Abstract
Introduction:
Nearly 40% of US women of childbearing age are obese. Obesity during pregnancy is associated with multiple risks for both the woman and fetus, yet clinicians often feel unprepared to provide optimal antepartum care for this group of women. We collected and reviewed current evidence concerning antepartum care of women who are obese during pregnancy.
Methods:
We conducted a systematic review using PRISMA guidelines. Current evidence relating to the pregnancy care of women with a pre-pregnancy body mass index of 30kg/m2 or higher was identified using MEDLINE databases via PubMed, Embase, and Web of Science Core Collection between January 2012 and February 2018.
Results:
A total of 354 records were located after database searches, of which 63 met inclusion criteria. Topic areas for of included studies were: pregnancy risk and outcomes related to obesity, communication between women and health care providers, gestational weight gain and activity/diet, diabetic disorders, hypertensive disorders, obstructive sleep apnea, mental health, pregnancy imaging and measurement, late antepartum care, and preparation for labor and birth.
Discussion:
Midwives and other health care providers can provide better antepartum care to women who are obese during pregnancy by incorporating evidence from the most current clinical investigations.
Keywords: obesity, pregnancy, evidence-based practice, antepartum care, person-centered care
INTRODUCTION
Nearly 40% of women are obese during their childbearing years, with 37.0% of US women between ages 20 and 39 years having a body mass index (BMI) of 30 kg/m2 or higher (95% CI, 33.9–40.3).1 In non-Hispanic black and Hispanic women, prevalence of obesity is even higher; 56.7% (95% CI, 48.6–64.6) of non-Hispanic black women and 43.3% (95% CI, 33.4–53.7) of Hispanic women have a BMI that is 30 kg/m2 or greater. The prevalence of obesity has increased by nearly 10 percentage points among adults in the United States over the past 2 decades, leaving many health care providers feeling overwhelmed and underprepared.2,3
During pregnancy, maternal obesity is associated with multiple risks for both women and their neonates. However, recommendations from professional organizations in the United States and globally that address care of these pregnant women differ.4–7 Moreover, research in this area is rapidly evolving, making it difficult for the busy clinician to stay abreast of the newest findings. The purpose of this review is to present the current evidence regarding antepartum care for women who are obese.
METHODS
We conducted a systematic review of current evidence on the subject of antepartum care for women who are obese. A health sciences library informationist developed and conducted extensive searches of the MEDLINE database via PubMed, Embase, and Web of Science Core Collection to identify potential titles. The following search concepts were combined using MeSH in PubMed, Emtree in Embase, and Topic tags in Web of Science: obese, weight gain, overweight or BMI AND pregnancy, labor, prenatal, antenatal, or antepartum. Within these searches, we focused on pregnancy complications commonly associated with maternal obesity from previous reviews of the literature, also including ovulation inhibition, ovulatory dysfunction, vitamin D deficiency, folic acid deficiency, dietary supplements/therapeutic use, congenital abnormalities, fetal version, depression, anxiety, sleep apnea, ultrasound, or poor pregnancy outcomes AND patient care or patient care management. Boolean conjunctions and additional keywords were added as appropriate. The searches were limited to humans, English language publications, and materials published between January 2012 and September 2017. Systematic reviews and clinical studies were included. All searches were completed in September 2017 and updated in February 2018. Results were exported to EndNote, then reviewed by other authors.
Studies were selected if they met our inclusion criteria of reporting outcomes that could influence the content of antepartum care and included analyses of women who were obese during pregnancy. We classified each included study using the Joanna Briggs Institute levels of evidence for effectiveness8 and excluded case reports, case series, nonsystematic review articles, and methods reports. Thus, we limited this review to studies rated as Joanna Briggs Institute Level 1 (experimental designs), Level 2 (quasi-experimental designs), Level 3 (observational analytic designs), or Level 4a-b (observational descriptive studies through cross-sectional designs). Titles and abstracts were screened by the first author, and full-text articles were reviewed if their abstracts did not supply enough information to interpret eligibility for inclusion. Then, articles were independently reviewed for eligibility in this review by the first and third authors. We excluded full-text articles that either did not focus on pregnancy, did not report results that were related to antepartum care, did not include groups or analyses by obesity, were Joanna Briggs Evidence Levels 4–5, or were reports of methodology only.
Data extraction from studies that met inclusion criteria was performed by the first and third authors. We grouped studies by their area of focus within antepartum care (ie, pregnancy risk and outcomes, communication, gestational weight gain and activity/diet, diabetic disorders, hypertensive disorders, obstructive sleep apnea [OSA], mental health, imaging and measurement during pregnancy, late antepartum/labor preparation).
RESULTS
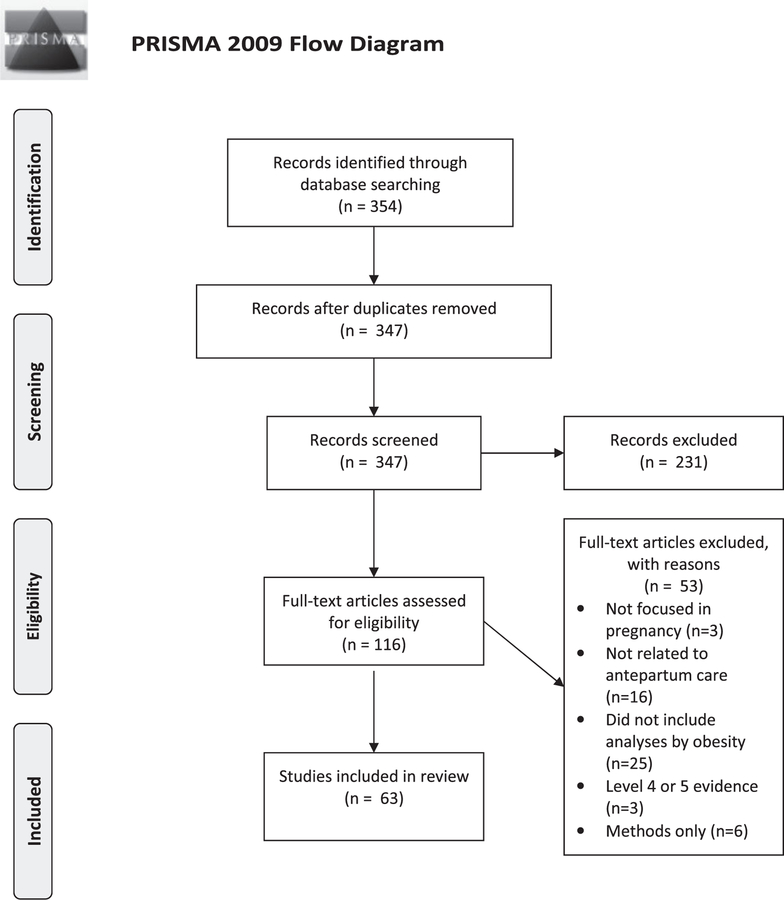
A total of 354 citations were retrieved and downloaded to EndNote. Figure 1 includes an accounting of each study, with reasons for inclusion or exclusion provided. After applying inclusion and exclusion criteria, we retained 63 studies for this review. Results are presented for each area of antepartum care.
Figure 1.
Flow Diagram Showing Identification and Screening of Included Studies
Techniques for Optimizing Communication between Health Care Providers and Women Who Are Obese
Interactions between health care providers and women who are obese during pregnancy involve topics that can be very sensitive. Evidence identified in this review reveals that, too often, health care providers are unsure about how to talk to women and instead tend to avoid or only vaguely cover important topics related to obesity.9 For example, in a mixed methods survey of women receiving antenatal care in the United Kingdom, women across all BMI categories expressed a desire for more engagement from their health care providers on the issue of body weight during pregnancy.10 However, nearly 70% of women who were obese (69.4%, 25/36 women) received no advice from their health care providers about recommended weight gain during pregnancy. In the United States, researchers found similar limitations in health care provider advice about gestational weight gain and physical activity.11 Among women with obesity, only 8.3% (2/24) received appropriate weight gain goals, and over half (14/24) were erroneously instructed to avoid exercise during pregnancy.11 Moreover, in a systematic review of qualitative evidence, women who were obese indicated that although their health care providers rarely communicated directly about weight, they nevertheless felt stigmatized during routine examinations.9 Health care providers require additional support with regard to both the content of antepartum care and techniques for working with women who are obese.
There is some evidence from a survey of Australian midwives (N = 335) that those using clinical guidelines in their care of women who are obese or overweight were more likely to consistently discuss weight issues (odds ratio [OR], 3.5; 95% CI, 1.9–6.4), to recommend referrals as suggested by guidelines (OR, 2.9; 95% CI, 1.2–3.4), and to feel more confident in their work with women compared with midwives who did not have a clinical guideline.12 Most major professional maternity care and gynecology associations now include separate guidelines for the care of women who are obese during the preconception, antepartum, intrapartum, and postpartum periods. Table 1 includes a comprehensive list of recommendations for the care of women who are obese during pregnancy, comparing professional organizations in the United States,13 United Kingdom,5 Australia and New Zealand,7 and Canada.6
Table 1.
Summary of Key Recommendations from Professional Organization Guidelines on the Antepartum Care of Women Who Are Obese
| Source of Guideline |
||||
|---|---|---|---|---|
| Recommendations | ACOG13 | RCOG5 | SOGC6 | RANZOG7 |
| First-Trimester Pregnancy Care | ||||
| Encourage women enter pregnancy with a BMI <30 kg/m2 through preconception counseling | X | X | X | X |
| Clear policies and guidelines should be available in all antepartum locations | X | |||
| Calculate BMI by first prenatal visit, document in health record, and use BMI to help guide care throughout pregnancy | X | X | X | X |
| Share risks associated with obesity and the limitations of ultrasounds for identifying structural anomalies | X | X | X | X |
| Recommend gestational weight gain on basis of initial visit BMI (if care initiated in first trimester; otherwise, use pre-pregnancy BMI), then track during pregnancy | X | X | X | X |
| Use motivational interviewing to promote dietary control, exercise, and behavior modification | X | |||
| Discourage weight loss during pregnancy | X | X | ||
| Recommend daily exercise to limit gestational weight gain and decrease risk of health complications | X | X | X | |
| Screen for depression and anxiety early and frequently; refer for psychological support as needed | X | |||
| Equipment: scales that can measure up to 200 kg, large blood pressure cuffs, appropriate sitting room chairs | X | X | X | |
| Screen obstructive sleep apnea at first antenatal visit in all women | X | |||
| Assess women with BMI ≥ 30 kg/m2 for risk of thromboembolism at first antenatal visit | X | |||
| Referrals | ||||
| Referral to nutritionist for counseling on nutrition and food choices | X | X | X | X |
| Refer to exercise physiologist | X | |||
| Refer women screening high-risk for obstructive sleep apnea for sleep study and consultation with sleep medicine specialist | X | |||
| Consider high-risk obstetric consultation and ultrasound, especially if local ultrasound does not have expertise in scanning for women who are obese | X | X | ||
| Refer women with history of bariatric surgery to dietitian, especially if she had malabsorptive surgery; consider additional supplementation of vitamin B12, iron, folate, vitamin D, and calcium | X | |||
| Supplements and medications to recommend | ||||
| High-dose folic acid 5 mg/day to decrease risk of neural tube defects if BMI >30 kg/m2, starting at least one month before pregnancy | X | X | ||
| Vitamin D 10 mcg/day (16,000 units/day) | X | |||
| Iodine supplement 150 mcg/day | X | |||
| Consider aspirin supplementation (75 mg/day from 12 weeks’ gestation until birth) for hypertensive disorder risk reduction | X | |||
| Imaging and laboratory tests | ||||
| Increased risk of congenital anomalies and neural tube defects: Encourage first-trimester options for genetic and congenital anomaly screening | X | X | X | |
| Test for vitamin D deficiency; supplement if needed | X | |||
| Baseline glucose intolerance screening in early pregnancy | Xa | Xb | ||
| Second-Trimester Pregnancy Care | ||||
| Track weight gain throughout pregnancy; discuss with woman | X | X | X | X |
| Surveillance for preeclampsia at every visit | X | |||
| Referrals | ||||
| Plan antenatal consultation with anesthesiologist to review analgesic options, discuss regional analgesia plan if desired, and make a plan to have needed staff and resources available if intubation is needed | X | Xc | X | X |
| Imaging and laboratory tests | ||||
| Encourage fetal anatomic assessment in second trimester between 20 and 22 weeks, rather than between 18 and 20 weeks, for better visualization | X | |||
| Repeat diabetes screening between 24 and 28 weeks | X | X | X | |
| Consider regular ultrasound assessments for fetal growth and well-being | X | |||
| Third-Trimester Pregnancy Care | ||||
| Weekly visits for preeclampsia surveillance | X | |||
| Use woman’s initial-visit BMI to help individualize counseling for vaginal birth after cesarean, given evidence of decreased success rates and increased complications with trial of labor after cesarean birth | X | X | X | X |
| Discuss with woman that if her BMI >40 kg/m2, intravenous access during labor is recommended | X | X | ||
| Make a plan with the woman for venous thromboembolism prevention, including weight-based thromboprophylaxis if BMI >40 kg/m2 | X | X | X | X |
| If the woman is planning cesarean birth, coordinate with the hospital to verify they have operating room, surgical instruments, and other equipment and staff available to accommodate women with weight >205 kg | X | X | X | X |
| Referrals | ||||
| Recommend visit with lactation consultant to begin education and support for initiation and continuation of breastfeeding, which is more difficult in women who are obese | X | X | ||
| If BMI ≥ 30 kg/m2, have informed discussion with obstetrician about intrapartum complications, including slow labor, shoulder dystocia, emergency cesarean birth, postpartum hemorrhage, and more difficult cesarean birth | X | |||
| Imaging and laboratory tests | ||||
| Continue regular ultrasound assessments for fetal growth and well-being | X | |||
Abbreviations: ACOG, American College of Obstetricians and Gynecologists; BMI, body mass index; RANZOG, Royal Australian and New Zealand College of Obstetricians and Gynaecologists; RCOG, Royal College of Obstetricians and Gynaecologists; SOGC, Society of Obstetricians and Gynaecologists of Canada.
For women with BMI ≥ 30 kg/m2.
For women with BMI ≥ 25 kg/m2.
For women with BMI ≥ 40 kg/m2.
Authors of some clinical guidelines and research studies are shifting to using person-centered language to support women.14 In person-centered language, women are not labeled as obese (condition first: obese woman, morbidly obese woman), but are instead described in terms of their weight or condition (person first: woman with obesity, woman with weight in high range). Likewise, in person-centered care, health care providers initiate partnerships with women based on their circumstances and motivations (Figure 2).15 When individuals are addressed using person-centered language and work with health care providers who focus on positive actions to limit their risks, they may feel less stigmatized and more empowered to optimize their health.
Figure 2.
Principles of Person-Centered Antepartum Care with Example
Adapted from Ekman et al.15
Pregnancy Risks and Outcomes Related to Obesity
The extant literature includes several investigations that add to our understanding of the risks associated with extra weight in women who are pregnant (Table 2). Among maternal outcomes associated with elevated BMI, researchers working with a cohort of 6674 women in Canada found a dose-dependent relationship between a woman’s pre-pregnancy BMI and her risk of hypertensive disorders of pregnancy, gestational diabetes mellitus (GDM), labor induction, and cesarean birth.16 Likewise, in a 2015 review of 22 systematic reviews, pregnant women with obesity were at increased risk for GDM, gestational hypertension and preeclampsia, mental ill health including anxiety and depression, postpartum depression, cesarean birth, and instrumental birth and were also at greater risk of having neonates who were born preterm, large-for-gestational-age, with congenital anomalies, and with higher risks of dying in the perinatal period.17 Another meta-analysis corroborated many of these findings, showing higher rates of infant death in women who were obese during pregnancy, with higher BMIs being linked to successively higher risks of infant death.18 Compared with outcomes from women with normal weight, infant mortality ranged from a 42% increased risk in women with a BMI between 30 and 34.99 kg/m2 (OR, 1.42; 95% CI, 1.24–1.63; P < .001; 11 studies) to a risk more than 2-fold higher among women with a BMI of 35 kg/m2 (OR, 2.03; 95% CI, 1.61–2.56; P < .001).
Table 2.
Short- and Long-Term Increased Risks Associated with Maternal Obesity
| Risks for the Woman Associated with Maternal Obesity | Risks for the Fetus or Neonate Associated with Maternal Obesity |
|---|---|
| Short-term outcomes | |
| Pregnancy loss83 | Congenital anomalies17,87 |
| Gestational diabetes16,17,26,44,84,85 | Stillbirth, infant mortality17,18 |
| Gestational hypertension16,17,26,84,85 | Preterm birth17,88: spontaneous preterm (22–27 weeks’ gestation) and induced preterm (22–36 weeks’ gestation) |
| Preeclampsia16,17,26,52,84,85 | |
| Anxiety73,86 | Large-for-gestational-age neonate17,23,25 |
| Depression17,86 | Shoulder dystocia89 |
| Obstructive sleep apnea64 | NICU admission26 |
| Gestational weight gain beyond recommended levels23 | Lower breastfeeding initiation17 |
| Inpatient care during pregnancy84 | Early breastfeeding cessation17 |
| Labor induction16 | Neonatal hypoglycemia26 |
| Longer labor duration80,81 | |
| Cesarean birth16,17 | |
| Surgical site infections17 | |
| Long-term outcomes | |
| Cardiovascular morbidity90 | Increased risk of poor cardiometabolic profiles into adulthood93 |
| Persistent obesity91 | Increased risk of obesity into adulthood93 |
| Infertility, longer time to conception92 |
Abbreviation: NICU, neonatal intensive care unit.
These risks are specific to maternal obesity during pregnancy and do not include other long-term health risks associated with obesity regardless of pregnancy status.
Professional organizations in the United States and internationally recommend that women who are obese decrease their pregnancy risks by losing weight before conception,4–7 including consideration of bariatric surgery (Table 1). In a recent study in which researchers conducted a meta-analysis matching women who did or did not have a history of bariatric surgery before pregnancy by their BMI, women who had surgery had lower risks of preeclampsia (OR, 0.45; 95% CI, 0.25–0.80; P = .007), GDM (OR, 0.47; 95% CI, 0.40–0.56; P < .001), and newborns who were large for gestational age (OR, 0.46; 95% CI, 0.34–0.62; P < .001).19 However, women with a history of bariatric surgery also had a higher incidence of preterm birth (OR, 1.31; 95% CI, 1.08–1.58; P = .006), small neonates (OR, 1.93; 95% CI, 1.52–2.44; P < .001), admission of their neonates to intensive care (OR, 1.33; 95% CI, 1.02–1.72; P < .03), and maternal anemia (OR, 3.41; 95% CI, 1.56–7.44; P = .002).19 Laparoscopic adjustable gastric banding procedures did not appear to have the same risks as other types of bariatric surgeries with regard to the rate of intrauterine growth restriction, small for gestational age (SGA) neonates, or low-birth-weight (LBW) neonates (OR, 1.93; 95% CI, 1.52–2.44; P < .001).19
Gestational Weight Gain in Women with Obesity
Professional guidelines from different countries include a variety of recommended gestational weight gain limits for women who are obese during pregnancy, ranging from 11 to 20 pounds in the US guidelines,7,13,20,21 approximately 15 pounds (7 kg) in Canadian guidelines,6 to no formal recommendations in the United Kingdom guidelines.5,22 In addition, some have questioned the lack of more specific gestational weight gain recommendations for women in higher obesity groups. Evidence identified for this review includes several studies that have explored the positive and negative effects of different degrees of gestational weight gain on maternal and neonatal outcomes. In a systematic review of 10 observational studies (N = 740,000 women with obesity), the lowest combined risk for poor maternal and fetal outcomes was achieved when women gained different amounts of weight according to their degree of obesity.23 Optimal gestational weight gain for women with a pre-pregnancy BMI between 30 and 34.99 kg/m2 was 5 to 9 kg. For women with a prepregnancy BMI of 35.00 to 39.99 kg/m2, the optimal weight gain was 1 to 5 kg, and no weight gain was optimal in this investigation for women with a prepregnancy BMI of at least 40 kg/m2.
Stratification of weight gain by women’s degree of obesity was also the recommendation of researchers who conducted a retrospective cohort analysis of maternal and neonatal outcomes of pregnant women with obesity (N = 18,053) in Belgium.24 They found that weight loss and low weight gain (0–5 kg) during pregnancy were associated with reduced rates of large-for-gestational-age as well as macrosomic neonates across all classes of obesity, with more profound decreases in large-for-gestational-age newborns among women with a BMI of 40 kg/m2 or more. They also found benefits for maternal health from low or no gestational weight gain among women with obesity. For example, women with prepregnancy BMIs between 30.00 and 34.99 kg/m2 who had small gestational weight gains during pregnancy (0–5 kg) were 54% less likely to develop gestational hypertension (OR, 0.46; 95% CI, 0.21–0.99) than women gaining 5 to 9 kg, as recommended by the Institute of Medicine.21 Lower gestational weight gain was also associated with reductions in unplanned cesarean birth.24 Importantly, these researchers did not find associations between gestational weight loss or low weight gain and rates of LBW neonates, SGA neonates, or neonatal intensive care unit (NICU) admissions among women with pre-pregnancy obesity.
Researchers have also attempted to better understand how prepregnancy BMI and gestational weight gain interact with other metabolic factors to change perinatal outcomes. Excess neonatal birth weight is a known risk associated with maternal obesity. Retnakaren and colleagues recently examined how prepregnancy cardiometabolic factors such as maternal blood pressure, glucose levels, lipid profiles, and excess gestational weight gain during pregnancy contributed to higher neonatal birth weights.25 In a prospective observational cohort study conducted in China (N = 1484), researchers found that the only independent predictors for having a large-for-gestational-age neonate were prepregnancy BMI (OR, 1.21 per kg/m2; 95% CI, 1.07–1.37) and gestational weight gain (OR, 1.10 per kg/m2; 95% CI, 1.06–1.14).25
This finding of larger birth weights among neonates born to women with higher prepregnancy weight or larger gestational weight gains is logical, given evidence in another recent study that maternal glucose profiles are elevated in women who are obese during pregnancy, even when they do not have diabetes diagnoses.26 Among 1736 women, Suk et al saw stepwise increases in neonatal birth weight with increasing class of maternal obesity. The mean neonatal birth weight among women with a normal BMI was 3262 g, which increased across weight categories with the highest mean birth weight of 3440 g occurring in women with a BMI greater than 39.99 kg/m2 (P < .05). In addition, neonatal hyperglycemia explained the increase in NICU admissions in neonates born to women who were obese. Neonates born to women with BMIs of 32.5 kg/m2 or higher at the time of labor had the highest risks of neonatal hyperglycemia.26
Based on these studies, clinicians should emphasize the importance of preconception weight loss in women with obesity. Gestational weight gain goals should be reviewed early in pregnancy, and clinicians should share with women information about how they might customize their gestational weight gain based on their starting BMI to decrease risks of developing comorbidities or having a surgical birth and decrease the risk that their neonate will be large for gestational age or need special care at birth. Unfortunately, women who are obese tend to have higher gestational weight gains than current guidelines recommend, regardless of the recommended gestational weight gain range.23 In the following sections, we review evidence on exercise and dietary interventions to decrease gestational weight gain in women with obesity.
Gestational Weight Gain and Exercise
Exercise during pregnancy is one strategy for decreasing gestational weight gain and is endorsed by most professional organizations in their pregnancy care guidelines for women with obesity (Table 1). In the National Institute for Health and Care Excellence (NICE) weight management guidelines, antenatal care providers are cautioned to be specific when recommending exercise by telling women that activities such as swimming, brisk walking, or strength conditioning are safe and that the aim should be to stay fit, not to reach peak fitness.22 The NICE guidelines also recommend giving a woman information about how much she should exercise depending on her beginning level of fitness. For example, women who have not exercised before pregnancy should limit their exercise sessions to 15 minutes, 3 times per week, increasing to daily 30-minute sessions. Several professional organizations emphasize the importance of telling women that being sedentary during pregnancy is likely to increase their risk for adverse outcomes.7,22
Several groups of researchers have tested exercise interventions to see if they help women with overweight or obese prepregnant BMIs to lower their gestational weight gain. Unfortunately, there is no evidence to date that exercise interventions improve laboratory results, physical measurements, or birth outcomes, perhaps in part because study participants poorly used the exercise and lifestyle changes tested in these studies.27–31 In fact, in one randomized controlled trial (RCT) involving a home-based antenatal exercise intervention, only 33% of exercise sessions were completed by participants based on data from heart rate monitors.32 By contrast, in another RCT in which Hispanic women did exercise vigorously after a culturally and linguistically targeted exercise intervention, those who were overweight and obese had reductions in insulin resistance, gestational weight gain, and newborn birth weights compared with women in the standard care group.33
Building upon this evidence that regular, vigorous exercise during pregnancy is necessary for women with obese or overweight BMIs to see improvements in pregnancy out-comes, several recent trials showed positive pregnancy out-comes after supervised exercise sessions during pregnancy. For example, in a trial involving a 12-week supervised exercise program from 15 to 27 weeks’ gestation, women with obesity gained less weight than similar women who received standard care (mean difference in weight change between groups was −0.1 kg/week; 95% CI, −0.2 to −0.02; P = .016) and maintained higher levels of cardiovascular fitness across pregnancy (difference in fitness change between groups was 8.1%, 95% CI, 0.7–9.5; P = .041).34 In another trial, 300 Chinese women with obese or overweight prepregnancy BMIs who were randomized to a cycling exercise program (at least 30 minutes, 3 times per week, starting in the first trimester, continued until 37 weeks’ gestation) not only gained significantly less weight during pregnancy (8.38 [3.65] kg vs 10.47 [3.33] kg; P < .001) but also had lower rates of GDM (22.0% vs 40.6%; P < .001), compared with similar women in standard care.35
Some health care providers are reluctant to encourage antenatal exercise for fear of poor fetal or neonatal outcomes. Encouragingly, researchers found no evidence that supervised exercise throughout pregnancy increased rates of preterm birth in the Chinese cycling trial mentioned earlier.35 Also, in a meta-analysis of 9 RCTs (N = 1502 women with prepregnancy BMIs of 25 kg/m2 or higher), aerobic exercise (30–60 min, 3–7 times per week) starting in early pregnancy actually lowered the incidence of preterm birth (RR, 0.62; 95% CI, 0.41–0.95) and GDM (RR, 0.61; 95% CI, 0.41–0.90). Further-more, this intervention was not associated with any difference in birth weight or stillbirth compared with standard care.36 In summary, regular vigorous exercise during pregnancy provides health benefits to women with prepregnancy overweight or obesity and is not associated with adverse effects, but adherence to such regimens may be difficult to maintain.
Gestational Weight Gain and Dietary Interventions
Research has shown disappointing results when testing the effects of dietary advice interventions on gestational weight gain in women who are obese during pregnancy. In the MOM-FIT trial, weekly coaching of 281 women who were over-weight or obese about a customized Dietary Approaches to Stop Hypertension (DASH)-style diet did not result in differences for pregnancy outcomes.37 In another RCT involving 382 women who were obese or overweight during pregnancy, serial weighing and dietary advice compared with standard antenatal care did not change gestational weight gain, comorbidities, or birth outcomes.38 An intervention involving a series of 4 face-to-face counseling sessions about weight, physical activity, and diet during pregnancy also did not show significant effects on gestational weight gain in 219 women with prepregnancy overweight or obesity.39 Similarly, in the RCT that involved a 4-session lifestyle intervention in which women were supported and empowered to optimize their lifestyle and gestational weight gain, investigators saw no difference in gestational weight gain among women with prepregnancy obesity. However, this study did find decreased gestational weight gain among women who started pregnancy overweight compared with women in the control group (7.8 [3.4] kg vs 6.0 [2.2] kg, P < .05).40
This finding that lifestyle interventions during pregnancy may not be as effective in women with obese pre-pregnancy BMIs compared with women with lower BMIs was corroborated in other studies. In an RCT by Hui and colleagues,41 women with normal pre-pregnancy BMIs who received a lifestyle intervention demonstrated lower rates of gestational weight gain and neonatal birth weight than the controls. However, these differences were not seen in women with above-normal pre-pregnancy BMIs. In another study by Peccei and colleagues,42 women with overweight or obese pre-pregnancy BMIs (N = 300) showed no differences in gestational weight gain after randomization to an intensive nutrition counseling intervention. Interestingly, when these researchers focused on women who actually completed the lifestyle intervention throughout their pregnancy, they found that women with overweight BMIs gained significantly less gestational weight than women who did not participate in the intervention (mean difference of −5.3 lb; 95% CI, −10.0 to −0.6), whereas women who were obese had no reduction in gestational weight gain regardless of their level of participation.
In summary, lifestyle interventions to encourage improved diet or activity during pregnancy appear to benefit women with normal or overweight pre-pregnancy BMIs but may not be sufficient to prevent adverse perinatal outcomes in women with pre-pregnancy obesity. It is unclear if these disappointing results are caused by poor adherence or metabolic differences that make weight loss more difficult for women who are obese. Encouragingly, researchers in England found that targeting dietary interventions to the culture and environment of women with obesity succeeded in changing food eating patterns.43 More research is needed on culturally targeted lifestyle interventions to improve pregnancy outcomes for women with obesity.
Screening for Diabetes and Interventions for Prevention of GDM
An elevated BMI during pregnancy is an independent risk factor for insulin resistance and GDM, as well as pre-existing diabetes. Several studies included in this review provided information about first-trimester screening strategies to identify women with diabetes. In one study, researchers found that a random blood glucose test (135 mg/dL cutoff) at 12 to 16 weeks’ gestation performed better than maternal pre-pregnancy BMI or age alone at predicting GDM later in pregnancy.44 In another study, 5.4% of women with pre-pregnancy BMIs greater than 34.9 kg/m2 were diagnosed with GDM at 20 weeks’ gestation, prompting those researchers to recommend this BMI level as a cutoff for first-trimester screening.45 Until the evidence is stronger regarding the best strategies for early pregnancy glucose intolerance testing, clinicians may wish to follow the recommendations of leading professional organizations (Table 1). The American College of Obstetricians and Gynecologists (ACOG)13 recommends a pre-pregnancy BMI cutoff of 30 kg/m2, whereas the Royal Australian and New Zealand College of Obstetricians and Gynaecologists5 recommends a cutoff of 25 kg/m2 for early pregnancy glucose intolerance testing using oral glucose tolerance tests.
Moving beyond screening for glucose intolerance, other research is focusing on the utility of early pregnancy lifestyle changes to prevent GDM from developing during pregnancy in women with pre-pregnancy obesity. Unfortunately, the additive metabolic changes present in women with both GDM and obesity appear to be resistant to exercise and weight restriction interventions. For example, women with normal-range BMIs but elevated hemoglobin A1c of 5.7% to 6.4% at less than 14 weeks’ gestation who were randomized to an intervention involving diet changes, blood glucose monitoring, and insulin treatment reduced their risk of developing GDM by 50%, but these interventions had no benefit in the 40% of women in this trial who were obese.46 Similarly, Casey et al conducted a secondary analysis of 958 women with GDM enrolled in an RCT designed to explore the influence of BMI on outcomes such as neonatal birth weight and fat mass.47 They found that formal nutritional counseling, diet therapy, daily self-monitoring of blood glucose, and insulin as needed, in women with BMIs of 40 kg/m2 or more, did not improve outcomes of excess neonatal weight or fat mass, whereas these interventions did reduce the incidence of excessive neonatal weight in women with lower BMI ranges (25.00–39.99 kg/m2).47
Metformin (Glucophage), which decreases inflammation, has been proposed as a therapeutic intervention for pregnant women with obesity to improve pregnancy outcomes. However, early results from the EMPOWaR study, a randomized, double-blind trial that compared the use of metformin (500 mg, increasing to a maximum of 2500 mg, dosed from 12–16 weeks’ gestation until birth) with placebo in 449 pregnant women with obesity and normal glucose tolerance, were not encouraging.48 Women taking metformin did not differ from women taking placebo with regard to birth weight or maternal or neonatal adverse outcomes.
Although early treatments and lifestyle modification in women who are obese have not yet been shown to be effective, other therapeutic targets are encouraging. For example, one study found that elevations in maternal triglycerides predicted large-for-gestational-age neonates in women who were overweight or obese and had GDM but maintained good glucose control during pregnancy.49 In addition, magnesium levels below the median in women with abdominal obesity were associated with abnormal oral glucose tolerance tests, insulin resistance, and proinflammatory oxidative stress.50 These or other laboratory markers may be tracked and/or targeted in the future to decrease outcomes of insulin resistance and hyperglycemia in women who are obese during pregnancy.
Interventions for Hypertensive Disorders of Pregnancy
Evidence-based adjustments in antenatal care strategies to help women with obesity avoid hypertension are critical to ensure a healthy pregnancy and reduce the risk of hypertensive disorders of pregnancy, preterm birth, and abruption.51 In a meta-analysis of 23 studies (N = 1,387,599 women), maternal obesity was associated with more than a 3-fold in-creased risk of preeclampsia (OR, 3.15; 95% CI, 2.96–3.35).52 When women who are obese during pregnancy have hypertensive disorders, they are more likely to die as a result of cardiovascular disease.53 Inadequate clinical care of these women has been cited as a key contributor to their deaths.53
Studies have identified behavioral risk factors and underlying biological mechanisms that increase the risk for hypertensive disorders of pregnancy in women who are obese. For example, in a recent study of women who were obese (N = 27,898), the participants were classified according to their reported gestational weight gain ranging from losing weight to excessive weight gain per the Institute of Medicine gestational weight gain guidelines.21 As expected, the rates of gestational hypertension and preeclampsia increased with each increasing BMI group (11.1% in women with BMI 30–34.9 kg/m2 vs 27.4% in women with BMI above 49.9 kg/m2).54 In addition, in women with pre-pregnancy obesity, excessive gestational weight gain was further associated with a higher incidence of gestational hypertension or preeclampsia compared with women with obesity who had a gestational weight gain within the recommended weight gain range (OR, 1.42; 95% CI, 1.30–1.55; P < .001).54 In contrast, in a recent multicenter RCT, antenatal lifestyle intervention in 1951 women who were overweight or obese did not result in differences in cardiometabolic or inflammatory biomarkers.55 Together, these findings suggest that although excessive gestational weight gain increases the risk of the development of a hypertensive disorder during pregnancy, lifestyle interventions that change women’s diet, physical activity, and other behaviors may not be sufficient to change the underlying biology in women who have pre-pregnancy obesity. The adipose and placental tissues of women who are obese are metabolically active sites with marked production of proinflammatory mediators,56 so it is possible that behavioral interventions may be insufficient to promote enough physiologic change to alter perinatal outcomes in some women who are obese.
Studies that have focused on hypertensive disorders of pregnancy in women who are obese have also explored the pathophysiology that may underlie these comorbid conditions. A recent prospective cohort study found that pregnant women who were obese demonstrated impaired myocardial function, measured by end-systolic wall stress, left ventricle cardiac index, and left ventricle mass index, compared with women who were not obese.57 These researchers also showed that women with obesity had higher blood pressure readings, systemic vascular resistance measures, and pulsatility index scores than the control group, particularly between 25 and 30 weeks’ gestation. In another study, researchers followed 614 women who initiated recommended aspirin therapy before 16 weeks’ gestation for preeclampsia prevention.58 They found that women who were obese in their first trimester were 80% more likely than women who were not obese to develop preeclampsia despite aspirin prophylaxis (OR, 1.8; 95% CI, 1.1–3.1; P = .034).58 Finally, women’s gut microbes may play a role in the link between high blood pressure measures and obesity during pregnancy. Women with the gut microbe Odoribacter had lower systolic blood pressures and lower levels of plasminogen activator inhibitor, a factor known to be involved in vascular epithelial injury.59,60 Taken together, these studies suggest that hypertensive disorders in women who are obese during pregnancy involve greater and varied systemic effects, with implications for the decreased efficacy of commonly used antepartum prophylactic measures like aspirin and exercise.
Complications related to obesity during pregnancy may also worsen outcomes in women who have hypertension. In a recent study of 54 pregnant women with obesity, preeclampsia, and/or hypertension, investigators evaluated the women’s risk of OSA.61 Women with obesity and preeclampsia had a higher risk of OSA. This link between maternal obesity and OSA was also seen in a large, multi-institutional cohort (N = 1,169,211) that additionally found an increased risk for peripartum cardiomyopathy within the first 180 days after birth (P < .001)62 in pregnant women with obesity, hypertension, and symptoms of OSA.
More studies are needed to elucidate the underlying biological mechanisms underpinning hypertension among women who are obese during pregnancy. However, literature included in this review indicates that exploring biologic mechanisms, such as gut health and oxygen deprivation during sleep, may be a future target of antepartum care to decrease hypertensive disease risk in these women. In addition, these studies reveal the important role of antepartum care in identifying women who are obese and are also at particular risk for OSA or cardiovascular events so they can be referred for sleep studies and/or cardiovascular specialists as indicated.
OSA Screening and Treatment
Many prenatal care providers are not aware of the importance of screening women for symptoms of sleep-disordered breathing, including OSA. In pregnancy, OSA is associated with adverse perinatal outcomes, including GDM and preeclampsia, as well as long-term cardiometabolic complications in both women and their offspring via mechanisms including intermittent hypoxia, oxidative stress, endothelial dysfunction, and hypothalamic-pituitary-adrenal axis (HPA) dysfunction.61–63 When a woman has OSA, her oxygen saturations periodically decrease through the night, leaving her tired in the morning and exposing her body and her fetus to stress.64 In a recent meta-analysis of 33 studies (N = 963,310), women with sleep-disordered breathing during pregnancy were more likely to be overweight or obese than controls and were twice as likely to experience stillbirth or perinatal death (OR, 2.02; 95% CI, 1.25–3.28).65 During labor, women with sleep-disordered breathing were 2.52 times more likely to have an emergency cesarean birth (95% CI, 1.20–5.29), and their neonates were more likely than those born to normal controls to have lower Apgar scores or to be admitted to the NICU.65 Corroborating these findings, another large study using linked maternal and new-born hospital records (N = 1,423,099) found that women with OSA were more likely to be obese and their neonates were more likely to require resuscitation after birth (adjusted OR, 2.76; 95% CI, 1.35–5.64), to have longer hospital stays after birth (adjusted OR, 2.25; 95% CI, 1.85–2.65), and to have congenital anomalies (adjusted OR, 1.26; 95% CI, 1.11–1.43).63
OSA is common in women with pre-pregnancy obesity but is often ignored by clinicians because OSA symptoms are dismissed as normal in pregnancy. In surveys of pregnant women with a range of BMIs, more than one-third reported snoring and restless sleep.66 However, sleep-disordered breathing during pregnancy in a woman with pre-pregnancy obesity is frequently a symptom of OSA; in one recent investigation of 105 women with prepregnant obesity, 18% tested positive for OSA in the first trimester of pregnancy.67 In fact, the incidence and severity of OSA are linked in a dose-dependent fashion with higher levels of obesity.64 Therefore, all women should be screened for OSA during pregnancy, but women who are overweight or obese and especially women with higher levels of obesity should be screened throughout pregnancy for these symptoms.65 There is also some recent evidence that neck circumference greater than 35.5 cm is an additional risk factor for OSA.68
Sleep-disordered breathing may help explain poor fetal and neonatal outcomes among women with obesity and other comorbidities. For example, in a detailed examination of sleep quality and fetal movements, researchers saw inspiratory flow limitations, increased rates of oxygen desaturations during sleep, reduced total fetal movements, and reduced rate of fetal hiccups in women with preeclampsia compared with similar women without preeclampsia.69 Continuous positive airway pressure, the common treatment for OSA, increased the number of fetal movements as well as fetal hiccups in these women, suggesting that the careful screening and treatment of sleep-disordered breathing among pregnant women with obesity may be one technique for protecting fetal health in situations of maternal comorbidities.
One of the most commonly used screening tools for OSA in the general population is the STOP-Bang questionnaire.70 STOP-Bang was recently tested in women who were obese during pregnancy, demonstrating a specificity of 97.5% but a disappointing sensitivity of less than 17%.67 Thus, STOP-Bang is a good questionnaire to rule out OSA but has limited ability to identify women who have OSA. An alternative screening for OSA during pregnancy for high-risk women, suggested in 2012 by Facco and colleagues, incorporates questions about frequent snoring, chronic hypertension, age, and BMI (Table 3).71 The Facco model performed better than other screening tools in identifying pregnant women with higher risk for OSA who went on to be diagnosed with the condition during a sleep study (P > .001). Given the high overlap between obesity and OSA in women who are pregnant, along with the concerning outcomes for women and their fetuses and neonates associated with maternal OSA, ACOG recommends that health care providers should screen all women for OSA and order sleep studies on any women who screen positive.13
Table 3.
The Facco Pregnancy-Specific Screening Tool for Sleep Apnea
| Topic | Question | Pointsa |
|---|---|---|
| Snoring | Do you snore on 3 or more days per week? | Yes = 15 No = 0 |
| Chronic hypertension | Did you have high blood pressure diagnosed before pregnancy? | Yes = 15 No = 0 |
| BMI | What is your BMI? | Total BMI |
| Age | What is your age? | Age in years |
Abbreviation: BMI, body mass index.
Score 0–74: low-risk for obstructive sleep apnea; score ≥75: screen positive for obstructive sleep apnea.
Adapted from Facco et al.71
Mental Health Screening and Treatment
Despite the fact that only one of the leading professional guidelines that addresses antepartum care of women with obesity recommends paying special attention to depression and anxiety,7 several studies in this review found evidence that maternal obesity during pregnancy is associated with higher risk for both depression17,72 and anxiety.73 Although screening for depression during pregnancy is recommended for all women regardless of BMI, it is especially important that clinicians include these screenings when caring for women with pre-pregnancy obesity.
Research in this area of antepartum care has largely focused on targeted antenatal lifestyle interventions to improve mental health symptoms in women with pre-pregnancy obesity.73–75 In a trial involving motivational interviewing provided by a midwife to women who were obese, depression scores were not affected, but both gestational weight gain and levels of anxiety decreased (P = .02).73 However, in another RCT of dietary and lifestyle or exercise advice provided to women who were overweight or obese during pregnancy, there was no change in women’s depression, anxiety, or quality of life scores compared with women in standard care.74
Perhaps a key to lifestyle interventions that show improvements in women’s mental health is the extent to which they emphasize partnership between the woman and her health care providers to create positive change. In a pilot study using both motivational interviewing and a person-centered approach, 107 low-income women who had depressive symptoms and were overweight or obese during pregnancy demonstrated decreased depression scores at 6 weeks postpartum compared with similar women who did not complete the intervention program.75
Imaging and Fundal Height Measurement
Fundal height measurements are likely to overestimate gestational age in women who are obese and therefore need to be adjusted or individualized to ensure greater accuracy. In a multisite retrospective study, fundal height growth curves were on average 0.1 to 0.4 cm higher during gestational weeks 22 to 29 in women who were overweight or obese compared with women with normal range BMIs, but these differences were not statistically significant.76 However, measurement differences became larger between 32 and 40 weeks’ gestation, when fundal heights were on average 0.6 to 0.8 cm higher, depending on the week, in women who were overweight or obese compared with those with normal weight (P < .001 for difference in 6 of the weeks between 32 and 40 weeks’ gestation). Although these differences are not large objectively, given that fundal height measurement of 2 to 3 cm beyond the weeks of gestation is a commonly used indication for ultrasound evaluation of fetal size,77 adjustment of fundal height norms for women with overweight or obesity between 32 and 40 weeks’ gestation may prevent some unnecessary referrals. These researchers recommended the use of individualized fundal height growth curves by pre-pregnancy BMI.76 In contrast to findings that fundal heights tend to overestimate gestational age in women who are obese, Källén et al found that second-trimester ultrasounds tend to underestimate gestational age in these women.78
Late Antepartum Care to Prepare for Labor and Birth
A key educational component of antepartum care is guiding women to prepare for a safe labor and birth. Several studies included in this review provide information about women’s reactions to end-of-pregnancy guidance. Most professional societies recommend an antepartum referral for women who are obese to the anesthetic service at their local hospital (Table 1). In a study of 114 women with BMIs of 35 kg/m2 or higher who were planning vaginal births, decisional conflict scores after anesthesia consultation were lower, but women’s anxiety scores did not significantly change.79 Although more women planned to use epidural analgesia for labor after these consultations, only about 20% of them understood that their BMI placed them at higher risk for adverse events after the anesthesia consultation.79 It may be beneficial to standardize the content of antepartum anesthesia consultation to ensure relevant information is effectively communicated to women who are obese.
Beyond anesthesia consultations in preparation for birth, we located no published studies on other childbirth preparation interventions for women with obesity during pregnancy. Given the known risks for this group of women to have longer labor course,80,81 higher risk for unplanned cesarean birth,82 and more difficulty initiating and maintaining lactation,17 the content and usefulness of special childbirth and lactation preparation to improve key outcomes is an important area for future research.
CONCLUSION
Maternal obesity changes the physiology of both the woman and fetus during pregnancy. Clinicians require information from well-conducted studies to help guide their care of women with obesity during pregnancy. In this systematic review of the current evidence concerning antepartum care of women who are obese during pregnancy, there are several key findings for clinicians. First, partnering with women who are obese by using motivational interviewing techniques, culturally appropriate suggestions, and person-first language appears to improve the success of several diet, exercise, and lifestyle interventions on various maternal and neonatal out-comes. Second, health care providers feel more confident and provide better quality care when they follow evidence-based guidelines for their work with women who are obese during pregnancy. Third, optimal gestational weight gain ranges may be lower and more specific to the degree of pre-pregnancy obesity than those currently suggested by Institute of Medicine guidelines. Fourth, women with obesity have a higher risk for comorbidities such as OSA, depression, and anxiety that may further increase the risk for adverse outcomes in both women and their neonates if not detected and treated. Fifth, there is a critical lack of current information about the best ways to prepare women who are obese during pregnancy for labor, birth, and lactation. This is a critical area for future research. Until the worldwide obesity epidemic is reversed, specialized pregnancy care of women who are obese offers our best hope of improving outcomes for women, neonates, and future generations.
Quick Points.
-
✦
Midwives and other health care providers can decrease the stigmatization that some women feel when they are obese during pregnancy by partnering with women in their care and using person-first language.
-
✦
Current studies reveal the important role of antepartum care in identifying women who are obese and have symptoms of comorbidities like obstructive sleep apnea or hypertension for specialized follow-up to decrease the risk of poor health outcomes across the lifetimes of both the women and their infants.
-
✦
Midwives and other health care providers using clinical guidelines in their care of women who are obese or overweight are more likely to consistently discuss weight issues, to share recommendations with women, and to feel greater confidence in their work.
ACKNOWLEDGMENT
Dr. Nicole Carlson receives support from the National Institute of Nursing Research (1K01NR016984) for her project, “The Metabolomics of Labor Dysfunction in African-American Women.”
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
Contributor Information
Nicole S. Carlson, The Nell Hodgson Woodruff School of Nursing and is in clinical practice at Grady Memorial Hospital and the Atlanta Birth Center in Atlanta, GA..
Sharon Lynn Leslie, The Emory University Woodruff Health Sciences Center Library, where she specializes as the Nursing Informationist..
Alexis Dunn, The Nell Hodgson Woodruff School of Nursing and is in clinical practice at Northside Women’s Specialists in Atlanta, GA..
REFERENCES
- 1.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA 2016;315(21):2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmied VA, Duff M, Dahlen HG, Mills AE, Kolt GS. ‘Not waving but drowning’: a study of the experiences and concerns of midwives and other health professionals caring for obese childbearing women. Midwifery 2011;27(4):424–430. [DOI] [PubMed] [Google Scholar]
- 3.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity among Adults and Youth: United States, 2015–2016. Atlanta, GA: Centers for Disease Control and Prevention; 2017. https://www.cdc.gov/nchs/products/databriefs/db288.htm. Accessed February 12, 2018. [PubMed] [Google Scholar]
- 4.American College of Obstetricians and Gynecologists. ACOG Committee Opinion no. 549: obesity in pregnancy. Obstet Gynecol 2013;121(1):213–217. [DOI] [PubMed] [Google Scholar]
- 5.Centre for Maternal and Child Enquires; Royal College of Obstetricians and Gynaecologists. CMACE/RCOG Joint Guideline: Management of Women with Obesity in Pregnancy Centre for Maternal and Child Enquires and Royal College of Obstetricians and Gynaecologists; March 2010. https://www.rcog.org.uk/globalassets/documents/guidelines/cmacercogjointguidelinemanagementwomenobesitypregnancya.pdf. Accessed February 16, 2018. [Google Scholar]
- 6.Davies GA, Maxwell C, McLeod L, et al. ; Society of Obstetricians and Gynaecologists of Canada. SOGC clinical practice guidelines: obesity in pregnancy. No. 239, February 2010. Int J Gynaecol Obstet 2010;110(2):167–173. [DOI] [PubMed] [Google Scholar]
- 7.Royal Australian and New Zealand College of Obstetricians and Gynaecologists. Management of Obesity in Pregnancy, C-Obs 49 Victoria, Australia: Royal Australian and New Zealand College of Obstetricians and Gynaecologists; 2016. https://www.ranzcog.edu.au/RANZCOG_SITE/media/RANZCOG-MEDIA/Women%27s%20Health/Statement%20and%20guidelines/Clinical-Obstetrics/C-Obs_49_Management-of-Obesity-in-Pregnancy-Review-Sep-2013.pdf?ext=.pdf. Accessed February 16, 2018. [Google Scholar]
- 8.Joanna Briggs Institute. The JBI Approach: Levels of Evidence http://joannabriggs.org/jbi-approach.html#tabbed-nav=Levels-of-Evidence. South Australia, Australia: Joanna Briggs Institute, University of Adelaide; 2014. Accessed February 16, 2018. [Google Scholar]
- 9.Johnson M, Campbell F, Messina J, Preston L, Buckley Woods H, Goyder E. Weight management during pregnancy: a systematic review of qualitative evidence. Midwifery 2013;29(12):1287–1296. [DOI] [PubMed] [Google Scholar]
- 10.Swift JA, Pearce J, Jethwa PH, et al. Antenatal weight management: women’s experiences, behaviours, and expectations of weighing in early pregnancy. J Pregnancy 2016;2016:8454759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stengel MR, Kraschnewski JL, Hwang SW, Kjerulff KH, Chuang CH. “What my doctor didn’t tell me”: examining health care provider advice to overweight and obese pregnant women on gestational weight gain and physical activity. Womens Health Issues 2012;22(6):e535–e540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biro MA, Cant R, Hall H, Bailey C, Sinni S, East C. How effectively do midwives manage the care of obese pregnant women? A cross-sectional survey of Australian midwives. Women Birth 2013;26(2):119–124. [DOI] [PubMed] [Google Scholar]
- 13.ACOG Practice Bulletin no. 156: obesity in pregnancy. Obstet Gynecol 2015;126(6):e112–e126. [DOI] [PubMed] [Google Scholar]
- 14.Olander EK, Berg M, McCourt C, Carlstrӧm E, Dencker A. Person-centred care in interventions to limit weight gain in pregnant women with obesity—a systematic review. BMC Pregnancy Childbirth 2015;15:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ekman I, Swedberg K, Taft C, et al. Person-centered care-ready for prime time. Eur J Cardiovasc Nurs 2011;10(4):248–251. [DOI] [PubMed] [Google Scholar]
- 16.El-Chaar D, Finkelstein SA, Tu X, et al. The impact of increasing obesity class on obstetrical outcomes. J Obstet Gynaecol Can 2013;35(3):224–233. [DOI] [PubMed] [Google Scholar]
- 17.Marchi J, Berg M, Dencker A, Olander EK, Begley C. Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews. Obes Rev 2015;16(8):621–638. [DOI] [PubMed] [Google Scholar]
- 18.Meehan S, Beck CR, Mair-Jenkins J, Leonardi-Bee J, Puleston R. Maternal obesity and infant mortality: a meta-analysis. Pediatrics 2014;133(5):863–871. [DOI] [PubMed] [Google Scholar]
- 19.Galazis N, Docheva N, Simillis C, Nicolaides KH. Maternal and neonatal outcomes in women undergoing bariatric surgery: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol 2014;181:45–53. [DOI] [PubMed] [Google Scholar]
- 20.Share with women: weight gain during pregnancy. J Midwifery Womens Health 2010;55(6):605–606. [DOI] [PubMed] [Google Scholar]
- 21.Institute of Medicine. Weight Gain During Pregnancy: Reexamining the Guidelines Washington, DC: National Academy of Sciences; May 2009. http://www.nationalacademies.org/hmd/~/media/Files/Report%20Files/2009/Weight-Gain-During-Pregnancy-Reexamining-the-Guidelines/Report%20Brief%20-%20Weight%20Gain%20During%20Pregnancy.pdf. Accessed February 16, 2018. [Google Scholar]
- 22.National Institute for Health and Clinical Excellence. Weight Management Before, During and After Pregnancy (PH27) Manchester, UK: National Institute for Health and Clinical Excellence; July 2010. [Google Scholar]
- 23.Faucher MA, Barger MK. Gestational weight gain in obese women by class of obesity and select maternal/newborn outcomes: a systematic review. Women Birth 2015;28(3):e70–e79. [DOI] [PubMed] [Google Scholar]
- 24.Bogaerts A, Ameye L, Martens E, Devlieger R. Weight loss in obese pregnant women and risk for adverse perinatal outcomes. Obstet Gynecol 2015;125(3):566–575. [DOI] [PubMed] [Google Scholar]
- 25.Retnakaran R, Wen SW, Tan H, et al. Maternal pre-gravid cardiometabolic health and infant birthweight: a prospective pre-conception cohort study. Nutr Metab Cardiovasc Dis 2017;27(8): 723–730. [DOI] [PubMed] [Google Scholar]
- 26.Suk D, Kwak T, Khawar N, et al. Increasing maternal body mass index during pregnancy increases neonatal intensive care unit admission in near and full-term infants. J Matern Fetal Neonatal Med 2016;29(20):3249–3253. [DOI] [PubMed] [Google Scholar]
- 27.Oostdam N, van Poppel MN, Wouters MG, et al. No effect of the Fit-For2 exercise programme on blood glucose, insulin sensitivity, and birthweight in pregnant women who were overweight and at risk for gestational diabetes: results of a randomised controlled trial. BJOG 2012;119(9):1098–1107. [DOI] [PubMed] [Google Scholar]
- 28.Dodd JM, Cramp C, Sui Z, et al. The effects of antenatal dietary and lifestyle advice for women who are overweight or obese on maternal diet and physical activity: the LIMIT randomised trial. BMC Med 2014;12:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dodd JM, Kannieappan LM, Grivell RM, et al. Effects of an antenatal dietary intervention on maternal anthropometric measures in pregnant women with obesity. Obesity (Silver Spring) 2015;23(8):1555–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garnæs KK, Mørkved S, Salvesen Ø, Moholdt T. Exercise training and weight gain in obese pregnant women: a randomized controlled trial (ETIP trial). PLoS Med 2016;13(7):e1002079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garnæs KK, Nyrnes SA, Salvesen KÅ, Salvesen Ø, Mørkved S, Moholdt T. Effect of supervised exercise training during pregnancy on neonatal and maternal outcomes among overweight and obese women. Secondary analyses of the ETIP trial: a randomised controlled trial. PLoS One 2017;12(3):e0173937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seneviratne SN, Jiang Y, Derraik J, et al. Effects of antenatal exercise in overweight and obese pregnant women on maternal and perinatal outcomes: a randomised controlled trial. BJOG 2016;123(4):588–597. [DOI] [PubMed] [Google Scholar]
- 33.Hawkins M, Hosker M, Marcus BH, et al. A pregnancy lifestyle intervention to prevent gestational diabetes risk factors in overweight Hispanic women: a feasibility randomized controlled trial. Diabet Med 2015;32(1):108–115. [DOI] [PubMed] [Google Scholar]
- 34.Bisson M, Alméras N, Dufresne SS, et al. A 12-week exercise program for pregnant women with obesity to improve physical activity levels: an open randomised preliminary study. PLoS One 2015;10(9): e0137742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang C, Wei Y, Zhang X, et al. A randomized clinical trial of exercise during pregnancy to prevent gestational diabetes mellitus and improve pregnancy outcome in overweight and obese pregnant women. Am J Obstet Gynecol 2017;216(4):340–351. [DOI] [PubMed] [Google Scholar]
- 36.Magro-Malosso ER, Saccone G, Di Mascio D, Di Tommaso M, Berghella V. Exercise during pregnancy and risk of preterm birth in overweight and obese women: a systematic review and meta-analysis of randomized controlled trials. Acta Obstet Gynecol Scand 2017;96(3):263–273. [DOI] [PubMed] [Google Scholar]
- 37.Peaceman AM, Kwasny MJ, Gernhofer N, Vincent E, Josefson JL, Van Horn L. MOMFIT: a randomized clinical trial of an intervention to prevent excess gestational weight gain in overweight and obese women. AJOG 2017;216(1, suppl):S2–S3. [Google Scholar]
- 38.McCarthy EA, Walker SP, Ugoni A, Lappas M, Leong O, Shub A. Self-weighing and simple dietary advice for overweight and obese pregnant women to reduce obstetric complications without impact on quality of life: a randomised controlled trial. BJOG 2016;123(6):965–973. [DOI] [PubMed] [Google Scholar]
- 39.Althuizen E, van der Wijden CL, van Mechelen W, Seidell JC, van Poppel MN. The effect of a counselling intervention on weight changes during and after pregnancy: a randomised trial. BJOG 2013;120(1):92–99. [DOI] [PubMed] [Google Scholar]
- 40.Harrison CL, Lombard CB, Strauss BJ, Teede HJ. Optimizing healthy gestational weight gain in women at high risk of gestational diabetes: a randomized controlled trial. Obesity (Silver Spring) 2013;21(5):904–909. [DOI] [PubMed] [Google Scholar]
- 41.Hui AL, Back L, Ludwig S, et al. Effects of lifestyle intervention on dietary intake, physical activity level, and gestational weight gain in pregnant women with different pre-pregnancy body mass index in a randomized control trial. BMC Pregnancy Childbirth 2014;14: 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peccei A, Blake-Lamb T, Rahilly D, Hatoum I, Bryant A. Intensive prenatal nutrition counseling in a community health setting: a randomized controlled trial. Obstet Gynecol 2017;130(2):423–432. [DOI] [PubMed] [Google Scholar]
- 43.Flynn AC, Seed PT, Patel N, et al. ; UPBEAT Consortium. Dietary patterns in obese pregnant women; influence of a behavioral intervention of diet and physical activity in the upbeat randomized controlled trial. Int J Behav Nutr Phys Act 2016;13(1):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meek CL, Murphy HR, Simmons D. Random plasma glucose in early pregnancy is a better predictor of gestational diabetes diagnosis than maternal obesity. Diabetologia 2016;59(3):445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Dwyer V, Farah N, Hogan J, O’Connor N, Kennelly MM, Turner MJ. Timing of screening for gestational diabetes mellitus in women with moderate and severe obesity. Acta Obstet Gynecol Scand 2012;91(4):447–451. [DOI] [PubMed] [Google Scholar]
- 46.Osmundson SS, Norton ME, El-Sayed YY, Carter S, Faig JC, Kitzmiller JL. Early screening and treatment of women with prediabetes: a randomized controlled trial. Am J Perinatol 2016;33(2):172–179. [DOI] [PubMed] [Google Scholar]
- 47.Casey BM, Mele L, Landon MB, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Does maternal body mass index influence treatment effect in women with mild gestational diabetes? Am J Perinatol 2015;32(1):93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chiswick C, Reynolds RM, Denison F, et al. Effect of metformin on maternal and fetal outcomes in obese pregnant women (EMPOWaR): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2015;3(10):778–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olmos PR, Rigotti A, Busso D, et al. Maternal hypertriglyceridemia: a link between maternal overweight-obesity and macrosomia in gestational diabetes. Obesity (Silver Spring) 2014;22(10):2156–2163. [DOI] [PubMed] [Google Scholar]
- 50.Mostafavi E, Nargesi AA, Asbagh FA, et al. Abdominal obesity and gestational diabetes: the interactive role of magnesium. Magnes Res 2015;28(4):116–125. [DOI] [PubMed] [Google Scholar]
- 51.Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P; Canadian Hypertensive Disorders of Pregnancy Working Group. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. Pregnancy Hypertens 2014;4(2):105–145. [DOI] [PubMed] [Google Scholar]
- 52.Poorolajal J, Jenabi E. The association between body mass index and preeclampsia: a meta-analysis. J Matern Fetal Neonatal Med 2016;29(22):3670–3676. [DOI] [PubMed] [Google Scholar]
- 53.Hameed AB, Lawton ES, McCain CL, et al. Pregnancy-related cardiovascular deaths in California: beyond peripartum cardiomyopathy. Obstet Gynecol Surv 2016;71(2):63–65. [DOI] [PubMed] [Google Scholar]
- 54.Barton JR, Joy SD, Rhea DJ, Sibai AJ, Sibai BM. The influence of gesta-tional weight gain on the development of gestational hypertension in obese women. Am J Perinatol 2015;32(7):615–619. [DOI] [PubMed] [Google Scholar]
- 55.Moran LJ, Fraser LM, Sundernathan T, et al. The effect of an antenatal lifestyle intervention in overweight and obese women on circulating cardiometabolic and inflammatory biomarkers: secondary analyses from the limit randomised trial. BMC Med 2017;15(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stupin JH, Arabin B. Overweight and obesity before, during and after pregnancy: part 1: pathophysiology, molecular biology and epigenetic consequences. Geburtshilfe Frauenheilkd 2014;74(7):639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Golinska Grzybala K, Konduracka E, Nessler J. Cardiac function in obese pregnant women. Poster PI875 presented at the 4th World Congress on Acute Heart Failure; April 29, 2017 to May 2, 2017; Paris, France. [Google Scholar]
- 58.Block-Abraham DM, Turan OM, Doyle LE, et al. First-trimester risk factors for preeclampsia development in women initiating aspirin by 16 weeks of gestation. Obstet Gynecol 2014;123(3):611–617. [DOI] [PubMed] [Google Scholar]
- 59.Gomez-Arango LF, Barrett HL, McIntyre HD, et al. ; SPRING Trial Group. Increased systolic and diastolic blood pressure is associated with altered gut microbiota composition and butyrate production in early pregnancy. Hypertension 2016;68(4):974–981. [DOI] [PubMed] [Google Scholar]
- 60.Martin C, Cameron J, McGrath B. Mechanical and circulating biomarkers in isolated clinic hypertension. Clin Exp Pharmacol Physiol 2008;35(4):402–408. [DOI] [PubMed] [Google Scholar]
- 61.Khan A, Andersen T, Rajmohan D, et al. Associations of high-risk pregnancy with OSA. Chest 2016;150(4, suppl):1280A. [Google Scholar]
- 62.Bauer A, Sheyn D, Dawodu K, ElAmm C, Al-Kindi S, Hackney D. Is obstructive sleep apnea associated with an increased incidence of peripartum cardiomyopathy? AJOG 2017;216(1, suppl):S348. [Google Scholar]
- 63.Bourjeily G, Danilack V, Bublitz M, Lipkind H, Caldwell D, Muri J. A national cohort study of obstructive sleep apnea in pregnancy and adverse neonatal outcomes. Sleep 2017;40(suppl 1):A177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Amnakkittikul S, Chirakalwasan N, Saetung S, Panburana P, Bumrungpeutch S, Reutrakul S. Obstructive sleep apnea and gestational diabetes mellitus: prevalence and relationship with glycemic control [Endocrine Society’s 97th Annual Meeting and Expo, presentation SAT-606]. Endocr Rev 2015;36(2, suppl). [Google Scholar]
- 65.Brown NT, Turner JM, Kumar S. The intrapartum and perinatal risks of sleep-disordered breathing in pregnancy: a systematic review and meta-analysis. [published online ahead of print February 15, 2018] Am J Obstet Gynecol 10.1016/j.ajog.2018.02.004 [DOI] [PubMed]
- 66.Angras K, Lott M, Schulkin J, Mackeen AD. Symptoms of obstructive sleep apnea in pregnancy [ACOG Annual Meeting, abstract 17C]. Obstet Gynecol 2017;129(suppl 1):34S–35S. [Google Scholar]
- 67.Sequeira T, Bublitz M, Adodoadji E, Livingston Z, Bourjeily G. STOP-Bang questionnaire correctly detects the absence of obstructive sleep apnea in the first trimester of pregnancy. Sleep 2017;40(suppl 1): A186. [Google Scholar]
- 68.Wanitcharoenkul E, Ongphiphadhanakul B, Chirakalwasan N, et al. Obstructive sleep apnea in gestational diabetes: prevalence, predictive factors and the development of a screening tool [presentation SAT-594]. Endocr Rev 2017;38(3, suppl). [Google Scholar]
- 69.Blyton DM, Skilton MR, Edwards N, Hennessy A, Celermajer DS, Sullivan CE. Treatment of sleep disordered breathing reverses low fetal activity levels in preeclampsia. Sleep 2013;36(1):15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chung F, Abdullah HR, Liao P. STOP-Bang questionnaire: a practical approach to screen for obstructive sleep apnea. Chest 2016;149 (3):631–638. [DOI] [PubMed] [Google Scholar]
- 71.Facco FL, Ouyang DW, Zee PC, Grobman WA. Development of a pregnancy-specific screening tool for sleep apnea. J Clin Sleep Med 2012;8(4):389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Herrero T, Fratto VM, Nelson E, Hamlin A, Laurent L, Ramos GA. The rate of elevated antenatal Edinburgh depression score in pregnancies complicated by gestational diabetes [ACOG Annual Meeting, abstract 12Q]. Obstet Gynecol 2017;129(suppl 1):176S. [Google Scholar]
- 73.Bogaerts AF, Devlieger R, Nuyts E, Witters I, Gyselaers W, Van den Bergh BR. Effects of lifestyle intervention in obese pregnant women on gestational weight gain and mental health: a randomized controlled trial. Int J Obes (Lond) 2013;37(6):814–821. [DOI] [PubMed] [Google Scholar]
- 74.Dodd JM, Newman A, Moran LJ, et al. The effect of antenatal dietary and lifestyle advice for women who are overweight or obese on emotional well-being: the LIMIT randomized trial. Acta Obstet Gynecol Scand 2016;95(3):309–318. [DOI] [PubMed] [Google Scholar]
- 75.Olayiwola JN, Irizarry OC, O’Connell K, Milan S. Living smart, living fit: a patient-centered program to improve perinatal outcomes in a community health center population. J Prim Care Community Health 2013;4(1):31–35. [DOI] [PubMed] [Google Scholar]
- 76.Deeluea J, Sirichotiyakul S, Weerakiet S, Arora R, Patumanond J. Fundal height growth curve for underweight and overweight and obese pregnant women in Thai population. ISRN Obstet Gynecol 2013;2013:657692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.King TL, Brucker MC, Kriebs JM, Fahey JO, Gegor CL, Varney H. Varney’s Midwifery 5th ed. Burlington, MA: Jones & Bartlett Learning; 2015. [Google Scholar]
- 78.Källén B, Finnstrӧm O, Nygren KG, Olausson PO. Maternal and fetal factors which affect fetometry: use of in vitro fertilization and birth register data. Eur J Obstet Gynecol Reprod Biol 2013;170(2):372–376. [DOI] [PubMed] [Google Scholar]
- 79.Eley VA, Donovan K, Walters E, Brijball R, Eley DS. The effect of antenatal anaesthetic consultation on maternal decision-making, anxiety level and risk perception in obese pregnant women. Int J Obstet Anesth 2014;23(2):118–124. [DOI] [PubMed] [Google Scholar]
- 80.Carlhäll S, Källén K, Blomberg M. Maternal body mass index and duration of labor. Eur J Obstet Gynecol Reprod Biol 2013;171(1):49–53. [DOI] [PubMed] [Google Scholar]
- 81.Lassiter JR, Holliday N, Lewis DF, Mulekar M, Abshire J, Brocato B. Induction of labor with an unfavorable cervix: how does BMI affect success? J Matern Fetal Neonatal Med 2016;29(18):3000–3002. [DOI] [PubMed] [Google Scholar]
- 82.Ellekjaer KL, Bergholt T, Løkkegaard E. Maternal obesity and its effect on labour duration in nulliparous women: a retrospective observational cohort study. BMC Pregnancy Childbirth 2017;17(1): 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bhandari H, Quenby S. Obesity is associated with loss of empty gestational sac pregnancies and in recurrent miscarriage is associated with ‘superfertility’ [BFS Annual Meeting, abstract]. Human Fertility 2014;17(2):144–145. [Google Scholar]
- 84.Lindholm ES, Altman D, Norman M, Blomberg M. Health care consumption during pregnancy in relation to maternal body mass index: a Swedish population based observational study. J Obes 2015;2015:215683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sun D, Li F, Zhang Y, Xu X. Associations of the pre-pregnancy BMI and gestational BMI gain with pregnancy outcomes in Chinese women with gestational diabetes mellitus. Int J Clin Exp Med 2014;7(12):5784–5789. [PMC free article] [PubMed] [Google Scholar]
- 86.Molyneaux E, Poston L, Ashurst-Williams S, Howard LM. Obesity and mental disorders during pregnancy and postpartum: a systematic review and meta-analysis. Obstet Gynecol 2014;123(4):857–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stothard KJ, Tennant PW, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA 2009;301(6):636–650. [DOI] [PubMed] [Google Scholar]
- 88.Gould JB, Mayo J, Shaw GM, Stevenson DK ; March of Dimes Prematurity Research Center at Stanford University School of Medicine. Swedish and American studies show that initiatives to decrease maternal obesity could play a key role in reducing preterm birth. Acta Paediatr 2014;103(6):586–591. [DOI] [PubMed] [Google Scholar]
- 89.Zhang C, Wu Y, Li S, Zhang D. Maternal pre-pregnancy obesity and the risk of shoulder dystocia: a meta-analysis. BJOG 2018;125(4):407–413. [DOI] [PubMed] [Google Scholar]
- 90.Yaniv-Salem S, Shoham-Vardi I, Kessous R, Pariente G, Sergienko R, Sheiner E. Obesity in pregnancy: what’s next? Long-term cardiovascular morbidity in a follow-up period of more than a decade. J Matern Fetal Neonatal Med 2016;29(4):619–623. [DOI] [PubMed] [Google Scholar]
- 91.Fraser A, Tilling K, Macdonald-Wallis C, et al. Associations of gestational weight gain with maternal body mass index, waist circumference, and blood pressure measured 16 y after pregnancy: the Avon Longitudinal Study of Parents and Children (ALSPAC). Am J Clin Nutr 2011;93(6):1285–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gesink Law DC, Maclehose RF, Longnecker MP. Obesity and time to pregnancy. Hum Reprod 2007;22(2):414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Santos Ferreira DL, Williams DM, Kangas AJ, et al. Association of pre-pregnancy body mass index with offspring metabolic profile: analyses of 3 European prospective birth cohorts. PloS Med 2017;14(8):e1002376. [DOI] [PMC free article] [PubMed] [Google Scholar]