severity of E. histolytica infection
Neutrophils have not only protective roles during E. histolytica infection but can also exacerbate disease (Figure 1). In the early phase of infection innate immune responses, especially neutrophil mediated reactions, play a pivotal role in protecting against E. histolytica invasion. This was shown in a previous in vivo experiment where either antibody deletion of neutrophils or disruption of neutrophil recruitment in knockout mice resulted in exacerbation of tissue invasion by E. histolytica.17,18 In an in vitro study, it was shown that neutrophils activated by tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) have amebicidal activity through the release of reactive oxygen species (ROS).19 Furthermore, in in vivo experiments, it was shown that administration of the proinflammatory cytokine interleukin (IL)-17 has a protective role against E. histolytica infection via the induction of innate immune responses in the gut.20,21 However, once E. histolytica invades the submucosa, neutrophil mediated tissue damage contributes to the severity of E. histolytica infection. In a cohort study, it was reported that higher TNF-α production was shown to correlate with E. histolytica diarrheal disease in children.22 Notably, in in vivo experiments, it was shown that the anti-inflammatory cytokine IL-10 is protective in E. histolytica infection by counteracting an exaggerated proinflammatory immune response by inhibiting the production of proinflammatory mediators, such as TNF-α.23 Interestingly, E. histolytica secretes cytokine-like proteins such as prostagrandin-E224,25 and mammalian inflammatory factor during tissue invasion,26,27 which induces excessive proinflammatory responses by neutrophils at the infection site. Thus, neutrophils play a critical role both in the protection against tissue invasion by E. histolytica and in the promotion of tissue damage by E. histolytica.
Figure 1.
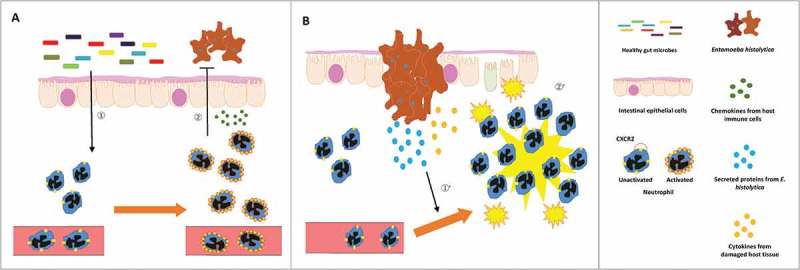
Protection from E. histolytica invasion by neutrophils activated by gut microbes at the early phase (A) and exacerbation of tissue damage by neutrophils excessively activated by E. histolytica secreted proteins at the later phase (B) of infection. (A) Neutrophils are continuously stimulated by host gut microbes in the resting state before infection (①). These neutrophils are potent in protecting host intestinal epithelial cells from E. histolytica invasion (②). (B) However, once E. histolytica invades the submucosa, neutrophils excessively activated by E. histolytica secreted proteins and host proinflammatory cytokines (①’) exacerbate tissue damage (②’).
Effects of the gut microbiome on neutrophil activation in a mouse model
We recently reported results from in vivo experiments using a mouse model of intestinal E. histolytica infection, which proposed a mechanism whereby gut microbiome-activated neutrophils protect host tissue from the invasion of external pathogens.28 In this paper, we first induced “dysbiosis” by the administration of an antibiotic cocktail, which is commonly used in studies for deleting normal gut flora.29–34 Thereafter, E. histolytica was directly injected into the cecum for challenge, then we assessed tissue invasion by E. histolytica and immune responses using cecal tissue from pretreated “dysbiosis” mice. As expected, damage to intestinal epithelial cells by E. histolytica was more severe, and E. histolytica clearance was delayed. In contrast, both reactive oxygen species activity, as assessed by fecal levels of lipocalin-2 and tissue myeloperoxidase (MPO) activity, and the number of neutrophils at the infection site, assessed by the number of Ly6Ghi and SiglecF− granulocytes in the cecum, were diminished in antibiotic pretreated “dysbiosis” mice, although proinflammatory cytokines (IL-1β) and chemokines (CXCL2/macrophage inflammatory protein (MIP)-2 and CXCL1/keratinocyte chemoattractant (KC)) from tissue macrophages were more highly elevated in these mice. These results strongly suggested that neutrophils in mice with dysbiosis have a lower potency of amebicidal activities, both qualitatively and quantitatively. In fact, we demonstrated that chemokine receptors on the surface of neutrophils were diminished after antibiotic induced dysbiosis, and that amebic colitis was more severe after blockade of CXCR2 by neutralizing antibody before E. histolytica challenge. Interestingly, the impact of CXCR2 blockade on tissue MPO activity was less profound than that seen by antibiotic pretreatment, despite the lower number of neutrophils at the site of infection after CXCR2 blocking. Furthermore, histopathological damage by E. histolytica-induced colitis was also less severe than that seen after antibiotic pretreatment. We concluded that the low expression of CXCR2 was an important determining factor for the higher susceptibility to E. histolytica infection in antibiotic pretreated “dysbiosis” mice, but not the sole explanation.
Phenotypic regulation of neutrophils (mainly activation) by the gut microbiome in an autoimmune disease model was already described prior to our report.35 The authors showed that the severity of sickle cell disease is relieved under antibiotic induced dysbiosis, due to fewer activated “aged neutrophils” which are characterized by the surface markers CD62Llow and CXCR4hi. They confirmed that the activated phenotype of neutrophils in sickle cell disease, as shown by aged neutrophils, have increased efficacy in attaching to red blood cells in in vitro experiments. They have also shown that there is a trend for higher expression of CXCR2 on aged neutrophils in an earlier paper.36 These results from previous papers are consistent with our results seen in phenotypic changes of neutrophils in E. histolytica infection. Furthermore, our report is the first showing that neutrophil activation by the gut microbiome contributes to the protection from infectious diseases.
Future perspectives
Previous works, including our recent paper, strongly suggest that it is possible to induce a protective neutrophil response to infectious diseases via the gut microbiome, possibly with probiotics. However, detailed molecular mechanisms for neutrophil activation by the gut microbiome or its derived molecules remain to be determined at present. Moreover, previous papers using human neutrophils show inconsistent results. The expression of chemokine receptors on neutrophils and monocytes of humans was downregulated by Toll-like receptor (TLR)-2 and -4 receptor agonists in in vitro experiments,37,38 whereas lipopolysaccharides (LPS) directly enhance amoebicidal activity of human neutrophils.39 Moreover, the species of commensal bacteria present in humans are different from those in mice. Therefore, in future studies, the molecular mechanisms of neutrophil activation by the gut microbiome should be investigated using human neutrophils and microbes from the human gut. Second, as described above, neutrophil mediated immune responses contribute not only to protection from E. histolytica invasion but also to the exacerbation of tissue damage by E. histolytica at the later phase of infection. Thus, investigations revealing the detailed mechanism of neutrophil activation in different situations will provide future directions for novel therapies based on the induction of neutrophil mediated protection in infectious diseases.
Funding Statement
This work was supported by the Emerging/Re-emerging Infectious Diseases Project of Japan from the Japan Agency for Medical Research and Development (AMED), a Grant for National Center for Global Health and Medicine (29-2013), and NIH grant R01 AI026649. The funders had no role in study design, data collection and interpretation.
Acknowledgments
We thank Simon Teteris, PhD, from the Edanz Group (www.edanzediting.com/ac), for editing the English text of a draft of this manuscript.
Competing interests
The authors report no conflicts of interest.
References
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haque R, Huston CD, Hughes M, Houpt E, Petri WA Jr. Amebiasis. N Engl J Med. 2003;348(16):1565–1573. [DOI] [PubMed] [Google Scholar]
- 3.Gathiram V, Jackson TF.. A longitudinal study of asymptomatic carriers of pathogenic zymodemes of Entamoeba histolytica. S Afr Med J. 1987;72(10):669–672. [PubMed] [Google Scholar]
- 4.Haque R, Ali IM, Petri WA Jr. Prevalence and immune response to Entamoeba histolytica infection in preschool children in Bangladesh. Am J Trop Med Hyg. 1999;60(6):1031–1034. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe K, Aoki T, Nagata N, Tanuma J, Kikuchi Y, Oka S, Gatanaga H. Clinical significance of high anti-entamoeba histolytica antibody titer in asymptomatic HIV-1-infected individuals. J Infect Dis. 2014;209(11):1801–1807. [DOI] [PubMed] [Google Scholar]
- 6.Irusen EM, Jackson TF, Simjee AE. Asymptomatic intestinal colonization by pathogenic Entamoeba histolytica in amebic liver abscess: prevalence, response to therapy, and pathogenic potential. Clin Infect Dis. 1992;14(4):889–893. [DOI] [PubMed] [Google Scholar]
- 7.Rani R, Murthy RS, Bhattacharya S, Ahuja V, Rizvi MA, Paul J. Changes in bacterial profile during amebiasis: demonstration of anaerobic bacteria in ALA pus samples. Am J Trop Med Hyg. 2006;75(5):880–885. [PubMed] [Google Scholar]
- 8.Verma AK, Verma R, Ahuja V, Paul J. Real-time analysis of gut flora in Entamoeba histolytica infected patients of Northern India. BMC Microbiol. 2012;12:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilchrist CA, Petri SE, Schneider BN, Reichman DJ, Jiang N, Begum S, Watanabe K, Jansen CS, Elliott KP, Burgess SL, et al. Role of the gut microbiota of children in Diarrhea Due to the Protozoan Parasite Entamoeba histolytica. J Infect Dis. 2016;213(10):1579–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ngobeni R, Samie A, Moonah S, Watanabe K, Petri WA Jr., Gilchrist C. Entamoeba Species in South Africa: correlations With the Host Microbiome, Parasite Burdens, and First Description of Entamoeba bangladeshi Outside of Asia. J Infect Dis. 2017;216(12):1592–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bracha R, Kobiler D, Mirelman D. Attachment and ingestion of bacteria by trophozoites of Entamoeba histolytica. Infect Immun. 1982;36(1):396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Padilla-Vaca F, Ankri S, Bracha R, Koole LA, Mirelman D. Down regulation of Entamoeba histolytica virulence by monoxenic cultivation with Escherichia coli O55 is related to a decrease in expression of the light (35-kilodalton) subunit of the Gal/GalNAc lectin. Infect Immun. 1999;67(5):2096–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galvan-Moroyoqui JM, Del Carmen Dominguez-Robles M, Franco E, Meza I. The interplay between Entamoeba and enteropathogenic bacteria modulates epithelial cell damage. PLoS Negl Trop Dis. 2008;2(7):e266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maeda Y, Takeda K. Role of gut microbiota in rheumatoid arthritis. J Clin Med. 2017;6(6), pii: E60. doi: 10.3390/jcm6060060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garrett WS, Gordon JI, Glimcher LH. Homeostasis and inflammation in the intestine. Cell. 2010;140(6):859–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sartor RB. Review article: the potential mechanisms of action of rifaximin in the management of inflammatory bowel diseases. Aliment Pharmacol Ther. 2016;43(Suppl 1):27–36. [DOI] [PubMed] [Google Scholar]
- 17.Asgharpour A, Gilchrist C, Baba D, Hamano S, Houpt E. Resistance to intestinal Entamoeba histolytica infection is conferred by innate immunity and Gr-1+ cells. Infect Immun. 2005;73(8):4522–4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naylor C, Burgess S, Madan R, Buonomo E, Razzaq K, Ralston K, Petri WA Jr. Leptin receptor mutation results in defective neutrophil recruitment to the colon during entamoeba histolytica infection. MBio. 2014;5(6). pii: e02046-14. doi: 10.1128/mBio.02046-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denis M, Chadee K. Human neutrophils activated by interferon-gamma and tumour necrosis factor-alpha kill Entamoeba histolytica trophozoites in vitro. J Leukoc Biol. 1989;46(3):270–274. [DOI] [PubMed] [Google Scholar]
- 20.Burgess SL, Buonomo E, Carey M, Cowardin C, Naylor C, Noor Z, Wills-Karp M, Petri WA Jr. Bone marrow dendritic cells from mice with an altered microbiota provide interleukin 17A-dependent protection against Entamoeba histolytica colitis. MBio. 2014;5(6):e01817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burgess SL, Saleh M, Cowardin CA, Buonomo E, Noor Z, Watanabe K, Abhyankar M, Lajoie S, Wills-Karp M, Petri WA Jr. Role of Serum Amyloid A, granulocyte-macrophage colony-stimulating factor, and bone marrow granulocyte-monocyte precursor expansion in segmented filamentous bacterium-mediated protection from entamoeba histolytica. Infect Immun. 2016;84(10):2824–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peterson KM, Shu J, Duggal P, Haque R, Mondal D, Petri WA Jr. Association between TNF-alpha and Entamoeba histolytica diarrhea. Am J Trop Med Hyg. 2010;82(4):620–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamano S, Asgharpour A, Stroup SE, Wynn TA, Leiter EH, Houpt E. Resistance of C57BL/6 mice to amoebiasis is mediated by nonhemopoietic cells but requires hemopoietic IL-10 production. J Immunol. 2006;177(2):1208–1213. [DOI] [PubMed] [Google Scholar]
- 24.Dey I, Chadee K.. Prostaglandin E2 produced by Entamoeba histolytica binds to EP4 receptors and stimulates interleukin-8 production in human colonic cells. Infect Immun. 2008;76(11):5158–5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lejeune M, Moreau F, Chadee K. Prostaglandin E2 produced by Entamoeba histolytica signals via EP4 receptor and alters claudin-4 to increase ion permeability of tight junctions. Am J Pathol. 2011;179(2):807–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moonah SN, Abhyankar MM, Haque R, Petri WA Jr. The macrophage migration inhibitory factor homolog of Entamoeba histolytica binds to and immunomodulates host macrophages. Infect Immun. 2014;82(9):3523–3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ngobeni R, Abhyankar MM, Jiang NM, Farr LA, Samie A, Haque R, Moonah SN. Entamoeba histolytica-encoded homolog of macrophage migration inhibitory factor contributes to mucosal inflammation during amebic colitis. J Infect Dis. 2017;215(8):1294–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watanabe K, Gilchrist CA, Uddin JM, Burgess SL, Abhyankar MM, Moonah SN, Noor Z, Donowitz JR, Schneider BN, Arju T, et al. Microbiome-mediated neutrophil recruitment via CXCR2 and protection from amebic colitis. PLoS Pathog. 2017;18(8):e1006513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, Iwasaki A. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci U S A. 2011;108(13):5354–5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118(2):229–241. [DOI] [PubMed] [Google Scholar]
- 31.Ivanov II, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4(4):337–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fagarasan S, Muramatsu M, Suzuki K, Nagaoka H, Hiai H, Honjo T. Critical roles of activation-induced cytidine deaminase in the homeostasis of gut flora. Science. 2002;298(5597):1424–1427. [DOI] [PubMed] [Google Scholar]
- 33.Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, Peaper DR, Bertin J, Eisenbarth SC, Gordon JI, et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145(5):745–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seo SU, Kamada N, Munoz-Planillo R, Kim YG, Kim D, Koizumi Y, Hasegawa M, Himpsl SD, Browne HP, Lawley TD, et al. Distinct commensals induce interleukin-1beta via NLRP3 inflammasome in inflammatory monocytes to promote intestinal inflammation in response to injury. Immunity. 2015;42(4):744–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang D, Chen G, Manwani D, Mortha A, Xu C, Faith JJ, Burk RD, Kunisaki Y, Jang JE, Scheiermann C, et al. Neutrophil ageing is regulated by the microbiome. Nature. 2015;525(7570):528–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casanova-Acebes M, Pitaval C, Weiss LA, Nombela-Arrieta C, Chevre R, N AG, Kunisaki Y, Zhang D, Van Rooijen N, Silberstein LE, et al. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell. 2013;153(5):1025–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sabroe I, Prince LR, Jones EC, Horsburgh MJ, Foster SJ, Vogel SN, Dower SK, Whyte MKB. Selective roles for Toll-like receptor (TLR)2 and TLR4 in the regulation of neutrophil activation and life span. J Immunol. 2003;170(10):5268–5275. [DOI] [PubMed] [Google Scholar]
- 38.Parker LC, Whyte MK, Vogel SN, Dower SK, Sabroe I. Toll-like receptor (TLR)2 and TLR4 agonists regulate CCR expression in human monocytic cells. J Immunol. 2004;172(8):4977–4986. [DOI] [PubMed] [Google Scholar]
- 39.Guerrant RL, Brush J, Ravdin JI, Sullivan JA, Mandell GL. Interaction between Entamoeba histolytica and human polymorphonuclear neutrophils. J Infect Dis. 1981;143(1):83–93. [DOI] [PubMed] [Google Scholar]


