Abstract
Juvenile idiopathic arthritis (JIA) is the most common chronic childhood arthritis; unfortunately, no diagnostic tool is available. Genetic disorders with musculoskeletal involvement that mimic chronic polyarthritis should be considered in the differential diagnostics of JIA. Normal inflammatory markers and characteristic radiological features are able to distinguish these disorders from JIA. Timely diagnosis of these disorders is crucial to offer the family proper genetic counseling and avoid inappropriate therapy. This review highlights selected noninflammatory disorders that often present with articular manifestations and that are often mislabeled as JIA. The focus is on the clinical, biochemical, and imaging features of these disorders.
Keywords: Juvenile idiopathic arthritis, Arthropathy, Multicentric osteolysis, Mucopolysaccharidosis, Dsyplasia
1. Introduction
Juvenile idiopathic arthritis (JIA) is the most common childhood chronic inflammatory arthritis. The nomenclature and classification of JIA are based on the International League of Associations for Rheumatology (ILAR) criteria [1,2]. JIA is a diagnosis of exclusion and represents a phenotypically heterogeneous group of arthritides; however, they have similar inflammatory articular changes. It is crucial to recognize arthritis, which is a clinical finding manifested as stiffness, particularly in the morning, effusion/swelling, and limited range of motion of the affected joints [3]. Many children are referred to pediatric rheumatology clinics with musculoskeletal manifestations such as joint contracture, swelling, or deformity without signs of inflammation. A spectrum of systemic noninflammatory disorders may masquerade as JIA [4]. Patients with noninflammatory disorders have arthropathy rather than arthritis. Arthropathy can arise from bony dysplasia, thickened synovium, or noninflammatory effusion [5,6]. Unfortunately, it is not uncommon for the presence of noninflammatory arthropathy to mimic juvenile arthritis, which delays the correct diagnosis and the appropriate management [7,8].
We believe that a complete history, with emphasis on the family history, and thorough musculoskeletal examination supported by basic laboratory tests, including tests for acute-phase reactants, and proper imaging studies are sufficient to identify such disorders and eventually initiate the proper management and avoidance of unnecessary treatment [9]. This review highlights selected noninflammatory disorders that often present with articular manifestations; it is not uncommon to mislabel such disorders as JIA. The focus is on the clinical, biochemical, and imaging features of these disorders.
2. Idiopathic multicentric osteolysis
Idiopathic osteolysis is a rare inherited heterogeneous group of disorders. The exact cause and pathogenesis are not well identified. There are different forms with different names; the frequency of these disorders worldwide is unknown [10,11]. The affected patients are young children, usually presenting with restricted movements of the wrist and ankle joints simulating arthritis but with rapid progressive resorption of carpal and tarsal bones ending with severe deformities and functional disabilities [12,13]. Patients have pain due to severe osteoporosis rather than synovitis.
The initial presentation mimics arthritis; so it is not uncommon to mislabel such conditions as JIA [14,15]. However, careful clinical assessment demonstrated that these patients do not have morning stiffness or joint effusion. Plain radiography of the hands and feet revealed early destructive osteolytic changes of the carpal and tarsal bones, which are not consistent with inflammatory arthritis. Furthermore, inflammatory markers, including erythrocyte sedimentation rate and C-reactive protein level, are typically within normal limits.
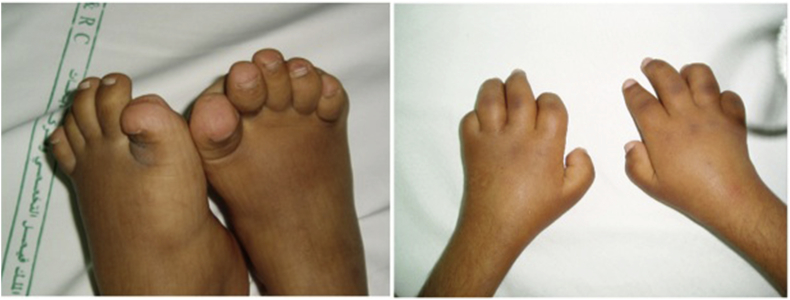
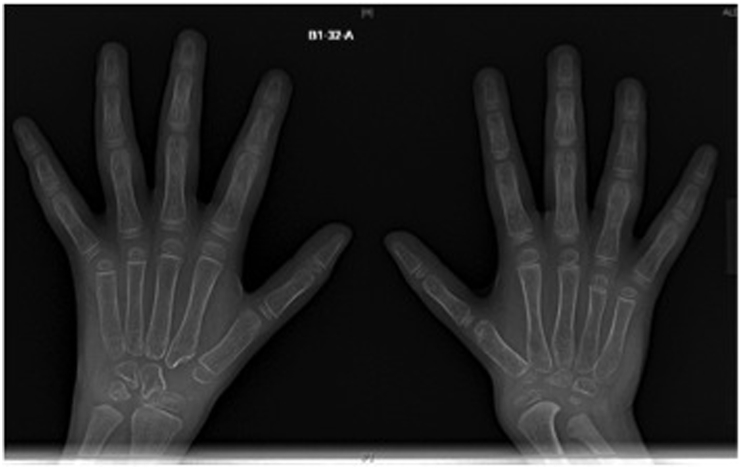
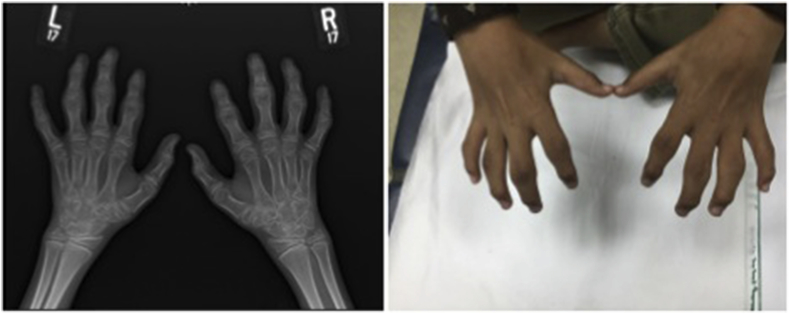
We described a large cohort of one of the idiopathic multicentric osteolysis disorders, named nodulosis, arthropathy, and osteolysis (NAO) syndrome [16]. NAO syndrome results from a mutation in the matrix metalloproteinase 2 gene (MMP2), at 16q12-21. Mutational inactivation of MMP2 creates an imbalance between bone synthesis and resorption [17]. The main clinical findings are arthropathy in the form of limitation of motion with contractures and deformities affecting both hands and feet in addition to nodular lesions in both palmar and plantar surfaces (Fig. 1). A few patients have involvement of other joints, including elbows, knees, and hips. Radiography of the hands and feet shows advanced osteolytic changes (Fig. 2).
Fig. 1.
Contractions of small joints of hands and feet in a patient with nodulosis, arthropathy, and osteolysis syndrome.
Fig. 2.
Bilateral hand radiograph of both hands showing generalized osteopenia and advanced osteolytic changes in a patient with nodulosis, arthropathy, and osteolysis syndrome.
Unfortunately, no effective treatment is available for these disorders. Supportive treatment in the form of calcium and vitamin D supplementation in addition to bisphosphonate might increase the bone density but does not change the disease course [18,19].
3. Mucopolysaccharidosis
Mucopolysaccharidoses (MPS) are a heterogeneous group of inborn metabolic disorders of glycosaminoglycan. There are different phenotypes depending on the mutations of certain genes encoding glycosaminoglycan-degrading enzymes, resulting in the inability to metabolize certain glycosaminoglycans. Overall the frequency of MPS differs in each population but the most common form is MPS type I [20,21]. Patients usually look normal at birth. However, as they get older, they show progressive clinical features affecting multiple organ systems. Severe MPS phenotypes are typically under the care of medical genetic specialists. The diagnostic approach and management of MPS are beyond the scope of this review. However, a general pediatrician or another specialists may suspect or see mild cases initially. Accordingly, he or she can refer the patient to a medical genetic specialist for more confirmatory testing and management.
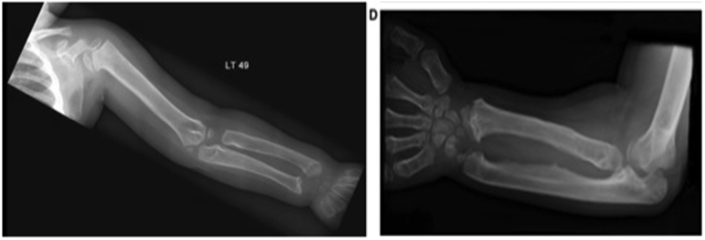
MPS type I has historically been delineated into three separate subtypes: Hurler syndrome (severe), Hurler-Scheie syndrome (intermediate), and Scheie syndrome (mild). Musculoskeletal manifestations such as stiffness and joint contractures are prominent in all forms of MPS; these manifestations include joint disorders that may mimic inflammatory arthritis and require consultation with a rheumatologist [7,22]. The milder phenotypes are more likely to be missed; unfortunately, diagnostic delays occur frequently in these patients. Rheumatologists and other specialists should be aware of the musculoskeletal manifestations of MPS. The proper history and physical examination should help in recognizing these cases. MPS should be considered a differential diagnosis in children with joint contractures in the absence of signs of inflammation [23]. Furthermore, certain radiographic findings, such as characteristic dysplasia and dysostosis multiplex, should raise suspicion of MPS (Fig. 3) [24,25].
Fig. 3.
Radiograph showing dysplasia and dysostosis multiplex of radius and ulna in a patient with mucopolysaccharidosis type I.
Early diagnosis and management are necessary to improve the outcome in affected patients. Enzyme activity assays based on cultured fibroblasts, leukocytes, plasma, or serum are considered the gold standard for diagnosis of MPS. Certain MPS disorders can be treated with hematopoietic stem cell transplantation or enzyme replacement therapy when this is available [25,26].
4. Camptodactyly-arthropathy–coxa vara–pericarditis syndrome
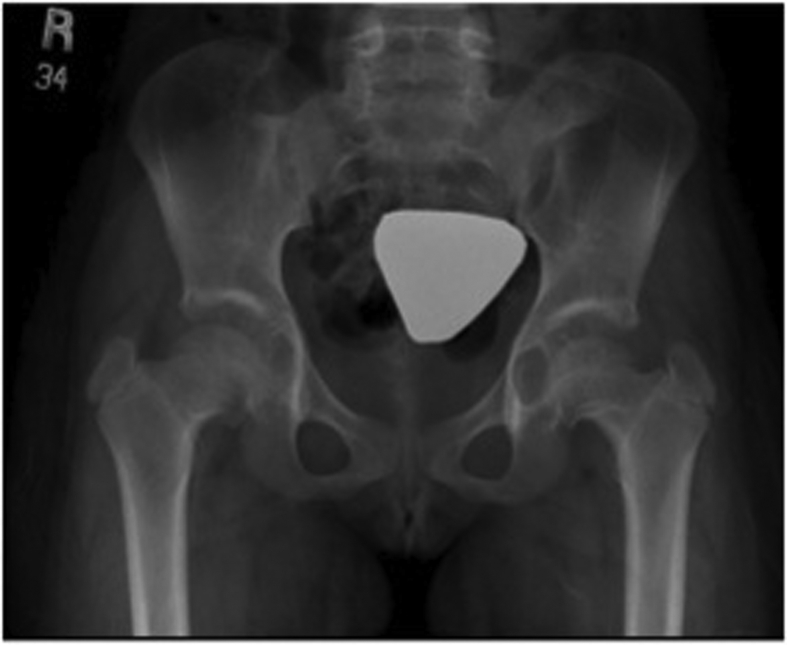
Camptodactyly-arthropathy–coxa vara–pericarditis (CACP) syndrome is a rare autosomal inherited disorder affecting mainly the joints. Usually, the affected individual does not report joint pain or morning stiffness. Typically, the manifestations start in the infancy period as camptodactyly of the fifth fingers. However, the hallmark features, such as swelling of interphalangeal joints, wrists, and knees, develop in early childhood. The swollen joints are due to synovial thickening and effusion, which are often associated with limited motion but without redness, tenderness, or hotness [8,27]. One of the important components of this disorder is femoral dysplasia; patients usually develop progressive coxa vara, some have other radiological findings such as acetabular cysts (Fig. 4). Ultrasonography is probably beneficial in differentiating CACP syndrome from inflammatory arthritis; CACP syndrome patients have prominent synovial proliferation with normal synovial vascularity [28,29].
Fig. 4.
Hip radiograph showing coxa vara and acetabular cysts.
Some patients may present with pericarditis, occasionally associated with effusion requiring pericardiocentesis.
All these manifestations are due to noninflammatory process. Characteristically, the levels of inflammatory markers are normal. The synovial disease is described as proliferative synovium with hypercellularity by infiltrating macrophages with a contribution by proliferating fibroblastic synoviocytes [30]. CACP syndrome occurs because of a defect in the main surface lubricant for the joints and tendons; this defect is caused by different mutations in the proteoglycan 4 gene (PRG4), which is located on 1q25-31. Several pathogenic mutations have been reported in this gene. However, no reports have elucidated the genotype-phenotype association of this rare syndrome [31,32]. Because of multiple swollen joints and the presence of joint effusion, CACP syndrome may be diagnosed inaccurately as JIA, which causes a delay in the diagnosis and management [9,33].
Unfortunately, no effective treatment is available for this rare disorder. Most CACP syndrome patients, as they were incorrectly classified as having JIA, received traditional and biological disease–modifying antirheumatic drugs during their disease course, with no beneficial therapeutic results.
5. Progressive pseudorheumatoid dysplasia
Progressive pseudorheumatoid dysplasia (PPRD) is an autosomal recessive disorder characterized by progressive arthropathy involving the small peripheral joints. Patients usually present in early childhood with progressive stiffness and flexion contractions of the interphalangeal joints associated with metaphyseal bony overgrowth of the metacarpals and phalanges. However, other musculoskeletal features gradually develop with time, and patients have abnormal posture, disproportionate short stature, kyphoscoliosis and hyperlordosis, abnormal gait due to axial skeleton involvement, and hip joint deformity and stiffness [34,35].
PPRD is a rare skeletal disorder frequently diagnosed among Arabs; it is attributed to the loss of function in the WNT1-inducible signaling pathway protein 3 gene (WISP3), which is essential for normal growth and function of joint cartilage [[35], [36], [37]]. All musculoskeletal changes result without evidence of inflammation. Typically, the levels of inflammatory markers are within the normal range, and radiological findings show dysplastic rather than arthritic changes; the characteristic findings include epimetaphyseal expansion and platyspondyly (Fig. 5).
Fig. 5.
Bilateral flexion contractions of the interphalangeal joints with metaphyseal bony overgrowth. Hand radiograph showing epimetaphyseal expansion.
Although the joint involvement is noninflammatory in nature, PPRD is frequently misdiagnosed as JIA, particularly in the early stages of the disease [38].
Unfortunately, there is no cure for this disorder, and treatment is only supportive. Occasionally, patients require analgesics or anti-inflammatory drugs, especially patients with osteoarthritis-like pain due to joint degeneration and bony dysplasia.
6. Conclusion
JIA is the most common chronic childhood arthritis; it is a diagnosis of exclusion. Unfortunately, no diagnostic tool is available, but the comprehensive history, including family history, and complete physical examination are the most helpful tools in differentiating the various causes of articular disorders in children. Genetic musculoskeletal disorders that mimic chronic polyarthritis should be considered in the differential diagnostics of JIA. Normal inflammatory markers and characteristic radiological features are able to distinguish these disorders from JIA. However, molecular genetic findings are the confirmatory test. Timely diagnosis of these disorders is crucial to offer the family proper genetic counseling and avoid inappropriate therapy.
Footnotes
Peer review under responsibility of King Faisal Specialist Hospital & Research Centre (General Organization), Saudi Arabia.
References
- 1.Duffy C., Collbert R., Laxer R., Schanberg L., Boweyer S. Nomenclature and classification in chronic childhood arthritis. Arthritis Rheum. 2005;52:382–385. doi: 10.1002/art.20815. [DOI] [PubMed] [Google Scholar]
- 2.Petty R., Southwood T., Manners P., Baum J., Glass D., Goldenberg J. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision. J Rheumatol. 2004;31:390–392. Edmonton, 2001. [PubMed] [Google Scholar]
- 3.Ansell B. Rheumatic disease mimics in childhood. Curr Opin Rheumatol. 2000;12:445–457. doi: 10.1097/00002281-200009000-00017. [DOI] [PubMed] [Google Scholar]
- 4.Spencer C., Patwardhan A. Pediatric rheumatology for the primary care clinicians-recognizing patterns of disease. Curr Probl Pediatr Adolesc Health Care. 2015;45:185–206. doi: 10.1016/j.cppeds.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Athreya B., Schumacher R. Pathologic features of a familial arthropathy associated with congenital flexion contractures of fingers. Arthritis Rheum. 1998;21:429–437. doi: 10.1002/art.1780210405. [DOI] [PubMed] [Google Scholar]
- 6.Al-Mayouf S.M. Familial arthropathy in Saudi Arabian children: demographic, clinical and biochemical features. Semin Arthritis Rheum. 2007;36:256–261. doi: 10.1016/j.semarthrit.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Morishita K., Petty R. Musculoskeletal manifestations of mucopolyscchraidosis. Rheumatology. 2011;50:9–25. doi: 10.1093/rheumatology/ker397. [DOI] [PubMed] [Google Scholar]
- 8.Albuhairan I., Al-Mayouf S.M. Camptodactyly-arthropathy-coxa vara-pericarditis syndrome in Saudi Arabia: clinical and molecular genetic findings in 22 patients. Semin Arthritis Rheum. 2013;43:292–296. doi: 10.1016/j.semarthrit.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Offiah A., Woo P., Prieur A., Hansson N., Hall C. Camptodactyly-arthropathy-coxa vara-pericarditis syndrome versus juvenile idiopathic arthropathy. Am J Roentgenol. 2005;185:522–529. doi: 10.2214/ajr.185.2.01850522. [DOI] [PubMed] [Google Scholar]
- 10.Naranjo A., Muniain M., Martin J., Vazquez J., Nunez J. Primary idiopathic osteolysis: description of a family. Ann Rheum Dis. 1992;51:1074–1078. doi: 10.1136/ard.51.9.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardegger F., Simpson L., Segmueller G. The syndrome of idiopathic osteolysis. J Bone Joint Surg Br. 1985;67:88–93. doi: 10.1302/0301-620X.67B1.3968152. [DOI] [PubMed] [Google Scholar]
- 12.Pai G., Macpherson R. Idiopathic multicentric osteolysis: report of two new cases and a review of the literature. Am J Med Genet. 1988;29:929–936. doi: 10.1002/ajmg.1320290425. [DOI] [PubMed] [Google Scholar]
- 13.Goldfarb C., Steffen J., Whyte M. Idiopathic multicentric osteolysis: upper extremity manifestations and surgical considerations during childhood. J Hand Surg Am. 2012;37:1677–1683. doi: 10.1016/j.jhsa.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Farber M., Verlaak R., Fiselier T., Hamel B., Franssen M., Gerrits G. Inherited multicentric osteolysis with carpal-tarsal localization mimicking juvenile idiopathic arthritis. Eur J Pediatr. 2004;163:612–618. doi: 10.1007/s00431-004-1502-1. [DOI] [PubMed] [Google Scholar]
- 15.Castberg F., Kjaergaard S., Mosig R., Lobi M., Martignetti C., Martignetti J. Multicentric osteolysis with nodulosis and arthropathy (MONA) with cardiac malformation, mimicking polyarticular juvenile idiopathic arthritis: case report and literature review. Eur J Pediatr. 2013;172(12):1657–1663. doi: 10.1007/s00431-013-2102-8. [DOI] [PubMed] [Google Scholar]
- 16.Al-Mayouf S.M., Majeed M., Hugosson C., Bahabri S. New form of idiopathic osteolysis: nodulosis, arthropathy and osteolysis (NAO) syndrome. Am J Med Genet. 2000;93:5–10. doi: 10.1002/1096-8628(20000703)93:1<5::aid-ajmg2>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 17.Martignetti J., Aqeel A., Sewairi W., Boumah C., Kambouris M., Al-Mayouf S. Mutation of the matrix metalloproteinase 2 gene (MMP2) causes a multicenteric osteolysis and arthritis syndrome. Nat Genet. 2001;28:261–265. doi: 10.1038/90100. [DOI] [PubMed] [Google Scholar]
- 18.Al-Mayouf S.M., Madi S., Bin-Abbas B. Cyclic intravenous pamidronate treatment in children with nodulosis, arthropathy and osteolysis syndrome. Ann Rheum Dis. 2006;65:1672–1673. doi: 10.1136/ard.2005.035725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee S., Whitewood C., Murray K. Inherited multicentric osteolysis: case report of three siblings treated with bisphosphonate. Pediatr Rheumatol Online J. 2010 Apr 17;8:12. doi: 10.1186/1546-0096-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muenzer J. Overview of the mucopolysaccharidoses. Rheumatol. (Oxf.) 2011;50:4–12. doi: 10.1093/rheumatology/ker394. [DOI] [PubMed] [Google Scholar]
- 21.Khan S., Peracha H., Ballhausen D., Wiesbauer A., Rohrbach M., Gautschi M. Epidemiology of mucopolysaccharidoses. Mol Genet Metabol. 2017;121:227–240. doi: 10.1016/j.ymgme.2017.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cimaz R., La Torre F. Mucopolysaccharidoses. Curr Rheumatol Rep. 2014 Jan;16(1):389. doi: 10.1007/s11926-013-0389-0. [DOI] [PubMed] [Google Scholar]
- 23.Chan M., Sen E., Hardy E., Hensman P., Wraith E., Jones S. Assessment of musculoskeletal abnormalities in children with mucopolysaccharidoses using pGALS. Pediatr Rheumatol Online J. 2014 Aug 1;12:32. doi: 10.1186/1546-0096-12-32. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White K., Karol L., White D., Hale S. Musculoskeletal manifestations of Sanfilippo syndrome (mucopolysaccharidosis type III) J Pediatr Orthop. 2011;31:594–598. doi: 10.1097/BPO.0b013e31821f5ee9. [DOI] [PubMed] [Google Scholar]
- 25.Williams N., Challoumas D., Ketteridge D., Cundy P., Eastwood D. The mucopolysaccharidosis: advances in medical care lead to challenges in orthopedic surgical care. Bone Joint Lett J. 2017;99-B:1132–1139. doi: 10.1302/0301-620X.99B9.BJJ-2017-0487. [DOI] [PubMed] [Google Scholar]
- 26.Muenzer J. Overview of the mucopolysaccharidoses. Rheumatology. 2011;50:4–12. doi: 10.1093/rheumatology/ker394. [DOI] [PubMed] [Google Scholar]
- 27.Bahabri S., Suwairi W., Laxer R., Polinkovsky A., Dalaan A., Warman M. The camptodactyly-arthropathy-coxa vara-pericarditis syndrome: clinical features and genetic mapping to human chromosome 1. Arthritis Rheum. 1998;41:730–735. doi: 10.1002/1529-0131(199804)41:4<730::AID-ART22>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 28.Vutukuru R., Reddy K. Pathognomonic acetabular cysts in camptodactyly-arthropathy-coxa vara- pericarditis (CACP) syndrome. Indian J Med Res. 2016;143:834–835. doi: 10.4103/0971-5916.192082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Mutairi M., AlIsmeal K., Almulhem A., Azzam H., Al-Mayouf S.M. Utility of ultrasonography in children with camptodactyly-arthropathy-coxa vara- pericarditis syndrome. Ann Paediatr Rheumatol. 2013;2:107–112. [Google Scholar]
- 30.Shavan K., Ho M., Edwards V., Laxer R., Thorner P. Synovial pathology in camptodactyly-artrhopathy-coxa vara- pericarditis syndrome. Pediatr Dev Pathol. 2005;8:26–33. doi: 10.1007/s10024-004-3035-z. [DOI] [PubMed] [Google Scholar]
- 31.Alazami A., Al-Mayouf S.M., Wyngaard C., Meyer B. Novel PRG4 mutations underlie CACP in Saudi families. Hum Mutat. 2006;27:213–216. doi: 10.1002/humu.9399. [DOI] [PubMed] [Google Scholar]
- 32.Ciullini Mannurita S., Vignoli M., Bianchi L., Kondi A., Gerloni V., Breda L. CACP syndrome: identification of five novel mutations and of the first case of UPD in the largest European cohort. Eur J Hum Genet. 2014;22:197–201. doi: 10.1038/ejhg.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Madhusudan S., Gupta A., Prakash M., Matta D., Suri D., Singh S. Camptodactyly-arthropathy-coxa vara-pericarditis (CACP) syndrome: a mimicker of juvenile idiopathic arthritis. Scand J Rheumatol. 2016;45:77–78. doi: 10.3109/03009742.2015.1085085. [DOI] [PubMed] [Google Scholar]
- 34.Wynne-Davies R., Hall C., Ansell B. Spondylo-epiphysial dysplasia trada with progressive arthropathy. A ‘new’ disorder of autosomal recessive inheritance. J Bone Joint Surg Br. 1982;64:442–445. doi: 10.1302/0301-620X.64B4.6807993. [DOI] [PubMed] [Google Scholar]
- 35.Garcia Segarra N., Mittaz L., Campos-Xavier A., Bartels C., Tuysuz B., Alanay Y. The diagnostic challenge of progressive pseudorheumatoid dysplasia (PPRD): a review of clinical features, radiographic features, and WISP3 mutations in 63 affected individuals. Am J Med Genet C Semin Med Genet. 2012;160:217–229. doi: 10.1002/ajmg.c.31333. [DOI] [PubMed] [Google Scholar]
- 36.El-Shanti H., Omari H., Qubain H. Progressive pseudorheumatoid dysplasia: report of a family and review. J Med Genet. 1997;34:559–563. doi: 10.1136/jmg.34.7.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teebi A., Al Awadi A. Spondyloepiphyseal dysplasia tarda with progressive arthropathy: a rare disorder frequently diagnosed among Arabs. J Med Genet. 1986;23:189–191. doi: 10.1136/jmg.23.2.189-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ekbote A., Danda D., Kumar S., Danda S., Madhuri V., Gibikote S. A descriptive analysis of 14 cases of progressive-psuedorheumatoid-arthropathy of childhood from south India: review of literature in comparison with juvenile idiopathic arthritis. Semin Arthritis Rheum. 2013;42:582–589. doi: 10.1016/j.semarthrit.2012.09.001. [DOI] [PubMed] [Google Scholar]