Abstract
Background
In Saudi Arabia, a diet for life policy has been adopted in the management of amino acid metabolism disorders for years. However, the specially designed low protein products/medical foods - which are one of the important treatment tools - were not available up until several years ago in Saudi Arabia (SA). Our aim was to measure the compliance and quality of life in patients affected with these disorders followed in the metabolic nutrition clinic at King Faisal Specialist Hospital & Research Centre (KFSH&RC), Riyadh, SA.
Methodology
We used a non-randomized retrospective/prospective study which utilized the growth parameters, biochemical data of patients plus questionnaires collected from patients and their family/caregivers. A total of n = 182 patients affected with selected amino acid metabolism disorders were enrolled. Some were excluded n = 84 for various reasons. Sample analyzed were: Phenylketonuria (PKU) (44), Maple Syrup Urine Disease (MSUD) (30), Tyrosinemia (TYR) (17) and Homocystinuria (HCU) (7). Tandem Mass Spectrometry (TMS) used to quantitate plasma amino acid concentrations. Data was obtained using (COMPLE) Microsoft-Access which was designed by the metabolic nutrition clinic at KFSH&RC-Riyadh. Student's paired t-test was used to investigate relationship between variables.
Results
The main findings were the improvement of selected amino acid levels pre and post the usage of medical foods. In PKU patients, the TMS Phenylalanine (PHE) levels post usage was significantly decreased (P value < .0001). This was also the case in MSUD patients with significant decrease in Leucine & Isoleucine levels (P value .0008) but not in Valine levels (P value .1148) as 36.7% of them received Valine supplements while enrolled in the study.
Conclusion
Low protein products availability was successful in improving outcomes for selected amino acid metabolic disorders. However, due to compliance issues and impracticality of the diet, the results were not significant in all enrolled patients.
Keywords: Amino acid metabolism, Low protein products, Phenylketonuria, Tyrosinemia, Maple Syrup Urine Disease, Homocystinuria, Saudi Arabia
1. Introduction
Disorders of protein and amino acid metabolism: Phenylketonuria (PKU), Maple Syrup Urine Disease (MSUD), Tyrosinemia (TYR), Homocystinuria (HCU) and Organic Acidemias (OA) are a class of inherited metabolic conditions that occur when certain amino acids either cannot be broken down or produced by the body, resulting in toxic accumulation of some substances and/or the deficiency of others. While Urea Cycle Disorder (UCD) defined as a genetic disorder caused by urea cycle enzymes deficiencies that result in waste nitrogen accumulating as ammonia and glutamine which are neurotoxin. These disorders are managed by life-long diet restriction. The aims of nutrition therapy in these disorders are: (a) reducing the production of toxic substances by restricting the offended nutrient in the patient's diet, (b) providing adequate calories, protein, vitamins and minerals which are necessary for optimal growth and development. To achieve this, amino acid metabolism disorders diet consists of:(a) synthetic amino-acid based formula,(b) calculated and measured amount of natural protein source, (c) carbohydrates and/or fat polymers are added to diet to build up calories and prevent catabolism, (d) specific calculated amino acid supplement as needed, (e) specially designed low protein foods/products. An individual approach combining all of the above will help in achieving expected outcomes. Specially designed low protein foods are products (i.e. low-protein pasta, rice, flour and bread) formulated to have ≤1 g protein per serving which are expected to provide 50–75% of estimated daily energy needs in patients with amino acid metabolism disorders [1]. For this reason patients are likely to need a large quantity and variety of products on an ongoing basis. They are substantially more expensive than regular food [2,3], and not readily available to purchase.
For patients with inherited metabolic disorder (IMD) the capacity to adhere to dietary restriction and overall management depends on both the patients and their caregivers [4]. Not adhering to the dietary restriction usually starts at school age (5–6 year old), leading to very poor compliance as children grow older (10–15 year old) [3,[5], [6], [7], [8]]. Reasons may be due to lack of dietary variation, cost and unavailability of specially designed low protein products/medical foods [9]. Additionally, feeding difficulties were common in children with IMD as reported in the literature [10,11]. It is also a dynamic process, with many patients varying in their levels of acceptance and willingness to adapt to such a strict diet or be selective in the adherence [4,12]. In addition to diet restriction, patients and caregivers usually complain about the frequent laboratory tests. It is also common that adult patients would improve their compliance just before lab test to ensure better results [3,8]. Furthermore, some of the amino acid metabolism disorders pose no risks of acute crisis e.g. PKU, HCU and TYR. While other disorders such as UCD, MSUD and OA could lead to frequent hospital admissions due to metabolic crisis secondary to poor dietary adherence [13]. Frequent admissions subsequently will negatively affect dietary adherence. As stated in the WHO report of adherence to long term therapies, compliance defined as (the extent to which a person's behavior – taking medication, following a diet, and/or executing lifestyle changes, corresponds with agreed recommendations from a health care provider) [12,14,15]. Therefore, no clear measurement of compliance has been defined [4]. The degree of dietary control required in these cases remains a controversial topic. However, from our practice, it is impossible to state that dietary liberalization is completely safe, equally it is futile to continue a strict dietary approach if our patients are refusing such a regimen.
Worldwide, a lot of research is done in regards to diet in the treatment of PKU which is the most common amino acid metabolism disorder in USA and UK. Most of these state a significant beneficial effect of phenylalanine restricted diet on intellectual achievement, neurologic status and behavioral improvement [8,[16], [17], [18], [19]]. However it could have a negative effect on physical growth [20,21] and an enhanced quality of life for both the patients and their caregivers in early and even late diagnosed patients [22]. Moreover, health related quality of life was reported to be compromised in IMD patients, which leads to increased demands of emotional and psychosocial support [23]. Most of the studies involved PKU patients with few papers published on other protein and amino acids metabolism disorders.
Growth parameter for these patients is usually compromised due to dietary restriction [24,25]. Lack of international publications related to growth and development of such patients impedes the comparison process. Vitamins and minerals deficiencies were reported in those groups of patients which can rapidly impair growth [7,26]. That could be due to the insufficient adherence to the synthetic amino-acid based formula provided or as consequences of the multiple dietary modifications that may place the patients at risk of macro and micronutrient deficiency [27,28]. Zinc, selenium, vitamin B12 and other deficiencies were reported long time ago mainly in PKU patients and other IMD [17,20,[29], [30], [31], [32], [33], [34], [35], [36]].
Saudi Arabia is one of the pioneers in treating patients affected with IMD around the world due to the high prevalence of these diseases (almost 4 times higher incidence than in USA) as per the Saudi national lab newborn screening program database [37,38]. This is related to our society's high rate of consanguinity and inter-caste marriages.
Dietary management is crucial for these patients through well trained metabolic dietitians which are unfortunately scarce in the rural areas of Saudi Arabia. Thus, such cases are referred to KFSH&RC-Riyadh from all over the Middle East, adding to the importance of this study.
2. Method
2.1. Study design
This is a non-randomized, retrospective/prospective study. Data before medical foods usage was retrospectively collected while post usage was prospectively gathered. Data were collected over around 2.6 year period, using (COMPLE) Microsoft access which was designed by the metabolic nutrition clinic at KFSH&RC-Riyadh. A written questionnaire was developed for this study. It was written in Arabic and also available in English, translated from Arabic to English by a professional translator. Questionnaire was validated through 3 well-educated mothers with affected patient/s, 4 clinical dietitians and 2 medical genetic physicians. It was developed covering socio-demographic data and technical challenges, and addressing the following topics: (a) compliance to dietary management and biochemical work-up from the view of patients and caregivers, (b) difficulties in choosing the appropriate food while following the restricted diet, (c) social life and academic performance, and (d) palatability and preparation of medical foods.
The study was conducted in accordance with the ethical principles contained in the Declaration of Helsinki (2000), the ICH Harmonized Tripartite Good Clinical Practice Guidelines, the policies and guidelines of the Research Advisory Council of KFSH&RC, and the laws of Saudi Arabia. A verbal informed consent was taken from the patients caregivers.
2.2. Participants
Inclusion criteria included (a) patients 2 years old and above, (b) affected with one of the selected amino acid metabolism disorders, (c) following up in the metabolic nutrition clinics at KFSH&RC-Riyadh, SA. Exclusion criteria comprised of (a) patients below 2 years old, (b) inability to obtain verbal consent, (c) total incompliance to the synthetic amino-acid based formula or restricted protein diet, and (d) patients with mild form of the disease (known mutation biochemically and phonetically) as per charted diagnosis. However, patients below 2 years old were included at the beginning of this study, and due to limitation of using medical foods during the weaning months, they were excluded.
In a median period of 1 year and 7 months, we approached 182 patients who met the inclusion criteria, all of which were affected with one of the selected amino acid metabolism disorders: PKU, TYR I & II, MSUD and HCU (Table 1). Biochemical data and growth parameters were taken as part of their routine metabolic-nutrition clinic visit. In addition, they were provided with a questionnaire with the patients and their caregivers prior to initiating the medical foods and after approximately 6 months. Post enrollment, 84 patients were withdrawn (Table 2) and the majority of them 69% (n = 58) were due to total incompliance or very poor compliance to synthetic mino-acid based formula and/or restricted protein diet or medical foods intake.
Table 1.
Characteristic of subjects completed the study.
| All n = 98 |
Pre low protein products usage | Post low protein products usage | P value | |
|---|---|---|---|---|
| Gender, n (%) | ||||
| Male | 44 (44.9) | |||
| Female | 54 (55.1) | |||
| Age at start low protein products, years, Median (range) | 8.7 (2.1–31) | |||
| Usage length of low protein products, years, Median (range) | 1.8 (0.7–2.6) | |||
| Weight kg, Mean (STD) | 34.2 (20.9) | 38.3 (22) | <.0001 | |
| Height cm, Mean (STD) | 127.1 (25.7) | 133.3 (22.4) | <.0001 | |
| BMI kg/m2, Mean (STD) | 19.1 (5.3) | 19.7 (6.0) | .0066 | |
| Diagnosis, n (%) | ||||
| PKU | 44 (44.9) | |||
| TYR | 17 (17.35) | |||
| MSUD | 30 (30.61) | |||
| HCU | 7 (7.14) | |||
Table 2.
List of causes for the excluded subjects from the selected amino acid metabolism disorders (PKU, TYR, HCU & MSUD) n = 84.
| Cause for exclusion | Number of subjects |
|---|---|
| Total incompliance, very poor compliance to medical formula or restricted protein diet/products | 58 |
| Below 2 years old | 8 |
| Poor oral intake or tube feeding (NGT/GT) dependent | 7 |
| Deceased | 2 |
| Did liver transplant | 1 |
| Inactive file | 7 |
| Stable patients/with mild form of the disease | 1 |
2.3. Anthropometric/demographic data
Demographic data were obtained from patient's medical and metabolic dietetic records, including gender, age at start of medical foods, length of usage, weight & height pre and post usage (Table 1). Body mass index was calculated for the given data pre and post medical foods usage, using the equation BMI = weight (kg) ÷ height (m2). Furthermore, BMI was compared with healthy peers and with general population standards using LMS growth excel add-in in order to obtain the international cut-offs that classify BMI in children age 2–18 years old depending on the child's age and gender and it was expressed as percentage (Fig. 1A and B) [39]. Growth parameters were plotted against National Center for Health Statistics reference data growth charts for all ≤ 18 year old patients and translated into Z-score correspond to the exact percentiles using LMS growth excel add-in Fig. 2 & Fig. 3(A and B,C&D) [40]. However, 13 patients classified as per WHO adult cut-offs due to their age, they were above 18 year old [41]. Total of (n = 37) boys and (n = 48) girls patients were analyzed at the baseline (pre medical foods usage) and at the end of the study (post medical foods usage) (Table 3).
Fig. 1.
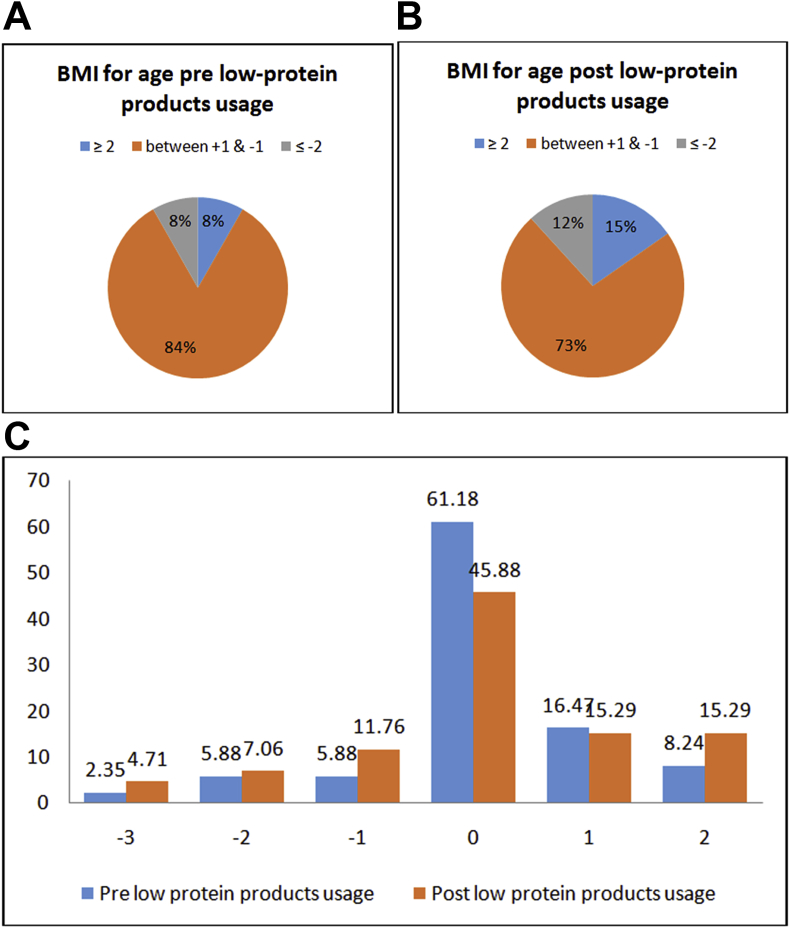
A and B. Percentage of children whose BMI for age Z-score is below or equal −2 (thinness) between −1 and +1 (normal), and above or equal 2 (obesity) 1C. Percentage of obesity=(2), overweight=(1), nor,al-(0) and thinness grade 1=(-1), 2=(-2) & 3=(-3) for children age 2–18 years based on the BMI international cut-offs.
Fig. 2.
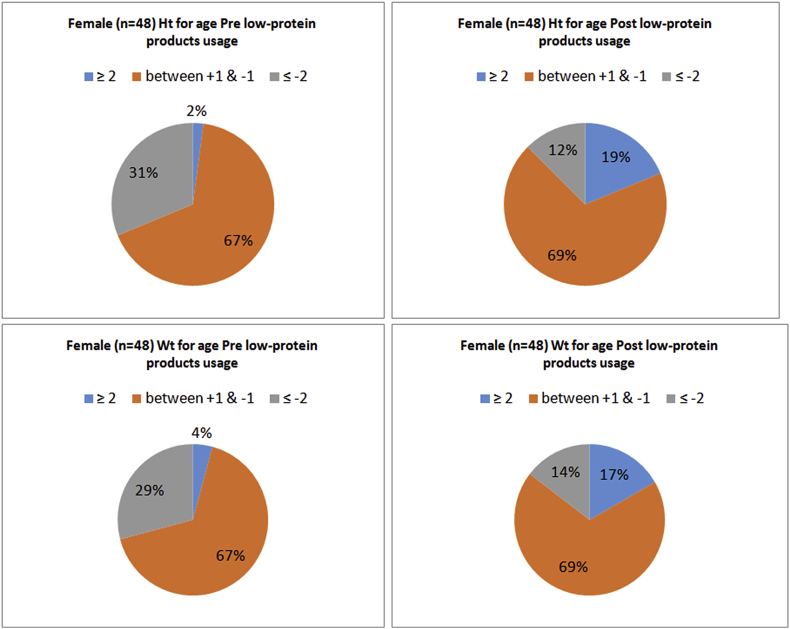
A–D Z-scores for height and weight for female patients, age 2–18 years, pre and post medical foods usage.
Fig. 3.
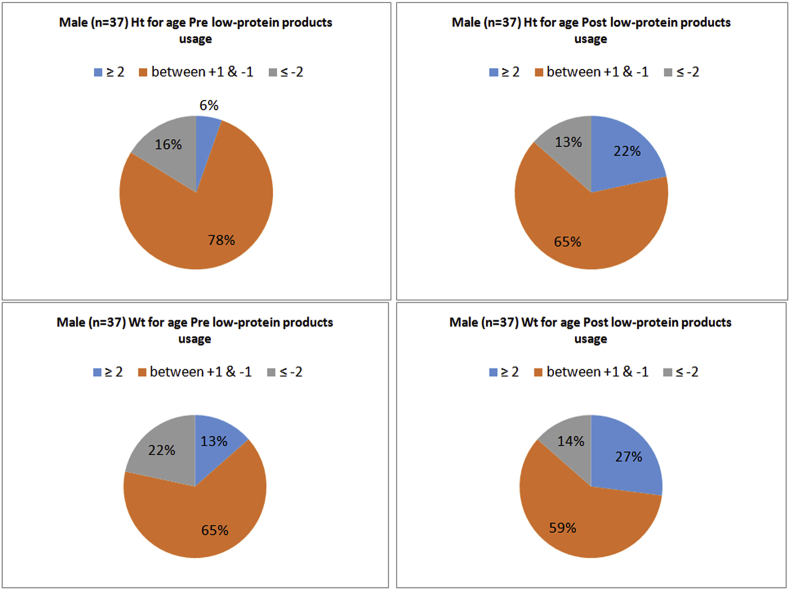
A–D. Z-scores for height and weight for male patients, age 2–18 years, pre and post medical foods usage.
Table 3.
NCHS z-scores for height and weight at the start and the end of the study for the analyzed boy's and girl's data.
| Weight for age |
Height for age |
|||
|---|---|---|---|---|
| Baseline (pre low protein usage) | End of study (post low protein usage) | Baseline (pre low protein usage) | End of study (post low protein usage) | |
| z-scores for Boys (n = 37), Mean (SD) | 0.003 (1.84) | 0.66 (2.236) | −0.675 (1.618) | 0.393 (2.076) |
| P < .0001 | P < .0001 | |||
| z-scores for Girls (n = 48), Mean (SD) | −0.873 (1.956) | 0.257 (1.904) | −1.317 (1.775) | 0.315 (2.069) |
| P < .0001 | P < .0001 | |||
2.4. Procedure
The first enrollment visit at the metabolic nutrition clinic at KFSHRC, Riyadh included: (a) thorough explanation of the importance of medical foods usage, value and effects, (b) completion of the pre-study questionnaire (consisting of multiple choices) by caregivers with the assistance of the metabolic dietitian present at that time, (c) comprehensive disease specific labs drawn as per the clinic routine follow up policy that included the following: Iron, Ferritin, CBC, Zinc, Selenium, Vitamin B12, Pre-albumin, Quantitative Amino Acid, TMS, Homocysteine level and others as needed(vitamin D levels were checked but not considered in the current study), (d) provision of low protein recipes booklet (developed by the metabolic dietitians at KFSR&RC-Riyadh) which contains amino acid analysis of each recipe, (e) free of charge issuance of the medical foods items (low protein flour, macaroni, spaghetti, rice, egg replacer, cake mix with two flavors, crackers and wafer, plus one item specifically for infant which is baby cereal,. (f) ensuring verbalized understanding of the given instructions, and follow up appointment after 3–6 months in the metabolic nutrition clinic as needed. Maximum of 4 clinic visits summary were collected and compared. In the period between the first and second clinic visits, adjustments of the synthetic amino-acid based formula were done as necessary according to the lab results or depending on clinical judgment. Additionally, medical genetic physician/s was contacted for a medication prescription to correct any nutritional deficiencies. Abnormal labs were repeated 3 months post the intervention or as required. Median results of TMS samples were ensured to be considered when the patient is stable (not sick) under similar circumstances, usually instructed to be drawn 3–4 h post feed, no fasting is recommended. The post-study questionnaire was completed almost at the third or the fourth clinic visit.
2.5. Statistical analysis
All the statistical analysis of data was done using the software package SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Descriptive statistics for the continuous variables were reported as mean ± standard deviation or (range) and categorical variables are summarized as frequencies and percentages.
All the continuous variables as appropriate for comparisons between subgroups of pre and post medical foods usage were compared by Student's paired t-test.
The level of statistical significance is set at P < .05.
3. Results
3.1. Subjects
Ninety eight patients (44 male and 54 female; median age 8.7 years [range from 2.1 to 31 years]) completed the study (Table 1). Their amino acid metabolism disorders were PKU (n = 44), TYR (n = 17), MSUD (n = 30) and HCU (n = 7) (Table 1). Dietary restrictions consisted mainly of synthetic amino-acid based formula, protein modified metabolic diet and medical foods which was issued to each patient at the first enrollment visit. The usage length period ranged from (0.7 year up to 2.6 years) with a median of 1.7 year. As per family responses, 29 (29.6%) patients had good tolerance to the taste of the medical foods, while 63 (64.3%) fairly tolerated one to two items, and 6 (6.1%) disliked them.
3.2. Biochemical status
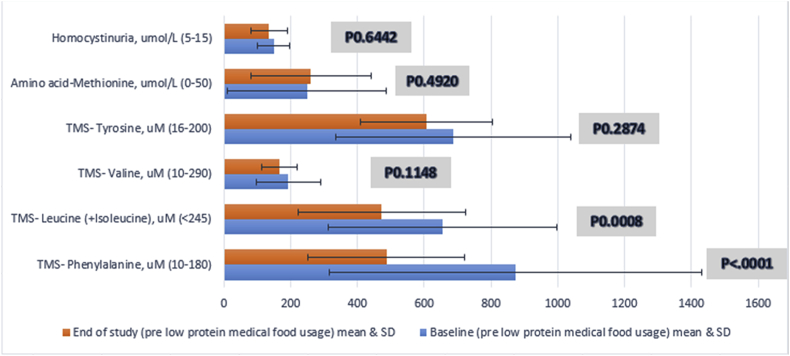
At the study period, there were significant improvement in Iron, Hemoglobin, zinc, Vitamin B12, TMS Phenylalanine and TMS Leucine &Isoleucine from the baseline pre using the medical foods to post usage checkup (Table 4). In PKU patients; the TMS Phenylalanine level post usage significantly decreased (P value < .0001). This was also the case in MSUD patients with significant decrease in Leucine &Isoleucine levels (P value = .0008) but not in Valine levels (P value = .1148) as 11 (36.7%) of them received Valine supplements while enrolled in the study. Additionally, all other biochemical markers had no significant differences as shown in the given table. However, there was a decrease in the percentage of selenium and pre-albumin below the reference range from the baseline to the end of the study (Fig. 4).
Table 4.
Percentage of subjects with blood measurements less than and above the plasma reference ranges and a comparison of blood marker levels at the start and the end of the study.
| Blood Marker (reference range) | Baseline % blood levels (number) < reference range | End of study % blood levels (number) < reference range | Baseline (pre low protein usage) mean (range) | End of study (post low protein usage) mean (range) | P value comparing mean blood levels |
|---|---|---|---|---|---|
| Iron, umol/L (6–7) | 11.46 (11) | 4.08 (2) | 13.6 (3.5–38.1) | 18.9 (5.7–170) | .0493 |
| Hemoglobin, g/L (110–150) | 5.15 (5) | 4.29 (3) | 129.2 (84–165) | 132 (100–164) | .0006 |
| Zinc, umol/L (10.6–19) | 37.76 (37) | 23.68 (18) | 11.2 (7.6–19.6) | 11.9 (6.5–29.8) | .0065 |
| Selenium, umol/L (0.89–1.52) | 3.61 (3) | 2.38 (1) | 1.3 (0.7–1.9) | 1.3 (0.7–1.7) | .0744 |
| Pre-albumin, mg/L (200–400) | 48.98 (48) | 48.95 (35) | 212.2 (37–441) | 208 (121–369) | .1229 |
| Vitamin B12, Pmol/L (145–637) |
4.76 (4) |
6.52 (3) |
751.3 (77–1476) |
792.3 (57–1476) |
.0349 |
|
Baseline % blood levels (number) > reference range |
End of study % blood levels (number) > reference range |
||||
| TMS- Phenylalanine, uM (10–180) | 93.18 (41) | 4.55 (2) | 873.2 (11–2719) | 487.5 (91–1134) | <.0001 |
| TMS- Leucine (+Isoleucine), uM (<245) | 86.67 (26) | 86.67 (26) | 655.6 (67–1499) | 472.5 (68–987) | .0008 |
| TMS- Valine, uM (10–290) | 13.33 (4) | 0 | 193.3 (32–415) | 166.6 (67–281) | .1148 |
| TMS- Tyrosine, uM (16–200) | 94.12 (16) | 100 (17) | 686.3 (107–1463) | 606.1 (294–947) | .2874 |
| Amino acid-Methionine, umol/L (0–50) | 80 (4) | 100 (7) | 248.6(35–616) | 259.9 (51–511) | .4920 |
| Homocystinuria, umol/L (5–15) | 100 (7) | 100 (7) | 149 (61–206) | 135 (49–204) | .6442 |
Fig. 4.
Comparison of amino acids measure * levels at the start and the end of the study.
*TMS was used for (Tyrosine, Valine, Leucine & Isoleucine and Phenylalanine) Methionine was measured using quantitative amino acid measure, plus homocysteine level.
3.3. Anthropometry and growth velocity
There were significant changes in weight and height between the baseline and the end of the study for all the enrolled subjects (Table 1). Additionally, the reported Z-scores for the analyzed (n = 48) girls and (n-37) boys, aged ≥ 2 years old to ≤ 18 years old were significantly different for weight and height pre and post the usage of medical foods (Table 3). Fig. 1 (A,B&C), Fig. 2 (A,B,C&D) and Fig. 3 (A,B,C&D) shows the growth indicators for all patients (male and female) pre and post the medical foods usage expressed as Z-scores according to the WHO definition. Sixteen percent of the male children were stunted and it was decreased to 13% at the end of the study. While in female children 31% were stunted and it was decreased to 12%. Additionally, 22% of the male children were underweight and it was decreased to 14% at the end of the study. While 29% of the female children were underweight and it was decreased to 14%. In the BMI reported for age (Fig. 1A&B), the percentage of obesity and over wt based on the international cut-offs to classify BMI in children age 2–18 years were increased by (7%) at the end of the study period. Moreover, with further analysis, 14 patients (16.5%) who reported to be obese or overweight at the end of the study period, were overweight or normal respectively previous the medical foods usage. Additionally, 12 patients (14%) continued to be overweight or obese through the study period. However, 10 patients (11.8%) who had normal BMI and became underweight at the end of the study period.
3.4. Questionnaire
All the 98 subjects filled the provided questionnaire; first section was pre initiating the medical foods and second section post usage. In the view of patients' and caregivers' opinion; 31 patients (31.6%) had an excellent compliance to the restricted diet regimen, 58 (59.2%) had good compliance and 9 (9.2%) were poorly compliant. In addition, 74 of the patients (75.5%) tolerated most of the medical foods items daily. Additionally, 71 (72.5%) were routinely doing the biochemical work-up. Interestingly, 91 (92.9%) of the caregivers reported difficulties in choosing the appropriate food pre introducing the medical foods . However, 84 (85.7%) reported positively that the products availability facilitated the adherence to the restricted diet. Additionally, 75 of the families (76.5%) reported that the social life of the affected member/s was influenced by the unavailability of the medical foods. While 52 (53%) noticed improvement in the social events after providing the products. Furthermore, 33 of the caregivers (33.7%) reported good academic performance among their affected children. And it continued to be 34 (34.7%) post the medical foods usage. Moreover, 43 of the families (43.9%) never tried any form of the medical foods pre-initiating the current study. In addition, in regards to the taste; post the medical foods usage 47 (47.9%) reported products tasted good, 42 (42.9%) reported acceptable taste, and 9 (9.2%) taste was unacceptable to them. Lastly, in regards to the preparation of medical foods; 30 (30.6%) found it easy, 51 (52%) found it moderate, and 17 (17.4%) found it difficult to prepare the products.
4. Discussion
4.1. Was it easy to introduce these products?
One of the factors associated with difficulty introducing the medical foods was the wide range age group. Although high percentage 75.5% of patients were obliged to consume the medical foods on daily basis, In some cases it was introduced as late as school age (median age was 8.7 years) at which incompliance to the dietary restriction is common. We theorize that tolerance of these foods would have been better if it was introduced as early as possible. Additionally, compliance of adult patients was more difficult to the restricted protein modified metabolic diet pre medical foods; what more when they had specially designed foods that could keep their meal away from the other family members. Supplies, transport, storage, preparation, time consuming, effort spent were taking long process that will not be repeated if the patient had poor tolerance to them [3].
4.2. Why children below two year were excluded?
These children are most likely to be unstable to wean or developmentally delayed. Furthermore, caregivers usually have anxiety regarding the quantity and quality of weaning food that should be used [5,42]. However, synthetic amino-acid based formula was found to almost provide 80–90% of their estimated daily needs, which consequently minimize the benefits of utilizing the medical foods at this stage [26]. In addition, most of the provided medical foods in the current study were not suitable as weaning food. There was only one product that would seldom be available.
Additionally, the substantial expensive value of the products was not applied as it was free of charge issued to each enrolled patient. However, as the awareness of these products are scarce, hospital administration and logistics had to argue the cost and take exception to consider the value to benefit ratio.
4.3. Why selected disorders?
Upon initiating of this research, methylmalonicacidemia (MMA), Propionic academia (PA) and UCD patients were given an opportunity to be part of this project aiming for equalization among all the protein and amino acid metabolism disorders. However, the selected amino acid metabolism disorders (PKU, TYR, HCU& MSUD) have a direct biochemical parameters that can be measured to check compliance and effectiveness of the medical foods, but on the other hand the other disorders (MMA, PA and UCD) don't have such parameters. Consequently, we believe that modification was done to provide validated proper results in the selected amino acid metabolism disorders. Additionally, the other included diagnoses were reflecting the high rate of incidence in our region. Adherence was more problematic with these chronic disorders which are more stable in regard to crisis and hospital admission (PKU, TYR & HCU). However, MSUD patients might be prone to crisis. This make the selected amino acid metabolism disorders a priority to control. However, most of the inherited metabolic disorders have several barriers to adherence to the restricted diet such as physical status, neuropsychological conditions and even social. In addition, the measurement of psychosocial development was excluded from the variance in the current study, as it could not be applied on patients because of unavailability of additional funding that was required for the parameter.
4.4. Is the long term dietary restriction affecting the quality of life?
The paradox of dietary managing amino acid metabolic disorders from our experience, that long term diet restriction is affecting the family as it becomes a way of living. This could easily keep the patient and his family in isolation. This will significantly reduce the quality of life as it been reported especially in adulthood period [43]. Moreover, 84 (85.7%) of the participants expressed that they had better dietary adherence post introducing the medical foods. However, almost half of the enrolled subjects (53%) reported positive improvement in the social life post introducing the medical foods. Therefore, steps towards improving the dietary adherence are counted as factors to improve the quality of life [44].
4.5. Did we reach metabolic control?
Metabolic controls were achieved in PKU and MSUD patients in regard to their biochemical measures post using the medical foods. However, Valine deficiency was more apparent which was expected due to lower Valine content of medical foods compared to regular food, which increased the consumption of Valine supplementation. Additionally, some patients reached normal stable levels with weekly doses, as in MSUD patients, to promote anabolism of Leucine, when Leucine blood concentration is high; additional supplementation of Valine and Isoleucine was often required. On the other hand, most of the TYR and HCU patients were towards adulthood when the medical foods introduced. And that made the compliance and adherence more challenging. Furthermore, the sample size of TYR and HCU patients who reached the end of the study period was small compared to the PKU and MSUD patients. This could be a reason for not reflecting the significant differences.
Regarding the other biochemical markers, it was reported frequently that amino acid metabolic disorder patients could show a specific nutrient deficiencies due to the diet restriction and poor tolerance of the synthetic amino-acid based formula [7]. However, monitoring these markers during the follow up clinic visits could significantly improve them. As noted in our study, micronutrients/and or vitamins oral supplementations were received to correct the reported deficiencies in zinc, Iron, Hgb, vitamin B12 but rarely selenium were used. Patients with borderline low levels of these markers were encouraged to consume the total prescribed volume of the synthetic amino-acid based formula and that was in charge of correcting them.
4.6. Any effect on growth parameters was noted?
The statistically significant differences in weight and height for male and female data, pre and post the medical foods could be referred to the rapid developmental years of age. During the period of the data collection; most of the enrolled subjects grew well and had significant improvement even if they are below the ideal developmental status compared to their healthy peers.
One other point worth to mention at this stage, is the percentage of obesity and overweight that increased post using the medical foods. This could be related to multiple reasons: as the patient had higher energy intake from the products that is freely allowed among their dietary restrictions. In addition, the decreased intake of fruits and vegetables in comparison to the starchy based food intake resulted in increased risk of developing obesity. And a conflict of evidence had been reported for PKU patients especially in regards to trend toward developing overweight and obesity [24,26,35,[45], [46], [47]].
4.7. Limitations
There are several limitations to the current study: nutrient analysis was not done by the investigators, and the intake of the synthetic amino-acid based formula was not considered as a variable. Outcome was not analyzed based on stratification (type of IMD). Other potential limitations of the study could be: a) illiterateness and un-cooperative care givers, b) inaccurate information, c) missed metabolic nutrition clinic appointments and irregular lab tests, d) picky eaters, e) possibility of disease crisis. These factors affected quality of data collected. Finally, worldwide use of quality of life measuring tools were not utilized in this paper.
4.8. Future research
Research is needed to evaluate the factors that affect the IMD patients' growth and development in an international study comparison. Further long term studies are needed to assess the vitamin and mineral status in those amino acid metabolic disorders patients. In addition, finding more practical ways to measure the compliance in long term dietary restriction needs to be investigated in order to gain acceptable dietary adherence and reach that level of concordance which is crucial for an optimal outcome.
4.9. Conclusion
The specially designed low protein products/medical foods availability was successful in improving selected amino acid metabolic disorder biochemical outcomes; especially in patients affected with PKU and MSUD. Additionally, growth parameters were improved, as the products contributed to the increased energy intake among the participants. Furthermore, this study highlighted the importance of vitamins and minerals supplementation as needed with individualized periodic adjustment to maintain adequate nutrition and physical development. However, due to compliance issues and impracticality of the diet, the results were not significant in all enrolled patients.
Conflicts of interest
The authors declare that they have no conflict of interest. No funding is declared.
Footnotes
Peer review under responsibility of King Faisal Specialist Hospital & Research Centre (General Organization), Saudi Arabia.
Contributor Information
Bedour Handoom, Email: Bhandoom@kfshrc.edu.sa.
Eman Megdad, Email: emegdad@kfshrc.edu.sa.
Dana Al-Qasabi, Email: dalqasabi@kfshrc.edu.sa.
Munirah Al Mesned, Email: malmesned@kfshrc.edu.sa.
Reem Hawary, Email: rhawary@kfshrc.edu.sa.
Samir Al-Nufiee, Email: doctor_aboferas@hotmail.com.
Zuhair Al-Hassnan, Email: zhassnan@kfshrc.edu.sa.
Moeenaldeen Dia Alsayed, Email: moeen@kfshrc.edu.sa.
Abdelmoneim Eldali, Email: aeldali@kfshrc.edu.sa.
References
- 1.Pena M., Almeida M., van Dam E., Ahring K., Bélanger-Quintana A., Dokoupil K. Special low protein foods for phenylketonuria: availability in Europe and an examination of their nutritional profile. Orphanet J Rare Dis. 2015;10(1) doi: 10.1186/s13023-015-0378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afroze B., Lakhani L., Naz F., Somani S., Yunus Z., Brown N. Challenges identified in the management of patients with inherited metabolic disorders – a five year experience from Pakistan. Egypt J Med Human Genet. 2016;17(3):259–264. [Google Scholar]
- 3.Bilginsoy C., Waitzman N., Leonard C., Ernst S. Living with phenylketonuria: perspectives of patients and their families. J Inherit Metab Dis. 2005;28(5):639–649. doi: 10.1007/s10545-005-4478-8. [DOI] [PubMed] [Google Scholar]
- 4.MacDonald A., Gokmen-Ozel H., van Rijn M., Burgard P. The reality of dietary compliance in the management of phenylketonuria. J Inherit Metab Dis. 2010;33(6):665–670. doi: 10.1007/s10545-010-9073-y. [DOI] [PubMed] [Google Scholar]
- 5.MacDonald A., Evans S., Cochrane B., Wildgoose J. Weaning infants with phenylketonuria: a review. J Hum Nutr Diet. 2011;25(2):103–110. doi: 10.1111/j.1365-277X.2011.01199.x. [DOI] [PubMed] [Google Scholar]
- 6.Rohde C., Thiele A., Och U., Schönherr K., Meyer U., Rosenbaum-Fabian S. Effect of dietary regime on metabolic control in phenylketonuria: is exact calculation of phenylalanine intake really necessary? Mol Genet Metabol Rep. 2015;5:36–41. doi: 10.1016/j.ymgmr.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shchelochkov O., Dickinson K., Scharschmidt B., Lee B., Marino M., Le Mons C. Barriers to drug adherence in the treatment of urea cycle disorders: assessment of patient, caregiver and provider perspectives. Mol Genet Metabol Rep. 2016;8:43–47. doi: 10.1016/j.ymgmr.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weglage J., Fünders B., Wilken B., Schubert D., Schmidt E., Burgard P.U. Psychological and social findings in adolescents with phenylketonuria. Eur J Pediatr. 1992;151(7):522–525. doi: 10.1007/BF01957759. [DOI] [PubMed] [Google Scholar]
- 9.Ho G., Ueda K., Houben R., Joa J., Giezen A., Cheng B. Metabolic Diet App Suite for inborn errors of amino acid metabolism. Mol Genet Metabol. 2016;117(3):322–327. doi: 10.1016/j.ymgme.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Evans S., Alroqaiba N., Daly A., Neville C., Davies P., MacDonald A. Feeding difficulties in children with inherited metabolic disorders: a pilot study. J Hum Nutr Diet. 2012;25(3):209–216. doi: 10.1111/j.1365-277X.2012.01229.x. [DOI] [PubMed] [Google Scholar]
- 11.MacDonald A., Harris G., Rylance G., Asplin D., Booth I. Abnormal feeding behaviours in phenylketonuria. J Hum Nutr Diet. 1997;10(3):163–170. [Google Scholar]
- 12.Playle J., Keeley P. Non-compliance and professional power. J Adv Nurs. 1998;27(2):304–311. doi: 10.1046/j.1365-2648.1998.00530.x. [DOI] [PubMed] [Google Scholar]
- 13.MacDonald A., van Rijn M., Feillet F., Lund A., Bernstein L., Bosch A. Adherence issues in inherited metabolic disorders treated by low natural protein diets. Ann Nutr Metabol. 2012;61(4):289–295. doi: 10.1159/000342256. [DOI] [PubMed] [Google Scholar]
- 14.De Geest S., Sabaté E. Adherence to long-term therapies: evidence for action. Eur J Cardiovasc Nurs. 2003;2(4) doi: 10.1016/S1474-5151(03)00091-4. 323–323. [DOI] [PubMed] [Google Scholar]
- 15.Haynes R.B. 1979. Introduction. In compliance in health care; p. 516. [Google Scholar]
- 16.Baumeister A.A., Baumeister A.A. Dietary treatment of destructive behavior associated with hyperphenylalaninemia. Clin Neuropharmacol. 1998;21:18–27. [PubMed] [Google Scholar]
- 17.Cleary M., Walter J. Assessment of adult phenylketonuria. Ann Clin Biochem. 2001;38(5):450–458. doi: 10.1177/000456320103800502. [DOI] [PubMed] [Google Scholar]
- 18.González M., Gutiérrez A., Gassió R., Fusté M., Vilaseca M., Campistol J. Neurological complications and behavioral problems in patients with phenylketonuria in a Follow-up Unit. Mol Genet Metabol. 2011;104:S73–S79. doi: 10.1016/j.ymgme.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 19.Koch R., Moseley K., Ning J., Romstad A., Guldberg P., Guttler F. Long-term beneficial effects of the phenylalanine-restricted diet in late-diagnosed individuals with phenylketonuria. Mol Genet Metabol. 1999;67(2):148–155. doi: 10.1006/mgme.1999.2863. [DOI] [PubMed] [Google Scholar]
- 20.Dobbelaere D., Michaud L., Debrabander A., Vanderbecken S., Gottrand F., Turck D. Evaluation of nutritional status and pathophysiology of growth retardation in patients with phenylketonuria. J Inherit Metab Dis. 2003 Jul 1;26(1) doi: 10.1023/a:1024063726046. 1–1. [DOI] [PubMed] [Google Scholar]
- 21.Huemer M., Huemer C., Möslinger D., Huter D., Stöckler-Ipsiroglu S. Growth and body composition in children with classical phenylketonuria: results in 34 patients and review of the literature. J Inherit Metab Dis. 2007;30(5):694–699. doi: 10.1007/s10545-007-0549-3. [DOI] [PubMed] [Google Scholar]
- 22.Pavone L., Meli C., Nigro F., Lisi R., Di-Raimondo S., Mollica F. Late diagnosed phenylketonuria patients Clinical presentation and results of treatment. Dev Brain Dysfunct. 1993;6(1–3):184–187. [Google Scholar]
- 23.Hatzmann J., Valstar M., Bosch A., Wijburg F., Heymans H., Grootenhuis M. Predicting health-related quality of life of parents of children with inherited metabolic diseases. Acta Paediatr. 2009;98(7):1205–1210. doi: 10.1111/j.1651-2227.2009.01269.x. [DOI] [PubMed] [Google Scholar]
- 24.Belanger-Quintana A., Martínez-Pardo M. Physical development in patients with phenylketonuria on dietary treatment: a retrospective study. Mol Genet Metabol. 2011;104(4):480–484. doi: 10.1016/j.ymgme.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 25.MacDonald A., Rocha J., van Rijn M., Feillet F. Nutrition in phenylketonuria. Mol Genet Metabol. 2011;104:S10–S18. doi: 10.1016/j.ymgme.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 26.Singh R., Rohr F., Frazier D., Cunningham A., Mofidi S., Ogata B. Recommendations for the nutrition management of phenylalanine hydroxylase deficiency. Genet Med. 2014;16(2):121–131. doi: 10.1038/gim.2013.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boyer S., Barclay L., Burrage L. Inherited metabolic disorders. Nutr Clin Pract. 2015;30(4):502–510. doi: 10.1177/0884533615586201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daly A., Evans S., Chahal S., Surplice I., Vijay S., Santra S. The challenges of vitamin and mineral supplementation in children with inherited metabolic disorders: a prospective trial. J Hum Nutr Diet. 2016;29(4):434–440. doi: 10.1111/jhn.12354. [DOI] [PubMed] [Google Scholar]
- 29.Acosta P., Fernhoff P., Warshaw H., Hambidge K., Ernest A., Mccabe E. Zinc and copper status of treated children with phenylketonuria. J Parenter Enteral Nutr. 1981;5(5):406–409. doi: 10.1177/0148607181005005406. [DOI] [PubMed] [Google Scholar]
- 30.Crujeiras V., Aldámiz-Echevarría L., Dalmau J., Vitoria I., Andrade F., Roca I. Micronutrient in hyperphenylalaninemia. Data Brief. 2015;4:614–621. doi: 10.1016/j.dib.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hvas A., Nexo E., Nielsen J. Vitamin B12 and vitamin B6 supplementation is needed among adults with phenylketonuria (PKU) J Inherit Metab Dis. 2006;29(1):47–53. doi: 10.1007/s10545-006-0108-3. [DOI] [PubMed] [Google Scholar]
- 32.Jochum F., Terwolbeck K., Meinhold H., Behne D., Menzel H., Lombeck I. Effects of a low selenium state in patients with phenylketonuria. Acta Paediatr. 1997;86(7):775–779. doi: 10.1111/j.1651-2227.1997.tb08587.x. [DOI] [PubMed] [Google Scholar]
- 33.Lombeck I., Kasperek K., Harbisch H., Becker K., Schumann E., Schröter W. The selenium state of children. Eur J Pediatr. 1978;128(4):213–223. doi: 10.1007/BF00445606. [DOI] [PubMed] [Google Scholar]
- 34.Robinson M., White F., Cleary M., Wraith E., Lam W., Walter J. Increased risk of vitamin B12 deficiency in patients with phenylketonuria on an unrestricted or relaxed diet. J Pediatr. 2000;136(4):545–547. doi: 10.1016/s0022-3476(00)90022-2. [DOI] [PubMed] [Google Scholar]
- 35.Shaw V. Wiley Blackwell; Chichester, West Sussex: 2015. Clinical paediatric dietetics. [Google Scholar]
- 36.Walter J. Vitamin B12 deficiency and phenylketonuria. Mol Genet Metabol. 2011;104:S52–S54. doi: 10.1016/j.ymgme.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 37.Afifi A.M., Abdul-Jabbar M.A. Saudi newborn screening. A national public health program: needs, costs, and challenges. Saudi Med J. 2007;28:1167–1170. [PubMed] [Google Scholar]
- 38.Alfadhel M., Al Othaim A., Al Saif S., Al Mutairi F., Alsayed M., Rahbeeni Z. Expanded newborn screening program in Saudi Arabia: incidence of screened disorders. J Paediatr Child Health. 2017;53(6):585–591. doi: 10.1111/jpc.13469. [DOI] [PubMed] [Google Scholar]
- 39.Child W.H.O., Standards G. World Health Organization. Training Course on Child Growth Assessment; Geneva: 2008. Training course on child growth assessment; pp. 1–116. WS 103. [Google Scholar]
- 40.Pan H., Cole T.J. 2012. LMSgrowth, a Microsoft Excel add-in to access growth references based on the LMS method. Version 2.77.http://www.healthforallchildren.co.uk/ [Google Scholar]
- 41.World Health Organization, Organization, W. H Public health Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Publ Health. 2004;363(9403):157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 42.Douglas J. Psychological treatment of food refusal in young children. Child Adolesc Ment Health. 2002;7(4):173–180. doi: 10.1111/1475-3588.00031. [DOI] [PubMed] [Google Scholar]
- 43.Cazzorla C., Del Rizzo M., Burgard P., Zanco C., Bordugo A., Burlina A. Application of the WHOQOL-100 for the assessment of quality of life of adult patients with inherited metabolic diseases. Mol Genet Metabol. 2012;106(1):25–30. doi: 10.1016/j.ymgme.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 44.Cotugno G., Nicolò R., Cappelletti S., Goffredo B., Dionisi Vici C., Di Ciommo V. Adherence to diet and quality of life in patients with phenylketonuria. Acta Paediatr. 2011;100(8):1144–1149. doi: 10.1111/j.1651-2227.2011.02227.x. [DOI] [PubMed] [Google Scholar]
- 45.Acosta P., Yannicelli S., Singh R., Mofidi S., Steiner R., DeVincentis E. Nutrient intakes and physical growth of children with phenylketonuria undergoing nutrition therapy. J Am Diet Assoc. 2003;103(9):1167–1173. doi: 10.1016/S0002-8223(03)00983-0. [DOI] [PubMed] [Google Scholar]
- 46.Burrage L., McConnell J., Haesler R., O'Riordan M., Sutton V., Kerr D., McCandless S. High prevalence of overweight and obesity in females with phenylketonuria. Mol Genet Metabol. 2012;107(1–2):43–48. doi: 10.1016/j.ymgme.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 47.Robertson L., McStravick N., Ripley S., Weetch E., Donald S., Adam S. Body mass index in adult patients with diet-treated phenylketonuria. J Hum Nutr Diet. 2013;26:1–6. doi: 10.1111/jhn.12054. [DOI] [PubMed] [Google Scholar]