ABSTRACT
Disruption of intestinal barrier homeostasis is an important pathogenic factor in conditions such as irritable bowel syndrome (IBS). Lactobacillus rhamnosus GG (LGG) improves IBS symptoms through unclear mechanisms. Previous studies utilizing colorectal adenocarcinoma cell lines showed that LGG metabolites prevented interferon gamma (IFN-gamma) induced barrier damage but the model employed limited these findings. We aimed to interrogate the protective effects of LGG on epithelial barrier function using human intestinal epithelial cultures (enteroids and colonoids) as a more physiologic model. To investigate how LGG affects epithelial barrier function, we measured FITC-Dextran (FD4) flux across the epithelium as well as tight junction zonula occludens 1 (ZO-1) and occludin (OCLN) expression. Colonoids were incubated with fecal supernatants from IBS patients (IBS-FSN) and healthy controls in the presence or absence of LGG to examine changes in gut permeability. Enteroids incubated with IFN-gamma demonstrated a downregulation of OCLN and ZO-1 expression by 67% and 50%, respectively (p<0.05). This was accompanied by increased paracellular permeability as shown by leakage of FD4. Pretreatment of enteroids with LGG prevented these changes and normalized OCLN and ZO-1 to control levels. These actions were independent of its action against apoptosis. However, these protective effects were not seen with LGG cell wall extracts, LGG DNA, or denatured (boiled) LGG. Intriguingly, IBS-FSN injected into colonoids increased paracellular permeability, which was prevented by LGG. LGG, likely due to secreted proteins, protects against epithelial barrier dysfunction. Bacterial-derived factors to modulate gut barrier function may be a treatment option in disorders such as IBS.
KEYWORDS: Lactobacillus rhamnosus GG metabolites, epithelial barrier function, human intestinal enteroids, human colonoids, tight junction, IFN-gamma, irritable bowel syndrome
Introduction
The primary functions of the human gastrointestinal tract are to absorb nutrients and serve as a protective barrier against luminal contents including food antigens and microbes. The intestinal barrier is comprised of a single layer of epithelial cells connected by tight junction proteins that modulate paracellular permeability.1,2 Tight junctions are highly dynamic structures which open and close continuously in response to various stimuli.3 For example, cytokines, such as tumor necrosis factor-alpha (TNF-alpha) and interferon-gamma (IFN-gamma), play a crucial role in regulating tight junction protein expression and gut barrier function.4,5
The gut microbiota comprises a diverse and complex community that closely interacts with the intestinal epithelium. There is a symbiotic host-microbe relationship with the microbiota providing essential functions, including antimicrobial protection as well as development and modulation of the gut immune system.6,7 The microbiota also play a critical role in gut barrier function. The microbiota interacts with the intestinal epithelium through release of microbe-associated molecular patterns which then bind to toll-like receptors (TLRs) and NOD-like receptors (NLRs).8,9 TLRs and NLRs, in turn, are key mediators in regulating intestinal epithelial barrier function.10–12 Microbes can further influence gut barrier function by release of peptides, toxins, or metabolites.13–16
Disruption of intestinal barrier integrity is an important factor in the pathogenesis of several highly prevalent and morbid diseases, including irritable bowel syndrome (IBS),17 and inflammatory bowel disease (IBD).18 Furthermore, there is accumulating evidence that alterations in gut microbiota are a key pathogenic factor linked to gut barrier dysfunction, increased intestinal permeability, and inflammation in these disorders.19–22 Although it is unclear whether alterations in gut microbiota lead to gut barrier dysfunction or vice versa, considerable attention is focused on modifying these pathogenic factors as potential therapeutic options. However, the ability to modulate intestinal barrier function remains elusive.
Probiotics are defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host.”23 Probiotics may have beneficial actions on the host by excluding or inhibiting pathogens, enhancing epithelial barrier function, or by modulating host immune responses.24 Lactobacillus rhamnosus Gorbach-Goldin (LGG) is one of the most widely used and studied probiotics and has several biological properties that make it potentially useful as a probiotic. LGG is resistant to acid and bile, adheres well to the human intestinal epithelium, and produces factors with activity against many bacterial species.25 Studies suggest that LGG is important in promoting gut immune development, protection against inflammation-induced damage and stimulating gut barrier function.26–29 Clinically, LGG has been shown to improve IBS symptoms,30 delay onset of pouchitis after surgery for ulcerative colitis,31 and maintain remission in ulcerative colitis.32 Previous studies using epithelial colorectal adenocarcinoma cell lines showed that LGG prevented IFN-gamma induced epithelial barrier damage.26–28,33,34 However, cancer cell lines are unable to mimic normal physiology. For example, it has been reported that norovirus can only infect and replicate in human epithelial cells derived from organoids and not from cancer cell lines.35
In this study, we employed human enteroids and colonoids, which are three-dimensional structures of human epithelium generated from isolated human intestinal crypts.36 We demonstrate that enteroids/colonoids are a physiologically relevant human model of gut barrier function and intestinal permeability that can be modulated by inflammatory cytokines. We implemented these systems to investigate whether pretreatment with LGG supernatant may prevent cytokine-evoked changes in tight junction protein expression and permeability in human enteroids. This may confirm or contradict findings using classical cell lines. To identify the component(s) of LGG responsible for stimulating tight junction protein expression, we investigated the effects of LGG extracted DNA, boiled LGG supernatant, LGG cell wall extract, and we compared the effect of LGG on tight junction protein expression and enteroid permeability to Lactobacillus crispatus. Lastly, we examined whether LGG may be useful for modulating gut barrier dysfunction seen in IBS by using human colonoids treated with fecal supernatants from diarrhea-predominant IBS (IBS-D) patients and healthy subjects in the presence or absence of LGG. We also investigated whether LGG regulates epithelial barrier function independent of its action against apoptosis.
Results
Human enteroid barrier function can be modulated by EGTA
We used human enteroids to evaluate paracellular barrier function under different conditions. FD4 was microinjected into the lumen of enteroids and images were obtained at different time points up to 20h. Under control conditions, the human enteroids retained 55% of FD4 over 20h, while treatment of the enteroids with 2mM EGTA for 2h to disrupt tight junctions and impair permeability resulted in 7% retention of the dye (Fig. 1). These results demonstrate that enteroids have an intact epithelial barrier, which can be modified by exogenous stimuli. We used this property of enteroids to measure epithelial barrier function and to monitor tight junction protein localization and mRNA expression over time in subsequent experiments.
Figure 1.
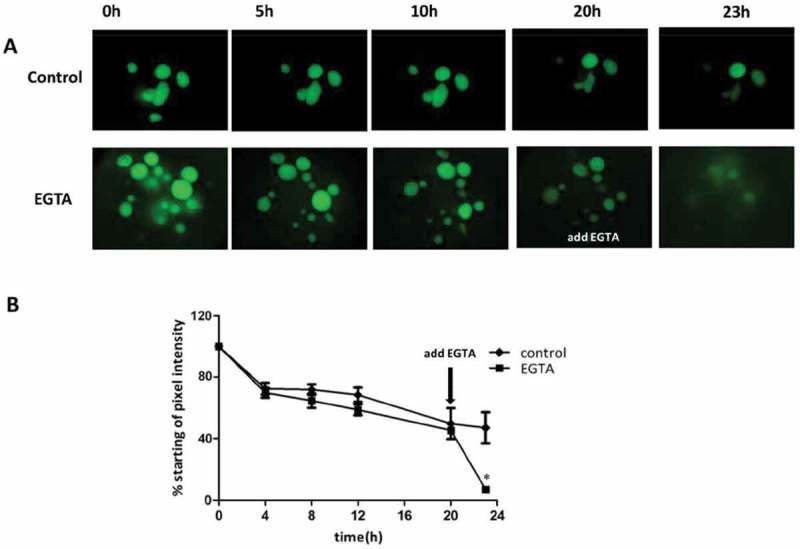
Human Intestinal Enteroids Have An Intact Intestinal Epithelium That Can Be Modified By EGTA (A) Representative images of FD4 dynamics in human enteroids. Human enteroids were injected with FD4 and imaged at 0, 5, 10, 20, and 23h postinjection. Control group retained the majority of injected FD4 within the lumen over 12 h. The addition of EGTA to the media 20h after injection resulted in the rapid loss of FD4 from the lumen, indicating the loss of epithelial paracellular barrier function. (B) Quantitation of barrier disruption by determination of the fraction of initial FD4 fluorescence retained over time. Points represent the medians, and bars represent the interquartile ranges. Control human enteroids retained 55% of FD4 over 20h, while the EGTA-treated enteroids retained 7% of FD4 over 20h (p < 0.01* by Mann-Whitney test, compared to control).
IFN-gamma disrupts epithelial barrier function in a concentration-dependent manner
Recent studies showed that IFN-gamma released from the colonic mucosa of IBS patients was elevated compared to healthy controls.37 Hence we used IFN-gamma to disrupt epithelial barrier function. Previous in vitro studies used concentrations ranging from 10ng/ml to 100ng/ml of IFN-gamma to induce epithelial barrier dysfunction.34,38,39 The physiological implication of these concentrations is not known since we do not know the exact local concentrations of IFN-gamma in the mucosa. In the present study, we performed a dose-response study (60-500 ng/ml) to determine the concentration of IFN-gamma required to induce epithelial dysunction in the enteroids. First, human enteroids were injected with FD4. Enteroids exposed to IFN-gamma showed a concentration- and time-dependent decrease in retention of FD4. Exposure to 500 ng/ml IFN-gamma for 20h resulted in a rapid loss of barrier integrity such that the fluorescence of the enteroids at 20h was 4% of the intensity at baseline. However, when enteroids were exposed to IFN-gamma at 60ng/ml, there was only 70% leakage of FD4 after 20h (Fig. 2A,2B).
Figure 2.
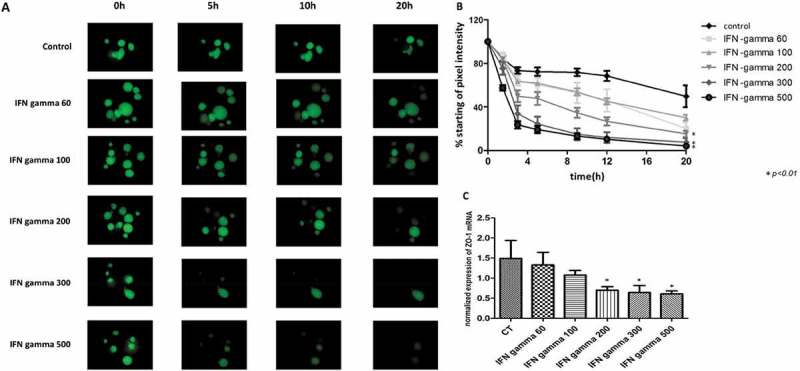
IFN-gamma disrupts intestinal epithelial barrier function and reduces gene expression of ZO-1 in a concentration-dependent manner (A) Representative images of FD4 leakage from the lumen of human enteroids treated with IFN-gamma compared with human enteroids under control conditions. The human enteroids were injected with the fluorescence dye FD4 and then exposed to IFN-gamma at 60, 100, 200, 300, and 500 ng/ml for 20h. (B) Quantitation of retention of FD4 fluorescence in human enteroids relative to time zero. The highest dose of IFN-gamma applied (500 ng/ml) resulted in a rapid loss with 20% retention of fluorescence by 3h after treatment, and 4% of fluorescence remaining at 20h. Meanwhile, the lowest dose of IFN-gamma (60 ng/ml) caused 70% loss of the fluorescence by 20h (*p<0.05, compared to control). (C) Treatment of human enteroids with IFN-gamma caused a dose dependent decrease of ZO-1 gene expression by qPCR.
We next evaluated gene expression of tight junction proteins in order to determine if IFN-gamma mediated disruption of barrier function is associated with altered tight junction expression. Incubation with increasing concentrations of IFN-gamma caused a progressive decrease in gene expression of zonula-occludens-1 (ZO-1) (p<0.05) (Fig. 2C). These results indicate that IFN-gamma causes a concentration-dependent decrease in tight junction gene expression and a corresponding increase in transepithelial permeability in human enteroids. IFN-gamma at a concentration of 200 ng/ml was chosen for subsequent experiments as this was the minimal concentration required for inducing reproducible epithelial barrier damage.
LGG specifically protects against enteroid barrier dysfunction
We next investigated whether modulation of barrier function induced by LGG was strain specific. To address this question, we compared its performance to Lactobacillus crispatus, another probotic, known to prevent and treat recurrent bacterial vaginosis. Enteroids were precultured with either LGG-conditioned media (LGG-CM) or L. crispatus-CM overnight and then exposed to IFN-gamma 200 ng/ml for 24h. Enteroids in the presence of LGG-CM and IFN-gamma showed expression of occludin (OCLN) and ZO-1 similar to control levels. This was not seen in enteroids exposed to both L. crispatus-CM and IFN-gamma or IFN-gamma alone (Fig. 3A). Consistent with qRT-PCR data, enteroids exposed to IFN-gamma caused a significant loss of barrier function whereas those exposed to both LGG-CM and IFN-gamma showed normalization of epithelial barrier function comparable to control levels. This rescue of barrier function was not seen in enteroids exposed to both L. crispatus-CM and IFN-gamma (Fig. 3B). This provided further evidence that bacterial components of LGG can inhibit cytokine-induced disruption of epithelial tight junction protein expression and intestinal mucosal barrier dysfunction. However, these beneficial actions were not seen in the presence of L. crispatus suggesting this protective property of LGG is species specific.
Figure 3.
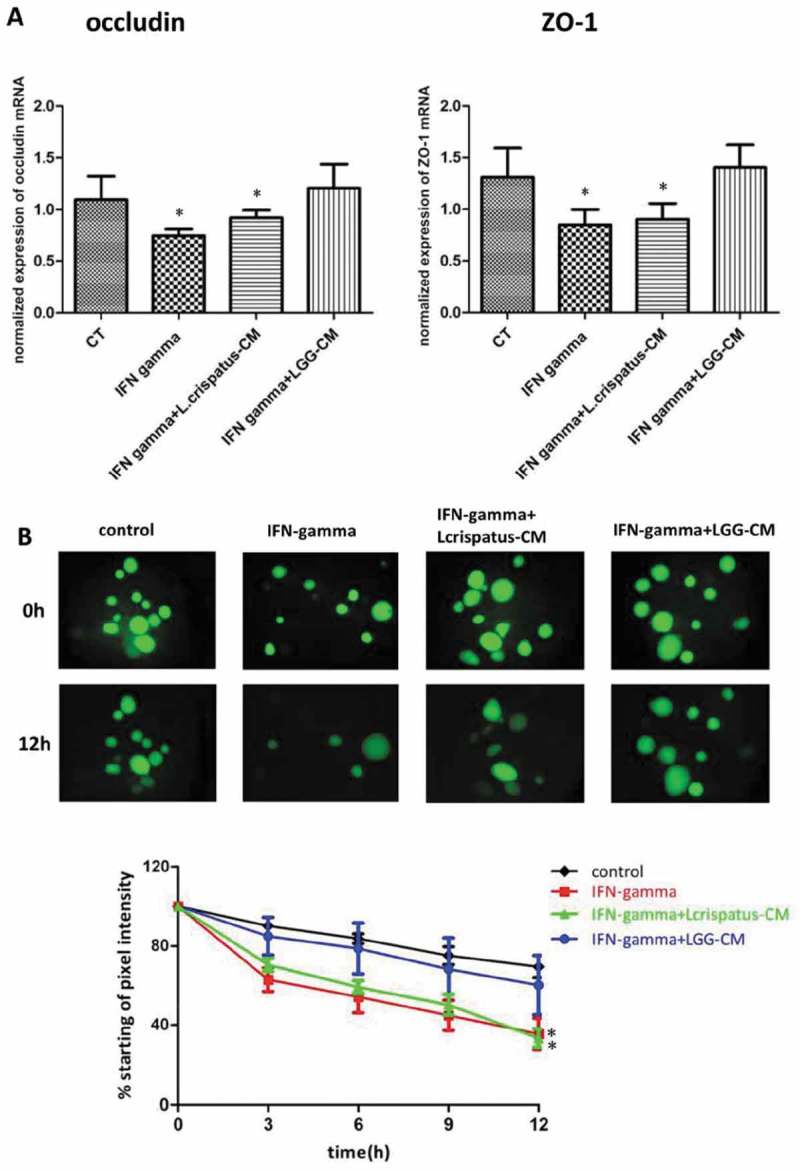
LGG specifically protects against human enteroid barrier dysfunction induced by IFN-gamma (A) Human enteroids were pretreated with LGG-CM or L. crispatus-CM overnight, and then they were exposed to IFN-gamma (200 ng/ml) for 24h. LGG-CM prevented IFN-gamma-induced downregulation of occludin and ZO-1 gene expression. However, this protective effect was not seen in enteroids incubated with L. Crispatus (*p<0.05, compared to control, CT). (B) Similarly, LGG-CM, but not L. Crispatus-CM, prevented leakage of the fluorescent dye FD4 induced by IFN-gamma. Under control conditions, the human enteroids retained 76% of FD4 at 12h. Treatment of the enteroids with IFN-gamma led to intestinal epithelial barrier dysfunction with only 35% of the dye retained at 12h. Administration of LGG, but not L. crispatus-CM, prevented leakage of dye evoked by IFN-gamma (*p<0.05, compared to control).
Proteins secreted by LGG prevents IFN-gamma induced epithelial barrier damage
We next investigated what components of LGG are responsible for preventing loss of mucosal barrier function. Enteroids were incubated for 24h with IFN-gamma with or without LGG-CM, LGG extracted DNA, boiled LGG-CM or LGG cell wall. Incubation of human enteroids with IFN-gamma caused 67% and 50% downregulation of OCLN and ZO-1 gene expression, respectively (p<0.05). Addition of LGG-CM prevented these changes and normalized OCLN and ZO-1 to control levels. However, addition of boiled LGG-CM, extracted LGG DNA or LGG cell wall abolished the protective effects of LGG-CM against IFN-gamma (Fig. 4A).
Figure 4.
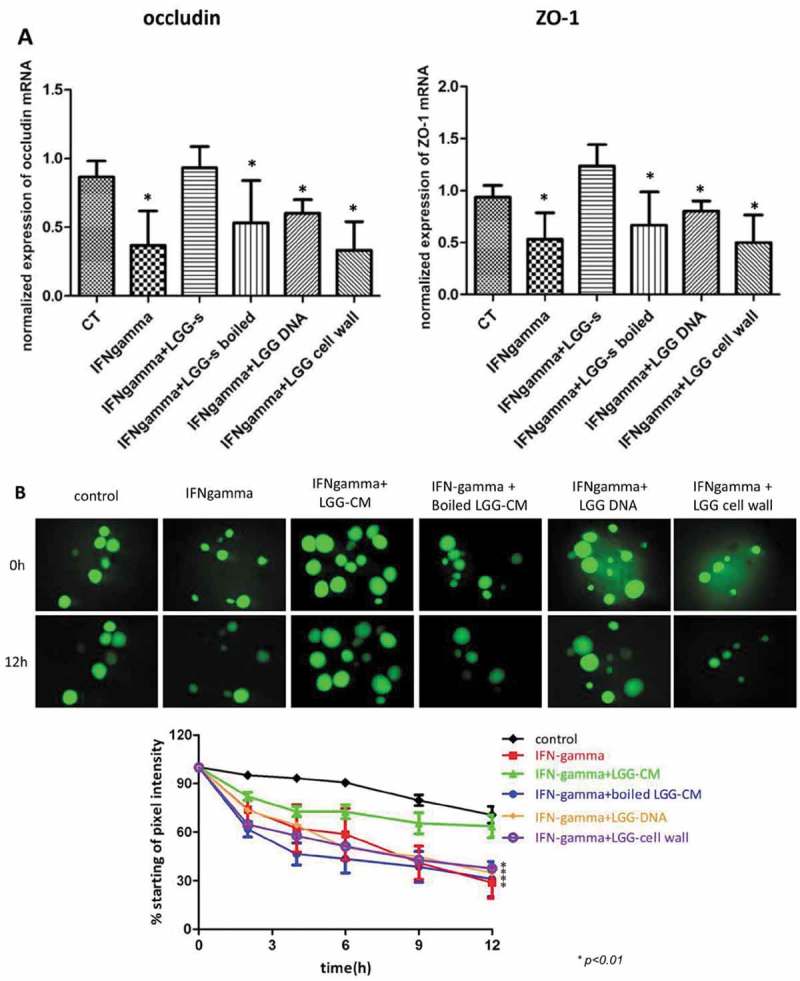
Protein metabolites of LGG prevent IFN-gamma-induced downregulation of tight junction gene expression and epithelial barrier dysfunction in human enteroids (A) Incubation of human enteroids with IFN-gamma (200 ng/ml) caused a 67% and 50% downregulation of gene expression of occludin and ZO-1, respectively (P<0.05). Pretreatment of enteroids with LGG-CM prevented these changes and normalized occludin and ZO-1 to control levels. In contrast, addition of LGG extracted DNA, boiled LGG-CM, or LGG cell wall led to expression of occludin and ZO-1 comparable to enteroids exposed to IFN-gamma (*p<0.05, compared to control, CT). (B) Under control conditions, the enteroids retained 70% of FD4 at 12 h, while treatment of the enteroids with IFN-gamma resulted in 30% retention of dye at 12 h. Administration of LGG-CM prevented leakage of dye induced by IFN-gamma. In contrast, LGG DNA, cell wall or boiled supernatant did not protect against barrier dysfunction evoked by IFN-gamma.
We then tested the permeability of the intestinal epithelium with or without LGG-CM, LGG extracted DNA, boiled LGG-CM or LGG cell wall. Under control conditions, the enteroids retained 70% of FD4 over 12h, while treatment of the enteroids with IFN-gamma impaired permeability resulting in 30% retention of dye after 12h. Administration of LGG-CM prevented leakage of dye evoked by IFN-gamma. In contrast, additions of extracted LGG DNA, cell wall or boiled supernatant were without effects (Fig. 4B).
We next performed immunofluorescence of the human enteroids. ZO-1 was normally present at the apical surface of the epithelium, whereas OCLN was located along the lateral/basal surface of the cell. Administration of IFN-gamma to human enteroids led to downregulation and disruption of ZO-1 and OCLN expression while pretreatment of enteroids with LGG-CM restored expression of ZO-1 and OCLN to control levels (Fig. 5). These observations indicate that protein metabolites secreted by LGG are responsible for preventing IFN-gamma induced epithelial barrier damage. This effect is mediated by normalizing the expression and localization of OCLN and ZO-1.
Figure 5.
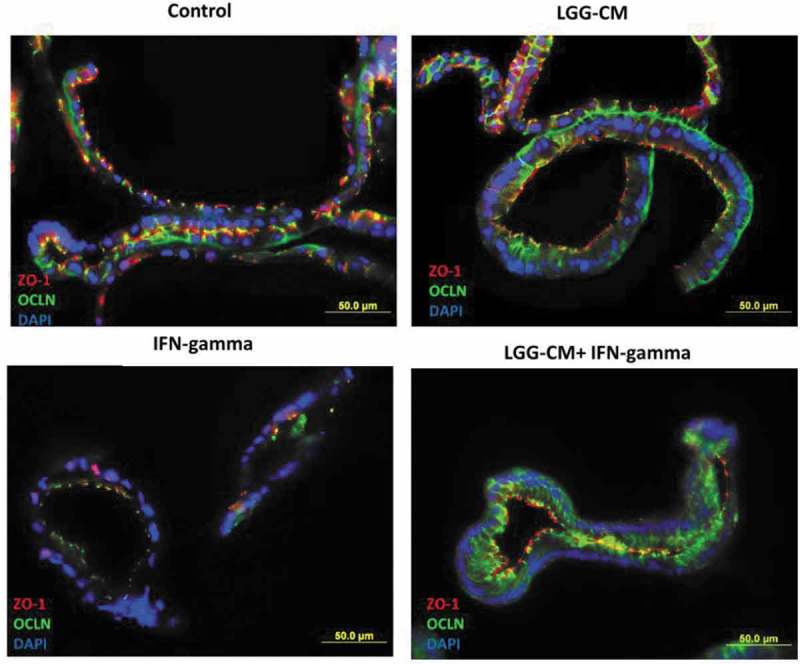
Lactobacillus rhamnosus GG prevents downregulation of ZO-1 and occludin expression induced by IFN-gamma Human enteroids were pretreated with or without LGG-CM overnight, and then exposed to IFN-gamma (200 ng/ml). After treatment with IFN-gamma for 20 h, the human enteroids were fixed and stained. ZO-1 is present at the tight junction near the apical surface of the epithelium, whereas occludin stained in green is seen at the tight junction and along the lateral surface of the cell in control human enteroids. In contrast, human enteroids treated with IFN-gamma, apical ZO-1 at the tight junction is lost and occludin is no longer restricted to the lateral surface of the epithelial cell. Meanwhile, pretreatment of human enteroids with LGG-CM have immunofluorescence for ZO-1 and occludin similar to those of the control.
LGG regulates epithelial barrier function independent of its action against apoptosis
Previous studies using cancer cell lines showed LGG is capable of preventing apoptosis.26 We next investigated whether the protective action of LGG on epithelial barrier function is dependent on its action against apoptosis. Human enteroids were treated with IFN-gamma at 200ng/ml, or the “cytokine mixture” consisting of IFN-gamma (1000ng/ml) and TNF-a (1000 ng/ml) for 24h in the presence or absence of LGG-CM. Immunofluorescence study showed that cleaved caspase-3 (green fluorescence) was not expressed in enteroids exposed to IFN-gamma at 200ng/ml. Treatment with the cytokines TNF-a and INF-gamma at 1000ng/ml induced apoptosis which was not prevented by pretreatment of LGG-CM, indicating that the protective effect of LGG occurs independent of its action against apoptosis (Fig 6A, B).
Figure 6.
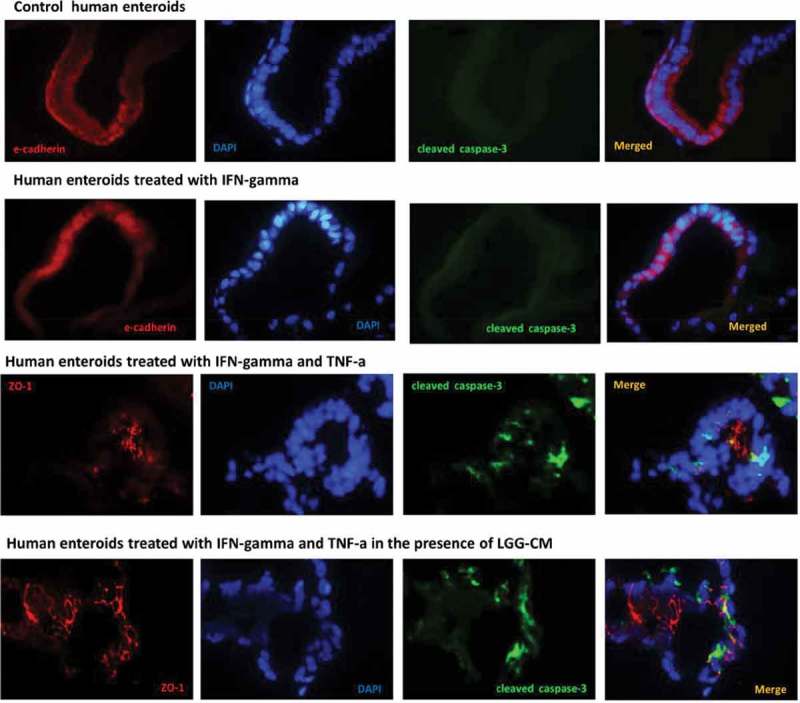
Protective effects of LGG-CM occur in the absence of apoptosis. Human enteroids were treated with IFN-gamma (200 ng/ml), or a “cytokine mixture” containing TNF-a (1000 ng/ml) and IFN-gamma (1000ng/ml) for 24 h in the presence or absence of 12h pretreatment with LGG-CM. Immunofluorescence study showed that expression of cleaved caspase-3 (green fluorescence) was absent in enteroids exposed to IFN-gamma at 200ng/ml and was similar to those of the control enteroids. Treatment with the cytokine mixture containing TNF-a and INF-gamma induced Caspase-3 activation. Immunofluorescence staining of enteroids treated with IFN-gamma in the presence of LGG-CM was not shown since caspase-3 immunoreactivities were not observed and were similar to control and treatment with IFN-gamma in the absence of LGG-CM. Cell death was found in enteroids treated with TNF-a and INF-gamma. Pretreatment with LGG-CM did not prevent apoptosis induced by the cytokine mixture IFN-gamma plus TNF-a.
The protective action of LGG on epithelial function is independent of MAPK/ERK pathway in human colonoids
Previous studies using cancer cell lines showed that LGG-derived soluble proteins protected against hydrogen peroxide-induced barrier dysfunction through a MAP kinase-dependent pathway.33 It is unknown whether a similar pathway is involved in the barrier protective actions of LGG in human colonoids. To examine this possibility human colonoids were treated with LGG-CM or epidermal growth factor (EGF) (100 ng/ml) for 90 min in the presence or absence of 1h pretreatment with (epidermal growth factor receptor) EGFR inhibitor, AG1478 (200nM). Cellular lysates were collected for Western blot analysis of total EGFR and extracellular signal-regulated kinase (ERK) levels and EGFR (Tyr-1068) and phosphorylated ERK. In human colonoids, LGG-CM treatment for 90min activated EGFR and its down-stream target, ERK. This was blocked by the EGFR inhibitor AG1478. In separate experiments, we examined whether the protective actions of LGG-CM on IFN-gamma induced barrier dysfunction was affected by inhibition of EGFR. As shown in Fig. 7, although AG1478 blocked the activation of EGFR and its downstream effects, it failed to affect the protective actions of LGG-CM on barrier dysfunction induced by IFN-gamma. This suggests that in humans, the actions of LGG on barrier function are mediated by mechanisms different from those observed in cell lines and animal models. Recently we showed that fecal supernatant of IBS-D patients impaired epithelial barrier function in human colonoids.40 We next investigated whether LGG can prevent barrier dysfunction induced by fecal supernatant on IBS-D patients in the human colonoids.
Figure 7.
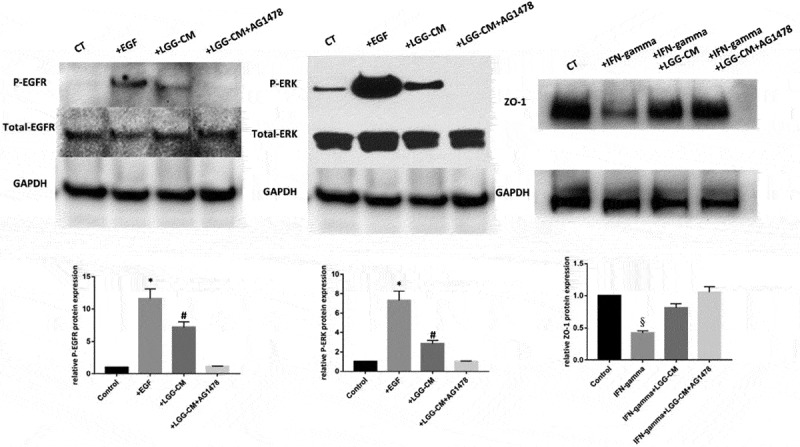
Protective actions of LGG on junction proteins were mediated by pathways independent of MAPK/ERK signaling cascade in human colonoids. Human colonoids were treated with LGG-CM or EGF (100 ng/ml) for 90 min in the presence or absence of 1-h pretreatment with an EGFR inhibitor, AG1478 (200nM). In separate experiments, human colonoids were treated with IFN-gamma (200 ng/ml) in the absence or presence of LGG. The protective action of LGG was examined in the presence of AG 1478 (200nM). Cellular lysates were prepared and immunoblotted for P-EGFR, total EGFR, P-ERK, total ERK, occludin, ZO-1 and GAPDH. Bands of GAPDH were used as control for an equal protein loading. The optical density is expressed in arbitrary units normalized against a control sample. Data in histograms represent means ± SE; n = 5 in each group (control group vs EGF group *p<0.05, control group vs LGG group #p<0.05, control group vs IFN-gamma group, §p<0.05).
LGG protects against barrier dysfunction in human colonoids induced by fecal supernatants from IBS-D patients
Fecal supernatants (FSN) from four IBS-D patients (IBS-FSN) as well as four healthy subjects were injected into human colonoids and retention of FD4 over time was measured. We observed little difference in the abilities to retain FD4 between colonoids from different healthy subjects. When exposed to FSN from healthy subjects (control) and IBS-D patients, the colonoids retained 80% of FD4 compared with 39% at 12 hours, respectively (p<0.05). Meanwhile, colonoids pretreated with LGG showed retention of FD4 similar to control levels, indicating a rescue of barrier function (Fig. 8).
Figure 8.
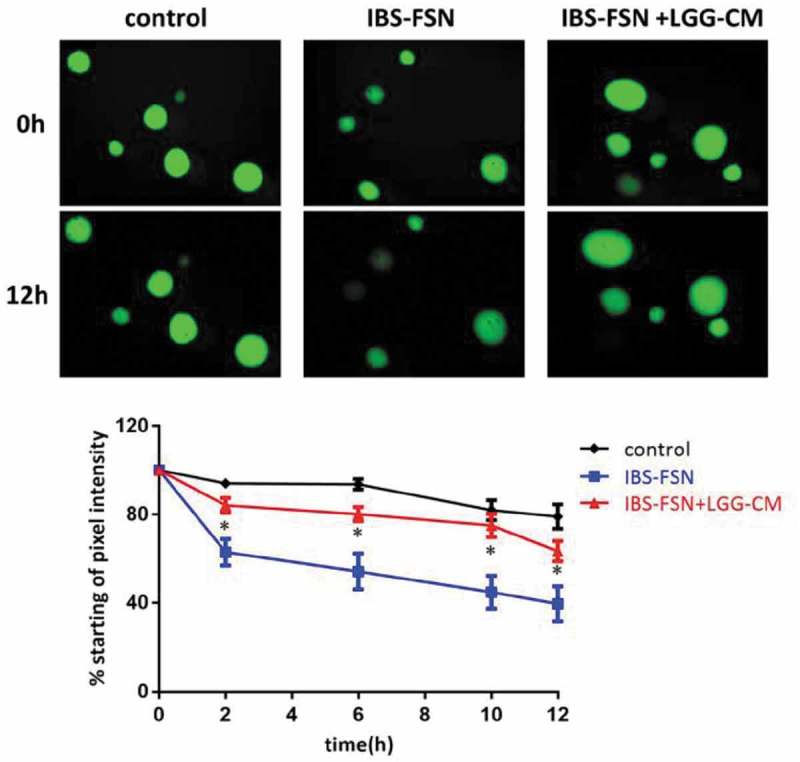
LGG-CM prevents barrier dysfunction in human colonoids induced by fecal supernatants from IBS-D patients. Fecal supernatants (FSN) from IBS patients (n = 4) were used to induce epithelial barrier damage. Human colonoids were incubated for 12 hr with FSN from healthy subjects (n = 4) and IBS patients (n = 4) with and without cell-free LGG supernatant. In the presence of FSN from healthy subjects, the colonoids retained 80% of FD4 at 12 hr. Treatment of the colonoids with IBS-FSN impaired permeability resulting in 39% retention of dye after 12 hr. Administration of LGG supernatant prevented leakage of dye evoked by IBS-FSN. * p<0.05 compared to control
Discussion
Although it has been demonstrated that LGG rescued barrier function and tight junction from cytokine-induced breakdown in mouse intestinal tissues and cancer cell lines, these models are unable to mimic normal physiology in humans. We demonstrated for the first time utilizing human enteroids and colonoids that secreted factors from LGG modulate epithelial barrier function and tight junction protein expression. We determined that barrier disruption induced by IFN-gamma was prevented by LGG-CM. We further showed that LGG normalizes tight junction expression and decreases mucosal permeability in the absence of apoptosis. In contrast to observations made in cancer cell lines, the action of LGG on barrier function is independent of activation of MAPK/ERK pathway. Finally, our results reveal that IBS-FSN disrupts intestinal barrier function in human colonoids which can be reversed by LGG. Our findings provide a molecular basis for therapeutic applications of LGG in gastrointestinal disorders, such as IBS-D.
Previous models of host-microbe interactions have utilized human epithelial colorectal adenocarcinoma cell lines, such as Caco-2 and HT29. A key difference in our model is the use of enteroids and colonoids, which are three dimensional structures derived from LGR5+ intestinal stem cells isolated from the small intestine or colon crypts41 rather than cancer-derived cell lines that are not physiologically relevant models of human intestinal function and structure. The human enteroids and colonoids have a single layer of epithelial cells with structural and planar cell polarity which assemble into polarized monolayers that separate central apical lumens from basal submucosa.42 This system is physiologically active which enables functional studies of tight junction expression and intestinal barrier function.43 We have confirmed the unique ability of enteroids and colonoids to study the complex host-microbe interactions.
We employed IFN-gamma to modulate tight junction protein expression and paracellular permeability. IFN-gamma is a pro-inflammatory cytokine that is elevated in the colonic mucosa of IBS patients37 and has been shown to directly decrease intestinal epithelial barrier function.4 We have demonstrated that IFN-gamma results in 40% reduction in gene expression of OCLN and ZO-1 in human enteroids which is consistent with previous studies.42,44 Immunofluorescence was employed to confirm decreased expression as well as abnormal subcellular localization of OCLN and ZO-1. This was associated with increased paracellular permeability of enteroids by microinjection of FD4.
We further demonstrated that IFN-gamma induced disruption of gut epithelial permeability was effectively inhibited when enteroids were pretreated with LGG-CM. This was accompanied by normalization of OCLN and ZO-1 gene expression. Our model suggests that LGG is unique in its ability to modulate epithelial barrier function as these protective effects were not seen when enteroids were pretreated with L. crispatus. L. crispatus is a probiotic capable of blocking uropathogens in vaginal epithelial cells and may prevent against recurrent urinary tract infections.45,46 In contrast to LGG, L. crispatus exacerbates murine colitis evoked by dextran sulfate sodium.47 Hence it was used as a negative control for our study.
Prior studies have demonstrated a cytoprotective effect of LGG via prevention against apoptosis. Yan et al. showed that LGG prevented cytokine induced apoptosis in intestinal epithelial cells through activation of Akt and inhibition of p38 activation.26 In contrast, our results indicate that LGG prevents cytokine-induced epithelial barrier damage by a mechanism independent from apoptosis.
LGG has been shown to be effective in treating gastrointestinal illnesses, such as acute gastroenteritis.48 However, the mechanisms by which LGG confer benefit are largely unknown. In addition, while probiotics are generally safe, there are potential risks in certain populations, such as immunocompromised or critically ill patients.49,50 Understanding the complex host-microbe interactions are still in its infancy, which is a major limitation for the prediction of efficacy, safety, and bioavailability of probiotics. One way to address these concerns is to understand how LGG interacts with the gut epithelium on a molecular level and then to isolate and purify the active factor(s) involved in these processes.
Although this study did not identify the specific factors responsible for LGG’s protective effects, we demonstrated that addition of extracted LGG DNA, LGG cell wall, and boiled LGG-CM did not alter intestinal epithelial permeability. This suggests that LGG exerts its effects on intestinal epithelial barrier function via secreted proteins. Prior studies have identified p40 and p75 as potential mediators of LGG-induced effects on epithelial barrier function. These two novel proteins secreted by LGG may attenuate hydrogen peroxide-induced disruption of barrier function in Caco-2 cell monolayers. These protective effects likely occur via PI3K/Akt signaling pathway and MAPK-dependent signaling.33 Further studies have demonstrated that p40 ameliorates intestinal injury and colitis by stimulating ADAM17 activity and EGFR activation in colonic epithelial cells51 which stimulates mucin production through transactivation of EGFR.52 Host-microbial interactions are complex and regulation of intestinal epithelial barrier function may be species and tissue specific. Actions observed in cancer cell lines may not be applicable to normal human epithelial lining. For example, we found that in contrast to cancer cell lines, the protective actions of LGG on tight junction proteins in human colonoids are not mediated by MAPK/ERK pathway. Furthermore, in human colonoids, LGG regulates epithelial barrier function independent of its action against apoptosis which is different from the observations made in HY-29 cell lines. Currently it is unclear whether p40 and p75 are responsible for normalizing expression of ZO-1 and OCLN. Future studies may determine whether p40 and p75 can be isolated from LGG-CM which may exert protective effects against inflammation-induced epithelial barrier dysfunction.
An intriguing finding in this study was that FSN taken from IBS-D patients, but not healthy controls, leads to impaired intestinal barrier function in human colonoids. However, pretreatment of colonoids with LGG prevented epithelial barrier dysfunction induced by IBS-FSN. De Palma et al. recently showed transfer of fecal samples from IBS-D patients to germ-free mice resulted in accelerated gastrointestinal transit and intestinal barrier dysfunction.53 Similar observations were made when fecal supernatant from IBS-D patients was administered into the colon of naïve rats.40 These findings indicate that soluble factors from IBS patients, possibly derived from gut microbiota, lead to pathophysiologic changes, including impaired gut barrier function. Furthermore, 39% of IBS-D patients have increased intestinal permeability as measured by the lactulose/mannitol ratio.54 These IBS patients demonstrate higher symptom scores and increased hypersensitivity to visceral nociceptive pain. Our findings indicate that LGG may directly improve mucosal barrier function by normalizing expression of junction proteins independent of immune modulation. This provides a rationale for using LGG to treat pain in IBS, which is a difficult symptom to treat. Future studies may investigate the potential of bacterial-derived factors to modulate intestinal barrier function in the treatment of conditions, such as IBS.
In conclusion, this study showed that LGG attenuates epithelial barrier dysfunction evoked by IFN-gamma in human enteroids. Furthermore, LGG normalizes tight junction protein expression which occurs in the absence of apoptosis. Our results demonstrate that protein metabolites secreted by LGG, but not bacterial DNA or cell wall, are responsible for preventing IFN-gamma induced epithelial barrier damage. Finally, our data indicate that IBS-FSN impaired intestinal barrier function in human colonoids, which was prevented by LGG. These findings support a potential application of bacterial components to prevent cytokine-mediated gastrointestinal injury and to treat epithelial barrier dysfunction in conditions, such as IBS. Identification of soluble factors mediating the beneficial effects of LGG may present an opportunity to understand their mechanism of action as well as to develop effective pharmacological strategies that may circumvent many of the problems posed by live bacterial therapies.
Methods
Human Specimens
Normal small intestinal and colonic tissue were obtained from patients undergoing surgical resection and colonoscopy, respectively, at the University of Michigan (UM). All human experiments were approved by the institutional review board at UM. Informed consent was obtained prior to acquisition of tissue.
LGG Conditioned Media Preparation
Lactobacillus rhamnosus GG (ATCC 53103) and Lactobacillus crispatus (ATCC 33820) (American Type Culture Collection (ATCC), Manassas, VA) were incubated at 37 °C for 24h, then diluted in MRS broth according to ATCC guidelines.
For LGG-CM, LGG were inoculated in 50ml Dulbecco’s modified Eagle medium (DMEM) at 37°C overnight to reach log phase with the density determined as 0.6 at A600. The media was centrifuged twice, adjusted to pH 7.4, then filtered through a 0.2um filter to remove live bacteria.
LGG cell wall isolation
LGG was incubated in Lactobacillus MRS broth at 37 °C to reach log phase.55 Cultures were harvested by centrifugation and the cells were washed with PBS at room temperature twice. Cell suspensions were pipetted into 4% boiling SDS to lyse the cells for 3h. Boiled cells were ultracentrifuged (400,000 × g, 20 min, room temperature). The supernatant was removed and the pellets were resuspended in room temperature ultrapure water. Centrifugation was repeated and the samples were washed until SDS had been fully removed. The samples were resuspended in 10 mM Tris-HCl (pH 7.2) + 0.06% w/v NaCl. One mg/ml activated Pronase E (100 µg/ml final concentration) was added to each sample and incubated at 60 °C for 2h. Next, 200 µl of 6% SDS was added to stop the Pronase E digestion. Centrifugation and washing was repeated until the SDS had been fully removed. Samples were then re-suspended in 50 mM sodium phosphate buffer.
LGG DNA Extraction
LGG was incubated for 24 h at 37 °C in the exponential phase growth according to ATCC guidelines. After centrifugation (12000 rpm, 10 min) the bacterial pellets were used for total DNA extraction.56 These pellets were washed with NaCl-EDTA (30 mM NaCl, 2 mM EDTA, pH = 8.0) and resuspended in lysis buffer (Tris-HCl 20 mM, EDTA 2 mM, pH = 8.0), lysozyme (20 mg/mL) and triton X-100 (1% v/v). After incubation for 2h at 37°C, proteinase K (20 mg/mL) and RNase A (0.2 mg/mL) were added and incubated for 1h at 55°C. DNA was purified by repeat extraction with solvent of phenol-chloroform-isoamyl alcohol (25:24:1, v/v), precipitated with sodium acetate and ethanol, and dissolved in TE buffer. The purity of DNA was checked by a Nanodrop spectrophotometer.
LGG boiled supernatant preparation
LGG were inoculated in DMEM at 37°C to reach log phase with the density determined as 0.6 at A600. LGG-CM were denatured by boiling for 10 min.
Human intestinal enteroid isolation and propagation
Human intestinal tissue was washed with ice-cold Dulbecco’s Phosphate buffered saline without Ca2+ and Mg2+ (DPBS), and secured on a silicone-coated glass Petri dish filled with ice-cold DPBS.57 The mucosa was dissected from the underlying sub-epithelial tissue, washed 3–4 times with ice-cold chelation buffer to remove villi and debris, digested with freshly prepared 8mM EDTA chelation buffer for 30 min on a horizontal orbital shaker at 4°C. Epithelial crypts that were released from the tissue were collected by centrifugation of the media at 5 min at 800rpm, 4°C. Crypts were resuspended and then cultured in human enteroid complete medium (AdDMEM/F12 medium composed 50% LWRN conditioned medium, HEPES, Glutamax, penicillin/streptomycin, N2, B27, N-acetyl-L-cysteine, epidermal growth factor) mixed with Matrigel as previously described.43,58,59 The medium was replaced every other day.
Enteroid treatment (IFN-gamma) and pretreatment (LGG-CM, L. crispatus-CM, cell wall, DNA, boiled LGG-CM)
Three days after splitting, enteroids were cultured in human enteroid complete medium containing cell-free LGG-CM (5% vol/vol), LGG extracted DNA (10 µg/ml), boiled LGG-CM (5% vol/vol), LGG cell wall (4 mg/ml) or L. Crispatus-CM (5% vol/vol) overnight before treatment with IFN-gamma (200 ng/mL).
Real-time quantitative polymerase chain reaction
Cellular RNA was extracted from the enteroids by using Trizol Reagent (Invitrogen, Carlsbad, CA, USA) and Qiagen (Hilden, Germany) RNeasy mini columns, which was then reverse-transcribed into first-strand cDNA according to the manufacturer’s recommendations (iScript™ cDNA Synthesis Kit, Bio Rad, USA).57 The resultant cDNAs were used for RT-PCR, with primer sets targeting ZO-1 and OCLN. GAPDH served as an endogenous housekeeping reference gene.
Immunofluorescent labeling
Double or triple immunofluorescent staining was performed against ZO-1 and OCLN as well as Caspase-3, a key protease that is activated during the early stages of apoptosis.43 Enteroids were collected and fixed in 4% paraformaldehyde at 4°C for 30 min, then washed 3 times with PBS, and soaked in 30% sucrose for 24–48h at 4°C. Enteroids were embedded in optimal-cutting-temperature compound (4583; Sakura) for 20 min and frozen at -80°C. Frozen sections were cut at 8 µm for immunostaining, followed by microscopy. The cryostat sections were next rehydrated in PBS and blocked by 5% normal donkey serum (1:10; Chemicon International, Temecula, CA, USA) containing 0.3% Triton X-100 for 30 min at room temperature. The preparations were incubated overnight at room temperature with anti-ZO-1 antibody (1:500, ThermoFisher Scientific, USA), anti-occludin antibody (1:500, Invitrogen, USA) or caspase-3 (1:500, cell signaling). After incubation with the primary antibodies, the preparations were washed three times in PBS and incubated for 1h at room temperature with secondary antibodies Cy3 (1:200; Jackson ImmunoResearch Laboratories, West Grove, PA), Alexa 488-conjugated goat anti-rabbit IgG (1:200; Molecular Probes, Life Sciences Solutions)). Sections were then washed three times in PBS, mounted in buffered glycerol, and observed under fluorescence microscopy (Olympus BX-51, Tokyo, Japan). Images were captured at the same time of exposure, gain and gamma adjustment for the control and experimental groups.
Human colonoids establishment
Human colonic crypts were isolated from biopsy samples taken from healthy subjects undergoing colonoscopy at UM. The biopsy samples were washed 3–4 times with ice-cold PBS buffer containing penicillin-streptomycin (Pen/Strep, 1x), gentamicin (50 μg/ml), normocin (100 g/ml), and amphotericin (2.5 μg/ml) to control contamination, as well as thiazovivin (2.5 μM), a ROCK inhibitor.60 Crypts were isolated from biopsies by EDTA chelation containing 8 mM EDTA with DTT for 15 minutes, and in 8 mM EDTA for 15 minutes for further digestion. Crypts were collected in LWRN complete medium (AdDMEM/F12 medium composed of 50% LWRN conditioned medium, HEPES, Glutamax, penicillin/streptomycin, N2, B27, N-acetyl-L-cysteine, and epidermal growth factor). The crypt pellets were resuspended in basement membrane matrix and maintained in culture for 2 weeks.57
Western blot analysis
To prepare total protein lysates, colonoids were harvested using BD Cell Recovery Solution in order to achieve Matrigel matrix depolymerization. The wells were washed with cold PBS and incubated for 60 minutes in cold BD Cell Recovery Solution. Once the colonoids were released, the cells were centrifuged at 800rpm for 5 minutes at 4°C. The pellet was homogenized in lysis buffer containing a mixture of proteinase and phosphatase inhibitors and then centrifuged at 15,000rpm for 15 min at 4°C. The protein concentration was determined using a BCA Protein Assay Kit (Pierce, Rockford, IL). Total protein was resolved on 4–12% precasted SDS-PAGE gels, then transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore, Bedford, MA). The PVDF membrane was blocked with 5% non-fat milk in PBS containing 0.1% Tween 20 for 2 h at room temperature and then incubated overnight at 4°C with primary antibodies. The following antibodies were used in this study: GAPDH, anti-EGFR (phosphor Y1068) (1:2000, abcam, USA), total-EGFR, P-ERK, total-EGFR antibody (1:1000, cell signaling, USA). After washing with TBST, the blots were incubated for 2h at room temperature with HRP-conjugated secondary antibody (1:5000; Amersham Biosciences, San Francisco, CA, USA), visualized by using Electro-Chemi-Luminescence (ECL) chemiluminescent detection system (Amersham Biosciences).
Fecal supernatant preparation
Four healthy subjects as well as four patients meeting Rome III criteria for IBS-D were recruited from outpatient clinics at UM. Subjects completed a 2 week screening period during which symptom severity was assessed based on 11-point numerical rating scale for abdominal pain, bloating and fecal urgency as well as stool consistency (Bristol stool form scale) and frequency.61,62 Six fecal samples were collected from each IBS-D patients and healthy subjects and stored at -80°C. Based on our recent studies, fecal samples were diluted (1 g fecal sample/5 ml PBS), homogenized on ice, and centrifuged (10,000 cpm, 10 minutes, 4°C).61,62 The supernatants were recovered, filtered on 0.22 μm filters to remove bacteria, and then stored at -80°C.
Microinjection of Enteroids and Colonoids
Each group of enteroids/colonoids was checked for integrity before injection. Thin-wall glass capillaries and tips were prepared as previously described.43 The capillaries were filled with 4kDa FITC-Dextran (FD4) and then loaded onto the microinjector (BRI XenoWorks analog microinjector; Sutter Instrument Company). Each enteroid/colonoid was injected with approximately 0.2 – 0.8ul of 1mMFD4 based on the volume of enteroids, as described previously.43 Human enteroids and colonoids were imaged using a fluorescent stereomicroscope (SZX16; Olympus) at 1x magnification. Images were taken at the indicated time points postinjection. Disruption of barrier integrity was determined by loss of FD4 from the lumen of the enteroids/colonoids.
Determining pixel intensity of FD4 in injected HIOs
ImageJ software was used to determine the starting and final pixel intensity of the human enteroids and colonoids.63 Both bright-field and fluorescent images were taken from each well. Using the bright-field image, the perimeter of the enteroids or colonoids were outlined manually, and this region was used to determine the mean gray value of the enteroids or colonoids in the fluorescent image. These steps were repeated for each enteroid or colonoid for all treatments. The percent pixel intensity is defined as the mean gray value of an enteroid or colonoid at a given time point divided by the mean gray value of that same organoids at time 0 (T = 0) multiplied by 100.43
Statistical analysis
All data were analyzed with SPSS 16.0 software (Chicago, IL). Differences between groups were compared by 2-tailed student’s t test or ANOVA for comparisons between 2 groups or more than 2 groups, respectively. Statistical significance was set at a P value of 0.05.
Funding Statement
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grants R01-DK048419 (C. Owyang) and P30-DK34933 (C. Owyang).
Competing interests
None declared.
Contributors
CO conceived and supervised the study. XU and CO designed and performed the experiments. XU, AL and CO wrote the manuscript. SH was involved in microinjection experiments. JG and JS were critical in human intestinal organoid culture and development. All authors discussed and interpreted the results.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Ethics approval
All procedures were approved by the institutional review board (University of Michigan).
Provenance and peer review
Not commissioned; externally peer reviewed.
Supplementary Material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.König J, Wells J, Cani PD, García-Ródenas CL, MacDonald T, Mercenier A, Whyte J, Troost F, Brummer R-J.. Human Intestinal Barrier Function in Health and Disease. Clin Transl Gastroenterol. 2016;7:e196. doi: 10.1038/ctg.2016.54 PMID:27763627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653 PMID:19855405 [DOI] [PubMed] [Google Scholar]
- 3.Arrieta MC, Bistritz L, JB Meddings. Alterations in intestinal permeability. Gut. 2006;55:1512–20. doi: 10.1136/gut.2005.085373 PMID:16966705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madara JL, Stafford J.. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. J Clin Invest. 1989;83:724–7. doi: 10.1172/JCI113938 PMID:2492310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mullin JM, Laughlin KV, Marano CW, Russo LM, Soler AP.. Modulation of tumor necrosis factor-induced increase in renal (LLC-PK1) transepithelial permeability. Am J Physiol. 1992;263:F915–924.PMID:1279987 [DOI] [PubMed] [Google Scholar]
- 6.Cash HL, Whitham CV, Behrendt CL, LV Hooper. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313:1126–30. doi: 10.1126/science.1127119 PMID:16931762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung H, Pamp SJ, Hill JA, Surana NK, Edelman SM, Troy EB, Reading NC, Villablanca EJ, Wang S, Mora JR, et al. Gut immune maturation depends on colonization with a host-specific microbiota. Cell. 2012;149:1578–93. doi: 10.1016/j.cell.2012.04.037 PMID:22726443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wells JM, Brummer RJ, Derrien M, MacDonald TT, Troost F, Cani PD, Theodorou V, Dekker J, Méheust A, de Vos WM, et al. Homeostasis of the gut barrier and potential biomarkers. Am J Physiol Gastrointest Liver Physiol. 2017;312:G171–93. doi: 10.1152/ajpgi.00048.2015 PMID:27908847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10:131–44. doi: 10.1038/nri2707 PMID:20098461 [DOI] [PubMed] [Google Scholar]
- 10.Wells JM, Loonen LMP, Karczewski JM.. The role of innate signaling in the homeostasis of tolerance and immunity in the intestine. Int J Med Microbiol IJMM. 2010;300:41–8. doi: 10.1016/j.ijmm.2009.08.008 PMID:19783476 [DOI] [PubMed] [Google Scholar]
- 11.Wells JM, Rossi O, Meijerink M, Baarlen P van. Epithelial crosstalk at the microbiota–mucosal interface. Proc Natl Acad Sci. 2011;108:4607–14. doi: 10.1073/pnas.1000092107 PMID:20826446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cario E, Gerken G, Podolsky DK.. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology. 2007;132:1359–74. doi: 10.1053/j.gastro.2007.02.056 PMID:17408640 [DOI] [PubMed] [Google Scholar]
- 13.Ewaschuk JB, Diaz H, Meddings L, Diederichs B, Dmytrash A, Backer J, Looijer-van Langen M, Madsen KL.. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1025–1034. doi: 10.1152/ajpgi.90227.2008 PMID:18787064 [DOI] [PubMed] [Google Scholar]
- 14.Fasano A, Fiorentini C, Donelli G, Uzzau S, Kaper JB, Margaretten K, Ding X, Guandalini S, Comstock L, Goldblum SE.. Zonula occludens toxin modulates tight junctions through protein kinase C-dependent actin reorganization, in vitro. J Clin Invest. 1995;96:710–20. doi: 10.1172/JCI118114 PMID:7635964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, Tobe T, Clarke JM, Topping DL, Suzuki T, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543–7. doi: 10.1038/nature09646 PMID:21270894 [DOI] [PubMed] [Google Scholar]
- 16.Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer R-J.. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27:104–19. doi: 10.1111/j.1365-2036.2007.03562.x PMID:17973645 [DOI] [PubMed] [Google Scholar]
- 17.Piche T, Barbara G, Aubert P, Bruley des Varannes S, Dainese R, JL Nano, Cremon C, Stanghellini V, De Giorgio R, JP Galmiche, et al. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut. 2009;58:196–201. doi: 10.1136/gut.2007.140806 PMID:18824556 [DOI] [PubMed] [Google Scholar]
- 18.Salim SY, Söderholm JD.. Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17:362–81. doi: 10.1002/ibd.21403 PMID:20725949 [DOI] [PubMed] [Google Scholar]
- 19.Camilleri M, Madsen K, Spiller R, Van Meerveld BG, Verne GN.. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil. 2012;24:503–12. doi: 10.1111/j.1365-2982.2012.01921.x PMID:22583600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chassaing B, Darfeuille-Michaud A.. The commensal microbiota and enteropathogens in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1720–8. doi: 10.1053/j.gastro.2011.01.054 PMID:21530738 [DOI] [PubMed] [Google Scholar]
- 21.Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, AT Gewirtz. Metabolic Syndrome and Altered Gut Microbiota in Mice Lacking Toll-Like Receptor 5. Science. 2010;328:228–31. doi: 10.1126/science.1179721 PMID:20203013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalliomäki M, Satokari R, Lähteenoja H, Vähämiko S, Grönlund J, Routi T, Salminen S.. Expression of Microbiota, Toll-like Receptors, and Their Regulators in the Small Intestinal Mucosa in Celiac Disease. J Pediatr Gastroenterol Nutr. 2012;54:727–32. doi: 10.1097/MPG.0b013e318241cfa8 PMID:22134550 [DOI] [PubMed] [Google Scholar]
- 23.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–14. doi: 10.1038/nrgastro.2014.66 PMID:24912386 [DOI] [PubMed] [Google Scholar]
- 24.Segers ME, Lebeer S.. Towards a better understanding of Lactobacillus rhamnosus GG–host interactions. Microb Cell Factories. 2014;13(Suppl 1):S7. doi: 10.1186/1475-2859-13-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doron S, Snydman DR, Gorbach SL.. Lactobacillus GG: Bacteriology and Clinical Applications. Gastroenterol Clin North Am. 2005;34:483–98. doi: 10.1016/j.gtc.2005.05.011 PMID:16084309 [DOI] [PubMed] [Google Scholar]
- 26.Yan F, Polk DB.. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J Biol Chem. 2002;277:50959–65. doi: 10.1074/jbc.M207050200 PMID:12393915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tao Y, Drabik KA, Waypa TS, Musch MW, Alverdy JC, Schneewind O, Chang EB, Petrof EO.. Soluble factors from Lactobacillus GG activate MAPKs and induce cytoprotective heat shock proteins in intestinal epithelial cells. Am J Physiol – Cell Physiol. 2006;290:C1018–30. doi: 10.1152/ajpcell.00131.2005 PMID:16306130 [DOI] [PubMed] [Google Scholar]
- 28.Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB.. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology. 2007;132:562–75. doi: 10.1053/j.gastro.2006.11.022 PMID:17258729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen R-C, Xu L-M, Du S-J, Huang S-S, Wu H, Dong J-J, Huang J-R, Wang X-D, Feng W-K, Chen Y-P.. Lactobacillus rhamnosus GG supernatant promotes intestinal barrier function, balances Treg and TH17 cells and ameliorates hepatic injury in a mouse model of chronic-binge alcohol feeding. Toxicol Lett. 2016;241:103–10. doi: 10.1016/j.toxlet.2015.11.019 PMID:26617183 [DOI] [PubMed] [Google Scholar]
- 30.Bauserman M, Bausserman M, Michail S.. The use of Lactobacillus GG in irritable bowel syndrome in children: A double-blind randomized control trial. J Pediatr. 2005;147:197–201. doi: 10.1016/j.jpeds.2005.05.015 PMID:16126049 [DOI] [PubMed] [Google Scholar]
- 31.Gosselink MP, Schouten WR, LMC van Lieshout, Hop WCJ, Laman JD, Ruseler-van Embden JGH.. Delay of the first onset of pouchitis by oral intake of the probiotic strain Lactobacillus rhamnosus GG. Dis Colon Rectum. 2004;47:876–84. doi: 10.1007/s10350-004-0525-z PMID:15108026 [DOI] [PubMed] [Google Scholar]
- 32.Zocco MA, dal Verme LZ, Cremonini F, Piscaglia AC, Nista EC, Candelli M, Novi M, Rigante D, Cazzato IA, Ojetti V, et al. Efficacy of Lactobacillus GG in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther. 2006;23:1567–74. doi: 10.1111/j.1365-2036.2006.02927.x PMID:16696804 [DOI] [PubMed] [Google Scholar]
- 33.Seth A, Yan F, Polk DB, Rao RK.. Probiotics ameliorate the hydrogen peroxide-induced epithelial barrier disruption by a PKC- and MAP kinase-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1060–9. doi: 10.1152/ajpgi.00202.2007 PMID:18292183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donato KA, Gareau MG, Wang YJJ, Sherman PM.. Lactobacillus rhamnosus GG attenuates interferon-{gamma} and tumour necrosis factor-alpha-induced barrier dysfunction and pro-inflammatory signalling. Microbiol Read Engl. 2010;156:3288–97. doi: 10.1099/mic.0.040139-0. [DOI] [PubMed] [Google Scholar]
- 35.Ettayebi K, Crawford SE, Murakami K, Broughman JR, Karandikar U, Tenge VR, Neill FH, Blutt SE, Zeng X-L, Qu L, et al. Replication of human noroviruses in stem cell–derived human enteroids. Science. 2016;353:1387–93. doi: 10.1126/science.aaf5211 PMID:27562956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–9. doi: 10.1038/nature09691 PMID:21151107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barbaro MR, Di Sabatino A, Cremon C, Giuffrida P, Fiorentino M, Altimari A, Bellacosa L, Stanghellini V, Barbara G.. Interferon-γ is increased in the gut of patients with irritable bowel syndrome and modulates serotonin metabolism. Am J Physiol Gastrointest Liver Physiol. 2016;310:G439–447. doi: 10.1152/ajpgi.00368.2015 PMID:26744473 [DOI] [PubMed] [Google Scholar]
- 38.Yang S, Yu M, Sun L, Xiao W, Yang X, Sun L, Zhang C, Ma Y, Yang H, Liu Y, et al. Interferon-γ-induced intestinal epithelial barrier dysfunction by NF-κB/HIF-1α pathway. J Interferon Cytokine Res. 2014;34:195–203. doi: 10.1089/jir.2013.0044. [DOI] [PubMed] [Google Scholar]
- 39.Alhouayek M, Rankin L, Gouveia-Figueira S, Fowler CJ.. Interferon γ treatment increases endocannabinoid and related N-acylethanolamine levels in T84 human colon carcinoma cells. Br J Pharmacol. 2018;doi: 10.1111/bph.14135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou S-Y, Gillilland M, Wu X, Leelasinjaroen P, Zhang G, Zhou H, Ye B, Lu Y, Owyang C.. FODMAP diet modulates visceral nociception by lipopolysaccharide-mediated intestinal inflammation and barrier dysfunction. J Clin Invest. 2018;128:267–80. doi: 10.1172/JCI92390 PMID:29202473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–5. doi: 10.1038/nature07935 PMID:19329995 [DOI] [PubMed] [Google Scholar]
- 42.Juuti-Uusitalo K, Klunder LJ, Sjollema KA, Mackovicova K, Ohgaki R, Hoekstra D, Dekker J, van Ijzendoorn SCD.. Differential effects of TNF (TNFSF2) and IFN-γ on intestinal epithelial cell morphogenesis and barrier function in three-dimensional culture. PloS One. 2011;6:e22967. doi: 10.1371/journal.pone.0022967 PMID:21853060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leslie JL, Huang S, Opp JS, Nagy MS, Kobayashi M, Young VB, Spence JR.. Persistence and toxin production by Clostridium difficile within human intestinal organoids result in disruption of epithelial paracellular barrier function. Immun Infect. 2015;83:138–45. doi: 10.1128/IAI.02561-14 PMID:25312952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Q, Zhang Q, Wang M, Zhao S, Ma J, Luo N, Li N, Li Y, Xu G, Li J.. Interferon-gamma and tumor necrosis factor-alpha disrupt epithelial barrier function by altering lipid composition in membrane microdomains of tight junction. Clin Immunol Orlando Fla. 2008;126:67–80. doi: 10.1016/j.clim.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 45.Osset J, Bartolomé RM, García E, Andreu A.. Assessment of the capacity of Lactobacillus to inhibit the growth of uropathogens and block their adhesion to vaginal epithelial cells. J Infect Dis. 2001;183:485–91. doi: 10.1086/318070 PMID:11133381 [DOI] [PubMed] [Google Scholar]
- 46.Stapleton AE, Au-Yeung M, Hooton TM, Fredricks DN, Roberts PL, Czaja CA, Yarova-Yarovaya Y, Fiedler T, Cox M, Stamm WE.. Randomized, Placebo-Controlled Phase 2 Trial of a Lactobacillus crispatus Probiotic Given Intravaginally for Prevention of Recurrent Urinary Tract Infection. Clin Infect Dis Off Publ Infect Dis Soc Am. 2011;52:1212–7. doi: 10.1093/cid/cir183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou F-X, Chen L, Liu X-W, Ouyang C-H, Wu X-P, Wang X-H, Wang C-L, Lu F-G.. Lactobacillus crispatus M206119 exacerbates murine DSS-colitis by interfering with inflammatory responses. World J Gastroenterol WJG. 2012;18:2344–56. doi: 10.3748/wjg.v18.i19.2344 PMID:22654425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Szajewska H, Kołodziej M.. Systematic review with meta-analysis: Lactobacillus rhamnosus GG in the prevention of antibiotic-associated diarrhoea in children and adults. Aliment Pharmacol Ther. 2015;42:1149–57. doi: 10.1111/apt.13404 PMID:26365389 [DOI] [PubMed] [Google Scholar]
- 49.Boyle RJ, Robins-Browne RM, Tang MLK.. Probiotic use in clinical practice: what are the risks? Am J Clin Nutr. 2006;83:1256–64–1447. [DOI] [PubMed] [Google Scholar]
- 50.Besselink MG, van Santvoort HC, Buskens E, Boermeester MA, van Goor H, Timmerman HM, Nieuwenhuijs VB, Bollen TL, van Ramshorst B, Witteman BJ, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet Lond Engl. 2008;371:651–9. doi: 10.1016/S0140-6736(08)60207-X. [DOI] [PubMed] [Google Scholar]
- 51.Yan F, Liu L, Dempsey PJ, Tsai Y-H, Raines EW, Wilson CL, Cao H, Z Cao, Liu L, Polk DB. A. Lactobacillus rhamnosus GG-derived soluble protein, p40, stimulates ligand release from intestinal epithelial cells to transactivate epidermal growth factor receptor. J Biol Chem. 2013;288:30742–51. doi: 10.1074/jbc.M113.492397 PMID:24043629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang L, Cao H, Liu L, B Wang, Walker WA, Acra SA, Yan F.. Activation of epidermal growth factor receptor mediates mucin production stimulated by p40, a Lactobacillus rhamnosus GG-derived protein. J Biol Chem. 2014;289:20234–44. doi: 10.1074/jbc.M114.553800 PMID:24895124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palma GD, MDJ Lynch, Lu J, Dang VT, Y Deng, Jury J, Umeh G, Miranda PM, Pastor MP, Sidani S, et al. Transplantation of fecal microbiota from patients with irritable bowel syndrome alters gut function and behavior in recipient mice. Sci Transl Med. 2017;9:eaaf6397. doi: 10.1126/scitranslmed.aaf6397 PMID:28251905 [DOI] [PubMed] [Google Scholar]
- 54.Zhou Q, Zhang B, Verne GN.. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain. 2009;146:41–6. doi: 10.1016/j.pain.2009.06.017 PMID:19595511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Desmarais SM, Cava F, de Pedro MA, Huang KC.. Isolation and Preparation of Bacterial Cell Walls for Compositional Analysis by Ultra Performance Liquid Chromatography. J Vis Exp JoVE [Internet] 2014. [cited 2017 August 8]; Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3987682/ doi: 10.3791/51183. [DOI] [PMC free article] [PubMed]
- 56.Alimolaei M, Golchin M.. An Efficient DNA Extraction Method for Lactobacillus casei, a Difficult-to-Lyse Bacterium. Int J Enteric Pathog. 2016;4:7–32472. doi: 10.17795/ijep32472. [DOI] [Google Scholar]
- 57.Mahe MM, Sundaram N, Watson CL, Shroyer NF, Helmrath MA.. Establishment of human epithelial enteroids and colonoids from whole tissue and biopsy. J Vis Exp JoVE. 2015;doi: 10.3791/52483 PMID:25866936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miyoshi H, Stappenbeck TS.. In vitro expansion and genetic modification of gastrointestinal stem cells as organoids. Nat Protoc. 2013;8:2471–82. doi: 10.1038/nprot.2013.153 PMID:24232249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miyoshi H, Ajima R, Luo CT-Y, Yamaguchi TP, Stappenbeck TS.. Wnt5a Potentiates TGF-β Signaling to Promote Colonic Crypt Regeneration after Tissue Injury. Science. 2012;338:108–13. doi: 10.1126/science.1223821 PMID:22956684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Nishikawa S, Muguruma K, et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–6. doi: 10.1038/nbt1310 PMID:17529971 [DOI] [PubMed] [Google Scholar]
- 61.Heaton KW, Radvan J, Cripps H, Mountford RA, Braddon FE, Hughes AO.. Defecation frequency and timing, and stool form in the general population: a prospective study. Gut. 1992;33:818–24. doi: 10.1136/gut.33.6.818 PMID:1624166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.SPIEGEL B, BOLUS R, HARRIS LA, LUCAK S, NALIBOFF B, ESRAILIAN E, CHEY WD, LEMBO A, KARSAN H, TILLISCH K, et al. Measuring IBS patient reported outcomes with an abdominal pain numeric rating scale: results from the proof cohort. Aliment Pharmacol Ther. 2009;30:1159–70. doi: 10.1111/j.1365-2036.2009.04144.x PMID:19751360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schneider CA, Rasband WS, Eliceiri KW. NIH. Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–5. doi: 10.1038/nmeth.2089 PMID:22930834 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


