Abstract
Deployment in southwest Asia is associated with a wide range of respiratory disorders related to tobacco use and to workplace and environmental exposures. Physicians should carefully consider deployment history when assessing and treating veterans with lung disorders.
Military deployed from World War II through the Vietnam War have had enough time for respiratory disorders with both short and long latencies to manifest. More recent deployments over the past 13 years to Iraq, Kuwait, Afghanistan, and other regions in southwest Asia (SWA) have been associated with a unique spectrum of respiratory disorders. The long-term respiratory effects of SWA deployments are unknown. This review will discuss deployment-related lung cancer and then focus primarily on the emerging respiratory disorders related to SWA deployment and case examples of deployment-related lung disease.
As the number of recent veterans in the VA health care system increases, primary care providers (PCPs) and specialists are increasingly faced with questions about potential hazards of deployment, referring patients to the VA Airborne Hazards and Open Burn Pit Registry, and evaluating patients with new-onset respiratory symptoms following deployment. Previous reviews and white papers have offered recommendations for evaluation and management; however, little has been reported in the form of case examples of patients with deployment-related lung disorders and their clinical course.1,2
DEPLOYMENT-ASSOCIATED LUNG CANCER
Lung cancer is the leading cause of cancer death in the U.S. and around the world.3 Lung cancer in the U.S. causes more deaths than does the combination of breast, prostate, colon, and rectal cancers. Lung cancer is the second most common cancer and causes more deaths than does any other cancer in the VHA.4 Most cancers with an environmental cause have a significant latent period of decades between the exposure and cancer incidence. Thus, although lung cancer risk is relatively low in active-duty military personnel, the rate of lung cancer in VA patients is nearly double that of the general population, suggesting causes associated with military service.5
Tobacco
The main cause of lung cancer is tobacco smoking, which accounts for 85% to 90% of lung cancer in the U.S. The latent period between initiation of tobacco smoking and lung cancer incidence is typically ≥ 30 years. Military service has long been associated with tobacco smoking, due to past practices that included the provision of free cigarettes, the availability of cigarettes at reduced cost, smoking breaks, perceived relief from both stress and boredom, and social factors.6 More recently, the adverse effects (AEs) of smoking on health and readiness have been appreciated, and many incentives encouraging tobacco smoking have been eliminated. In 2009, the Institute of Medicine called for a tobacco-free military, and both the Secretary of the Navy and Secretary of Defense have seriously considered this change.7
The additional effect of deployment on smoking has been reported.8 The longitudinal Millennium Cohort study compared several smoking measures between 55,021 deployers and nondeployers who completed both baseline (acquired July 2001–June 2003) and follow-up questionnaires (acquired June 2004–January 2006). Smoking initiation affected 2.3% of deployers and 1.3% of nondeployers; smoking resumption showed a similar pattern with an increase of 39.4% compared with 28.7%. The overall prevalence of smoking increased 44% among nondeployers and 57% among deployers. Those never smokers exposed to combat were 60% more likely to initiate smoking compared with noncombat deployers. Thus, it is clear that tobacco smoking should be considered a deployment-related exposure that contributes to lung cancer risk.
Asbestos
In 1955, Doll published an analysis associating asbestos exposure with risk for lung cancer.9 Many naval veterans and shipyard workers had asbestos exposure, resulting in a spectrum of asbestos-related diseases, including bronchogenic cancer.10
Depleted Uranium
Depleted uranium was used in munitions during the first Gulf War and more recently during military operations in SWA as a part of Operation New Dawn (OND), Operation Iraqi Freedom (OIF), and Operation Enduring Freedom (OEF). Because of concerns of military personnel having complex exposure to depleted uranium, including via inhalation, the VA established the Depleted Uranium Surveillance Program, which has followed a cohort of service members exposed to inhaled depleted uranium during friendly fire in 1991. No significant differences between individuals with high urinary uranium levels and low urinary uranium levels were found in self-reported respiratory symptoms and pulmonary function testing (PFT). Additionally, 20 years after exposure to depleted uranium, there was no statistically significant difference of low-dose chest computed tomography (CT) evidence of lung cancer in these 2 groups.11
Mustard Gas
Mustard gas is considered a definite lung carcinogen.12,13 Both long-term, low-dose and short-term, high-intensity exposures are known to cause human lung cancer.14 Mustard gas was first widely used in warfare in World War I. Mustard gas was used in training for World War II; training accidents resulted in acute toxicity even in lower exposures. It was later used as a chemical warfare agent in the Iran-Iraq conflict in the late 1980s and early 1990s. It is estimated that about 4,000 U.S. service members have been acutely exposed to high concentrations of mustard gas. Sulfur mustard may be incorporated into improvised explosive devices, and there is concern that troops in Iraq have been exposed to this agent in sites previously used for manufacturing and storage.15
Agent Orange
The herbicide Agent Orange is commonly contaminated with dioxin, which has been demonstrated to be a tumor promoter in animal studies. Agent Orange was used widely in the Vietnam War. The National Academy of Sciences issued a report in 2001 reviewing evidence for a link between Agent Orange and various neoplasms. Evidence was strongest for Hodgkin lymphoma and soft tissue sarcoma. The evidence of an association between Agent Orange exposure and lung cancer was deemed only suggestive.16
RESPIRATORY DISEASE ASSOCIATED WITH SOUTHWEST ASIA DEPLOYMENT
Over the past 14 years, > 2.5 million U.S. military personnel and civilian contractors have been deployed as part of 3 major military operations: OEF in Afghanistan (2001 to present), OIF in Iraq (2003 to 2010), and OND in Iraq (2010 to present).17,18 Deployed personnel encounter a wide variety of inhalational exposures that include desert dust particulate matter, burn pit combustion products, environmental tobacco smoke, vehicular diesel exhaust, debris from detonations and explosions, and other unique or specific job-related exposures (Table 1).19,20
Table 1.
|
A number of recent studies have helped identify and characterize an emerging spectrum of deployment-related lung disorders, including asthma, rhinosinusitis, emphysema, bronchiolitis, granulomatous pneumonitis, and less common conditions such as acute eosinophilic pneumonia and rapidly progressive pulmonary fibrosis (Table 2).20–30 Still, diagnosis of these conditions is often challenging, and traditional diagnostic tools such as PFT and chest radiography may be normal or mildly abnormal despite significant histopathologic abnormalities on surgical lung biopsy.24,30,31
Table 2.
|
Deployment-Related Exposures
As listed in Table 1, there are a number of other exposures that may be encountered during deployment. Environmental air sampling was conducted in several locations in Iraq, Afghanistan, and sites in SWA as part of the Enhanced Particulate Matter Survey. All sites were notable for air pollutant levels that exceeded 15 μg/m3, the military exposure guideline for fine particulate matter (PM2.5). The PM2.5 fraction comprised geologic dust, burn pit emissions, and the heavy metals aluminum, cadmium, and lead.32,33
Respiratory Disorders
Reports of deployers with respiratory symptoms during and after deployment surfaced as early as 2004.34 The Millennium Cohort study reported a 1.7-fold higher rate of new-onset respiratory symptoms that was independent of smoking status, such as cough and shortness of breath, in deployers compared with nondeployers. These increased symptom rates were associated with land-based deployment and longer deployment duration.35 A number of epidemiologic studies also demonstrated an association between respiratory symptoms and environmental exposures encountered during deployment.36–39
Respiratory diseases such as asthma, acute eosinophilic pneumonia, and constrictive bronchiolitis have been reported following deployment to SWA, but a review of the literature supports a more expansive list of deployment-related respiratory diseases (Table 2).20–30 The following case examples describe findings in veterans referred to the authors’ clinic for evaluation of chest symptoms associated with deployment.
OEF/OIF/OND CASE STUDIES
Case Study 1
A 42-year-old male never smoker presented to his VA PCP for evaluation of nonproductive cough, dyspnea on exertion, chest tightness, and recurrent episodes of bronchitis since 2004 when he was deployed to Afghanistan. He had no history of asthma or other chronic respiratory disease in childhood or adolescence.
The patient served as a Civil Affairs officer in the U.S. Army and was deployed to Bosnia in 1997, Afghanistan in 2004, and Camp Arif- Jan in Kuwait as well as Mosul, Iraq, in 2005. He was exposed to depleted uranium while serving in Bosnia. He also had exposures to sandstorms, desert dust, and burn pit combustion products while deployed to Afghanistan and Iraq. He developed symptoms of chest tightness and dyspnea on exertion during his 2004 deployment, with these symptoms persisting after returning home from deployment. His symptoms occurred frequently while running and limited his ability to pass his military physical fitness test requirements and train for marathons as he had done previously. He also had symptoms of chest tightness and excessive coughing at rest, which were treated with antibiotics by his medical provider as recurrent acute infectious/viral bronchitis.
The patient was medically discharged from the U.S. Army in July 2005, primarily due to musculoskeletal injuries. His past medical history was notable for PTSD, recurrent allergic rhinosinusitis, and lumbosacral back pain. Given persistent respiratory symptoms of dyspnea after walking 1 block, the patient presented to his VA PCP in early 2006.
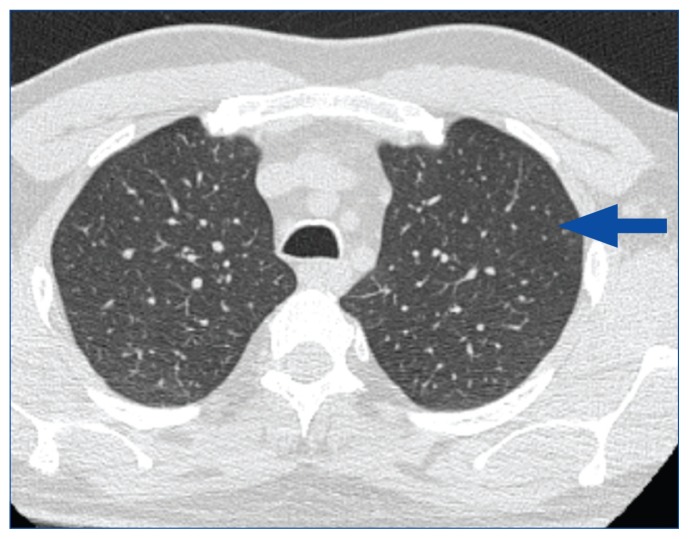
The patient’s vital signs and physical examination were normal. Spirometry showed a mixed restrictive and obstructive pattern, prompting referral for pulmonary consultation. Full PFT demonstrated an abnormally increased residual volume and mildly decreased diffusion capacity (Table 3). Laryngoscopy was negative for vocal cord dysfunction. A chest X-ray showed mild airway wall thickening bilaterally in the lower lung fields. Subsequent high-resolution CT of the chest demonstrated diffuse centrilobular nodularity (Figure 1). Serial spirometry measurements over 8 months showed severe and worsening airflow limitation despite treatment with inhaled bronchodilator and corticosteroid therapy. Seeking diagnostic clarity, the patient was referred for surgical lung biopsy via video-assisted thorascopic surgery (VATS) within 6 months of initial consultation.
Table 3.
Case Study 1: Pulmonary Function Testing
Prebronchodilator spirometry
|
Lung volumes
|
Diffusion testing
|
Abbreviations: DLCO, diffusing capacity of the lung for carbon monoxide; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity.
Figure 1.
Case Study 1: High-Resolution Computed Chest Tomography Demonstrating Diffuse Centrilobular Nodularity
Arrow illustrates a centrilobular nodule.
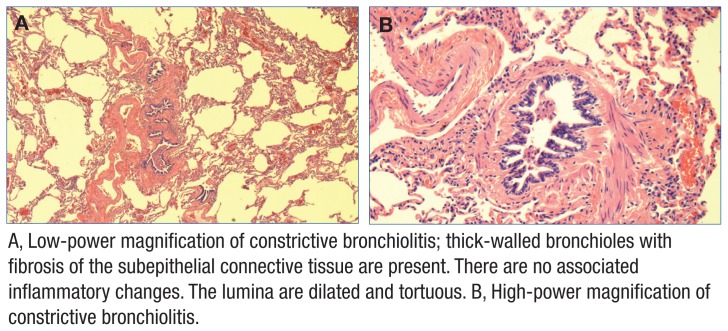
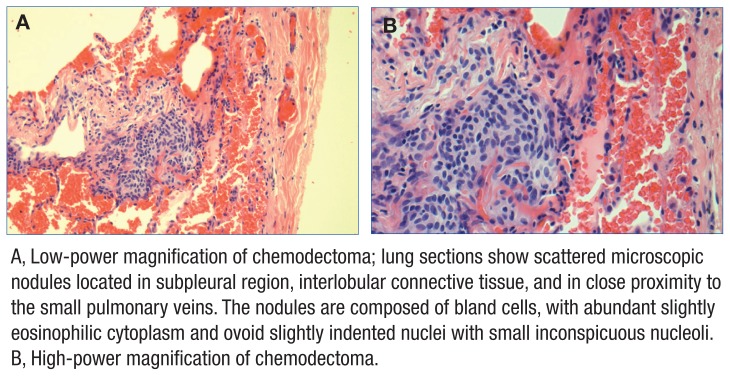
The patient’s lung biopsy demonstrated constrictive changes in bronchioles, hyperinflation, and multiple chemodectomas in all 3 lobes of the right lung (Figures 2 and 3). Three pulmonary pathologists reviewed the biopsy and confirmed findings of constrictive bronchiolitis. Serologies for connective tissue disease were negative, indicating no autoimmune cause of bronchiolitis.
Figure 2.
Constrictive Bronchiolitis Identified on Surgical Lung Biopsy in Case Study 1
A, Low-power magnification of constrictive bronchiolitis; thick-walled bronchioles with fibrosis of the subepithelial connective tissue are present. There are no associated inflammatory changes. The lumina are dilated and tortuous. B, High-power magnification of constrictive bronchiolitis.
Figure 3.
Chemodectomas Identified on Surgical Lung Biopsy in Case Study 1
A, Low-power magnification of chemodectoma; lung sections show scattered microscopic nodules located in subpleural region, interlobular connective tissue, and in close proximity to the small pulmonary veins. The nodules are composed of bland cells, with abundant slightly eosinophilic cytoplasm and ovoid slightly indented nuclei with small inconspicuous nucleoli. B, High-power magnification of chemodectoma.
As no specific etiology was identified, the patient was referred for a second opinion with a pulmonologist with expertise in interstitial lung disease. Finding no evidence of postinfectious or autoimmune bronchiolitis, the patient’s diagnosis of constrictive bronchiolitis was deemed to be idiopathic. A number of years later, following publication of a case series of 38 OEF/OIF deployers with biopsyproven constrictive bronchiolitis, the patient was referred for consultation to an occupational lung disease clinic.24 He subsequently was diagnosed with deployment-related lung disease, as his constrictive bronchiolitis was thought to be related to exposures encountered during his OEF/OIF deployments from 2003 to 2005.
The patient was monitored with spirometry over the next few months. After observing a 10% decline in forced expiratory volume in 1 second (FEV1) over 9 months despite stable lung volumes and diffusion capacity, the patient was started on macrolide therapy with erythromycin 500 mg daily. He was switched to azithromycin 250 mg daily due to gastrointestinal AEs of nausea and diarrhea while taking erythromycin. He continued use of an inhaled corticosteroid (ICS), as well as bronchodilator therapy with albuterol and formoterol and had stable dyspnea.
The patient was treated briefly with prednisone 40 mg, but he discontinued this medication after 5 days due to worsening anxiety and PTSD symptoms. Azithromycin therapy was discontinued after 4 years, because no significant improvement was noted in the patient’s lung function. Spirometry, lung volumes, and diffusion testing were unchanged for 2 years following discontinuation of azithromycin and continuing therapy with an ICS, long-acting beta-agonist, and albuterol. The patient has stable dyspnea on exertion but exercises regularly and recently was able to complete a marathon.
Case Study 2
A 43-year-old female ex-smoker presented to a VA chest clinic for evaluation of cough that started during a 2003 deployment to Iraq as well as dyspnea on exertion and chest tightness that had been present since her 2010 to 2011 deployment to Afghanistan. The patient had no history of asthma or other chronic respiratory disease during childhood.
She enlisted in the U.S. Navy in 1987 and later served as a medic while in the Navy Reserves. When she joined the U.S. Navy, she easily passed a 1.5-mile physical fitness readiness test run-time requirement with an 8.5-minute run time. She had no respiratory symptoms and ran in several marathons until her first SWA deployment in 2003.
In April 2003, she was deployed for 3 months to work as a combat medic near the Kuwait and Iraq border. She had frequent exposure to desert dust and recalled 5 sandstorms that appeared like a “wall of sand” coming toward the base. A few weeks into this deployment, the patient developed a nonproductive cough that persisted after returning to the U.S. She stopped smoking for a few months after returning home but continued to have a nonproductive cough. She did not seek further medical attention, because she had no exercise-limiting symptoms.
The patient joined the Army National Guard in 2006 and was activated in 2009 to deploy to Afghanistan from January 2010 through January 2011. She was stationed at Bagram Airbase for the entire deployment and worked as a military police officer in the prison. She had exposure to sandstorms and burn pit combustion products. The prison was about 2 miles downwind from a large burn pit.
In October 2010, she quit smoking again because of new-onset chest tightness and dyspnea on exertion. However, her symptoms did not abate, and she noted increased chest tightness and difficulty catching her breath when running near the burn pit. While she tried to avoid the burn pit, she participated in competitive races and a 10-mile run along paths that were near the burn pit.
After returning from deployment, the patient presented to her VA PCP for evaluation of persistent nonproductive cough, chest tightness, and dyspnea on exertion. She was not taking any respiratory or allergy medications at the time of evaluation. Initial chest X-ray and spirometry were normal, and she was referred to the chest clinic for consultation. At the time of pulmonary consultation, the patient had a total smoking history of 15 pack-years but had now abstained from smoking for about 2 years. She reported residential exposure to pet birds for > 20 years. High-resolution chest imaging and full PFT with lung volumes and diffusion capacity were performed to evaluate for hypersensitivity pneumonitis.
Her vital signs, physical examination pulmonary function testing with spirometry, lung volumes, and diffusion testing were all normal (Table 4). Bronchial challenge to methacholine demonstrated airways hyperresponsiveness at a PC[-20] FEV1 of 1.25 mg/mL. High-resolution chest CT did not demonstrate air trapping, centrilobular nodules, or other evidence of chronic interstitial lung disease. A cardiopulmonary maximum multistage exercise test with arterial line placement showed normal exercise tolerance with the patient achieving 109% of the maximum predicted workload and 90% of predicted VO2 max.
Table 4.
Case Study 2: Pulmonary Function Testing
Prebronchodilator spirometry
|
Lung volumes
|
Diffusion testing
|
Abbreviations: DLCO, diffusing capacity of the lung for carbon monoxide; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity.
The patient was diagnosed with deployment-related asthma based on the finding of airways hyperresponsiveness after bronchial challenge testing. Her asthma was considered deployment-related based on the temporal onset of cough and later chest tightness and dyspnea on exertion that occurred during deployment. Ongoing smoking cessation was emphasized.
The patient was started on bronchodilator therapy with albuterol prior to exercise and as needed, but she continued to have symptoms of chest tightness while exercising. Eventually, a low-dose ICS was initiated in conjunction with albuterol as needed. Her symptoms did not resolve with this regimen, but she did experience improvement in exertional chest tightness. This patient was not referred for biopsy given clinical findings of asthma. She will continue pulmonary monitoring every 6 months. However, if her symptoms worsen, she will undergo full PFT, which includes lung volumes and diffusion testing and possible repeat chest imaging.
CONCLUSION
These 2 cases are representative of the spectrum of deployment-related lung disease. This assessment requires a detailed chronologic occupational and environmental history, establishing a temporal link between respiratory symptoms and deployment exposures and evidence of lung disease on noninvasive testing (or confirmation by surgical lung biopsy in select cases) in which noninvasive testing is nondiagnostic.
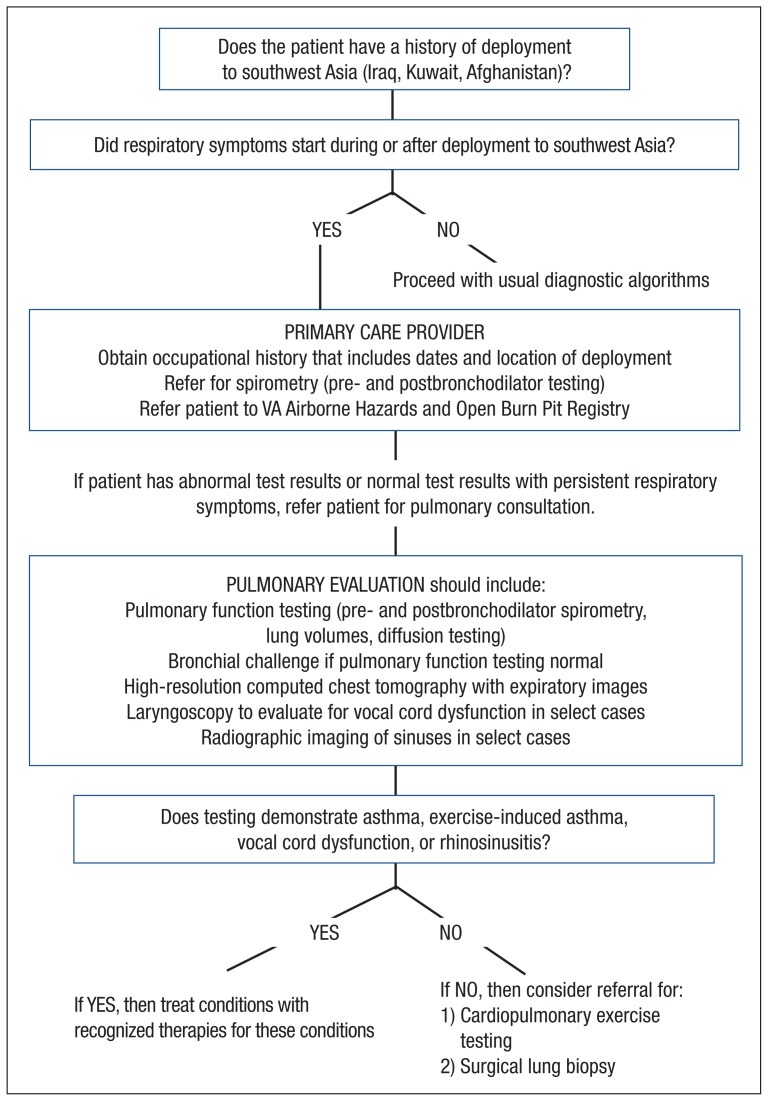
Referral for surgical lung biopsy was particularly helpful in the first case, because it ruled out other lung diseases that are more responsive to systemic therapy. However, referral for surgical lung biopsy is not recommended in all patients, and in-depth discussion of the risks and benefits associated with surgery is recommended. Although diagnostic clarity is a benefit of surgical lung biopsy, the authors also discuss with patients that there is no currently available therapy for deployment-related lung disease and thus management is unlikely to change after biopsy. The recommended approach to diagnostic evaluation is shown in Figure 4.
Figure 4.
Recommended Approach to Diagnostic Evaluation of Deployment-Related Respiratory Symptoms
In the authors’ experience, treatment of deployment-related asthma with standard asthma treatment usually improves or stabilizes respiratory symptoms but often does not result in complete resolution of symptoms. Improvement in lung function with systemic pharmacotherapy in the management of deployment-related lung diseases, such as constrictive bronchiolitis, respiratory bronchiolitis, emphysema, or granulomatous pneumonitis has not been observed. Although little is currently known about prognosis, utilization of data collected from the VA Airborne Hazards and Open Burn Pit Registry may contribute to the understanding of deployment exposures and long-term respiratory health effects.
Footnotes
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
REFERENCES
- 1.Rose C, Abraham J, Harkins D, et al. Overview and recommendations for medical screening and diagnostic evaluation for postdeployment lung disease in returning US warfighters. J Occup Environ Med. 2012;54(6):746–751. doi: 10.1097/JOM.0b013e31825297ba. [DOI] [PubMed] [Google Scholar]
- 2.Morris MJ, Lucero PF, Zanders TB, Zacher LL. Diagnosis and management of chronic lung disease in deployed military personnel. Ther Adv Respir Dis. 2013;7(4):235–245. doi: 10.1177/1753465813481022. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 4.Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693–701. doi: 10.7205/milmed-d-11-00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu K, Devesa SS, Wu H, et al. Cancer incidence in the U.S. military population: comparison with rates from the SEER program. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1740–1745. doi: 10.1158/1055-9965.EPI-09-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith EA, Jahnke SA, Poston WS, et al. Is it time for a tobacco-free military? N Engl J Med. 2014;371(7):589–591. doi: 10.1056/NEJMp1405976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bondurant S, Wedge R, editors. Combating Tobacco in Military and Veteran Populations. Washington, DC: National Academies Press; 2009. [PubMed] [Google Scholar]
- 8.Smith B, Ryan MA, Wingard DL, Patterson TL, Slymen DJ, Macera CA Millennium Cohort Study Team. Cigarette smoking and military deployment: a prospective evaluation. Am J Prev Med. 2008;35(6):539–546. doi: 10.1016/j.amepre.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Doll R. Mortality from lung cancer in asbestos workers. Br J Ind Med. 1955;12(2):81–86. doi: 10.1136/oem.12.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krstev S, Stewart P, Rusiecki J, Blair A. Mortality among shipyard Coast Guard workers: a retrospective cohort study. Occup Environ Med. 2007;64(10):651–658. doi: 10.1136/oem.2006.029652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hines SE, Gucer P, Kligerman S, et al. Pulmonary health effects in Gulf War I service members exposed to depleted uranium. J Occup Environ Med. 2013;55(8):937–944. doi: 10.1097/JOM.0b013e31829176c7. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization, International Agency for Research on Cancer. IARC monographs on the evaluation of the carcinogenic risk of chemicals to man: some aziridines, N-, S- & O-mustards and selenium. IARC Monogr Eval Carcinog Risk Chem Man. 1975;9:1–268. [PubMed] [Google Scholar]
- 13.Field RW, Withers BL. Occupational and environmental causes of lung cancer. Clin Chest Med. 2012;33(4):681–703. doi: 10.1016/j.ccm.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghanei M, Harandi AA. Lung carcinogenicity of sulfur mustard. Clin Lung Cancer. 2010;11(1):13–17. doi: 10.3816/CLC.2010.n.002. [DOI] [PubMed] [Google Scholar]
- 15.Chivers CJ. The secret casualties of Iraq’s abandoned chemical weapons. New York Times. Oct 14, 2014. [Accessed May 13, 2015]. http://www.nytimes.com/interactive/2014/10/14/world/middleeast/us-casualties-of-iraq-chemical-weapons.html?_r=0.
- 16.Institute of Medicine (US) Veterans and Agent Orange: Update 2000. Washington, DC: National Academies Press; 2001. Committee to Review the Health Effects in Vietnam Veterans of Exposure to Herbicides (Third Biennial Update) [PubMed] [Google Scholar]
- 17.How to help military & veteran families before, during, and after deployment. Military Family Research Institute Web site. [Accessed November 6, 2014]. https://www.mfri.purdue.edu/resources/public/hth/HowToHelp_FamilyFriendNeighbor.pdf.
- 18.Torreon BS. U.S. periods of war and dates of current conflicts. Washington, DC: Congressional Research Service Report for Congress; Dec 28, 2012. [Accessed November 6, 2014]. http://fas.org/sgp/crs/natsec/RS21405.pdf. [Google Scholar]
- 19.Rose CS. Military service and lung disease. Clin Chest Med. 2012;33(4):705–714. doi: 10.1016/j.ccm.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Szema AM. Occupational lung diseases among soldiers deployed to Iraq and Afghanistan. Occup Med Health Aff. 2013 doi: 10.4172/2329-6879.1000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris MJ, Dodson DW, Lucero PF, et al. Study of active duty military for pulmonary disease related to environmental deployment exposures (STAMPEDE) Am J Respir Crit Care Med. 2014;190(1):77–84. doi: 10.1164/rccm.201402-0372OC. [DOI] [PubMed] [Google Scholar]
- 22.Shorr AF, Scoville SL, Cersovsky SB, et al. Acute eosinophilic pneumonia among US Military personnel deployed in or near Iraq. JAMA. 2004;292(24):2997–3005. doi: 10.1001/jama.292.24.2997. [DOI] [PubMed] [Google Scholar]
- 23.Roop SA, Niven AS, Calvin BE, Bader J, Zacher LL. The prevalence and impact of respiratory symptoms in asthmatics and nonasthmatics during deployment. Mil Med. 2007;172(12):1264–1269. doi: 10.7205/milmed.172.12.1264. [DOI] [PubMed] [Google Scholar]
- 24.King MS, Eisenberg R, Newman JH, et al. Constrictive bronchiolitis in soldiers returning from Iraq and Afghanistan [published correction appears in N Engl J Med 2011;365(18):1749] N Engl J Med. 2011;365(3):222–230. doi: 10.1056/NEJMoa1101388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanders JW, Putnam SD, Frankart C, et al. Impact of illness and non-combat injury during Operations Iraqi Freedom and Enduring Freedom (Afghanistan) Am J Trop Med Hyg. 2005;73(4):713–719. [PubMed] [Google Scholar]
- 26.Stecker T, Fortney J, Owen R, McGovern MP, Williams S. Co-occurring medical, psychiatric, and alcohol-related disorders among veterans returning from Iraq and Afghanistan. Psychosomatics. 2010;51(6):503–507. doi: 10.1176/appi.psy.51.6.503. [DOI] [PubMed] [Google Scholar]
- 27.Szema AM, Peters MC, Weissinger KM, Gagliano CA, Chen JJ. New-onset asthma among soldiers serving in Iraq and Afghanistan. Allergy Asthma Proc. 2010;31(5):67–71. doi: 10.2500/aap.2010.31.3383. [DOI] [PubMed] [Google Scholar]
- 28.Scoville SL. Acute eosinophilic pneumonia (AEP) among U.S. military personnel in the U.S. Central Command Area of Responsibility (USCENTCOM AOR) USACHPPM Information Paper. [Accessed January 27, 2015]. http://www.pdhealth.mil/AEP_Info_paper_01oct09.pdf. Published October 1, 2009.
- 29.Zembrzuska H, Collen J, Roop S. Pulmonary fibrosis presenting at post-deployment health screening. Am J Respir Crit Care Med. 2011;183:A4780. Abstract. [Google Scholar]
- 30.Dhoma S, Gottschall B, Robinson M, et al. Lung disease in deployers returning from Afghanistan and Iraq. Am J Respir Crit Care Med. 2013;187:A3669. Abstract. [Google Scholar]
- 31.Dhoma S, Cox C, Chung JH, et al. Chest tomography may predict histopathologic abnormalities in symptomatic deployers returning from Iraq and Afghanistan. Am J Respir Crit Care Med. 2014;189:A5102. Abstract. [Google Scholar]
- 32.Engelbrecht JP, McDonald EV, Gillies JA, Javanty RK, Casuccio G, Gertler AW. Characterizing mineral dusts and other aerosols from the Middle East – part I: ambient sampling. Inhal Toxicol. 2009;21(4):297–326. doi: 10.1080/08958370802464273. [DOI] [PubMed] [Google Scholar]
- 33.Engelbrecht JP, McDonald EV, Gillies JA, Javanty RK, Casuccio G, Gertler AW. Characterizing mineral dusts and other aerosols from the Middle East – part 2: grab samples and re-suspensions. Inhal Toxicol. 2009;21(4):327–336. doi: 10.1080/08958370802464299. [DOI] [PubMed] [Google Scholar]
- 34.Helmer DA, Rossignol M, Blatt M, Agarwal R, Teichman R, Lange G. Health and exposure concerns of veterans deployed to Iraq and Afghanistan. J Occup Environ Med. 2007;49(5):475–480. doi: 10.1097/JOM.0b013e318042d682. [DOI] [PubMed] [Google Scholar]
- 35.Smith B, Wong CA, Smith TC, Boyko EJ, Gackstetter GD, Ryan MAK for the Millennium Cohort Study Team. Newly reported respiratory symptoms and conditions among military personnel deployed to Iraq and Afghanistan: a prospective population-based study. Am J Epidemiol. 2009;170(11):1433–1442. doi: 10.1093/aje/kwp287. [DOI] [PubMed] [Google Scholar]
- 36.Abraham JH, DeBakey SF, Reid L, Zhou J, Baird CP. Does deployment to Iraq and Afghanistan affect respiratory health of US military personnel? J Occup Environ Med. 2012;54(6):740–745. doi: 10.1097/JOM.0b013e318252969a. [DOI] [PubMed] [Google Scholar]
- 37.McAndrew LM, Teichman RF, Osinubi OY, Jasien JV, Quigley KS. Environmental exposure and health of Operation Enduring Freedom/Operation Iraqi Freedom veterans. J Occup Environ Med. 2012;54(6):665–669. doi: 10.1097/JOM.0b013e318255ba1b. [DOI] [PubMed] [Google Scholar]
- 38.Quigley KS, McAndrew LM, Almeida L, et al. Prevalence of environmental and other military exposure concerns in Operation Enduring Freedom and Operation Iraqi Freedom veterans. J Occup Environ Med. 2012;54(6):659–664. doi: 10.1097/JOM.0b013e3182570506. [DOI] [PubMed] [Google Scholar]
- 39.Teichman R. Exposures of concern to veterans returning from Afghanistan and Iraq. J Occup Environ Med. 2012;54(6):677–681. doi: 10.1097/JOM.0b013e318259c1ce. [DOI] [PubMed] [Google Scholar]