Abstract
Background
Anaerobic meningitis is mainly caused by Bacteroides fragilis and it is rarely detected in children. Few cases have been reported and there is usually an underlying cause. The timing of early recognition is crucial because any delay in the diagnosis and initiation of appropriate antimicrobial therapy has a devastating outcome. Only 14 cases have been reported in 50 years. To the best of our knowledge, the present case is the first to be reported in Saudi Arabia with no underlying etiology.
Case presentation
We describe a 35-day-old male infant with culture-negative pyogenic meningitis who did not show satisfactory response to the empirical antibiotics, consequently, he developed severe subdural/epidural empyema and ventriculitis. When the drained empyema was cultured anaerobically, B. fragilis was detected and the patient improved after treatment with metronidazole combined with adjuvant surgical drainage of the empyema, and he finally had hydrocephalus. No underlying etiology was found to explain his infection.
Conclusion
B. fragilis is an uncommon cause of meningitis that requires a high index of clinical suspicion. Any pyogenic cerebrospinal fluid with negative culture should draw the attention of physicians to an unusual organisms such as anaerobes because early identification and initiation of appropriate antimicrobials can prevent long-term morbidity and mortality.
Keywords: Anaerobe, Bacteroides fragilis, Meningitis, Metronidazole, Saudi Arabia
1. Introduction
Bacteroides species are pleomorphic, non-spore-forming, facultative anaerobic Gram-negative bacilli. Bacteroides spp. are part of the normal flora of the mouth, gastrointestinal tract, and female genital tract, but they can cause infections as opportunists [1,2]. Endogenous infection can occur due to aspiration, bowel perforation, and damage to mucosal surfaces caused by surgery or chemotherapy [3]. Infections are usually polymicrobial [4]. Invasion of the bloodstream can lead to meningitis, endocarditis, and osteomyelitis. Neonatal infections including conjunctivitis, pneumonia, and meningitis are rare occurrences, where the predisposing factors include maternal chorioamnionitis, gastrointestinal sepsis or surgeries, congenital anomalies such as meningomyelocele, anorectal anomalies or fistulas, ventriculoperitoneal shunt infection, and upper respiratory infections, including otitis media.
Diagnosis is based on presumptive clinical suspicion and Gram staining findings, and the confirmation of diagnosis relies on culture under anaerobic conditions [4,5]. Bacteroides spp. can be reliably identified with new techniques such as 16S ribosomal ribonucleic acid gene sequencing [5]. Metronidazole, a beta lactam/lactamase inhibitor combination, and carbapenem are the preferred therapies [3,6,7]. Abscesses should be drained when feasible and necrotizing soft tissue should be debrided surgically. Cure rates of up to 80% have been reported [5].
In this study, we describe a case of B. fragilis meningitis in an infant from Saudi Arabia with severe complications. This report should alert physicians to this unusual treatable conditions in order to prevent long-term morbidity and mortality.
2. The case
A 35-day-old infant was admitted to King Fahad Medical City; Children Specialized Hospital with a history of fever for 7 days duration, which was low grade initially and treated only with an antipyretic. One day before admission, the fever became high grade, where it was associated with poor feeding and reduced activity, but no other constitutional symptoms. The infant is a product of full-term spontaneous vaginal delivery and he was admitted to the nursery for a few days as a case of neonatal jaundice, which was treated only with phototherapy. The maternal history included recurrent urinary tract infections (UTI), which were not documented as positive urine culture and they were treated with oral antibiotics. The group B streptococcus test result was not known for the mother. There was no history of maternal fever, prolonged rupture of membrane (PROM), or chorioamnionitis during the last trimester.
Examination upon admission revealed a sick-looking infant who was febrile, conscious, and alert. The anterior fontanelle was at the level and no focal neurological deficits were detected. A subsequent clinical examination obtained unremarkable findings.
2.1. Laboratory findings
Full blood count: white blood cell count (WBC) = 14.3 × 109, hemoglobin = 9.9 g/l, platelets = 677 × 109, C-reactive protein = 56 mg/l, erythrocyte sedimentation rate = 25 mm/hour.
The infant's renal profile and other chemistry findings were within normal limits.
A full septic workup was conducted and lumbar puncture detected cerebrospinal fluid (CSF), which was turbid in color, where WBC = 1641 cells and these were predominantly neutrophils (60%) and CSF glucose 1.8 mmol/l protein = 2.9 g/l. CSF culture was negative after three days. Blood culture and urine culture were also negative.
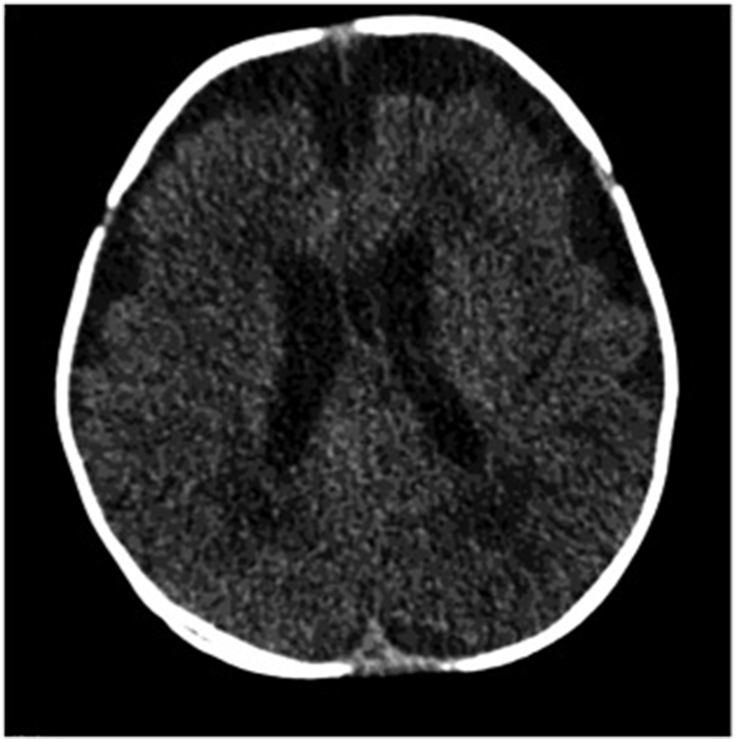
The infant was started empirically on ampicillin and cefotaxime. At 3 days after admission, his temperature continued to spike and frequent attacks of focal seizures started, so antiepileptic medication was provided. The Infectious Diseases (ID) service was consulted and they advised vancomycin and computed tomography, with suspicions of complicated meningitis, but no intracerebral collections or abnormal enhancement were detected Fig. 1.
Fig. 1.
No definite intracranial abnormality and no definite abnormal enhancement or intracranial collection.
Repeated lumbar puncture on day 7 determined: WBC = 4 cells, glucose <0.11 mmol/l, protein = 2.03 g/l, and negative culture again. The infant's condition did not show any improvement in terms of the fever pattern or seizures. Cefotaxime was replaced with meropenem according to advice from the ID service.
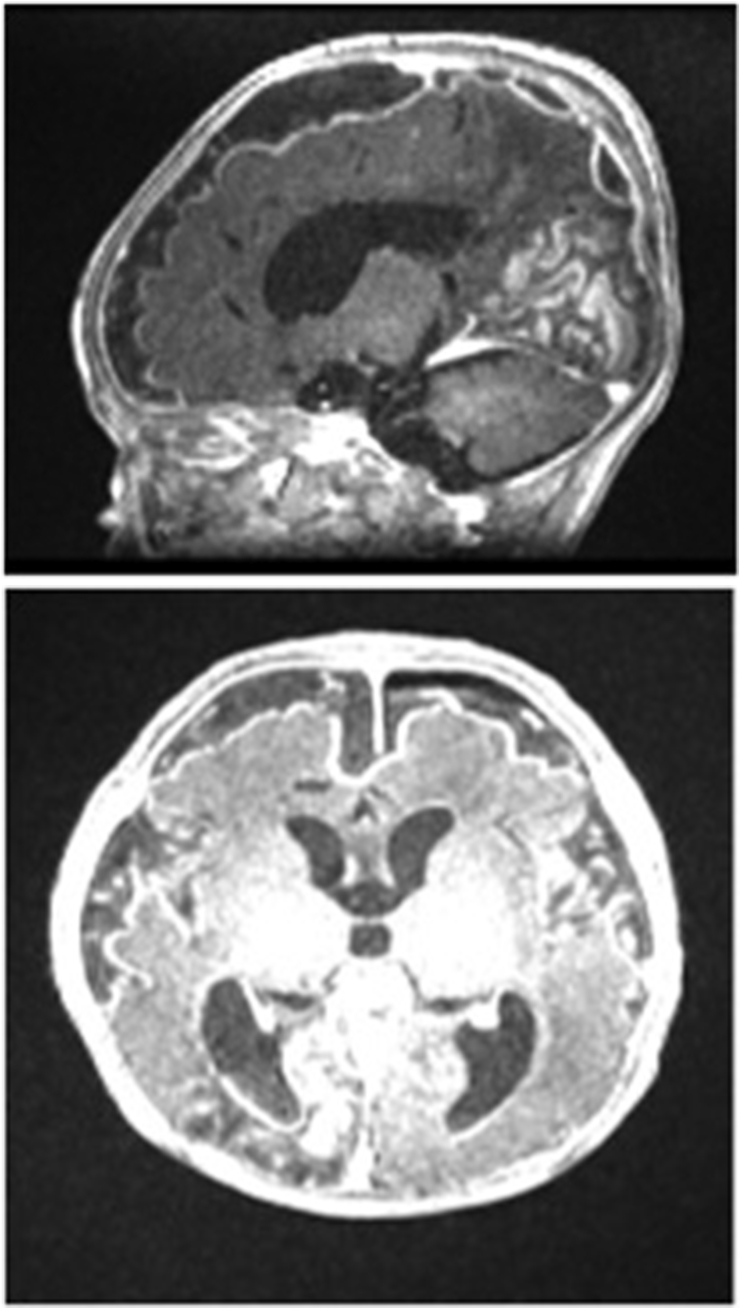
Magnetic resonance imaging was conducted on day 10, and extensive ventriculitis and subdural empyema were detected (Fig. 2).
Fig. 2.
Extensive bilateral subdural empyema along bilateral cerebral hemispheres and localized pockets of subdural empyema along the posterior fossa adjacent to the right sigmoid sinus as well as in the right posterior parasagittal region along the occipital lobe, and extensive ventriculitis with pus within the occipital horns of the bilateral lateral ventricles.
Empyema was drained by a neurosurgeon through a burr hole and pus culture again failed to isolate any organism. The infant remained afebrile for 5 days post-drainage, but his temperature spiked again with the recurrence of empyema. A second evacuation was planned plus insertion of external ventricular drainage. The ID team suggested sending the pus specimen in a blood culture bottle to increase the culture yield and to request anaerobic culture on this occasion. Two days later, the culture was positive for anaerobic Gram-negative bacilli and Bacteroides fragilis was identified. The isolated was metronidazole sensitive, beta lactamase positive, and intermediately sensitive to meropenem. Thus, metronidazole was added to the previous antibiotics (meropenem and vancomycin). After 1 week, the infant underwent a third evacuation of the septated empyema via craniotomy.
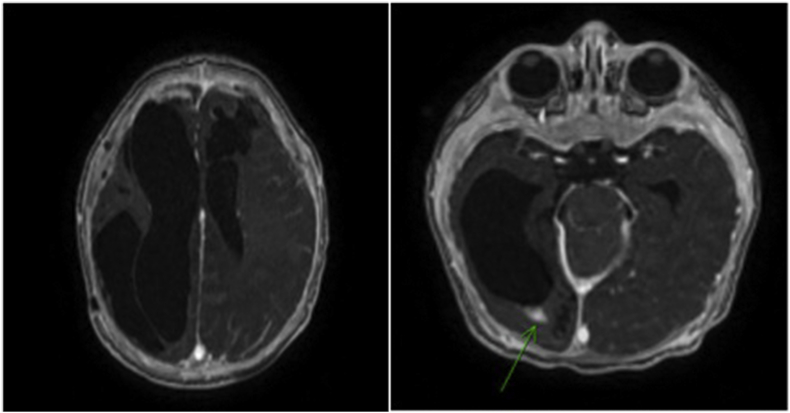
After starting metronidazole and the third evacuation, the fever subsided, activity improved, and the seizures were controlled. Antimicrobial therapy was continued for a total of 8 weeks. Repeated brain magnetic resonance imaging at the end of treatment indicated significant improvement with resolution of most of the extra-axial and subdural empyema. Only thick reactive dural enhancement, small remnant foci with ependymal enhancement at the occipital horn of the right lateral ventricle, and a large pro-encephalic cyst on the left frontal lobe remained, which resulted from ischemic changes in the posterior cerebral artery (Fig. 3) (see Fig. 4).
Fig. 3.
Overall improvement in the appearance of the brain with marked resolution of the multiple extra-axial subdural collections. Only thick dural enhancement could be seen along the calvarium. No foci of diffusion restriction were seen. The diffuse leptomeningeal enhancement was stable and probably reactive in nature. The persistent focus of enhancement in the occipital horn of the right lateral ventricle with no diffusion restriction probably represented persistent ventriculitis.
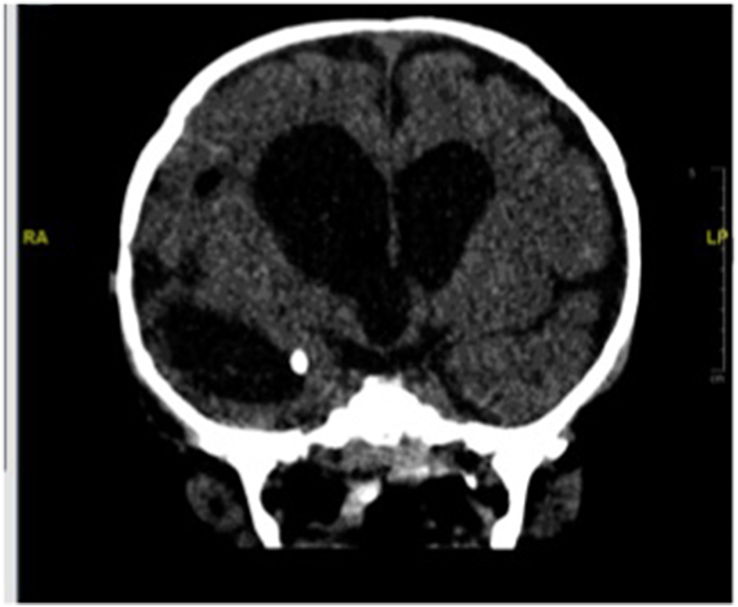
Fig. 4.
Ventriculoperitoneal shunt was inserted plus endoscopic fenestration of the cyst.
The infant was assessed by the immunology team to exclude any immunological disorders and his primary immune workup was unremarkable.
Finally, a ventriculoperitoneal shunt (V-P) was inserted plus endoscopic fenestration of the cyst (Fig. 4). A hearing assessment was conducted at the end of therapy and near normal hearing was found in both ears. The infant was discharged as stable and planned for follow-up by a multidisciplinary team, including those from infectious diseases, neurosurgery, neurology, physical therapy, and audiology subspecialties. He was seen regularly in planned clinics. At the age of 1 year, he passed all hearing assessments and his development was consistent with 9 months of age. He had hydrocephalus with a functioning V-P shunt.
3. Discussion
Subdural empyema, ventriculitis, and intracranial abscesses are the most common complications of meningitis caused by anaerobic organisms such as B. fragilis. Less than 100 cases of B. fragilis meningitis have been reported previously in adults and children [5,8]. Most of the reported cases were from the 1970s up to the 1990s, with few in the last decade, which might be explained by the widespread use of carbapenems such as meropenem (which obtained United States Food and Drug Administration approval in 1996) as empirical therapies for treating critically ill patients. Thus, the missed anaerobic cases were treated empirically and passed as undetected. Unfortunately, our reported case had intermediate sensitivity to meropenem, which led to the failure of empiric treatment. The underreporting of such cases might have contributed to their scarcity in previous studies.
Over a period of 40 years, only 20 cases of anaerobic meningitis were described in a review by Tarnvik in 1986, where eight of them were caused by B.fragilis. [9].
Similarly, Feder reviewed nine cases of B. fragilis meningitis reported before 1987, where seven of them were premature infants, two died, four ended with neurological complications, and only three survived with no sequelae [6].
Between 1963 and 2010, only 14 cases of B. fragilis meningitis were reported and nine (75%) had significant neurological sequelae [3,7].
In Saudi Arabia, the first case of B. fragilis was mentioned in a study performed during 1981 in a tertiary hospital in Riyadh, where the etiological causes of neonatal septicemia were described over a period of 4 years from 1976 to 1980 [10]. A premature neonate with PROM for more than 36 hours and birth asphyxia developed B. fragilis septicemia and meningitis. Based on previous reports, the current case is the second to be reported with B. fragilis meningitis and the first with no predisposing factors.
In most of the reported cases, an underlying etiology that predisposed toward B. fragilis infection was determined, such as abdominal sepsis and perforation, V-P shunt insertion [6], or post-meningocele repair [1]. By contrast, no clear focus of infection was identified in our case, i.e., no abdominal source of infection, no anatomical defects according to detailed clinical examinations, and no immunological disorders, and the only risk factor was the presumed maternal UTI.
However, anaerobic infections of the central nervous system still need to be considered, even in very young infants without predisposing factors, as described in some case reports.
Carapetis reported a similar case to our case, where an infant with B. fragilis meningitis and brain abscess presented with fever and convulsion. No clear origin of infection was found [7]. The second case reported by Law was a 12-year-old boy who was previously healthy, but developed anaerobic meningitis complicated by brain abscess caused by Streptococcus species, and no primary source was identified based on repeated evaluations [8].
Infections are sometimes polymicrobial. Thus, Ganeshalingham described a case with Escherichia coli meningitis who failed to respond to antibiotics, but the culture-negative purulent CSF alerted them to the presence of an anaerobic infection, thereby prompting a search for poly-microbial meningitis. Unfortunately, the infant passed away and B. fragilis was isolated 3 days post-death [4]. In another tragedy, Odugbemi reported a 6-year-old boy with chronic otitis media who was admitted with meningitis and ear discharge. He had pyogenic, culture-negative CSF, and was positive for Providencia rettgeri from the ear. He was treated with cefotaxime and gentamicin but with no response. On day 13, a second CSF was obtained and cultured anaerobically, which yielded B. fragilis, so he was started on metronidazole but unfortunately died on the second day after starting on metronidazole [11].
Therapy should be broadened to cover other polymicrobial possibilities when detecting anaerobes. In our case, despite the detection of B. fragilis, treatment with meropenem and vancomycin continued but with the addition of metronidazole.
The timing of early recognition and initiation of appropriate therapy is crucial for preventing complications. Most cases ended with hydrocephalus and V-P shunt insertion even after starting treatment with metronidazole, which is similar to our case scenario.
By contrast, early detection of the organism and the initiation of metronidazole lead to full recovery with no significant morbidity or mortality. Law reported a preterm neonate with a B. fragilis scalp infection and meningitis who was successfully treated by the early administration of metronidazole and no neurological disorders were noted on follow-up [12]. This case emphasizes the importance of keeping a high index of clinical suspicion in such cases because CSF is not routinely cultured for anaerobes.
In terms of management, all previous studies agreed on metronidazole as the drug of choice because of its bactericidal effect, and the fact that it reliably penetrates CSF and abscesses [8,[12], [13], [14]]. Other treatment options include carbapenems or a beta lactam/beta lactamase inhibitor combination. Carbapenems are highly effective with only 1.1–2.5% resistance by B. fragilis in a multicenter survey conducted in the United States, where only one case was found to be resistant to metronidazole [15]. A Canadian surveillance study reported 99.7% susceptibility to metronidazole and 97.7% to imipenem [16]. The duration of treatment is usually 14 days for uncomplicated meningitis but longer for ventriculitis or abscesses at up to 6–8 weeks, and ideally until complete resolution of the abscess contents.
The outcome is usually variable if anaerobes are not identified early. In a review, Tarnvik reported that 25% died, 30% recovered entirely, and the remainder ended with significant sequelae. The anaerobic culture of CSF should be considered if no growth occurs in aerobic culture, particularly if accompanied by an inadequate response to standard empirical therapy for meningitis, or if meningitis occurs as a complication of otitis media or sinusitis or in a compromised newborn [9].
Mortality and long-term morbidity can be reduced through early recognition and the initiation of appropriate therapy combined with necessary surgical interventions.
4. Conclusion
Anaerobic meningitis is rare but it seems to be underdiagnosed and it should be considered in the differential diagnosis when faced with an unusual presentation or inconclusive microbiological results, such as unexplained culture-negative pyogenic meningitis.
Ethical approval
This case report was ethically approved by institutional review board – KFMC.
IRB log Number: 16-376.
Competing interests
No conflict of interest declared.
Authors' contributions
All authors contributed equally to this case management and report writing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgment
D. Mutaz Abdelbagi H. Mohammed.
Footnotes
Peer review under responsibility of King Faisal Specialist Hospital & Research Centre (General Organization), Saudi Arabia.
References
- 1.Goyo-Rivas J., Salgar N.T., Pulido O., Urdaneta M.T., Torres E. Meningitis caused by Bacteroides fragilis in children. Bol Med Hosp Infant Mex. 1989 Nov;46(11):724–727. [PubMed] [Google Scholar]
- 2.Parmar M.S. Pneumocephalus associated with Bacteroides fragilis meningitis. J Postgrad Med. 2004;50(4):272. [PubMed] [Google Scholar]
- 3.Ziebold C. Delayed etiologic diagnosis of meningitis in an extremely low birth weight newborn. Pediatr Infect Dis J. 2010;29(4):383–388. doi: 10.1097/INF.0b013e3181d58216. [DOI] [PubMed] [Google Scholar]
- 4.Ganeshalingham A., Buckley D., Shaw I., Freeman J.T., Wilson F., Best E. Bacteroides fragilis concealed in an infant with Escherichia coli meningitis. J Paediatr Child Health. 2014 Jan 1;50(1):78–80. doi: 10.1111/jpc.12394. [DOI] [PubMed] [Google Scholar]
- 5.Ramakrishnan S., Krishnan P., Chandra P.S., VeenaKumari H.B., Nagarathna S. Pneumocephalus in mixed aerobic and anaerobic (Bacteroides fragilis) meningitis. Indian J Pathol Microbiol. 2014 Jan 1;57(1):160. doi: 10.4103/0377-4929.130940. [DOI] [PubMed] [Google Scholar]
- 6.Feder H.M., Jr. Bacteroides fragilis meningitis. Rev Infect Dis. 1987;9(4):783–786. doi: 10.1093/clinids/9.4.783. [DOI] [PubMed] [Google Scholar]
- 7.Carapetis J., Anderson K., McLellan J., Grimwood K. An infant with fever and convulsions. Eur J Pediatr. 1996 Jun 30;155(6):517–518. doi: 10.1007/BF01955194. [DOI] [PubMed] [Google Scholar]
- 8.Law D.A., Aronoff S.C. Anaerobic meningitis in children: case report and review of the literature. Pediatr Infect Dis J. 1992;11(11):968–971. [PubMed] [Google Scholar]
- 9.Tärnvik A. 1986. Anaerobic meningitis in children; pp. 271–274. [DOI] [PubMed] [Google Scholar]
- 10.Ohlsson A., Serenius F. Neonatal septicemia in Riyadh, Saudi Arabia. Acta Paediatr. 1981;70(6):825–829. doi: 10.1111/j.1651-2227.1981.tb06234.x. [DOI] [PubMed] [Google Scholar]
- 11.Odugbemi T., Jatto S., Afolabi K. Bacteroides fragilis meningitis. J Clin Microbiol. 1985;21(2):282–283. doi: 10.1128/jcm.21.2.282-283.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Law B.J., Marks M.I. Excellent outcome of Bacteroides meningitis in a newborn treated with metronidazole. Pediatrics. 1980;66(3):463–465. [PubMed] [Google Scholar]
- 13.CCL N. Bacteroides fragilis meningitis. Singap Med J. Jun 1994;35(3):283–285. [PubMed] [Google Scholar]
- 14.Berman B.W., King F.H., Jr., Rubenstein D.S., Long S.S. Bacteroides fragilis meningitis in a neonate successfully treated with metronidazole. J Pediatr. 1978;93(5):793–795. doi: 10.1016/s0022-3476(78)81080-4. [DOI] [PubMed] [Google Scholar]
- 15.Snydman D.R., Jacobus N.V., McDermott L.A., Golan Y., Goldstein E.J., Harrell L. Update on resistance of Bacteroides fragilis group and related species with special attention to carbapenems 2006–2009. Anaerobe. 2011 Aug 1;17(4):147–151. doi: 10.1016/j.anaerobe.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 16.Karlowsky J.A., Walkty A.J., Adam H.J., Baxter M.R., Hoban D.J., Zhanel G.G. Prevalence of antimicrobial resistance among clinical isolates of Bacteroides fragilis group in Canada in 2010–2011: CANWARD surveillance study. Antimicrob Agents Chemother. 2011 Mar Dec 27;56(3):1247–1252. doi: 10.1128/AAC.05823-11. AAC–05823. [DOI] [PMC free article] [PubMed] [Google Scholar]