Abstract
Zebrafish (Danio rerio) are a widely used as an experimental model for a wide range of retinal diseases. Previously, optical coherence tomography (OCT) was introduced for quantitative analysis of the zebrafish cone photoreceptor cell mosaic, however no data exists on the intersession reproducibility or intrasession repeatability of such measurements. We imaged 14 wild-type (WT) fish three times each, with 48 hours between each timepoint. En face images of the UV cone mosaic were generated from the OCT volume scans at each timepoint. These images were then aligned and the overlapping area cropped for analysis. Using a semi-automated cone-counting algorithm, a single observer identified each cone to calculate the cone density for every image, coutning each image twice (84 total counts). The OCT cone density measurements were found to have an intersession reproducibility of 0.9988 (95% CI = 0.9978 – 0.9999) and a intrasession repeatability of 136.0±10.5 cones/mm2 (about 0.7%), Factors affecting image quality include gill movement during acquisition of the OCT volume and variable inclusion of non-UV cone mosaics in the contours used to generate the en face images.
XX.1. Introduction
Zebrafish (Danio rerio) are widely used to model human development and disease. Zebrafish have orthologs to many disease-causing proteins, whose functional domains are often similar to those found in human proteins (Langheinrich 2003; Howe et al. 2013). Zebrafish are especially valuable as an ocular model system since their cone rich retinas mimic the human retina (Allison et al. 2004) and multiple zebrafish models exist for ocular diseases (Link and Collery 2015). In addition, zebrafish are a cost-effective model for drug development, and have been used to accurately predict mammalian teratogenicity (Van Leeuwen et al. 1990) as well as drug oculotoxicty (Deeti et al. 2014), which is especially important given the number of drugs with ocular side effects (Santaella and Fraunfelder 2007). Finally, zebrafish mature quickly and breed prolifically, making them well-suited for high-throughput screening (Schutera et al. 2016; Truong et al. 2016).
Optical coherence tomography (OCT) is a non-invasive, high resolution imaging modality that has previously been used to examine zebrafish retina, lens, and anterior segment (Rao et al. 2006; Bailey et al. 2012; Collery et al. 2014). Recently, an OCT-based method for deriving in vivo estimates of cone density in the adult zebrafish retina was introduced (Huckenpahler et al. 2016). This method could allow researchers to quantify photoreceptor changes in response to disease or drug treatment, though the repeatability of these measurements has not been studied. Here we assess the intrasession repeatability and intersession reproducibility of OCT-based measurements of UV cone density in the adult zebrafish.
XX.2. Methods
Volumetric images (1000 A-scans/B-scan, 1000 B-scans, nominal scan width of 1.2×1.2 mm) of the zebrafish retina were obtained using a Bioptigen Envisu R2200 SD-OCT (Research Triangle Park, NC) equipped with a 186.3 nm bandwidth SLD (center wavelength = 878.4 nm). Fourteen WT zebrafish were imaged three times each, with 48 hours between imaging sessions. En face images of the UV cone mosaic were generated from the OCT volumes as previously described (Huckenpahler et al. 2016). The en face images for each fish were manually aligned using Photoshop (Adobe Photosystems, San Jose, CA) and a common region of interest (ROI) was cropped (ROI size varied from 12.18–102*10−3 mm2). A low pass filter was applied to each ROI, and cones were identified using semi-automated cone counting software (Garrioch et al. 2012; Cooper et al. 2016a). Axial length values were obtained as previously described,(Collery et al. 2014) and used to calculate the lateral scale of each en face image (Huckenpahler et al. 2016), thus enabling measurements of cone density. Cone density was taken as the number of cones with bound Voronoi cells divided by the total area of the Voronoi cells (Cooper et al. 2013).
To assess the intrasession repeatability, the cropped ROI from each imaging session was counted twice. The within subject standard deviation (Sw) was taken as the square root of the average variance across all fish (Bland and Altman 1996). The repeatability is calculated by multiplying Sw by 2.77 and the 95% confidence interval calculated with the formula:
| (1) |
Where n is the number of zebrafish imaging sessions (42), and m is the number of counts for each image (2). The measurement error can also be estimated as Sw × 1.96 (Bland and Altman 1996).
To calculate the intersession reproducibility of the cone density measurements, both cone density estimates at each time point were averaged and used to calculate the intraclass correlation coefficient (ICC) using R statistical package (The R Foundation for Statistical Computing, Vienna, Austria).
XX.3. Results
Qualitatively, we observed variability in image quality across fish and imaging sessions. Factors such as shadowing from the overlying blood vessels and movement of the fish during scan acquisition can result in distortions or missing data in the resultant en face images of the cone mosaic (Figure XX.1), though this rarely affected the ability to identify whether a cone was present or not. Across the 14 fish, the average cone density was found to be 20,588 cones/mm2, with densities ranging from 12,326 to 39,254 cones/mm2 (Table XX.1). Differences between repeated measures of cone density ranged from 0–246 cones/mm2 with an average within-fish standard deviation (Sw) of 49.1 cones/mm2. Intrasession repeatability was 136.0±10.5 cones/mm2 (about 0.7%). This means that the difference between two measurements for the same fish would be expected to be less than this value for 95% of pairs of measurements. The measurement error for this same data was determined to be 96.2 cones/mm2 (about 0.5%), meaning that the difference between a given measurement of cone density and the true value would be expected to be less than this value for 95% of observations. Examining the cone density measurements over time, we found the ICC to be 0.9988 (95% CI = 0.9978 – 0.9999), indicating that 99.88% of the total variance is due to real differences in cone density across fish.
Fig. XX.1.
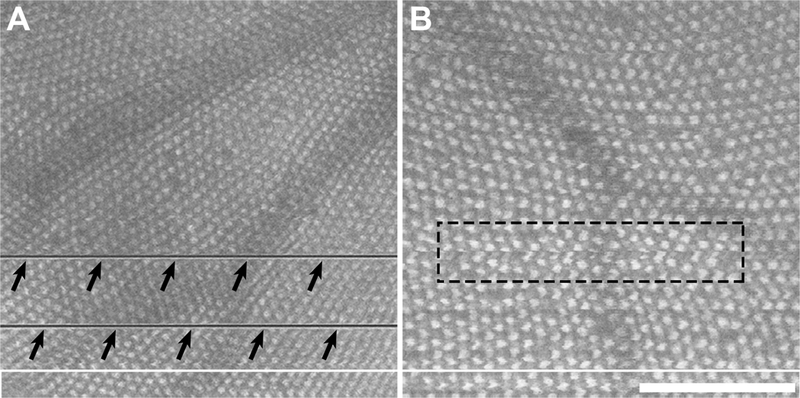
Disruptions in en face images of the cone mosaic derived from OCT volume scans. (A) Significant gill movements during the process of scanning result in missing data in the en face image (arrows). (B) Subtler gill movements and/or errors in contour placement can result in localized distortions (dashed rectangle). While these distortions may not affect the ability to identify the cones in the images, they would affect measurements of mosaic geometry (Cooper et al. 2016b). In addition, shadowing from overlying retinal vasculature was seen in many images. Scale bar = 100 μm.
Table XX.1.
Zebrafish scaling information and density measurements
| Fish | Axial Length (mm) |
Region of Interest (μm2) |
Timepoint 1 (cones/mm2) |
Timepoint 2 (cones/mm2) |
Timepoint 3 (cones/mm2) |
|||
|---|---|---|---|---|---|---|---|---|
| 1 | 1.41 | 49,644 | 20,527 | 20,482 | 20,632 | 20,683 | 19,496 | 19,401 |
| 2 | 1.60 | 12,185 | 19,980 | 19,986 | 20,614 | 20,734 | 20,684 | 20,893 |
| 3 | 1.91 | 42,025 | 12,453 | 12,479 | 12,578 | 12,593 | 12,563 | 12,526 |
| 4 | 1.88 | 29,649 | 18,589 | 18,557 | 19,047 | 19,170 | 18,711 | 18,664 |
| 5 | 1.39 | 50,392 | 26,185 | 26,231 | 25,810 | 25,756 | 26,400 | 26,439 |
| 6 | 1.56 | 98,882 | 15,238 | 15,224 | 15,450 | 15,427 | 15,248 | 15,281 |
| 7 | 1.60 | 38,325 | 12,711 | 12,645 | 12,435 | 12,373 | 12,761 | 12,802 |
| 8 | 1.56 | 64,947 | 18,939 | 18,926 | 18,669 | 18,687 | 18,792 | 18,756 |
| 9 | 1.75 | 102,133 | 12,339 | 12,394 | 12,472 | 12,447 | 12,368 | 12,326 |
| 10 | 1.69 | 75,105 | 13,038 | 13,031 | 13,477 | 13,495 | 13,260 | 13,260 |
| 11 | 1.66 | 50,265 | 15,067 | 15,089 | 15,071 | 15,068 | 14,875 | 14,817 |
| 12 | 1.26 | 24,073 | 28,636 | 28,513 | 28,113 | 28,095 | 28,016 | 27,993 |
| 13 | 1.11 | 20,022 | 35,651 | 35,405 | 35,508 | 35,531 | 35,828 | 35,836 |
| 14 | 1.22 | 35,429 | 38,598 | 38,587 | 39,229 | 39,254 | 38,717 | 38,666 |
XX.4. Discussion
Our data demonstrate excellent repeatability and reproducibility of in vivo measurements of cone density, using en face images of the UV cone mosaic derived from volumetric OCT scans. However, this study has some limitations. First, the repeatability of the OCT density measurements will depend on the quality of the en face images. A low quality OCT scan with multiple breathing artifacts or low signal will produce a low quality en face image of the cone mosaic and subsequently impact the repeatability of the cone density measurements. Secondly, the repeatability and reproducibility measurements were performed by an expert who was proficient in both acquiring OCT images and generating en face images. For other observers, the repeatability and reliability of this technique may be worse (Bartlett and Frost 2008; Liu et al. 2014). Finally, our analysis was limited to the UV-cone submosaic- as other submosaics are more difficult to visualize (Huckenpahler et al. 2016); thus our results should not be assumed to apply to the R/G- or S-cone submosaic.
Adaptive optics scanning light ophthalmoscopy (AOSLO) is another technique capable of providing high-resolution in vivo images of the cone mosaic (Williams 2011), and has been used for examining cone density in a wide range of retinal diseases (Carroll et al. 2013; Roorda and Duncan 2015). Semi-automated techniques have shown an intrasession repeatability of 2.7% for measures of parafoveal cone density (Garrioch et al. 2012). Fully-automated methods have been shown to have excellent reproducibility, with one study reporting an ICC of 0.989 (Chui et al. 2013). Thus, the repeatability of our OCT-derived cone density measurements in the zebrafish retina are at least as good (if not slightly better) than AOSLO-derived measurements from the human retina. This may be due to the increased regularity of the zebrafish mosaic compared to humans, making it easier for the observer to see when the automated algorithm has missed a cone. In conclusion, with the non-invasive nature of the technique and availability of quantitative tools for analyzing images, the OCT-based method described here could be used to quantitatively study ocular disease, monitor retinal development, and facilitate drug discovery in zebrafish with high reproducibility and repeatability.
Acknowledgments
The authors would like to thank Christine Skumatz and Alexis Visotcky for their assistance with this study and Robert Cooper for providing the cone counting software. Research reported in this publication was supported by the National Eye Institute and the National Institute of General Medical Sciences of the NIH under award numbers R01EY016060, T32EY014537, P30EY001931, T32GM080202. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- Allison WT, Haimberger TJ, Hawryshyn CW et al. (2004) Visual pigment composition in zebrafish: Evidence for a rhodopsin-porphyropsin interchange system. Vis Neurosci 21:945–952 [DOI] [PubMed] [Google Scholar]
- Bailey TJ, Davis DH, Vance JE et al. (2012) Spectral-domain optical coherence tomography as a noninvasive method to assess damaged and regenerating adult zebrafish retinas. Invest Ophthalmol Vis Sci 53:3126–3138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett JW, Frost C (2008) Reliability, repeatability and reproducibility: Analysis of measurement errors in continuous variables. Ultrasound Obstet Gynecol 31:466–475 [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG (1996) Statistics notes: Measurement error. Br Med J 313:744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll J, Kay DB, Scoles D et al. (2013) Adaptive optics retinal imaging-clinical opportunities and challenges. Curr Eye Res 38:709–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chui TYP, Gast TJ, Burns SA (2013) Imaging of vascular wall fine structure in human retina using adaptive optics scanning laser ophthalmoscopy. Invest Ophthalmol Vis Sci 54:7115–7124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collery RF, Veth KN, Dubis AM et al. (2014) Rapid, accurate, and non-invasive measurement of zebrafish axial length and other eye dimensions using SD-OCT allows longitudinal analysis of myopia and emmetropization. PLoS One 9:e110699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper RF, Wilk MA, Tarima S et al. (2016a) Evaluating descriptive metrics of the human cone mosaic. Invest Ophthalmol Vis Sci 57:2992–3001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper RF, Sulai YN, Dubis AM et al. (2016b) Effects of intraframe distortion on measures of cone mosaic geometry from adaptive optics scanning light ophthalmoscopy. Transl Vis Sci Technol 5:e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper RF, Harvey Z, Dubow M et al. (2013) The effect of AOSLO image distortion on metrics of mosaic geometry. Invest Ophthalmol Vis Sci 54:5546 [Google Scholar]
- Deeti S, O’Farrell S, Kennedy BN (2014) Early safety assessment of human oculotoxic drugs using the zebrafish visualmotor response. J Pharmacol Toxicol Methods 69: [DOI] [PubMed] [Google Scholar]
- Garrioch R, Langlo C, Dubis AM et al. (2012) Repeatability of in vivo parafoveal cone density and spacing measurements. Optom Vis Sci 89:632–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe K, Clark MD, Torroja CF et al. (2013) The zebrafish reference genome sequence and its relationship to the human genome. Nature 496:498–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckenpahler AL, Wilk MA, Cooper RF et al. (2016) Imaging the adult zebrafish cone mosaic using optical coherence tomography. Vis Neurosci in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langheinrich U (2003) Zebrafish: anew model on the pharmaceutical catwalk. Bioessays 25:904–912 [DOI] [PubMed] [Google Scholar]
- Link BA, Collery RF (2015) Zebrafish models of retinal disease. Annu Rev Vis Sci 1:125–153 [DOI] [PubMed] [Google Scholar]
- Liu B, Tarima S, Visotcky A et al. (2014) The reliability of parafoveal cone density measurements. Br J Ophthalmol 98:1126–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao KD, Verma Y, Patel HS et al. (2006) Non-invasive ophthalmic imaging of adult zebrafish eye using optical coherence tomography. Curr Sci 90:1506–1510 [Google Scholar]
- Roorda A, Duncan JL (2015) Adaptive optics ophthalmoscopy. Annu Rev Vis Sci 1:19–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santaella RM, Fraunfelder FW (2007) Ocular adverse effects associated with systemic medications: recognition and management. Drugs 67:75–93 [DOI] [PubMed] [Google Scholar]
- Schutera M, Dickmeis T, Mione M et al. (2016) Automated phenotype pattern recognition of zebrafish for high-throughput screening. Bioengineered 7:261–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong L, Bugel SM, Chlebowski A et al. (2016) Optimizing multi-dimensional high-throughput screening using zebrafish. Reprod Toxicol 65:139–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leeuwen CJ, Grootelaar EM, Niebeek G (1990) Fish embryos as teratogenicity screens: a comparison of embryotoxicity between fish and birds. Ecotoxicol Environ Saf 20:42–52 [DOI] [PubMed] [Google Scholar]
- Williams DR (2011) Imaging single cells in the living retina. Vision Res 51:1379–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]


