Abstract
Welding fume is a complex mixture of different potentially cytotoxic and genotoxic metals, such as chromium (Cr), manganese (Mn), nickel (Ni), and iron (Fe). Documented health effects have been observed in workers exposed to welding fume. The objective of the study was to use an animal model to identify potential biomarkers of epigenetic changes (e.g., changes in telomere length, DNA methylation) in isolated peripheral blood mononuclear cells (PBMCs) after exposure to different welding fumes. Male Sprague-Dawley rats were exposed by intratracheal instillation (ITI) of 2.0 mg/rat of gas metal arc-mild steel (GMA-MS) or manual metal arc-stainless steel (MMA-SS) welding fume. Vehicle controls received sterile saline by ITI. At 4 h, 14 h, 1 d, 3 d, 10 d, and 30 d, bronchoalveolar lavage (BAL) was performed to assess lung inflammation. Whole blood was collected, and PBMCs were isolated. Dihydroethidium (DHE) fluorescence and 4-hydroxylnonenal protein adduct (P-HNE) formation were measured in PBMCs to assess reactive oxygen species (ROS) production. DNA alterations in PBMCs were determined by evaluating changes in DNA methylation and telomere length. Metal composition of the two fumes was different: MMA-SS (41 % Fe, 29 % Cr, 17 % Mn, 3 % Ni) versus GMA-MS (85 % Fe, 14 % Mn). The more soluble and chemically complex MMA-SS sample induced a more persistent and greater inflammatory response compared to the other groups. Also, oxidative stress markers increased at 24 h in the PBMCs recovered from the MMA-SS group compared to other group. No significant differences were observed when comparing DNA methylation between the welding fume and control groups at any of the time points, whereas the MMA-SS sample significantly increased telomere length at 1 and 30 d after a single exposure compared to the other groups. These findings suggest that genotoxic metals in MMA-SS fume (e.g., Cr and Ni), that are absent in the GMA-MS fume, may enhance lung toxicity, as well as induce markers of oxidative stress and increase telomere length in PBMCs. Importantly, the measurement of telomere length in cells isolated from peripheral blood may serve as a potential biomarker of response in the assessment of toxicity associated with welding fumes.
Keywords: welding fume, chromium, nanoparticles, reactive oxygen species, telomere length
1. INTRODUCTION
Electric arc welding is the most common industrial process used to join metals. By application of intense heat, metal at a joint between two parts is melted and caused to intermix with an intermediate molten filler metal (The James F. Lincoln Arc Welding Foundation, 2000). The extreme heat (> 5,000°C) needed to melt metal is produced by an electric arc between the work to be welded and an electrode that is moved along the joint. Upon cooling and solidification, a metallurgical bond results. The welding process produces aerosol by-products composed of a mixture of metal oxides volatilized from the welding electrode/rod or the flux material incorporated within the electrode (Zimmer and Biswas, 2001). The formed welding fume is vaporized metal that has reacted with air to generate incidental nanometer-sized primary particles that can quickly form into chain-like agglomerates of larger particles (Antonini et al., 2011a). The fume formed during electric arc welding is mostly respirable, having a mass median aerodynamic diameter (MMAD) that is typically <0.5 μm (Antonini et al., 2006; Jenkins et al., 2005; Zimmer and Biswas, 2001). Arc welding processes generate complex aerosols that are composed of potentially hazardous metals, such as chromium (Cr), manganese (Mn), iron (Fe) and nickel (Ni).
Approximately 400,000 workers were employed full-time as welders in the U.S. in 2014 (Bureau of Labor Statistics, 2016). It has been estimated that additional workers, believed to be in the millions worldwide, perform duties related to welding but are not classified as full-time welders, such as construction workers, boilermakers, shipbuilders, automotive workers, pipefitters, and farmers. Employment of welders in the U.S. is expected to increase over the next several years, mostly reflecting the need for welders in the manufacturing industry due to the importance and versatility of welding as a manufacturing process. Also, because of the aging workforce and the nation’s deteriorating infrastructure, additional jobs will be needed for repair and rebuilding of bridges, highways, and buildings as well as in the construction of new power generation facilities and specifically, pipelines for transport of natural gas and oil (Bureau of Labor Statistics, 2016).
The health of welders has been difficult to study because of differences in welding processes and materials used, work area ventilation, worker populations, and other occupational exposures (Antonini, 2003). Most worker studies have evaluated the pulmonary effects of welding fume exposure. Many full-time welders have experienced some type of respiratory disorder during their employment (Antonini, 2014; Martin et al., 1997). Acute pulmonary effects have included metal fume fever, siderosis, transient changes in lung function, and an increased susceptibility to upper and lower respiratory infections. Chronic pulmonary effects include bronchitis and the possible development of lung cancer. Less is known regarding the effects of welding fume inhalation on non-pulmonary organ systems of the body, such as the central nervous and cardiovascular systems. Studies have indicated that welders are at an increased risk for ischemic heart disease (Ibfelt et al., 2010; Sjogren et al., 2002). Also, because of the presence of manganese in most welding fumes, long-time welders may be susceptible to manganism, a Parkinson’s Disease-like irreversible condition of the brain characterized by the accumulation of elevated manganese levels localized to the basal ganglia of the brain (Racette, 2014; Ellison et al., 2008; Racette et al., 2001).
Because of the potential adverse health effects associated with welding fumes, the objective of the current study was to identify potential biomarkers of response using an animal model to investigate epigenetic changes in isolated peripheral blood mononuclear cells (PBMCs) after exposure to different welding fumes. PBMCs can be easily isolated from collected blood of human subjects, and have been used to assess cellular, molecular, and epigenetic changes related to welding fume exposure (du Plessis et al., 2010; Sardas et al., 2010; Rim et al., 2007). Male Sprague-Dawley rats were exposed by intratracheal instillation (ITI) of 2.0 mg/rat of gas metal arc-mild steel (GMA-MS) or manual metal arc-stainless steel (MMA-SS) welding fume. Vehicle controls received sterile saline by ITI. At 4 h, 14 h, 1 d, 3 d, 10 d, and 30 d, bronchoalveolar lavage (BAL) was performed to assess lung toxicity. Whole blood was collected, and PBMCs were isolated. Production of reactive oxygen species (ROS) by PBMCs was assessed by evaluating dihydroethidium (DHE) fluorescence and 4-hydroxynonenal protein adduct (PHNE) formation. DNA alterations in PBMCs were determined by evaluating changes in DNA methylation and telomere length.
2. MATERIALS AND METHODS
2.1. Animals
Male Sprague-Dawley rats from Hilltop Lab Animals (Scottdale, PA), weighing 250–300 g and free of viral pathogens, parasites, mycoplasmas, Helicobacter, and CAR Bacillus, were used. Total 64 rats were used in this study, 16 rats (6 control, 5 GMA-MS and 5 MMA-SS) for each time point. The rats were acclimated for one week after arrival and were provided HEPA-filtered air, irradiated Teklad 2918 diet, and tap water ad libitum. All animal procedures used during the study were reviewed and approved by the NIOSH Animal Care and Use Committee. The animal facilities are specific pathogen-free, environmentally controlled, and accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
2.2. Welding Fume Characterization
The two welding fume samples were kindly generated and provided by Lincoln Electric Co. (Cleveland, OH). Bulk samples of the welding fumes were generated in an open front fume chamber (volume = 1 m3) by a welder using an appropriate electrode and collected on 0.2 μm Nuclepore filters (Nuclepore Co., Pleasanton, CA). The fume samples were generated by: (1) gas metal arc welding with a mild steel E70S-3 electrode (GMA-MS) with argon and CO2 as shielding gases and (2) shielded manual metal arc welding with a stainless steel ER308-16-1 electrode (MMA-SS).
Welding particle composition.
Analysis of elements present in the welding fume samples (Table 1) was performed as using inductively coupled plasma-atomic emission spectroscopy (ICP-AES) using NIOSH method 7300 modified for microwave digestion (NIOSH, 1994). In addition, portions of the different welding fume samples were suspended in distilled water, pH 7.4, and sonicated for 1 min with a Sonifier 450 Cell Disruptor (Branson Ultrasonics, Danbury, CT, USA) to determine particle/metal solubility. The particle suspensions (total samples) were incubated for 24 h at 37° C, and the samples were centrifuged at 12,000 g for 30 min. The supernatants of the samples (soluble fraction) were recovered and filtered with a 0.22 μm filter (Millipore Corp., Bedford, MA, USA). The pellets (insoluble fraction) were re-suspended in water. The sample suspensions (total, soluble, and insoluble fractions) were digested, and the metals analyzed by ICP-AES according to NIOSH method 7300 (NIOSH, 1994).
Table 1.
Welding Fume Characterization
| Sample | Metal Composition (weight %)a |
Soluble/Insoluble Ratio |
|---|---|---|
| gas metal arc-mild steel | 14 % Mn | 0.020 |
| manual metal arc-stainless steel | 28 % Cr 17 % Mn 3 % Ni |
0.345 (87 % Cr, 11 % Mn) |
Relative to all metals analyzed.
Welding particle size.
The aggregate size of the particles dispersed in water (hydrodynamic diameter, dH) was measured using dynamic light scattering (DLS) with a Malvern Zetasizer Nano-ZS instrument (Malvern Instruments Ltd., Worcestershire, UK). Particle stock was prepared by initially dispersing particles at 5 mg/ml concentration in 1 mg/ml bovine serum albumin. DLS measurements were performed in water by diluting the stock solution to 50 μg/ml in ultra-pure sterile water. Table 2 shows average and standard deviation data from six independent samples.
Table 2.
Welding Particle Size in Solution
| Sample | Z-Average (d.nm) | Polydispersity Index (Pdl) | Peak 1 Size Intensity d.nm (%) | Peak 2 Size Intensity d.nm (%) |
|---|---|---|---|---|
|
GMA-MS: gas metal arc-mild steel |
300 ± 7 | 0.2 ± 0.02 | 319 ± 20 (99%) | 3479 ± 2696 (1%) |
|
MMA-SS: manual metal arc-stainless steel |
604 ± 24 | 0.4 ± 0.02 | 592 ± 69 (94%) | 5109 ± 419 (6%) |
Note. Measurements were performed at particle concentration of 50 μg/ml. Data represents average and standard deviations from six independent samples. d.nm is the hydrodynamic diameter in nanometers. The peak size distribution suggest most of the particles are around 300 and 600 nm in size with a few micron-sized agglomerates.
Welding particle morphology.
Welding samples were dispersed onto 47-mm Nuclepore polycarbonate filters (Whatman, Clinton, PA). The filters were mounted onto aluminum stubs with silver paste. The welding particles were viewed using a Hitachi S4800 field emission scanning electron microscope (Bruker, Madison, WI).
2.3. Welding Fume Exposure
The welding fume samples were prepared in sterile saline and sonicated to disperse the particulates. Rats were lightly anesthetized by an intraperitoneal injection of 25mg/kg body weight of methohexital sodium (Brevital 500 mg; JHP Pharmaceuticals, LLC, Rochester, MI, USA) and exposed by ITI with 2.0 mg/rat of GMA-MS or MMASS welding fume in 300 μl of sterile phosphate buffered saline (PBS). Vehicle control animals were received 300 μl of sterile PBS by ITI. Saline solutions and anesthetics were USP grade.
To estimate how the ITI particle dose used in the study correlated with a “real world” worker exposure to welding fumes, the exposure was calculated as previously described (Sriram et al., 2010). Importantly, the calculations made here do not account for particle clearance, but provides an estimate of the plausible welder exposure concentrations that our exposure paradigm mimics. The daily lung burden of a welder was estimated, assuming 8 hours of continuous welding, a worker minute ventilation of 20 L/min, a particle deposition efficiency in the alveolar region of 15 % (ICRP, 1994), and a fume concentration of 5 mg/m3 (previous Threshold Limit Values for 8-hour day for welding fume). The following calculations were used:
Next, assuming an average worker weighed 75 kg and the average weight of the rats used in the study was 0.40 kg:
From the dose used in the study, 2.0 mg:
2.4. Bronchoalveolar Lavage
At 4 h, 14 h, 1 d, 3 d, 10 d, and 30 d after exposure, bronchoalveolar lavage (BAL) was performed to assess lung injury and inflammation. Animals were euthanized with an intraperitoneal injection of sodium pentobarbital (>100 mg/kg body weight, IP; Fatal-Plus Solution, Vortech Pharmaceutical, Inc., Dearborn, MI, USA) and then exsanguinated by severing the abdominal aorta. The lungs were first lavaged with a 1 ml/100 g body weight aliquot of calcium- and magnesium-free PBS, pH 7.4. The first fraction of recovered bronchoalveolar lavage fluid (BALF) was centrifuged at 500 × g for 10 minutes, and the resultant cell-free supernatant was analyzed for lactate dehydrogenase as a marker for lung cell damage. The lungs were further lavaged with 6-ml aliquots of PBS until 30 ml were collected. These samples also were centrifuged for 10 min at 500 × g and the cell-free BALF discarded. The cell pellets from all washes for each rat were combined, washed, and re-suspended in 1 ml of PBS buffer and counted and differentiated.
2.5. Lung Injury and Inflammation
Total cell numbers recovered by BAL were determined using a Coulter Multisizer II and AccuComp software (Coulter Electronics, Hialeah, FL, USA). Cells were differentiated using a Cytospin 3 centrifuge (Shandon Life Sciences International, Cheshire, England). Cell suspensions (5 × 104 cells) were spun for 5 min at 800 rpm and pelleted onto a slide. Cells (200/rat) were identified after labeling with Leukostat stain (Fisher Scientific, Pittsburgh, PA, USA) as lung macrophages, neutrophils, lymphocytes, and eosinophils. Using the acellular first fraction of BALF, LDH (lactate dehydrogenase) activity was determined by measuring the oxidation of lactate to pyruvate coupled with the formation of NADH at 340 nm. Measurements were performed with a COBAS MIRA auto-analyzer (Roche Diagnostic Systems, Montclair, NJ, USA) and expressed as units/liter (U/L).
2.6. Isolation of PBMCs
At each time point after exposure, whole blood was drawn using an 18-gauge needle from the abdominal vena cava and collected in BD Vacutainer tubes (Becton, Dickinson, and Co., Franklin Lakes, NJ). The PBMCs were isolated from heparinized blood collected from each animal using Accuspin 12-ml tubes (Sigma-Aldrich Co., St. Louis, MO, USA) containing Histopaque-1083 (Sigma-Aldrich Co., St. Louis, MO) by centrifuging at 1250 × g for 30 min. PBMCs were collected in 15-ml falcon tubes and washed once with PBS at 450 × g for 20 min. Finally, PBMCs were re-suspended in 1 ml PBS (Lonza, Walkersville, MD, USA) for cell counting and genomic DNA (gDNA) isolation. All steps were performed at room temperature.
2.7. Epigenetic Effects in PBMCs
Reactive oxygen species generation.
Isolated PBMCs from whole blood of welding fume-exposed animals were re-suspended in PBS, spun down with the Cytospin 3 centrifuge, fixed in 10 % neutral buffered formalin (Fisher Scientific, Fair Lawn, NJ, USA), rinsed with PBS, and incubated with dihydroethidium (DHE; 2.5 μmol/L; Molecular Probes, Eugene, OR, USA) at 37°C for 10–15 min. Cells were washed in PBS twice and mounted using ProLong Gold (Molecular Probes, Eugene, OR).
P-HNE adduct formation.
Isolated PBMCs from whole blood of animals exposed to welding fumes were re-suspended with PBS, spun down with the Cytospin 3 centrifuge, fixed in 10 % formalin, rinsed with PBS, blocked (2% BSA and 0.3% Triton X) for 2–3 h, and incubated with P-HNE antibodies (Ab46545, Abcam, Cambridge, UK) overnight. Cells then were washed once with PBS and incubated with florescent-tagged secondary antibodies (Ab150077, Abcam, Cambridge, UK) for 45 min. Finally, the cells were washed in PBS twice and mounted using ProLong Gold.
DNA Isolation and Telomere Length Analysis by qPCR.
gDNA was extracted from the PBMCs isolated from whole blood using DNeasy Blood & Tissue Kit (Qiagen Sciences Inc., Germantown, MD, USA). DNA concentration was measured using the Nano-Drop 2000 spectrophotometer. Samples were diluted to a final concentration of 25 ng/1.5 μl to measure telomere length. Quantitative PCR was performed using the SYBR Select Master Mix (Life Technologies, Carlsbad, CA, USA) with a step one plus real time PCR system (Applied Biosystems, Foster City, CA, USA). The parameters used were as follows: 95°C for 10 min (enzyme activation), 95°C for 15 sec (denaturing), and 60°C for 60 sec (annealing), 60 cycles. Primers used were as follows: Tel rat-F 5’-GGT TTT TGA GGG TGA GGG TGA GGG TGA GGG TGA GGG t-3’, and Tel rat-R 5’-TCC CGA CTA TCC CTA TCC CTA TCC CTA TCC CTA TCC CTA-3’; AT1 rat-F 5’-ACG TGT TCT CAG CAT CGA CCG CTA CC-3’ and AT1 rat-R 5’-AGA ATG ATA AGG AAA GGG AAC AAG AAG CCC-3’ (Invitrogen Corporation, Carlsbad, CA, USA). The relative telomere length was measured by comparing the ratio of telomere repeat copy number (T as Tel1) and single gene copy number (S as AT1), expressed as telomere length (T/S) ratio. Each individual values obtained by qPCR were processed through the formula T/S= 2ˉΔCT, where ΔCT = CTtelomere – CTAT1. This ratio was then compared with the ratio of the reference DNA. Each DNA sample collected was measured in duplicate.
DNA Methylation.
DNA Colorimetric Quantification Kit (Abcam, Cambridge, UK) was used to determine gDNA methylation according to manufacturer instructions. Briefly, binding buffer was added to each well then a negative control, positive control, or 400 ng of gDNA per reaction was added. The plate was incubated for 90 min, washed, and incubated with capture antibody for 60 min. Then, washed and incubated with detection antibody after which an enhancer solution was added. Finally, the plate was washed, and developing solution was added followed by stop solution. Absorbance was read at 450 nm.
2.8. Statistics
Results are means ± standard error of measurement. Statistical analysis was performed using SigmaStat (Systat Software, Inc., San Jose, CA). The significance of difference between exposure groups within a time point was analyzed using a one-way analysis of variance (ANOVA) and the Tukey post-hoc test. The criterion of significance was set at p<0.05.
3. RESULTS
3.1. Particle Characterization
Elemental analysis of the welding fume samples was performed by ICP-AES (Table 1). The composition of the two fumes was different as the GMA-MS sample was composed of Fe (85 %) and Mn (14 %), whereas the MMA-SS sample was composed of similar Mn (17 %) levels but less Fe (41 %). Measureable amounts of Cr (28 %) and Ni (3 %) were present in the MMA-SS sample, but not in the GMA-MS sample. In addition, the GMA-MS sample was mostly insoluble with a soluble-to-insoluble ratio of 0.020, whereas the MMA-SS sample was significantly more soluble with a soluble-to-insoluble ratio of 0.345. The soluble fraction of the MMA-SS fume was composed of Cr (87 %) and Mn (11 %).
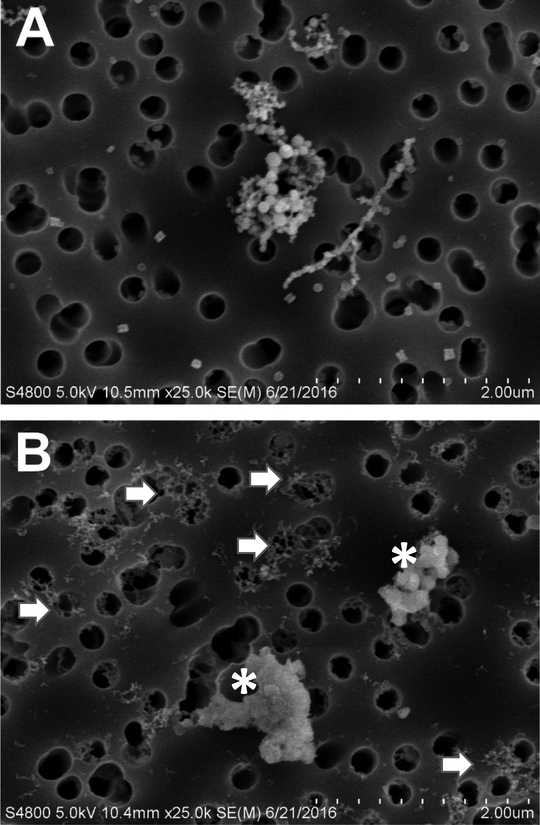
Particle morphology for the two welding samples as assessed by scanning electron microscopy appeared different. The GMA-MS particles were agglomerates of submicron-sized primary particles arranged as chain-like structures (Figure 1A). The GMA-MS primary particles were mostly spherical and homogenous in size. On the other hand, the MMA-SS particles were a combination of amorphous material (asterisks) and much smaller and finer chain-like agglomerates (arrows) as compared to the GMA-MS particles (Figure 1B). DLS analysis indicated MMASS welding fume particles agglomerated to a greater extent than GMA-MS welding fume particles in solution with average dH of 604 nm and 300 nm, respectively (Table 2).
Figure 1.
Scanning electron micrographs of (A) GMA-MS and (B) MMA-SS welding particles dispersed onto filters; asterisks indicate amorphous material, arrows indicate small, very fine chain-like agglomerates. Dashed micron bar equals 2 μm.
3.2. Lung Injury and Inflammation
In the assessment of lung injury, BALF LDH was significantly elevated compared to control at all time points after MMA-SS exposure except at 30 d. Similarly, BALF LDH was significantly elevated by MMA-SS compared to GMA-MS exposure at 14 h, 1 d, 3 d, and 10 d (Figure 2). GMA-MS exposure significantly increased LDH at the early time points compared to saline but returned to control levels by 10 d.
Figure 2.
LDH activity in BALF at 4 h, 14 h, 1 d, 3 d, 10 d, and 30 d after 2.0 mg/rat ITI exposure to MMA-SS or GMA-MS welding fumes. Vehicle controls received saline. (n = 4–6; values are means ± standard error; *significantly different from saline control, p<0.05; #significantly different from saline control and GMA-MS groups, p<0.05).
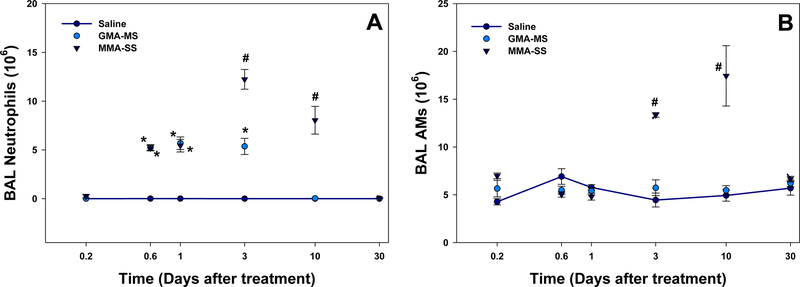
In regards to lung inflammation, neutrophils recovered by BAL were significantly elevated after MMA-SS exposure at 14 h, 1 d, 3 d, and 10 d compared to control and at 3 d and 10 d compared to GMA-MS (Figure 3A). GMA-MS significantly increased neutrophils at 14 h, 1 d, and 3 d compared to saline but PMNs returned to control levels by 10 d. Lung AMs were significantly elevated at 3 d and 10 d after MMA-SS exposure (Figure 3B). GMA-MS had no effect on the number of AMs recovered by BAL.
Figure 3.
(A) Neutrophils and (B) AMs recovered by BAL at 4 h, 14 h, 1 d, 3 d, 10 d, and 30 d after 2.0 mg/rat ITI exposure to MMA-SS or GMA-MS welding fumes. Vehicle controls received saline. (n = 4–6; values are means ± standard error; *significantly different from saline control, p<0.05; #significantly different from saline control and GMA-MS groups, p<0.05).
3.3. Epigenetic Changes in PBMCs
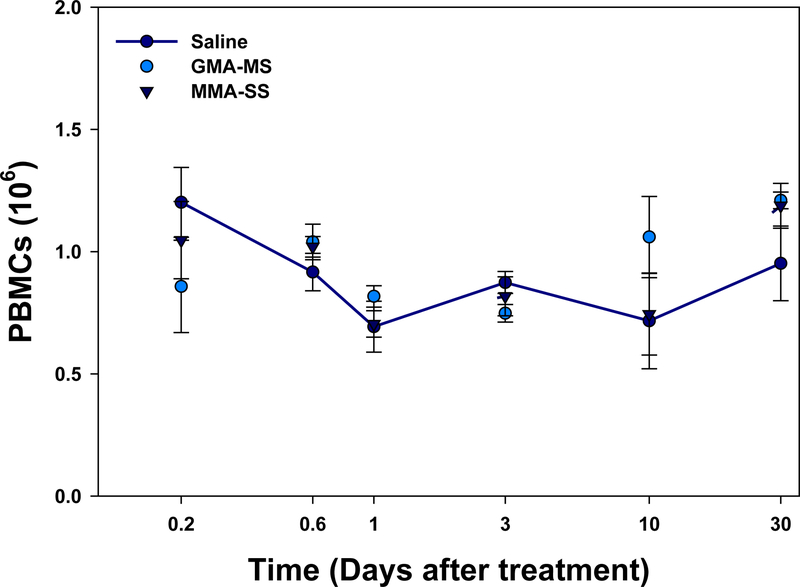
In the assessment of the number of PBMCs isolated from the whole blood, there was no significant difference in PBMC number observed at any time point after exposure when comparing either welding fume with control (Figure 4).
Figure 4.
PBMCs isolated at 4 h, 14 h, 1 d, 3 d, 10 d, and 30 d after ITI exposure to 2.0 mg/rat of MMA-SS or GMAMS welding fumes. Vehicle controls received saline. (n = 4–6; values are means ± standard error).
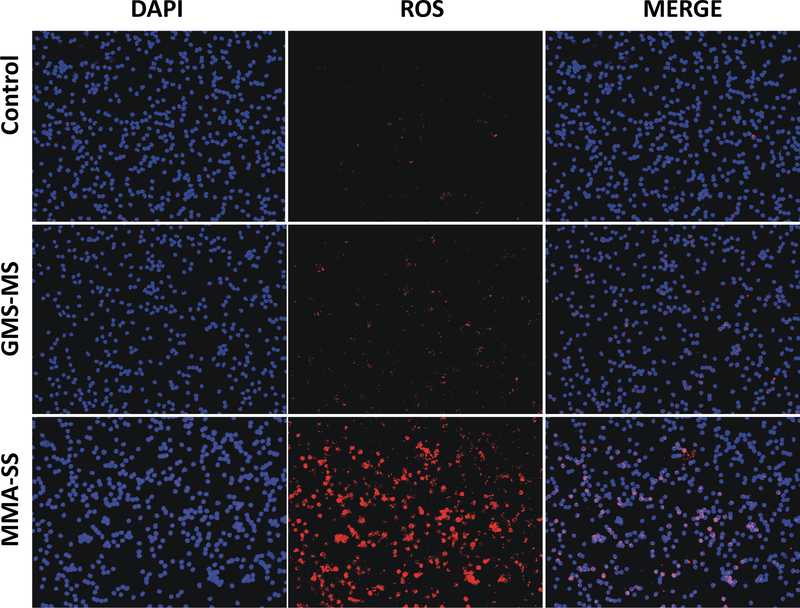
Because of the inflammatory response in the lungs after ITI exposure to the welding fume samples, markers of oxidative stress were examined in isolated PBMCs. As observed by the amplified red fluorescence of DHE, the welding fume samples induce ROS generation in PBMCs at 24 h after exposure (Figure 5). However, comparatively less DHE fluorescence was observed in PBMCs from GMA-MS welding fume exposed rats as compared to MMA-SS exposed rats, suggesting a potential mechanism related to the differences observed in lung injury and inflammation (Figures 5). Because ROS generation can lead to the formation of proteins adducts of the lipid peroxidation product 4-hydroxynonenal, P-HNE was examined in PBMCs isolated from animals exposed to the different welding fumes (Figure 6). ITI exposure to MMA-SS welding fume increased P-HNE adduct formation (green fluorescence) in PBMCs at 24 h as compared to the GMA-MS fume and control. The PBMCs isolated from the GMA-MS group had slightly increased P-HNE adducts when compared to the controls.
Figure 5.
ROS generation (DHE; red fluorescence) by PBMCs isolated from Sprague-Dawley rats at 24 h after 2.0 mg/rat ITI exposure to MMA-SS or GMA-MS welding fumes. Vehicle controls received saline.
Figure 6.
P-HNE adduct formation (green fluorescence; FTIC) in PBMCs isolated from Sprague-Dawley rats at 24 h after ITI exposure to 2.0 mg/rat of MMA-SS or GMA-MS welding fumes. Vehicle controls received saline. DAPI blue fluorescence indicates nucleus staining.
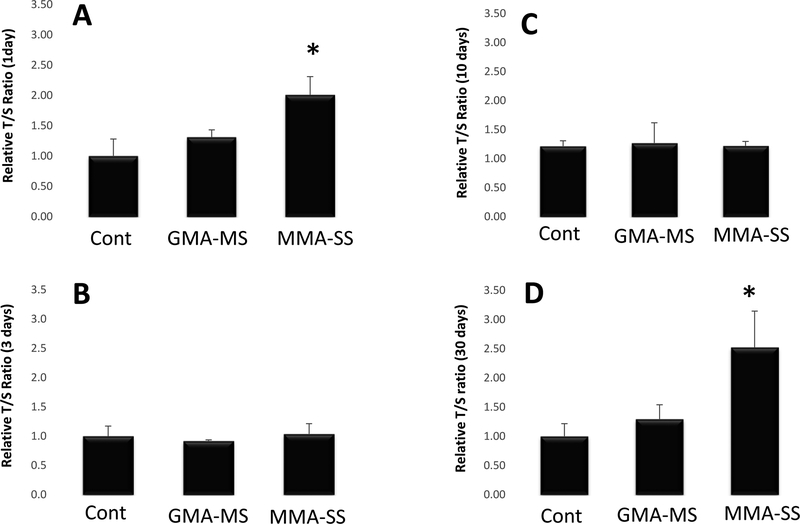
DNA methylation is a common epigenetic biomarker that has been associated with airborne particulate exposure. DNA methylation of PBMCs was assessed at each of the time points after welding fume exposure. No significant differences were observed when comparing DNA methylation between the welding fume and control groups at any of the time points assessed (Figure 7). However, PBMCs from MMA-SS exposed rtas had significantly increased telomere length ratios at 1 and 30 d after a single exposure compared to the GMA-MS and control groups (Figure 8). No significant differences were observed in PBMC telomere length ratio when comparing the GMA-MS group with control at any of the time points.
Figure 7.
DNA methylation was measured by the production of 5-methylcytosine (5-mC) in PBMCs isolated at (A) 1 d, (B) 3 d, (C) 10 d, and (D) 30 d after ITI exposure to 2.0 mg/rat of MMA-SS or GMA-MS welding fumes. Vehicle controls received saline. (n = 4–6; values are means ± standard error).
Figure 8.
Telomere length of PBMCs isolated at (A) 1 d, (B) 3 d, (C) 10 d, and (D) 30 d after ITI exposure to 2.0 mg/rat of MMA-SS or GMA-MS welding fumes. Vehicle controls received saline. (n = 4–6; values are means ± standard error; *significantly different from saline control and GMA-MS groups, p<0.05). The relative telomere length was measured by comparing the ratio of telomere repeat copy number (T) and single gene copy number (S), expressed as telomere length (T/S) ratio.
4. DISCUSSION
The goal of the study was to use an animal model to assess the effect of pulmonary exposure to welding fumes on potential epigenetic changes in isolated PBMCs. We examined changes in telomere length, alterations in DNA methylation and biomarkers of oxidative stress in PBMCs isolated at different time points after exposure to two common, but chemically distinct, welding fumes. The GMA-MS fume was relatively insoluble and composed of mostly Fe and with a small percentage of Mn, whereas the MMA-SS fume was highly water-soluble and composed of significant amounts of Cr and Ni in addition to Fe and Mn. Both particle samples were respirable in size and displayed the typical agglomerated chain-like structures that are commonly observed with particles formed during welding.
A differential lung injury and inflammation response was observed when comparing the two welding fumes. The more soluble and chemically complex MMA-SS sample induced a more persistent and greater inflammatory response as evidenced by significant elevations in lung LDH and neutrophil influx above the GMAMS sample and control group. This elevation remained elevated beyond 10 d after exposure. ITI GMA-MS fume exposure significantly increased injury and inflammation at early time points compared to control but had no significant effect by 10 d. These results correlated well with earlier studies comparing the toxicity of mild steel and stainless steel welding fumes after exposure by a single ITI (Antonini et al., 1996; 1997) or exposure by inhalation (Antonini et al., 2011b). This confirmed that the responses seen in the current study were those anticipated in this established model.
In workers, welding fume inhalation has been reported to cause oxidative stress in PBMCs. In isolated PBMCs from fifteen welders, du Plessis et al. (2010) observed increases of 87 % and 96 % in ROS and lipid peroxidation levels, respectively compared to non-exposed controls. Unfortunately, as is the case with most welder studies, a limitation of the study was the absence of information on the specific type of welding fume exposure. According to the description of the study population, the workers were involved ‘with various welding-related processes under various conditions’. Our study suggests that different types of welding fumes vary in their effects on PBMC in exposed rats. Neither welding fume affected the number of PBMCs that were isolated at any time point. In our rat model at 24 hr after exposure, both GMA-MS and MMA-SS increased PBMC P-HNE adducts and DHE which are biomarkers of oxidative stress. However, MMA-SS was significantly more effective than GMAMS in causing oxidative stress in the PBMCs.
One potential mechanism by which particle-induced oxidative stress can influence transcriptional regulation is through epigenetic modification. Epigenetics refers to persistent alterations in gene regulation that do not involve changes to the DNA sequence (Feil and Fraga, 2012). A commonly studied epigenetic modification is DNA methylation, described as the covalent addition of a methyl group to cytosine primarily in the context of a cytosine-guanine dinucleotide. DNA methylation modulates gene expression and varies in response to different external stimuli (Feinberg, 2007). Jiang et al. (2014) demonstrated that short-term inhalation exposure to diesel exhaust resulted in DNA methylation changes at sites involved with inflammation and oxidative stress. However, no significant differences were observed when comparing DNA methylation between the welding fume and control groups at any of the time points assessed in the current study. In agreement, Wong et al. (2014a) and Li et al. (2015) did not see any significant associations between DNA methylation and cumulative particulate matter exposure in a longitudinal study of a cohort of welding fume-exposed boilermakers and respirable dust in group of Swedish welders, respectively. Also, Erdely et al. (2014) observed that in whole blood MMA-SS exposure diminished the inflammatory protein response to lipopolysacchide but that the transcription of associated genes was unaffected. This finding agrees with the observation of a lack of an acute impact of DNA methylation in the current study. Furthermore, a positive effect on DNA methylation may reflect long-term alterations, whereas the current study used an acute, single exposure.
It has been hypothesized that oxidative stress may be the primary mechanism responsible for the changes observed in telomere length (von Zglinicki, 2002). Telomeres are complexes of tandem repetitive structures of DNA (5’-TTAGGG-3’, 6–15 kb) that stabilize ends of chromosomes by preserving genetic information and preventing DNA degradation (Shaw et al., 2014; Hug and Lingner, 2006). Telomeres shorten with age, and their length may be affected by life experiences, such as stress, diet, chronic inflammation, physical activity, and environmental exposures (Patel et al., 2016; Zhang et al., 2013). Occupational exposure to different pollutants, including inhaled particulate matter, has been associated with telomere length changes (Hou et al., 2012). Telomere integrity is mostly maintained by telomerase activity by which TTAGGG repeats are added on chromosomal ends to compensate for continual erosion of telomeres (Hug and Lingner, 2006). Telomerase activity is highest in germline and cancer cells, but also is quantifiable in some normal somatic cells, such as PBMCs (Marion and Blasco, 2010). It has been estimated that the reduction rate of telomere repeats in PBMCs is approximately 84 bp per year along with a progressive decrease in telomerase activity in healthy individuals (Iwama et al., 1998). Because of the ease of isolation from whole blood samples, PBMCs may serve as a valuable cell type in the study of telomere length as a possible biomarker to assess past occupational exposure as well as predict future disease. Both shorter (Ma et al., 2011; Jang et al., 2008) and longer (Seow et al., 2014; Lan et al., 2013) telomeres in peripheral blood have been associated with increased risk of various cancer types, including lung cancer.
Based on previous welder studies, it was hypothesized that pulmonary exposure to welding fume would decrease telomere length. In peripheral blood from a cohort of welders in Sweden, Li et al. (2015) observed that shorter telomeres were associated for every working year as a welder. Although there were no clear associations between concentrations of respirable dust and different biomarkers in peripheral blood, the study observed modest signs of associations between oxidative stress, telomere alterations, and exposure to low-to-moderate levels in welding fumes. Also, in a small cohort of boilermakers exposed to welding fumes, Wong et al. (2014b) observed that increased cumulative exposure to PM2.5 in the month prior to measurement, as opposed to exposures extending further into the past, was associated with decreased telomere length in peripheral blood leukocytes. However, in both studies, it was impossible to separate welding fume exposure of the workers from exposure to other occupational and environmental substances and lifestyle factors that potentially may cause epigenetic changes in DNA.
Interestingly, the MMA-SS fume sample in the current study significantly increased telomere length of isolated PBMCs at 1 and 30 d after ITI exposure compared to the GMA-MS fume and control. No alterations in telomere length were observed when comparing the GMA-MS and control groups at any time point. Although not expected, the result of an increase in telomere length for the MMA-SS welding fume, which was more reactive and inflammatory than the GMA-MS fume, is not surprising. Exposure to stressors, including inhaled particulates, have been shown to increase telomere length. In an evaluation of steel workers exposed to metal-rich particulates near Brescia, Italy, Dioni et al. (2011) observed a rapid and significant increase in telomere length after a short-term exposure of just three days. The authors believed that acute inflammation, an important process in mediating health effects associated with particulate exposure, could be the mechanism causing the increase in telomere length. In agreement, Colicino et al. (2016) observed that leukocytes with longer telomeres due to a 1-year exposure to carbon black, a marker of traffic-related air pollution, were more responsive to inflammatory stimuli. Additionally, Hou et al. (2012) observed an increased association between increased telomere length with elemental carbon and personal PM2.5 levels during work hours on examination days. However, this association of an increase in telomere length was lost at later time points after exposure. This observation may help explain the results in the current study that showed an increase in telomere length in PBMCs isolated at 1 d from the MMA-SS group when lung injury and inflammation were the highest. Toxico-kinetic studies indicate that the soluble Cr from stainless steel welding fumes quickly translocate to the circulation and are significantly elevated at 1 d after exposure compared to mild steel fumes (Antonini et al., 2010). Also, reactive oxygen species potential has been observed to be the greatest in the chromium-rich, soluble fraction of MMA-SS welding fume (Taylor et al., 2003).
As the response subsided, PBMC telomere length ratio in the MMA-SS group returned to control levels at 3 and 10 d before increasing at 30 d, suggesting either a biphasic response or changes in cell populations. In other types of lung injury, bone marrow-derived progenitor cells have been observed to move into the lungs and are involved in lung repair (Serikov et al., 2008; Yamada et al., 2004). The influx of bone marrow-derived, stem-like cells may have altered overall telomere length. An examination of this hypothesis as well as the study of the biological reactivity of the serum and the presence of specific metals and inflammatory mediators in samples collected from the animals in the study at different time points is ongoing in an attempt to explain the mechanism for the increase in telomere length at 30 d.
5. CONCLUSIONS
A well-controlled animal study was performed to determine the effects of welding fumes with different chemical and toxicity profiles on oxidative stress, DNA methylation, and telomere length in PBMCs isolated at different time points after exposure. The welding fume that was more soluble, inflammatory, and contained genotoxic metals enhanced markers of oxidative stress and increased telomere length as compared to other fume and control. No differences were observed when comparing DNA methylation between the welding fume and control groups. Importantly, telomere length increased in PBMCs from MMA-SS exposed rats, suggesting the need for additional investigation of telomere changes in studies of acute welding fume exposure.
Footnotes
Publisher's Disclaimer: DISCLAIMER: The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
REFERENCES
- Antonini JM. Health effects associated with welding In: Bassim N, editor. Comprehensive Materials Processing. Oxford, UK: Elsevier Ltd; 2014, [Google Scholar]
- Antonini JM, Keane M, Chen BT, et al. , 2011a. Alterations in welding process voltage affect the generation of ultrafine particles, fume composition, and pulmonary toxicity. Nanotoxicol. 5, 700–710. [DOI] [PubMed] [Google Scholar]
- Antonini JM, Roberts JR, Stone S, et al. , 2011b. Persistence of deposited metals in the lungs after stainless steel and mild steel welding fume inhalation in rats. Arch. Toxicol 85, 487–498. [DOI] [PubMed] [Google Scholar]
- Antonini JM, Roberts JR, Chapman RS, et al. , 2010. Pulmonary toxicity and extrapulmonary tissue distribution of metals after repeated exposure to different welding fumes. Inhal. Toxicol 22, 805–816. [DOI] [PubMed] [Google Scholar]
- Antonini JM, Afshari AA, Stone S, et al. , 2006. Design, construction, and characterization of a novel robotic welding fume generator and inhalation exposure system for laboratory animals. J. Occup. Environ. Hyg 3, 194–203. [DOI] [PubMed] [Google Scholar]
- Antonini JM, 2003. Health effects of welding. Crit. Rev. Toxicol 33, 61–203. [DOI] [PubMed] [Google Scholar]
- Antonini JM, Krishna Murthy GG, Brain JD, 1997. Responses to welding fumes: lung injury, inflammation, and the release of tumor necrosis factor-α and interleukin-1b. Exp. Lung Res 23, 205–227. [DOI] [PubMed] [Google Scholar]
- Antonini JM, Krishna Murthy GG, Rogers RA, Albert R, Ulrich GD, Brain JD, 1996. Pneumotoxicity and pulmonary clearance of different welding fumes after intratracheal instillation in the rat. Toxicol. Appl. Pharmacol 140, 188–199. [DOI] [PubMed] [Google Scholar]
- Bureau of Labor Statistics, Occupational Outlook Handbook Employment: Welders, Cutter, Solders, and Brazers, U.S. Department of Labor. 2016. http://www.bls.gov/ooh, Accessed 14 July 2016.
- Colicino E, Wilson A, Frisardi MC, et al. , 2016. Telomere length, long-term black carbon exposure and cognitive function in a cohort of older men: the VA normative aging study. Envion. Health Perspect. 10.1289/EHP241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dioni L, Hoxha M, Nordio F, et al. , 2011. Effects of short-term exposure to inhalable particulate matter on telomere length, telomerase expression, and telomerase methylation in steel workers. Environ. Health Perspect 119, 622–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Plessis L, Laubscher P, Jooste J, et al. , 2010. Flow cytometric analysis of the oxidative status in human peripheral blood mononuclear cells of workers exposed to welding fumes. J. Occup. Environ. Hyg 7, 367–374. [DOI] [PubMed] [Google Scholar]
- Ellingsen DG, Konstantinov R, Bast-Pettersen R, et al. , 2008. A neurobehavioral study of current and former welders exposed to manganese. Neurotoxicol. 29, 48–59. [DOI] [PubMed] [Google Scholar]
- Erdely A, Antonini JM, Young S-H, et al. , 2014. Oxidative stress and reduced responsiveness of challenged circulating leukocytes following pulmonary instillation of metal-rich particulate matter in rats. Part. Fibre Toxicol 11, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil R, Fraga M, 2012. Epigenetics and the environment: emerging patterns and implications. Nature Rev. Genet 13, 97–109. [DOI] [PubMed] [Google Scholar]
- Feinberg AP, 2007. Phenotypic plasticity and the epigenetics of human disease. Nature. 447, 433–440. [DOI] [PubMed] [Google Scholar]
- Hou L, Wang S, Dou C, et al. , 2012. Air pollution exposure and telomere length in highly exposed subjects in Beijing, China: a repeated-measure study. Environ. Int 48C, 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hug N, Lingner J, 2006. Telomere length homeostasis. Chromosoma. 115, 413–425. [DOI] [PubMed] [Google Scholar]
- Ibfelt E, Bonde JP, Hansen J, 2010. Exposure to metal welding fume particles and risk for cardiovascular disease in Denmark: a prospective cohort study. Occup. Environ. Med 67, 772–777. [DOI] [PubMed] [Google Scholar]
- ICRP., 1994. Human respiratory tract model for radiological protection: a report of a task group of the international commission on radiological protection. Annals ICRP. 24 (1–3), 267–272. [PubMed] [Google Scholar]
- Iwama H, Ohyashiki K, Ohyashiki JH, et al. , 1998. Telomeric length and telomerase activity vary with age in peripheral blood cells obtained from normal individuals. Hum. Genet 102, 397–402. [DOI] [PubMed] [Google Scholar]
- The James F. Lincoln Arc Welding Foundation, Arc-welding fundamentals, in: The Procedure Handbook of Arc Welding, Fourteenth Edition, (The James F. Lincoln Arc Welding Foundation, Ed.), Cleveland, OH, 2000, p. 1.3–1. [Google Scholar]
- Jang JS, Choi YY, Lee WK, et al. , 2008. Telomere length and the risk of lung cancer. Cancer Sci. 99, 1385–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins NT, Pierce WM-G, Eagar TW, 2005. Particle size distribution of gas metal and flux cored arc welding fumes. Welding J. 84, 156s–163s. [Google Scholar]
- Jiang R, Jones MJ, Sava F, 2014. Short-term diesel exhaust inhalation in a controlled human crossover study is associated with changes in DNA methylation of circulating mononuclear cells in asthmatics. Part. Fibre Toxicol 11, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Q, Cawthorn R, Gao Y, et al. , 2013. Longer telomere length in peripheral white blood cells is associated with risk of lung cancer and the rs2736100 (CLPTM1L-TERT) polymorphism in a prospective cohort study among women in China. PLoS One. 8, e59230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Hedmer M, Wojdacz T, 2015. Oxidative stress, telomere shortening, and DNA methylation in relation to low-to-moderate occupational exposure to welding fumes. Environ. Mol. Mutagen 56, 684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Zhou Z, Wei S, et al. , 2011. Shortened telomere length is associated with increased risk of cancer: a meta-analysis. PLoS One. 6, e20466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion RM, Blasco MA, 2010. Telomeres and telomerase in adult stem cells and pluripotent embryonic stem cells. Adv. Exp. Med. Biol 695, 118–131. [DOI] [PubMed] [Google Scholar]
- Martin CJ, Guidotti TL, Langard S, 1997. Respiratory hazards of welding. Clin. Pulm. Med 4, 194–204. [Google Scholar]
- NIOSH: Elements (ICP): Method 7300. In NIOSH Manual of Analytical Methods. 4th Edition, Issue 2, U.S. Department of Health and Human Services, Publication No. 98–119. Washington, DC: NIOSH; 1994. [Google Scholar]
- Patel CJ, Manrai AK, Corona E, Kohane S, 2016. Systematic correlation of environmental exposure and physiological and self-reported behavior factors with leukocyte telomere length. Int. J. Epidemiol advanced access epub, doi: 10.1093/ije/dyw043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racette BA, 2014. Manganism in the 21st century: the Hanninen lecture. Neurotoxicol. 45, 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racette BA, McGee-Minnich L, Moerlein SM, et al. , 2001. Welding-related parkinsonism: clinical features, treatment, and pathophysiology. Neurol. 56, 8–13. [DOI] [PubMed] [Google Scholar]
- Rim TR, Park KK, Kim YH, et al. , 2007. Gene-expression profiling of human mononuclear cells from welders using cDNA microarray. J. Toxicol. Environ. Health, Part A 70, 1264–1277. [DOI] [PubMed] [Google Scholar]
- Sardas S, Omurtag GZ, Tozan A, Gul H, Beyoglu D, 2010. Evaluation of DNA damage in construction-site workers occupationally exposed to welding fume and solvent-based paints in Turkey. Toxicol. Ind. Health 26, 601–608. [DOI] [PubMed] [Google Scholar]
- Seow WJ, Cawthorn RM, Purdue MP, et al. , 2014. Telomere length in white blood cell DNA and lung cancer: a pooled analysis of three prospective cohorts. Cancer Res. 74, 4090–4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serikov VB, Mikhaylov VM, Krasnodembskay AD, Matthay MA, 2008. Bone marrow-derived cells participate in stromal remodeling of the lung following acute bacterial pneumonia in mice. Lung. 186, 179–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw JG, Vaughan A, Dent AG, et al. , 2014. Biomarkers of progression of chronic obstructive pulmonary disease (COPD). J. Thorac. Dis 6, 1532:1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjogren B, Fossum T, Lindh T, Weiner J, 2002. Welding and ischemic heart disease. Int. J. Occup. Environ. Health 8, 309–311. [DOI] [PubMed] [Google Scholar]
- Sriram K, Lin GX, Jefferson AM, et al. , 2010. Mitochondrial dysfunction and loss of Parkinson’s disease-linked proteins contribute to neurotoxicity of manganese-containing welding fumes. FASEB J. 24, 4989–5002. [DOI] [PubMed] [Google Scholar]
- Taylor MD, Roberts JR, Leonard SS, et al. , 2003. Effects of welding fumes of differing composition and solubility on free radical production and acute lung injury and inflammation in rats. Toxicol. Sci 75, 181–191. [DOI] [PubMed] [Google Scholar]
- Von Zglinicki T, 2002. Oxidative stress shortens telomeres. Trends Biochem. Sci 27, 339–344. [DOI] [PubMed] [Google Scholar]
- Weng NP, Hathcock KS, Hodes RJ, 1998. Regulation of telomere length and telomerase in T and B cells: a mechanism for maintaining replicative potential. Immunity. 9, 151–157. [DOI] [PubMed] [Google Scholar]
- Wong JYY, De Vivo I, Lin X, et al. , 2014a. The association between global DNA methylation and telomere length in a longitudinal study of boilermakers. Genet. Epidemiol 38, 254–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JYY, De Vivo I, Lin X, Christiani DC, 2014b. Cumulative PM2.5 exposure and telomere length in workers exposed to welding fumes. J. Toxicol. Environ. Health, Part A 77, 441–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Kubo H, Kobayashi S, et al. , 2004. Bone marrow-derived progenitor cells are important for lung repair after lipopolysaccharide-induced lung injury. J. Immunol 172, 1266–1272. [DOI] [PubMed] [Google Scholar]
- Zhang X, Lin S, Funk WE, Hou L, 2013. Environmental and occupational exposure to chemicals and telomere length in human studies. Occup. Environ. Med 70, 743–749. [DOI] [PubMed] [Google Scholar]
- Zimmer AT, Biswas P, 2001. Characterization of the aerosols resulting from arc welding processes. J. Aerosol. Sci 32, 993–1008. [Google Scholar]