ABSTRACT
Background: This exploratory analysis was conducted to characterize the level of HPV types 6/11 antibodies in peripartum maternal blood and in cord blood of infants born to women who received 9-valent HPV (9vHPV) vaccine or quadrivalent HPV (qHPV) vaccine in a pivotal efficacy study (V503-001, NCT 00543543).
Methods: A total of 21 mother-infant pairs had evaluable HPV 6/11 results available for analysis. HPV6/11 antibodies were assessed using competitive Luminex immunoassay. The distribution of the ratios of infant to mother anti-HPV antibodies (i.e., infant-anti-HPV/mother- anti-HPV) was summarized.
Results: All mothers and infants were seropositive to HPV 6 and HPV 11. Anti-HPV 6/11 geometric mean titers (GMTs) in peripartum maternal blood and in cord blood of infant born to study participants were highly correlated. A 100% of infants born to seropositive mothers were also seropositive. The GMT ratios of peripartum maternal blood vs. those in cord blood were HPV 6: 1.23 [0.43, 3.49] and HPV 11: 1.29 [0.54, 3.07] in the 9vHPV vaccine group and HPV 6: 1.33 [0.41, 4.29] and HPV 11: 1.19 [0.45, 3.13] in the qHPV vaccine group, respectively.
Conclusions: These results indicate that antibodies induced by the 9vHPV vaccine cross the placenta, which could potentially be beneficial against HPV6/11 infection and related disease such as recurrent respiratory papillomatosis.
Keywords: human papillomavirus, 9vHPV vaccine, recurrent respiratory papillomatosis, clinical trial, geometric mean titers (GMTs)
Introduction
A 9-valent HPV (types 6/11/16/18/31/33/45/52/58) vaccine (9vHPV vaccine, Gardasil 9TM, Merck & Co., Inc., Kenilworth, NJ, USA) has been licensed for the prevention of cervical, vulvar, vaginal and anal cancers and precancers related to HPV16/18/31/33/45/52/58 and genital warts due to HPV6/11. In a clinical study conducted in young women, 16–26 years of age at enrollment, the 9vHPV vaccine prevented HPV31/33/45/52/58-related high-grade cervical, vulvar and vaginal disease, and induced non-inferior HPV6/11/16/18 antibody responses compared to the previously licensed quadrivalent HPV (types 6/11/16/18) (qHPV) vaccine [1, 2]. One exploratory immunogenicity objective of the study was to assess transfer of HPV antibodies from mother to infant, by measuring HPV6/11 antibodies in maternal serum and cord blood samples. The results of these analyses are reported here.
Methods
Study design
These data were derived from an exploratory immunogenicity objective of a randomized, double-blinded, and controlled with qHPV vaccine, dose-ranging, tolerability, immunogenicity, and efficacy study of the 9vHPV vaccine in 16- to 26-year-old women (Protocol V503-001; NCT00543543). This study enrolled ~ 14,000 women between the ages of 16 and 26 years from 105 study sites located in 18 countries. An institutional review board for each study site approved the protocol and written informed consent was obtained from all participants. Participants were randomized to receive 3 doses of 9vHPV or qHPV vaccine at day 1, month 2 and month 6, and were subsequently followed for efficacy and immunogenicity. The design and the key primary and secondary results of the study have been reported.1,2
As part of the enrollment criteria for the study, participants were not pregnant at the time of enrollment, and had to use an effective contraception method during the vaccination period (from Day 1 through Month 7). Participants could become pregnant during the post-vaccination follow-up period. Participants who inadvertently became pregnant prior to receiving the 3 doses of vaccine did not receive additional doses until ≥ 4 weeks after resolution of pregnancy and normalization of β-hCG levels. Participants, who became pregnant during the study could be considered for these analyses if they were followed by a study investigator through their pregnancy and delivery, and provided consent to participate in maternal blood draw and cord blood collection.
Sample collection and immunogenicity assessment
Maternal serum samples (“mother anti-HPV result”) were obtained at the time of infant delivery from a study participant who became pregnant during the study and gave birth to an infant who contributed a cord blood sample (“infant anti-HPV result”). These samples were referred to as a “mother-infant pair”. Mother-infant pair sample levels of vaccine-type epitope-specific neutralizing anti-HPV 6/11 antibodies were analyzed with a competitive Luminex based Immunoassay (cLIA) developed by Merck Research Laboratories, as described previously.3
Data analysis
All mother-infant pairs with valid anti-HPV 6 or anti-HPV 11 antibody measurements at the time of birth were included in these analyses. Seropositivity rates for HPV 6/11 are presented descriptively. Geometric mean titers (GMTs) and GMT ratios (mother/infant) with associated 95% confidence intervals were computed. All of these evaluations were exploratory in nature; therefore, no statistical tests of hypotheses were performed. Non overlapping 95% confidence intervals were used as indicators of differences of immune response. Two-dimensional scatter plots were generated to show graphically the mother (vertical axis) and infant (horizontal axis) pair of anti-HPV 6 and 11 antibodies. The concordance between the mother and infant anti-HPV antibodies was measured using the Pearson correlation coefficient.
Results
There were a total of 21 participants (9vHPV vaccine: 9 pairs; qHPV vaccine: 12 pairs) with available mother-infant pair serology data that were considered for the antibody analysis. Of the available 21 mother-infant pairs, 18 mother-infant pairs (n = 7: 9vHPV vaccine and n = 11: qHPV vaccine, respectively) had evaluable results for HPV type 6 and all 21 mother-infant pairs had evaluable results for HPV type 11. Most participants to this analysis became pregnant after receiving dose 3. However, one participant became pregnant shortly after vaccination dose 1, delivered the infant 9 months after dose 1, resumed vaccination thereafter, and completed the third vaccination dose approximately 5 months after the infant was born. Among the participants whose mother-infant pairs were used in the correlation analysis, the median time after vaccine dose 3 when the maternal serum and cord blood samples were obtained was 16 months (range, -5 to 29 months; interquartile range, 10–21 months). Relative to day 1, the median time after vaccine dose 1 when the maternal serum and cord blood samples were obtained was 22 months (range, 9–34 months; interquartile range, 15–27 months).
Table 1 shows the summary of immunogenicity for mothers and infants by vaccination group. All mothers who received the (9vHPV or qHPV) vaccine were seropositive to HPV 6 and 11 and all the paired infant samples were seropositive to those HPV types as well. Thus, 100% of the immunized mother-infant pairs were seropositive to HPV types 6 and 11. GMTs for the mothers were not significantly different from the GMTs for the infants for both HPV types and vaccine groups. GMTs were comparable between the two vaccine groups, for mothers, as well as for infants. The 95% confidence intervals of associated GMT ratios that compare the fold difference between maternal and infant GMTs included 1 for both vaccine groups, which illustrates the concordant mother and infant HPV 6 and 11 antibody titers with both vaccines.
Table 1.
Summary of geometric mean titers (GMTs) and mother-infant pair serostatus by vaccination group.
| Mother (Comparison group A) |
Infant (Comparison group B) |
Estimated fold difference |
Mother – Infant pair |
Infants serostatus* |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | HPV type | n | GMT (95%C.I.) | n | GMT (95%C.I.) | Group A/Group B (95%C.I.) | n | Mothers Serostatus* | Positive m (%) | Negative m (%) |
| 9vHPV (N = 7099) | HPV 6 | 9 |
170.3 (85.4, 339.5) |
7 |
138.4 (63.3, 302.8) |
1.23 (0.43, 3.49) |
7 | Positive | 7 (100) | 0 (0) |
| Negative | 0 (0) | 0(0) | ||||||||
| HPV11 | 9 |
138.5 (73.7, 260.3) |
10 |
107.5 (59.1, 195.6) |
1.29 (0.54, 3.07) |
9 | Positive | 9 (100) | 0(0) | |
| Negative | 0(0) | 0(0) | ||||||||
| qHPV (N = 7105) | HPV 6 | 11 |
288.4 (122.0, 681.9) |
13 |
216.2 (98.0, 477.2) |
1.33 (0.41, 4.29) |
11 | Positive | 11 (100) | 0(0) |
| Negative | 0 (0) | 0(0) | ||||||||
| HPV11 | 12 |
110.9 (55.1, 223.1) |
13 |
93.3 (47.7, 182.7) |
1.19 (0.45, 3.13) |
12 | Positive | 12 (100) | 0(0) | |
| Negative | 0 (0) | 0(0) | ||||||||
*Serostatus HPV 6 and 11 was evaluated using HPV cLIA; seropositive ≥ 30 and 16 milliMerck units/mL, respectively.
cLIA, competitive Luminex Immunoassay.
N = Number of subjects randomized to the respective vaccination group that received at least 1 dose of the vaccine (9vHPV or qHPV).
n = Number of mother- infant pairs contributing to the analysis (i.e., number of mother-infant pairs with a valid cLIA result for both mother and infant for the HPV type being analyzed).
m = Number of subjects seropositive to the HPV type being analyzed).
Percent = 100 x (m/n).
Figure 1 shows the 2-dimensional scatter plot of the HPV cLIA data points (x = cord blood anti-HPV result, y = maternal serum anti-HPV result) for each of HPV types 6 and 11 by vaccine group. The data indicate that HPV titers in maternal serum samples are highly positively correlated with anti-HPV titers from infant cord blood samples. Pearson correlation coefficients of 0.94 and 0.93 were calculated for HPV-6 and 11 in the 9vHPV vaccine group and 0.99 and 0.77 for HPV 6 and 11 in qHPV vaccine group.
Figure 1.
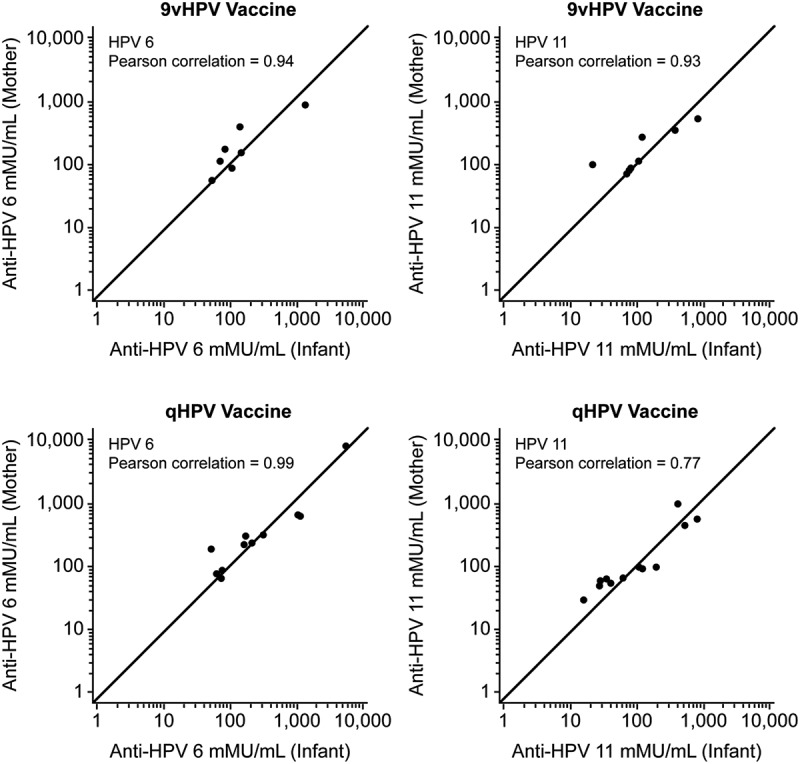
Scatter plots of the mother- infant HPV cLIA data points (x = cord blood anti-HPV result, y = maternal serum anti-HPV result) by vaccine group. Pearson correlation coefficients of 0.94 and 0.93 were calculated for HPV 6 and 11 in the 9vHPV vaccine group and 0.99 and 0.77 for HPV 6 and 11 in the qHPV vaccine group.
Discussion
The results of this exploratory analysis indicate that anti-HPV type 6 and type 11 antibodies induced by the prophylactic HPV (9vHPV and qHPV) vaccination are transferred transplacentally from vaccinated mother to the unborn baby. For each newborn infant whose mother was vaccinated with either the 9vHPV or qHPV vaccine, 100% of the cord blood samples were positive to HPV types 6 and 11 antibodies. Additionally, the evaluation of anti-HPV 6 and anti-HPV 11 GMTs showed a highly positive correlation between antibody levels in peripartum maternal blood and in cord blood of infant born to study participants. These results demonstrate a similar immunogenicity profile for the 2 vaccines. Similar findings were previously reported in a previous publication for mothers-infant pairs after administration of the qHPV vaccine.4
HPV 6 and HPV 11 are the causative agents of anogenital warts and recurrent respiratory papillomatosis (RRP).5,6 Anogenital warts are a very common sexually transmitted disease with an annual incidence of 0.1% to 0.2% in developed countries, and possibly higher in developing countries.7 RRP is a rare disease characterized by exophytic lesions in the upper airway that can cause severe speech and respiratory impairment, can be life-threatening, and require multiple surgical interventions.8 Most cases of RRP are juvenile onset (JORRP), occurring mostly between 2- 5 years of age with an estimated incidence of 4 per 100,000.8–10 HPV infection of children generally occurs at birth during the passage through the birth canal of infected mothers, although transmission prior to birth through the placenta can occur in some cases.8 In clinical studies, the qHPV vaccine was shown to prevent HPV 6/11-related persistent infection11 and genital warts.12 A clinical study to directly quantify the efficacy of the 9vHPV vaccine in preventing RRP would be prohibitively large, owing to the rare occurrence of JORRP and was out of scope for this exploratory immunogenicity objective.
An estimation of the impact of prophylactic vaccination in preventing HPV 6/11 related warts and RRP is available from real-world studies. A 90% decline in the prevalence of genital warts was observed four years after the implementation of the qHPV vaccination program in Australia;13 moreover, a 90% decrease of the prevalence of vaccine HPV types was reported within 9 years of implementation of the vaccination program.14 A recent study also from Australia documents a significant decline in the rates of new diagnosis of JORRP in Australia in the 5–10 year period following the implementation of the National HPV Vaccination program.15 In this study all incident cases of JORRP were from mothers who were not vaccinated prior to pregnancy (there were no incident cases in vaccinated mothers). Even though there are no data to indicate that maternal antibodies will be effective in preventing RRP, it is reasonable to speculate that maternal transfer of HPV specific immunity could be a mechanism of protection. In further support of this idea, maternal immunization and transplacental transfer of antibody from mother to fetus has been shown to protect newborns and infants from many infections including those of the upper airway, for example: respiratory syncytial virus, measles, Streptococcus pneumoniae and Hemophilus influenza.16–18
Previous studies1,2 as well as this study have demonstrated that the 9vHPV vaccine and the qHPV vaccine have similar immunogenicity profiles with respect to the shared HPV types 6/11/16/18. Therefore, it is reasonable to assume that prophylactic immunization with the 9vHPV vaccine will result in protection similar to qHPV vaccine for HPV 6/11-related diseases in real-world scenarios. Demonstration of highly correlated antibody titers in peripartum maternal blood and cord blood following 9vHPV or qHPV vaccination of the mother prior to pregnancy provides evidence for trans-placental transfer of anti HPV types 6 and 11 antibodies to the new born.
As a precautionary note, these results should not be considered as a basis for a maternal immunization program with HPV vaccine. The qHPV and 9vHPV vaccine clinical programs were not designed to provide a systematic assessment of the vaccine in pregnant women; therefore, neither vaccine is indicated in pregnant women (even though results from clinical studies of the qHPV and 9vHPV vaccines19,20 and post-licensure studies of the qHPV vaccine21–24 have not shown evidence of adverse pregnancy outcomes following inadvertent HPV vaccine administration in pregnant women). Moreover, these results should not be considered as a basis for HPV vaccination in neonates. HPV vaccination is not indicated in children less than 9 years of age and has not been studied in young children.
Durable effectiveness and sustained immunogenicity was observed in long-term follow-up studies following HPV vaccine administration, thus indicating persistence of protective antibody levels over time.25–27 Thus, female subjects who received HPV vaccination during adolescence should be able to transfer transplacentally protective levels of anti-HPV antibodies when they become pregnant later in life. Such transplacental transfer of neutralizing antibodies against HPV types 6 and 11 may potentially provide benefit against JORRP.
Funding Statement
Funding for this research was provided by Merck & Co., Inc., Kenilworth, NJ, USA.
Disclosure of potential conflicts of interest
AMG: Received research grants from Merck Sharp & Dohme, Corp (MSD) disbursed through his institution.
ES: Received research grants from MSD disbursed through his institution.
AV: Received research grants from MSD disbursed through his institution.
HN: Received speakers' and advisory board fees; travel and accommodation support and honoraria from MSD.
ALH: Received speakers' and advisory board fees; travel and accommodation support and honoraria from MSD.
EF: Received research grants from MSD disbursed through his institution.
OB, CS, AJ and AL are employees of Merck Sharp & Dohme, Corp, a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA and may hold stock/stock options.
Acknowledgments
The authors graciously acknowledge the contribution of all the participants and their caregivers involved with this study. We also wish to acknowledge the expert assistance of Jennifer Pawlowski, M.S. and Karyn Davis, B.S. of Merck & Co., Inc., Kenilworth, NJ, USA for editorial support.
References
- 1.Huh WK, Joura EA, Giuliano AR, Iversen O-E, Andrade RP, Ault KA, Bartholomew D, Cestero RM, Fedrizzi E, Hirschberg AL, et al. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16–26 years: a randomized, double-blind trial. Lancet. 2017;390(10108): 2143–2159.doi: 10.1016/S0140-6736(17)31821-4. [DOI] [PubMed] [Google Scholar]
- 2.Joura EA, Giuliano AR, Iversen O-E, Bouchard C, Mao C, Mehlsen J, Moreira ED, Ngan Y, Petersen LK, Lazcano-Ponce E, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8): 711–723.doi: 10.1056/NEJMoa1405044. [DOI] [PubMed] [Google Scholar]
- 3.Roberts C, Green T, Hess E, Matys K, Brown MJ, Haupt RM, Luxembourg A, Vuocolo S, Saah A, Antonello J.. Development of a human papillomavirus competitive immunoassay for nine HPV types. Hum Vaccines Immunother. 2014;10(8):2168–2174. doi: 10.4161/hv.29205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matys K, Mallary S, Bautista O, Vuocolo S, Manalastas R, Pitisuttithum P, Saah A. Mother-infant transfer of anti-human papillomavirus (HPV) antibodies following vaccination with the quadrivalent HPV (type 6/11/16/18) virus-like particle vaccine. Clin Vacc Immunol. 2012;19(6):881–885. doi: 10.1128/CVI.00002-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garland SM, Steben M, Sings HL, James M, Lu S, Railkar R, Barr E, Haupt RM, Joura EA. Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J Infect Dis. 2009;199(6):805–814. doi: 10.1086/597071. [DOI] [PubMed] [Google Scholar]
- 6.Dickens P, Srivastava G, Loke SL, Larkin S. Human papillomavirus 6 and 11 in laryngeal papillomas. J Pathol. 1991;165(3):243–246. doi: 10.1002/path.1711650308. [DOI] [PubMed] [Google Scholar]
- 7.Forman D, De Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, Bruni L, Vignat J, Ferlay J, Bray F, Plummer M, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30(suppl 5): F12–23.doi: 10.1016/j.vaccine.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 8.Fortes HR, von Ranke FM, Escuissato DL, Araujo Neto CA, Zanetti G, Hochhegger B, Souza CA, Marchiori E. Recurrent respiratory papillomatosis: A state-of-the-art review. Respir Med. 2017;126:116–121. doi: 10.1016/j.rmed.2017.03.030. [DOI] [PubMed] [Google Scholar]
- 9.Carifi M, Napolitano D, Morandi M, Dall’Olio D. Recurrent respiratory papillomatosis: current and future perspectives. Ther Clin Risk Manag. 2015;11:731–738. doi: 10.2147/TCRM.S81825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reeves WC, Ruparelia SS, Swanson KI, Derkay CS, Marcus A, Unger ER. National registry for juvenile onset recurrent respiratory papillomatosis. Arch Otolaryngologye Head Neck Surg. 2003;129(9):976–982. doi: 10.1001/archotol.129.9.976. [DOI] [PubMed] [Google Scholar]
- 11.Villa LL, Costa RLR, Petta CA, Andrade RP, Ault KA, Giuliano AR, Wheeler CM, Koutsky LA, Malm C, Lehtinen M, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005;6(5): 271–278.doi: 10.1016/S1470-2045(05)70101-7. [DOI] [PubMed] [Google Scholar]
- 12.Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, Tang GW, Ferris DG, Steben M, Bryan J, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19): 1928–1943.doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 13.Garland SM, Kjaer SK, Muñoz N, Block SL, Brown DR, DiNubile MJ, Lindsay BR, Kuter BJ, Perez G, Dominiak-Felden G, et al. Impact and effectiveness of the quadrivalent human papillomavirus vaccine: a systematic review of 10 years of Real-world experience. Clin Infect Dis. 2016;63(4): 519–527.doi: 10.1056/NEJMoa061760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Machalek DA, Garland SM, Brotherton JML, Bateson D, McNamee K, Stewart M, Skinner R, Liu B, Cornall AM, Kaldor JM, et al. Very low prevalence of vaccine human papillomavirus (HPV) types among 18 to 25 year old Australian women, nine years following implementation of vaccination. J Infect Dis. 2018;217(10): 1590–1600.doi: 10.1093/infdis/jiy075. [DOI] [PubMed] [Google Scholar]
- 15.Novakovic D, Cheng ATL, Zurynski Y, Booy R, Walker PJ, Berkowitz R, Harrison H, Black R, Perry C, Vijayasekaran S, et al. A prospective study of the incidence of juvenile-onset recurrent respiratory papillomatosis after implementation of a national HPV vaccination program. J Infect Dis. 2018;217(2): 208–212.doi: 10.1093/infdis/jix498. [DOI] [PubMed] [Google Scholar]
- 16.Chu H, Steinhoff C, Margaret A, Zaman K, Roy E, Langdon G, Formica MA, Walsh EE, Englund JA. Respiratory syncytial virus transplacental antibody transfer and kinetics in mother –infant pairs in Bangladesh. J Infect Dis. 2014;210(10):1582–1589. doi: 10.1093/infdis/jiu316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta A, Mathad J, Yang WT, Singh HK, Gupte N, Mave V, Bharadwaj R, Zaman K, Roy E, Bollinger RC, et al. Maternal pneumococcal capsular IgG antibodies and transplacental transfer are low in South Asian HIV-infected mother-infant pairs. Vaccine. 2014;32(13): 1466–1472.doi: 10.1016/j.vaccine.2014.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keller-Stanislawski B, Englund JA, Kang G, Mangtani P, Neuzil K, Nohynekf H, Pless R, Lambachh P, Zuber P. Safety of immunization during pregnancy: a review of the evidence of selected inactivated and live attenuated vaccines. Vaccine. 2014;32(52): 7057–7064.doi: 10.1016/j.vaccine.2014.09.052. [DOI] [PubMed] [Google Scholar]
- 19.Garland SM, Ault KA, Gall SA, Paavonen J, Sings HL, Ciprero KL, Saah A, Marino D, Ryan D, Radley D, et al. Quadrivalent human papillomavirus vaccine phase III Investigators. Pregnancy and infant outcomes in the clinical trials of a human papillomavirus type 6/11/16/18 vaccine: a combined analysis of five randomized controlled trials. Obstet Gynecol. 2009;114(6): 1179–1188.doi: 10.1097/AOG.0b013e3181c2ca21. [DOI] [PubMed] [Google Scholar]
- 20.Moreira EDJ, Block SL, Ferris D, Giuliano AR, Iversen OE, Joura EA, Kosalaraksa P, Schilling A, Van Damme P, Bornstein J, et al. Safety profile of the 9-valent HPV vaccine: a combined analysis of 7 phase III clinical trials. Pediatrics. 2016;138:e 20154387. doi: 10.1542/peds.2015-4387. [DOI] [PubMed] [Google Scholar]
- 21.Goss MA, Lievano F, Buchanan KM, Seminack MM, Cunningham ML, Dana A. Final report on exposure during pregnancy from a pregnancy registry for quadrivalent hu-man papillomavirus vaccine. Vaccine. 2015;33(29):3422–3428. doi: 10.1016/j.vaccine.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 22.Scheller NM, Pasternak B, Molgaard-Nielsen D, Svanström H, Hviid A. Quadrivalent HPV vaccination and the risk of adverse pregnancy outcomes. N Engl J Med. 2017;376(13):1223–1233. doi: 10.1056/NEJMoa1612296. [DOI] [PubMed] [Google Scholar]
- 23.Lipkind HS, Vazquez-Benitez G, Nordin JD, Romitti PA, Naleway AL, Klein NP, Hechter RC, Jackson ML, Hambidge SJ, Lee GM, et al. Maternal and infant outcomes after human papillomavirus vaccination in the periconceptional period or during pregnancy. Obstet Gynecol. 2017;130(3): 599–608.doi: 10.1097/AOG.0000000000002191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sy LS, Meyer KI, Klein NP, Chao C, Velicer C, Cheetham TC, Ackerson BK, Slezak JM, Takhar HS, Hansen J, et al. Postlicensure safety surveillance of congenital anomaly and miscarriage among pregnancies exposed to quadrivalent human papillomavirus vaccine. Hum Vaccin Immunother. 2018;14(2):412–419. doi: 10.1080/21645515.2017.1403702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nygard M, Saah A, Munk C, Tryggvadottir L, Enerly E, Hortlund M, Sigurdardottir LG, Vuocolo S, Kjaer SK, Dillner J. Evaluation of the long-term anti-human papillomavirus 6 (HPV6), 11, 16, and 18 immune responses generated by the quadrivalent HPV vaccine. Clin Vaccine Immunol. 2015. August;22(8):943–948. doi: 10.1128/CVI.00133-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kjaer SK, Nygard M, Dillner J, Brooke Marshall J, Radley D, Li M, Munk C, Hansen BT, Sigurdardottir LG, Hortlund M, et al. A 12-year follow-up on the long-term effectiveness of the quadrivalent human papillomavirus vaccine in 4 Nordic countries. Clin Infect Dis. 2018;66(3): 339–345.doi: 10.1093/cid/cix797. [DOI] [PubMed] [Google Scholar]
- 27.Ferris D, Samakoses R, Block S, Lazcano-Ponce E, Restrepo JA, Mehlsen J, Chatterjee A, Iversen O-E, Joshi A, Chu JL, et al. Quadrivalent human papillomavirus vaccine in preadolescents and adolescents after 10 years. Pediatrics. 2017;140(6): pii:e20163947.doi: 10.1542/peds.2016-3947. [DOI] [PubMed] [Google Scholar]


