Abstract
There was no statistically significant difference between amoxicillin and nitrofurantoin for the treatment of ampicillin-resistant Enterococcus faecium urinary tract infections.
Enterococcus species account for about 110,000 urinary tract infections (UTIs) annually in the U.S.1 The most common species isolated are Enterococcus faecalis and Enterococcus faecium (E faecium). Amoxicillin is the drug of choice for the treatment of enterococcal UTIs. Second-line therapies include vancomycin and nitrofurantoin. Alternative therapies include daptomycin and linezolid; however, these newer agents ideally would be reserved for more serious infections to preserve activity.2
Increased E faecium resistance to ampicillin and vancomycin has limited the therapeutic options. The results of a study by Zhanel and colleagues assessed the prevalence of resistant enterococcal urine isolates in North America.3 Of the 658 E faecium urine isolates, about 96% were resistant to ampicillin and 94% were resistant to vancoymcin.3 Nitrofurantoin has much lower resistance rates; however, its use is contraindicated in patients with a creatinine clearance (CrCl) < 60 mL/min.4 Data supporting the contraindication are limited, but the results of a study by Oplinger and Andrews suggested that using nitrofurantoin in patients with a CrCl ≥ 40 mL/min may be safe and effective.5 A therapeutic dilemma may occur when resistant E faecium UTIs are encountered and viable treatment options are limited due to intolerances, administration difficulties, lack of susceptibility data, or cost.
Based on the current Clinical and Laboratory Standards Institute standard, Enterococcus species with a minimal inhibitory concentration (MIC) ≥ 16 μg/mL are considered ampicillin resistant. Microbiology laboratories use the same breakpoint regardless of the site of infection.6 Amoxicillin concentrates in the urine; therefore, urinary concentrations are much higher than serum concentrations. The mean serum peak concentration after a single dose of oral amoxicillin 500 mg is 7.6 μg/mL.7 After a single dose of oral amoxicillin 500 mg, the average concentration in pooled urine collected over 6 hours was 1,100 μg/mL.8
In 2002, Williamson and colleagues analyzed 30 ampicillin-resistant E faecium urine isolates. Reported MICs were 128 μg/mL (30%), 256 μg/mL (60%), and 512 μg/mL (10%).9 A more recent retrospective analysis analyzed 234 ampicillin-resistant E faecium urine isolates. The MIC ranged from 32 to 1,024 μg/mL, with a median MIC of 256 μg/mL. Only 5 isolates had an MIC value > 1,000 μg/mL, but each of these isolates was within 1 dilution of 512 μg/mL.10 Because penicillins exhibit time-dependent killing, an optimal response will occur as long as the urine concentration is above the MIC for at least 50% of the dosing interval. 11 Therefore, therapeutic doses of amoxicillin are expected to produce urine concentrations that exceed the MIC of resistant E faecium urine isolates. The purpose of this study was to determine if amoxicillin was a viable treatment option for ampicillin-resistant E faecium UTIs based on this in vitro theory.
METHODS
Veterans aged ≥ 18 years with a positive urine culture for ampicillin-resistant E faecium who received antibiotic therapy for cystitis at the Jesse Brown VA Medical Center (JBVAMC) from January 1, 2005, through June 22, 2010, were evaluated in this retrospective cohort study. Exclusion criteria were the presence of any other organisms in the initial urine culture, prostatic involvement, and the presence of E faecium in a blood culture. Subjects treated with multiple antibiotics concurrently and with sequential treatment of different antibiotics with no evaluation of efficacy between courses were also excluded.
All included subjects were evaluated for resolution of symptoms; improvement in leukocyte esterase count and white blood cell (WBC) count from urine analysis (UA); and eradication of E faecium from a repeat urine culture. The response to treatment was classified as cure, presumed cure, or failure. The criteria for cure were based on the following: resolution of symptoms if present at baseline; repeat UA indicating improvement from the initial positive UA (if obtained); and eradication of E faecium in a repeat urine culture (if obtained).
At least 1 of the aforementioned criteria must have been met to be classified as cure. If more than 1 of the aforementioned criteria was present, then each one must have been met to be classified as cure. To be evaluated for presumed cure, the subject must have had symptoms at baseline. No documentation of ongoing symptoms in subjects who had an appropriate follow-up but did not have a repeat UA or urine culture indicated presumed cure. Persistence or worsening of pretreatment symptoms, a repeat UA without improvement from the initial positive UA, or a repeat urine culture demonstrating continued presence of E faecium indicated failure. The primary endpoint for the study was to determine whether amoxicillin was effective for the management of ampicillin-resistant E faecium UTIs. This study was conducted in compliance with the University of Illinois at Chicago Institutional Review Board and JBVAMC Human Subjects Research Committee requirements.
RESULTS
This study included 20 positive urine cultures for ampicillin-resistant E faecium in 19 subjects. Nine cases were treated with amoxicillin, and 11 cases were treated with nitrofurantoin. At baseline, the mean age was 75 years, mean duration of therapy was 14 days, and all the subjects were male. The baseline characteristics of the 2 groups were similar with the exception of an older population, shorter duration of therapy, and increased incidence of chronic kidney disease in the amoxicillin treatment group, P = .02, .03, and .01, respectively.
Symptoms were documented in 8 of 9 (89%) cases at the time of the positive culture in the amoxicillin treatment group and 5 of 11 (45%) cases in the nitrofurantoin treatment group (Table). The asymptomatic amoxicillin treatment group case and 5 of the 6 nitrofurantoin treatment group asymptomatic cases received treatment prior to a urologic procedure in accordance with the Infectious Diseases Society of America (IDSA) guidelines for the treatment of asymptomatic bacteriuria. The urologic procedures included transurethral resection of a bladder tumor, cystoscopy, urethral dilation, cystometrogram, and transurethral resection of the prostate. One asymptomatic subject in the nitrofurantoin group did not have any documentation to support an appropriate indication for treatment. All positive cultures were > 100,000 colonies/mL except for 1 culture in the nitrofurantoin treatment group, which was 45,000 colonies/mL, but because the subject was symptomatic, treatment was administered and a repeat urine culture was negative.
Table.
Baseline Characteristics of Subjects Included Per Protocol
| Amoxicillin 500 mg tid, n (%)a,b | Nitrofurantoin 100 mg bid, n (%)c,d | P value | |
|---|---|---|---|
|
| |||
| Age | 82 ± 4 years | 74 ± 9 years | .02 |
|
| |||
| Gender | 100% male | 100% male | |
|
| |||
| Duration of therapy | 9 ± 3 days | 13 ± 6 days | .03 |
|
| |||
| Benign prostatic hyperplasia | 7 (78) | 8 (73) | .01 |
|
| |||
| Chronic kidney disease (stage 3, 4, or 5) | 9 (100) | 5 (45) | .01 |
|
| |||
| Complicating factors | 3 (33) | 4 (36) | .99 |
| Foley catheter | 2 | 2 | |
| Urethral/prostatic stent | 0 | 0 | |
| Kidney stones | 0 | 1 | |
| Nephrostomy tube | 0 | 0 | |
| Neurogenic bladder | 1 | 1 | |
| Ileal conduit | 0 | 0 | |
|
| |||
| Treatment | |||
| Symptomatic bacteriuria | 8 (89) | 5 (45) | |
| Asymptomatic bacteriuria | 1 (11) | 6 (55) | |
One subject was dosed at amoxicillin 500 mg bid.
N = 9.
Two subjects were dosed at 100 mg qid.
N = 11.
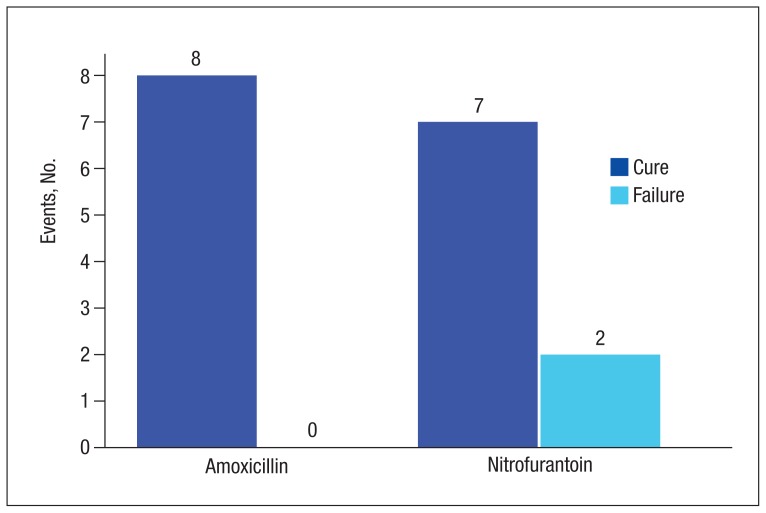
There were 8 cases classified as cure, 1 presumed cure, and no failures in the amoxicillin group. In the nitrofurantoin group, 7 cases were classified as cure, 1 presumed cure, and 3 failures. The presumed cures were excluded from the statistical analysis due to inability to ensure these cases were truly cured. Also excluded from the statistical analysis was one of the failures in the nitrofurantoin group, because the subject was asymptomatic with no known indication for treatment. This left 8 cases classified as cure and no failures in the amoxicillin group compared with 7 cases classified as cure and 2 failures in the nitrofurantoin group, P = .47 (Figure). Statistical analysis was performed using the Fisher exact test.
Figure.
Urinary Tract Infection Treatment Resultsa
aP = .47.
DISCUSSION
There was no statistically significant difference between amoxicillin and nitrofurantoin for the treatment of ampicillin-resistant E faecium UTIs. There were no failures in the amoxicillin group despite all isolates displaying resistance based on current breakpoints, supporting the theory that higher urine concentrations of amoxicillin may overcome the MIC of resistant isolates.
Of the 11 cases treated with nitrofurantoin, 3 were classified failures. The first failure in the nitrofurantoin group was an asymptomatic subject who did not have a repeat urine culture but had a repeat UA, which showed a persistent elevation in WBC and leukocyte esterase count. This subject was removed from the statistical analysis, as treatment was not indicated per IDSA guidelines. No reason could be identified for the second failure, as a repeat culture demonstrated continued presence of E faecium. Chronic kidney disease (CKD) contributed to the third failure in the nitrofurantoin treatment group; the subject’s CrCl was about 17 mL/min. After treatment, the subject had a repeat urine culture, which indicated the continued presence of E faecium. The subject was later successfully treated with amoxicillin. Both cultures in the same subject were included in the final analysis per protocol, as the subject had an adequate evaluation of efficacy between courses. Four additional cases with CKD were treated with nitrofurantoin; however, their CrCl ranged from 40 to 55 mL/min, and all were classified cure or presumed cure.
LIMITATIONS
There were several limitations to this study. Due to the strict inclusion and exclusion criteria, a limited number of subjects were evaluated. Given that this was a retrospective study, it is possible that symptoms were reported by a subject but not appropriately documented. Another significant limitation of this trial was that MICs were not determined due to the retrospective nature of the study. External validity was also limited due to a predominately elderly and male population. Safety data regarding different therapies were not collected, as this study evaluated only the efficacy of therapies.
CONCLUSION
Although this was a very small retrospective analysis, to the authors knowledge this is the first clinical study supporting the in vitro theory that amoxicillin (500 mg every 8 hours) may overcome the MIC of resistant isolates due to achievement of higher urinary concentrations. Because this was a small retrospective analysis, more prospective evidence is needed to confirm these results.
Acknowledgements
Heather Kim, biostatistician, University of Illinois at Chicago. CCTS Support: UL1RR029879.
Footnotes
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
REFERENCES
- 1.Huycke MM, Sahm DF, Gilmore MS. Multipledrug resistant enterococci: the nature of the problem and an agenda for the future. Emerg Infect Dis. 1998;4(2):239–249. doi: 10.3201/eid0402.980211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heintz BH, Halilovic J, Christensen CL. Vancomycin-resistant enterococcal urinary tract infections. Pharmacotherapy. 2010;30(11):1136–1149. doi: 10.1592/phco.30.11.1136. [DOI] [PubMed] [Google Scholar]
- 3.Zhanel GG, Laing NM, Nichol KA, et al. NAVRESS Group. Antibiotic activity against urinary tract infection (UTI) isolates of vancomycinresistant enterococci (VRE): results from the 2002 North American Vancomycin Resistant Enterococci Susceptibility Study (NAVRESS) J Antimicrob Chemother. 2003;52(3):382–388. doi: 10.1093/jac/dkg352. [DOI] [PubMed] [Google Scholar]
- 4.Macrobid [package insert] Pine Brook, NJ: Almatica Pharma; 2013. [Google Scholar]
- 5.Oplinger M, Andrews CO. Nitrofurantoin contraindicated in patients with a creatinine clearance below 60 mL/min: looking for the evidence. Ann Pharmacother. 2013;47(1):106–111. doi: 10.1345/aph.1R352. [DOI] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Seventeenth Informational Supplement M100-S17. Wayne, PA: Clinical and Laboratory Standards Institute; 2007. [Google Scholar]
- 7.Gordon RC, Regamey C, Kirby WM. Comparative clinical pharmacology of amoxicillin and ampicillin administered orally. Antimicrob Agents Chemother. 1972;1(6):504–507. doi: 10.1128/aac.1.6.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sutherland R, Croydon EA, Rolinson GN. Amoxycillin: a new semi-synthetic penicillin. Br Med J. 1972;3(5817):13–16. doi: 10.1136/bmj.3.5817.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williamson JC, Craft DW, Butts JD, Raasch RH. In vitro assessment of urinary isolates of ampicillin-resistant enterococci. Ann Pharmacother. 2002;36(2):246–250. doi: 10.1345/aph.1A085. [DOI] [PubMed] [Google Scholar]
- 10.Dumkow LE, Perri MB, Zervos M. Time to stop using alternatives to ampicillin for enterococcal UTIs? In-vitro susceptibility trends for enterococcus urinary isolates over a one-year period in Detroit. Poster presented at: 53rd Interscience Conference of Antimicrobial Agents and Chemotherapy (ICAAC); September 10–13, 2013; Denver, CO. [Google Scholar]
- 11.Quintiliani R. Using pharmacodynamics and pharmacokinetics concepts to optimize treatment of infectious diseases. Infect Med. 2004;21(5):219–232. [Google Scholar]