ABSTRACT
The rational for designing dendritic cell (DC)-targeted immunotherapies is their central role in orchestrating immunity. Most studies addressing antigen-targeting to DCs for eliciting T cell responses have employed ex-vivo matured DCs derived from monocytes or myeloid DCs isolated from peripheral blood. More recently, also plasmacytoid DCs (pDCs) emerged as attractive targets that can be readily isolated and activated ex vivo. pDCs are known as key effectors of innate and adaptive immunity due to their exquisite ability to produce large amounts of type-1 interferons upon signaling via TLR7 or TLR9 intracellular receptor for viral RNA or bacterial DNA, respectively. In this study, we describe and characterize the immune modulating and targeting module of a composite human specific vaccine platform for active immunotherapy. This module, called warhead (WH), is composed of a single-chain variable fragment (scFv) and CpG-C type oligonucleotides (ODNs) that are covalently coupled. The scFv mediates specific binding to FcγRII/CD32 on APCs and internalization of the ODNs which stimulate TLR9-expressing B cells and pDCs. Furthermore, the scFv in the WH is extended with a five-time heptad repeat (EVSALEK) alpha helix which allows for a coiled-coil complex formation with any immunogen also extended with another five-time heptad (KVSALKE) repeat. WH elicits fast and robust pDC activation as evidenced by the release of interferon-α, TNF-α and IL-6. The WH thus takes advantage of the key features of human pDCs for immunostimulation and can be a versatile tool for antigen-specific vaccination with a variety of proteins or peptides.
KEYWORDS: vaccine, immunotherapy, CpG, CD32, plasmacytoid dendritic cells
Introduction
The ability to induce immune responses with guaranteed predicted outcome is a holy grail in immunology and immunotherapy. Although there is no such thing as a guarantee, new approaches that reduce the risk of uncontrolled random responses would be very welcome. Especially in the field of allergy and cancer immunotherapy such approaches are needed. This may be achieved by eliciting specific immunity through targeting disease-related antigens to a particular subset of dendritic cells and simultaneously activate them. The present study describes a versatile molecular construct for vaccination by targeting and activating plasmacytoid dendritic cells (pDCs). The human pDC subset comprises specialized antigen-presenting cells normally found in peripheral blood or diverse epithelial tissues such as mucosa and skin. Phenotypically, pDCs have been described as lineage-negative, IL-3Rα+/CD123+, ILT3+, ILT1−, CD11c− cells, that can be further identified by their unique expression of BDCA-2 (CD303) and BDCA-4.1,2 Due to the expression of endosomal Toll-like receptor (TLR) 7 and TLR9, which sense viral RNA and DNA respectively, pDC mount a rapid and massive type I interferon (IFN) response to nucleic acids derived from virus, bacteria or dead cells.3,4 Following activation of TLR9 with the ligand CpG,5 they mature into potent antigen presenting cells and acquire the ability to regulate T cell-mediated adaptive immunity by preferentially triggering Th1 responses.6,7 Therefore, it appears attractive to translate these functions into a therapeutic approach that aims to trigger Th1 cell responses in an antigen-specific way. The first application of this idea was to use CpG as an adjuvant mixed with the antigen into one vaccine formulation.8 To further enhance the efficiency of inducing an immune response, the vaccine should be targeted to the most important antigen presenting cells. The natural receptor on DCs for antigen/antibody complexes is the FcγRII (CD32) enhancing the efficiency for antigen presentation by a factor 1,000 or 10,000 compared to pinocytosis.9 Fc-Receptors such as CD32a are internalized for unloading of cargo into the endosomal/lysosomal compartment.10 Stimulating pDCs through e.g. TLR9, however, impairs antigen uptake while the cells mature. Hence, pDCs activated by the adjuvant CpG greatly enhance the ability to boost antigen-specific T cell responses but at the same time their function to efficiently internalize and present new antigens is strongly impaired.11 The concentration range of CpG allowing optimal pDC activation is another critical aspect. Stimulation of purified human pDCs in vitro with different concentrations of CpG resulted in a dose response curve with an optimum,12,13 indicating that higher or lower concentrations of CpG will result in less activation of the pDC. By using CpG as adjuvant there is little control over the local concentration of CpG at the injection site. Hence it seems very unlikely that the optimal CpG concentration would be delivered to pDCs simultaneously with antigen. The solution to this dilemma could be to physically combine antigen and CpG. An additional improvement would be to direct antigen/CpG complex to antigen presenting cells through targeting CD32.
The other cell type in humans that constitutively expresses CD32(b) and TLR9 is the B cells. In B cells, co-crosslinking CD32(b) with the B cell receptor (BCR) will prevent antibody production.14 However, TLR9 activation has been shown to overrule this negative feedback loop.15
Previously, we reported that targeting CpG ODNs to the Fc receptor CD32 on human and monkey pDCs efficiently activated the secretion of IFNα aside other inflammatory cytokines and mediated their maturation.16 In the present study we describe the construction of a single-chain Fv (scFv) molecule derived from the well characterized monoclonal antibody IV.3 that specifically binds to human and non-human primate (NHP) CD32a on pDCs and CD32b on B cells and induces uptake by receptor mediated endocytosis.17,18 For optimal DC activation and induction of IFNα secretion, the TLR9 ligand M362 CpG was covalently linked to the scFv.12 Further, scFv domains were extended to incorporate five repeats of a pepE alpha-helical heptad motif (EVSALEK) that allows the co-delivery of an antigenic peptide/protein linked to five repeats of the pepK alpha-helical heptad motif (KVSALKE) endowed with the intrinsic function to form a coiled coil complex with the scFv module.19 The extended scFv coupled with CpG is called warhead (WH) and is generic, meaning that in all vaccines generated by this technology, the WH-module is the same, yet the immunogen will vary depending on the indication. Although not shown in the present study, WH plus pepK extended antigen, called immunogen, can be mixed in 1:1 molar ratio forming a complexed-vaccine molecule. The combination of the generic module (e.g. WH) with different disease-related molecules (e.g. immunogens) thus creates a human-specific vaccine platform. As shown recently in a non-human primate allergic asthma model, vaccine complexes of this type induced Th1 responses against house dust mite peptides as the immunogen.20 Moreover, such vaccine modules also induced strong immune responses against tumor associated (auto)-antigens,21 which are generally prevented by checkpoint control mechanisms.22,23
Results
Characterization of the CD32-binding scFv module
Dendritic cells use Fc-receptors such as CD32 for internalization of antibody-antigen complexes for subsequent processing and presentation to specific T cells.9,24,25 For efficient stimulation of type-1 helper or cytotoxic T cells, however, DCs need to be further activated by non-specific danger signals such as pathogen-associated molecular patterns (PAMPs). Previously, we could show that sequential treatment of plasmacytoid dendritic cells (pDCs) with streptavidin-conjugated anti-CD32 antibody and biotinylated CpG ODN oligonucleotides induced their maturation and triggered the release of type-1 interferon (e.g. IFNα) and other cytokines and was significantly more effective that the same amount of CpG alone.16 As the logical next step we prepared a single new molecule which binds to CD32 and delivers the TLR9 ligand CpG ODN and the relevant antigen to target pDCs so that it could be used in human vaccines. For this purpose, we constructed a scFv derived from the monoclonal antibody IV.3,17 extended by an alpha-helix forming heptad repeat motif referred to as pepE which is able to form high affinity hetero coiled-coil dimers with the counter alpha helix “pepK”.19 As pepK will be covalently linked to the antigen(s) against which the immune response should be induced, this modular approach allows for maximal flexibility to design new different vaccines and is the basis for a novel human and NHP-specific vaccine platform. The modified antibody fragment (Figure 1), referred to as scFv-coil1v5, is produced in CHO cells. Although the gene expressing the scFv-coil1v5 harbours a basic unit composed of a variable heavy chain (VH) linked to a variable light chain (VL) fragment and a coil, the protein can adopt dimeric conformations (diabody) and/or other orders of associations, in addition to the monomeric scFv-coil1v5 unit.26 Indeed, preferably dimers (~ 70%) but also monomers (25%) and multimers (5%) can be purified from the culture supernatant (Figure 1S; supplemental file online).
Figure 1.

Sequence of the scFv-coil1 construct.
Amino acid sequence of the scFv-coil1v5 construct. In the box, the amino acids correspond to the signal peptide which will be trimmed before secretion of the scFv. Underlined, the linkers between the different scFv motifs and in brackets, the five-times repeated heptad carboxy-terminal E-peptide motif ending with the extra alanine.
Specific binding of scFv-coil1v5 to CD32 isoforms was confirmed by incubation with immobilized low and high affinity Fc-receptors CD16 and CD64, respectively and the CD32 isoforms a and b using an ELISA format. The data in Figure 2 show binding of scFv-coil1v5 to CD32 isoforms with an almost 5-times higher binding affinity to CD32a as compared to CD32b. After separation and purity assessment of the monomeric and dimeric forms of scFv-coil1v5 by HPLC and PAGE (Figure 2S, supplemental file online), their binding affinity to Fc-receptor isoforms was assayed by biolayer interferometry. For monomers (scFv-coil1v5m), the estimated Kd for binding to CD32a and CD32b was 12 ± 3nM and 470 ± 50nM, respectively, while the fraction enriched for dimers (scFv-coil1v5s) bound with a Kd of 5 ± 2nM and 20 ± 7nM, respectively. No binding could be detected to CD16 or CD64. The preferential binding of dimers versus monomers to immobilized CD32a was further demonstrated by assessment of CD32a binding kinetics using biotinylated pepK ligand in a specifically designed ELISA (Figure 3S, supplemental file online).
Figure 2.
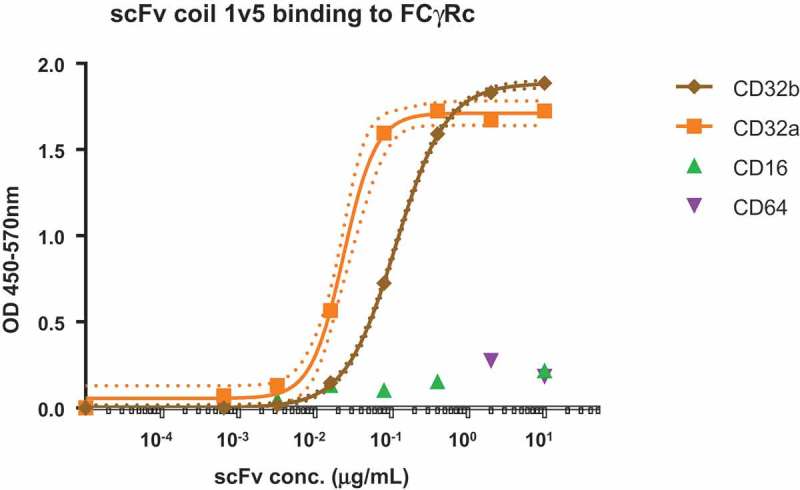
Binding of scFv construct to immobilized Fc-receptors.
Dose response ELISA of scFv-coil1 version5 binding different FcγRcs. In the graph the EC50 of CD32a binding is 4.8 times higher than the one observed to CD32b. There is no binding to CD16 or CD64. Dot lines indicate the 99% confidence interval determined by the Graphpad Prism 6.0 software.
Characterization of the warhead construct
The next step towards the construction of a targeting and immunostimulatory molecule required the coupling of the CD32-binding scFv-coil1v5 to a PAMP motif for which we selected a TLR9 ligand CpG-C type ODN (M362), previously used in the pilot study.16 Coupling of the latter to scFv was achieved by using the heterobifunctional amine-to-sulfhydryl crosslinker sulfo-SMCC, which is also used in registered products such as Kadcyla.27 By this method, multiple CpG-ODN oligonucleotides could be directly linked to each scFv-coil1v5 molecule, creating the immunostimulatory WH constructs WHv5s.1 (dimeric scFv-coil1) and WHv5m.1 (monomeric scFv-coil1). The average number of CpG-ODN per WH molecule and their protein concentration is depicted in Table 1. Data of the biochemical analysis of the WH-constructs can be found in supplementary materials. Next, both WH-batches were compared with respect to their binding to immobilized CD32a and CD32b followed by pepK-biotin/streptavidin detection as described before. Figure 3 shows that monomeric and dimeric WH-constructs bind to the same CD32 isoform with identical saturation kinetics. Superior binding to CD32a as compared to CD32b was revealed by the difference of EC50 values. This was confirmed by the biolayer interferometry analysis demonstrating a lower apparent Kd of WHv5s.1 and WHv5m.1 for binding to CD32a as compared to CD32b (Table 2). WH-mixture showed slightly lower Kd values probably due to the formation of higher association states of the scFv-coil1v5 in the WH preparation. Differences in binding affinities to CD32a or CD32b isoforms between scFv-coil1v5 and the corresponding WH-form are likely due to the palindromic nature of type C-CpG sequences that may mediate temperature sensitive dynamic intermolecular interactions (dsDNA) thereby increasing binding avidity.
Table 1.
Protein concentration and average molar ratios of CpG ODN/WH molecule.
| Sample | WHv5s.1 | WHv5m.1 |
|---|---|---|
| Conc. BCA (mg/mL) | 1.12 ± 0.04 | 1.24 ± 0.05 |
| Conc. CpG (mg/mL) | 1.61 ± 0.04 | 1.98 ± 0.01 |
| CpG/WH ratio | 5.35 ± 0.12 | 5.97 ± 0.01 |
Figure 3.
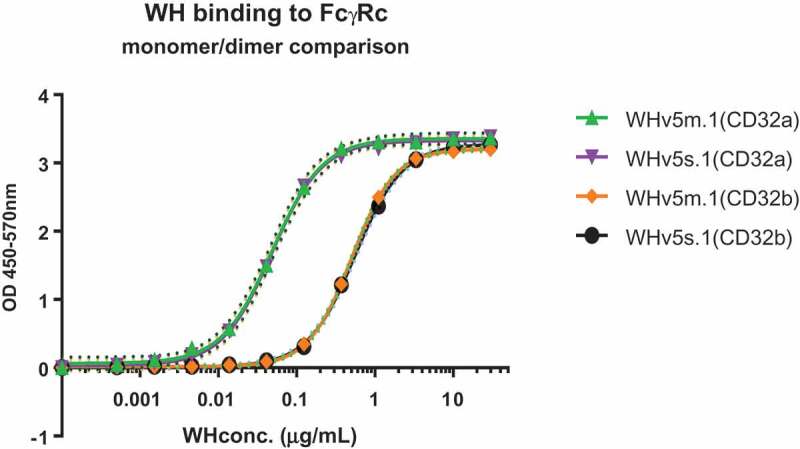
Binding properties of WH preparations.
Functional comparison of WH.V5m.1 batch 1 and WH.V5s.1 batch 1. CD32a or CD32b coated wells were incubated with WHv5 and bound material detected with pepK-biotin as described in M&M. EC50 were calculated by interpolating the Vmax values in the curve using Graphpad Prism 6.0 software. Both WHv5 batches showed identical binding to a given isoform, with an EC50 for CD32a around 10x lower than CD32b. Dot lines indicate the 99% confidence interval determined by the Graphpad Prism 6.0 software.
Table 2.
Biolayer Interferometry analysis of WH – IgG-Fc-receptor binding.
| BLI (Octet) Kd estimation (nM) |
|||
|---|---|---|---|
| FcγRc | WH mix | WHv5s.1 | WHv5m.1 |
| CD16 | No Binding | ||
| CD32a | 3.2 ± 0.3 | 5.8 ± 0.4 | 3.9 ± 0.1 |
| CD32b | 3.5 ± 0.2 | 13.0 ± 1.6 | 17.0 ± 4.1 |
| CD64 | No Binding | ||
Functional profile of WH constructs
After having shown high affinity binding to CD32 isoforms, it was important to demonstrate the functionality of WH constructs. Plasmacytoid DCs are known to express TLR9, the intracytoplasmic receptor for single stranded oligonucleotides such as CpG-ODN. WH was compared to the CD32-binding scFv construct and to free CpG-ODN with respect to the potency of activating peripheral blood derived pDC. Superior potency of WH as compared to free CpG-ODN in terms of inducing secretion of IFNα, TNFα and IL-6 was confirmed by the use of pDCs in a dose-dependent manner (Figure 4a). Moreover, Figure 4b shows the selective IFNα release by pDCs but not pDC-depleted PBMC upon CpG and WH stimulation. ScFv used at molar concentrations that corresponded to its WH-linked counterparts did not induce any cytokine production. Significantly lower levels of cytokine release were induced in pDCs by free CpG-ODN at molar concentration comparable to the WH using the same conditions. Finally, a direct comparison of monomeric (WHv5m.1) and dimeric WH (WHv5s.1) forms proved both to be equally effective to stimulate cytokine secretion from purified pDC (Figure 5).
Figure 4.
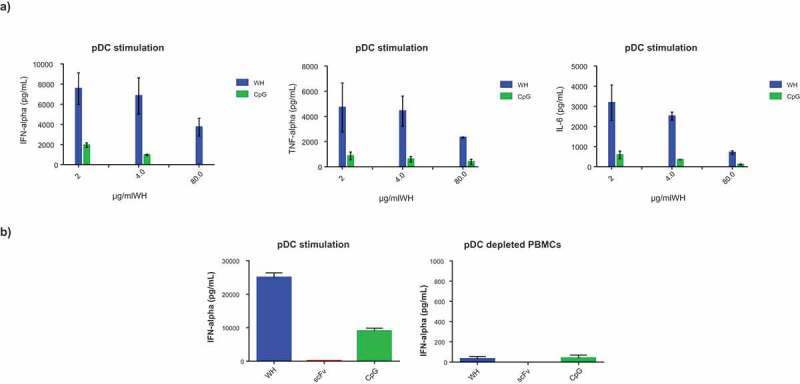
WH-mediated stimulation of cytokine release by pDC.
Cytokine levels produced by human pDCs and/or pDC depleted-PBMCs after stimulation with WH, scFv or CpG. Samples were incubated with pDCs of three different donors for 15 minutes at room temperature with the removal of not-bound material (a) or overnight without any washing step (b). The columns represent the mean of replicates with a SD calculated from Graphpad 6.0 software. Donors of the two experiments (a and b) are different and the free CpG was used at molar concentrations comparable to those in the WH preparations. In panel b, the scale of the y-axis is different showing the background secretion of IFNa by pDC depleted PBMCs.
Figure 5.
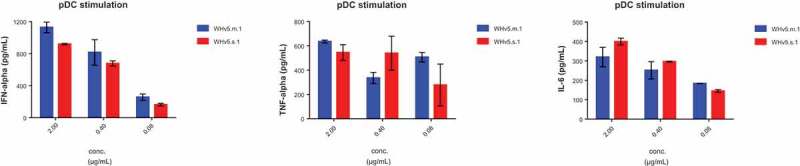
Comparison of WH preparations for induction of cytokines in pDC.
Cytokine levels produced by human pDCs after stimulation with WHv5s.1 and WHv5m.1. WH incubation with pDCs were performed for 15 minutes at room temperature and not-bound material was removed. After 24h the supernatant was evaluated for cytokine content.
Purification of pDCs from fresh blood samples is very labor intensive and expensive. Moreover, the number of cells obtained is too low to be used for routine batch release testing and in addition the donor to donor variation is so high (see also Figure 4a) that it is not possible to compare the quality of different batches. Therefore, a reporter cell line (UTLR9-6) was established as potential replacement for the use of primary pDC. UTLR9-6 is a CD32a-positive, TLR9 expressing cell line that carries the luciferase gene under the control of the NFkB promoter. Extensive testing of this cell line confirmed data obtained with fresh pDCs but with a much lower inter-assay variability. Figure 6a shows the superiority of WH preparations over either free CpG-ODN, scFv-coil, or the combination of CpG and scFv samples in terms of inducing NFkB regulated genes, such as type-I interferons and other inflammatory cytokines.28 Moreover, pre-incubation of UTLR9 cells for 15 min using the CD32-binding scFv module effectively reduced subsequent WH-induced stimulation by > 60% as measured in the luciferase reporter assay (Figure 6b).
Figure 6.
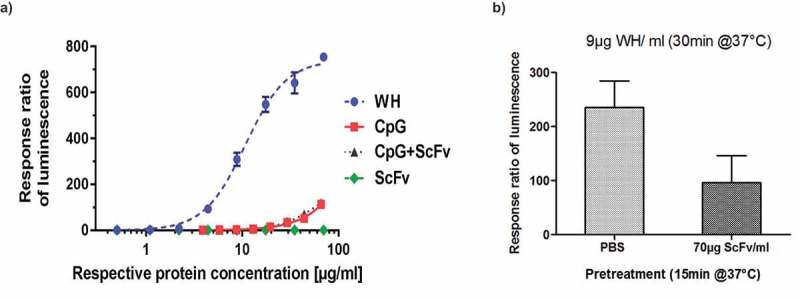
Reporter gene assay for WH potency evaluations.
Warhead stimulation using a TLR9 reporter cell line (UTLR9-6).(a) UTLR9- 6 cells were stimulated by WH, CpG, CpG+ScFv and scFv inducing the NFkB promoter-controlled luciferase expression measured by luminometry. ScFv was used at the same protein concentration range as the WH, and DNA concentrations in the CpG containing samples are equivalent to those in the WH sample for all data points. (b) Competition of WH uptake by U-TLR9-6 cells using scFv: U-TLR9-6 cells were pretreated with scFv and subsequently incubated with WH and compared to non-pretreated cells. Ratios of luminescence for WH-stimulation over scFv-treated cells are shown.
Discussion
Intensive research during more than two decades has led to a clearer understanding of the pivotal role of DCs in the induction and orchestration of adaptive immunity. So far, numerous studies have unraveled the existence of diverse DC subsets and characterized their plasticity regarding antigen-presentation in the context of class I and II MHC molecules and the ability to activate and modulate T cell responses (for a review, see Ref. 29). DC subsets in lymphoid organs or in the periphery are distinguished by the expression of specific surface molecules, some of which can be targeted to achieve controlled delivery of antigens and/or adjuvants. This has inspired investigations into DC-vaccination approaches aimed at activating the desired type T and B cell responses.30,31 Examples for efficient MHC class-II mediated presentation and induction of T cell immunity have been reported by targeting C-type lectin receptors (CLR), such as DEC-205/CD205 in mice and humans.32–34 However, in contrast to rodents where CD205 is predominantly found on DC, its human counterpart is found on several leukocytes including myeloid blood DC and monocytes, B cells, NK cells, pDCs and T cells.35 Other CLRs that have been targeted to induce Ag-specific naïve and memory T cell responses include DC-SIGN/CD209, DCIR/Clec4a/CD367, Clec9A/CD370, Clec12A/MICL/CD371 and recently the DC-specific chemokine receptor XCR.36–41 However, potential issues associated with targeting aforementioned CLRs for DC-based vaccination approaches include very broad leukocyte expression profile (most CLRs), promiscuous ligand-binding as observed for CD205,42,43 negative interference with DC activation and/or type-1 interferon synthesis as reported for BDCA-2 and DCIR,1,38 or context-dependent mediation of inhibitory or activating functions, as shown for CD371, also known as MICL or Clec12A.40,44,45 The applicability of XCR1 as targeting receptor of myeloid CD141+ DC to promote antigen-specific responses of T cells has so far been demonstrated in transgenic mice and awaits further confirmation in man.41
pDCs appear as an attractive cellular target for specific vaccination approaches as they represent a unique population at the crossroad of innate and adaptive immunity.46 In contrast to most myeloid DC, pDCs circulate in the bloodstream and enter peripheral tissues only at sites of infection to take up antigens, migrate to lymph nodes and present the encountered antigens to specific T cells. The unique capability of pDCs to produce large amounts of type I IFNs (IFN-α/β) in response to pathogen recognition is based on their expression of TLR7 and TLR9 located in intracellular endosomes and lysosomes. In the absence of the respective TLR ligands, pDCs maybe inferior as compared to myeloid DC subsets for T cell priming and activation.47 Upon TLR-mediated stimulation, however, pDCs proved to be potent activators of antigen-experienced T cells as shown by their ability to prime influenza virus-specific CD4+ and CD8+ T cells and promote the Th1 differentiation of memory CD4+ T cells.48,49 In melanoma patients vaccinated with KLH-pulsed autologous monocyte-derived DC, pDCs were able to capture and internalize KLH-antibody complexes via their low-affinity FcγRIIa for presentation to antigen-experienced CD4+ T cells.50 Moreover, in metastatic melanoma patients who received intranodal injections of pDCs activated and loaded with tumor antigen-associated peptides ex vivo, pDCs induced anti-vaccine CD4+ and CD8+ T-cell responses.51
The basic concept that guided the design of the WH molecule described in this study was to enable targeting of a natural antigen-internalization receptor combined with the simultaneous activation of pDCs. Importantly, pDCs only express the activating isoform of the intermediate-affinity IgG receptor and can use FcγRIIa/CD32a for efficient uptake of exogenous antigens.10 The scFv sequence within WH mediates specific and high-affinity binding to both isoforms of CD32 with a Kd of ~3nM (as determined for the mixture of dimers and monomers). The functionality in terms of pDC activation and induction of interferon and cytokine release is attributed to six or seven covalently linked CpG motifs as ligand for TLR9. The WH mediated the induction of IFNα, TNFα and IL-6 release in pDC populations and activation of the TLR9 reporter gene in the cell line UTLR9. The C-terminal extension by five heptad-repeats of the pepE-coil motif allows for complex formation with pepK-conjugated peptide or whole protein cargos as antigen thus building a WH-based human and NHP-specific vaccine platform for rapid generation of new vaccines with similar immune modulating characteristics.
Stimulation of TLR9 through CpG ODNs activates the accessory functions of pDCs and boost the generation of humoral and cellular vaccine-specific immune responses as demonstrated experimentally in mice and more recently in human clinical studies. 51,52 These effects become optimized when ODNs and vaccines are delivered together via an internalization receptor expressed by DC.16,53 The WH fulfills this requirement and can be seen as a versatile tool to directly target pDCs via FcγRIIa in vivo and simultaneously deliver TLR9 agonist and antigenic cargos linked by coiled-coil peptide complex formation. Indeed, using the WH as CD32 targeting module with intrinsic adjuvant activity, three different vaccines against house dust mite (SG100),20 little gastrin or G17 (TYG100)21 and OQR200 against HER2/neu (manuscript in preparation) have achieved in vivo proof of concept in NHP. Ex vivo antigen specific stimulation of T cells from treated animals only showed INFγ production in the absence of any IL-4, confirming text book knowledge concerning TLR9 activation through CpG. Indeed, even in highly allergic animals20 no vaccine specific IgE responses were seen (data not shown). Noteworthy, very strong antibody responses seen in non-human primates immunized with TYG100 and OQR200 confirm that the negative effects from co-cross-linking CD32b and BCR are overruled by CpG in the WH (manuscript in preparation). Importantly, activation of the immune system through the WH in these studies did not lead to adverse effects other than an occasional transient injection site reaction.
Materials and methods
Construction and purification of the CD32-binding scFv module
The scFv-coil1 consists of an Fv fragment derived from the monoclonal anti CD32 antibody IV.3,17 connected by a polypeptide linker and a five-times repeated heptad carboxy-terminal E-peptide motif tag (pepE).19,54 An alanine residue at the C’-terminus was added to prevent proteolytic degradation of the molecule.55 A monoclonal Chinese Hamster Ovary-K1 (CHO-K1) cell line harboring this gene was generated by Polymun (Klosterneuburg, Austria). CHO-K1 cells expressing the scFv were expanded at a concentration of 3 × 105 cells/ml in culture (CD-CHO medium supplemented with 0.5mg/ml G418, 100U/mL penicillin, 100µg/mL streptomycin and 8mM L-Glutamine (all reagents from Gibco). After a culture period of 10 days, cells were collected by centrifugation and supernatants (SN) cleared by filtration through 0.2µm stericup devices (Merck) followed by addition of 0.1mM PMSF (Sigma). SN aliquots were stored at −20°C for at least 3 days before downstream processing.
The scFv was purified on an affinity column prepared by mixing 1mL Streptavidin-Agarose Ultra Performance resin (SoluLink) and 1.74 mg of pepK(4)biotin (Lifetein LCC) in 1 ml PBS for 1h at RT with shaking. PepK(4), was used instead of the pepK(5) (five times repeated sequence conjugated to immunogen sequences to form vaccines) allowing for milder elution conditions due to lower affinity for the pepE(5). The resin beads were washed with 10 volumes of PBS to remove any traces of free peptide, and incubated overnight at 4°C with rotation. Subsequently, bound scFv proteins were separated from the bead-pepK(4)-biotin mixture in a 1.5 x 15 cm column by two and three washing cycles with 1 ml PBS/0.05% Tween20 and 3 ml PBS, respectively and elution of bound material by adding 8 volumes of 0.1M glycine, pH 3.5. Fractions of 1 mL were collected in tubes containing 100 µl 1M Tris-HCl, pH 8.0 and tested for protein content by spectrophotometry (A280) and molecular size on SDS-PAGE (Thermo Fisher).
For separation of dimers and monomers, samples were filtrated through 0.22µm and 4ml aliquots of up to 10mg/ml processed on Superdex 200 prep grade 26/60 columns (GE Healthcare) using Dulbecco’s phosphate-buffered saline (DPBS) as running buffer at a flow rate of 2mL/min. Fractions of 4 mL are then analyzed by HPLC and native-PAGE (Thermo Fisher) following the manufacturer instructions.
The dimeric and monomeric forms of the scFv-coil1 were quantified by HPLC on a LC-20ADXR Liquid Chromatograph (Shimadzu) equipped with a SPD-M20A UV/VIS Photodiode Array Detector (Shimadzu). As running buffer DPBS containing 200 mM NaCl was used with a Superdex 200 10/300 column.
From each sample 50 μg of protein was injected. As reference, a gel filtration standard was also run through the column. The A280 values from the UV/VIS detector were exported and analyzed in Microsoft Excel.
Fcgamma-receptor ELISA
FcγReceptors (CD32a, CD32b, CD16 and CD64) fused to a His tag (all from SINO Biological) were coated on His-Sorb plates (Qiagen) at a concentration of 2 µg/ml o/n at 4°C. On the next day following triple washing with PBST (PBS + 0.05% Tween-20), wells were incubated for 1h at room temperature (RT) with different concentrations of scFv-coil1. After washing 3x, an incubation with 100 ng/ml of a biotinylated five-times repeated heptad amino acid sequence (KVSALKE), called pepK(5)-biotin followed for 1h at RT. The complex consisting of CD32a–scFv-coil1–pepK(5)-biotin was detected with Streptavidin-HRP (GE Healthcare) at a 1:5000 dilution. Colour development was performed using colorimetric TMB-substrate. Absorbance was measured at 405 nm and 570 nm as reference wavelength.
Warhead preparation
2.5 mg of M362 CpG-SH (8 kDa), was dissolved in 25 mM phosphate buffer at pH 6.5 to obtain a final concentration of 1–2.5 mg/ml and incubated with 2 mM Tris(2-carboxyethyl)phosphine (TCEP) for 15–20 min at room temperature.12 The solution was then desalted by running over a Zeba desalting spin column (2 or 5 ml; Thermo Scientific), pre-equilibrated in the same buffer without TCEP, according to manufacturer’s instructions. Subsequently, 0.5–0.75 mg sulfo-SMCC (sulfosuccinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate, stock concentration 4mg/ml) in ddH2O was added to obtain a 4- to 6-fold excess over CpG-SH, mixed and incubated at 25°C under agitation for 30 min. Upon completion of the reaction (no color change to yellow upon addition of 1 mM solution of 2,2′-dithiobis(5-nitropyridine), DTNP), 3 M sodium acetate pH 5.6 (1/9 of the total reaction volume) was added and the DNA-linker conjugate was precipitated in three or four equal portions by adding 2.5–3 volumes of ethanol. The precipitate was collected by centrifugation at maximum speed and pellets were washed with 70% ethanol. After subsequent centrifugation, ethanol was removed and the pellets were air-dried by inverting centrifuge tubes. Dry pellets can be used immediately or stored at −20°C for at least 72 hours. The resulting product is CpG-sulfo-SMCC.
One of the CpG-sulfo-SMCC pellets was dissolved in a solution of scFv-coil1 (1 mg; at a concentration of 3 mg/ml) in PBS buffer supplemented with 1/9 volume of 1 M phosphate buffer pH 7.5 and incubated at 38°C for one hour under agitation. The entire reaction mixture was transferred to a tube with a new pellet of CpG-sulfo-SMCC and incubation at 38°C continued. The process was repeated until the addition of the last pellet. Then the incubation was extended for two hours to make sure that reaction proceeds to completion. The resulting product is Warhead (WH) which contained an approximately 10x molar excess of CpG over 1 mg of scFv-coil1. The crude warhead preparation (1mg aliquots) was loaded on Superdex-75 column (16x600 mm, GE Healthcare) pre-equilibrated in PBS. Separation was achieved by running in PBS at 1 ml/min over 1 column volume.
To determine the protein concentration of WHv5m.1 and WHv5s.1 the micro-BCA (bicinchoninic acid) protein assay kit (Thermo Scientific) was used because this assay was shown to be compatible with the DNA contained in the WH molecule. The assay was performed according to the manufacturer’s instruction. On the other hand, the determination of CpG content in WH, different concentrations of synthesized CpG molecules were measured at an absorbance of 260 nm using a Nanodrop 1000 (Thermo Scientific). The extinction coefficient for translating OD values into concentrations was determined by dividing the absorbance at 260 nm (A260) by the CpG concentration provided by the manufacturer. The average extinction coefficient of 52µg/mL was based on 18 independent measurements. The CpG content of WH was then estimated by correcting the A260 value with the factor 52 µg/mL. In order to calculate the molar ratio between protein and DNA, the protein concentration (µg/mL) and CpG concentration (µg/mL) of the WH were converted to molarity based on their molecular weight (30,835.4 Da and 8261 Da, respectively).
Purification of pDCs from human PBMCs
The pDC fraction from PBMCs was isolated by positive magnetic selection using the human CD304 (BDCA-4/Neuropilin) MicroBead Kit from Miltenyi Biotec as indicated by the manufacturer. Usually, cell numbers between 1 × 106 and 2 × 106 pDCs were obtained, which was donor dependent. The pDC frequency in the initial PBMC preparation and pDC purities after the first and the second MACS column were assessed by flow cytometry. For this, cell samples of 80 µl are incubated with 20 µl FcγR blocking reagent, 10 µl anti-CD303 (BDCA-2)-APC antibody and 5 µl anti-CD123-FITC antibody (Miltenyi Biotech) for 10 min at 4°C in the dark. After washing, samples were analyzed on a Beckman Coulter Gallios Flow Cytometer using 7-AAD for discrimination between live and dead cells. Routinely, purity between 80% and 95% is reached.
Stimulation of purified human pDCs with WH or free CpG
After MACS purification of pDCs (Miltenyi Biotech) and cell counting, cells are centrifuged for 10 min at 300xg at 4°C and resuspended either in PBS containing 0.5% BSA for preincubation with the stimuli and subsequent washing steps or directly in growth medium (X-VIVO 15 (LONZA), 2% human serum (LONZA), 10 ng/ml IL3 (eBioscience)) for overnight incubations without washing. Cell concentrations are adjusted to yield final cell numbers of 0.5 – 1 × 105 cells per sample in 200 µl. For preincubation samples, WH is added to the cells at different end concentrations ranging from 0.1 µg/ml to 10 µg/ml. Samples are incubated for 15 min at room temperature, followed by two washes with 4 ml cold PBS/BSA to remove unbound WH or CpG. After centrifugation, supernatants are removed carefully with a pipette to minimize loss of cells during the washing steps. After the second wash, cells are re-suspended in 200 µl growth medium. Alternatively, test items were incubated with the cells overnight without washing. The CpG concentrations selected for this stimulation assay were published previously.22 All samples are incubated in 96-well round bottom plates in a humidified incubator at 37°C, 5% CO2. After 24 hours, plates are centrifuged for 10 min at 300xg at room temperature and supernatants are harvested without disturbing the cells and frozen.
Analysis of cytokine production by pDCs upon stimulation
For the determination of titres of IFNα, IL-6 and TNFα in pDC supernatants, quantitative ELISA kits from eBioscience were used according to the manufacturer’s instructions. Absorbance at 450 nm was measured on a Tecan Infinite M1000 microplate reader and analyzed with Magellan software.
Reporter-gene assay
To test CD32-mediated uptake and stimulation of cells by WH-modules the reporter cell line U-TLR9-6 bearing the luciferase gene under control of the NFkB promoter was incubated for 15 min at 37°C in the presence of absence of scFv. Subsequently, cells were treated for 15 min with WH and compared to non-pretreated cells. After washing twice with culture medium, cells were further incubated in culture medium (RPMI 1640 (LONZA) supplemented with 10% FCS (SAFC)) for 3h at 37°C. Samples were tested in triplicates and stimulation ratios obtained by diving absolute luminescence readouts after stimulation by results of non-treated cells.
Biolayer interferometry
Binding affinities of purified WH preparations were determined by Bio-layer Interferometry (ForteBio OctetQK machine) using Ni-NTA biosensors for kinetic measurements (ForteBio). First, biosensors were equilibrated in DPBS + 0.02% Tween-20 for 15 minutes, which represents the buffer for all steps in this experiment. Concentrations of 0.5 – 2µg/ml of His-tagged Fcγ receptors (Sino Biologics) were used for loading the sensors aiming for same immobilization relative units after 15 minutes. Association and Dissociation rate of WH were measured in a range of 0nM to 100nM for 15 and 30 minutes, respectively. A steady state analysis was applied for calculating the Kd values obtained from the Data Analysis 6.4 software (ForteBio).
Disclosure of potential conflicts of interest
Dr. Geert Mudde is 50% shareholder of both companies and the co-authors work for either OncoQR ML GmbH or S-TARget therapeutics GmbH, with the exception of K. Fink who was a graduate student at OncoQR ML.
Funding Statement
This work was supported by the Austria Wirtschaftsservice Gesellschaft mbH [P0701197-SZL02];Austria Wirtschaftsservice Gesellschaft mbH [R100003];Österreichische Forschungsförderungs-Gesellschaft [848330];Austria Wirtschaftsservice Gesellschaft mbH [P1204389-SZL01];
Acknowledgments
Special thanks go to Frank Kalthoff for his dedicated support in preparation of the manuscript. This work was funded by a Pre-seed Grant from the Austria Wirtschaftsservice Gesellschaft mbH (project numbers: R100003, P0701197-SZL02 and P1204389-SZL01) and by the Österreichische Forschungsförderungs-gesellschaft (project number: 848330).
Supplementary data
Supplemental data for this article can be accessed here.
References
- 1.Dzionek A, Inagaki Y, Okawa K, Nagafune J, Röck J, Sohma Y, Winkels G, Zysk M, Yamaguchi Y, Schmitz J.. Plasmacytoid dendritic cells: from specific surface markers to specific cellular functions. Hum Immunol. 2002;63(12):1133–1148. doi: 10.1016/S0198-8859(02)00752-8. [DOI] [PubMed] [Google Scholar]
- 2.Colonna M, Trinchieri G, Liu YJ.. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5(12):1219–1226 10.1038/ni1141 [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Swiecki M, McCartney SA, Colonna M. dsRNA sensors and plasmacytoid dendritic cells in host defense and autoimmunity. Immunol Rev. 2011;243:.74–90. doi: 10.1111/j.1600-065X.2011.01049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bao M, Liu YJ. Regulation of TLR7/9 signaling in plasmacytoid dendritic cells. Protein Cell. 2013;4(1):40–52. doi: 10.1007/s13238-012-2104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klinman DM, Currie D, Gursel I, Verthelyi D. Use of CpG oligodeoxynucleotides as immune adjuvants. Immunol Rev. 2004;199:.201–216. doi: 10.1111/j.0105-2896.2004.00148.x. [DOI] [PubMed] [Google Scholar]
- 6.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nature Immunology. 2005;6(8):769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sittig SP, Bakdash G, Weiden J, Sköld AE, Tel J, Figdor CG, De Vries IJ, Schreibelt G. A comparative study of the T cell stimulatory and polarizing capacity of human primary blood dendritic cell subsets. Mediators Inflamm. 2016:3605643. doi: 10.1155/2016/3605643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov. 2006;5(6):471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 9.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179(4):1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Båve U, Magnusson M, Eloranta ML, Perers A, Alm GV, Rönnblom L. Fc gamma RIIa is expressed on natural IFN-alpha-producing cells (plasmacytoid dendritic cells) and is required for the IFN-alpha production induced by apoptotic cells combined with lupus IgG. J Immunol. 2003;171(6):3296–3302. doi: 10.4049/jimmunol.171.6.3296. [DOI] [PubMed] [Google Scholar]
- 11.Benitez-Ribas D, Tacken P, Punt CJ, De Vries IJ, Figdor CG. Activation of human plasmacytoid dendritic cells by TLR9 impairs Fc gammaRII-mediated uptake of immune complexes and presentation by MHC class II. J Immunol. 2008;181(8):5219–5224. doi: 10.4049/jimmunol.181.8.5219. [DOI] [PubMed] [Google Scholar]
- 12.Hartmann G, Battiany J, Poeck H, Wagner M, Kerkmann M, Lubenow N, Rothenfusser S, Endres S. Rational design of new CpG oligonucleotides that combine B cell activation with high IFN-alpha induction in plasmacytoid dendritic cells. Eur J Immunol. 2003;33(6):1633–1641. doi: 10.1002/eji.200323813. [DOI] [PubMed] [Google Scholar]
- 13.Ida JA, Shrestha N, Desai S, Pahwa S, Hanekom WA, Haslett PA. A whole blood assay to assess peripheral blood dendritic cell function in response to Toll-like receptor stimulation. J Immunol Methods. 2006;310(1–2):86–99. doi: 10.1016/j.jim.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8(1):34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 15.Karnell JL, Dimasi N, Karnell FG 3rd, Fleming R, Kuta E, Wilson M, Wu H, Gao C, Herbst R, Ettinger R. CD19 and CD32b differentially regulate human B cell responsiveness. J Immunol. 2014;192(4):1480–1490. doi: 10.4049/jimmunol.1301361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tel J, Beenhakker N, Koopman G, Hart B, Mudde GC, De Vries IJ. Targeted delivery of CpG ODN to CD32 on human and monkey plasmacytoid dendritic cells augments IFNα secretion. Immunobiology. 2012;217(10):1017–1024. doi: 10.1016/j.imbio.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 17.Looney RJ, Abraham GN, Anderson CL. Human monocytes and U937 cells bear two distinct Fc receptors for IgG. J Immunol. 1986;136(5):1641–1647 http://www.jimmunol.org/content/136/5/1641 [PubMed] [Google Scholar]
- 18.Horejs-Hoeck J, Hren A, Mudde GC, Woisetschläger M. Inhibition of immunoglobulin E synthesis through Fc gammaRII (CD32) by a mechanism independent of B-cell receptor co-cross-linking. Immunology. 2005;115(3):407–415. doi: 10.1111/j.1365-2567.2005.02162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chao H, Bautista DL, Litowski J, Irvin RT, Hodges RS. Use of a heterodimeric coiled-coil system for biosensor application and affinity purification. J Chromatogr B Biomed Sci Appl. 1998;715(1):307–329. doi: 10.1016/S0378-4347(98)00172-8. [DOI] [PubMed] [Google Scholar]
- 20.Reece SW, Wardle RL, Gaier S, Pichler J, Chandler J, Kravchuk AV, Sepulveda JA, Putnam BD, Katwa LC, Olmstead SG, et al. Vaccination with SG100 attenuates aeroallergen-induced early and late phase asthmatic responses in house dust mite sensitive non-human primates In: editors, Maurer M, Behrendt H. Allergies: current challenges and solutions (Proceedings of the 30th Symposium of the Collegium Internationale Allergologicum (CIA)). Munich, Germany: Pacini Medicina Editore S.r.l; 2016. p. 215–221. http://www.pacinimedicina.it/allergies-current-challenges-and-solutions/ [Google Scholar]
- 21.Broome P, Wardle RL, Wang X, Gaier S, Pichler J, Chandler JH, Katwa LC, Reece RC, Laing P, Mudde GC, and others . Primate study of TYG100 a novel rationally-designed recombinant vaccine for gastrin sensitive cancers: immunogenicity and tolerability in a species-homologous mode. In: Proceedings of the 105th Annual Meeting of the American Association for Cancer Research; 2014. April 5-9; San Diego (CA. Philadelphia (PA)): AACR; Cancer Res 2014; 74(19Suppl):Abstract nr 2886. doi: 10.1158/1538-7445.AM2014-2886 [DOI] [Google Scholar]
- 22.Zhang X, Munegowda MA, Yuan J, Wei Y, Xiang J. Optimal TLR9 signal converts tolerogenic CD4-8- DCs into immunogenic ones capable of stimulating antitumor immunity via activating CD4+ Th1/Th17 and NK cell responses. J Leukoc Biol. 2010;88(2):393–403. doi: 10.1189/jlb.0909633. [DOI] [PubMed] [Google Scholar]
- 23.Peng SL. Signaling in B cells via Toll-like receptors. Curr Opin Immunol. 2005;17(3):230–236. doi: 10.1016/j.coi.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Mudde GC, Van Reijsen FC, Boland GJ, De Gast GC, Bruijnzeel PL, Bruijnzeel-Koomen CA. Allergen presentation by epidermal Langerhans’ cells from patients with atopic dermatitis is mediated by IgE. Immunology. 1990;69(3):335–341 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1385948/ [PMC free article] [PubMed] [Google Scholar]
- 25.Santamaria LF, Bheekha R, van Reijsen FC, Perez Soler MT, Suter M, Bruijnzeel-Koomen CA, Mudde GC. Antigen focusing by specific monomeric immunoglobulin E bound to CD23 on Epstein-Barr virus-transformed B cells. Hum Immunol. 1993;37(1):23–30. doi: 10.1016/0198-8859(93)90139-R. [DOI] [PubMed] [Google Scholar]
- 26.Le GF, Kipriyanov SM, Moldenhauer G, Little M. Di-, tri- and tetrameric single chain Fv antibody fragments against human CD19: effect of valency on cell binding. FEBS Lett. 1999;453(1–2):164–168. doi: 10.1016/S0014-5793(99)00713-9. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, Kim MT, Zheng L, Deperalta G, Jacobson F. Structural Characterization of Cross-Linked Species in Trastuzumab Emtansine (Kadcyla). Bioconjug Chem. 2016;27(9):2037–2047. doi: 10.1021/acs.bioconjchem.6b00316. [DOI] [PubMed] [Google Scholar]
- 28.Gohda J, Matsumura T, Inoue JI. Cutting edge: TNFR-associatedfactor (TRAF) 6 is essential for MyD88-dependent pathway but not Toll/IL-1 receptor domain-containing adaptor-inducing IFN-β(TRIF)-dependent pathway in TLR signaling. J Immunology. 2004;173(5):2913–2917. doi: 10.4049/jimmunol.173.5.2913. [DOI] [PubMed] [Google Scholar]
- 29.Boltjes A, van Wijk F. Human dendritic cell functional specialization in steady-state and inflammation. Front Immunol. 2014;5:.131. doi: 10.3389/fimmu.2014.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lehmann CH, Heger L, Heidkamp GF, Baranska A, Lühr JJ, Hoffmann A, Dudziak D. Direct Delivery of Antigens to Dendritic Cells via Antibodies Specific for Endocytic Receptors as a Promising Strategy for Future Therapies. Vaccines (Basel). 2016;4(2):8. doi: 10.3390/vaccines4020008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macri C, Dumont C, Johnston AP, Mintern JD. Targeting dendritic cells: a promising strategy to improve vaccine effectiveness. Clin Transl Immunology. 2016;5(3):e66. doi: 10.1038/cti.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonifaz LC, Bonnyay DP, Charalambous A, Darguste DI, Fujii S, Soares H, Brimnes MK, Moltedo B, Moran TM, Steinman RM. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med. 2004;199(6):815–824. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Birkholz K, Schwenkert M, Kellner C, Gross S, Fey G, Schuler-Thurner B, Schuler G, Schaft N, Dörrie J. Targeting of DEC-205 on human dendritic cells results in efficient MHC class II-restricted antigen presentation. Blood. 2010;116(13):2277–2285. doi: 10.1182/blood-2010-02-268425. [DOI] [PubMed] [Google Scholar]
- 34.Stoitzner P, Schaffenrath S, Tripp CH, Reider D, Komenda K, Del Frari B, Djedovic G, Ebner S, Romani N. Human skin dendritic cells can be targeted in situ by intradermal injection of antibodies against lectin receptors. Exp Dermatol. 2014;23(12):909–915. doi: 10.1111/exd.12573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kato M, McDonald KJ, Khan S, Ross IL, Vuckovic S, Chen K, Munster D, MacDonald KP, Hart DN. Expression of human DEC-205 (CD205) multilectin receptor on leukocytes. Int Immunol. 2006;18:.857–869. doi: 10.1093/intimm/dxl022. [DOI] [PubMed] [Google Scholar]
- 36.Tacken PJ, De Vries IJ, Gijzen K, Joosten B, Wu D, Rother RP, Faas SJ, Punt CJ, Torensma R, Adema GJ; others . Effective induction of naive and recall T-cell responses by targeting antigen to human dendritic cells via a humanized anti-DC-SIGN antibody. Blood. 2005;106(4):1278–1285. doi: 10.1182/blood-2005-01-0318. [DOI] [PubMed] [Google Scholar]
- 37.Kretz-Rommel A, Qin F, Dakappagari N, Torensma R, Faas S, Wu D, Bowdish KS. vivo targeting of antigens to human dendritic cells through DC-SIGN elicits stimulatory immune responses and inhibits tumor growth in grafted mouse models. J Immunother. 2007;30(7):715–726. doi: 10.1097/CJI.0b013e318135472c. [DOI] [PubMed] [Google Scholar]
- 38.Meyer-Wentrup F, Benitez-Ribas D, Tacken PJ, Punt CJ, Figdor CG, De Vries IJ, Adema GJ. Targeting DCIR on human plasmacytoid dendritic cells results in antigen presentation and inhibits IFN-alpha production. Blood. 2008;111(8):4245–4253. doi: 10.1182/blood-2007-03-081398. [DOI] [PubMed] [Google Scholar]
- 39.Tullett KM, Leal Rojas IM, Minoda Y, Tan PS, Zhang JG, Smith C, Khanna R, Shortman K, Caminschi I, Lahoud MH, et al.; others . Targeting CLEC9A delivers antigen to human CD141+ DC for CD4+ and CD8+T cell recognition. JCI Insight. 2016;1(7):e87102. doi: 10.1172/jci.insight.87102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marshall AS, Willment JA, Lin HH, Williams DL, Gordon S, Brown GD. Identification and characterization of a novel human myeloid inhibitory C-type lectin-like receptor (MICL) that is predominantly expressed on granulocytes and monocytes. J Biol Chem. 2004;279(15):14792–14802. doi: 10.1074/jbc.M313127200. [DOI] [PubMed] [Google Scholar]
- 41.Hartung E, Becker M, Bachem A, Reeg N, Jäkel A, Hutloff A, Weber H, Weise C, Giesecke C, Henn V, et al.; others . Induction of potent CD8 T cell cytotoxicity by specific targeting of antigen to cross-presenting dendritic cells in vivo via murine or human XCR1. J Immunol. 2015;194(3):1069–1079. doi: 10.4049/jimmunol.1401903. [DOI] [PubMed] [Google Scholar]
- 42.Lahoud MH, Ahmet F, Zhang JG, Meuter S, Policheni AN, Kitsoulis S, Lee CN, O’Keeffe M, Sullivan LC, Brooks AG; others . DEC-205 is a cell surface receptor for CpG oligonucleotides. Proc Natl Acad Sci USA. 2012;109:16270–16275. doi: 10.1073/pnas.1208796109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shrimpton RE, Butler M, Morel AS, Eren E, Hue SS, Ritter MA. CD205 (DEC-205): a recognition receptor for apoptotic and necrotic self. Mol Immunol. 2009;46:.1229–1239. doi: 10.1016/j.molimm.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neumann K, Castiñeiras-Vilariño M, Höckendorf U, Hannesschläger N, Lemeer S, Kupka D, Meyermann S, Lech M, Anders HJ, Kuster B; others . Clec12a is an inhibitory receptor for uric acid crystals that regulates inflammation in response to cell death. Immunity. 2014;40(3):389–399. doi: 10.1016/j.immuni.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 45.Hutten TJ, Thordardottir S, Fredrix H, Janssen L, Woestenenk R, Tel J, Joosten B, Cambi A, Heemskerk MH, Franssen GM; others . CLEC12A-mediated antigen uptake and cross-presentation by human dendritic cell subsets efficiently boost tumor-reactive T cell responses. J Immunol. 2016;197(7):2715–2725. 10.4049/jimmunol.1600011. [DOI] [PubMed] [Google Scholar]
- 46.Macagno A, Napolitani G, Lanzavecchia A, Sallusto F. Duration, combination and timing: the signal integration model of dendritic cell activation. Trends Immunol. 2007;28(5):227–233. doi: 10.1016/j.it.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 47.Krug A, Veeraswamy R, Pekosz A, Kanagawa O, Unanue ER, Colonna M, Cella M. Interferon-producing cells fail to induce proliferation of naive T cells but can promote expansion and T helper 1 differentiation of antigen-experienced unpolarized T cells. J Exp Med. 2003;197(7):899–906. doi: 10.1084/jem.20021091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fonteneau JF, Gilliet M, Larsson I, Dasilva I, Münz C, Liu YJ, Bhardwaj N. Activation of influenza virus-specific CD4+ and CD8+ T cells: a new role for plasmacytoid dendritic cells in adaptive immunity. Blood. 2003;101:.3520–3526. doi: 10.1182/blood-2002-10-3063. [DOI] [PubMed] [Google Scholar]
- 49.Langenkamp A, Nagata K, Murphy K, Wu L, Lanzavecchia A, Sallusto F. Kinetics and expression patterns of chemokine receptors in human CD4+ T lymphocytes primed by myeloid or plasmacytoid dendritic cells. Eur J Immunol. 2003;33(2):474–482. doi: 10.1002/immu.200310023. [DOI] [PubMed] [Google Scholar]
- 50.Benitez-Ribas D, Adema GJ, Winkels G, Klasen IS, Punt CJ, Figdor CG, De Vries IJ. Plasmacytoid dendritic cells of melanoma patients present exogenous proteins to CD4+ T cells after Fc gamma RII-mediated uptake. J Exp Med. 2006;203(7):1629–1635. doi: 10.1084/jem.20052364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tel J, Aarntzen EH, Baba T, Schreibelt G, Schulte BM, Benitez-Ribas D, Boerman OC, Croockewit S, Oyen WJ, van Rossum M; others . Natural human plasmacytoid dendritic cells induce antigen-specific T-cell responses in melanoma patients. Cancer Res. 2013;73(3):1063–1075. doi: 10.1158/0008-5472.CAN-12-2583. [DOI] [PubMed] [Google Scholar]
- 52.Stern BV, Boehm BO, Tary-Lehmann M. Vaccination with tumor peptide in CpG adjuvant protects via IFN-gamma-dependent CD4 cell immunity. J Immunol. 2002;168(12):6099–6105. doi: 10.4049/jimmunol.168.12.6099. [DOI] [PubMed] [Google Scholar]
- 53.Krishnamachari Y, Salem AK. Innovative strategies for co-delivering antigens and CpG oligonucleotides. Adv Drug Deliv Rev. 2009;61(3):205–217. doi: 10.1016/j.addr.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huston JS, Levinson D, Mudgett-Hunter M, Tai MS, Novotný J, Margolies MN, Ridge RJ, Bruccoleri RE, Haber E, Crea R; others . Protein engineering of antibody binding sites: recovery of specific activity in an anti-digoxin single-chain Fv analogue produced in Escherichia coli. Proc Natl Acad Sci USA. 1988;85(16):5879–5883. doi: 10.1073/pnas.85.16.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harris RJ. Processing of C-terminal lysine and arginine residues of proteins isolated from mammalian cell culture. J Chromatogr A. 1995;705(1):129–134. doi: 10.1016/0021-9673(94)01255-D. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


