Abstract
Background:
The ABC-stroke and ABC-bleeding risk scores incorporate clinical variables and cardiovascular biomarkers to estimate risk of stroke or systemic embolic events (S/SEE) and bleeding, respectively, in patients with atrial fibrillation (AF). These scores have been proposed for routine clinical use but their performance in external cohorts remains uncertain.
Methods:
ENGAGE AF-TIMI 48 was a multinational, randomized trial of the oral factor Xa inhibitor edoxaban in patients with AF and CHADS2 score ≥2. We performed a nested prospective biomarker study in 8705 patients, analyzing baseline high-sensitivity troponin T (hsTnT), N-terminal B-type natriuretic peptide (NT-proBNP), and growth differentiation factor (GDF)-15, as well as in serial samples after 12 months. The ABC-stroke (age, prior stroke/transient ischemic attack, hsTnT, NT-proBNP) and ABC-bleeding (age, prior bleeding, hemoglobin, hsTnT, and GDF-15) scores were tested. Hazard ratios were adjusted for estimated glomerular filtration rate and the components of the CHA2DS2-VASc and HAS-BLED scores, respectively. Discrimination and reclassification were compared with these established scores.
Results:
Median baseline hsTnT, NT-proBNP, and GDF-15 levels were 13.7 ng/L (25th–75th percentiles, 9.6–20.4 ng/L), 811 pg/mL (386–1436 pg/L), and 1661 pg/mL (1179–2427 pg/mL), respectively. Elevated hsTnT, NT-proBNP, and GDF-15 were independently associated with higher rates of S/SEE, and elevated hsTnT and GDF-15 were independently associated with higher rates of major bleeding (p<0.001 for each). The ABC-stroke and ABC-bleeding scores were well-calibrated and yielded higher c-indices than the CHA2DS2-VASc score for S/SEE (0.67 [95% CI, 0.65 – 0.70] vs. 0.59 [95% CI, 0.57 – 0.62]; p<0.001) and HAS-BLED score for major bleeding (0.69 [95% CI, 0.66 – 0.71] vs. 0.62 [95% CI, 0.60 – 0.64]; p<0.001), respectively. The ABC-stroke and ABC-bleeding scores stratified patients within CHA2DS2-VASc and HAS-BLED risk categories (p<0.001 for both). Patients with ABC-bleeding scores predicting a high 1-year risk of bleeding (>2%) derived greater benefit from treatment with edoxaban compared with warfarin.
Conclusions:
The ABC-stroke and ABC-bleeding scores evaluated in this anticoagulated clinical trial cohort were well-calibrated and outperformed the CHA2DS2-VASc and HAS-BLED scores, respectively. These scores may help identify patients most likely to derive a benefit from treatment with non-vitamin K antagonist oral anticoagulants (NOACs).
Clinical Trial Registration:
URL: https://www.clinicaltrials.gov. Unique identifier: NCT00781391.
Keywords: Atrial fibrillation, biomarkers, risk score, stroke, bleeding
Introduction
Stroke and systemic embolism are morbid and often fatal complications of atrial fibrillation (AF).1 Oral anticoagulation in patients with AF significantly reduces the risk of stroke and systemic embolic events (S/SEE) but increases the risk of major bleeding. Current guidelines recommend systematically evaluating the absolute risks of S/SEE and bleeding estimated using clinical risk scores such as the CHA2DS2-VASc and HAS-BLED scores, respectively, when making decisions regarding oral anticoagulation.2–4 Despite the availability of these scores and guidelines for treatment, a surprisingly high proportion of patients with AF go untreated, in particular because of concern regarding the risk of bleeding.5–7
In research dating back for more than a decade, multiple studies have suggested that circulating biomarkers of cardiovascular disease may improve prediction of stroke or systemic embolic events (S/SEE) and bleeding in patients with AF compared with established clinical risk scores.8–13 In an analysis of a cohort from the ENGAGE-TIMI 48 trial, we previously demonstrated that incorporating a conventional assay for cardiac troponin I, N-terminal B-type natriuretic peptide (NT-proBNP), and D-dimer into a multimarker risk score for S/SEE and death significantly enhanced prognostic accuracy compared with or when added to the CHA2DS2-VASc score.14
The novel ABC (age, biomarker, clinical history)-stroke and ABC-bleeding risk scores also incorporate biomarkers, along with clinical variables, to estimate risk of S/SEE and bleeding, respectively.15, 16 The components of the ABC-stroke score include age, NT-proBNP, high-sensitivity cardiac troponin T (hsTnT), and prior stroke/transient ischemic attack, while the components of the ABC-bleeding score include age, growth differentiation factor-15 (GDF-15), hsTnT, hemoglobin, and history of bleeding.15, 16
Although the ABC-stroke and ABC-bleeding scores outperformed the CHA2DS2-VASc and HAS-BLED scores, respectively, in their predictive accuracy in the derivation cohorts,15, 16 their performance in external cohorts remains uncertain.17, 18 Given the current equipoise regarding clinical use of the biomarker-based ABC scores, we designed a nested biomarker study to independently evaluate the prognostic performance and identify novel applications of these risk scores in patients with AF in a well characterized cohort from a large, multinational clinical trial.19
Methods
Study population and design
The Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation—Thrombolysis in Myocardial Infarction 48 (ENGAGE AF-TIMI 48) trial was a multinational, randomized, double-blind trial of the oral factor Xa inhibitor edoxaban versus warfarin for the prevention of stroke and systemic embolism in 21,105 patients with AF and CHADS2 score ≥2.19 Patients randomized to edoxaban received either a higher-dose edoxaban regimen (HDER) with edoxaban 60/30 mg daily or a lower-dose edoxaban regimen (LDER) with edoxaban 30/15 mg daily. Patients randomized to warfarin were dosed to reach a target INR of 2.0–3.0. The median follow-up was 2.8 years. Participation in a prospective nested biomarker substudy was offered to all enrolled patients at sites that elected to participate in the biomarker substudy until approximately 9000 patients were recruited. For this analysis, collected samples were available for 8705 patients from the time of trial enrollment (baseline), and for 6806 patients at 12 months following trial enrollment. All patients provided written informed consent. The protocol was approved by ethics committees at each center. We encourage parties interested in collaboration and data sharing to contact the corresponding author directly for further discussions.
Biomarkers
Baseline blood samples were collected on the day of randomization, which was the same day that the first dose of study drug was administered. Samples were collected in EDTA anticoagulant tubes, and isolated plasma was stored at −20°C or colder until shipped to the central laboratory on dry ice, where plasma was stored at −70°C or colder until thawed for analysis at the TIMI Clinical Trials Laboratory (Boston, MA). hsTnT, NT-proBNP, and GDF-15 concentrations were measured with immunoassays on the Cobas e601 (Roche Diagnostics) (Biomarker assay parameters in Supplemental Material). Hemoglobin was measured separately in the commercial core laboratory during conduct of the trial. The creatinine clearance was estimated using the Cockroft-Gault equation.
Clinical Endpoints
The primary efficacy endpoint was the time to first adjudicated stroke (ischemic or hemorrhagic) or systemic embolic event. The primary safety endpoint was major bleeding, which was adapted from the International Society on Thrombosis and Hemostasis definition. This endpoint included (1) fatal bleeding; (2) bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intra-articular or pericardial, or intramuscular with compartment syndrome; and/or (3) bleeding causing a fall in hemoglobin level of ≥2 g/dL (adjusted for transfusion), or leading to transfusion of two or more units of whole blood or red cells. The primary net clinical outcome (NCO) was the composite of stroke, systemic embolic event, major bleeding, or death from any cause. An independent clinical events committee, blinded to study assignment, adjudicated all outcomes.20
Statistical Methods
Baseline characteristics stratified by pre-specified biomarker categories for hsTnT, NT-proBNP, and GDF-15 were summarized. Univariate associations between individual biomarkers and the clinical outcomes of S/SEE and major bleeding were assessed using a Cox proportional hazard model with the biomarker as the independent variable. These analyses were performed with the biomarker modeled both as continuous and categorical variables using a priori thresholds. Based on the distribution of biomarker values, continuous data were log transformed. Event rates were estimated and displayed using annualized event rates.
Adjusted estimates of the association between individual biomarkers and S/SEE were calculated using a Cox proportional hazard model with the biomarker as an independent variable along with estimated glomerular filtration rate (eGFR) and each of the elements of the CHA2DS2-VASc score (age, sex, history of heart failure, history of hypertension, history of known atherosclerosis, diabetes mellitus, and history of stroke or TIA). Similarly, adjusted estimates of the association between individual biomarkers and major bleeding were calculated using a Cox proportional hazard model with the biomarker as an independent variable along with eGFR and each of the elements of the HAS-BLED score (age, history of hypertension, history of abnormal renal or liver function, history of stroke or TIA, history of major bleeding, medication use predisposing to bleeding, and alcohol use). INR lability (a component of the HAS-BLED score) was not included, because there were no available INR data prior to randomization and 40% of patients enrolled in the trial were naïve to vitamin K antagonists.
The univariate and multivariable analyses were repeated using the absolute biomarker values at 12 months as well as the absolute change (i.e., delta) in biomarker values from randomization to 12 months using a priori thresholds. For these analyses, a landmark analysis of S/SEE and bleeding outcomes starting at 12 months was performed.
Multivariable analyses were performed assessing the ABC-stroke and ABC-bleeding risk score variables in a Cox proportional hazard model with eGFR and each of the risk score components (ABC-stroke: age, NT-proBNP, hsTnT, and prior stroke/TIA; ABC-bleeding: age, GDF-15, hsTnT, hemoglobin, and history of bleeding). The discriminatory performance was assessed using Harrell’s c-index21–23 for the CHA2DS2-VASc and HAS-BLED scores, for each biomarker alone, for the ABC-stroke and ABC-bleeding risk scores using both coefficients for the regression models derived in ENGAGE AF-TIMI 48 as well as those from the original derivation cohorts, and for comprehensive clinical and biomarker models for S/SEE and major bleeding tested during the previous derivation of the scores.12–13 The predictive performance (i.e., C-indices) of these correlated models were compared using the approach described by Kang.24 To estimate the relative prognostic information provided by the ABC-stroke and ABC-bleeding risk models compared to the comprehensive S/SEE and bleeding models, respectively, we approximated the comprehensive models using ordinary least squares models in which the estimated linear predictors from the full Cox model were the outcome variables and the components of the ABC-stroke and ABC-bleeding risk scores were the covariates. Reclassification was assessed by calculating the categorical Net Reclassification Improvement (NRI) at 1 year with the ABC-stroke vs. CHA2DS2-VASc scores and ABC-bleeding vs. HAS-BLED scores using prespecified categorical subgroups defined by 1-year predicted risks of S/SEE and major bleeding (<1%, 1–2%, >2%). These thresholds were selected based on previous evaluations of the ABC risk score performance in AF. In addition, we performed a sensitivity analysis for the NRI of the ABC-stroke vs. CHA2DS2-VASc scores, using 0.75% as a single threshold for 1-year risk of S/SEE.
Annualized S/SEE event rates were described according to: (1) categorical subgroups defined by 1-year S/SEE risk predicted by the ABC-stroke score (<1%, 1–2%, >2%) and categorical subgroups defined by the CHA2DS2-VASc score (≤3, 4, ≥5); and (2) categorical subgroups defined by 1-year S/SEE risk predicted by the ABC-stroke score (<1%, 1–2%, >2%) and categorical subgroups defined by a previously reported TIMI-AF clinical risk score (low, intermediate, high).25 Similarly, annualized major bleeding event rates were described according to: (1) categorical subgroups defined by 1-year major bleeding risk predicted by the ABC-bleeding score (<1%, 1–2%, >2%) and categorical subgroups defined by the HAS-BLED score (0–1, 2, 3, ≥4); and (2) categorical subgroups defined by 1-year major bleeding risk predicted by the ABC-bleeding score (<1%, 1–2%, >2%) and categorical subgroups defined by the TIMI-AF clinical risk score (low, intermediate, high).25
Calibration of the ABC-stroke and ABC-bleeding risk scores was assessed by categorizing patients into strata using the estimated 1-year S/SEE and bleeding risks from the ABC-stroke and ABC-bleeding scores and comparing the predicted risk in each group with the observed rate, as well as by calculating the Nam-D’Agostino statistic.26
To test for interaction between treatment effect of edoxaban vs. warfarin and baseline ABC-stroke and ABC-bleeding risk scores, Cox regression was performed with the main effects and interaction terms. The proportional hazards assumption was confirmed using statistical tests and visual inspection based on the scaled Schoenfeld residuals. Pairwise comparisons between each of the two edoxaban exposure groups (HDER and LDER) and warfarin were performed.
Data were analyzed with R version 3.5.0.
Results
Baseline characteristics
The median baseline hsTnT, NT-proBNP, and GDF-15 values in the 8705 patients in the nested biomarker analysis were 13.7 ng/L (25th–75th percentiles, 9.6–20.4 ng/L), 811 pg/mL (386–1436 pg/L), and 1661 pg/mL (1179–2427 pg/mL), respectively. Baseline characteristics stratified by pre-specified biomarker categories for hsTnT (<7 ng/mL, 7-<14 ng/mL, ≥14 ng/mL), NT-proBNP (<450 pg/mL, 450–<900, ≥900 pg/mL), and GDF-15 (<1200 pg/mL, 1200–<1800 pg/mL, ≥1800 pg/mL) are shown in Table 1 (baseline characteristics for the full cohort are shown in Supplemental Table 1). Patients in the highest categories of hsTnT, NT-proBNP, and GDF-15 were older, had worse renal function, and had a higher prevalence of coronary artery disease at the time of trial enrollment (all p<0.001).
Table 1.
Baseline characteristics stratified by prespcecified biomarker categories.
| hsTnT (ng/L) | NT-proBNP (pg/mL) | GDF-15 (pg/mL) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <7 (N=794) |
7–<14 (N=3688) |
≥14 (N=4223) |
P-value | <450 (N=2536) |
450–<900 (N=2236) |
≥900 (N=3958) |
P-value | <1200 (N=2261) |
1200–<1800 (N=2593) | ≥1800 (N=3851) | P-value | |
| Age (year) | 62.0 (57.0–70.0) | 70.0 (63.0–76.0) | 75.0 (68.0–80.0) | <0.001 | 69 (61.0–76.0) | 71 (63.0–77.0) | 74 (67.0–79.0) | <0.001 | 65 (59.0–72.0) | 73 (65.0–78.0) | 75 (68.0–80.0) | <0.001 |
| Female sex (%) | 459 (57.8%) | 1662 (45.1%) | 1254 (29.7%) | <0.001 | 980 (39.0%) | 790 (35.3%) | 1605 (40.6%) | <0.001 | 903 (39.9%) | 1070 (41.3%) | 1402 (36.4%) | <0.001 |
| White race (%) | 682 (85.9%) | 3253 (88.2%) | 3795 (89.9%) | <0.001 | 2222 (88.5%) | 2006 (89.7%) | 3502 (88.5%) | 0.135 | 1995 (88.3%) | 2304 (88.9%) | 3431 (89.1%) | 0.884 |
| BMI (kg/m2) | 29.6 (26.3–34.0) | 29.1 (25.8–33.2) | 29.1 (25.8–33.2) | 0.016 | 29.6 (26.3–33.9) | 29.9 (26.4–33.9) | 28.5 (25.4–32.2) | <0.001 | 29.6 (26.5–33.6) | 29.1 (25.8–33.0) | 28.9 (25.6–33.2) | <0.001 |
| CrCl (ml/min) | 94.3 (72.3–118.7) | 76.7 (60.0–97.7) | 64.2 (49.6–84.1) | <0.001 | 80.6 (62.4–105.2) | 77.9 (59.7–100.8) | 63.7 (49.6–82.5) | <0.001 | 90.3 (71.3–112.9) | 73.0 (58.1–92.1) | 61.1 (47.7–80.5) | <0.001 |
| AF type Paroxysmal | 326 (41.1%) | 1050 (28.5%) | 964 (22.8%) | <0.001 | 1399 (55.8%) | 421 (18.8%) | 520 (13.1%) | <0.001 | 649 (28.7%) | 729 (28.1%) | 962 (25.0%) | 0.008 |
| Persistent | 168 (21.2%) | 847 (23.0%) | 953 (22.6%) | 503 (20.0%) | 481 (21.5%) | 984 (24.9%) | 504 (22.3%) | 559 (21.6%) | 905 (23.5%) | |||
| Permanent | 300 (37.8%) | 1790 (48.5%) | 2305 (54.6%) | 607 (24.2%) | 1334 (59.7%) | 2454 (62.0%) | 1108 (49.0%) | 1305 (50.3%) | 1982 (51.%) | |||
| Hx Heart failure (%) | 489 (61.6%) | 2086 (56.6%) | 2600 (61.6%) | <0.001 | 1251 (49.8%) | 1297 (58.0%) | 2627 (66.4%) | <0.001 | 1459 (64.5%) | 1463 (56.4%) | 2253 (58.5%) | <0.001 |
| Hx Hypertension (%) | 489 (61.6%) | 2086 (56.6%) | 4033 (95.5%) | 0.181 | 2392 (95.3%) | 2140 (95.7%) | 3784 (95.6%) | 0.726 | 2150 (95.1%) | 2470 (95.3%) | 3696 (96.0%) | 0.196 |
| Hx T2DM (%) | 263 (33.1%) | 1253 (34.0%) | 1705 (40.4%) | <0.001 | 1017 (40.5%) | 892 (39.9%) | 1312 (33.1%) | <0.001 | 595 (26.3%) | 838 (32.3%) | 1788 (46.4%) | <0.001 |
| Hx Stroke/TIA (%) | 263 (33.1%) | 1253 (34.0%) | 1148 (27.2%) | 0.037 | 696 (27.7%) | 618 (27.6%) | 1123 (28.4%) | 0.772 | 703 (31.1%) | 743 (28.7%) | 991 (25.7%) | <0.001 |
| Hx CAD (%) | 199 (25.1%) | 1082 (29.3%) | 1750 (41.5%) | <0.001 | 754 (30.0%) | 747 (33.4%) | 1530 (38.7%) | <0.001 | 502 (29.4%) | 607 (31.7%) | 1263 (39.7%) | <0.001 |
| Hx PAD (%) | 13 (1.6%) | 118 (3.2%) | 254 (6.0%) | <0.001 | 94 (3.7%) | 98 (4.4%) | 193 (4.9%) | 0.097 | 51 (2.3%) | 95 (3.7%) | 239 (6.2%) | <0.001 |
| Hx Non-ICH bleed (%) | 58 (7.3%) | 368 (10.0%) | 485 (11.5%) | 0.001 | 260 (10.4%) | 232 (10.4%) | 419 (10.6%) | 0.945 | 166 (7.3%) | 272 (10.5%) | 473 (12.3%) | <0.001 |
| Hx Aortic valve dz (%) | 64 (8.1%) | 517 (14.1%) | 753 (17.9%) | <0.001 | 287 (11.5%) | 315 (14.1%) | 732 (18.6%) | <0.001 | 251 (11.1%) | 395 (15.3%) | 688 (17.9%) | <0.001 |
| Hx Mitral valve dz (%) | 229 (29.0%) | 1261 (34.3%) | 1626 (38.7%) | <0.001 | 722 (28.9%) | 803 (36.0%) | 1591 (40.4%) | <0.001 | 722 (32.1%) | 906 (35.1%) | 1488 (38.8%) | <0.001 |
| Current smoker (%) | 53 (6.7%) | 269 (7.3%) | 285 (6.7%) | 199 (7.9%) | 156 (7.0%) | 252 (6.4%) | <0.001 | 134 (5.9%) | 189 (7.3%) | 284 (7.4%) | ||
| LVEF <30% | 18 (2.3%) | 88 (2.4%) | 240 (5.7%) | <0.001 | 45 (1.8%) | 56 (2.5%) | 245 (6.2%) | <0.001 | 73 (3.2%) | 78 (3.0%) | 195 (5.1%) | <0.001 |
| 30–39% | 33 (4.2%) | 200 (5.4%) | 400 (9.5%) | 92 (3.7%) | 161 (7.2%) | 380 (9.6%) | 146 (6.5%) | 167 (6.4%) | 320 (8.3%) | |||
| 40–49% | 104 (13.1%) | 496 (13.4%) | 645 (15.3%) | 285 (11.4%) | 324 (14.5%) | 636 (16.1%) | 349 (15.4%) | 336 (13.0%) | 560 (14.5%) | |||
| ≥50% | 434 (54.7%) | 1900 (51.5%) | 1876 (44.4%) | 1433 (57.1%) | 1113 (49.8%) | 1664 (42.0%) | 1083 (47.9%) | 1323 (51.0%) | 1804 (46.8%) | |||
| Unknown | 205 (25.8%) | 1004 (27.2%) | 1062 (25.1%) | 656 (26.1%) | 582 (26.0%) | 1033 (26.1%) | 610 (27.0%) | 689 (26.6%) | 972 (25.2%) | |||
| OAC use at rando | 7 (0.9%) | 21 (0.6%) | 27 (0.6%) | 0.600 | 23 (0.9%) | 15 ( 0.7%) | 17 (0.4%) | 0.053 | 13 (0.6%) | 22 (0.8%) | 20 (0.5%) | 0.243 |
| ASA use at rando (%) | 225 (28.3%) | 1080 (29.3%) | 1369 (32.4%) | 0.003 | 794 (31.6%) | 666 (29.8%) | 1214 (30.7%) | 0.386 | 638 (28.2%) | 766 (29.5%) | 1270 (33.0%) | <0.001 |
| CHADS2 score | 2.0 (2.0–3.0) | 2.0 (2.0–3.0) | 3.0 (2.0–4.0) | <0.001 | 2.0 (2.0–3.0) | 3.0 (2.0–3.0) | 3.0 (2.0–4.0) | <0.001 | 2.0 (2.0–3.0) | 3.0 (2.0–3.0) | 3.0 (2.0–4.0) | <0.001 |
| HAS-BLED score | 2.0 (1.0–3.0) | 2.0 (2.0–3.0) | 3.0 (2.0–3.0) | <0.001 | 2.0 (2.0–3.0) | 2.0 (2.0–3.0) | 3.0 (2.0–3.0) | <0.001 | 2.0 (2.0–3.0) | 2.0 (2.0–3.0) | 3.0 (2.0–3.0) | <0.001 |
Categorical variables are shown as counts and percentages based on the number of patients with available data for that parameter; continuous variables are shown as median (25th–75th percentiles). Differences in the baseline characteristics between biomarker strata were evaluated using Pearson’s chi-square test for categorical variables and Kruskal-Wallis test for continuous variables. AF indicates atrial fibrillation; ASA, acetylsalicylic acid (aspirin); BMI, body-mass index; CAD, coronary artery disease; CrCl, creatinine clearance; GDF-15, growth differentiation factor-15; hsTnT, high-sensitivity troponin T; Hx, history; ICH, intracranial hemorrhage; LVEF, left ventricular ejection fraction; NSAID, nonsteroidal anti-inflammatory drug; NT-proBNP, N-terminal B-type natriuretic peptide; OAC, oral anticoagulant; PAD, peripheral artery disease; T2DM, type 2 diabetes mellitus.
Individual biomarkers at baseline and one year
After adjusting for the effects of eGFR and each of the elements of the CHA2DS2-VASc score (age, sex, history of heart failure, history of hypertension, history of known atherosclerosis, diabetes mellitus, and history of stroke or TIA), comparing the highest vs. lowest biomarker category for each biomarker, hsTnT, NT-proBNP, and GDF-15 were each independently associated with a more than 2-fold higher rate of S/SEE (p<0.001; Figure 1). After adjusting for the effects of eGFR and each of the elements of the HAS-BLED score (age, history of hypertension, history of abnormal renal or liver function, history of stroke or TIA, history of major bleeding, medication use predisposing to bleeding, and alcohol use), comparing the highest vs. lowest biomarker category for each biomarker, hsTnT and GDF-15 were each independently associated with a more than 2.5-fold higher rate of bleeding (p<0.001), while NT-proBNP was not (Figure 1). When analyzed as a continuous variable, each biomarker was independently associated with both outcomes (Supplemental Table 2). In multivariable models including all three biomarkers, GDF-15 was no longer independently associated with S/SEE and NT-proBNP was no longer independently associated with major bleeding.
Figure 1. Cardiovascular biomarkers and annualized rate of stroke or systemic embolism and major bleeding.
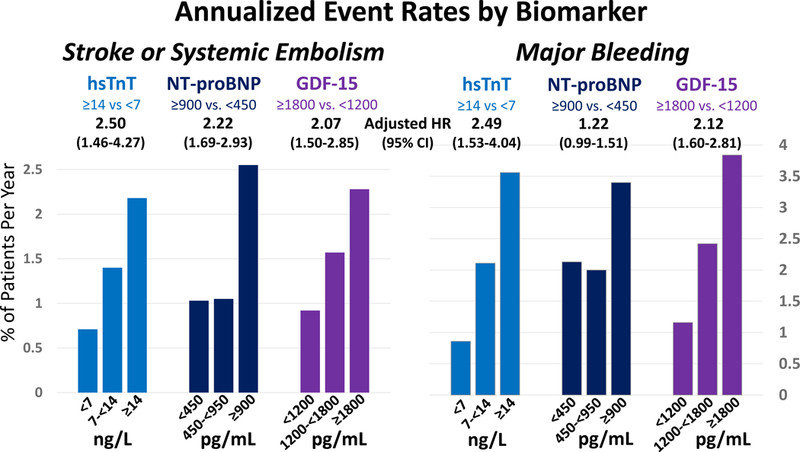
High-sensitivity troponin T (hsTnT), N-terminal B-type natriuretic peptide (NT-proBNP), and growth differentiation factor-15 (GDF-15) were each independently associated with higher rates of S/SEE. hsTnT and GDF-15 were both independently associated with higher rates of bleeding, but NT-proBNP was not. GDF-15 indicates growth differentiation factor-15; hsTnT, high-sensitivity troponin T; HR, hazard ratio; Int, interval; ng/L, nanograms/liter; NT-proBNP, N-terminal B-type natriuretic peptide; pg/mL, picograms/milliliter.
The median hsTnT, NT-proBNP, and GDF-15 values at 12 months following trial enrollment among the 6806 patients with available data from the nested biomarker cohort were 13.8 ng/L (25th–75th percentiles, 9.6–20.7 ng/L), 773 pg/mL (367–1362 pg/L), and 1711 pg/mL (1199–2563 pg/mL), respectively. From a landmark of 12 months following trial enrollment, hsTnT, NT-proBNP, and GDF-15 remained independently associated with higher rates of subsequent S/SEE, and GDF-15 remained independently associated with higher rates of major bleeding, but hsTnT did not (Supplemental Table 3).
Among patients with serial biomarker measurements, a >6 ng/mL increase in the value of hsTnT, a >800 pg/mL increase in the value of NT-proBNP, and a >1200 pg/mL increase in the value of GDF-15 between randomization and 12 months were each independently associated with higher rates of S/SEE (Supplemental Table 3). Similarly, a >6 ng/mL increase in the value of hsTnT and a >1200 pg/mL increase in the value of GDF-15 between randomization and 12 months were each independently associated with higher rates of major bleeding (Supplemental Table 3).
ABC-stroke and ABC-bleeding scores
Each clinical variable and biomarker modeled in the ABC-stroke and ABC-bleeding derivation cohorts15, 16 was reassessed in our cohort (Figure 2). Consistent with the findings in the original derivation cohort, the strongest predictors of stroke and systemic embolic events were NT-proBNP, age, history of stroke/TIA, and hsTnT, mirroring the components of the ABC-stroke score (Figure 2a). The ABC-stroke score variables accounted for 94.3% of the prognostic information provided by the clinical and biomarker variables included in a comprehensive model in the derivation cohort. Moreover, including additional cardiovascular biomarkers (GDF-15, cystatin C, and D-dimer) in the model for S/SEE did not improve the prognostic performance of the ABC-stroke risk score (Table 2).
Figure 2. Relative importance of each variable in the risk models for stroke or systemic embolism and major bleeding.
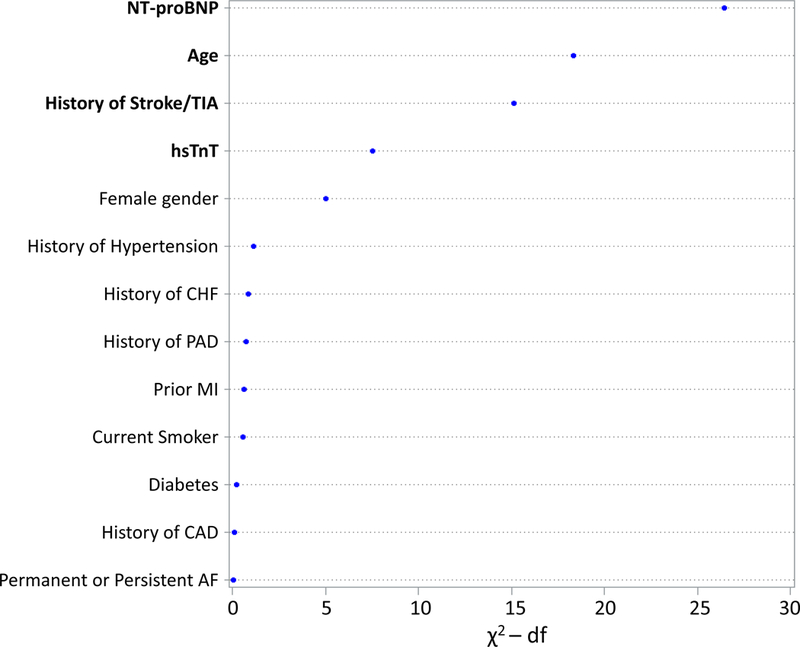
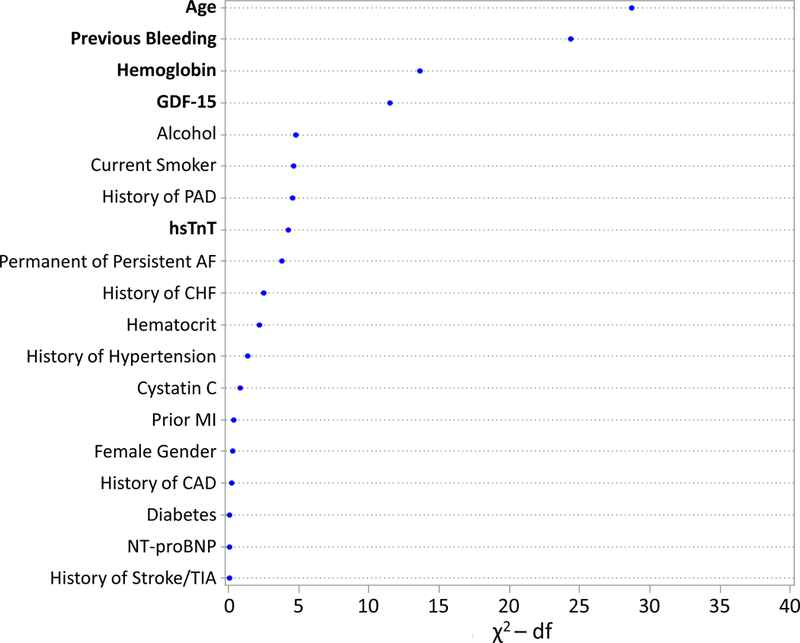
The relative contributions were assessed using the partial Wald χ2 minus the predictor degrees of freedom. The components of the (A) ABC-stroke and (B) ABC-bleeding risk scores are bolded. AF indicates atrial fibrillation; CAD, coronary artery disease; CHF, congestive heart failure; df, degrees of freedom; GDF-15, growth differentiation factor-15; hsTnT, high-sensitivity troponin T; MI, myocardial infarction; NT-proBNP, N-terminal B-type natriuretic peptide; PAD, peripheral artery disease; TIA, transient ischemic attack.
Table 2.
Comparison of predictive models for stroke or systemic embolism and major bleeding.
| Risk Model | Harrell’s C-Index (95% CI) |
|---|---|
| Models for Stroke or Systemic Embolism | |
| CHA2DS2-VASc | 0.59 (0.57 – 0.62) |
| ABC-Stroke (Parameter estimates from ENGAGE AF-TIMI 48) | 0.67 (0.65 – 0.70) |
| ABC-Stroke (Parameter estimates from original derivation cohort) | 0.66 (0.63 – 0.68) |
| Comprehensive Model for S/SEE* | 0.67 (0.65 – 0.70) |
| Comprehensive Model for S/SEE plus GDF-15, cystatin C, and D-dimer† | 0.68 (0.65 – 0.70) |
| Models for Major Bleeding | |
| HAS-BLED | 0.62 (0.60 – 0.64) |
| ABC-Bleeding (Parameter estimates from ENGAGE AF-TIMI 48) | 0.69 (0.66 – 0.71) |
| ABC-Bleeding (Parameter estimates from original derivation cohort) | 0.67 (0.65 – 0.70) |
| Comprehensive Model for Major Bleeding* | 0.70 (0.68 – 0.72) |
The components of the CHA2DS2-VASc score include age, sex, history of heart failure, history of hypertension, history of known atherosclerosis, diabetes mellitus, and history of stroke or TIA. The components of the HAS-BLED include age, history of hypertension, history abnormal renal or liver function, history of stroke or TIA, history of major bleeding, medication use predisposing to bleeding, and alcohol use (INR lability not included). The components of the ABC-stroke score include age, NT-proBNP, hsTnT, and prior stroke/TIA. The components of the ABC-bleeding score include age, prior bleeding, hemoglobin, hsTnT, and GDF-15. The “comprehensive models” include the all of the clinical and biomarker variables that were included in the comprehensive models from the derivation cohort15, 16 (*) (shown Figure 2). Notably, GDF-15 and cystatin C were included in the full bleeding model but not in the comprehensive S/SEE model in the derivation cohort.15, 16 In addition, D-dimer, which had been included in a previous multimarker risk score for S/SEE and death derived in ENGAGE AF-TIMI 48,14 was not included in the comprehensive S/SEE model in the derivation cohort.15 Therefore, the C-index for an additional model that included the full model for S/SEE in the derivation cohort plus GDF-15, cystatin C, and D-dimer is shown (†). CI indicates confidence interval; GDF-15, growth differentiation factor-15; hsTnT, high-sensitivity troponin T; INR, international normalized ratio; NT-proBNP, N-terminal B-type natriuretic peptide; S/SEE, stroke or systemic embolic event; TIA, transient ischemic attack.
The strongest predictors of major bleeding were age, history of bleeding, hemoglobin, and GDF-15. In our cohort, hsTnT was a statistically significant but less strong predictor of bleeding, with an effect size similar to alcohol use, current smoking, and history of peripheral artery disease (Figure 2b). The ABC-bleeding score variables accounted for 90.3% of the prognostic information provided by the clinical and biomarker variables included in a comprehensive model in the derivation cohort.
The ABC-stroke risk score applied using model coefficients derived in our cohort yielded a c-index of 0.67 (95% CI, 0.65 – 0.70) for prediction of S/SEE, compared with a c-index of 0.59 (95% CI, 0.57 – 0.62) for the CHA2DS2-VASc score (p<0.001). The ABC-stroke risk score discrimination applied using the coefficients from the original derivation model was highly consistent with a c-index of 0.66 (95% CI, 0.63 – 0.68) for prediction of S/SEE. The overall net reclassification improvement (NRI) at 1 year was 25.2% (95% CI, 13.1% – 46.1%), with similar proportions of correct upward and downward reclassification (Table 3). In a sensitivity analysis using 0.75% as a single threshold for 1-year risk of S/SEE, the NRI at 1 year was 11.7% (95% CI, 7.0% – 18.6%).
Table 3. Net Reclassification Improvement.
| Stroke or Systemic Embolism (Number of Patients with Events) | ||||
|---|---|---|---|---|
|
1-year Risk of S/SEE
Predicted
by CHA2DS2-VASc Model |
1-year Risk of S/SEE Predicted by ABC-Stroke Model | |||
| <1% | 1–2% | >2% | ||
| <1% | 1 | 1 | 1 | |
| 1–2% | 14 | 37 | 41 | |
| >2% | 3 | 12 | 29 | |
| Stroke or Systemic Embolism (Number of Patients with No Events) | ||||
|
1-year Risk of S/SEE
Predicted
by CHA2DS2-VASc Model |
1-year Risk of S/SEE Predicted by ABC-Stroke Model | |||
| <1% | 1–2% | >2% | ||
| <1% | 517 | 113 | 5 | |
| 1–2% | 1868 | 2870 | 1188 | |
| >2% | 124 | 604 | 966 | |
| Risk Score Comparison | ||||
| ABC-Stroke vs. CHA2DS2-VASc Risk Scores | Percent Reclassification (n) | |||
| Correctly Upclassified | 31.9% (43) | |||
| Incorrectly Downclassfied | 20.9% (29) | |||
| Incorrectly Upclassified | 15.8% (1306) | |||
| Correctly Downclassified | 31.4% (2596) | |||
| Net Reclassification Improvement at 3 years | 25.2% (95% CI, 13.1% – 46.1%) | |||
| Major Bleeding (Number of Patients with Events) | ||||
|
1-year Risk of Major
Bleeding Predicted by HAS-BLED Model |
1-year Risk of Major Bleeding Predicted by ABC-Bleeding Model | |||
| <1% | 1–2% | >2% | ||
| <1% | 0 | 0 | 0 | |
| 1–2% | 1 | 6 | 3 | |
| >2% | 0 | 32 | 209 | |
| Major Bleeding (Number of Patients with No Events) | ||||
|
1-year Risk of Major
Bleeding Predicted by HAS-BLED Model |
1-year Risk of Major Bleeding Predicted by ABC-Bleeding Model | |||
| <1% | 1–2% | >2% | ||
| <1% | 0 | 0 | 0 | |
| 1–2% | 287 | 512 | 147 | |
| >2% | 290 | 1460 | 4268 | |
| Risk Score Comparison | ||||
| ABC-Bleeding vs. HAS-BLED Risk Scores | Percent Reclassification (n) | |||
| Correctly Upclassified | 1.2% (3) | |||
| Incorrectly Downclassfied | 13.1% (33) | |||
| Incorrectly Upclassified | 2.1% (147) | |||
| Correcty Downclassified | 29.2% (2037) | |||
| Net Reclassification Improvement at 3 years | 13.8% (95% CI, 8.0% – 22.8%) | |||
CI indicates confidence interval; S/SEE, stroke or systemic embolic event.
The ABC-bleeding risk score in our cohort yielded a c-index of 0.69 (95% CI 0.66 – 0.71) for prediction of major bleeding, compared with a c-index of 0.62 (95% CI, 0.60 – 0.64) for the HAS-BLED score (p<0.001). Similarly, the ABC-bleeding risk score applied using model coefficients from the original derivation cohort yielded a c-index of 0.67 (95% CI, 0.65 – 0.70) for prediction of major bleeding. The overall NRI at 1 year was 13.8% (95% CI, 8.0% – 22.8%) with predominantly correct downward reclassification (Table 3).
Moreover, the ABC-stroke and ABC-bleeding risk scores accurately stratified patients irrespective of CHA2DS2-VASc (ptrend <0.001) and HAS-BLED risk score categories (ptrend <0.001), respectively (Figure 3); this pattern was consistent across all treatment groups (Supplemental Figure 1). In addition, the ABC-stroke and ABC-bleeding risk scores further stratified S/SEE and bleeding risk within categories of the previously reported TIMI-AF clinical risk score,25 though it should be noted that the TIMI-AF risk score was designed to predict net clinical outcomes rather than S/SEE or bleeding alone (Supplemental Figure 2).
Figure 3. Annualized rates of (A) stroke or systemic embolic event stratified by the CHA2DS2-VASc and ABC-stroke risk scores and (B) major bleeding stratified by the HAS-BLED and ABC-bleeding risk scores.
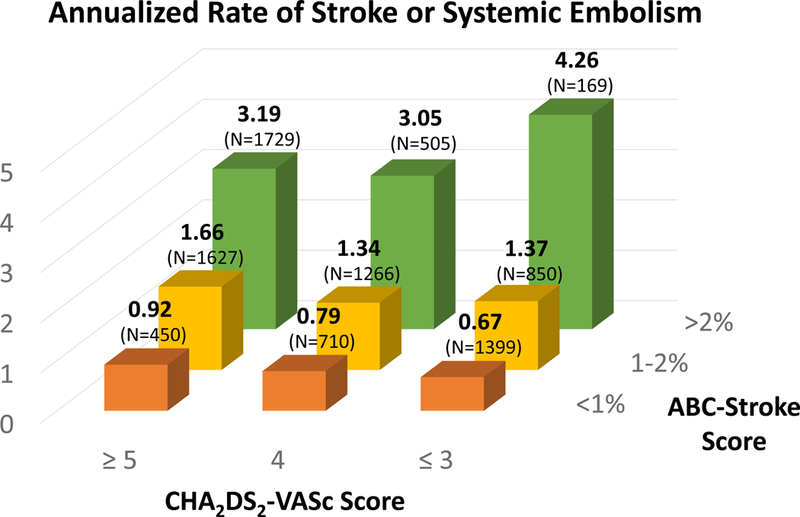
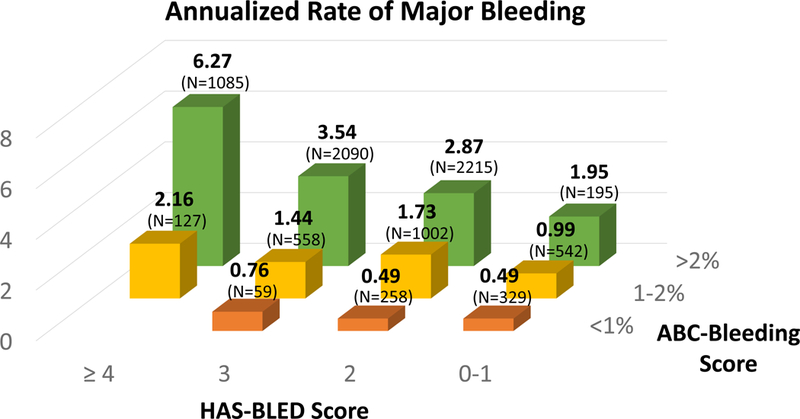
Observed annualized event rates are shown as percent of patients at-risk. (A) The ABC-stroke risk score accurately stratified patients irrespective of CHA2DS2-VASc risk score categories (ptrend <0.001). (B) The ABC-bleeding risk score accurately stratified patients irrespective of HAS-BLED risk score categories (ptrend <0.001).
The ABC-stroke and ABC-bleeding risk scores were well calibrated, with observed cumulative incidence rates of S/SEE and major bleeding within each stratum of risk matching the 1-year risks predicted by the ABC-stroke and ABC-bleeding scores using both the coefficients for the regression models from ENGAGE AF-TIMI 48 (Figure 4) as well as those from the original derivation cohort (Supplemental Figure 3); this pattern was consistent across all treatment groups (Supplemental Figure 4). Furthermore, the Nam-D’Agostino statistics for calibration (non-significant p-values indicate adequate calibration) for the ABC-stroke and ABC-bleeding scores at 3 years were 14.0 (p=0.12) and 14.6 (p=0.10), respectively (Supplemental Figure 5). This was also consistent across all treatment groups (Supplemental Figure 6).
Figure 4. Cumulative incidence of stroke or systemic embolism and major bleeding stratified by 1-year risk estimates from the ABC scores.
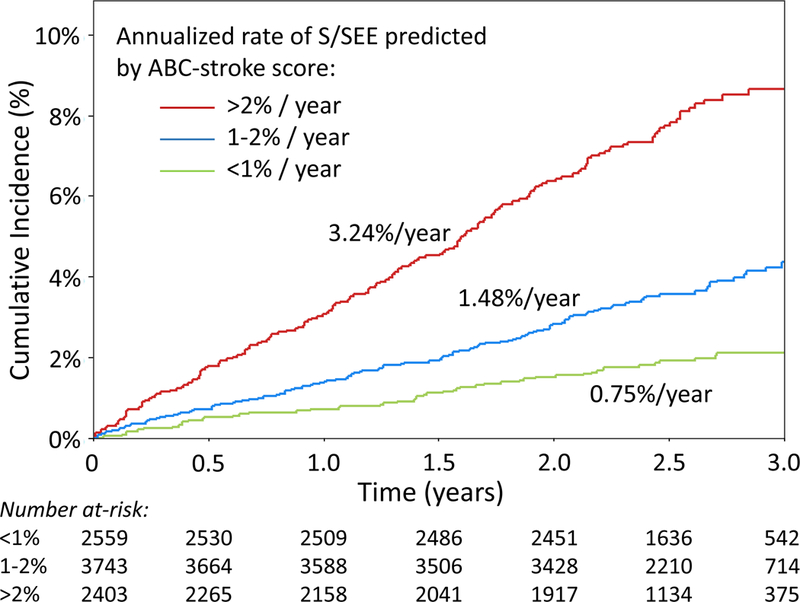
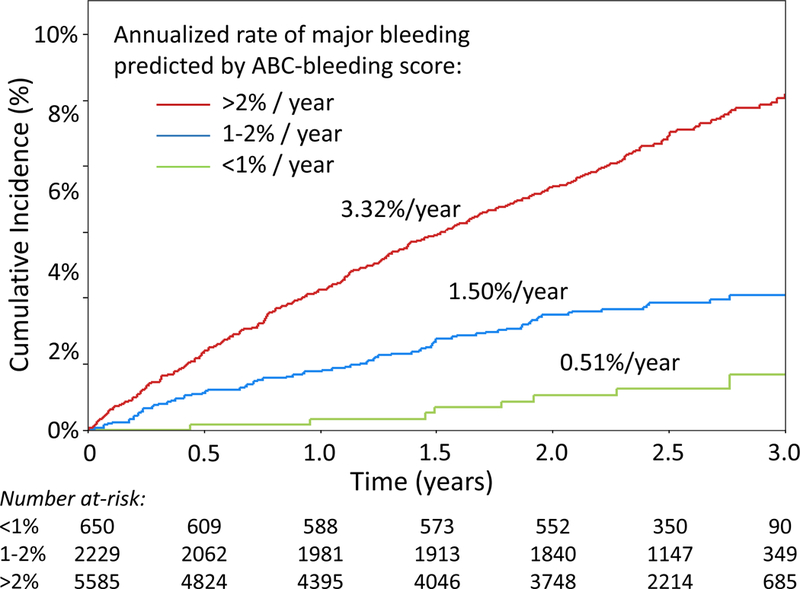
(A) The observed cumulative incidence of S/SEE over the first three years following trial enrollment was stratified by the 1-year risk of S/SEE predicted by the ABC-stroke score (<1%, 1–2%, >2%). The observed annualized event rates (shown for each risk group) were significantly different across strata (p<0.001). (B) The observed cumulative incidence of major bleeding over the first three years following trial enrollment was stratified by the 1-year risk of major bleeding predicted by the ABC-bleeding score (<1%, 1–2%, >2%). The observed annualized event rates (shown for each risk group) were significantly different across strata (p<0.001). S/SEE indicates stroke or systemic embolic event.
Treatment interactions
In comparisons of each edoxaban treatment group vs. warfarin, there was no heterogeneity in the performance of either the ABC-stroke score (HDER vs. warfarin, p-interaction=0.99; LDER vs. warfarin, p-interaction=0.94) or the ABC-bleeding score (HDER vs. warfarin, p=0.84; LDER vs. warfarin, p=0.95) (Supplemental Table 4).
For patients with ABC-bleeding scores predicting 1-year major bleeding risks of <1% (low), 1–2% (intermediate), and >2% (high), observed annualized major bleeding rates were 0.37%, 1.54%, and 3.92% in the HDER arm, and 0.43%, 1.01%, and 2.66% in the LDER arm, respectively. Compared with patients in the warfarin arm, the absolute reduction in annualized major bleeding rates were 0.35%, 0.40%, and 0.72% for patients in the HDER arm with low, intermediate, and high risk of major bleeding, and 0.29%, 0.93%, and 1.98% for patients in the LDER arm.
For patients with ABC-stroke and ABC-bleeding scores predicting 1-year S/SEE and major bleeding risks of <1%, the rates of the NCO between patients treated with edoxaban vs. warfarin were very similar (HDER, 1.48%/year; LDER, 1.43%/year; warfarin, 1.38%/year) (Table 4). However, for patients with ABC-bleeding scores predicting 1-year major bleeding risks of >2%, observed rates of the NCO were more favorable in both the HDER and LDER arms compared to warfarin regardless of stroke risk. Among patients with elevated ABC-bleeding scores (>2%), those with elevated ABC-stroke scores (>2%) derived the greatest benefit from HDER (NCO: HDER, 8.31%/year; LDER, 9.22%/year; warfarin, 10.91%/year), and those with low ABC-stroke scores (<1%) derived the greatest benefit from LDER (NCO: HDER, 3.57%/year; LDER, 2.44%/year; warfarin, 4.25%/year) (Table 4).
Table 4. Annualized event rates for clinical outcomes in the higher-dose edoxaban, lower-dose edoxaban, and warfarin arms stratified by ABC-stroke and ABC-bleeding risk scores.
| 1-year Risk of Bleeding Predicted by ABC-Bleeding Model | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| < 1 % | 1–2 % | > 2 % | |||||||||
| LDER | HDER | WAR | LDER | HDER | WAR | LDER | HDER | WAR | |||
|
1-year Risk of S/SEE
Predicted by ABC-Stroke Model |
<1% | N | 175 | 195 | 208 | 390 | 386 | 388 | 269 | 237 | 239 |
| Stroke/SEE | 0.43 | 0.77 | 0.18 | 0.38 | 0.78 | 0.87 | 1.26 | 0.48 | 1.09 | ||
| Bleeding | 0.48 | 0.42 | 0.59 | 0.84 | 1.61 | 1.89 | 1.46 | 2.44 | 3.50 | ||
| NCO | 1.43 | 1.48 | 1.38 | 1.67 | 2.80 | 2.64 | 2.44 | 3.57 | 4.25 | ||
| 1–2% | N | 18 | 28 | 19 | 307 | 308 | 322 | 884 | 904 | 844 | |
| Stroke/SEE | 0.00 | 2.84 | 2.02 | 1.11 | 1.62 | 0.97 | 1.91 | 1.16 | 1.66 | ||
| Bleeding | 0.00 | 0.00 | 2.19 | 0.96 | 1.58 | 2.18 | 2.17 | 3.68 | 4.05 | ||
| NCO | 0.00 | 4.71 | 2.19 | 2.35 | 3.59 | 4.12 | 4.86 | 5.62 | 5.96 | ||
| >2% | N | 1 | 3 | 3 | 47 | 29 | 52 | 737 | 732 | 739 | |
| Stroke/SEE | 0.00 | 0.00 | 0.00 | 2.68 | 2.77 | 4.62 | 3.58 | 2.92 | 3.21 | ||
| Bleeding | 0.00 | 0.00 | 0.00 | 3.14 | 0.00 | 0.86 | 3.85 | 4.83 | 5.85 | ||
| NCO | 0.00 | 0.00 | 0.00 | 4.18 | 4.91 | 5.30 | 9.22 | 8.31 | 10.91 | ||
The net clinical outcome is a composite of stroke, systemic embolic event, major bleeding, and all-cause mortality. HDER indicates high-dose edoxaban regimen; LDER, low-dose edoxaban regimen; NCO, net clinical outcome; S/SEE, stroke or systemic embolic event; WAR, warfarin.
Discussion
In this nested prospective biomarker substudy of the ENGAGE AF-TIMI 48 trial, we demonstrated that hsTnT, NT-proBNP, and GDF-15—three circulating biomarkers of underlying cardiovascular disease—were independently associated with risk of S/SEE, and that hsTnT and GDF-15 were independently associated with risk of major bleeding in patients with AF. Moreover, we independently confirmed the prognostic performance of the ABC-stroke and ABC-bleeding risk scores for the prediction of S/SEE and major bleeding, respectively, in a well-characterized anticoagulated cohort from a large multinational clinical trial. New compared with previous work, we found that correct upward and downward reclassification of stroke risk and predominantly correct downward reclassification of bleeding risk may identify patients whose risk-benefit profile more clearly favors treatment, ameliorating the current treatment gap that exists in patients with AF. While a high bleeding risk in itself should not automatically result in the decision not to anticoagulate as stroke risk tracks along with bleeding risk, using the ABC-bleeding score, which provides a more precise estimate of bleeding risk, may empower physicians and patients to make better informed treatment decisions. In addition, we showed that simultaneous application of the ABC-stroke and ABC-bleeding risk score scores can identify patients who are most likely to derive a benefit from treatment with NOACs as compared to warfarin. Lastly, extending beyond prior work, we demonstrated the stable prognostic contribution of the biomarkers after 1 year of clinical follow-up while continuing anticoagulation, and found that increases in these biomarkers over a 12-month period were independently associated with increased risk of S/SEE and major bleeding. These data support the concept that biomarker-based risk scores improve prediction of S/SEE and major bleeding in patients with AF as compared to established clinical risk scores, may be useful for therapeutic decision-making, and warrant consideration in management guidelines.
Potential for Application of the ABC Scores in Clinical Practice
Although risk scores based on clinical variables only, including the CHA2DS2-VASc and HAS-BLED scores, are widely used by clinicians, multiple studies have demonstrated modest prognostic performance.14–16 In addition, several prior studies have established that incorporating multiple as opposed to single biomarkers of cardiovascular disease into risk prediction tools enhances their discriminatory performance, perhaps by capturing orthogonal information about distinct pathways implicated in thrombosis and vascular integrity. For example, in a previous analysis from the ENGAGE-TIMI 48 trial, incorporating a conventional assay for cardiac troponin I, NT-proBNP, and D-dimer into a multimarker risk score for S/SEE and death significantly enhanced prognostic accuracy of the CHA2DS2-VASc score.14 Among the risk prediction tools that have been developed for patients with AF, the ABC-stroke and ABC-bleeding risk scores have been proposed for routine clinical use27; however, their performance in validation cohorts has been variable. Specifically, while the ABC-stroke and ABC-bleeding risk scores outperformed the CHA2DS2-VASc and HAS-BLED scores in the original validation cohorts derived from the STABILITY (n=1400) and RE-LY (n=8468) trials, respectively,15, 16 the prognostic performance of a modified ABC-bleeding score was not significantly better than the HAS-BLED risk score in a smaller non-clinical trial cohort from the Murcia Atrial Fibrillation Project (n=1120).17, 18 Although the differential performance of the modified ABC-bleeding score in this non-clinical trial cohort may have been related to incomplete biomarker data (GDF-15 was not included in the risk score) and/or inadequate power, it has also been suggested that it may have been related to the higher prevalence of non-AF comorbidities in this population.17, 18 Since the circulating biomarkers in the ABC scores are elevated in multiple different disease states,28, 29 it is possible that patients with more non-AF comorbidities have serum biomarker levels that do not necessarily reflect their thrombotic or bleeding risk. Although this analysis was performed in a clinical trial population, ENGAGE AF-TIMI 48 enrolled a higher risk population with a greater burden of comorbid diseases compared to the clinical trial populations in which the ABC scores were derived and initially validated.30–32 The results of this analysis therefore represent an important step forward in establishing a robust evidence-base toward potential clinical use of the ABC scores by providing additional realistic estimates of their discriminatory and reclassification performance.
Although there was some variability in the relative effect sizes of the individual components of the ABC-bleeding score in our cohort compared with the original derivation cohort, the overall discriminatory performance of both the ABC-stroke and ABC-bleeding scores was similar between cohorts. Moreover, when evaluated collectively, the ABC-stroke and ABC-bleeding score variables accounted for 94.3% and 90.3% of the prognostic information provided by comprehensive models for S/SEE and major bleeding,15, 16 respectively. These data indicate that traditional clinical risk factors other than age and prior history of S/SEE and bleeding add very little additional discriminatory information beyond what is provided by the cardiovascular biomarkers included in the ABC-stroke and ABC-bleeding scores.
The implications of these findings are several-fold. First, given the reproducible performance of the ABC-stroke and ABC-bleeding risk scores for predicting S/SEE and major bleeding in patients with AF on anticoagulaton, clinicians should consider using these biomarker-based risk scores rather than traditional clinical risk scores when making decisions about oral anticoagulation. For example, simultaneous application of these instruments may help to identify patients at increased risk for S/SEE and relatively lower risk for major bleeding in whom anticoagulation would be favored. Importantly, however, there are no data yet available regarding the performance of the ABC risk scores in a population of AF patients not receiving anticoagulation, where application of the ABC scores would be particularly useful. Since all patients in the ENGAGE AF-TIMI 48 trial received anticoagulation, the results reported in this analysis should not be interpreted as identifying patients at sufficiently low risk of S/SEE to defer anticoagulation. Additional validation of the ABC scores in a lower risk population and in patients not on anticoagulation would be necessary to establish the evidence base to support such application of the ABC-stroke score. Second, given the relative stability of the biomarker values over a 12-month period, and the consistent risk prediction between the primary analysis of baseline biomarker values and the landmark analysis using biomarker values at 12 months post-randomization, the ABC scores appear to provide steady and robust estimates of risk. Finally, the ABC risk scores seem to perform equally well in patients treated with warfarin and edoxaban, helping to identify patients who derive the greatest absolute benefit from treatment with edoxaban compared to warfarin. Specifically, among patients with ABC-bleeding scores predicting 1-year major bleeding risk of >2%, those with high ABC-stroke scores (i.e., predicting 1-year S/SEE risk >2%) derive the greatest net clinical benefit from HDER, and those with low ABC-stroke scores (<1%) derive the greatest net clinical benefit from LDER. This finding illustrates the potential of the ABC-stroke score to identify low-risk individuals who are candidates for less intensive anticoagulation. By enhancing precision of estimated stroke and bleeding risks, and directly informing treatment decisions, the ABC scores support the movement towards precision medicine. Nevertheless, the decision to use any biomarker-based tool for risk stratification might be individualized and weighed against the ease of using traditional clinical risk scores, which do not require laboratory testing. For example, it may not be necessary to perform additional testing for risk stratification when the risk-benefit calculus clearly favors oral anticoagulation even though application of a biomarker-based score may provide a more precise estimate of risk.
Limitations
Several limitations of this analysis should be acknowledged. All patients in the ENGAGE AF-TIMI 48 trial received anticoagulation, whereas the CHA2DS2-VASc score was developed in patients who were not receiving anticoagulants. Despite this difference, prior studies of the CHA2DS2-VASc score have shown similar discriminatory performance in patients receiving8, 9 and not receiving anticoagulation.33, 34 Moreover, in our analysis, the ABC-stroke score outperformed the CHA2DS2-VASc score among patients treated with LDER (a less effective regimen for S/SEE prevention than HDER or warfarin). These data suggest that the ABC-stroke score is robust across varying levels of anticoagulation exposure. Still, it should be clear that our analysis provides a better estimate of major bleeding risk on anticoagulation than of S/SEE risk off anticoagulation. Since this is the primary clinical question of interest to clinicians, it would be ideal to assess the performance of the ABC-stroke score in a population of AF patients not receiving anticoagulation as well. Given the widespread use of anticoagulation in all but the lowest-risk patients in the modern era, it may not be feasible or ethical to conduct such an analysis in this population; however, doing so in a low-risk population not on anticoagulation in future validation studies would be useful for addressing this knowledge gap.
In addition, this validation was performed in a clinical trial cohort, which may affect the generalizability of the findings. Although ENGAGE AF-TIMI 48 enrolled a higher risk population with a greater burden of comorbid diseases compared to the clinical trial populations in which the ABC scores were derived,30–32 additional well-powered studies in non-clinical trial cohorts would be helpful to confirm whether the ABC risk scores will improve discrimination of S/SEE and bleeding outcomes when applied to unselected populations in clinical practice.
Other limitations of our analysis deserve mention. First, since the ENGAGE AF-TIMI 48 trial excluded patients at low risk for S/SEE (i.e., CHADS2 0–1), the performance of the ABC scores in those patients cannot be assessed. Second, by excluding one year of events, our power was diminished in the landmark analyses performed starting at 12 months. Third, these results do not address whether analytical variability in assay performance in the general population may have any meaningful impact on the estimation of risk. Finally, this analysis does not address the cost effectiveness of using the ABC scores over traditional clinical risk scores to estimate risk of S/SEE and major bleeding; this is an important question for the field.
Conclusion
The ABC-stroke and ABC-bleeding risk scores re-estimated in this anticoagulated clinical trial cohort were well-calibrated and had significantly better discriminatory performance than the CHA2DS2-VASc and HAS-BLED clinical risk scores for the prediction of S/SEE and major bleeding, respectively. Irrespective of the CHA2DS2-VASc and HAS-BLED risk scores, the ABC-stroke and ABC-bleeding risk scores accurately stratified risk of these clinical outcomes. Application of these scores may help to identify patients who are most likely to derive a benefit from treatment with NOACs compared to warfarin. Incorporating these biomarker-based risk scores into clinical practice may significantly improve the risk-benefit assessment of patients with AF considering anticoagulation.
Supplementary Material
Clinical Perspective.
What is new?
In this independent external validation, the ABC-stroke and ABC-bleeding risk scores performed well for stratifying the risk of stroke or systemic embolic events (S/SEE) and major bleeding in a well-characterized anticoagulated cohort from a large multinational trial.
Compared with the CHA2DS2-VASc score, the ABC-stroke score provides both correct upward and downward reclassification of S/SEE risk. Compared with the HAS-BLED score, the ABC-bleeding score results in predominantly correct downward reclassification of bleeding risk.
Increases in high-sensitivity troponin T (hsTnT), N-terminal-B-type natriuretic peptide (NT-proBNP), and growth differentiation factor (GDF)-15 over 12-months were independently associated with increased risk of S/SEE and major bleeding.
What are the clinical implications?
Our analysis suggests that incorporating the biomarker-based ABC-stroke and ABC-bleeding risk scores into clinical practice may improve the risk-benefit assessment of patients with AF considering anticoagulation.
Application of these scores may help to identify patients who are most likely to derive a clinical benefit from treatment with non-vitamin K antagonist oral anticoagulants (NOACs) compared with warfarin.
Acknowledgments
Sources of Funding
D.D.B. is supported by a T32 postdoctoral training grant from the National Heart, Lung, and Blood Institute (T32 HL007604). The ENGAGE AF-TIMI 48 study was supported by Daiichi Sankyo Pharma Development. The present analysis was supported by a grant from Roche Diagnostics.
Disclosures
D.D.B. has nothing to disclose. C.T.R. reports research grants from Daiichi Sankyo, Boehringer-Ingelheim, and consulting fees from Daiichi Sankyo, Boehringer Ingelheim, Bayer, Portola, and Janssen. P.J. reports research grants from Abbott Laboratories, Amgen, AstraZeneca, Daiichi Sankyo, GlaxoSmithKline, Merck & Co, Roche Diagnostics, Takeda Global Research and Development Center, and Waters Technologies Corporation, and consulting fees from Roche Diagnostics. R.P.G. reports research grants from Amgen, and Merck; honoraria from Amgen, Daiichi Sankyo, Merck; and consulting fees from Amarin, Amgen, Boehringer-Ingelheim, Bristol-Myers-Squibb, CVS Caremark, Daiichi Sankyo, GlaxoSmithKline, Lexicon, Merck, Portola, and Pfizer. F.N. has nothing to disclose. H.J.L. reports employment by Daiichi Sankyo. M.F.M. reports past employment by Daiichi Sankyo. M.G. reports employment by Daiichi Sankyo. E.M.A. reports research grants from Eli Lilly. E.B. reports research grants from Daichi Sankyo. D.A.M. reports research grants from Abbott Labs, Amgen, Astra Zeneca, BRAHMS, Eisai, GSK, Medicines Co, Merck, Novartis, Pfizer, Roche, Takeda, and consulting fees from Modest, Abbott Labs, Astra Zeneca, InCardia, Peloton, Roche, Verseon, Aralez, and Bayer.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Statistics C and Stroke Statistics S. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Jr., Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW and Members AATF. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014;130:2071–2104. [DOI] [PubMed] [Google Scholar]
- 3.Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, Haeusler KG, Oldgren J, Reinecke H, Roldan-Schilling V, Rowell N, Sinnaeve P, Collins R, Camm AJ, Heidbuchel H and Group ESCSD. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J 2018;39:1330–1393. [DOI] [PubMed] [Google Scholar]
- 4.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GY, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL and Zeppenfeld K. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016;18:1609–1678. [DOI] [PubMed] [Google Scholar]
- 5.Wilke T, Groth A, Mueller S, Pfannkuche M, Verheyen F, Linder R, Maywald U, Kohlmann T, Feng YS, Breithardt G and Bauersachs R. Oral anticoagulation use by patients with atrial fibrillation in Germany. Adherence to guidelines, causes of anticoagulation under-use and its clinical outcomes, based on claims-data of 183,448 patients. Thromb Haemost 2012;107:1053–1065. [DOI] [PubMed] [Google Scholar]
- 6.Lubitz SA, Khurshid S, Weng LC, Doros G, Keach JW, Gao Q, Gehi AK, Hsu JC, Reynolds MR, Turakhia MP and Maddox TM. Predictors of oral anticoagulant non-prescription in patients with atrial fibrillation and elevated stroke risk. Am Heart J 2018;200:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waldo AL, Becker RC, Tapson VF, Colgan KJ and Committee NS. Hospitalized patients with atrial fibrillation and a high risk of stroke are not being provided with adequate anticoagulation. J Am Coll Cardiol 2005;46:1729–1736. [DOI] [PubMed] [Google Scholar]
- 8.Hijazi Z, Oldgren J, Andersson U, Connolly SJ, Ezekowitz MD, Hohnloser SH, Reilly PA, Vinereanu D, Siegbahn A, Yusuf S and Wallentin L. Cardiac biomarkers are associated with an increased risk of stroke and death in patients with atrial fibrillation: a Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY) substudy. Circulation 2012;125:1605–1616. [DOI] [PubMed] [Google Scholar]
- 9.Hijazi Z, Oldgren J, Siegbahn A, Granger CB and Wallentin L. Biomarkers in atrial fibrillation: a clinical review. Eur Heart J 2013;34:1475–1480. [DOI] [PubMed] [Google Scholar]
- 10.Hijazi Z, Siegbahn A, Andersson U, Granger CB, Alexander JH, Atar D, Gersh BJ, Mohan P, Harjola VP, Horowitz J, Husted S, Hylek EM, Lopes RD, McMurray JJ, Wallentin L and Investigators A. High-sensitivity troponin I for risk assessment in patients with atrial fibrillation: insights from the Apixaban for Reduction in Stroke and other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial. Circulation 2014;129:625–634. [DOI] [PubMed] [Google Scholar]
- 11.Hijazi Z, Wallentin L, Siegbahn A, Andersson U, Alexander JH, Atar D, Gersh BJ, Hanna M, Harjola VP, Horowitz JD, Husted S, Hylek EM, Lopes RD, McMurray JJ, Granger CB and Investigators A. High-sensitivity troponin T and risk stratification in patients with atrial fibrillation during treatment with apixaban or warfarin. J Am Coll Cardiol 2014;63:52–61. [DOI] [PubMed] [Google Scholar]
- 12.Hijazi Z, Wallentin L, Siegbahn A, Andersson U, Christersson C, Ezekowitz J, Gersh BJ, Hanna M, Hohnloser S, Horowitz J, Huber K, Hylek EM, Lopes RD, McMurray JJ and Granger CB. N-terminal pro-B-type natriuretic peptide for risk assessment in patients with atrial fibrillation: insights from the ARISTOTLE Trial (Apixaban for the Prevention of Stroke in Subjects With Atrial Fibrillation). J Am Coll Cardiol 2013;61:2274–2284. [DOI] [PubMed] [Google Scholar]
- 13.Lip GY, Lane D, Van Walraven C and Hart RG. Additive role of plasma von Willebrand factor levels to clinical factors for risk stratification of patients with atrial fibrillation. Stroke 2006;37:2294–2300. [DOI] [PubMed] [Google Scholar]
- 14.Ruff CT, Giugliano RP, Braunwald E, Murphy SA, Brown K, Jarolim P, Mercuri M, Antman EM and Morrow DA. Cardiovascular Biomarker Score and Clinical Outcomes in Patients With Atrial Fibrillation: A Subanalysis of the ENGAGE AF-TIMI 48 Randomized Clinical Trial. JAMA Cardiol 2016;1:999–1006. [DOI] [PubMed] [Google Scholar]
- 15.Hijazi Z, Lindback J, Alexander JH, Hanna M, Held C, Hylek EM, Lopes RD, Oldgren J, Siegbahn A, Stewart RA, White HD, Granger CB, Wallentin L, Aristotle and Investigators S. The ABC (age, biomarkers, clinical history) stroke risk score: a biomarker-based risk score for predicting stroke in atrial fibrillation. Eur Heart J 2016;37:1582–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hijazi Z, Oldgren J, Lindback J, Alexander JH, Connolly SJ, Eikelboom JW, Ezekowitz MD, Held C, Hylek EM, Lopes RD, Siegbahn A, Yusuf S, Granger CB, Wallentin L, Aristotle and Investigators R-L. The novel biomarker-based ABC (age, biomarkers, clinical history)-bleeding risk score for patients with atrial fibrillation: a derivation and validation study. Lancet 2016;387:2302–2311. [DOI] [PubMed] [Google Scholar]
- 17.Esteve-Pastor MA, Rivera-Caravaca JM, Roldan V, Vicente V, Valdes M, Marin F and Lip GYH. Long-term bleeding risk prediction in ‘real world’ patients with atrial fibrillation: Comparison of the HAS-BLED and ABC-Bleeding risk scores. The Murcia Atrial Fibrillation Project. Thromb Haemost 2017;117:1848–1858. [DOI] [PubMed] [Google Scholar]
- 18.Rivera-Caravaca JM, Roldan V, Esteve-Pastor MA, Valdes M, Vicente V, Lip GYH and Marin F. Long-Term Stroke Risk Prediction in Patients With Atrial Fibrillation: Comparison of the ABC-Stroke and CHA2DS2-VASc Scores. J Am Heart Assoc 2017;6:e006490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Spinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM and Investigators EA-T. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 20.Ruff CT, Giugliano RP, Antman EM, Crugnale SE, Bocanegra T, Mercuri M, Hanyok J, Patel I, Shi M, Salazar D, McCabe CH and Braunwald E. Evaluation of the novel factor Xa inhibitor edoxaban compared with warfarin in patients with atrial fibrillation: design and rationale for the Effective aNticoaGulation with factor xA next GEneration in Atrial Fibrillation-Thrombolysis In Myocardial Infarction study 48 (ENGAGE AF-TIMI 48). Am Heart J 2010;160:635–641. [DOI] [PubMed] [Google Scholar]
- 21.Harrell FE, Califf RM, Pryor DB, Lee KL and Rosati RA. Evaluating the yield of medical tests. JAMA 1982;247:2543–2546. [PubMed] [Google Scholar]
- 22.Harrell FE, Lee KL, Califf RM, Pryor DB and Rosati RA. Regression modelling strategies for improved prognostic prediction. Statistics in Medicine 1984;3:143–152. [DOI] [PubMed] [Google Scholar]
- 23.Harrell FE, Lee KL and Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Statistics in Medicine 1996;15:361–387. [DOI] [PubMed] [Google Scholar]
- 24.Kang L, Chen W, Petrick NA and Gallas BD. Comparing two correlated C indices with right-censored survival outcome: a one-shot nonparametric approach. Statistics in Medicine 2015;34:685–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fanola CL, Giugliano RP, Ruff CT, Trevisan M, Nordio F, Mercuri MF, Antman EM and Braunwald E. A novel risk prediction score in atrial fibrillation for a net clinical outcome from the ENGAGE AF-TIMI 48 randomized clinical trial. Eur Heart J 2017;38:888–896. [DOI] [PubMed] [Google Scholar]
- 26.D’Agostino RBB-HN. Evaluation of the performance of survival analysis models: discrimination and calibration measures. Handbook of statistics 2003;23:1–25. [Google Scholar]
- 27.Hijazi Z, Oldgren J, Siegbahn A and Wallentin L. Application of Biomarkers for Risk Stratification in Patients with Atrial Fibrillation. Clin Chem 2017;63:152–164. [DOI] [PubMed] [Google Scholar]
- 28.Sharma A, Stevens SR, Lucas J, Fiuzat M, Adams KF, Whellan DJ, Donahue MP, Kitzman DW, Pina IL, Zannad F, Kraus WE, O’Connor CM and Felker GM. Utility of Growth Differentiation Factor-15, A Marker of Oxidative Stress and Inflammation, in Chronic Heart Failure: Insights From the HF-ACTION Study. JACC Heart Fail 2017;5:724–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schernthaner C, Lichtenauer M, Wernly B, Paar V, Pistulli R, Rohm I, Jung C, Figulla HR, Yilmaz A, Cadamuro J, Haschke-Becher E, Pernow J, Schulze PC, Hoppe UC and Kretzschmar D. Multibiomarker analysis in patients with acute myocardial infarction. Eur J Clin Invest 2017;47:638–648. [DOI] [PubMed] [Google Scholar]
- 30.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L, Committees A and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 31.Investigators S, White HD, Held C, Stewart R, Tarka E, Brown R, Davies RY, Budaj A, Harrington RA, Steg PG, Ardissino D, Armstrong PW, Avezum A, Aylward PE, Bryce A, Chen H, Chen MF, Corbalan R, Dalby AJ, Danchin N, De Winter RJ, Denchev S, Diaz R, Elisaf M, Flather MD, Goudev AR, Granger CB, Grinfeld L, Hochman JS, Husted S, Kim HS, Koenig W, Linhart A, Lonn E, Lopez-Sendon J, Manolis AJ, Mohler ER, 3rd, Nicolau JC, Pais P, Parkhomenko A, Pedersen TR, Pella D, Ramos-Corrales MA, Ruda M, Sereg M, Siddique S, Sinnaeve P, Smith P, Sritara P, Swart HP, Sy RG, Teramoto T, Tse HF, Watson D, Weaver WD, Weiss R, Viigimaa M, Vinereanu D, Zhu J, Cannon CP and Wallentin L. Darapladib for preventing ischemic events in stable coronary heart disease. N Engl J Med 2014;370:1702–1711. [DOI] [PubMed] [Google Scholar]
- 32.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L, Committee R-LS and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 33.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW and Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001;285:2864–2870. [DOI] [PubMed] [Google Scholar]
- 34.Lip GY, Nieuwlaat R, Pisters R, Lane DA and Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


