ABSTRACT
T cell-based immunotherapies have revolutionized the treatment against cancer. But complete and long-lasting efficacy is only observed in a fraction of the patient population. One of the suspected causes is the inability of cytotoxic T cells, endowed with tumor killing ability, to reach their malignant targets. Using dynamic fluorescence imaging to study the dynamic of T cells in tumors from patients with lung cancer, we have recently demonstrated that macrophages trap the T lymphocytes, which are not longer able to contact the tumor cells. In murine models of breast cancer, we could show that the depletion of macrophages allows T cells to interact with tumor cells, a process which enhances anti-PD-1 immunotherapy. These findings illustrate the relevance of current clinical trials combining a strategy that deplete or modulate macrophages with anti-PD-1 immunotherapy.
Keywords: Cancer, T cells, immunotherapy, migration, macrophages, anti-PD-1
Introduction
Cancer immunotherapies are making the headline these days. This is due to very promising clinical results obtained in a number of malignancies. Among the different novel strategies, two approaches that target T cells, key players in the fight against cancer, appear particularly efficient, namely immune checkpoint inhibitors, such as anti-PD-1 antibodies, and chimeric antigen receptor (CAR) T cells.
Although some patients treated with T-cell based immunotherapies achieve long-term disease-free survival, a still elevated percentage of them do not respond clinically. This highlights the presence of resistance mechanisms that one needs to identity in order to propose more powerful strategies.1,2
In order for cytotoxic CD8 T cells to perform their task, namely the killing of tumor cells, they need to respond adequately to tumor antigens and also to make a direct physical contact with malignant cells. This cell-cell interaction is the end result of a series of stepwise events during which T cells rapidly migrate. It is believed that, like any immune response, tumor-specific T cells are primed in the tumor-draining lymph node by dendritic cells which have captured the antigen at the tumor site. During this activation step T cell acquire their effector functions but also their ability to migrate towards tumors by expressing novel chemokine receptors and adhesion molecules. Then, T cells home to the tumors by extravasation, navigate within the tumor environment, make contact with stromal cells and stromal components and eventually interact with tumor cells. Thus, an active cellular motility is considered of crucial importance for T cell anti-tumoral activities.2
In cancer patients, one or several of this migration steps do not operate optimally. So far, a lot of attention has been paid to defects in the entry of T cells into the tumors, presumably due to abnormal vessel structures and reduced expression of chemokines and/or adhesion molecules.3,4 Nevertheless, even when T cell succeed in crossing the blood vessels they are, in a wide range of human malignancies, rarely in contact with tumor cells but instead enriched in the surrounding microenvironment called the stroma that is composed, among others, of activated fibroblasts and extracellular matrix components.5
This defect in T cell-tumor cell interaction can contribute to explain the persistent growth of some tumors but also why several immunotherapy protocols, e.g anti-PD-1 antibodies and CAR T cells, do not always give the expected results in solid tumors. As a matter of fact, results from a clinical trial indicate that patients who respond well to anti-PD-1 therapy exhibit, prior to the treatment, many CD8 T cells in contact with tumor cells.6 Conversely, in cancer patients refractory to immune checkpoint inhibitors two different profiles have been reported. In some patients, tumor biopsies are devoid of T lymphocytes, most likely because tumor cells are not recognized by the immune system in so called “non-immunogenic” tumors. Another profile is characterized by the infiltration of numerous T cells in the tumor, but not in contact with malignant cells in a profile named “immune-excluded”.2 These results provoke a number of questions: why in many human tumors are T cells enriched in the stroma, unable to reach malignant cells? Is it possible to design innovative strategies enabling T cells to enter tumor islets and and kill tumor cells?
Macrophages impede T cells from reaching tumor cells
To address some of these issues, we decided to track CD8 T cells in tumor biopsies. An experimental system based on thick slices made from fresh tumor biopsies combined with fluorescent imaging microscopy enabled us to monitor the migration of CD8 T cells within an intact tumor environment. This approach has been instrumental in demonstrating an important role of several stromal elements in controlling T cell migration within human lung and ovarian tumors. We first reported a detrimental impact of a dense extracellular matrix (ECM) on the migration of T cells and their ability to contact tumor cells.7,8 Growing tumors are characterized by an excessive accumulation of collagen-1 likely to favor tumor progression and prevent antitumor T cell functions.
Progressing tumors are also enriched in macrophages which have been associated with a bad outcome in many cancers. These tumor-associated macrophages (TAM) can promote tumor growth by a variety of mechanisms, including a suppression of T cell antitumor activities.9 Yet, the mechanisms by which macrophages negatively control T cells are not completely understood. We therefore decided to explore whether macrophages were also able to affect the migration of T cells in tumors. Our recent results obtained in human and murine tumors evidenced the presence of stable conjugates formed between TAM and CD8 T cells in the stroma (Figure 1), suggesting that macrophages could contribute to sequestering lymphocytes away from tumor cells.10 Remarkably, in mouse mammary tumor models we found that the depletion of TAM with pexidartinib, an inhibitor of the colony stimulating factor 1 receptor (CSF1R), increased the motility of CD8 T cells and their ability to reach tumor cells. This macrophage-depletion strategy, which by itself has a minor effect on the tumor growth, also improved the effect of an anti-PD-1 treatment.10 Altogether, these data suggest that TAM, very abundant cells in the tumor microenvironment, participate to the exclusion of CD8 T cells from the vicinity of cancer cells.
Figure 1.
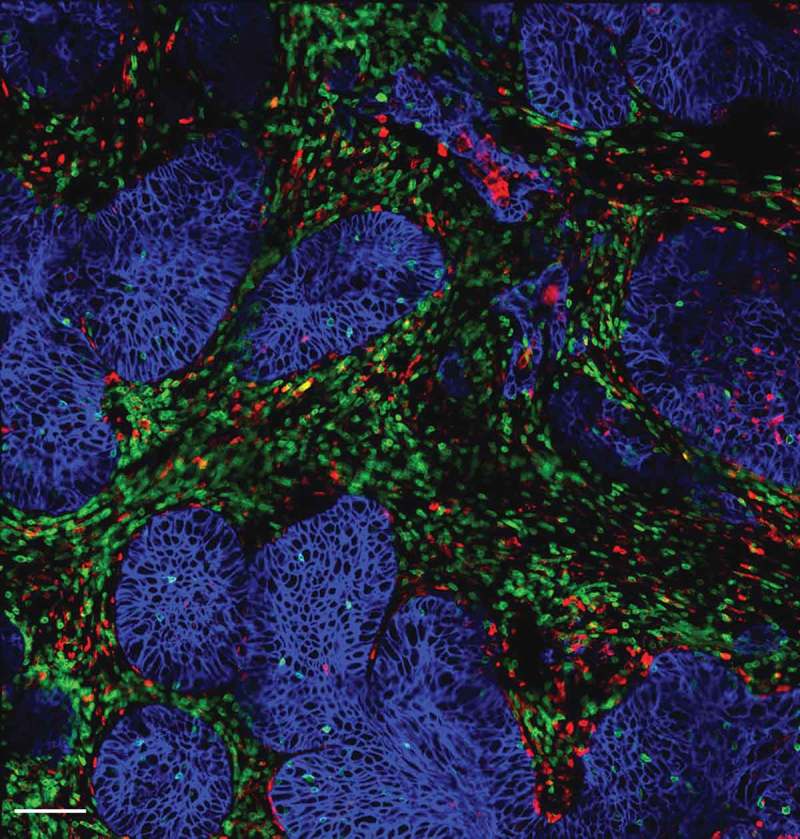
Distribution of T cells, macrophages and tumor cells within a human lung tumor. Tumor cells (blue, stained for EpCAM) are organized into compact islets surrounded by T cells (green, stained for CD3) and macrophages (red, stained for CD206) which are often in contact. Bar, 100 µm.
The mechanisms by which TAM prevent CD8 T cells from reaching tumor cells is not known at the moment. We favor an adhesion process between both cell types triggered by an effective antigen recognition. This would be in line with data showing antigen-dependent interactions between CD8 T cells and TAM in a spontaneous mammary carcinoma murine model.11 However, the nature of the adhesion molecules involved in such cell-cell conjugates needs to be further investigated.
An effect of macrophages on environmental factors controlling the motility of T cells cannot be ruled out. Studies performed over the last few years have provided evidence for a role of the structure of the tissue and the presence of chemokines in regulating the migration of T cells.12 In mice harboring mammary tumors, we found that the depletion of TAM resulted in more inflammatory chemokines, such as CCL2 and CXCL10, which are likely to enhance the entry of T cells into the tumor and their intratumoral migration.10 In some situations like breast development, macrophages actively participate to the construction of the tissue.13 In addition, evidence indicates a role of TAM in ECM production within the tumor.14 Interestingly, a recent study demonstrates that TAM activate carcinoma-associated fibroblasts to produce excessive amount of the ECM, excluding T cells from tumor cells, through the secretion of granulin, a growth factor belonging to the epithelin family.15
However, in the murine models we studied10 the loss of macrophages in the tumor microenvironment though CSF1R inhibition did not significantly altered tumor architecture, at least histologically. It would be interesting to explore whether ECM remodeling in tumors can be observed with other TAM targeting therapies.
Therapeutic strategies to target tumor-associated macrophages
Knowing the pro-tumoral and immunosuppressive functions of macrophages found in solid malignancies, several therapeutic applications to impair their recruitment or survival are either entering or have entered clinical trials.9 CSF1R inhibitors are currently being tested, the most advanced being the small-molecule Pexidartinib.16 However, CSF1R inhibitors have shown very limited antitumor effects in patients as single agents, suggesting the need to combine these inhibitors with other approaches, including immune checkpoint inhibitors. Such combination strategies are ongoing in a number of solid malignancies (NCT02452424, NCT02713529).
Macrophages also use the CCL2/CCR2 axis to enter into tumors. Thus, anti-CCR2 approaches are being developed to reduce the number of immunosuppressive macrophages into solid malignancies.17
Macrophages are plastic cells, which can be divided into several subtypes, including a pro-inflammatory, tumoricidal type (also referred to as M1-like) and an immunosuppressive type (also referred to as M2-like).18 Moreover, we and other have observed that different subsets of TAM coexist in the tumor microenvironment, bearing different markers and localization. These observations suggests that more specific targeting of M2-like TAM could be conceived in order to selectively deplete the most suppressive population and the one that interacts preferentially with T cells, limiting their migration. Interestingly, a peptide able to target CD206+ macrophages at the tumor site has been recently developed and is able to transport drug-loaded nanoparticles to these cells.19
As the depletion of macrophages other than M2-like TAMs might be detrimental for the host, an alternative and perhaps more efficient approach would be the reprogramming of immunosuppressive macrophages toward an anti-tumor phenotype that would support T cell migration and function.20 Recent evidence suggests that in certain contexts macrophages can cooperate with T cells to promote tumor regression.21,22 Although the basis of this cell-cell cooperation is not known for the moment, the CD40-CD40 ligand axis appears as a promising target. CD40 is a member of the tumor necrosis factor receptor family and is mainly expressed on antigen presenting cells, including macrophages. Under normal conditions, CD40 ligand on activated T cells interacts with antigen-presenting cells via CD40, resulting in T cell and macrophage activation.23 Interestingly, CD40 agonists in combination with gemcitabine induce clinical responses in patients with surgically incurable pancreatic cancer.24 Apart from CD40 agonists, several other approaches are currently being develop to reprogram macrophages and boost T cell immunity, including class IIa HDAC inhibitors25 and PI3Kγ inhibitors.26 It would be interesting to understand whether the reprogramming of TAM also results in differences in the interactions of these cells with lymphocytes and, thus, in the T cell migratory behavior.
Conclusion
Developing therapeutic strategies that help overcome a lack of T cell unresponsiveness to tumor antigen and a lack of infiltration of T cells into tumor islets is critical in order to increase the fraction of patients responding to immunotherapy.
Our findings suggest that targeting TAM may serve as a potential therapeutic strategy to improve CD8 T cell interaction with malignant cells, thereby converting “immune-excluded” tumors, which are refractory to immune checkpoint inhibitors, into tumors that respond to anti-PD-1 antibodies. Overall, these results support the rational for targeting TAM in combination with immune checkpoint blockers for the treatment of solid tumors.
Funding Statement
Our work is supported by grants from the French Ligue Nationale contre le Cancer (Equipes labellisées), Plan Cancer (Tumor heterogeneity and ecosystem program) (ED), AIRC (EP), CARPEM (Cancer Research for Personalized Medicine) (EP) and Fondation de France (EP).
Abbreviations
- CAF
carcinoma-associated fibroblasts
- CAR
chimeric antigen receptor
- CSF1R
colony stimulating factor 1 receptor
- ECM
extracellular matrix
- TAM
tumor-associated macrophages
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the authors.
References
- 1.Anderson KG, Stromnes IM, Greenberg PD.. Obstacles posed by the tumor microenvironment to T cell activity: a case for synergistic therapies. Cancer Cell. 2017;31:311–325. doi: 10.1016/j.ccell.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–330. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 3.Debets R, Donnadieu E, Chouaib S, Coukos G. TCR-engineered T cells to treat tumors: seeing but not touching? Semin Immunol. 2016;28:10–21. doi: 10.1016/j.smim.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Melero I, Rouzaut A, Motz GT, Coukos G. T-cell and NK-cell infiltration into solid tumors: a key limiting factor for efficacious cancer immunotherapy. Cancer Discov. 2014;4:522–526. doi: 10.1158/2159-8290.CD-13-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348:74–80. doi: 10.1126/science.aaa6204. [DOI] [PubMed] [Google Scholar]
- 6.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bougherara H, Mansuet-Lupo A, Alifano M, Ngo C, Damotte D, Le Frere-Belda MA, Donnadieu E, Peranzoni E. Real-time imaging of resident T cells in human lung and ovarian carcinomas reveals how different tumor microenvironments control T lymphocyte migration. Front Immunol. 2015;6:500. doi: 10.3389/fimmu.2015.00500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salmon H, Franciszkiewicz K, Damotte D, Dieu-Nosjean MC, Validire P, Trautmann A, Mami-Chouaib F, Donnadieu E. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J Clin Invest. 2012;122:899–910. doi: 10.1172/JCI45817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14:399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peranzoni E, Lemoine J, Vimeux L, Feuillet V, Barrin S, Kantari-Mimoun C, Bercovici N, Guérin M, Biton J, Ouakrim H, et al. Macrophages impede CD8 T cells from reaching tumor cells and limit the efficacy of anti-PD-1 treatment. Proc Natl Acad Sci U S A. 2018;115:E4041–E50. doi: 10.1073/pnas.1720948115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engelhardt JJ, Boldajipour B, Beemiller P, Pandurangi P, Sorensen C, Werb Z, Egeblad M, Krummel MF. Marginating dendritic cells of the tumor microenvironment cross-present tumor antigens and stably engage tumor-specific T cells. Cancer Cell. 2012;21:402–417. doi: 10.1016/j.ccr.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krummel MF, Bartumeus F, Gerard A. T cell migration, search strategies and mechanisms. Nat Rev Immunol. 2016;16:193–201. doi: 10.1038/nri.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ingman WV, Wyckoff J, Gouon-Evans V, Condeelis J, Pollard JW. Macrophages promote collagen fibrillogenesis around terminal end buds of the developing mammary gland. Develop Dynamics Am Assoc Anatomists. 2006;235:3222–3229. doi: 10.1002/dvdy.20972. [DOI] [PubMed] [Google Scholar]
- 14.Afik R, Zigmond E, Vugman M, Klepfish M, Shimshoni E, Pasmanik-Chor M, Shenoy A, Bassat E, Halpern Z, Geiger T, et al. Tumor macrophages are pivotal constructors of tumor collagenous matrix. J Exp Med. 2016;213:2315–2331. doi: 10.1084/jem.20151193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quaranta V, Rainer C, Nielsen SR, Raymant ML, Ahmed MS, Engle DD, Taylor A, Murray T, Campbell F, Palmer DH, et al. Macrophage-derived granulin drives resistance to immune checkpoint inhibition in metastatic pancreatic cancer. Cancer Res. 2018;78:4253–4269. doi: 10.1158/0008-5472.CAN-17-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cannarile MA, Weisser M, Jacob W, Jegg AM, Ries CH, Ruttinger D. Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J Immunother Cancer. 2017;5:53. doi: 10.1186/s40425-017-0257-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nywening TM, Wang-Gillam A, Sanford DE, Belt BA, Panni RZ, Cusworth BM, Toriola AT, Nieman RK, Worley LA, Yano M, et al. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet Oncol. 2016;17:651–662. doi: 10.1016/S1470-2045(16)00078-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 19.Scodeller P, Simon-Gracia L, Kopanchuk S, Tobi A, Kilk K, Saalik P, Kurm K, Squadrito ML, Kotamraju VR, Rinken A, et al. Precision targeting of tumor macrophages with a CD206 binding peptide. Sci Rep. 2017;7:14655. doi: 10.1038/s41598-017-14709-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schultze JL. Reprogramming of macrophages–new opportunities for therapeutic targeting. Curr Opin Pharmacol. 2016;26:10–15. doi: 10.1016/j.coph.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Saha D, Martuza RL, Rabkin SD. Macrophage polarization contributes to glioblastoma eradication by combination immunovirotherapy and immune checkpoint blockade. Cancer Cell. 2017;32:253–67 e5. doi: 10.1016/j.ccell.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thoreau M, Penny HL, Tan K, Regnier F, Weiss JM, Lee B, Johannes L, Dransart E, Le Bon A, Abastado J-P, et al. Vaccine-induced tumor regression requires a dynamic cooperation between T cells and myeloid cells at the tumor site. Oncotarget. 2015;6:27832–27846. doi: 10.18632/oncotarget.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoves S, Ooi CH, Wolter C, Sade H, Bissinger S, Schmittnaegel M, Ast O, Giusti AM, Wartha K, Runza V, et al. Rapid activation of tumor-associated macrophages boosts preexisting tumor immunity. J Exp Med. 2018;215:859–876. doi: 10.1084/jem.20171440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guerriero JL, Sotayo A, Ponichtera HE, Castrillon JA, Pourzia AL, Schad S, Johnson SF, Carrasco RD, Lazo S, Bronson RT, et al. Class IIa HDAC inhibition reduces breast tumours and metastases through anti-tumour macrophages. Nature. 2017;543:428–432. doi: 10.1038/nature21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Henau O, Rausch M, Winkler D, Campesato LF, Liu C, Cymerman DH, Budhu S, Ghosh A, Pink M, Tchaicha J, et al. Overcoming resistance to checkpoint blockade therapy by targeting PI3Kgamma in myeloid cells. Nature. 2016;539:443–447. doi: 10.1038/nature20554. [DOI] [PMC free article] [PubMed] [Google Scholar]


