ABSTRACT
The reemergence of pertussis in the last two decades led to the introduction of adolescents and adults immunization strategies of tetanus–diphtheria–acellular pertussis vaccines (Tdap) in several countries. The health authorities must consider economic aspects when deciding to recommend and fund new programs. Here we present a systematic review of worldwide full economic evaluations of pertussis vaccination targeting adolescents or adults published from 2000. Studies were identified by searching MEDLINE, Excerpta Medica, CRD, and Lilacs databases. Twenty-seven economic evaluations of different strategies with Tdap were identified. Booster vaccination for adolescents and adults were the most frequent, followed by cocooning and pregnant women vaccination. Strategies performance varied considerably among different studies. Assumptions regarding underreporting correction, herd protection and vaccine coverage were crucial to cost-effectiveness results. Understanding the model and the parameters used is essential to understand the results, and identify the major issues important to public health decisions.
Keywords: Adolescent, adult, economic evaluation, maternal immunization, pertussis vaccination, systematic review
Introduction
Pertussis is a highly contagious respiratory disease mainly caused by Bordetella pertussis.1,2 It causes uncontrollable violent coughing for long periods, most commonly affects infants and young children, and can be fatal, especially in infants up to 6 months of age.1,2 Childhood immunization with whole-cell pertussis (wP) containing vaccines led to important reduction in pertussis incidence in countries that achieved high vaccine coverage.2,3
However, a global reemergence of pertussis has been observed in the last 20 years, in spite of sustained high vaccine coverage.1-3 Hypotheses to explain this reemergence are post-vaccination waning immunity; reduced effectiveness of acellular vaccines; implementation of molecular methods for diagnosis; improvement of surveillance systems; enhanced disease awareness; and genetic changes in the pathogen.2 The reemergence of epidemics, severe infections in very young not yet vaccinated infants, and pertussis in older children, adolescents and adults, resulted in renewed attention of the public health authorities to further improve pertussis control and optimize protection through vaccination.2
Tetanus–diphtheria–acellular pertussis vaccines (Tdap) for adolescents and adults were licensed in 2005 and additional immunization strategies were proposed: 1) booster doses for adolescents and adults; 2) cocooning strategy; 3) pregnant women and post-partum maternal vaccination; and 4) vaccination of healthcare workers.2
Pertussis among infants frequently presents as severe cases, with higher hospitalization and case-fatality rates.4-6 Cocooning and pregnant women vaccination aim to avoid pertussis among infants aged less than one year, particularly infants younger than two months, who have not received any vaccine dose. Cocooning strategy is vaccinating neonates’ contacts, potentially reducing household transmission and preventing infant infection. Pregnant women vaccination results in direct newborn protection through transplacental antibody transfer from mother to infant.7-9
Even though the health benefits of some of these pertussis vaccination strategies have been demonstrated,7,8,10,11 national health authorities must also consider economic aspects when deciding to recommend and fund new programs. Economic evaluation of vaccination programs may support decision-making, and is considered an essential tool in a context of rising budget constraints.12
Health economic evaluation depends on good quality data of the disease epidemiology, not easily available in this case. Pertussis burden is underestimated by the surveillance systems, due to limited demand/access to healthcare, cases’ underrecognition or misdiagnosis, and underreporting.5 Higher rates of underreporting have been observed in older children, adolescents and adults.13
Despite the methodological difficulties, the efficiency of pertussis vaccination strategies has been evaluated in several studies worldwide. The objectives of this article are to provide a critical literature review of economic evaluations of adolescents and adults’ pertussis vaccination, to investigate the studies results’ disparity and the reasons for such differences, and to identify most cost-effective vaccination strategies. This review attempts to provide guidance and suggestions for improvement, contributing to future economic evaluations.
Results
Search results
The initial searches identified 1,318 articles. After duplicates removal and the titles and abstracts reading, 33 studies were considered potentially relevant and retrieved in full text. After reading the full text, 27 studies met the eligibility criteria and were included in this review (Figure 1).
Figure 1.
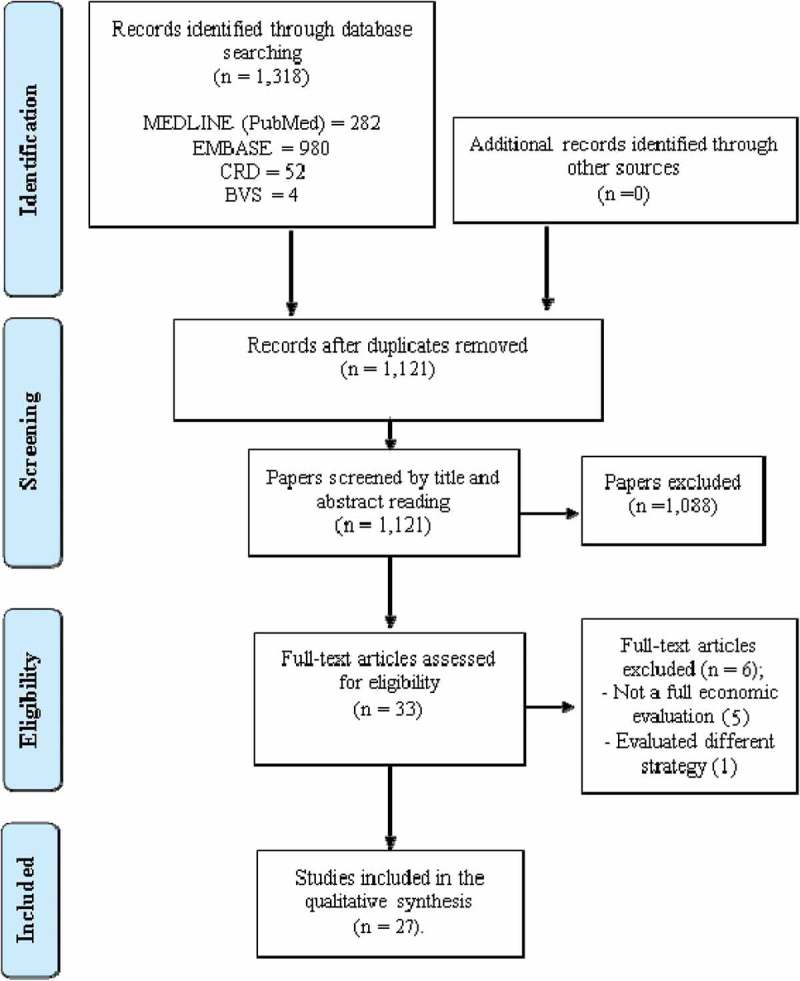
Flowchart of selection of studies included in the review.
Methodological studies characteristics
Table 1 describes the methodological characteristics of economic evaluations of pertussis immunization programs for adolescents and adults. Most studies considered developed countries: 12 in the United States of America, four in the Netherlands, three in Canada, two in England, and one each in Australia, Germany, Italy, Japan, and Spain. Only one referred to a developing country (Brazil).
Table 1.
Methodological characteristics of the economic evaluations of pertussis vaccination for adolescents and adults.
| Study/ Country | Year | Targeted population | Vaccination strategies compared | Type of study | Perspective | Model | Time horizon | Monetary unit / year | Health outcomes | Discount rate |
|---|---|---|---|---|---|---|---|---|---|---|
| Edmunds et al./ England and Wales14 |
2002 | Pre-school Adolescents |
(0) no vaccination; (1) vaccination at 4 years of age; (2) Adolescents vaccination | CEA | Healthcare provider and societal |
Dynamic | Lifetime | UK£, 1999/2000 | LYG | C and B – 3% |
| Scuffham e McIntyre/ Australia15 | 2004 | Both parents after birth (cocooning) and neonates | (0) childhood vaccination; (1) at-birth immunization; (2) 1-month vaccination; (3) Cocooning | CEA | Healthcare system |
Markov | 6 months | AUS$, 2000 | DALY | B – 3% |
| Purdy et al./ USA16 | 2004 | Adolescents, adults (different ages) and healthcare workers | (0) no vaccination; (1) Adolescents vaccination; (2) Adults aged >20 years; (3) Adults aged >50 years; (4) Adults aged ≥18 years with chronic obstructive pulmonary disease; (5) Adults aged ≥15 years caretakers of infants <1 year of age; (6) Healthcare workers vaccination; (7) 10-year boosters | CBA | Societal | NR | 10 years | US$, 2002 | Cases prevented | C and B – 3% |
| Iskedjian et al./ Canada (Ontario)17 | 2004 | Adolescents (12 years of age) | (0) No vaccination; (1) Adolescents vaccination | CEA | Ministry of Health and societal |
Dynamic | 10 years | CAD$, 2003 | Cases prevented | C – 3% |
| Caro et al./ USA18 | 2005 | Adolescents (11–18 years) |
(0) no vaccination; (1) Adolescents vaccination | CEA | Healthcare payer and societal |
Cohort Simulation |
Lifetime | US$, 2002 | LYG | B – 3% |
| Iskedjian et al./ Canada (Quebec)19 | 2005 | Adolescents (14 years of age) |
(0) no vaccination; (1) Adolescents vaccination | CEA | Ministry of Health and societal |
Dynamic | 10 years | CAD$, 2003 | Cases prevented | C – 3% |
| Lee et al./ USA20 | 2005 | Adolescents (11 years of age); adults (20 years of age); mothers immediately after birth and all other close contacts before the birth (cocooning) | (0) no vaccination; (1) Adolescent vaccination; (2) one-time adult vaccination; (3) adult vaccination with 10-year boosters; (4) Adolescent + adult + 10-year boosters; (5) Cocooning | CEA | Healthcare payer and societal |
Markov | Lifetime | US$, 2004 | Cases prevented and QALY | C and B – 3% |
| Calugar et al./ USA21 | 2006 | Healthcare workers | (0) No vaccination; (1) Healthcare workers vaccination | CBA | Hospital perspective | Dynamic | 10 years | US$, 2004 | Exposures to pertussis cases prevented | C and B – 3% |
| Lee et al./ USA22 | 2007 | Adults | (0) No vaccination; (1) Adult vaccination; (2) 10-year boosters | CEA | Societal | Markov | Lifetime | US$, 2005 | Cases prevented and QALY | C and B – 3% |
| Lee et al./ Germany23 | 2008 | Adults | (0) No vaccination; (1) One-time adult vaccination at 20–64 years of age; (2) 10-year boosters | CEA | Healthcare payer and societal |
Markov | Lifetime | €, 2006 | Cases prevented and QALY | C and B – 3% |
| Coudeville et al./ USA/ 200924 | 2009 | Adolescents, adults and both parents after birth (cocooning) | (0) No vaccination; (1) Adolescents vaccination; (2) Adolescents vaccination + cocooning; (3) Adolescents + adult + cocooning; (4) Adolescents + adults + 10-year boosters | CEA | Societal | Dynamic | 100 years | US$, 2006 | LYG | C and B – 3% |
| Westra et al./ Netherlands25 | 2010 | Father before and mother after the delivery (cocooning), pregnant women and neonates | (0) No vaccination; (1) Vaccination at birth; (2) Cocooning; (3) Pregnant women; (4) combining (2)+(3) | CEA | Healthcare payer and societal |
Decision-tree | 8 years | € and US$, 2008 (1 € = 1.4 US$) | QALY | C – 4%, B – 1.5% |
| de Vries et al./ Netherlands26 | 2010 | Adolescents (12 years of age) |
(0) No vaccination (1) Adolescents vaccination |
CEA | Societal | Dynamic | 25 years | €, 2008 | QALY | C – 4%, B – 1.5% |
| Greer and Fisman/ Canada27 | 2011 | Healthcare workers | (0) No vaccination; (1) Healthcare workers vaccination | CEA | Societal | Markov | 10 years | US$, 2008 | QALY | B – 3% |
| Rozenbaum et al./ Netherlands28 | 2012 | Adolescents and adults | (0) No vaccination; (1) A single dose for adolescents or adults; (2) single dose for both adolescents and adults vaccination; (3) booster doses with 10-year intervals | CEA | Societal | Dynamic | 25 years | €, 2011 | QALY | C – 1.5%, B – 4% |
| Itatani et al./ Japan29 | 2013 | Adolescents, adults and both parents after birth (cocooning) | (0) No vaccination; (1) Adolescents vaccination; (2) Adolescents + 10-year booster; (3) Adolescents + cocooning |
CEA | Societal | Markov | 40 years | Yen | QALY | C and B – 3% |
| Meregaglia et al./ Italy30 | 2013 | Both parents and other close contacts during pregnancy or immediately after delivery (cocooning) | (0) No vaccination; (1) Cocooning | CEA CBA |
National Health Service | NR | 1 year | €, 2011 | Cases prevented | Not considered |
| Ding et al./ USA31 | 2013 | Mothers after birth | (0) No vaccination; (1) Postpartum maternal vaccination |
CBA | Healthcare system and societal |
Decision tree | 10 years | US$, 2012 | Cases and deaths prevented | C – 3% |
| Terranella et al./ USA32 | 2013 | Mothers immediately after birth, all other close contacts vaccinated before the birth (cocooning) and pregnant women | (0) No vaccination; (1) Pregnant women; (2) Postpartum maternal vaccination; (3) Cocooning |
CEA | Societal | Markov | 1 year | US$, 2011 | Cases averted, QALY and LYG |
C and B – 3% |
| Lugnér et al./ Netherlands33 | 2013 | Both parents after birth (cocooning), pregnant women and neonates | (0) No vaccination; (1) neonate immunization at birth; (2) cocooning; (3) Pregnant women. |
CEA | Societal | NR | 10 years | €, 2009 | QALY | C – 4%, B – 1.5% |
| McGarry et al./ USA34 | 2013 | Adults aged ≥65 years | (0) No vaccination; (1) Adults vaccination | CEA | Healthcare payer and societal |
Decision tree | 35 years | US$, 2010 | Cases prevented and QALY | C and B – 3% |
| McGarry et al./ USA35 | 2014 | Adults aged ≥65 years | (0) No vaccination; (1) Adults vaccination | CEA | Healthcare payer and societal |
Dynamic | Lifetime | US$, 2010 | QALY | B – 3% |
| Fernández-Canoa et al./ Spain36 | 2015 | Both parents after birth (cocooning) and pregnant women | (0) No vaccination; (1) Cocooning; (2) Pregnant women | CBA | Healthcare system |
Decision tree | 1 year | €, 2012 | Hospitalizations and cases prevented | Not considered |
| Kamiya et al./ USA37 | 2016 | Adolescents and adults | (0) No revaccination; (1) Adolescent revaccination; (2) Adult revaccination | CEA | Society | Decision tree | 20 years | US$, 2010 | Cases prevented and QALY | C and B – 3% |
| Atkins et al./ USA38 | 2016 | Adults, both parents (cocooning) and pregnant women | (0) No vaccination; (1) Adult vaccination; (2) Mother antepartum and (3) postpartum vaccination; (4)Both parents antepartum and (5) postpartum | CEA | Healthcare provider |
Dynamic | 20 years | US$, 2013 | QALY | C and B – 3% |
| Sartori et al./ Brazil39 | 2016 | Pregnant women | (0) No vaccination; (2) Pregnant women | CEA | Healthcare system and societal |
Decision tree | 1 year | US$, 2011 | Cases and deaths prevented and LYG | Not considered |
| Hoek et al./ England40 | 2016 | Pregnant women | (1) No vaccination; (2) Pregnant women | CEA | Healthcare payer |
Dynamic | 5, 10, 30 and 200 years | UK£ (reference year not reported) | QALY | C -1.5%, B - 3.5% |
C: Costs; B: Benefits; CEA: cost-effectiveness analyses; CBA: cost-benefit analyses; CUA: cost-utility analyses; LYG: life year gained; QALY: quality-adjusted life year; LYG: Life of years gained; NR: Not Reported
The studies evaluated 7 different strategies involving Tdap vaccine: 1) adolescents vaccination; 2) adults vaccination; 3) healthcare workers vaccination and its impact on hospital outbreak control; 4) cocooning; 5) pregnant women vaccination; 6) postpartum maternal vaccination; and 7) adults with chronic obstructive pulmonary disease.
Adolescents and adults immunization were the most frequent strategies evaluated: 11/27 each. The adults vaccination strategies were: one-time vaccination at 20 to 64 years of age (8/11), decennial boosters (6/11), and vaccination at 65 years of age or older (2/11). Six studies evaluating adolescents’ immunization were published from 2002 to 2005.
The economic evaluations of Tdap considered different strategies as cocooning: vaccinating both parents immediately after birth; or assuming that fathers would be vaccinated during the pregnancy and mothers would be vaccinated immediately after delivery; or vaccinating mothers and another adult caregiver after birth; or vaccinating mothers immediately after birth and all other close contacts before the birth; or vaccinating both parents and other close contacts during pregnancy or immediately after delivery.
The first study evaluating pregnant women immunization was published in 2010. This strategy and cocooning were the most frequent strategies evaluated since then (7 studies each), followed by adult immunization (6), adolescents (4), postpartum maternal vaccination (3), and health professionals (1).
Ten studies used dynamic models, which were more frequently used to evaluate adolescents vaccination (6), followed by adults vaccination (4), cocooning (2), pregnant women (2), and health professionals vaccination (1). Thirteen studies used static models: seven used Markov, and six used decision tree. Most of them evaluated cocooning, and/or adults vaccination (6 each), followed by pregnant women vaccination (4), adolescents (3), postpartum maternal vaccination (2), and health professionals (1). Three papers did not report the model used and one used cohort simulation.
Vaccines and vaccination programs assumptions
Vaccines and vaccination programs data are presented in Table 2.
Table 2.
Characteristics of vaccines and vaccination programs used in the economic evaluations of pertussis vaccination for adolescents and adults.
| Study | Vaccine coverage | Vaccine efficacy /effectiveness | Adverse events following immunization | Duration of protection / Waning Immunity | Herd protection |
|---|---|---|---|---|---|
| Edmunds et al.14 | 84% | 95% | NC | 5 years | Yes |
| Scuffham e McIntyre15 | 95% (adults) | Adults 75%; At birth 67%; 1-month 75% | NC | NC | No |
| Purdy et al.16 | 40% (adolescents and adults) | 88% | 1% | 10 years | No |
| Iskedjian et al.17 | 95% | 85% | NC | NC | No |
| Caro et al.18 | 80% | 85% | 2% | 10 years | Yes |
| Iskedjian et al.19 | 85% | 85% | NC | NC | Not |
| Lee et al.20 | Coverage by age: 10 years 76%; 20 years 36%; 30 years 34%; 40 years 29%; 50 years 21%; 60 years 14%; 70 years 5%; postpartum 66% | 100% | Local reaction 2%; Systemic reaction 1%; Anaphylaxis 0.0001% | 15 years | Yes |
| Calugar et al.21 | 66% | 71.4% | Anaphylaxis 0.0001% | 10 years | No |
| Lee et al.22 | 20–49 years of age: 66%; 50–64 years of age: 57% | 87% | Local reactions: 2%; Systemic reactions: 1%; Anaphylaxis: 0,0001% |
15 years | Yes |
| Lee et al.23 | Coverage by age: 20 years 82%; 30 years 58%; 40 years 40%; 50 years 75%; 60 years 62% | 87% | Local reactions 2%. Systemic reactions 1%. Anaphylaxis 0,0001% |
15 years | Yes |
| Coudeville et al.24 | Adolescents 75%; adults 40%; cocooning 65% | 92% | Additional medical consultations for AEFI (2%) in vaccination cost | 12 years | Yes |
| Westra et al.25 | 96% | 89% | NC | 4 months (persistence of maternal antibodies in infants) | No |
| de Vries et al.26 | 96% | 89% | NC | Two scenarios: 8 and 15 years | Yes |
| Greer and Fisman27 | 25 to 95% | 100% | Anaphylaxis 0.00001% | NC | No |
| Rozenbaum et al.28 | 70% | 89% | NC | 10 years | Yes |
| Itatani et al.29 | 11–12 years of age 70%; >12 years 20% |
85% | Severe (anaphylaxis) 0.0001%; moderate 2% | 10 years | No |
| Meregaglia et al.30 | NR | 89% | NC | NC | No |
| Ding et al.31 | 25 to 60% | 80% | Local reaction 2%; Systemic reaction 1%; Anaphylaxis 0.0001% |
10 years | No |
| Terranella et al.32 | 72% | Adults vaccination: 85%; Efficacy of maternal vaccination on newborn protection 60% | NC | 2 months (persistence of maternal antibodies in infants) | No |
| Lugnér et al.33 | 75% | 89% | NC | 5 years | No |
| McGarry et al.34 | 10% | 89% | Included in the vaccine cost | 8 years | No |
| McGarry et al.35 | 10% (at 65 years of age) | 89% | Included in the vaccine cost | 8 years | Yes |
| Fernández-Canoa et al.36 | 50%, 80% and 100% | Adults vaccination: 85% Efficacy of maternal vaccination on newborn protection 60% |
NC | 2 months (persistence of maternal antibodies in infants) | No |
| Kamiya et al.37 | Coverage by age: 11 years 78%; 16 years 50%; 21 years 64% | 74% | Medically-attended allergic reactions 0.003%; Anaphylaxis 0.00006% |
15% decrease of vaccine effectiveness each year post-vaccination | No |
| Atkins et al.38 | 75% | Adults vaccination: 100%; Maternal vaccination on newborn protection 89% |
US$0.93 added to vaccination cost | 2.7 years | Yes |
| Sartori et al.39 | 57% | 78% | NC | 6 months (duration of maternal antibody protection); 4 months in SA | No |
| Hoek et al.40 | 60% | Infants 91%; Mother 89% |
NC | 3 months (persistence of maternal antibodies); 5 years among adults |
No |
NC – Not Considered; NR – not reported: SA – Sensitivity analyses; AEFI - adverse event following vaccination
Ten studies that evaluated adolescents or adults vaccination considered vaccine coverage >50%, six of them considered >80%. Coverage varied from 20 to 96%, for cocooning, and from 57 to 96%, for pregnant women vaccination.
Ten studies clearly stated they incorporated herd protection in the model. Among them, six evaluated adolescent immunization, seven adult immunization, three cocooning, one pregnant immunization, one postpartum maternal immunization. Seven studies that evaluated adolescents and/or adults strategies did not consider herd protection.
Three studies used Markov model to evaluate adolescent and adult vaccination and considered herd protection applying a reduction factor on pertussis incidence in unvaccinated infants and adults or in the base case analysis. Caro et al. used a cohort simulation to evaluate adolescent vaccination and considered indirect impact on other age groups and on unvaccinated adolescents. Four studies that used dynamic models did not clearly state they incorporated herd protection in the model.
McGarry et al. used a dynamic model age-structured with compartments repeated for each month of age below 1 year and 1-year age groups from 1 to 99 years old. This model made possible evaluate a Tdap vaccination of adults aged 65 years in addition to DTaP vaccination from age 2 months to 4–6 years, and one dose of Tdap once to individuals 11–64 years of age in place of the decennial Td booster.
Studies that evaluated pregnant women immunization made different assumptions regarding efficacy of maternal antibodies in infant protection and duration of protection of maternal antibodies (Table 2). One study considered that 60% of maternal antibodies would pass through placenta. Duration of maternal antibodies protection was assumed as two months, three months, four months and six months. Just one study considered interference of maternal antibodies in the infant response to active pertussis vaccination, assuming a negative impact (10% reduction) in the infant responses to the second and third vaccine doses.
Fourteen studies included adverse events following immunization in the model (Table 2). Just three studies considered vaccine wastage rate in the model, assumed as 15%, 10% and 5%.
Epidemiological estimates
Table 3 shows pertussis incidence estimates used in the studies. Pertussis incidences among adolescent or adults were considered in 20 of 27 studies, and 18 of them used some strategy to correct pertussis underreporting.
Table 3.
Pertussis incidence and underreporting correction factor used in economic evaluations of pertussis vaccination for adolescents and adults.
| Study | Incidence rates by age groups | Source of incidence data | Strategies to account for underreporting | Source of correction factor |
|---|---|---|---|---|
| Edmunds et al.14 | Consultation rates: <3 months: 38.58/100,000; 3 months to 4 years: 107.88/100,000; 5 to 14 years: 49.27/100,000; 15 to 44 years: 5.33/100,000; >45 years: 2.21/100,000 | Royal College of General Practioners Weekly Returns Service (RCGP); Hospital Episode Statistics (HES);Office of National Statistics (ONS) | Used correction factor of 2.5 | Authors’ assumption |
| Scuffham e McIntyre15 | 5.171 notified cases / 100,000 infants per week | Health Outcomes Information Statistical Toolkit of the New South Wales Department Australian Childhood Immunization register | Not considered | Not considered |
| Purdy et al.16 | Adolescents and adults: 450/100,000 person-years; distribution by age: 10–19 years: 41%; 20–29 years: 7%; 30–39 years: 17%; 40–49 years: 28% | Centers for Disease Control and Prevention; Acellular Pertussis Vaccine Trial (APERT) (clinical trial). | Children aged 0–9 years: correction factor of 2 | Authors’ assumption |
| Iskedjian et al.17 | Adolescents aged 12–17 years: 511/100,000; Adults aged 18–21 years: 65/100,000 | Health Canada | Adolescents: correction factor of 9 | Enhanced surveillance with serosurvey |
| Caro et al.18 | 0.2–57/100,000 (age-specific rates used) | Centers for Disease Control and Prevention | Used correction factor of 7.6 | Local study using capture-recapture methods to analyze morbidity data from independent surveillance systems |
| Iskedjian et al.19 | Adolescents (14–17 years) 511/100,000; Adults (18–24 years) 65/100,000 | Health Canada | Used correction factor of 9 | Enhanced surveillance with serosurvey |
| Lee et al.20 | Infants 58.5/100,000; Adolescents 155/100,000; Adults 11/100,000 | Massachusetts Department of Public Health (pertussis surveillance data) | Not considered | Not considered |
| Calugar et al.21 | Proportion of infections in healthcare workers: 6.75% | Two local studies | Not considered | Not considered |
| Lee et al.22 | Incidence in adults ranged from 10 to 500/100,000; Infants 58.5/100,000 |
Infants: 2 local studies; Adults: Massachusetts Department of Public Health (official data) | Range of incidences | Tdap efficacy study; 3 studies of pertussis prevalence among persons with cough |
| Lee et al.23 | Adults 165/100,000; Adolescents 95/100,000; Infants 22/100,000. | Adults: Local study (17160764); Adolescents and infants: governmental epidemiological data | Adults’ Incidence varied from 50 to 500 / 100,000 in sensitivity analysis | Studies in Europe and USA |
| Coudeville et al.24 | Adult cases requiring medical care 90/ 100,000 | Acellular Pertussis Vaccine Trial (APERT) and Centers for Disease Control and Prevention (CDC) | Children data were adjusted using age-specific underreporting estimates (data not shown) | Capture-recapture study |
| Westra et al.25 | Incidence in infants <1 year of age: 129/100,000. Distribution of cases among infants <1 year: 0 months: 7.0%; 1 month: 21.4%; 2 months: 18.1%; 3 months: 11.2%; 4 months: 5.3%; 5 months: 2.7%; 6 months: 7.8%; 7 months: 4.2%; 8 months: 8.0%; 9 months: 5.5%; 10 months: 5.0%; 11 months: 3.8%. Incidence in adults 25–34 years of age: 17.9/100,000 |
Centre for Infectious Disease Control of the Dutch National; Institute for Public Health and the Environment | Adults: correction factor of 200 Children: no correction |
Serological survey and dynamic transmission model study |
| de Vries et al.26 | Age specific (data not shown) | RIVM report – Rijksinstituut voor Volksgezondheid en Milieu | Age specific correction factor of (up to 660) | Serological survey |
| Greer and Fisman27 | Average number of exposures/case: 8.73 Symptomatic adults: 40% | Data from a real outbreak | Not considered | Not considered |
| Rozenbaum et al.28 | <1 year: 200/100,000 5 years: 100/100,000 ≥15 years: 50/100,000 |
Surveillance data from 1996 to 2001 | Used correction factor of 600 | Serological survey |
| Itatani et al.29 | Incidence rates ranged from 25 to 250/100,000 person-years | Japan’s Infectious Disease Surveillance Centre | Range of incidences | Previous studies from USA, Germany and Canada |
| Meregaglia et al.30 | Infants: 54/100,000 hospitalizations/year | Regional hospital discharge database | Not considered | Not considered |
| Ding et al.31 | Mothers – 450/100,000. Infants aged <6 months – 71.6/100,000 |
Mothers: local study. Infants: Surveillance data (California Department of Public Health and Centers for Disease Control and Prevention (CDC) |
Not considered | Not considered |
| Terranella et al.32 | <1 year: 62.6/100,000; Incidence by month of age (/100,000) < 1: 12.4; 1: 18.9; 2: 15.3; 3: 8.9; 4: 5.7; 5: 3.2; 6: 2.4; 7: 1.6; 8: 1.5; 9: 1.4; 10: 1.1; 11: 1.4 | National Notifiable Diseases Surveillance System (NNDSS), 2000 – 2007. | Increase of 15% | Authors’ assumption |
| Lugnér et al.33 | <5 years-old: 130/100,000; 20 – 40 years-old women: 2,606/100,000 |
Statistics Netherlands | 100 x the surveillance data | Serological surveys |
| McGarry et al.35 | Different incidence rates were considered: 25, 50, 100, 150, and 200/100,000 | Centers for Disease Control and Prevention, California Department of Public Health Pertussis Report 2011, Washington State Department of Health (2012) | Different incidence rates were used | Tdap efficacy study; 3 studies of pertussis prevalence among persons with cough |
| McGarry et al.35 | Incidence rate by age (/100,000) <1 year: 435.00; 1–6 years: 61.8; 7–9 years: 67.3; 10–18 years: 49.0; 19–64 years: 124.15; ≥65 years: 86.08 |
California Department of Public Health surveillance data; Centers for Disease Control and Prevention (CDC). | For adults ≥65 years, data was inflated, assuming 1% reporting |
2009 Centers for Disease Control and Prevention (CDC) |
| Fernández-Canoa et al.36 | Hospitalization by age-group 0–2 months – 119/100,000; 3–4 months – 26/100,000 ; 5–6 months – 5/100,000; 7–11 months – 4/100,000; <1 year – 153/100,000 |
Hospitalization data of Spanish Government (MBDS), from 2009 to 2011. | Not considered | Not considered |
| Kamiya et al.37 | Age-specific; 11–30 years (data not shown) | National Notifiable Diseases Surveillance System – NNDSS 2002–2011 | Used correction factor of 20–200 in sensitivity analysis | Several studies and authors’ assumptions |
| Atkins et al.38 | The model was calibrated to USA incidence data from 2003–2012 (data not shown) | Centers for Disease Control and Prevention (CDC) | The authors combined data of reported case, hospitalization rates, reporting rates for hospitalized cases, and active surveillance of non-hospitalized cases (data not shown) | CDC, Wisconsin Department of Health Services, and local study |
| Sartori et al.39 | Children aged <1 year-old: 55.407/100,000 | National Notifiable Diseases Information System (Sistema de Informação de Agravos de Notificação, SINAN) | Not considered | Not considered |
| Hoek et al.40 | Infants aged <3 months: ~ 0.5 to ~ 45/100,000; Women aged 20 – 44 years: 0 to ~ 40/100,000 | Number of hospitalization from 2010 to 2012 (NHS) | Not considered | Not considered |
Ten studies used official incidence data multiplied by a correction factor, which varied from 2.5 to 660; four studies considered a range of incidences for adults or adolescents; and four studies derived pertussis incidence from local studies data. The approaches for estimating pertussis incidences among adolescents and adults and the correction factor were based on serological surveys (8 studies), clinical trials (6), authors’ assumption (2), capture-recapture studies (1), enhanced surveillance (1), and compilation of data from previous dTpa economic studies (1). One study applied the infants’ disease incidence to women of childbearing age. Lee et al. (2005) estimated the burden of disease among adolescents and adults in the USA based on 2003 Massachusetts State incidence data. Massachusetts was the only state in the USA that had a single-serum enzyme-linked immunosorbent assay for IgG anti-pertussis toxin available as a diagnostic test, which allows enhanced disease detection among adolescents and adults. Two studies corrected disease data for infants to take underreporting into account, using an underreporting factor of 2 and 1.15.
Pertussis incidence rates varied from 22 to 435 per 100,000, for infants, from 10 to 511 per 100,000, for adolescents, and from 5.33 to 2,606 per 100,000, for adults.
Supplementary Table 1 shows outpatient cases, hospitalizations, complications and case-fatality rates estimates. Even after correcting underreporting, most studies considered that all pertussis cases among adolescents and adults use health care services, resulting in cost. Among adolescents and adults, mild outpatient cases varied from 1% to 79.3%, while severe cases ranged from <1% to 66%. Caro et al. and McGarry et al. assumed that 70% of unreported cases would be significantly milder than typical cases. Coudeville et al. considered 2% of infected adults would be asymptomatic and calibrated the model for their potential infectiousness. Three studies explored the impact of including asymptomatic infections in the disease transmission dynamic model.
Cost estimates
The elements of costs considered in the reviewed studies are described in Supplementary Table 2. All studies included direct medical costs and the vaccination program costs, and 23 included indirect costs. Calugar et al. evaluated the healthcare workers vaccination from the hospital perspective and included productivity loss as indirect cost. Atkins et al. included indirect costs in the sensitivity analysis. All the studies used local data to estimate direct medical costs, except Itatani et al., who assumed the values.
Eight studies considered public health response as part of the direct medical costs. The studies considered costs of health surveillance, contact tracing and prophylactic measures.
Seven studies that evaluated strategies focused on protecting infants (pregnant women vaccination, maternal postpartum vaccination or cocooning) considered caregivers loss of productivity.
Results of the analyses
Table 4 shows the summary measures presented in the results of the analyses.
Table 4.
Summary measures (Incremental Cost-Effectiveness Ratio, ICER, or Cost-Benefit ratio) presented in results of economic evaluations of pertussis vaccination for adolescents and adults, according to the perspective.
| Study | Societal* | Health care provider* | Sponsor |
|---|---|---|---|
| Edmunds et al.14 | 9,278.21/LYG | 18,047.45/LYG | Medical Research Council |
| Scuffham e McIntyre15 | Not considered | 1,562,146.18/DALY | Commonwealth Department of Health and Ageing |
| Purdy et AL.16 | Cost preventable (billions of US$)/ break-even (US$) Adolescent vaccination: US$0.4 to 2.1 billons/ US$49.12 |
Not considered | GlaxoSmithKline |
| Iskedjian et al.17 | Cost-saving | 274.77/case prevented | Sanofi-Pasteur |
| Caro et al.18 | 6,322.19/LYG | 29,310.66/ LYG | NR |
| Iskedjian et al.19 | 374.13/ case prevented | 476.35/case prevented | Sanofi-Pasteur |
| Lee et al.20 | Adolescents vaccination: 25,244.96/QALY; | Adolescents vaccination: 29,031.70/QALY; | National Immunization Program, Centers for Disease Control and Prevention, Association of Teachers of Preventive Medicine, National Vaccine Program Office Agency for Healthcare Research and Quality |
| Calugar et al.21 | Not considered | Cost-benefit ratio: 3 | Centers for Disease Control and Prevention and St. Luke’s Hospital |
| Lee et al.22 | Adult vaccination: 13,539.39/ QALY; 10-year boosters: 14,770.25/QALY | Not considered | Agency for Healthcare Research and Quality, National Immunization Program, Centers for Disease Control and Prevention Association of Teachers of Preventive Medicine |
| Lee et al.23 | Adult vaccination: 8,796.50/QALY. 10-year boosters: 10,919.79 /QALY | Adult vaccination: 31,919.79 /QALY. 10-year boosters:40,949.25/QALY | Agency for Healthcare Research and Quality, US Department of Health and Human Services |
| Coudeville et al.24 | Cost-saving | Not considered | Sanofi-Pasteur |
| Westra et al.25 | Cost-saving | Cocooning: 6,234.84/QALY; Pregnant women: 4,743.90/QALY | GlaxoSmithKline |
| de Vries et al.26 | 5,988.16/QALY and 8,635.27/QALY (for duration of protection after vaccination of 8 and 15 years, respectively) | Not considered | GlaxoSmithKline |
| Greer and Fisman27 | Cost-saving | Not considered | Ontario Early Researcher Award Sanofi-Pasteur |
| Rozenbaum et al.28 | Single (3rd) booster for adolescents or adults: 7,292.58/QALY; Adolescent + adult vaccination: 13,022.47/QALY. 10-year booster: 21,971.53 /QALY | Not considered | NR |
| Itatani et al.29 | Adolescents’ vaccination: 36.24/QALY; Adolescents + 10-year boosters: dominated; Adolescents + cocooning: 2,432.54/QALY | Adolescents vaccination: 51.21/QALY; Adolescents + 10-year boosters: dominated; Adolescents + cocooning: 2,496.61/QALY | NR |
| Meregaglia et al.30 | Not considered | 32%: 246,490.46/case prevented | NR |
| Ding et al.31 | Expected Net of Benefit US$61.25/vaccinated mother | Expected Net of Benefit US$37.25/ vaccinated mother | Centers for Disease Control and Prevention, U.S. Dept. of Health and Human Services |
| Terranella et al.32 | Pregnant women: 439,708.46/QALY. Cocooning: 2,127,816.28/QALY | Not considered | NR |
| Lugnér et al.33 | Cocooning: 120,828.15/QALY; Pregnant women: 171,060.07/QALY. | Not considered | National Institute for PublicHealth and the Environment, Bilthoven, Netherlands |
| McGarry et al.34 | ICER per disease incidence (/100.000): 25: 369,229.63/QALY; 100: 68,896.32/QALY; 200: 18,675.26/QALY. | “similar results” | GlaxoSmithKline |
| McGarry et al.35 | Cost-saving | Cost-saving | GlaxoSmithKline |
| Fernández-Canoa et al.36 | Not considered | Benefit-to-cost ratio: Cocooning: 0.4; Pregnant women: 0.15. | NR |
| Kamiya et al.37 |
Adolescents vaccination: 21,672,785.63/QALY Adult vaccination: 28,752,816.66/QALY |
Not considered | Centers for Disease Control and Prevention |
| Atkins et al.38 | Not considered | Pregnant women: 116,902.95/QALY; Both parents: 835,056,23/QALY (antepartum) and dominated (postpartum); Adult vaccination: dominated | Notsew Orm Sands Foundation (Houston, Texas) and Sanofi-Pasteur |
| Sartori et al.39 | 17,217.25/LYG | 17,237.13/LYG | Brazilian Ministry of Health/Pan American Health Organization |
| Hoek et al.40 | Not considered | 60,619.60/QALY | National Institute for Health Research Health Protection Research Unit |
*Summary measures were adjusted to 2016 values and then converted to international dollar units using Purchasing Power Parity (PPP).
NR: Not reported; LYG: Life years gained; QALY: Quality adjusted life years; DALY: Disability adjusted life year
Two studies showed that adolescents’ vaccination strategy was cost-saving at society perspective. Other seven studies had incremental cost-effectiveness ratio considered cost-effective or highly cost-effective and recommended it as a good strategy. Adolescent vaccination presented unsatisfactory results in only one study.
Among 11 studies that evaluated adults vaccination strategy, the program was considered cost-effective in six and cost-saving in two.
Cocooning strategy performance diverged among studies. It was cost-saving in two studies, and not cost-effective in the other studies, with ICER ranging from U$112,091/QALY to U$2,005,940/QALY.
Seven studies evaluated pregnant women vaccination and the ICER varied from cost-saving to not cost-effective (US$ 439,708.46/QALY). When compared with cocooning, pregnant women vaccination had better economic performance in four of five studies.
Ten studies declared sponsorship by pharmaceutical industry; eleven by public institutions and six did not report sponsorship. All studies sponsored by pharmaceutical industry showed good results for Tdap vaccination, except two that evaluated cocooning, pregnant women and elderly vaccination. All cost-saving studies were in this group.
All studies conducted some Sensitivity Analyses (Supplementary Table 3). The parameters with the greatest impact on the results were pertussis incidence, followed by vaccine efficacy and vaccine price.
Discussion
The first Tdap economic evaluation was published in 2002, when a significant increase in pertussis incidence among unvaccinated infants, adolescents and adults became a problem in developed countries and new immunization strategies for older age groups became available.14,41 Adolescents and adults vaccination were the first strategies introduced in developed countries, such as Australia, Canada, France, Germany and the USA,2 and also the first economically evaluated.
In general, the studies found favorable cost-effectiveness ratio for adolescents and adults vaccination, particularly for adolescents’ vaccination. Assumptions regarding underreporting correction, herd protection and vaccine coverage were crucial to cost-effectiveness results of adolescents and adults vaccination.
In general, pertussis is considered a childhood disease and goes unnoticed among adolescents and adults. Adolescents and adults usually have milder symptoms, similar to viral infections, making pertussis diagnosis difficult.42,43 Mostly, only culture-positive cases or cases with typical symptoms are reported. Underreporting is an issue since asymptomatic infections are transmissible.5,43 Most studies on the cost-effectiveness of adolescent and adults vaccination explicitly took underreporting into account, increasing the incidence detected by regular health surveillance from 2.5 to 600 times. Serological surveillance studies, capture-recapture studies, enhanced surveillance data and author assumption were the source for correction factor. Increasing the incidence has a positive impact on the performance of the strategies evaluated.17,19,20,26,28
Some studies considered that all pertussis cases used health services resulting in direct costs. Assuming that undiagnosed or unreported cases are just as severe and costly as reported cases probably overestimates pertussis-related health resource utilization and costs. Few studies considered asymptomatic cases and recognized their importance in the transmission of the disease.24,26,28,34,35
Eleven studies that evaluated adolescents or adults vaccination considered herd protection.14,17,19,20,22,24,26,28,29,35,38 Eight of them used dynamic models and three studies used static models and included herd protection as a correction factor. Herd protection refers to protection of susceptible individuals due to decreased transmission of the pathogen, i.e., reduction in the force of infection, when a high proportion of the population is immunized. Dynamic models allow projecting changes in transmission patterns, taking herd protection into account. Adolescents and adults are the main source of pertussis infection for infants.2,4,44-48 Considering herd protection for adolescents and adults vaccination would result in averted cases among infants. However, recent studies showed the lack of sterilizing mucosal immunity following aP vaccination.49 The vaccinated could be colonized by Bordetella pertussis and transmit the disease, lacking herd protection of adolescents and adults vaccination.49
Some economic evaluations of adolescents and adults vaccination overestimated vaccine coverage, reaching 96%, which contributed to the good performance of the program. Vaccination coverage among adolescents and adults is low for many vaccines in most countries. In the USA, Tdap vaccine coverage among adults aged 19–64 years was 24.7%, in 2014–2015.50 According to the Vaccine European New Integrated Collaboration Effort consortium, adult vaccination coverage for tetanus and diphtheria ranged from 61% to 74%, in 2010–2011.51 In Brazil, dT coverage among adults is approximately 33% per year (Immunization Division, São Paulo State) and Tdap coverage among pregnant women was 40.3%, in 2015.
The primary objective of the cocooning and pregnant women vaccination is to reduce transmission to infants. The first economic study of cocooning was published in 2004.15 Cocooning was introduced in developed countries, such as Australia, France, Germany and the USA, in the early 2000s.2 This review showed that cocooning performance diverges among studies. The economic evaluations with higher effectiveness for cocooning,23,33 even to the point of cost-saving,24 assumed that the mother was the only source of pertussis for the infants, overestimating the impact of postpartum maternal vaccination.
Cocooning effectiveness/impact also diverged among different studies, and there is evidence that the strategy is inefficient to reduce hospitalizations and deaths among infants in settings with low pertussis incidence. In Canada, it would be necessary to vaccinate more than 10,000 people to prevent one hospitalization, and vaccinate at least 1 million to prevent one death of infant <1 year of age, in a setting with 57 hospitalizations per 100,000 inhabitants and risk of parents-to-infant transmission of 35%.52 In the USA, a study of a postpartum vaccination program did not show any beneficial effect.53
After a frustrating performance of the previous strategies, pregnant women vaccination was introduced in USA, in 2011, and UK, in 2012. The first economic evaluation was published in 2010, when many countries reported further increase of pertussis incidence in infants.3,54,55 Pregnant women vaccination was demonstrated efficacious, had good economic performance and became the main strategy of adults’ pertussis vaccination to protect infants.
The overall impact and cost-effectiveness of cocooning are likely to be substantially lower than pregnant women vaccination, which requires only one dose, whereas cocooning requires, as a minimum, multiple doses for parents and family members. Implementing an effective cocooning strategy with high coverage has also proved challenging in several countries.2
Pertussis incidence was one of the parameters that mostly influenced the results of pregnant women vaccination programs. Westra et al. (2010)25 reported that ICERs increased 6x and 3x for cocooning and pregnant women vaccination, respectively, when unreported cases were not taken into account in the analysis. Van Hoek et al.40 show that pregnant women pertussis immunization would be highly cost effective if the peak incidence of infant disease at the time the program was introduced continues (ICER ~ 17,000 during incidence peak). However, the ICER was highly dependent on the future incidence of pertussis in infants under 3 months of age and it will vary over time considering the cyclical pattern of the disease.40
The number of vaccinees in cocooning and pregnant women vaccination does not allow the development of herd protection and static models are adequate to evaluate these strategies.25,39
Only eight studies included the public health response in the direct costs.17-19,21,27,32,36,39 In one study in the USA, the epidemiological investigation of household contacts, laboratory testing the symptomatic contacts and antibiotic treatment for contacts positive for B. pertussis cost US$2,269/case, being an important component of costs.32 Another study, in Brazil, estimated that surveillance costs per case were higher than the outpatient care costs per case.39 Many countries have long-standing surveillance systems for pertussis.56 The case reporting results in a public health response, including cases interviews, contacts testing (PCR or culture), identification of symptomatic contacts, and treatment of symptomatic contacts or chemoprophylaxis for all contacts. The U.S. Centers for Disease Control and Prevention recommends post exposure prophylaxis for all household contacts of a pertussis case.57 In Brazil, the MoH recommends nasopharyngeal swab for diagnostic tests for all domiciliary contacts of pertussis cases.58 The contact tracing results in costs that should be considered in economic evaluation of pertussis vaccination programs.
Just one study referred to a developing country.54 Pregnant women vaccination was shown a cost-effective intervention for preventing pertussis cases and deaths in infants in Brazil. Brazil, Argentina and Chile reported significant increase in pertussis incidence rates in recent years, despite pertussis childhood vaccination with whole-cell vaccines and have already introduced pregnant women vaccination with Tdap.3,54
This systematic review and synthesis of the results of the articles included in the analysis of economic evaluations of pertussis vaccination strategies in adults presented more challenges than usual in this type of study due the large number of different strategies, and methodological differences of the studies. The strategies performance and economic evaluation conclusions varied considerably among different studies. Variations were due to different assumptions on epidemiological parameters, health service utilization and costs made in the studies from different countries. Understanding the model and all the parameters used in the economic analysis is essential to understand the results, and identify the major issues important to public health decisions.
Methods
Protocol and registration
This systematic review has been conducted based on the Centre for Reviews and Dissemination (CRD) guidelines12. A protocol was developed before initiating this review but it was not registered in the international prospective register of systematic reviews (PROSPERO).
Literature search
A search of studies published from January 1st, 2000 to July 15th, 2016 was conducted in four databases: MEDLINE (via PubMed), Excerpta Medica, CRD and Latin-American and Caribbean Health Sciences Literature (LILACS). It was deemed appropriate to narrow the search to this timeframe because Tdap was licensed in 2005.The following terms were used: ‘pertussis’ and ‘pertussis vaccine’ in combination with any of the following: ‘economics’, ‘pharmaceutical’, ‘cost analysis’, ‘cost of illness’, ‘cost(-) benefit’, ‘health care cost”, ‘cost(-) effectiveness’, ‘cost(-)utility’, ‘cost’ and/or (pharmaco) economic evaluation. The search was limited to full economic evaluations on pertussis vaccination of adolescents (≥10 years of age) and adults. The Appendix 1 shows the electronic search strategies created for each database.
Searching other sources
The reference lists of all included studies identified in the electronic databases were reviewed to identify further studies.
Eligibility criteria
The eligibility criteria were defined based on the components of the PICOS approach:
Population: adolescents (≥10 years of age) and adults (including healthcare workers, pregnant women, cocooning and any other vaccination strategies targeting adolescents or adults);
Intervention: pertussis vaccination;
Comparators: no vaccination and strategies of pertussis vaccination of adolescents and adults;
Outcome: incremental cost-effectiveness ratio (ICER) or cost-benefit ratio;
Study design: full economic evaluation, defined as a comparative analysis of costs and consequences of two alternative healthcare interventions; including cost-minimization analysis, cost-effectiveness analysis, cost-utility analysis, and cost-benefit analysis.
Study selection
One reviewer (EGF) screened all titles and abstracts of studies retrieved by the search and selected them using the eligibility criteria. Any doubts during this process were resolved by discussion with another reviewer (PCS).
Data extraction
A predefined data extraction form was calibrated amongst the two reviewers (EGF and CCMR) using a random sample of five included studies. After this, data was independently extracted by the two reviewers (EGF and CCMR) and checked by them. The divergences between the data that the reviewers extracted were resolved by discussion or by arbitrage of a third reviewer (PCS).
Data collected
Methodological characteristics: type of study, perspective, model, herd protection, time horizon, number of cohorts, currency and year of costs, discount rate, sensitivity analysis, and parameters varied in the sensitivity analysis;
Estimates of key parameters: epidemiological data (pertussis incidence, disease severity, and case fatality rate); vaccine related data (vaccination schedule, coverage, efficacy, adverse events, and waning immunity rate); costs (direct and indirect), and summary measures (incremental cost-effectiveness ratios (ICERs) or cost-benefit ratio);
Research funding sources.
To improve comparability between studies results, all summary measures presented in different currencies were adjusted to 2016 value (latest price year used in included studies) using consumer price index [59]. Afterwards, they were converted to international dollar units using Purchasing Power Parity (PPP), the exchange-rate equivalent to an identical basket of goods and services in countries (Organization for Economic Co-operation and Development.60
Synthesis of results
The more relevant results were summarized as a narrative synthesis. The methodological characteristics and key variables estimates are shown in summary tables.
Appendix 1. Search strategy per database
| Database | Search strategy |
|---|---|
| MEDLINE | ((((((((((((((((((((((((((Economics[MeSH Terms]) OR Economics, Pharmaceutical[MeSH Terms]) OR ((cost and cost analysis[MeSH Terms]))) OR cost of illness[MeSH Terms]) OR cost benefit analyses[MeSH Terms]) OR health care cost[MeSH Terms]) OR analyses, cost benefit[MeSH Terms])) OR ““analysis cost-benefit”“) OR ““cost benefit analysis”“) OR ““analyses, cost benefit”“) OR ““analysis, cost benefit”“) OR ““cost benefit analyses”“) OR ““cost effectiveness”“) OR ““effectiveness, cost”“) OR ““cost-utility analysis”“) OR ““analysis, cost-utility”“) OR ““cost utility analysis”“) OR ““economic, evaluation”“) OR ““evaluation economic”“) OR ““cost benefit”“) OR ((““cost and benefit”“))) OR ((““benefit and cost”“))) OR ““cost effectiveness-analysis”“) OR ““analysis, cost-effectiveness”“) OR ““cost effectiveness analysis”“)) AND (“pertussis” OR pertussis[MeSH Terms] OR pertussis vaccine[MeSH Terms] OR “pertussis vaccine” OR “Diphtheria-Tetanus-acellular Pertussis Vaccines” OR ““Diphtheria-Tetanus-acellular Pertussis Vaccines”“[MeSH Terms]) |
| EMBASE | (‘diphtheria pertussis tetanus vaccine’ OR ‘pertussis’ OR ‘pertussis vaccination’ OR ‘pertussis vaccine’) AND (‘biomedical technology assessment’/exp OR ‘cost utility analysis’/exp OR ‘cost of illness’/exp OR ‘cost minimization analysis’/exp OR ‘pharmacoeconomics’/exp OR ‘cost benefit analysis’/exp) |
| CDR | (PERTUSSIS) OR (PERTUSSIS VACCINE) OR (Diphtheria-Tetanus-acellular Pertussis Vaccines) IN DARE, NHSEED, HTA |
| Lilacs | ((tw:(Pertussis Vaccine)) OR (tw:(Diphtheria-Tetanus-Pertussis Vaccine)) OR (tw:(Whooping Cough)) OR (tw:(Diphtheria-Tetanus-acellular Pertussis Vaccines)) AND (tw:(Pharmaceutical Economics)) OR (tw:(Pharmacoeconomics)) OR (tw:(Cost-Benefit Analysis)) OR (tw:(Cost-Effectiveness Evaluation)) OR (tw:(cost effectiveness))) |
Funding Statement
No funding was secured for this study.
Footnotes
PCS, HMDN and AMCS are researchers from the IATS, National Institute of Science and Technology for Health Technology Assessment (IATS) – CNPq/Brazil.
Disclosure of potential conflicts of interest
The authors report no conflict of interest.
Supplementary Material
Supplemental data for this article can be accessed here.
References
- 1.Mattoo S, Cherry JD.. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin Microbiol Rev. 2005;18(2):326–382. PubMed PMID: 15831828; PubMed Central PMCID: PMC1082800. doi: 10.1128/CMR.18.2.326-382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pertussis vaccines: WHO position paper - September 2015. Wkly Epidemiol Rec. 2015;90(35):433–458. PubMed PMID: 26320265. WHO. [PubMed] [Google Scholar]
- 3.Falleiros Arlant LH, de Colsa A, Flores D, Brea J, Avila Aguero ML, Hozbor DF. Pertussis in Latin America: epidemiology and control strategies. Expert Rev Anti Infect Ther. 2014;12(10):1265–1275. PubMed PMID: 25139010. doi: 10.1586/14787210.2014.948846. [DOI] [PubMed] [Google Scholar]
- 4.Bisgard KM, Pascual FB, Ehresmann KR, Miller CA, Cianfrini C, Jennings CE, Rebmann CA, Gabel J, Schauer SL, Lett SM. Infant pertussis: who was the source? Pediatr Infect Dis J. 2004;23(11):985–989. Epub 2004/ 11/17 PubMed PMID: 15545851. [DOI] [PubMed] [Google Scholar]
- 5.Crowcroft NS, Pebody RG. Recent developments in pertussis. Lancet. 2006;367(9526):1926–1936. Epub 2006/ 06/13 PubMed PMID: 16765762. doi: 10.1016/S0140-6736(06)68848-X. [DOI] [PubMed] [Google Scholar]
- 6.Heininger U, Klich K, Stehr K, Cherry JD. 1997. Clinical findings in Bordetella pertussis infections: results of a prospective multicenter surveillance study. Pediatrics. 100(6):E10 Epub 1998/ 05/19PubMed PMID: 9382911. doi: 10.1542/peds.100.6.e10. [DOI] [PubMed] [Google Scholar]
- 7.Dabrera G, Amirthalingam G, Andrews N, Campbell H, Ribeiro S, Kara E, Fry NK, Ramsay M. A case-control study to estimate the effectiveness of maternal pertussis vaccination in protecting newborn infants in England and Wales, 2012-2013. Clin Infect Dis. 2015;60(3):333–337. PubMed PMID: 25332078. doi: 10.1093/cid/ciu821. [DOI] [PubMed] [Google Scholar]
- 8.Amirthalingam G, Andrews N, Campbell H, Ribeiro S, Kara E, Donegan K, Fry NK, Miller E, Ramsay M. Effectiveness of maternal pertussis vaccination in England: an observational study. Lancet. 2014;384(9953):1521–1528. PubMed PMID: 25037990. doi: 10.1016/S0140-6736(14)60686-3. [DOI] [PubMed] [Google Scholar]
- 9.Hardy-Fairbanks AJ, Pan SJ, Decker MD, Johnson DR, Greenberg DP, Kirkland KB, Talbot EA, Bernstein HH. Immune responses in infants whose mothers received Tdap vaccine during pregnancy. Pediatr Infect Dis J. 2013;32(11):1257–1260. PubMed PMID: 23799518. doi: 10.1097/INF.0b013e3182a09b6a. [DOI] [PubMed] [Google Scholar]
- 10.Ward JI, Cherry JD, Chang SJ, Partridge S, Lee H, Treanor J, Greenberg DP, Keitel W, Barenkamp S, Bernstein DI, et al. Efficacy of an acellular pertussis vaccine among adolescents and adults. N Engl J Med. 2005;353(15):1555–1563. PubMed PMID: 16221778. doi: 10.1056/NEJMoa050824. [DOI] [PubMed] [Google Scholar]
- 11.Rank C, Quinn HE, McIntyre PB. Pertussis vaccine effectiveness after mass immunization of high school students in Australia. Pediatr Infect Dis J. 2009;28(2):152–153. PubMed PMID: 19106780. doi: 10.1097/INF.0b013e318185608e. [DOI] [PubMed] [Google Scholar]
- 12.Centre for Reviews and Dissemination Systematic Reviews: CRD’s guidance for undertaking reviews in health care. [Internet] CRD. [accessed 2018 Ap 11]. https://www.york.ac.uk/media/crd/Systematic_Reviews.pdf.
- 13.de Melker HE, Versteegh FG, Schellekens JF, Teunis PF, Kretzschmar M. The incidence of Bordetella pertussis infections estimated in the population from a combination of serological surveys. J Infect. 2006;53(2):106–113. PubMed PMID: 16352342. doi: 10.1016/j.jinf.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 14.Edmunds WJ, Brisson M, Melegaro A, Gay NJ. The potential cost-effectiveness of acellular pertussis booster vaccination in England and Wales. Vaccine. 2002;20(9–10):1316–1330. PubMed PMID: 11818150 [DOI] [PubMed] [Google Scholar]
- 15.Scuffham PA, McIntyre PB. Pertussis vaccination strategies for neonates–an exploratory cost-effectiveness analysis. Vaccine. 2004;22(21–22):2953–2964. PubMed PMID: 15246632. doi: 10.1016/j.vaccine.2003.11.057. [DOI] [PubMed] [Google Scholar]
- 16.Purdy KW, Hay JW, Botteman MF, Ward JI. Evaluation of strategies for use of acellular pertussis vaccine in adolescents and adults: a cost-benefit analysis. Clin Infect Dis. 2004;39(1):20–28. PubMed PMID: 15206048. doi: 10.1086/421091. [DOI] [PubMed] [Google Scholar]
- 17.Iskedjian M, Walker JH, Hemels ME. Economic evaluation of an extended acellular pertussis vaccine programme for adolescents in Ontario, Canada. Vaccine. 2004;22(31–32):4215–4227. PubMed PMID: 15474711. doi: 10.1016/j.vaccine.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 18.Caro JJ, Getsios D, El-Hadi W, Payne K, O’Brien JA. Pertussis immunization of adolescents in the United States: an economic evaluation. Pediatr Infect Dis J. 2005;24(5 Suppl):S75–82. PubMed PMID: 15876932 [DOI] [PubMed] [Google Scholar]
- 19.Iskedjian M, Walker JH, De Serres G, Einarson TR. Economic evaluation of an extended acellular pertussis vaccine program for adolescents in Quebec, Canada. Paediatr Drugs. 2005;7(2):123–136. PubMed PMID: 15871632 [DOI] [PubMed] [Google Scholar]
- 20.Lee GM, Lebaron C, Murphy TV, Lett S, Schauer S, Ta L. Pertussis in adolescents and adults: should we vaccinate? Pediatrics. 2005;115(6):1675–1684. PubMed PMID: 15930232. doi: 10.1542/peds.2004-2509. [DOI] [PubMed] [Google Scholar]
- 21.Calugar A, Ortega-Sanchez IR, Tiwari T, Oakes L, Jahre JA, Murphy TV. Nosocomial pertussis: costs of an outbreak and benefits of vaccinating health care workers. Clin Infect Dis. 2006;42(7):981–988. PubMed PMID: 16511764. doi: 10.1086/500321. [DOI] [PubMed] [Google Scholar]
- 22.Lee GM, Murphy TV, Lett S, Cortese MM, Kretsinger K, Schauer S, Lieu TA. Cost effectiveness of pertussis vaccination in adults. Am J Prev Med. 2007;32(3):186–193. PubMed PMID: 17296470. doi: 10.1016/j.amepre.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 23.Lee GM, Riffelmann M, Wirsing von Konig CH. Cost-effectiveness of adult pertussis vaccination in Germany. Vaccine. 2008;26(29–30):3673–3679. PubMed PMID: 18538901. doi: 10.1016/j.vaccine.2008.04.068. [DOI] [PubMed] [Google Scholar]
- 24.Coudeville L, Van Rie A, Getsios D, Caro JJ, Crepey P, Nguyen VH. Adult vaccination strategies for the control of pertussis in the United States: an economic evaluation including the dynamic population effects. PloS One. 2009;4(7):e6284 PubMed PMID: 19606227; PubMed Central PMCID: PMC2707617. doi: 10.1371/journal.pone.0006284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Westra TA, de Vries R, Tamminga JJ, Sauboin CJ, Postma MJ. Cost-effectiveness analysis of various pertussis vaccination strategies primarily aimed at protecting infants in the Netherlands. Clin Ther. 2010;32(8):1479–1495. PubMed PMID: 20728761. doi: 10.1016/j.clinthera.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 26.de Vries R, Kretzschmar M, Schellekens JF, Versteegh FG, Westra TA, Roord JJ, Postma MJ, Jefferson T. Cost-effectiveness of adolescent pertussis vaccination for the Netherlands: using an individual-based dynamic model. PloS One. 2010;5(10):e13392 PubMed PMID: 20976213; PubMed Central PMCID: PMC2955521. doi: 10.1371/journal.pone.0013392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greer AL, Fisman DN. Use of models to identify cost-effective interventions: pertussis vaccination for pediatric health care workers. Pediatrics. 2011;128(3):e591–9. PubMed PMID: 21844056. doi: 10.1542/peds.2010-0796. [DOI] [PubMed] [Google Scholar]
- 28.Rozenbaum MH, De Cao E, Postma MJ. Cost-effectiveness of pertussis booster vaccination in the Netherlands. Vaccine. 2012;30(50):7327–7331. PubMed PMID: 22749838. doi: 10.1016/j.vaccine.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 29.Itatani T, Shimizu S, Iwasa M, Ohkusa Y, Hayakawa K. Cost-effectiveness analysis of a pertussis vaccination programme for Japan considering intergenerational infection. Vaccine. 2013;31(27):2891–2897. PubMed PMID: 23570987. doi: 10.1016/j.vaccine.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 30.Meregaglia M, Ferrara L, Melegaro A, Demicheli V. Parent “cocoon” immunization to prevent pertussis-related hospitalization in infants: the case of Piemonte in Italy. Vaccine. 2013;31(8):1135–1137. PubMed PMID: 23306370. doi: 10.1016/j.vaccine.2012.12.061. [DOI] [PubMed] [Google Scholar]
- 31.Ding Y, Yeh SH, Mink CA, Zangwill KM, Allred NJ, Hay JW. Cost-benefit analysis of hospital based postpartum vaccination with combined tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap). Vaccine. 2013;31(22):2558–2564. PubMed PMID: 23583811. doi: 10.1016/j.vaccine.2013.03.053. [DOI] [PubMed] [Google Scholar]
- 32.Terranella A, Asay GR, Messonnier ML, Clark TA, Liang JL. Pregnancy dose Tdap and postpartum cocooning to prevent infant pertussis: a decision analysis. Pediatrics. 2013;131(6):e1748–56. PubMed PMID: 23713104. doi: 10.1542/peds.2012-3144. [DOI] [PubMed] [Google Scholar]
- 33.Lugner AK, van der Maas N, van Boven M, Mooi FR, de Melker HE. Cost-effectiveness of targeted vaccination to protect new-borns against pertussis: comparing neonatal, maternal, and cocooning vaccination strategies. Vaccine. 2013;31(46):5392–5397. PubMed PMID: 24075918. doi: 10.1016/j.vaccine.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 34.McGarry LJ, Krishnarajah G, Hill G, Skornicki M, Pruttivarasin N, Masseria C, Arondekar B, Pelton SI, Weinstein MC, van Baal PHM. Cost-effectiveness analysis of Tdap in the prevention of pertussis in the elderly. PloS One. 2013;8(9):e67260 PubMed PMID: 24019859; PubMed Central PMCID: PMC3760878. doi: 10.1371/journal.pone.0067260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGarry LJ, Krishnarajah G, Hill G, Masseria C, Skornicki M, Pruttivarasin N, Arondekar B, Roiz J, Pelton SI, Weinstein MC, et al. Cost-effectiveness of Tdap vaccination of adults aged 65 years in the prevention of pertussis in the US: a dynamic model of disease transmission. PloS One. 2014;9(1):e72723 PubMed PMID: 24416118; PubMed Central PMCID: PMC3886978. doi: 10.1371/journal.pone.0072723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernandez-Cano MI, Armadans Gil L, Campins Marti M. Cost-benefit of the introduction of new strategies for vaccination against pertussis in Spain: cocooning and pregnant vaccination strategies. Vaccine. 2015;33(19):2213–2220. PubMed PMID: 25825331. doi: 10.1016/j.vaccine.2015.03.045. [DOI] [PubMed] [Google Scholar]
- 37.Kamiya H, Cho BH, Messonnier ML, Clark TA, Liang JL. Impact and cost-effectiveness of a second tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine dose to prevent pertussis in the United States. Vaccine. 2016;34(15):1832–1838. PubMed PMID: 26899377. doi: 10.1016/j.vaccine.2016.02.027. [DOI] [PubMed] [Google Scholar]
- 38.Atkins KE, Fitzpatrick MC, Galvani AP, Townsend JP. Cost-Effectiveness of Pertussis Vaccination During Pregnancy in the United States. Am J Epidemiol. 2016;183(12):1159–1170. PubMed PMID: 27188951; PubMed Central PMCID: PMC4908210. doi: 10.1093/aje/kwv347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sartori AMC, de Soarez PC, Fernandes EG, Gryninger LCF, Viscondi JYK, Novaes HMD. Cost-effectiveness analysis of universal maternal immunization with tetanus-diphtheria-acellular pertussis (Tdap) vaccine in Brazil. Vaccine. 2016;34(13):1531–1539. PubMed PMID: 26899375. doi: 10.1016/j.vaccine.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 40.van Hoek AJ, Campbell H, Amirthalingam G, Andrews N, Miller E. Cost-effectiveness and programmatic benefits of maternal vaccination against pertussis in England. J Infect. 2016;73(1):28–37. PubMed PMID: 27108802. doi: 10.1016/j.jinf.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 41.Witt MA, Katz PH, Witt DJ. Unexpectedly limited durability of immunity following acellular pertussis vaccination in preadolescents in a North American outbreak. Clin Infect Dis. 2012;54(12):1730–1735. PubMed PMID: 22423127. doi: 10.1093/cid/cis287. [DOI] [PubMed] [Google Scholar]
- 42.Klein NP, Bartlett J, Rowhani-Rahbar A, Fireman B, Baxter R. Waning protection after fifth dose of acellular pertussis vaccine in children. N Engl J Med. 2012;367(11):1012–1019. PubMed PMID: 22970945. doi: 10.1056/NEJMoa1200850. [DOI] [PubMed] [Google Scholar]
- 43.Wendelboe AM, Van Rie A, Salmaso S, Englund JA. Duration of immunity against pertussis after natural infection or vaccination. Pediatr Infect Dis J. 2005;24(5 Suppl):S58–61. Epub 2005/05/07 PubMed PMID: 15876927 [DOI] [PubMed] [Google Scholar]
- 44.Castagnini LA, Munoz FM. Clinical characteristics and outcomes of neonatal pertussis: a comparative study. J Pediatr. 2010;156(3):498–500. Epub 2010/ 01/09. PubMed PMID: 20056236. doi: 10.1016/j.jpeds.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 45.Wendelboe AM, Njamkepo E, Bourillon A, Floret DD, Gaudelus J, Gerber M, Grimprel E, Greenberg D, Halperin S, Liese J, et al. Transmission of Bordetella pertussis to young infants. Pediatr Infect Dis J. 2007;26(4):293–299. Epub 2007/ 04/07 PubMed PMID: 17414390. doi: 10.1097/01.inf.0000258699.64164.6d. [DOI] [PubMed] [Google Scholar]
- 46.Baptista PN, Magalhaes V, Rodrigues LC, Rocha MA, Pimentel AM. Source of infection in household transmission of culture-confirmed pertussis in Brazil. Pediatr Infect Dis J. 2005;24(11):1027–1028. Epub 2005/11/12 PubMed PMID: 16282950 [DOI] [PubMed] [Google Scholar]
- 47.Baptista PN, Magalhaes VS, Rodrigues LC. The role of adults in household outbreaks of pertussis. Int J Infect Dis: IJID: Off Publ Int Soc Infect Dis. 2010;14(2):e111–4. PubMed PMID: 19559636. doi: 10.1016/j.ijid.2009.03.026. [DOI] [PubMed] [Google Scholar]
- 48.Berezin EN, de Moraes JC, Leite D, Carvalhanas TR, Yu AL, Blanco RM, Rodrigues M, Almeida FJ, Bricks LF. Sources of pertussis infection in young babies from Sao Paulo State, Brazil. Pediatr Infect Dis J. 2014;33(12):1289–1291. PubMed PMID: 25386966. doi: 10.1097/INF.0000000000000424. [DOI] [PubMed] [Google Scholar]
- 49.Lapidot R, Gill CJ. The Pertussis resurgence: putting together the pieces of the puzzle. Trop Dis, Travel Med Vaccines. 2016;2:26 PubMed PMID: 28883970; PubMed Central PMCID: PMC5530967. doi: 10.1186/s40794-016-0043-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Williams WW, Lu PJ, O’Halloran A, Kim DK, Grohskopf LA, Pilishvili T, Skoff TH, Nelson NP, Harpaz R, Markowitz LE, et al. Surveillance of Vaccination Coverage among Adult Populations - United States, 2015. Morb Mortal Wkly Rep Surveill Summaries. 2017;66(11):1–28. PubMed PMID: 28472027. doi: 10.15585/mmwr.ss6611a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee HJ, Choi JH. Tetanus-diphtheria-acellular pertussis vaccination for adults: an update. Clin Exp Vaccine Res. 2017;6(1):22–30. PubMed PMID: 28168170; PubMed Central PMCID: PMC5292353. doi: 10.7774/cevr.2017.6.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Skowronski DM, Janjua NZ, Tsafack EP, Ouakki M, Hoang L, De Serres G. The number needed to vaccinate to prevent infant pertussis hospitalization and death through parent cocoon immunization. Clin Infect Dis. 2012;54(3):318–327. PubMed PMID: 22156850. doi: 10.1093/cid/cir836. [DOI] [PubMed] [Google Scholar]
- 53.Healy CM, Rench MA, Wootton SH, Castagnini LA. Evaluation of the impact of a pertussis cocooning program on infant pertussis infection. Pediatr Infect Dis J. 2015;34(1):22–26. PubMed PMID: 24992123. doi: 10.1097/INF.0000000000000486. [DOI] [PubMed] [Google Scholar]
- 54.Guimaraes LM, Carneiro EL, Carvalho-Costa FA. Increasing incidence of pertussis in Brazil: a retrospective study using surveillance data. BMC Infect Dis. 2015;15:442 PubMed PMID: 26498058; PubMed Central PMCID: PMC4619034. doi: 10.1186/s12879-015-1222-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gkentzi D, Katsakiori P, Marangos M, Hsia Y, Amirthalingam G, Heath PT, Ladhani S. Maternal vaccination against pertussis: a systematic review of the recent literature. Arch Dis Childhood Fetal Neonatal Ed. 2017;102(5):F456–F63. PubMed PMID: 28468899. doi: 10.1136/archdischild-2016-312341. [DOI] [PubMed] [Google Scholar]
- 56.Guiso N, Wirsing von Konig CH. Surveillance of pertussis: methods and implementation. Expert Rev Anti Infect Ther. 2016;14(7):657–667. PubMed PMID: 27224518. doi: 10.1080/14787210.2016.1190272. [DOI] [PubMed] [Google Scholar]
- 57.Centers for Disease Control and Prevention Pertussis (Whooping cough): postexposure Antimicrobial Prophylaxis 2013 [accessed 2013 December13]. https://www.cdc.gov/pertussis/outbreaks/pep.html.
- 58.Novas Recomendações para Vigilância Epidemiológica da Coqueluche [Internet]. 2014 [accessed 2017 June08]. www.portalarquivos.saude.gov.br/images/pdf/2014/julho/15/Coq-NI-Novas-Recomenda—-es-02-06-2014-FINAL.pdf.
- 59.Inflation Calculator [accessed 2017 September20]. http://fxtop.com/en/inflation-calculator.php.
- 60.Organisation for Economic Co-operation and Development [accessed 2017 September20]. http://www.oecd.org/std/ppp.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


