The ethylene precursor aminocyclopropane-1-carboxylic acid (ACC) positively regulates the symmetric division of stomatal guard mother cells in a manner that is dependent on CDKB1s and CYCA2s.
Keywords: 1-Aminocyclopropane-1-carboxylic acid (ACC), ethylene, guard mother cell, stomata, symmetric division
Abstract
Stomata have a critical function in the exchange of gases and water vapor between plants and their environment. Stomatal development is under the rigorous control of many regulators. The last step of development is the terminal division of guard mother cells (GMC) into two guard cells (GC). It is still unclear how the symmetric division of GMCs is regulated. Here, we show that the ethylene precursor aminocyclopropane-1-carboxylic acid (ACC) is required for the symmetric division of GMCs into GCs in Arabidopsis. Exogenous application of the ACC biosynthesis inhibitor aminoethoxyvinylglycine (AVG) induced the formation of single guard cells (SGCs). Correspondingly, an acs octuple-mutant with extremely low endogenous ACC also developed SGCs, and exogenous ACC dramatically decreased the number of SGCs in this mutant whereas exogenous ethephon (which is gradually converted into ethylene) had no effect. Furthermore, neither blocking of endogenous ethylene synthesis nor disruption of ethylene signaling transduction could induce the production of SGCs. Further investigation indicated that ACC promoted the division of GMCs in fama-1 and flp-1myb88 mutants whereas AVG inhibited it. Moreover, ACC positively regulated the expression of CDKB1;1 and CYCA2;3 in the fama-1 and flp-1myb88 mutants. The SGC number was not affected by ACC or AVG in cdkb1;11;2 and cyca2;234 mutants. Taken together, the results demonstrate that ACC itself, but not ethylene, positively modulates the symmetric division of GMCs in a manner that is dependent on CDKB1s and CYCA2s.
Introduction
Stomata play a key role in controlling gas exchange between plants and the atmosphere. A stoma forms after at least one asymmetric division as well as a single symmetric division. Each asymmetric division produces one smaller cell (meristemoid) and one larger cell. The larger cell further divides unequally to extend the stem cell lineage. Meristemoids acquire a guard mother cell (GMC) fate after several rounds of asymmetric divisions. Stomatal development ends after the terminal division of a GMC, each of whose daughter cells terminally differentiates into a guard cell (Bergmann and Sack, 2007; Pillitteri and Torii, 2012). Guard cells exit the cell cycle at the G2-to-M stage. In Arabidopsis, A-type cyclins and B-type cyclin-dependent kinases function synergistically in the G2-to-M transition of GMCs (Boudolf et al., 2004a, 2004b; Vanneste et al., 2011). Transgenic Arabidopsis plants overexpressing a dominant negative allele of CDKB1;1 (CDKB1;1.N161) have a block in GMC division and form single guard cells (SGCs). SGCs are also found on the epidermis of cdkb1;11;2 and cyca2;234 mutants. Although no phenotype is observed in the cdkb1;1 single-mutant, the quadruple-mutant cdkb1;1cyca2;234 displays more SGCs than the triple-mutant cyca2;234. CDKA;1 activity is also required for GMC division as evidenced by the fact that arrested GMC division is also found in the cdka;1 null mutant (Weimer et al., 2012). Expression of CDKA;1 in the stomatal lineage driven by the TOO MANY MOUTHS (TMM) promoter can partially rescue cdkb1;11;2 stomata defects, suggesting functional redundancy between CDKA;1 and CDKB1s. CYCD3;2 and CDKA;1 under the control of the FAMA (bHLH097) promoter can stimulate extra symmetric GC subdivisions, and their synergistic effect on promoting GC subdivision requires functional RETINOBLASTOMA RELATED (RBR) (Borghi et al., 2010; Yang et al., 2014).
FAMA and FOUR LIPS (FLP)/MYB88 are two classes of regulators for the symmetric division of GMCs. FAMA restricts the GMCs to a single division. Loss-of-function of FAMA causes cell clusters without GC identity. FAMA negatively regulates the expression of CDKB1;1 and CYCA2;3 via direct promoter binding (Hachez et al., 2011; Lee et al., 2014). Loss-of-function of FLP and MYB88 also induces clusters of four or more guard cells (Lai et al, 2005). FLP/MYB88 restricts the symmetric division of GMCs to one event only by directly targeting the core cell cycle genes CDKB1;1, CYCA2;3 and CDKA;1 (Xie et al., 2010; Vanneste et al., 2011;Yang et al., 2014). RBR is a negative regulator of cell cycle gene expression and cell proliferation (Inzé and De Veylder, 2006; Lee et al., 2014). Both FAMA and FLP/MYB88 interact with RBR to form a complex to bind the upstream regulatory sequences of CDKB1;1 (Lee et al., 2014; Matos et al., 2014).
Phytohormones play important roles in the growth and development of plants, and several regulate stomatal development, including brassinosteroids (BRs), abscisic acid (ABA), auxin, gibberellins (GAs), and jasmonates (JAs), as well as ethylene (Kieber et al., 1993; Saibo et al., 2003; Gudesblat et al., 2012; Kim et al., 2012; Khan et al., 2013; Tanaka et al., 2013; Balcerowicz et al., 2014; Le et al., 2014; Zhang et al., 2014; Han et al., 2018b).
The synthesis of ethylene starts from the conversion of methionine into S-adenosylmethionie, which is then converted by the enzyme 1-aminocyclopropane-1-carboxylate synthase (ACS) into methylthioadenosine (MTA) and aminocyclopropane-1-carboxylic acid (ACC). Ethylene is synthesized through the oxidation of ACC by ACC oxidase (ACO) (Dong et al., 1992). Among the 12 genes (annotated as ACS1–12) in the Arabidopsis genome, ACS3 is a pseudogene, and ACS10 and ACS12 encode aminotransferases (Yamagami et al., 2003). Nine authentic ACS genes (ACS1, ACS2, ACS4–9, and ACS11) have been identified in the Arabidopsis genome (Tsuchisaka et al., 2009). Each of these nine genes can contribute to ethylene production. The elimination of all of them results in embryonic lethality, but an acs octuple-mutant can survive (Tsuchisaka et al., 2009).
In Arabidopsis, exogenous ACC induces a high density and different spacing of stomata on the epidermis of the cotyledon whilst the inhibition of ACC synthesis by aminovihylglycine (AVG) has the opposite effect (Serna and Fenoll, 1996). In addition, ACC treatment results in more stomata on the hypocotyls of Arabidopsis grown on low-nutrition medium (Saibo et al., 2003). However, it is unclear whether ACC is related with the division processes of GMCs. Here, we show that ACC is required for the symmetric division of GMCs in Arabidopsis. Blocking ACC synthesis genetically or chemically resulted in the inhibition of GMC division and the formation of SGCs on the epidermis of cotyledons, leaves, and hypocotyls. Further analysis indicated that ACC-mediated GMC division is independent of ethylene and its signaling components, suggesting an independent role of ACC in the symmetric division in Arabidopsis. The positive role of ACC in the symmetric division of GMCs can be antagonized by FAMA and FLP/MYB88. Moreover, ACC functions in the division of GMCs in a manner that is dependent on CDKB1s and CYCA2s.
Materials and methods
Plant materials and growth conditions
Arabidopsis thaliana plants were grown on half-strength Murashige and Skoog (MS) medium at 22 °C under a 16/8 h light/dark photoperiod. Transgenic plants with GC-specific E1728, pCDKB1;1::GFP, or pCYCA2;3::GFP were obtained from Professor Fred Sack (University of British Columbia). The acs octuple-mutant harboring E1728 was generated by genetic crossing using standard techniques, and the mutant seedlings were confirmed by PCR-based genotyping and YFP (Tsuchisaka et al., 2009). Col-0 was used as the wild-type. Plants were sampled at 14 d after germination. In an additional experiment, plants were grown on low-nutrition medium (Smalle et al., 1997) in the dark for 21 d to produce etiolated seedlings.
Chemical treatments
Stock solutions were prepared by dissolving the following in DMSO: ACC at 10 mM; ethephon at 10 mM; AVG at 25 mM; α-aminoisobutyric acid (AIB) at 10 mM; and Co2+ at 25 mM (all chemicals obtained from Sigma). All the stock solutions were stored at –20 °C, and diluted with sterilized medium to the final experimental concentration: 10 μM for ACC, 10 μM for ethephon, 25 μM for AVG, 10 μM for AIB, and 25 μM for Co2+. Seeds were surface-sterilized and germinated on the treated medium or on control medium. Plants were then grown at 22 °C under a 16/8 h light/dark photoperiod.
Microscopy
Images of stomata were obtained from samples stored in Hoyer’s Solution and visualized using differential interference contrast microscopy on a Nikon D-ECLIPSE C1 microscope. The cotyledons were observed at 14 d after germination. Samples were taken and placed in 70% ethanol, cleared overnight at room temperature, and then stored in Hoyer’s Solution. To visualize cell walls, freshly dissected leaves were stained for 3 min in an aqueous solution containing 2 mg ml−1 propidium iodide (Sigma-Aldrich). The leaves were then rinsed and mounted in distilled water. The abaxial side of leaves was viewed with a 3100 oil objective (Nikon Plan Fluor NA 1.3) using a Nikon PCM 2000 confocal laser-scanning microscope equipped with 488 nm argon and 543 nm helium–neon lasers. A Nikon D-ECLIPSE C1 laser confocal scanning microscope was used for green fluorescent protein (GFP) fluorescence images and propidium iodide staining.
DAPI staining and measurement of DNA content
For analysis of nuclei in GCs, cotyledons were dissected and fixed in 70% ethanol for 3 h, incubated in DAPI staining solution for at least 30 min, and excited by UV fluorescence. Relative DNA levels were measured following the protocol described previously by Yang et al. (2014).
Stomatal counts
Samples were fixed in Hoyer’s Solution and visualized using an Olympus BX51 microscope with a DP73 CCD camera. To determine numbers of SGCs, 50 cotyledons from 25 plants were counted for each genotype.
RNA extraction and qRT-PCR
Total RNA was extracted from 4-d-old seedlings using Trizol reagent (Invitrogen). First-strand cDNA was synthesized from 1.5 μg DNase-treated RNA in a 20-μl reaction volumes using M-MuLV reverse transcriptase (Fermentas, EU) with an oligo(dT)18 primer. qRT-PCR was performed using 2× SYBR Green I Master Mix on a Roche Light Cycler 480 real-time PCR machine, according to the manufacturer’s instructions. At least three biological replicates for each sample were used for qRT-PCR analysis and at least three technical replicates were analysed for each biological replicate. The KAT1 gene was used as an internal control (Nakamura et al., 1995; Lee et al., 2014). The primers used in qRT-PCR are listed in Supplementary Table S1 at JXB online.
Accession numbers
The Arabidopsis Genome Initiative numbers for the genes considered in this article are as follows: ACS1, AT2G43750; ACS2, AT1G01480; ACS3, AT5G28360; ACS4, AT2G22810; ACS5, AT5G65800; ACS6, AT4G11280; ACS7, AT4G26200; ACS8, AT4G37770; ACS9, AT3G49700; ASC10, AT1G62960; ACS11, AT4G08040; ETO1, AT3G51770; FAMA, AT3G24140; FLP, AT1G14350; MYB88, AT2G02820; CDKB1;1, AT3G54180; CDKB1;2, AT2G38620; CYCA2;2, AT5G11300; CYCA2;3, AT1G15570; CYCA2;4, AT1G80370; and KAT1, AT5G46240.
Results
AVG treatment results in the disruption of GMC terminal division
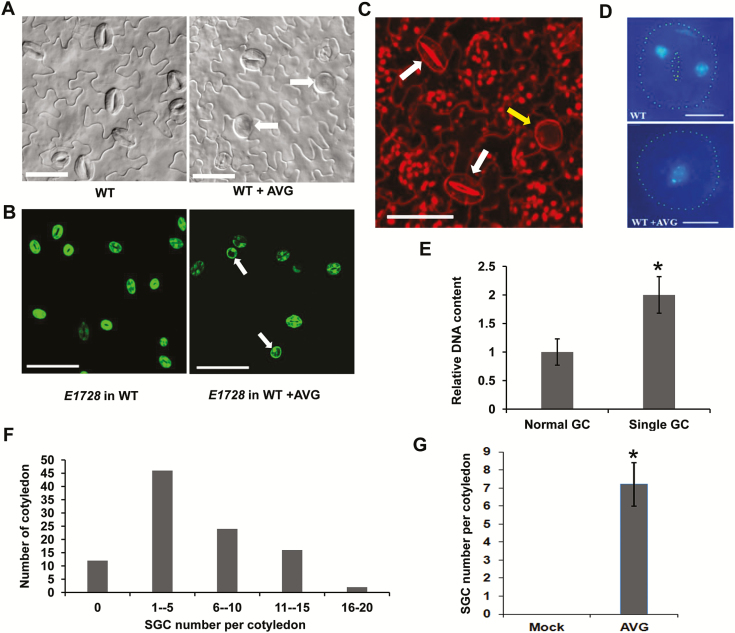
It is known that ACC positively affects stomatal density (Serna and Fenoll, 1996; Saibo et al., 2003); however, it is unknown whether it affects the division processes. Seedlings were therefore grown for 14 d on media with or without supplementary AVG, an ACC biosynthesis inhibitor. The seedlings grown with AVG developed some stomatal cells without a central pore (Fig. 1A, Supplementary Fig. S1), which are very similar to typical single guard cells (SGCs). To confirm whether these stomatal cells had the GC fate, wild-type seedlings harboring the GC fate marker E1728er::YFP (E1728) were treated with AVG, which could be seen to be expressed in the stomatal cells (Fig. 1B). We used propidium iodide to stain plant cell walls, which indicated that these abnormal stomatal cells had no internal cell wall (Fig. 1C). In addition, we used DAPI to examine the number and size of the nuclei, and found that they had only one nucleus (Fig. 1D) with nearly double the DNA content of normal GCs, as deduced from the fluorescent intensity of the DAPI-stained nuclei (Fig. 1E). All these results supported the identity of the cells as SGCs. To quantify the frequency of SGCs induced by AVG, we counted the numbers of SGCs and normal stomata on the abaxial epidermis of the cotyledons. No SGCs were found on the epidermis with stomata in any of the control seedlings. After AVG treatment, the number of SGCs varied from 0 to 16 per cotyledon (Fig. 1F) with an average number of 7.2 (Fig. 1G).
Fig. 1.
Treatment with aminoethoxyvinylglycine (AVG) results in the formation of single guard cells (SGCs). (A) Abaxial epidermis of cotyledons in the wild-type (WT) with or without AVG treatment. Arrows indicate SGCs. Scale bars are 20 μm. (B) E1728 expression in the abaxial epidermis of cotyledons in the wild-type before and after AVG treatment. Arrow indicates SGCs. Scale bars are 100 μm. (C) Abaxial epidermis of a cotyledon in the wild-type with AVG treatment. The confocal z-projection was visualized using propidium iodide staining with a confocal laser-scanning microscope. The yellow arrow indicates a SGC without a central cell wall. White arrows indicate normal stomata with two GCs and a thickened central cell wall. The scale bar is 50 μm. (D) GCs and SGCs with DAPI fluorescence. Scale bars are 5 μm. (E) Quantitative analysis of DAPI fluorescence intensity in GCs and SGCs. The data are means (±SD) (n=10) and the significant difference between SGCs and GCs was determined using Student’s t-test: *P<0.01. (F) Frequency of cotyledons with different numbers of SGCs on the abaxial epidermis of AVG-treated wild-type plants. A total of 50 cotyledons from 25 seedlings were examined. (G) Mean number of SGCs per cotyledon in the wild-type with or without (Mock) AVG treatment. The significant difference between the means was determined using Student’s t-test: *P<0.01. A total of 50 cotyledons from 25 seedlings were examined.
Loss-of-function of ACS genes causes production of SGCs
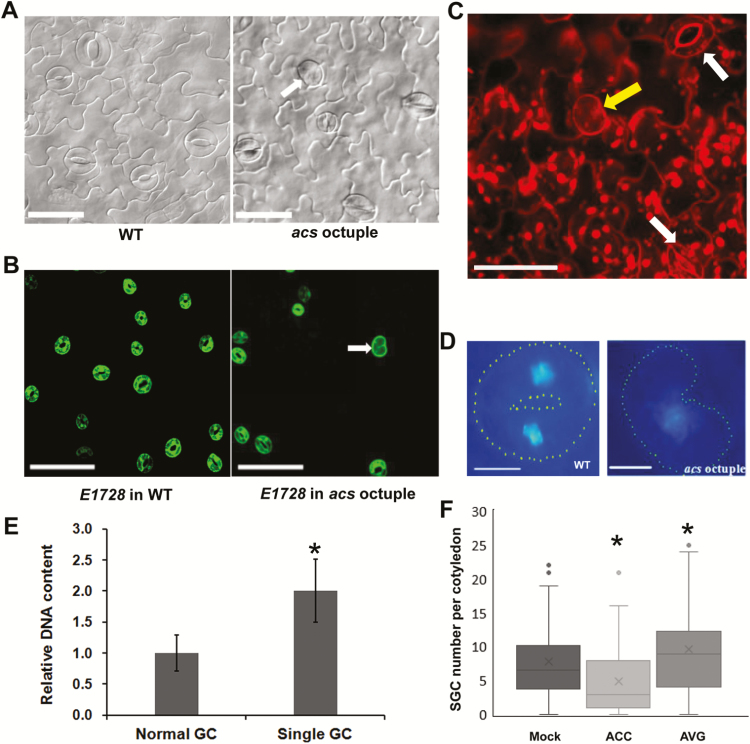
To further investigate the functions of ACC, an acs octuple-mutant (CS16651: acs2-1/4-1/5-2/6-1/7-1/9-1 amiRacs8 acs11) with rather low endogenous ACC was used for analysis. As expected, putative SGCs were found on the epidermis of cotyledons, true leaves, and hypocotyls in this mutant (Fig. 2A, Supplementary Fig. S2). These abnormal stomatal cells were further identified as SGCs according to their DNA content, GC fate, and cell walls (Fig. 2B–E). The number of SGCs on the abaxial epidermis of cotyledons ranged from 0 to 25 in the acs octuple mutant (Fig. 2F).
Fig. 2.
Defect of guard mother cell (GMC) division in the Arabidopsis asc octuple-mutant. (A) Abaxial epidermis of cotyledons of the wild-type (WT) and acs octuple-mutant. The arrow indicates a single guard cell (SGC). Scale bars are 20 μm (B) E1728 expression in the abaxial epidermis of cotyledons of the wild-type and acs octuple-mutant. The arrow indicates a SGC. Scale bars are 100 μm. (C) Abaxial epidermis of a cotyledon of the acs octuple-mutant. The confocal z-projection image was visualized using propidium iodide staining with a confocal laser-scanning microscope. The yellow arrow indicates a SGC without a central cell wall. White arrows indicate normal stomata with two GCs and a thickened central cell wall. Scale bar is 50 μm. (D) GCs and SGCs imaged with DAPI fluorescence. Sacle bars are 5 μm. (E) Quantitative analysis of DAPI fluorescence intensity in GCs and SGCs. The data are means (±SD) (n=10) and the significant difference between SGCs and GCs was determined using Student’s t-test: *P<0.01. (F) Number of SGCs per cotyledon in the acs octuple-mutant in control (Mock) plants and plants treated with aminocyclopropane-1-carboxylic acid (ACC) and aminoethoxyvinylglycine (AVG). The data are means (±SD) and significant differences compared with the Mock were determined using Student’s t-test: *P<0.01. A total of 50 cotyledons from 25 seedlings were analysed.
Given the low level of ACC in the acs octuple-mutant, we then sought to determine whether addition of ACC could rescue the defect of GMC division. As expected, exogenous ACC treatment effectively reduced the number of SGCs on the abaxial epidermis of the cotyledons (Fig. 2F). In contrast, AVG treatment increased the number of SGCs. These data suggest that ACC is required for the division of GMCs into GCs.
Blocking ethylene synthesis or signaling fails to cause SGC formation
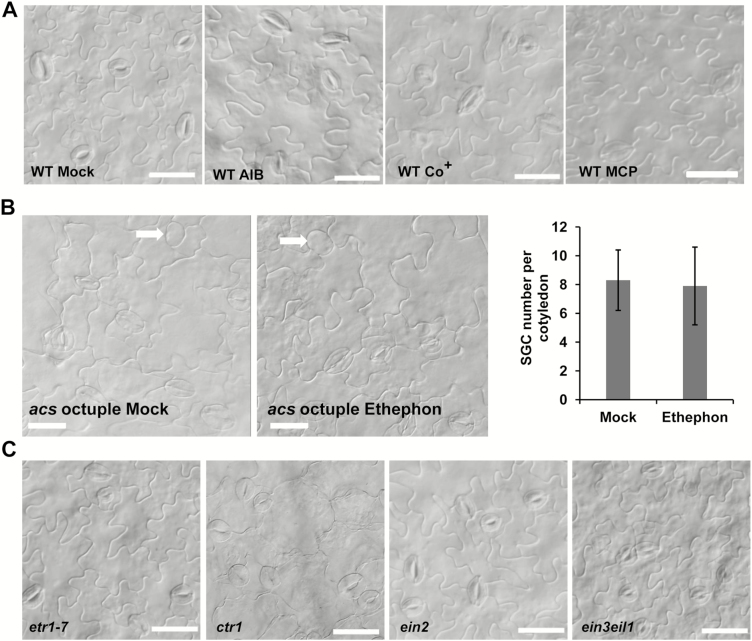
Having confirmed that ACC was required for the terminal division of GMCs, we investigated whether ethylene also played a role similar to ACC by using ethylene-synthesis inhibitors to block its production. ACO catalyses the oxidation of ACC into ethylene and this reaction can be inhibited by α-aminoisobutyric acid (AIB). Low concentrations of AIB can efficiently inhibit the ACC binding protein while millimolar concentrations can block ACC oxidase (Xu et al., 2008). To our surprise, AIB treatment failed to result in SGC production in the wild-type seedlings, even under a high concentration of 10 mM (Fig. 3A). Similar to AIB, Co2+ is also an inhibitor of ethylene synthesis, and no SGCs were found in the wild-type seedlings treated with Co2+ (Fig. 3A). These data suggested that inhibition of ethylene synthesis through ACC oxidation has no contribution to the formation of SGCs.
Fig. 3.
Blocking ethylene synthesis and signaling has no effect on guard mother cell division in Arabidopsis wild-type (WT) and acs octuple-mutant plants. (A) Abaxial epidermis of the wild-type after treatment with α-aminoisobutyric acid (AIB), Co2+, or 1-methycyclopropene (1-MCP) compared with untreated controls (Mock). Scale bars are 20 μm. (B) Numbers of single guard cells (SGCs) of abaxial epidermis of the acs octuple-mutant after treatment with ethephon. Arrow indicates SGCs. Scale bars are 20 μm. Data are are means (±SD). (C) Abaxial epidermis of the ethylene signaling-associated mutants etr1-7, ctr1, ein2, and ein3eil1. Scale bars are 20 μm.
Given that ACC could partially rescue the stomatal division defect in the acs octuple-mutant, we examined whether ethylene could also play a similar role. Ethephon (a widely used chemical replacement for ethylene) was used to treat seedlings. As expected, ethephon treatment caused the typical triple response that is inductive of high levels of ethylene (data not shown); however, the number of SGCs in the acs octuple-mutant was not significantly affected (Fig. 3B).
Next, we sought to determine whether ethylene signaling was required for the terminal division of GMCs. 1-methycyclopropene (1-MCP) is a highly specific and competitive inhibitor of ethylene binding to receptors (Hall et al., 2000; Sisler and Serek, 2006). As shown in Fig. 3A, no SGCs were found on the epidermis of seedlings subjected to 1-MCP treatment. To further confirm that ethylene signaling was not necessary for the symmetric division of GMCs, we checked the shape of stomata in several ethylene-signaling mutants. ETR1 (ETHYLENE RECEPTOR 1) is an ethylene receptor, CTR1 is a negative regulator of the ethylene response pathway, and EIN2 (ETHYLENE INSENSITIVE 2) and EIN3 (ETHYLENE INSENSITIVE 3)/EIL1 (ETHYLENE-INSENSITIVE3-LIKE 1) mediate the ethylene signaling transduction (Chang et al., 1993; Kieber et al., 1993; Hua and Meyerowitz, 1998; Hua et al., 1998; Sakai et al., 1998). As expected, no SGCs were observed in the ethylene-signaling mutants etr1-7, ctr1, ein2, and ein3eil1 (Fig. 3C). These data suggested that neither ethylene itself nor ethylene-signaling components affect the terminal division of GMCs.
ACC fails to induce extra divisions of the GMCs in wild-type Arabidopsis
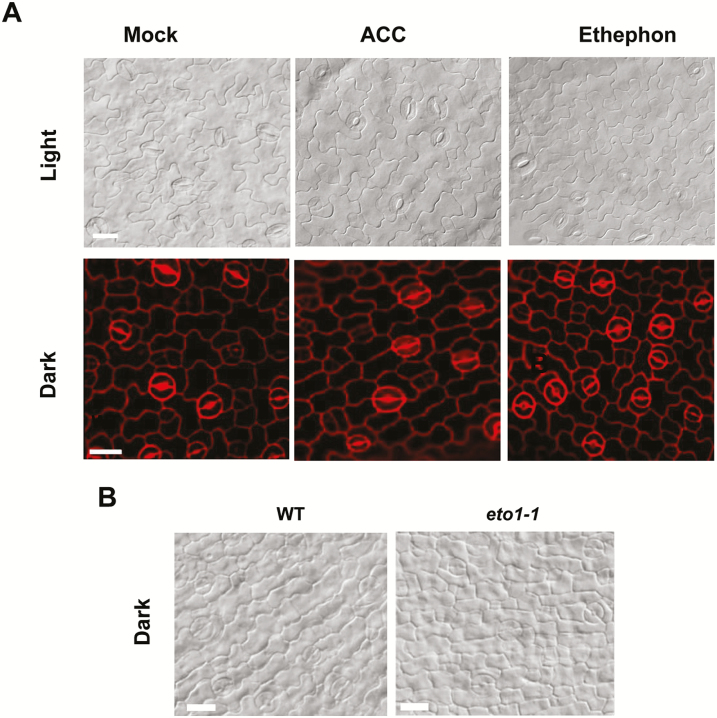
Having confirmed that ACC was required for the last symmetric division of GMCs, we examined whether elevated ACC would lead to extra divisions of GMCs. We treated wild-type seedlings with ACC, as well as with ethephon. No extra divisions of GMCs were found in either treatment for seedlings grown under a 16/8 h light/dark photoperiod (Fig. 4A, Supplementary Fig. S3).
Fig. 4.
Elevation of aminocyclopropane-1-carboxylic acid (ACC) and ethylene fails to induce extra division of guard mother cells in wild-type (WT) Arabidopsis seedlings. (A) Abaxial epidermis of cotyledons. Seedlings were grown on media with ACC or ethephon under a 16/8 h photoperiod or under constant darkness. Scale bars are 20 μm. (B) Abaxial epidermis of cotyledons of the wild-type and the eto1-1 mutant grown under constant darkness. Scale bars are 20 μm.
A previous study found that exogenous ethylene treatment causes ectopic division of GMCs on the hypocotyl epidermis of etiolated cucumber seedlings grown on low-nutrient medium (Kazama et al., 2004). Therefore, to exclude the possible effect of light, we examined the stomatal lineage cells of etiolated seedlings grown on low-nutrient medium with ACC or ethephon. We did not find extra divisions of GMCs (Fig. 4A, Supplementary Fig. S3). These results suggested that exogenous ACC has no effect on the terminal division of GMCs in Arabidopsis.
We then examined whether an increase of endogenous ACC or ethylene would cause ectopic division of GMCs by using, the ethylene over-accumulating mutant eto1 (ethylene overproducer1), which has enhanced ACS activity and over-produces both ACC and ethylene (Guzmán and Ecker, 1990; Woeste et al., 1999). We found that all the stomata on the epidermis of both the cotyledons and the hypocotyls consisted of two symmetric GCs without extra division (Fig. 4B; Supplementary Fig. S4). Taken together, these data suggested that a high level of ACC is not sufficient for the induction of extra symmetric divisions of the GMCs.
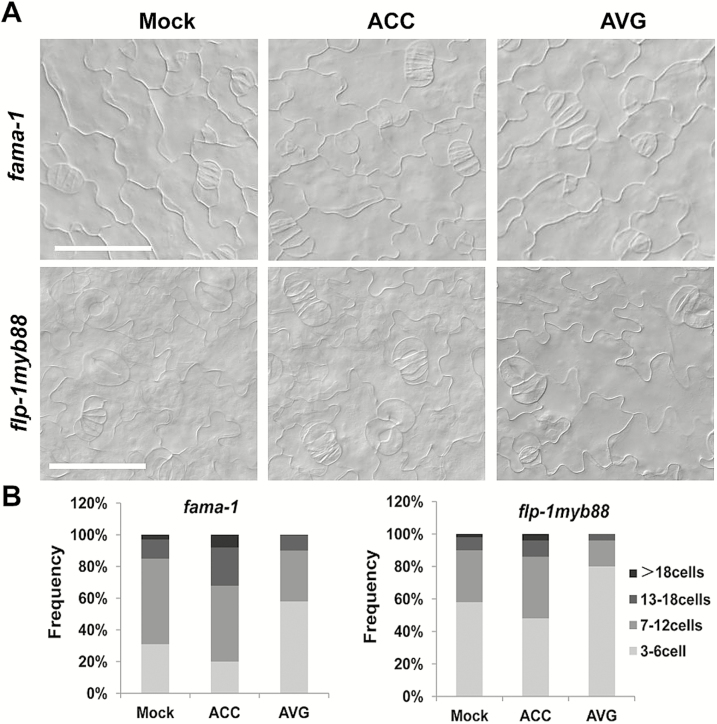
ACC causes extra divisions of the GMCs in fama-1 and flp-1myb88 mutants
Failure of ACC to induce more divisions of GMCs indicated that a rigid genetic mechanism existed to restrict these division. In the known genetic regulation network for stomatal development in Arabidopsis, FAMA and FLP/MYB88 are the key regulators of GMC division. FAMA is required to control the transition from GMCs to GCs and to halt division at the end of the stomatal lineage. The fama-1 mutant lacks recognizable stomata and instead develops clusters of small, narrow epidermal cells where they would normally be located (Ohashi-Ito and Bergmann, 2006). We therefore examined whether ACC could promote the symmetric division of GMCs in the fama-1 mutant. We found that the stomatal clusters of fama-1 contained more cells after ACC treatment (Fig. 5A, B), suggesting that it promoted the division of GMCs. When AVG was applied, the stomatal clusters developed fewer cells, suggesting that it suppressed the division of GMCs. In addition to FAMA, FLP and MYB88 also restrict the symmetric division of GMCs (Lai et al., 2005). To further investigate whether FLP and MYB88 are required for the role of ACC in the division of GMCs, the flp-1myb88 mutant was used for analysis. Loss-of-function of FLP/MYB88 induced excessive GMC divisions and produced many stomatal cell clusters (Fig. 5A, B). When the flp-1myb88 mutant was treated with ACC, more cells occurred in the stomatal clusters, whilst in contrast AVG treatment inhibited the excessive divisions and decreased the number of cells in the clusters. When ethephon, AIB, and Co2+ were used to treat seedlings, the division of stomatal clusters was not affected in the fama-1 and flp-1myb88 mutants (Supplementary Fig. S5). The promotion of division of stomatal clusters in the fama-1 and flp-1myb88 mutants by ACC was not observed in the wild-type, implying that functional FAMA and FLP/MYB88 antagonize the positive role of ACC in controlling the division of GMCs.
Fig. 5.
Aminocyclopropane-1-carboxylic acid (ACC) promotes symmetric division of guard mother cells in Arabidopsis fama-1 and flp-1myb88 mutants. (A) Abaxial epidermis of cotyledons of the mutants treated with ACC or aminoethoxyvinylglycine (AVG) compared with the control (Mock). Scale bars are 20 μm. (B) Frequency of cell clusters containing different numbers of cells on the abaxial epidermis of fama-1 and flp-1myb88 mutants treated with ACC or AVG.
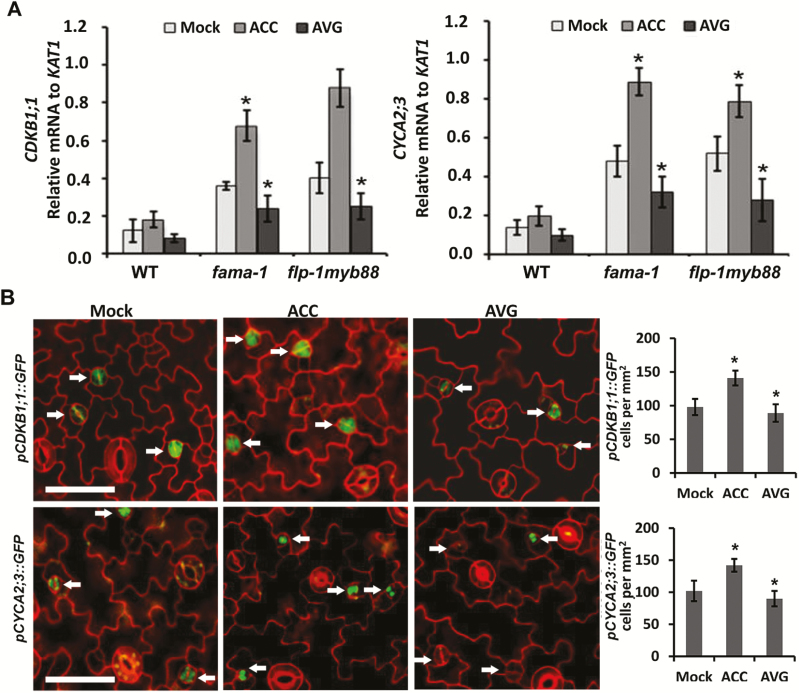
ACC promotes the expression of CDKB1 and CYCA2
It is well known that FAMA and FLP/MYB88 can restrict the division of stomatal lineage cells by negatively regulating the expression of CDKB1;1 and CYCA2;3 (Xie et al., 2010; Hachez et al., 2011; Vanneste et al., 2011; Lee et al., 2014). We hypothesized that ACC might stimulate the expression of CDKB1;1 and CYCA2;3 and thus promote the division of GMCs. To test this, we determined the expression of CDKB1;1 and CYCA2;3 in the wild-type, and fama-1 and flp-1myb88 mutants after ACC or AVG treatment (Fig. 6A). Under control conditions, the expression of CDKB1;1 and CYCA2;3 was significantly higher in fama-1 and flp-1myb88 than in the wild-type, agreeing with negative regulation by FAMA and FLP/MYB88. After ACC treatment, the expression of CDKB1;1 showed no significant change in wild-type, but increased in fama-1 and flp-1myb88 (by 2.4- and 2.5-fold, respectively). A similar effect was observed for the expression of CYCA2;3 after ACC treatment. In contrast, AVG inhibited the expression of CDKB1;1 and CYCA2;3 in the fama-1 and flp-1myb88 mutant plants. Correspondingly, we also detected GFP signals in pCDKB1;1::GFP and pCYCA2;3::GFP plants, with the signal increasing after ACC treatment and decreasing after AVG treatment (Fig. 6B). These data suggested that ACC stimulates the expression of CDKB1;1 and CYCA2;3.
Fig. 6.
Aminocyclopropane-1-carboxylic acid (ACC) positively regulates expression of CDKB1;1 and CYCA2;3 in Arabidopsis. (A) Relative mRNA levels of CDKB1;1 and CYCA2;3 (normalized to KAT1) in the wild-type (WT) and fama-1 and flp-1myb88 mutants treated with ACC or aminoethoxyvinylglycine (AVG) compared with the control (Mock). Data are means (±SD) of three biological replicates. Significant differences compared with the corresponding Mock value were determined using Student’s t-test: *P<0.01. Three independent experiments were performed. (B) Expression of pCDKB1;1::GFP and pCYCA2;3::GFP. Representative images are shown. Arrows indicate newly formed GCs. The graphs show quantitative analyses of GFP-positive cells. Data are means (±SD) of three biological replicates. Significant differences compared with the Mock were determined using Student’s t-test: *P<0.01. A total of 50 cotyledons from 25 seedlings were analysed. Three independent experiments were performed.
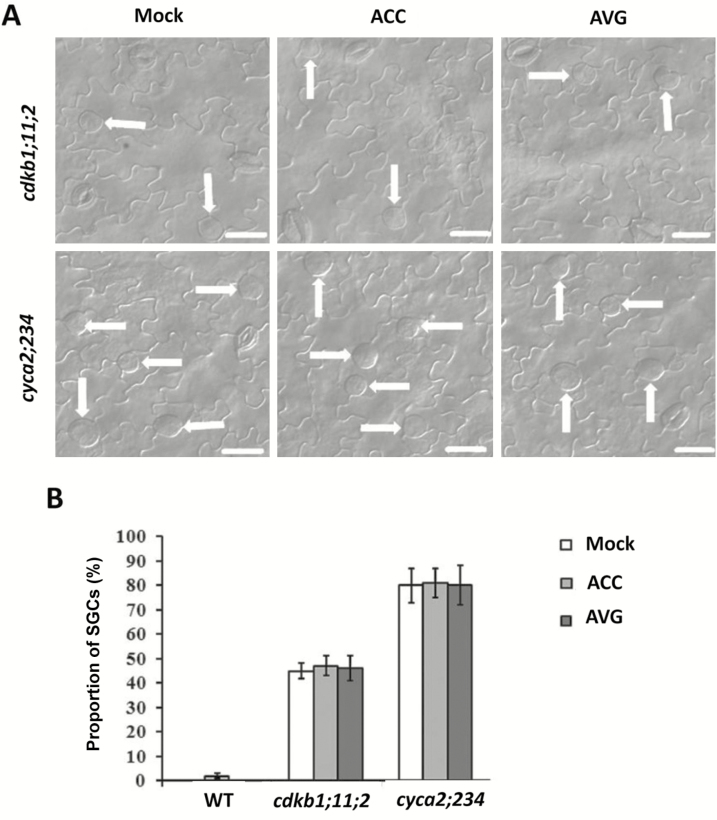
ACC has no effect on the division of GMCs in cdkb1;11;2 and cyca2;234 mutants
Given the fact that CDKB1;1 and CYCA2;3 were induced by ACC treatment, we were interested to determine whether the function of ACC in promoting the division of GMCs was dependent on CDKB1s and CYCA2s. We therefore used the cdkb1;11;2 and cyca2;234 mutants for further analysis. Under normal growth conditions, SGCs accounted for about 45% of stomata on the epidermis of cotyledon in the cdkb1;11;2 mutant (Fig. 7A, B) and we found that the number and proportion were not affected by ACC and AVG. On the abaxial epidermis of cotyledons of the cyca2;234 mutant, about 80% of stomata were SGCs and again neither ACC nor AVG treatment changed the proportion (Fig. 7A, B). These data suggested that CDKB1s and CYCA2s are required for the function of ACC in promoting the division of GMCs.
Fig. 7.
The cdkb1;11;2 and cyca2;234 mutants are insensitive to treatment with aminocyclopropane-1-carboxylic acid (ACC) and aminoethoxyvinylglycine (AVG). (A) Abaxial epidermis of cotyledons of the cdkb1;11;2 and cyca2;234 mutants treated with ACC or AVG compared with the control (Mock). The arrows indicate single guard cells (SGCs). Scale bars are 20 μm. (B) Proportion of SGCs in the cdkb1;11;2 and cyca2;234 mutants and the wild-type (WT) treated with either ACC or AVG. Data are means (±SD) (n=10).
Discussion
ACC, but not ethylene, is required for the terminal division of GMCs
Ethylene is a pivotal plant hormone that regulates multiple processes of plant growth and development. ACC is the direct precursor of ethylene production; however, it has also been identified as a primary regulator of plant growth and development (Vanderstraeten and Van Der Straeten, 2017). For example, in contrast to the viability of null mutations in key components of the ethylene-signaling pathways, such as the ein2 and ctr1 mutants (Kieber et al., 1993; Roman et al., 1995; Alonso et al., 1999), the acs null mutant is lethal, strongly supporting the hypothesis that ACC, but not ethylene, is required for Arabidopsis viability (Tsuchisaka et al., 2009). In addition, ACC is the primary regulator responsible for cell expansion mediated by the SALT OVERLY SENSITIVE 5 (SOS5)/FEI pathway in Arabidopsis roots. The cell expansion defect in the roots of fei1fei2 is suppressed by the inhibition of ACC synthase, but not by the inhibition of ACC oxidase or by disruption of the ethylene response pathway, indicating that the FEI proteins regulate cell extension via an ACC-mediated signal (Xu et al., 2008; Tsang et al., 2011).
Although ethylene and ACC have been reported to regulate stomatal development, it is still unclear whether they affect the division of stomatal lineage cells. AVG is a direct inhibitor of the conversion of S-adenosylmethionie to ACC. When AVG was applied, SGCs formed on the epidermis of wild-type plants (Fig. 1). Correspondingly, the acs octuple-mutant in which ACC production is severely blocked also developed SGCs (Fig. 2). It is noteworthy that ACC, but not ethephon, reduced the number of SGCs in the acs octuple-mutant (Figs 2F, 3B) These data suggest that ACC is crucial for the normal division of GMCs. In contrast, the direct block of ethylene synthesis by AIB or Co2+ did not result in the formation of SGCs (Fig. 3A), implying that ethylene is not involved in the control of GMC division. In agreement with these results, the disruption of the binding of ethylene with its receptors by 1-MCP also failed to induce SGC formation (Fig. 3A). Similarly, no SGCs were found in the etr1-7, ctr1, ein3, and ein3eil1 mutants, which are defective in ethylene perception or signaling (Fig. 3C). Taken together, these data suggest that ACC, but not ethylene, is required for the symmetric division of GMCs.
Antagonistic roles between ACC and FAMA/FLP/MYB88 in the symmetric division of GMCs in Arabidopsis
The production of ACC and ethylene is greatly inhibited in all multiple acs mutants including the octuple mutant. Ethylene production is decreased by 92% and 86% in seedlings and mature plants, respectively, of the acs octuple-mutant (Tsuchisaka et al., 2009). We only found SGCs in the acs octuple-mutant and not in other lower-multiple mutants, including a variety of double-, quadruple-, pentuple-, hexuple-, and even heptuple-mutants (Supplementary Fig. S6), which suggests that a rather low level of endogenous ACC is sufficient for the division of GMCs. We observed that the frequency of SGCs was quite low in the octuple-mutant plants (Fig. 2F), which means that a large portion of GMCs undergo normal symmetric division. It is likely that a complete absence of endogenous ACC would result in more SGCs; however, the exclusion of all the nine functional ACS genes causes embryo lethality (Tsuchisaka et al., 2009). We also cannot exclude the possibility that other factor(s) might function in positively regulating the terminal division of GMCs in Arabidopsis.
Loss-of-function of FAMA or FLP/MYB88 increased the division frequencies of GMCs in Arabidopsis, resulting in more cells in the clusters (Fig. 5). ACC treatment increased the division frequencies and the cell number of clusters in both the fama-1 and flp-1myb88 mutants. In contrast, ACC had no effect on the division of GMCs in the wild-type (Fig. 4). Therefore, FLP/MYB88 and FAMA antagonized the positive role of ACC in promoting the division of GMCs. The expression of CDKB1;1 and CYCA2;3 in GMCs was under the control of FAMA, FLP, and MYB88. FLP/MYB88 and FAMA directly bind to the promoter sequences of CDKB1;1 and CYCA2;3 and inhibit their expression in the newly formed GCs after the terminal division, limiting the division to a single event (Xie et al., 2010; Vanneste et al., 2011; Lee et al., 2014). In contrast to the negative roles of FLP/MYB88 and FAMA, ACC positively regulated the expression of CDKB1;1 and CYCA2;3 (Fig. 6). It has previously been confirmed that CDKB1;1 and CYCA2;3 are needed for the GMCs to progress through the G2-to-M transition (Porceddu et al., 2001; Boudolf et al., 2004a; Vanneste et al., 2011). After AVG treatment, the division of GMCs halted and caused the formation of SGCs (Fig. 1A, B), in agreement with the negative regulation of CDKB1;1 and CYCA2;3 by AVG (Fig. 6). ACC partially rescued the defect of GMC division in the acs octuple-mutant (Fig. 2F), but not that of the cdkb1;11;2 and cyca2;234 mutants (Fig. 7), which suggests that the promotion of GMC division by ACC depends on CDKB1;1 and CYCA2;3. CDKB1;1 and CYCA2;3 are specifically expressed in the stomatal lineage cells, with a maximum expression level during the symmetric division. We found that ACC enhanced the expression of CDKB1;1 whereas AVG weakened its expression in the newly formed GCs (Fig. 6B). CYCA2;3 displayed expression patterns similar to CDKB1;1 after ACC and AVG treatments. In contrast, the expression of CDKB1;1 and CYCA2;3 did not change with or without treatment with ethephon, AIB, or Co2+ in either the wild-type or the ethylene signaling mutants ein2 and ein3eil1 (Supplementary Fig. S7).These data suggest that ACC bypasses the ethylene-signaling pathway to promote the division of GMCs via the activation of CDKB1;1 and CYCA2;3. Further investigation is required to identify the unknown transcription factors that are responsible for the up-regulation of CDKB1;1 and CYCA2;3 by ACC.
In cucumber, extra divisions of GMCs have been observed on the hypocotyl epidermis after ethylene treatment (Kazama et al., 2004). However, we found that this phenomenon occurred only in Arabidopsis without functional FAMA or FLP/MYB88 (Fig. 5). The different roles of ACC in Arabidopsis and cucumber may result from their different stomatal development processes. For example, in Arabidopsis the stomatal lineage precursor cells undergo at least one asymmetric division to produce a GMC, while in cucumber no asymmetric division is needed for the formation of GMCs (Kazama et al., 2004). We hypothesize that this species-specific stomatal development processes may contribute to their different responses to ACC.
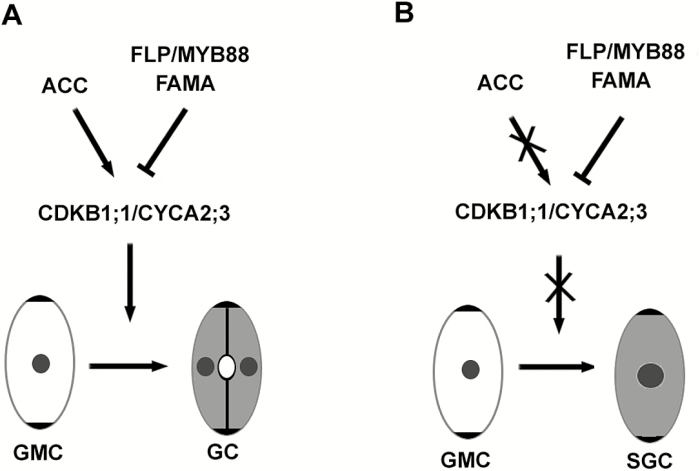
A working model for ACC mediation of GMC division in Arabidopsis
Our study has shown that ACC functions as an independent signal molecule to regulate the division of GMCs in Arabidopsis. Based on our results, we present a working model for ACC-mediated GMC division (Fig. 8). In this model, ACC is crucial for the symmetric division of GMCs into GCs. ACC positively regulates and FAMA/FLP/MYB88 negatively regulates the expression of CDKB1;1 and CYCA2;3, which are responsible for the division of GMCs. Under normal conditions (Fig. 8A), ACC and FAMA/FLP/MYB88 maintain the appropriate expression of CDKB1;1 and CYCA2;3 in the GMCs and ensure their symmetric division into GCs. When ACC synthesis is inhibited (Fig. 8B), the expression of CDKB1;1 and CYCA2;3 is down-regulated, which disrupts the symmetric division of GMCs and then leads to the formation of SGCs.
Fig. 8.
Model of the function of aminocyclopropane-1-carboxylic acid (ACC) in the symmetric division of guard mother cells (GMCs). (A) Under normal conditions, ACC and FAMA/FLP/MYB88 maintain the appropriate expression of CDKB1;1 and CYCA2;3 in GMCs and ensure their symmetric division into GCs. (B) When ACC synthesis is blocked, the expression of CDKB1;1 and CYCA2;3 is inhibited, which disrupts the symmetric division of GMCs and then leads to the formation of single guard cells (SGCs).
A recent report has demonstrated that the bHLH protein MUTE directly binds to the promoters of CDKB1;1 and CYCAs and up-regulates their gene expression (Han et al., 2018a). Here, we found that ACC is a positive regulator of CDKB1;1 and CYCAs. It is unclear whether MUTE functions downstream of ACC. Further investigation of the signal components responsible for ACC-mediated GMC division will provide new insights into the mechanisms underlying the terminal division of GMCs.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. AVG treatment results in SGC formation in the hypocotyl epidermis of wild-type Arabidopsis.
Fig. S2. SGCs in the acs octuple-mutant.
Fig. S3. ACC and ethephon treatment fail to induce SGCs in the hypocotyl epidermis of the wild-type.
Fig. S4. No extra division of GMCs is observed in the hypocotyl epidermis of etiolated eto1-1 seedlings.
Fig. S5. Ethephon, AIB and Co2+ have no effect on the division of GMCs in the fama-1 and flp-1myb88 mutants.
Fig. S6. There are no SGCs in the acs double-, quadruple-, pentuple-, hexuple-, and heptuple-mutants.
Fig. S7. Ethylene signaling has no effect on the expression of CDKB1;1 and CYCA2;3.
Table S1. Primers used for qRT-PCR in this study.
Author Contributions
JY, XZ, and XC designed the experiments; XZ, JY, and YW performed the experiments; XZ, JY, GZ, and XC analysed the data; XZ, GZ, GL, and XC wrote the manuscript; all the authors read and approved the final manuscript.
Acknowledgments
This work was supported by the Natural Science Foundation of China (31560078 and 31260283 to XC) and the Youth Innovation Promotion Association of CAS (to GL). We thank the Nottingham Arabidopsis Stock Centre (NASC) for sharing the acs mutants, Professor Fred Sack (University of British Columbia) for providing the flp-1myb88, fama-1, cdkb1;11;2, cyca2;234, E1728 enhancer trap line, pCDKB1::GFP, and pCYCA2;3::GFP lines, and Dr. Qikuang Wen (Institute of Plant Physiology and Ecology) for providing the eto1-1, etr1-7, ctr1, ein2, and and ein3eil1 lines. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR. 1999. EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284, 2148–2152. [DOI] [PubMed] [Google Scholar]
- Balcerowicz M, Ranjan A, Rupprecht L, Fiene G, Hoecker U. 2014. Auxin represses stomatal development in dark-grown seedlings via Aux/IAA proteins. Development 141, 3165–3176. [DOI] [PubMed] [Google Scholar]
- Bergmann DC, Sack FD. 2007. Stomatal development. Annual Review of Plant Biology 58, 163–181. [DOI] [PubMed] [Google Scholar]
- Borghi L, Gutzat R, Fütterer J, Laizet Y, Hennig L, Gruissem W. 2010. Arabidopsis RETINOBLASTOMA-RELATED is required for stem cell maintenance, cell differentiation, and lateral organ production. The Plant Cell 22, 1792–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudolf V, Barrôco R, de Almeida Engler J, Verkest A, Beeckman T, Naudts M, Inzé D, De Veylder L. 2004a. B1-type cyclin-dependent kinases are essential for the formation of stomatal complexes in Arabidopsis thaliana. The Plant Cell 16, 945–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudolf V, Vlieghe K, Beemster GT, Magyar Z, Torres Acosta JA, Maes S, Van Der Schueren E, Inzé D, De Veylder L. 2004b. The plant-specific cyclin-dependent kinase CDKB1;1 and transcription factor E2Fa-DPa control the balance of mitotically dividing and endoreduplicating cells in Arabidopsis. The Plant Cell 16, 2683–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Kwok SF, Bleecker AB, Meyerowitz EM. 1993. Arabidopsis ethylene-response gene ETR1: similarity of product to two-component regulators. Science 262, 539–544. [DOI] [PubMed] [Google Scholar]
- Dong JG, Ferna′ndez-Maculet JC, Yang SF. 1992. Purification and characterization of 1-aminocyclopropane-1-carboxylate oxidase from apple fruit. Proceedings of National Academy of Science, USA 89, 9789–9793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudesblat GE, Schneider-Pizoń J, Betti C, Mayerhofer J, van Dongen W, Boeren S, Zhiponova M, de Vries S, Jonak C, Russinova E. 2012. SPEECHLESS integrates brassinosteroid and stomata signalling pathways. Proceedings of National Academy of Science, USA 14, 548–554. [DOI] [PubMed] [Google Scholar]
- Guzmán P, Ecker JR. 1990. Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. The Plant Cell 2, 513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachez C, Ohashi-Ito K, Dong J, Bergmann DC. 2011. Differentiation of Arabidopsis guard cells: analysis of the networks incorporating the basic helix-loop-helix transcription factor, FAMA. Plant Physiology 155, 1458–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AE, Findell JL, Schaller GE, Sisler EC, Bleecker AB. 2000. Ethylene perception by the ERS1 protein in Arabidopsis. Plant Physiology 123, 1449–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SK, Qi X, Sugihara K, Dang JH, Endo TA, Miller KL, Kim ED, Miura T, Torri KU. 2018a. MUTE directly orchestrates cell-state switch and the single symmetric division to create stomata. Developmental Cell 45, 303–315.e5. [DOI] [PubMed] [Google Scholar]
- Han X, Hu Y, Zhang G, Jiang Y, Chen X, Yu D. 2018b. Jasmonate negatively regulates stomatal development in Arabidopsis cotyledons. Plant Physiology 176, 2871–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua J, Meyerowitz EM. 1998. Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell 94, 261–271. [DOI] [PubMed] [Google Scholar]
- Hua J, Sakai H, Nourizadeh S, Chen QG, Bleecker AB, Ecker JR, Meyerowitz EM. 1998. EIN4 and ERS2 are members of the putative ethylene receptor gene family in Arabidopsis. The Plant Cell 10, 1321–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzé D, De Veylder L. 2006. Cell cycle regulation in plant development. Annual Review of Genetics 40, 77–105. [DOI] [PubMed] [Google Scholar]
- Kazama H, Dan H, Imaseki H, Wasteneys GO. 2004. Transient exposure to ethylene stimulates cell division and alters the fate and polarity of hypocotyl epidermal cells. Plant Physiology 134, 1614–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M, Rozhon W, Bigeard J, Pflieger D, Husar S, Pitzschke A, Teige M, Jonak C, Hirt H, Poppenberger B. 2013. Brassinosteroid-regulated GSK3/Shaggy-like kinases phosphorylate mitogen-activated protein (MAP) kinase kinases, which control stomata development in Arabidopsis thaliana. The Journal of Biological Chemistry 288, 7519–7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR. 1993. CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the Raf family of protein kinases. Cell 72, 427–441. [DOI] [PubMed] [Google Scholar]
- Kim TW, Michniewicz M, Bergmann DC, Wang ZY. 2012. Brassinosteroid regulates stomatal development by GSK3-mediated inhibition of a MAPK pathway. Nature 482, 419–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai LB, Nadeau JA, Lucas J, Lee EK, Nakagawa T, Zhao L, Geisler M, Sack FD. 2005. The Arabidopsis R2R3 MYB proteins FOUR LIPS and MYB88 restrict divisions late in the stomatal cell lineage. The Plant Cell 17, 2754–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le J, Liu XG, Yang KZ, et al. . 2014. Auxin transport and activity regulate stomatal patterning and development. Nature Communications 5, 3090. [DOI] [PubMed] [Google Scholar]
- Lee E, Lucas JR, Sack FD. 2014. Deep functional redundancy between FAMA and FOUR LIPS in stomatal development. The Plant Journal 78, 555–565. [DOI] [PubMed] [Google Scholar]
- Matos JL, Lau OS, Hachez C, Cruz-Ramírez A, Scheres B, Bergmann DC. 2014. Irreversible fate commitment in the Arabidopsis stomatal lineage requires a FAMA and RETINOBLASTOMA-RELATED module. elife 3, e03271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura RL, McKendree WL Jr, Hirsch RE, Sedbrook JC, Gaber RF, Sussman MR. 1995. Expression of an Arabidopsis potassium channel gene in guard cells. Plant Physiology 109, 371–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi-Ito K, Bergmann DC. 2006. Arabidopsis FAMA controls the final proliferation/differentiation switch during stomatal development. The Plant Cell 18, 2493–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillitteri LJ, Torii KU. 2012. Mechanisms of stomatal development. Annual Review of Plant Biology 63, 591–614. [DOI] [PubMed] [Google Scholar]
- Porceddu A, Stals H, Reichheld JP, Segers G, De Veylder L, De Pinho Barrôco R, Casteels P, Van Montagu M, Inzé D, Nironov V. 2001. A plant-specific cyclin-dependent kinase is involved in the control of G2/M progression in plants. Jounal of Biological Chemistry 276, 36354–36360. [DOI] [PubMed] [Google Scholar]
- Roman G, Lubarsky B, Kieber JJ, Rothenberg M, Ecker JR. 1995. Genetic analysis of ethylene signal transduction in Arabidopsis thaliana: five novel mutant loci integrated into a stress response pathway. Genetics 139, 1393–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saibo NJ, Vriezen WH, Beemster GT, Van Der Straeten D. 2003. Growth and stomata development of Arabidopsis hypocotyls are controlled by gibberellins and modulated by ethylene and auxins. The Plant Journal 33, 989–1000. [DOI] [PubMed] [Google Scholar]
- Sakai H, Hua J, Chen QG, Chang C, Medrano LJ, Bleecker AB, Meyerowitz EM. 1998. ETR2 is an ETR1-like gene involved in ethylene signaling in Arabidopsis. Proceedings of National Academy of Science, USA 95, 5812–5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serna L, Fenoll C. 1996. Ethylene induces stomata differentiation in Arabidopsis. International Journal of Development Biology 40, S123–S124. [PubMed] [Google Scholar]
- Sisler EC, Serek M. 2006. Inhibitors of ethylene response in plants at the receptor level: recent developments. Physilogia Plantarum 100, 577–582. [Google Scholar]
- Smalle J, Haegman M, Kurepa J, Van Montagu M, Van Der Straeten D. 1997. Ethylene can stimulate Arabidopsis hypocotyl elongation in the light. Proceedings of National Academy of Science, USA 94, 2759–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Nose T, Jikumaru Y, Kamiya Y. 2013. ABA inhibits entry into stomatal-lineage development in Arabidopsis leaves. The Plant Journal 74, 448–457. [DOI] [PubMed] [Google Scholar]
- Tsang DL, Edmond C, Harrington JL, Nühse TS. 2011. Cell wall integrity controls root elongation via a general 1-aminocyclopropane-1-carboxylic acid-dependent, ethylene-independent pathway. Plant Physiology 156, 596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchisaka A, Yu G, Jin H, Alonso JM, Ecker JR, Zhang X, Gao S, Theologis A. 2009. A combinatorial interplay among the 1-aminocyclopropane-1-carboxylate isoforms regulates ethylene biosynthesis in Arabidopsis thaliana. Genetics 183, 979–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderstraeten L, Van Der Straeten D. 2017. Accumulation and transport of 1-aminocyclopropane-1-carboxylic acid (ACC) in plants: current status, considerations for future research and agronomic applications. Frontiers in Plant Science 8, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste S, Coppens F, Lee E, et al. . 2011. Developmental regulation of CYCA2s contributes to tissue-specific proliferation in Arabidopsis. EMBO Journal 30, 3430–3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimer AK, Nowack MK, Bouyer D, Zhao X, Harashima H, Naseer S, De Winter F, Dissmeyer N, Geldner N, Schnittger A. 2012. RETINOBLASTOMA RELATED1 regulates asymmetric cell divisions in Arabidopsis. The Plant Cell 24, 4083–4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woeste KE, Ye C, Kieber JJ. 1999. Two Arabidopsis mutants that overproduce ethylene are affected in the posttranscriptional regulation of 1-aminocyclopropane-1-carboxylic acid synthase. Plant Physiology 119, 521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Lee E, Lucas JR, Morohashi K, Li D, Murray JA, Sack FD, Grotewold E. 2010. Regulation of cell proliferation in the stomatal lineage by the Arabidopsis MYB FOUR LIPS via direct targeting of core cell cycle genes. The Plant Cell 22, 2306–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SL, Rahman A, Baskin TI, Kieber JJ. 2008. Two leucine-rich repeat receptor kinases mediate signaling, linking cell wall biosynthesis and ACC synthase in Arabidopsis. The Plant Cell 20, 3065–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagami T, Tsuchisaka A, Yamada K, Haddon WF, Harden LA, Theologis A. 2003. Biochemical diversity among the 1-amino-cyclopropane-1-carboxylate synthase isozymes encoded by the Arabidopsis gene family. The Journal of Biological Chemistry 278, 49102–49112. [DOI] [PubMed] [Google Scholar]
- Yang K, Wang H, Xue S, Qu X, Zou J, Le J. 2014. Requirement for A-type cyclin-dependent kinase and cyclins for the terminal division in the stomatal lineage of Arabidopsis. Journal of Experimental Botany 65, 2449–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JY, He SB, Li L, Yang HQ. 2014. Auxin inhibits stomatal development through MONOPTEROS repression of a mobile peptide gene STOMAGEN in mesophyll. Proceedings of National Academy of Science, USA 111, E3015–E3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.