Abstract
Communication between the brain and peripheral mediators of systemic inflammation is implicated in numerous psychological, behavioral, and physiological processes. Functional neuroimaging studies have identified brain regions that associate with peripheral inflammation in humans, yet there are open questions about the consistency, specificity, and network characteristics of these findings. The present systematic review provides a meta-analysis to address these questions. Multilevel kernel density analysis of 24 studies (37 statistical maps; 264 coordinates; 457 participants) revealed consistent effects in the amygdala, hippocampus, hypothalamus, striatum, insula, midbrain, and brainstem, as well as prefrontal and temporal cortices. Effects in some regions were specific to particular study designs and tasks. Spatial pattern analysis revealed significant overlap of reported effects with limbic, default mode, ventral attention, and corticostriatal networks, and co-activation analyses revealed functional ensembles encompassing the prefrontal cortex, insula, and midbrain/brainstem. Together, these results characterize brain regions and networks associated with peripheral inflammation in humans, and they provide a functional neuroanatomical reference point for future neuroimaging studies on brain-body interactions.
Keywords: brain, functional magnetic resonance imaging, immunity, inflammation, meta-analysis, multilevel kernel density analysis, neuroimaging, positron emission tomography, stress
Introduction
The brain and peripheral immune system communicate continuously through bidirectional signaling pathways. This communication provides a basis for complex interactions that contribute to a range of physiological processes that are coordinated with behavioral states in health and disease. In particular, interactions between the brain and peripheral mediators of acute and chronic systemic inflammation are implicated in adaptive behavioral and physiological processes, including defending against infectious pathogens (Ben-Shaanan et al., 2016; Tracey, s2009), maintaining physiological homeostasis during experiences of psychological stress (Pfau and Russo, 2015), and altering social behaviors according to context (Filiano et al., 2016). Moreover, dysregulated communication between the brain and peripheral mediators of systemic inflammation may play a role in the pathophysiology of diverse neurological, neuropsychiatric, and physical health conditions, including Alzheimer’s disease (Glass et al., 2010), major depressive disorder (Dantzer et al., 2008), and cardiovascular disease (Gianaros et al., 2014).
To better understand the mechanisms that support brain-inflammation interactions in humans, a growing number of functional neuroimaging studies have focused on characterizing correlations between neural activity and peripheral inflammatory physiology. Other studies have examined manipulations of peripheral inflammatory physiology, including endotoxin and typhoid vaccination exposure, and their effects on neural activity (e.g., Eisenberger et al., 2009; Harrison et al., 2009). However, results from existing functional neuroimaging studies of brain-inflammation interactions have not yet been empirically summarized to identify brain regions that are consistently reported in the literature. Identifying and characterizing these spatially convergent regions has the potential to advance conceptual frameworks of brain-body and brain-inflammation interactions, and to further enable future efforts toward the more precise brain-based classification and prediction of health and disease outcomes that involve the brain and peripheral inflammation (Miller et al., 2017).
Accordingly, in this review, we first provide an overview of the signaling pathways that allow for communication between peripheral inflammation and the brain. Then, we describe candidate brain regions and networks that are thought to be important for representing and regulating peripheral inflammatory physiology. Finally, we describe some of the functional neuroimaging methods used for elucidating these brain regions in humans. We then present results from a quantitative meta-analysis of human functional neuroimaging studies on this topic, with the aims of identifying brain regions that are consistently reported across the literature, examining the specificity of reported regions to experimental factors (e.g., methodological design and psychological tasks), and describing how regions are organized into functional networks and ensembles.
Peripheral inflammatory physiology in health and disease
Inflammation is a complex innate immunologic response that provides the host organism with defense against infectious pathogens or tissue injury. The acute inflammatory response is primarily initiated via local activation of macrophages and production and release of cell-signaling proteins called pro-inflammatory cytokines that serve a broad array of functions in the body. In addition to mediating the acute inflammatory response, cytokines, such as interleukin-6 (IL-6), enter the bloodstream and facilitate the production and release of acute phase proteins, such as C-reactive protein (CRP), by the liver. Following the acute inflammatory response and upon clearing the pathogen, levels of inflammation typically decrease. However, sustained low-level elevations of inflammatory mediators are considered to be detrimental to long-term health, comprising a condition termed chronic or systemic inflammation (Nathan and Ding, 2010). Chronic inflammation may confer risk for several neuropsychiatric, neurodegenerative, and physical health outcomes. Importantly, there are appreciable and stable individual differences in both acute inflammatory responses and chronic inflammation which are determined by multiple and interacting demographic, psychosocial, and physiological factors (Marsland et al., 2017b; McDade et al., 2006; Prather et al., 2009).
Acute inflammatory responses and related aspects of chronic inflammation may increase risk for disease outcomes not only due to effects in the periphery, but also due to interactions with the central nervous system (CNS). It is well established, for example, that inflammatory processes in the periphery are involved in the pathogenesis of coronary artery disease (Libby et al., 2002), hypertension (Vaziri and Rodríguez-Iturbe, 2006), and the metabolic syndrome (Hotamisligil, 2006). In addition, because experiences of acute psychological stress and chronic life event stress associate with reliable increases in peripheral inflammatory physiology in the absence of injury (Kiecolt-Glaser et al., 2003; Marsland et al., 2017b), it is thought that inflammation may comprise an intermediate pathway linking brain-based processes, such as psychological stress, with risk for physical disease outcomes, including those noted above (Gianaros and Wager, 2015). Moreover, inflammatory processes are implicated in the pathogenesis and prognosis of several disorders of the brain that are highly co-morbid with heart disease and other chronic illnesses, including major depression (Raison and Miller, 2011; Wohleb et al., 2016), post- traumatic stress disorder (Lindqvist et al., 2014), chronic pain (Watkins and Maier, 2000), Alzheimer’s disease (Heppner et al., 2015), and premature cognitive decline (Weaver et al., 2002). Accordingly, the frequent comorbidity of these neuropsychiatric conditions with chronic illnesses raises the possibility that altered or dysregulated relationships between peripheral inflammation and the brain may represent a shared pathophysiological mechanism (Anisman and Hayley, 2012).
Pathways linking peripheral inflammatory physiology and the brain
Peripheral inflammatory physiology interacts with the brain via bidirectional signaling pathways [see (Dantzer et al., 2000; Prinz and Priller, 2017; Quan and Banks, 2007) for review]. More specifically, inflammatory processes in the periphery signal the brain via viscerosensory or bottom-up pathways, and are also regulated by the brain via visceromotor or top-down pathways. Within the brain are several brainstem, limbic, basal ganglia, and cortical regions, reviewed below, that have been reported to be jointly involved in these viscerosensory and visceromotor pathways. Their involvement in both pathways suggests that they comprise critical nodes within the brain for exchanging inflammatory and other immune-related signals between the brain and body. Hence, when activated in sequence, these viscerosensory and visceromotor signaling pathways are thought to form reflex loops that involve specific brain regions and networks capable of sensing changes in peripheral inflammatory activity and making adjustments to regulate peripheral inflammation (Tracey, 2009).
Several viscerosensory signaling pathways link peripheral inflammation to the brain. First, peripheral inflammatory cytokines can cross the blood-brain barrier by way of an active transport mechanism (Banks and Kastin, 1991). Second, cytokines such as interleukin-1 (IL-1) can passively cross the blood-brain barrier in areas of increased diffusivity, such as the circumventricular organs and choroid plexus (Blatteis et al., 1983). Third, peripheral inflammation can activate the vagus nerve (Ek et al., 1998), a major immunosensory nerve that in turn propagates inflammatory signals into the CNS via its projections to the nucleus tractus solitarius and ventrolateral medulla, subsequently stimulating a central immune response in the brain (Bluthé et al., 1994; Rinaman, 2007).
In addition to viscerosensory signaling pathways, there are multiple visceromotor signaling pathways by which the brain modulates peripheral inflammatory activity [see (Eisenberger and Cole, 2012; Pavlov and Tracey, 2017) for review]. Such top-down pathways principally include neuroendocrine and autonomic effector mechanisms. Activation of the neuroendocrine effector mechanism chiefly involves the peripheral actions of glucocorticoids controlled by the hypothalamic- pituitary-adrenal (HPA) axis. Glucocorticoids (e.g., cortisol in humans) act via glucocorticoid receptors in immune cells to modulate proinflammatory gene expression, serving to alter (e.g., downregulate) inflammation in homeostatic conditions (Eisenberger and Cole, 2012; Sternberg, 2006). Autonomic effector mechanisms involve activation of the sympathetic and parasympathetic nervous system, the former influencing proinflammatory gene transcription and cytokine production via beta-adrenergic receptor actions (Johnson et al., 2005; Maestroni and Mazzola, 2003), and the latter down-regulating peripheral inflammatory activity via cholinergic outflow of the vagus nerve (Borovikova et al., 2000).
In interim, viscerosensory and visceromotor mechanisms comprise bidirectional pathways and feedback loops by which the brain interacts with peripheral inflammation to shape diverse processes in health and disease. What is currently lacking, however, is a complete understanding of the specific human brain regions and networks that are involved in these loops.
Brain regions and networks implicated in peripheral inflammation
Within the brain, inflammatory signals from the periphery influence local physiological processes such as neurotransmitter metabolism, long term potentiation, and synaptic plasticity [see (Yirmiya and Goshen, 2011) for review]. These signals can also induce a central neuroinflammatory cascade via the activation of microglia (Kreisel et al., 2013). Behaviorally, inflammatory signals and their effects in the brain promote “sickness behaviors”, which are a constellation of cognitive and affective changes that include social withdrawal, reduced nutrient intake, altered reward processing, impaired cognitive capacities, and disturbed sleep (Dantzer et al., 2008; Hart, 1988; Reichenberg et al., 2001). There is substantial overlap between symptoms of sickness behaviors and some core cognitive and affective disturbances ssreported in neuropsychiatric disorders, consistent with the view that dysregulated interactions between peripheral inflammation and the brain may be involved in the pathogenesis of some of these disorders (Dantzer et al., 2008).
A substantial line of animal research has identified candidate brain regions that process viscerosensory signals and control visceromotor pathways in the context of peripheral inflammation. Discrete nuclei in the pons, medulla, and brainstem are implicated in transmitting inflammatory signals between the periphery and higher brain regions. Foremost are the ventrolateral medulla, parabrachial nucleus, and nucleus of the solitary tract (Critchley and Harrison, 2013; Dantzer et al., 2008; Rinaman, 2007). In turn, more rostral limbic structures are involved in sensing these afferent visceral signals and initiating efferent neuroendocrine and autonomic responses. These limbic include the paraventricular nucleus of the hypothalamus, amygdala, hippocampus, bed nucleus of stria terminalis, striatum, and thalamus (Frenois et al., 2007; Heimer and Van Hoesen, 2006; Wrona, 2006). Broadly, neuroimaging studies (reviewed in more detail below) suggest that these limbic, midbrain, and brainstem regions are similarly involved with peripheral inflammation. in human samples (Critchley and Harrison, 2013; Hannestad, 2013; Miller et al., 2013). Moreover, some neuroimaging studies have identified additional striatal and nigral regions of the basal ganglia, as well as networked cortical regions within the insula, anterior cingulate cortex (ACC), and prefrontal cortex (PFC), as being involved as well (Capuron and Miller, 2011; Harrison, 2017; Thayer and Sternberg, 2010). Taken together, animal models and human neuroimaging studies suggest that particular brainstem, limbic, basal ganglia, and cortical regions may be involved in mediating a range of homeostatic, behavioral, and psychological consequences of peripheral inflammation.
In addition to the role of individual brain regions in peripheral inflammation, it is also clear that many of these regions are anatomically and functionally connected, forming brain networks that sense and regulate peripheral inflammation. In one well-characterized example of these networks, inflammatory signals reach the brain via the parabrachial nucleus to the hypothalamus, which in turn initiates a neuroendocrine reflex loop whereby corticotrophin-releasing hormone is secreted to the pituitary gland (Sternberg, 2006). In addition, several of these networks are classified based on their cortical, limbic, (i.e., corticolimbic) and striatal (i.e., corticostriatal) interconnections, and are implicated in several psychological process that are altered in the context of peripheral inflammation, including psychological stress (Myers et al., 2016) and reward processing (Harrison et al., 2009a; Nusslock and Miller, 2016). Finally, large-scale intrinsic networks consist of anatomically and functionally connected brain regions that exhibit coherent activity as measured by neuroimaging. Several of these intrinsic networks, including the “limbic,” “default mode,” and “ventral attention” networks, are comprised of previously described brain regions and are further implicated in core behavioral, psychological, and disease processes that are known to associate with peripheral inflammation (Barrett and Satpute, 2013; Kleckner et al., 2017; Yeo et al., 2011).
Human neuroimaging studies of brain – peripheral inflammation interactions
An emerging line of human neuroscience research has recently begun to translate animal model findings on brain-inflammation interactions. This translational research is made possible by noninvasive functional neuroimaging methods, of which functional magnetic resonance imaging (fMRI) and fludeoxyglucose positron emission tomography (FDG-PET) are the two most widely utilized. These methods enable measurement of activity in the entire brain simultaneously, which is not possible with many invasive animal study designs. As a result, functional neuroimaging permits the study of the neural correlates of peripheral inflammation throughout the brain in diverse human samples and across a range of behavioral states and psychological processes.
To date, fMRI and FDG-PET studies on neural correlates of peripheral inflammation have mostly been conducted using two general classes of study design, which we here term inflammatory manipulation studies and inflammatory observational studies. In the former, the effect of peripheral inflammatory physiology on the brain is examined by stimulating (inducing) a peripheral inflammatory response and evaluating behavioral and brain responses via self-report, performance on behavioral tasks, and concurrent functional brain imaging. Several inflammatory manipulation studies have been used in humans [see (Hannestad, 2013) for review]. The effect of acute inflammatory manipulation on the brain is investigated, for example, via administration of the Salmonella typhi vaccine, which provokes transient increases in peripheral proinflammatory cytokines (Hingorani et al., 2000), as well as changes in cognitive and affective behaviors (Harrison et al., 2009a). Separately, the effect of chronic inflammation on the brain is investigated, for example, via administration of interferon-alpha (IFN-α) over the course of several weeks, as part of treatment for hepatitis C or melanoma. This treatment produces reports of fatigue in patients, and functional neuroimaging studies suggest that these effects are mediated via altered function in the striatum (Capuron et al., 2012, 2007). The effect of an inflammatory manipulation on the brain is statistically evaluated using within-participant designs (e.g., comparing responses to vaccine versus placebo across different sessions) or between-participant designs (e.g., comparing patients undergoing IFN- α treatment to wait-list controls, or correlating individual differences in stimulus-evoked peripheral inflammatory reactivity). Together, the importance of inflammatory manipulation studies lies in their ability to reveal the effects of induced peripheral inflammation on multiple psychological phenomena, including reward processing (Eisenberger et al., 2010), memory (Harrison et al., 2014), and social cognition (Kullmann et al., 2014). Moreover, neuroimaging studies using these inflammatory manipulation protocols have the ability to identify functional changes in brain regions and networks that in turn are thought to mediate the effect of peripheral inflammation on changes in cognitive and affective behaviors (Harrison et al., 2014, 2009a).
In contrast to study designs using inflammatory manipulations, designs using inflammatory observational designs measure correlations between brain activity and peripheral inflammatory physiology during rest or in response to a task. The latter measures of peripheral inflammatory physiology are measured across various tissues, organ systems, etc., including blood (e.g., IL-6, CRP), saliva, (e.g., soluble tumor necrosis factor-alpha receptor type II), and the airway (e.g., fractional exhaled nitric oxide). It should be noted here that because inflammatory observational studies typically adopt a between-participant or individual differences study design, other factors (e.g., age, adiposity) may complicate or confound interpretations of findings. Hence, in comparison to results from inflammatory manipulations, the strength and reliability of relationships between neuroimaging responses and markers of inflammation in inflammatory observational studies may be less clear. Despite this distinction, inflammatory observational designs are clinically relevant insofar as they use markers of peripheral inflammation that are known to track demographic, psychosocial, and physiological gradients that predict health outcomes (McDade et al., 2006).
Moreover, there is some evidence suggesting that study designs involving inflammatory manipulation and those involving inflammatory correlational observations may reveal similar or common brain regions in association with inflammatory physiology, yet the precise degree of similarity is unknown. For instance, studies using each kind of design have previously reported associations between peripheral inflammation and the amygdala (Inagaki et al., 2012; Swartz et al., 2017), hippocampus (Harrison et al., 2014; Wik et al., 1998), insula (Hannestad et al., 2012b; Tashiro et al., 2001), and ACC (Harrison et al., 2009b; Slavich et al., 2010). Yet, beyond these and other individual sets of findings, it is empirically unknown at present whether peripheral inflammation consistently engages similar brain regions in these two classes of study design.
Aim of the present meta-analysis
Although functional neuroimaging studies have begun to describe the brain regions and networks where activity patterns associate with peripheral inflammation in humans, there is not yet a precise and empirical synthesis of these studies. Several statistical issues, methodological limitations, and sources of heterogeneity across individual studies in this literature support the importance of such a synthesis. First, several studies in this literature include small sample sizes, which makes them subject to low statistical power, questionable reliability, and potentially inflated false positive results (Button et al., 2013; Cremers et al., 2017). Second, there is appreciable heterogeneity in the use of study designs, psychological tasks, and participant samples. As described above, it is unknown whether results from different study designs yield systematically consistent results or identify spatially convergent brain regions and networks. Similarly, these studies employ a range of psychological tasks during neuroimaging assessment and moreover include diverse participant samples, ranging from healthy college students to adults undergoing cancer treatment (see Table 1). Hence, it is unclear whether brain regions reported in these studies are generalizable across factors and people, or instead might be relatively specific to particular contexts and populations. Third, while individual brain regions have been identified in association with inflammatory physiology, what is less clear is the extent to which these regions are encompassed by intrinsic brain networks that are thought to be jointly involved in peripheral physiological control and psychological and behavioral processes impacted by inflammation. Although some emerging neuroimaging studies have begun to identify brain networks and network- level patterns of activity that associate with peripheral inflammation [e.g., (Dipasquale et al., 2015; Harrison et al., 2009a; Marsland et al., 2017a)], these patterns of activity have not yet been described at the meta-analytic level. Indeed, results from neuroimaging meta-analyses can be characterized based on how they spatially correspond with intrinsic brain networks. They can also be used to identify co-activated brain regions across studies, which permits a characterization of spatially distributed functional ensembles whose activity consistently covaries within in the brain (Etkin and Wager, 2007; Kober et al., 2008; Wager et al., 2009a)
Table 1.
Descriptive information of included studies.
| PMID | First Author |
Year | Imagin g |
N | Clinical Sample |
Design | Inflammatory Stimulant |
Inflammatory Marker |
# Contrasts |
# Foci |
Task Type |
Task |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 11140330 | Juengling | 2000 | PET | 11 | hepatitis C | MAN | IFN-α | - | 1 | 7 | REST | - |
| 11747066 | Tashiro | 2001 | PET | 8 | cancer | OBS | - | NK cell | 1 | 12 | REST | - |
| 16084839 | Capuron | 2005 | fMRI | 12 | hepatitis C | MAN | IFN-α | - | 1 | 1 | COG | visuospatial attention |
| 17327884 | Capuron | 2007 | PET | 12 | melanoma | MAN | IFN-α | - | 1 | 5 | REST | - |
| 17913515 | Ohira | 2008 | PET | 11 | - | OBS | - | NK cell | 2 | 10 | COG | mental arithmetic |
| 17977695 | Matsunaga | 2008 | PET | 12 | - | OBS | - | NK cell | 1 | 10 | EMO | viewing emotional films |
| 18242584 | Brydon | 2008 | fMRI | 16 | - | MAN | typhoid | IL-6 | 2 | 3 | COG | color-word Stroop, visual stimulation |
| 19376240 | Eisenberger | 2009 | fMRI | 39 | - | MAN | endotoxin | IL-6 | 2 | 43 | EMO | cyberball |
| 19409533 | Harrison | 2009 | fMRI | 16 | - | MAN | typhoid | - | 2 | 18 | COG | color-word Stroop |
| 19427910 | Ohira | 2009 | PET | 16 | - | OBS | - | NK cell | 2 | 18 | COG | stochastic learning |
| 19481155 | O’Connor | 2009 | fMRI | 18 | - | OBS | - | IL-1β, sTNFαRII |
4 | 22 | EMO | grief induction |
| 20679216 | Slavich | 2010 | fMRI | 31 | - | OBS | - | sTNFαRII | 1 | 19 | EMO | cyberball |
| 20719303 | Eisenberger | 2010 | fMRI | 39 | - | MAN | endotoxin | - | 1 | 10 | EMO | Monetary Incentive Delay |
| 22414635 | Hannestad | 2012 | PET | 9 | - | MAN | endotoxin | - | 1 | 10 | REST | - |
| 22461242 | Kullmann | 2013 | fMRI | 18 | - | MAN | endotoxin | - | 1 | 9 | EMO | viewing emotional pictures |
| 22870208 | Rosenkranz | 2012 | fMRI | 28 | asthma | MAN | allergen | EOS | 1 | 5 | EMO | viewing emotional & disease-relevant words |
| 23416033 | Harrison | 2013 | PET | 20 | - | MAN | typhoid | - | 1 | 2 | REST | - |
| 23547245 | Kullmann | 2014 | fMRI | 18 | - | MAN | endotoxin | - | 1 | 22 | COG | Reading the Mind in the Eyes Test |
| 23684123 | Ohira | 2013 | PET | 10 | - | OBS | - | NK cell | 1 | 3 | COG | stochastic learning |
| 23835929 | Pomykala | 2013 | PET | 23 | breast cancer |
OBS | - | CRP, IL-1Ra, IL-6, sTNFαRII |
4 | 7 | REST | - |
| 24534013 | Harrison | 2014 | PET | 13/7 | - | MAN | typhoid | - | 2 | 7 | REST | - |
| 25016200 | Muscatell | 2015 | fMRI | 31 | - | OBS | - | IL-6 | 1 | 3 | EMO | social feedback |
| 25154706 | Harrison | 2015 | fMRI | 16 | - | MAN | typhoid | - | 1 | 12 | COG | novelty processing |
| 27039241 | Rosenkranz | 2016 | PET | 30 | asthma | OBS | - | FeNO | 2 | 6 | EMO | Trier social stress test |
CRP: C-reactive protein; EOS: eosinophil; FeNO: fractional exhaled nitric oxide; fMRI: functional magnetic resonance imaging; IFN-α: interferon alpha; IL-1β: interleukin-1 beta; IL-1ra: interleukin-1 receptor antagonist; IL-6: interleukin-6; MAN: inflammatory manipulation design; MKDA: multilevel kernel density analysis; NK: natural killer; OBS: inflammatory observational study design; PET: positron emission tomography; PMID: PubMed (www.pubmed.gov) identification; REST: resting-state; sTNFαRII: soluble tumor necrosis factor-alpha receptor type II.
Accordingly, we conducted a meta-analysis of the human functional neuroimaging literature on brain-immune interactions, with a focus on identifying the functional neural correlates of peripheral inflammatory physiology. The first aim was to empirically examine the consistency of reported brain regions in this literature. Brain regions identified by this analysis may be implicated in regulating and responding to peripheral inflammation across a variety of study designs, psychological processes, and participant samples (Kober and Wager, 2010). The second aim was to examine whether reports of any of these brain regions are specific to particular study designs, psychological tasks, or participant samples. The third aim was to describe the network composition and organization of reported brain regions, using two approaches. First, the spatial pattern of the consistency analysis results was compared with known intrinsic brain networks, striatum subdivisions, and corticostriatal loops; second, patterns of co-activated regions across studies were grouped into connections that might reflect functional brain networks or ensembles that are commonly implicated in peripheral inflammation. Our approach and procedures, detailed below, closely conform to recently published guidelines on conducting neuroimaging meta-analyses (Müller et al., in press).
Methods
Study selection
In accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Moher et al., 2009), studies considered here were identified via a systematic search of the PubMed database through April 20161. Search parameters included: “brain” AND (“immune” OR “inflammation” OR “cytokine”) AND (“neuroimaging” OR (“fMRI” OR “functional magnetic resonance imaging”) OR (“PET” OR “positron emission tomography”) OR (“MEG” OR “magnetoencephalography”) OR (“SPECT” OR “single-photon emission computed tomography”)). In addition, reference sections of relevant review papers (Hannestad, 2013; Haroon et al., 2012; Thayer and Sternberg, 2010) were searched. Two investigators (TEK and PJG) screened titles and abstracts of identified records, doubly verifying that records (1) were not review papers, (2) studied humans, and (3) used fMRI or FDG-PET. Duplicate records were removed, and full texts of eligible articles were examined to verify that studies (1) conducted whole-brain analyses, excluding studies using region-of-interest (ROI) analyses or restricted search space, (2) reported coordinates of activation results using a standard template space (i.e., Montreal Neurological Institute (MNI) or Talairach), and (3) used a study design that either stimulated peripheral inflammation (i.e., inflammatory manipulation design) or measured a peripheral marker of inflammatory physiology along with neuroimaging (i.e., inflammatory observational design).
For each study, we considered analyses that described either a main effect of inflammation or an interaction between inflammation and a task condition. As a concrete example of our literature search process, a study by Kullmann and colleagues (Kullmann et al., 2013) reported activations that reflected the main effect of task (i.e., emotional vs neutral visual stimuli) along with another set of activations that reflected the interaction between inflammatory state and task. We only considered the latter set of activations, which reflect brain regions wherein manipulating peripheral inflammatory physiology changes brain function. The former set of activations, on the other hand, does not account for changes or manipulations in peripheral inflammatory physiology, and was therefore not appropriate for the meta-analysis.
We recorded various characteristics of each study, including imaging methodology (e.g., fMRI), study design (i.e., inflammatory manipulation, inflammatory observational), inflammatory stimulus (e.g., typhoid vaccine), and peripheral measure of inflammatory physiology (e.g., circulating IL-6). Because the choices of task paradigms varied substantially, we classified study tasks into broader categories of cognitive (e.g., visuospatial attention, color-word Stroop), emotion (e.g., viewing emotional pictures, grief induction), or resting-state paradigms (see Table 1). Finally, summary results from each study were recorded from reported peak activation coordinates (foci).
Some studies and analyses were excluded based on additional a priori criteria (see Figure 1). Analyses measuring immune markers not clearly related to inflammation (e.g., helper T cell count) were excluded. Results of higher-ordered statistical interactions were not included, because there were too few studies reporting interactions to adequately group with other studies in the meta-analysis. Specifically, we did not include analyses that reported brain activity differences reflecting clinical status (e.g., Rosenkranz et al., 2012), sex differences (e.g., Eisenberger et al., 2009), or individual differences in behavioral (e.g., mood) responses to inflammation (e.g., Harrison et al., 2009). However, studies using clinical samples or combinations of clinical and nonclinical samples were included.
Figure 1. Flow diagram depicting study selection and screening procedures.
Several studies identified in the literature search did not report whole-brain results (Figure 1), and instead conducted ROI analyses. Specifically, these studies conducted ROI analyses using three methods: (a) reducing the analytic search space, e.g., using small volume correction, (b) using functionally-defined (e.g., task-evoked) ROIs, and (c) using anatomically-defined ROIs. We did not include results from these studies in the main analyses because they may bias meta-analytic results by increasing the false positive and false negative rate (Costafreda, 2009). Such studies, however, were incorporated in an ancillary set of consistency analyses (see supplemental material for additional reporting). For studies using reduced search spaces or functionally-defined ROIs, reported activation coordinates were recorded. For studies using anatomical ROIs for which sufficient details were provided, masks were constructed, and center-of-mass coordinates of the resultant masks were recorded.
Consistency analyses: Multilevel kernel density analysis (MKDA)
The consistency analysis used multilevel kernel density analysis [MKDA; (Wager et al., 2009a, 2007)] to identify brain regions that are consistently reported across studies. This procedure nests peak activation coordinates within individual analytic maps, which reduces bias from studies that report more coordinates or use less stringent statistical testing thresholds. Prior to MKDA procedures, coordinates reported in Talairach space were transformed into MNI space using the Brett tal2mni transformation (Brett et al., 2001). The MKDA steps were as follows: (1) construct contrast indicator maps (CIM), which are binary voxel-wise maps that describe, for each voxel, whether an particular study contrast reported an effect within 20 mm; (2) compute an overall density map from weighted averages of each CIM, weighting by the square root of the sample size for each CIM; and (3) perform Monte Carlo simulations using 5000 iterations to compare the overall density map to a null distribution derived from randomly distributed supra-threshold blobs throughout the brain, preserving the spatial structure inherent in each study (i.e., smooth blobs when multiple nearby local peaks are reported).
Significant clusters were identified correcting for multiple statistical testing at a family-wise error rate (FWER) threshold of p < 0.05 while adapting to characteristics of the data. Specifically, we used an extent-based threshold to identify clusters that exceeded chance expectations with respect to the null distribution of cluster size for a given voxel-wise alpha level. Clusters were identified using a primary extent-based threshold with a voxel-wise alpha level of 0.01 to maintain cluster-level FWER p < 0.05 correction. In addition to this primary threshold, some regions surpassed a more stringent, extent-based threshold using alpha of 0.001, as well as a height-based, voxel-wise FWER corrected (p < 0.05) threshold. We note that extent-based thresholds determined by Monte Carlo simulation respect the underlying distributions of the data when constructing the null distribution of cluster size, and hence do not suffer from inflated false positive rates reported in parametric forms of cluster-based extent thresholding (Eklund et al., 2016).
Specificity analyses: Chi-square (χ2) and conjunction
We took clusters identified in the previous analysis (using the primary cluster- based extent threshold) and subsequently conducted three voxel-wise chi-square (χ2) analyses to evaluate the specificity of reported activations in these clusters to three different factors: study design, task paradigm, and sample clinical status (Wager et al., 2009a). Each χ2 analysis tests, at each voxel, whether there are significant differences in the absolute proportion of effects (e.g., activation) associated with a given factor (Agresti, 2002). Analyses of study design grouped studies into inflammatory manipulation or inflammatory observational designs. Analyses of task paradigm grouped studies into emotional, cognitive, and resting state paradigms as described above. Analyses of sample clinical status considered whether the study sample was derived from a clinical population. Because these specificity analyses were secondary to the main consistency analyses, and because there were a low number of studies across the different factors, the results from these specificity analyses were evaluated using a more lenient cluster-based threshold of p < 0.005, 10 voxel extent.
In addition to the χ2 analysis of study design, we were interested in whether studies using inflammatory manipulations and inflammatory observations can independently report effects that are consistent with the pooled, primary MKDA results. To this end, we repeated the MKDA procedure separately for each set of studies and examined the combined spatial overlap of their thresholded results maps using a conjunction analysis (Nichols et al., 2005). Details of this conjunction analysis are in the supplemental material.
Network analyses: Spatial similarity and co-activation
To examine whether regions reported in the consistency analyses reflect large-scale brain networks, we conducted spatial similarity analyses by comparing their profiles with 7 known intrinsic brain networks derived from 1000 individuals using resting-state functional connectivity MRI (Yeo et al., 2011). Specifically, we computed point-biserial correlations between each CIM with maps for each of the 7 intrinsic brain networks, and determined statistical significance using one-sample t- tests on Fisher r-to-z transformed values
We then repeated these spatial similarity analyses using striatal subdivisions and corticostriatal loops derived from a recent meta-analysis of over 5000 neuroimaging studies (Pauli et al., 2016). This large-scale meta-analysis was chosen to serve as an additional reference point for our network analyses, for three reasons. First, Pauli et al. (2016) used meta-analytic co-activation analyses (see below) to derive striatal subdivisions and corticostriatal networks, an approach that may yield networks that are more comparable to our meta-analytic procedures than are resting-state fMRI-derived networks. Second, Pauli et al. (2016) used data-driven term-based analyses to identify associations between their networks and the psychological and behavioral concepts described in the text of constituent studies, presumably providing an unbiased source for characterizing our MKDA results in psychological terms. Third, we were interested in the striatum and its cortical connections because it was strongly implicated in results of the consistency and specificity analyses, and because corticostriatal networks have been implicated in inflammatory physiology and processes (Felger et al., 2016; Harrison et al., 2009a).
Finally, to examine the network organization of reported regions, we tested how patterns of reported co-activation across different brain regions in the sample of 24 neuroimaging studies (37 CIMs) may form functional groupings (Etkin and Wager, 2007; Kober et al., 2008). Here, patterns of reported co-activation between two regions indicate a meta-analytic index of connectivity. First, clusters identified in the consistency analyses were anatomically parcellated to reduce their dimensionality to individual brain regions. Anatomical boundaries for this step were derived from a combination of atlases measuring 91 cortical, subcortical, thalamic, cerebellar, and brainstem regions (Diedrichsen et al., 2009; Johansen-Berg et al., 2005; Shattuck et al., 2008). From this, we created an indicator matrix, in which rows represent each of the 37 CIMs and columns represent each of the 55 atlas regions, and each element coded whether the study contrast map reported an effect within the atlas region. This indicator matrix was then empirically grouped using multivariate dimensionality reduction followed by hierarchical clustering. Briefly, this step used nonmetric multidimensional scaling (NMDS), which is similar to a principal component analysis, but does not assume that the distances in activation across regions are in Euclidean space; thus, it allows for nonlinear monotonic relationships (Shepard, 1980). Solutions of varying numbers of clusters were then computed, and the optimal number of clusters was determined using nonparametric permutation testing. From here, a new indicator matrix was created, with rows representing the 37 CIMs and columns representing the 7 regions defined by the optimal clustering solution.
We repeated the multivariate dimensionality reduction and clustering step to find functional groupings across regions, yet this analysis yielded two functional groupings: a grouping containing the dorsomedial PFC (dmPFC) and dorsal ACC (dACC; see Figure 5, light blue), and another grouping containing all other regions. Given this, we evaluated co-activations among the 7 identified regions by computing Kendall’s Tau-b between each pair of regions using the 37-CIM-by-7-region adjacency matrix. Here, Kendall’s Tau-b reflects the degree to which one brain region is co-activated with another brain region across the 37 CIMs, and hence reflects a meta-analytic index of functional connectivity (Gibbons, 1993; Kober et al., 2008). We adjusted for multiple comparisons at this step using the false discovery rate, q < 0.05 corrected, (Benjamini and Hochberg, 1995) and further removed connections that were statistically mediated by another region (Baron and Kenny, 1986).
Figure 5. Co-activation of identified brain regions forming functionally connected ensembles.
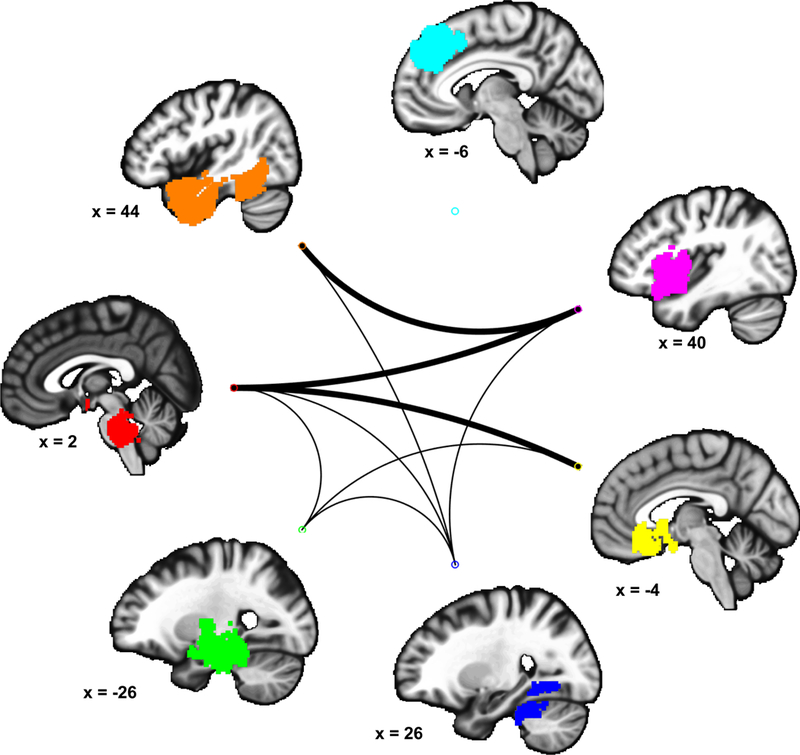
Regions identified by the MKDA results map were grouped into functionally connected ensembles. Lines between two regions indicates they were significantly co-activated (Kendall’s tau-b) across studies. Thick lines: significant co-activation with correction for multiple comparisons, FDR q < 0.05, and not mediated by another brain region. Thin lines: significant co-activation, p < 0.05 uncorrected.
Results
Included studies
A flowchart of the database search and text screening procedure is in Figure 1. Following the literature search and text screening, 24 studies met inclusion criteria for the meta-analysis. In total, the included studies reported 37 analytic maps, 264 coordinates, and 457 participants. Included studies and their attributes are described in Table 1. Seven studies examined clinical samples; these included participants with hepatitis C infection (Capuron et al., 2005; Juengling et al., 2000), melanoma (Capuron et al., 2007), asthma (Rosenkranz et al., 2016, 2012), breast cancer (Pomykala et al., 2013), and multiple cancer types (Tashiro et al., 2001). No included studies examined psychiatric or neurological patient samples. In terms of study design, there were comparable numbers of studies using inflammatory manipulations and inflammatory observations. As expected, the types of psychological tasks administered during scanning varied across studies, yet our task classifications yielded comparable numbers of resting state, emotion, and cognitive studies.
Results of consistency analyses: MKDA
Figure 2 displays the clusters that were consistently reported across studies, identified using our primary, extent-based threshold with a voxel-wise alpha level of 0.01 (purple). In addition to this primary threshold, clusters surpassing a more stringent, extent-based threshold using alpha of 0.001, as well clusters exceeding a height-based, voxel-wise FWER corrected (p < 0.05) threshold are displayed using orange and yellow, respectively. Descriptive statistics of clusters identified using these thresholds are in Table 2.
Figure 2. Consistently reported activations.
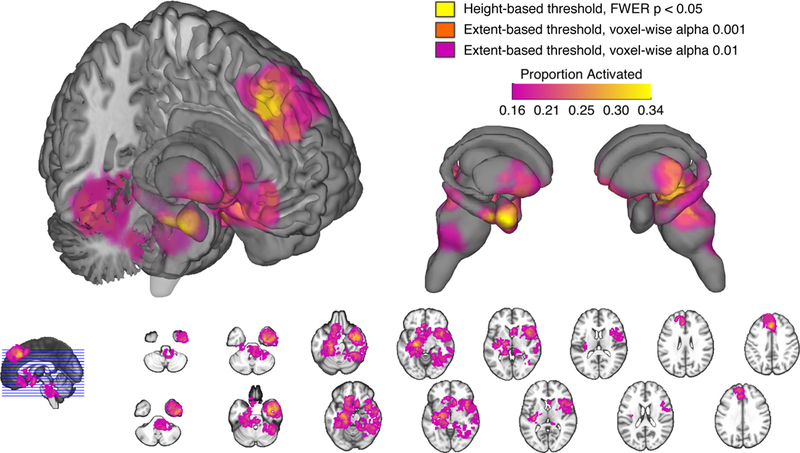
Multilevel kernel density analysis identified brain regions consistently reported across included studies. Results are thresholded according to height-based and extent-based methods. Color bar describes the proportion of studies activating in a given voxel.
Table 2.
Significant clusters from multilevel kernel density analysis (MKDA) results.
| Regions | Side | Center Coordinates (MNI) | Size (voxels) |
Mean Proportion Activated |
|||
|---|---|---|---|---|---|---|---|
| x | y | Z | |||||
|
Height-based Threshold (FWER p < 0.05) |
Amygdala, Hippocampus, Parahippocampal gyrus, Superior temporal gyrus |
R | 36 | 0 | −24 | 75 | 0.31 |
| Lentiform nucleus, Parahippocampal gyrus | L | −22 | −16 | −10 | 26 | 0.31 | |
| Dorsal medial prefrontal cortex, Dorsal anterior cingulate cortex |
L / R | −2 | 28 | 40 | 32 | 0.31 | |
|
Extent-based threshold (alpha 0.001) |
Insula, Inferior temporal cortex, Superior temporal cortex, Temporal pole |
R | 40 | 4 | −20 | 3194 | 0.23 |
| Hippocampus, Parahippocampal gyrus, Fusiform gyrus, Thalamus, Cerebellum, Midbrain |
L | −22 | −22 | −10 | 1739 | 0.23 | |
| Inferior temporal gyrus, Fusiform gyrus, Cerebellum |
R | 42 | −44 | −20 | 356 | 0.20 | |
| Hypothalamus, Putamen, Subgenual anterior cingulate cortex, Orbitofrontal cortex, Ventral medial prefrontal cortex |
L | −10 | 16 | −14 | 628 | 0.22 | |
| Dorsal medial prefrontal cortex, Dorsal anterior cingulate cortex |
L / R | −4 | 34 | 40 | 944 | 0.23 | |
|
Extent-based Threshold (alpha 0.01) |
Amygdala, Hippocampus, Hypothalamus, Thalamus, Parahippocampal gyrus, Caudate Putamen, Insula, Anterior cingulate cortex, Orbitofrontal cortex, Ventral medial prefrontal cortex, Cerebellum, Culmen, Midbrain, Pons |
L / R | 20 | −18 | −18 | 13977 | 0.18 |
| Dorsal medial prefrontal cortex, Dorsal anterior cingulate cortex |
L / R | −8 | 40 | 42 | 2176 | 0.18 | |
FWE: Familywise error; MNI: Montreal Neurological Institute
This analysis revealed consistent effects in several limbic and basal ganglia regions, including the right amygdala, bilateral hippocampus, hypothalamus, and bilateral striatum (caudate and putamen). Within these regions, two clusters encompassing the right amygdala / hippocampus as well as the left lentiform nucleus (bordering the hippocampus) survived the most stringent, voxel-wise FWER corrected threshold. Midbrain and brainstem regions included a large cluster covering several nuclei, including the parabrachial complex, powntine tegmentum, locus coeruleus, substantia nigra, and rostral ventral medulla. Finally, this analysis revealed several cortical regions, including a cluster containing the dACC and dmPFC, which also survived the most stringent, voxel-wise FWER corrected threshold. Another cortical cluster encompassed a large territory of the ventromedial PFC (vmPFC). Remaining clusters were localized to orbitofrontal, temporal, insular, and cerebellar cortices.
Results of specificity analyses: Chi-square (χ2) and conjunction
We conducted three χ2 analyses to examine the specificity of reported effects according to study design, task paradigm, and sample clinical status. The first χ2 analysis of study design revealed a cluster in the right insula (MNI center coordinates [50 4 −6], 572 voxels), as well as a cluster in the right superior temporal gyrus (MNI center coordinates [58 −32 4], 71 voxels). In these clusters, study designs using an inflammatory manipulation reported significantly greater proportions of activation than observational designs (e.g., 45.7% vs 19.2% activating the insula, Figure 3, left). The second χ2 analysis of task paradigm revealed one cluster that included the caudate, subgenual ACC (sgACC), and medial orbitofrontal cortex (OFC, MNI center coordinates [2 20 −6], 430 voxels). Here, studies that employed emotion tasks reported significantly greater proportions of activation (65.5% activating) compared to cognitive (23.9%) and resting state (6.5%) task paradigms (Figure 3, right). The third χ2 analysis of sample clinical status revealed two small clusters in the dmPFC; in these clusters, studies using clinical samples reported significantly greater proportions of activation than studies using nonclinical samples (>42.7% vs <7.6% activating; see supplemental material).
Figure 3. Activations specific to study design and task type.
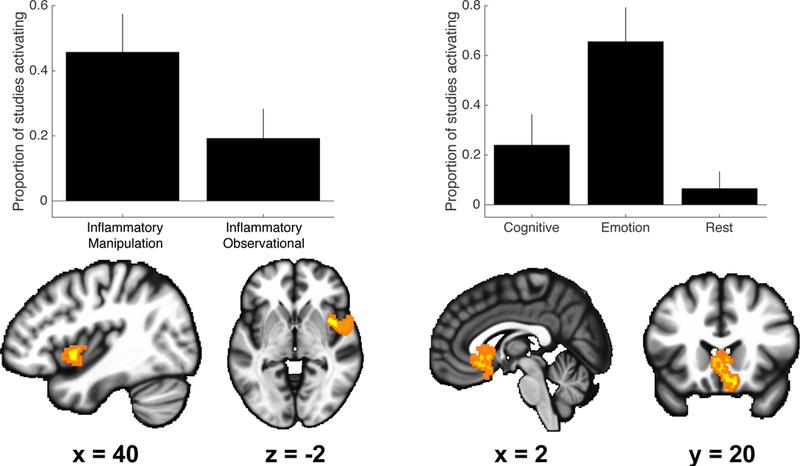
Left: voxel-wise chi-square analysis revealed a cluster encompassing the anterior insula that was specific to study deign. Here, inflammatory manipulation designs reported greater absolute proportions of activations than inflammatory observational designs. Right: voxel-wise chi-square analyses revealed a cluster encompassing the ventral striatum, subgenual anterior cingulate and orbitofrontal cortex that was specific to task type. Here, emotion tasks reported greater absolute proportions of activations than cognitive or resting state tasks. Results presented using p < 0.005 uncorrected threshold. Results of specificity analyses regarding clinical status are described in the supplemental material.
When performing separate consistency analyses for each type of study design (i.e., inflammatory manipulations and observational), a conjunction analysis of their respective thresholded MKDA maps indicated that each study design reported overlapping clusters of consistent effects in the amygdala, hippocampus, striatum, thalamus, posterior insula, dorsal and sgACC, dmPFC, and temporal cortex (Figure S1, magenta), which overlapped considerably with regions identified in the MKDA results that were pooled across study designs. Several regions previously identified by the pooled MKDA results were not observed in both study designs; these include the insula, midbrain, and brainstem (consistently activated by inflammatory manipulations only, yellow) and rostral medial and ventrolateral PFC (consistently activated by observational designs only, blue). Consistent with these findings, statistically comparing the unthresholded results for inflammatory manipulation designs and inflammatory observational designs indicated the pattern of their results were moderately correlated (rho = 0.55) suggesting that both study designs converge on similar patterns of brain regions.
Results of network analyses: Spatial similarity and co-activation
Comparing the spatial similarity of individual study contrast maps to maps reflecting 7 intrinsic brain networks (Yeo et al., 2011) revealed significant overlap with the so-called default mode, limbic, and ventral attention networks (Figure 4, top panel, mean r’s > 0.02, T’s > 2.89, p’s < 0.05). In contrast, individual study contrast maps showed little specificity in their overlap with striatum sub-regions (Pauli et al., 2016), showing significant overlap with all sub-regions except the posterior caudate (Figure 4, bottom left panel, mean r’s > 0.01 T’s > 2.07, p’s < 0.05). Finally, individual study contrast maps exhibited some overlap with corticostriatal loops, (Pauli et al., 2016), particularly ventral striatum, posterior putamen, and anterior caudate loops (mean r’s > 0.02, T’s > 2.30, p’s < 0.03). None of the point-biserial correlations between individual CIMs and the significant network maps were statistically associated with the sample size reported in each CIM (p’s > 0.05, Table S1).
Figure 4. Spatial similarity of identified brain regions to large-scale brain networks.
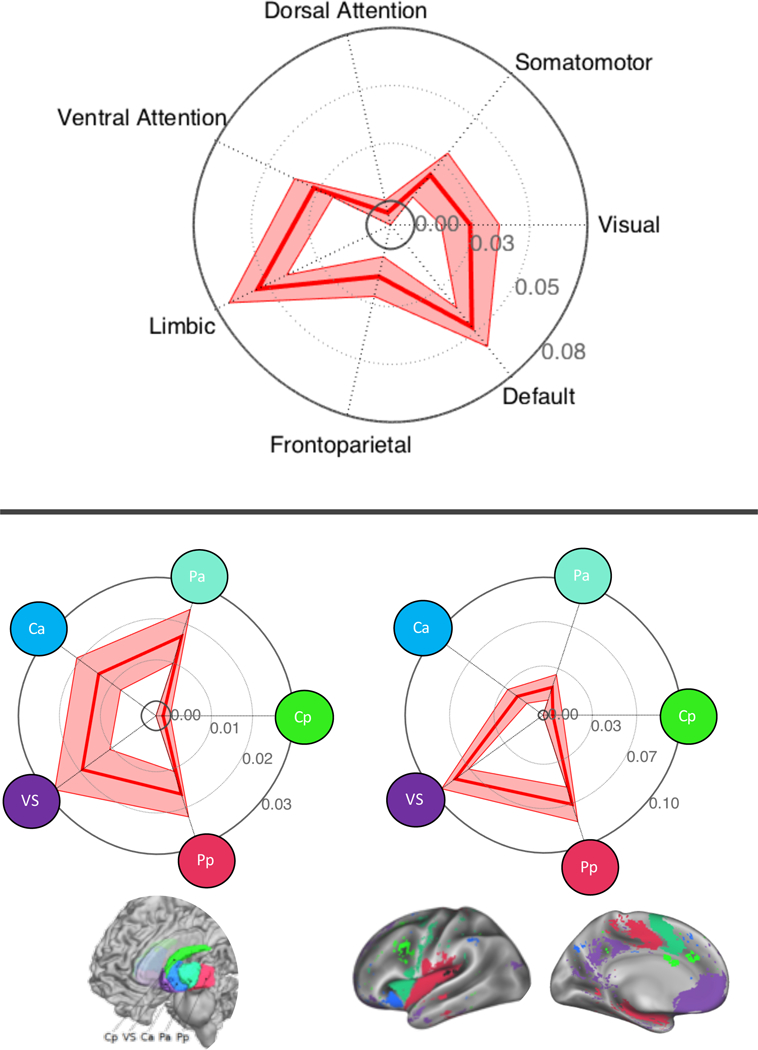
Top panel: Associations (mean point-biserial correlation ± standard error) describing the similarity of contrast indicator maps (CIMs) to intrinsic brain networks derived from resting-state fMRI (Yeo et al., 2011). Bottom panel: Associations describing the similarity of CIMs to striatum subdivisions (left) and corticostriatal loops (right) [from (Pauli et al., 2016)]. VS: ventral striatum; Ca: anterior caudate; Pp: posterior putamen; Pa: anterior putamen; Cp: posterior caudate.
For the network co-activation analyses, we parcellated the MKDA map (cluster-based threshold p < 0.01) into separate anatomical regions as previously described (Kober et al., 2008). Studies reported effects in 55 anatomical parcels; NMDS dimension loadings were computed from these parcels, and a range of clustering solutions were considered. Of these, the 7-region solution was optimal (mean silhouette value = 0.38, 18.88 standard deviation improvement over permuted data, p < 0.001). A statistic of co-activation (Kendall’s Tau-b) was computed for each pair of these 7 regions; significant co-activations are shown in Figure 5. As shown, these functional ensembles of regions included brainstem, limbic, and cortical regions, most notably involving significant connections between the brainstem / pons (red), right anterior insula (magenta), sgACC / vmPFC (yellow), and right amygdala / parahippocampal gyrus (orange). In comparison, the left parahippocampal gyrus (green) and right temporal gyrus (blue) had connections that were significant at an uncorrected threshold (p < 0.05). As described previously, repeating the NMDS and cluster analysis procedure on this 7-region set revealed two functional groupings. The circle plot in Figure 5 effectively illustrates the nature of these groupings: the first consists of the dACC/dmPFC (light blue), and the second consists of all other regions.
Ancillary analyses including ROI-based studies
In a set of ancillary and post-hoc analyses, ROI-based studies were incorporated into the dataset of activation foci, adding 11 studies and 43 coordinates (see supplemental material). The MKDA procedures reported above were repeated on this expanded dataset and thresholded in accordance with the original consistency analysis. The results of this ancillary consistency analysis are provided in Figure S3 in supplemental material. Results revealed comparatively stronger effects for the ‘core’ inflammation-related regions identified in the a priori MKDA results, including the amygdala, striatum, DMPFC, VMPFC, and insula. Notably, however, adding the additional ROI coordinates to the dataset changed the required proportion threshold to achieve family-wise error-rate correction for multiple testing. As a result, other largely posterior brain areas originally identified (e.g., brainstem, occipital cortex) were no longer statistically detectable. Importantly, the unthresholded MKDA map for this ancillary consistency analysis was highly correlated with the original unthresholded MKDA map (Spearman’s rho = 0.96). Taken together, these ancillary analyses indicate that adding the ROI-based studies, while potentially biased (Costafreda, 2009), do agree with and appear to reinforce several of the original key findings.
Discussion
The present meta-analysis provides three sets of novel findings that inform our understanding of brain regions and networks associated with peripheral inflammatory physiology. First, we observed consistently reported effects in limbic, basal ganglia, and brainstem regions, including the amygdala, hippocampus, hypothalamus, striatum, midbrain, and pons, as well as effects in cortical regions spanning the medial prefrontal and temporal cortices. Second, reported effects in some of these brain regions appeared to be specific to particular study designs and psychological tasks: inflammatory manipulation designs and emotional tasks activated some brain regions more consistently than inflammatory observational designs and other types of tasks. Third, patterns of consistent effects overlapped significantly with known intrinsic brain networks, such as the limbic, default mode, and ventral attention networks, as well as corticostriatal loops implicated in stimulus value, sensorimotor control, and action value. Furthermore, co-activation patterns across studies suggest that some of the limbic and cortical regions may be co- activated as ensembles in the context of peripheral inflammatory activity, plausibly constituting functionally connected pathways implicated in brain-inflammation and brain-immune interactions.
To our knowledge, this is the first meta-analysis of existing functional neuroimaging studies of peripheral inflammation. Interestingly, several brain regions and networks identified in our meta-analysis appear consistent with prior meta- analyses of neuroimaging studies of peripheral autonomic and cardiovascular physiology (Beissner et al., 2013; Gianaros and Sheu, 2009; Ruiz Vargas et al., 2016; Thayer et al., 2012). These meta-analyses similarly identified limbic, basal ganglia, and cortical regions such as the amygdala, hippocampus, hypothalamus, striatum, anterior insula, and ACC. The similarity of our meta-analysis with these findings raises the possibility that brain regions and networks characterized here are not specific to peripheral inflammation, but rather more broadly associate with brain systems that integrate and regulate physiological information that is transferred between the brain and internal organs and tissues of the body (Cameron, 2009). While we did not explicitly test whether our results were specific to inflammation, future work could examine how our observed regions and networks encode and regulate common and distinct features of visceral information (e.g., immune, autonomic, pain) [e.g., (Eisenbarth et al., 2016; Gianaros et al., 2017; Wager et al., 2013)].
A contribution of our meta-analysis is that it empirically supports a role for limbic and basal ganglia regions, including the amygdala, hippocampus, hypothalamus, and striatum, in peripheral inflammatory physiology across a variety of human neuroimaging studies. The latter would appear to corroborate animal models that show effects of peripheral inflammation on viscerosensorysss processes in these regions (Frenois et al., 2007; Stone et al., 2006). Additionally, animal stimulation and lesion studies [reviewed in (Wrona, 2006)] implicate these regions in visceromotor control of the peripheral immune response. Moreover, several other neuroimaging studies that use ROI analyses (and hence are not eligible for the present meta-analysis) report associations between peripheral inflammation and activity in these regions (Muscatell et al., 2016a; Swartz et al., 2017; Wik et al., 1998). Finally, network similarity analyses indicated that the MKDA results were similar to a broader “limbic” network that comprises the amygdala, hippocampus, as well as cortical regions including the vmPFC, parahippocampal gyrus, and inferior temporal gyrus (Figure 4, top panel). Taken together, these findings are consistent with animal models that implicate the limbic network and its components in brain–inflammation interactions (Haas and Schauenstein, 1997). The physiological and behavioral significance of these limbic regions in the context of peripheral inflammation are wide-ranging and continue to be defined and debated. Broadly, functional viscerosensory changes in these regions are thought to mediate the effects of inflammation on sickness behaviors and other related affective changes (Dantzer et al., 2008), while some particular limbic regions (e.g., amygdala, hippocampus) are additionally implicated in visceromotor control over neuroendocrine and autonomic outflow (Ménard et al., 2017b). Taken together, the results of our consistency and network similarity analyses highlight and reinforce views on limbic involvement in peripheral inflammatory physiology.
The consistency analysis also identified several midbrain and brainstem regions, including the parabrachial complex, pontine tegmentum, locus coeruleus, substantia nigra, and rostral ventral medulla. These regions extended spatially into an area consistent with the habenula, which is increasingly recognized as important for negative mood states, aversive learning, and hormonal regulation of behavior (Hikosaka, 2010). Interestingly, the midbrain periaqueductal gray was not revealed by the consistency analysis, although there is ample evidence that this region is implicated in the regulation of peripheral physiology (Napadow et al., 2008; Wager et al., 2009b) and is also detectable in neuroimaging studies (Buhle et al., 2012; Linnman et al., 2012; Satpute et al., 2013) and meta-analyses (Kober et al., 2008). Lower medullary regions including the rostral ventolateral medulla and nucleus tractus solitarius are known to associate with peripheral inflammation, yet were also not activated (Berntson et al., 2003; Gaykema and Goehler, 2011; Goehler et al., 2000). This pattern of null results may be due to noise heterogeneity, methodological factors, or spatial convergence in studies included in the meta-analysis.
In addition to the above limbic and brainstem areas, the consistency analysis identified several cortical regions. In particular, the cluster encompassing the dACC/dmPFC accords with a considerable literature linking this region to attentional control, arousal, and subjective anxiety in the context of peripheral inflammation (Miller et al., 2013). Specifically, it is thought that during states of infection or peripheral inflammation, this region supports hypervigilance towards environmental external threats (Capuron et al., 2005; Muscatell et al., 2016b; Slavich et al., 2010). Moreover, the ACC and mPFC, in addition to other prefrontal and somatomotor cortical regions, issue multi-synaptic projections to the adrenal medulla (Dum et al., 2016), consistent with a role for this region in regulating autonomic and neuroendocrine outflow, and thereby a neural basis to influence inflammation in the periphery. Interestingly, the MKDA results did not include the dorsolateral prefrontal cortex or more rostral portions of the ACC and mPFC. This suggests that, although some individual studies report associations within these regions (Eisenberger et al., 2009; Gianaros et al., 2014; Matsunaga et al., 2008; Ohira et al., 2013), results are not consistent across the literature. Taken together, our meta-analysis findings corroborate multiple hypotheses relating the dorsal and ventral (but not rostral) medial components of the PFC to peripheral inflammation.
The consistency analysis also identified the insula, which is consistent with two hypotheses regarding this complex region’s role in peripheral inflammation. First, the insula is involved homeostatic control via the autonomic nervous system, (Oppenheimer and Cechetto, 2016) one of the several visceromotor pathways that influence peripheral inflammatory physiology (Pavlov and Tracey, 2017). Second, the insula receives and integrates a broad array of visceral signals that convey the status of internal physiology, mediated via sensory afferents projecting through the lamina I spinothalamic tract (Craig, 2009, 2002). Substantial work has focused on the role of this pathway in viscerosensation, thermosensation, and nociception (Cechetto and Saper, 1987; Krushel and van Der Kooy, 1988; Segerdahl et al., 2015). Interestingly, the consistency analysis identified clusters in left posterior and right anterior subdivisions of the insula (see Figure 2 montage, row 2, images 4–5), which is somewhat consistent with Craig’s proposed neuroanatomical model of visceral afference (Craig, 2009). This model describes the processing of viscerosensory signals along a posterior-to-anterior pathway, wherein the posterior insula processes lower- level sensory components, and the anterior insula integrates these signals with higher level subjective and motivational context (Craig, 2009). Moreover, results of the specificity analyses according to study design may provide additional support for this model. Compared to inflammatory observational designs, a greater proportion of studies using inflammatory manipulations reported effects in a cluster encompassing the right insula. From a conceptual perspective, it is plausible that studies using the latter design may preferentially target viscerosensory pathways as compared to visceromotor pathways (Schedlowski et al., 2014), which in turn would conform with a hypothesized role for the insula in inflammatory viscerosensation. Finally, results of this specificity analysis suggest that future studies aimed at examining insula function in the context of peripheral inflammatory physiology may be better suited to incorporating inflammatory manipulations into their study design. Taken together, the results of this meta-analysis add to prior literature highlighting the insula as a core brain region involved in peripheral inflammation.
In contrast to the insula, the consistency of reported activity changes in other brain regions did not appear to differ statistically by study design. Indeed, when conducting the consistency analyses for each study design separately, and subsequently examining their conjunction, both study designs yielded consistent reports of activity changes in core ‘inflammation-related’ regions, including the hippocampus, striatum, and dmPFC. This pattern of findings could suggest that one study design might not necessarily yield more reliable changes in activity in these brain regions. However, these ancillary specificity analyses relied on a small number of studies coded according to design. As a result, our speculative conclusions should be interpreted as provisional. Along these lines, the specificity analyses of clinical status revealed a cluster in the dmPFC—raising the possibility that links between peripheral inflammation and activity in this region may be more consistently engaged or ‘upregulated’ in disease states (Figure S4). Again, we interpret this ancillary finding with caution owing to sample size considerations. In these regards, future work on brain-inflammation interactions should continue to investigate the modifying influences of study design and clinical status in larger neuroimaging datasets with diverse samples to more precisely the generalizability of the specificity findings reported in this meta-analysis.
It is also noteworthy that the results of the specificity analysis indicated that, compared to cognitive and resting-state tasks, a greater proportion of studies using emotional tasks reported effects in a cluster containing the ventral striatum, sgACC, and medial OFC. Together, these regions comprise a corticostriatal network that purportedly supports mood and reward processing (Lindquist et al., 2012; Pauli et al., 2016). This network is also broadly implicated in mood-related psychiatric disorders, such as depression, anxiety, and post-traumatic stress disorder (Etkin and Wager, 2007; Groenewold et al., 2013). Notably, the specificity analysis did not test for differences between emotional and other processes, but rather for differences in their interaction with peripheral inflammation. In this way, the findings appear to agree with the hypothesis that peripheral inflammation may influence emotional, stressful, or reward-related processes by affecting corticostriatal function (Felger and Treadway, 2017), possibly via effects on blood-brain barrier permeability (Ménard et al., 2017a) and dopamine synthesis and release (Capuron et al., 2012). Interestingly, inflammation-related functional changes in the corticostriatal network were documented in a separate neuroimaging study not included in the meta-analysis (Harrison et al., 2009a). Here, mood change in response to typhoid vaccination was associated with altered activity and functional connectivity within a sgACC – ventral striatum network. A recent study similarly observed altered corticostriatal functional connectivity in adults with major depressive disorder who also had elevated markers of circulating inflammation (Felger et al., 2016). Finally, our results and these studies conform with recent theoretical perspectives suggesting that a “neuroimmune network”, comprising peripheral inflammation and brain networks including these reward pathways, may be involved in linking adversity (e.g., during early life) to physical and mental health outcomes linked to inflammation (Hostinar et al., 2017; Nusslock and Miller, 2016). Taken together, our results reinforce prior suggestions that a corticostriatal network may be particularly relevant to peripheral inflammatory physiology in the context of emotional processes and disorders.
Comparing the spatial similarity of the MKDA results to intrinsic brain networks (Yeo et al., 2011) revealed the strongest similarity with the “default mode network” (DMN), which is consistent with recent reports linking circulating levels of IL-6 with resting state functional connectivity of the DMN (Dev et al., 2017; Marsland et al., 2017a). Alterations in the DMN have been reported in neuropsychiatric and neurodegenerative disorders that are thought to involve inflammatory mechanisms, most notably major depression (Hamilton et al., 2015; Kaiser et al., 2015) and Alzheimer’s disease (Greicius et al., 2004). Finally, the MKDA results were similar to the “ventral attention network”, which is also termed the “cingulo-opercular” (Dosenbach et al., 2007) or “salience” (Seeley et al., 2007) network, and primarily comprises the dACC and anterior insula. This network consists of extensive cortical- subcortical anatomical connections (Ongür and Price, 2000) and may be involved in detecting environmental stimuli relevant to survival, as well as the predictive regulation over internal physiology (Barrett and Simmons, 2015; Ginty et al., 2017; Hermans et al., 2011). In the context of our meta-analysis results, we speculate that peripheral inflammation might be an important physiological mediator that relays visceral signals to and from the so-called ventral attention network (Barrett and Satpute, 2013; Critchley and Harrison, 2013).
The above findings regarding intrinsic brain networks were corroborated by a follow-up set of spatial similarity analyses to corticostriatal loops. Specifically, the MKDA results were similar to the ventral striatum, posterior putamen, and anterior caudate loops, which have previously been associated with distinct psychological terms via data-driven analyses on over 5000 neuroimaging studies (Pauli et al., 2016). According to Pauli et al., (2016), the ventral striatum loop anatomically comprises the vmPFC, OFC, and posterior cingulate, and may be important for evaluating the value and motivational qualities of different stimuli, associating with psychological terms such as “reward,” “losses,” and “craving.” The posterior putamen loop comprises the sensorimotor cortex, mid and posterior insula, operculum, and medial temporal lobes, and is implicated in sensorimotor processes, associating with psychological terms such as “foot,” “noxious,” and “taste.” The anterior caudate loop comprises the dmPFC, lateral PFC, anterior insula, and inferior parietal cortex, and is implicated in evaluating the value of different actions, associating with psychological terms such as “grasping,” “reaching,” and “reinforcement.” It should be noted that, although these corticostriatal loops appear to partially overlap with components of the default mode, limbic, and ventral attention networks, their relation to the striatum as well as their meta-analytic derivation significantly adds to a network- based interpretation of our meta-analysis results. Specifically, these results point to a role for peripheral inflammatory processes across various motivational and sensorimotor contexts (Felger and Treadway, 2017).
Indeed, while many of these intrinsic networks and corticostriatal loops are thought to be “independent” insofar as they frequently exhibit stronger within- network coherence than between-network coherence, their components nonetheless interact in a variety of processes. In particular, these networks are thought to contribute to a broader “allostatic-interoceptive” network that is implicated in sensing, representing, and predicting sensory inputs from the body’s internal organs and viscera (Kleckner et al., 2017). In addition to inflammatory physiology, other viscerosensory signals that are thought to be relevant to the allostatic-interoceptive network include autonomic, endocrine, vascular, temperature, and pain signals. Future studies should investigate how these networks, as well as other networks derived from alternative approaches, interact in the context of peripheral inflammation in health and disease.
The network co-activation analyses, suggestive of a meta-analytic index of functional connectivity, identified connections linking several pairs of brain regions, plausibly forming functionally connected ensembles of regions across studies. Two of the most significant connections (FDR corrected p < 0.05) involved mPFC- brainstem and insula-brainstem pathways, consistent with animal models and human neuroimaging studies highlighting interconnections between these regions and their role in regulating peripheral physiology. Specifically, the infralimbic cortex, a rodent homologue of the vmPFC, issues projections to the amygdala, hypothalamus, and parabrachial and solitary nuclei of the brainstem (Gabbott et al., 2005; Vertes, 2004). Separately, the anterior insula receives projections from the parabrachial nuclei in the brainstem by way of the ventromedial thalamic nuclei (Craig, 2003; Saper, 2002). Prior neuroimaging studies confirm that both pathways are functionally implicated in regulating autonomic (i.e., visceromotor) outflow (Gianaros et al., 2012b; Vertes, 2004; Wager et al., 2009b, 2009c). Finally, the co-activation analyses identified the brainstem region as exhibiting the most significant connections with other regions across studies, emphasizing its role as a potential hub for transmitting and modulating inflammation-related neural activity. While our meta-analysis is poorly powered to characterize the co-activation profiles of individual regions within the midbrain and brainstem, future neuroimaging studies that utilize improved data acquisition methods and physiological correction techniques could focus on these individual regions and their connections to higher-level limbic and cortical regions in the context of peripheral inflammation (Bär et al., 2016). Finally, we note that some results of the co-activation analyses were inconsistent with prior reports: in particular, the dACC/dmPFC was not significantly connected with any limbic or brainstem areas (p’s > 0.3) for reasons that are unclear.
In addition to fMRI and FDG-PET methods represented in this meta-analysis, other neuroimaging methods examine structural and functional properties of the brain. Moreover, many of these methods have been used to examine relationships with peripheral inflammation, including structural MRI (Marsland et al., 2008), diffusion MRI (Verstynen et al., 2013), functional connectivity MRI (Lekander et al., 2015), magnetic resonance spectroscopy (Haroon et al., 2014), and F-dopa PET (Capuron et al., 2012). However, this literature is relatively small in comparison to the fMRI and FDG-PET literature on activation patterns or activity levels. In the context of the present meta-analysis, these methods do not provide clearly comparable neurobiological interpretations to fMRI and PET, which is necessary when systematically combining results across multiple studies. Therefore, studies using these methods were not included in the quantitative meta-analysis of this review.
However, our results may be informed by related studies that link peripheral inflammation to structural and morphological features of the brain. For instance, cross-sectional studies have linked circulating inflammation to cortical and subcortical grey matter morphology (Marsland et al., 2015, 2008), as well as white matter integrity (Gianaros et al., 2012a; Verstynen et al., 2013), but to our knowledge no studies have demonstrated effects of transient, experimentally-manipulated changes in inflammatory physiology on these aspects of brain structure. Despite this, some studies showed effects of peripheral inflammation on brain microstructure, which were in part related to brain function (Dowell et al., 2016; Harrison et al., 2015b), highlighting a potential structural pathway linking peripheral inflammatory activity to altered brain function. It is not currently clear whether reported changes in brain microstructure are directly comparable to changes in brain function as measured with fMRI or PET; however, these and other structural neuroimaging methods might identify structural pathways that underlie changes in brain function, and may in turn inform our co-activation results.
Another candidate mechanism linking peripheral inflammation to brain function as reported in these studies may involve central neuroinflammatory processes. Specifically, peripheral inflammatory signals can activate microglia within the CNS, setting off a cascade that influences neuronal function (Kreisel et al., 2013). PET radioligands have recently been developed to noninvasively measure central microglia activity by targeting the translocator protein (TSPO) (Sandiego et al., 2015). However, the generalizability of these methods is limited insofar as there is appreciable genetically-determined individual variation in radioligand binding affinity across individuals (Fujita et al., 2008; Owen et al., 2011). Moreover, it is somewhat unclear whether activity as reported by these methods can be combined with well- validated functional methods that are reviewed here. Nonetheless, emerging work suggests some correspondence between central microglia activation as measured with PET and peripheral immune markers in both human (Kanegawa et al., 2016) and nonhuman primate (Hannestad et al., 2012a) samples. Future studies might incorporate these measures of central neuroinflammatory processes to examine whether they are localized to regions similar to those identified in our meta-analysis.
Limitations
A number of limitations warrant attention. First, we did not include studies or analyses that were restricted to ROIs or used small volume correction, which could have resulted in excluding studies that examined certain hypothesis-driven regions or tested smaller effect sizes. Ancillary analyses incorporating these studies, however, appeared to reinforce and agree with the results of the primary, unbiased consistency analysis (see Figure S3). Nonetheless, we interpret these ancillary findings with caution, owing to their potentially problematic introduction of biases. Second, our meta-analysis was performed using reported coordinates in the published literature, which makes several assumptions about reported activity changes, including cluster size, effect size, and effect direction. Indeed, regarding the latter factor, peripheral inflammation can plausibly relate to opposite directions of activity in the same brain region depending on contextual factors, such as stimulus type or clinical status. For example, increases in peripheral inflammation (via endotoxin administration) have been observed to decrease ventral striatum response during anticipation of monetary rewards (Eisenberger et al., 2010), yet increase ventral striatum response to images of social support figures (Inagaki et al., 2015).
These and other contextual influences on the direction of activity changes within the brain are of clear importance to future research. Nonetheless, the results of our meta-analysis provide converging and new evidence that inflammation associates consistently with changes in the activity of a reliably detected group of brain regions, without regard to the direction of the activity change. Yet, we note that our approach using reported coordinates is in contrast to the less frequently employed, yet gold standard practice of evaluating consistency using unthresholded statistical images from studies (Salimi-Khorshidi et al., 2009). Hence, improved data-sharing practices could allow for this approach in future meta-analyses of the literature. Third, it is likely that other factors could explain heterogeneity of reported effects across individual studies, including inflammatory stimulant (e.g., IFN-α, typhoid vaccination), inflammatory marker of interest (e.g., IL-6, CRP), sample biological attributes (e.g., age, body mass index), and sample psychosocial attributes (e.g., socioeconomic status, psychological stress), yet our small sample of studies precludes exploring these and other factors.
Conclusion and Future Directions
To summarize, we empirically evaluated the consistency of brain regions involved in peripheral inflammatory physiology across several human neuroimaging studies, confirming the role of the amygdala, hippocampus, hypothalamus, striatum, midbrain, brainstem, as well as prefrontal, insular, and temporal cortices. Further, we identified regions that may be specific to some contextual factors, such as psychological task, study design, and sample clinical status. Finally, we described the similarity of reported patterns to known intrinsic networks and corticostriatal loops, as well as show how reported regions group together to reflect functionally connected pathways implicated in brain-inflammation interactions. Given the present meta-analysis, future work might continue to examine the connections and interactions within and across these brain systems, as measured by structural and functional connectivity, in the context of peripheral inflammatory and other immune physiology. Future work could also expand the psychological and health contexts of these results by studying associations between peripheral inflammatory physiology and brain function across novel samples of participants. To these ends, we provide masks of the empirically-derived brain regions and clusters that can be used for future analyses, made publicly accessible on NeuroVault (Gorgolewski et al., 2015).
Supplementary Material
Highlights:
Review of brain regions and networks associated with peripheral inflammation.
Meta-analysis of 24 neuroimaging studies revealed consistently reported regions.
Some regions were specific to study design and task.
Patterns of regions resembled intrinsic brain networks and corticostriatal loops.
Co-activated regions formed prefrontal-brainstem and insula-brainstem ensembles.
Acknowledgments
Funding Sources: This work was supported by the National Institutes of Health (NHLBI T32 HL007560 and NHLBI R01 HL089850).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors attest that they have no conflicts of interest.
included in meta-analysis
We repeated this search in October 2017 after the completion of the review and meta- analysis, and we identified several papers that were potentially relevant to update the meta-analysis. Upon further inspection, however, all were deemed ineligible. Hence, we retained the original counts depicted in the CONSORT diagram (Figure 1).
References
- Agresti A, 2002. Inference for contingency tables. Categ. Data Anal Second Ed. 70–114.
- Anisman H, Hayley S, 2012. Inflammatory Factors Contribute to Depression and Its Comorbid Conditions. Sci. Signal 5, pe45–pe45. 10.1126/scisignal.2003579 [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, 1991. Blood to brain transport of interleukin links the immune and central nervous systems. Life Sci 48, PL117–121. [DOI] [PubMed] [Google Scholar]
- Bär K-J, de la Cruz F, Schumann A, Koehler S, Sauer H, Critchley H, Wagner G, 2016. Functional connectivity and network analysis of midbrain and brainstem nuclei. NeuroImage 134, 53–63. 10.1016/j.neuroimage.2016.03.071 [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA, 1986. The moderator–mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol 51, 1173. [DOI] [PubMed] [Google Scholar]
- Barrett LF, Satpute AB, 2013. Large-scale brain networks in affective and social neuroscience: towards an integrative functional architecture of the brain. Curr. Opin. Neurobiol 23, 361–372. 10.1016/j.conb.2012.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Simmons WK, 2015. Interoceptive predictions in the brain. Nat. Rev.Neurosci 16, 419–429. 10.1038/nrn3950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beissner F, Meissner K, Bär K-J, Napadow V, 2013. The Autonomic Brain: An Activation Likelihood Estimation Meta-Analysis for Central Processing of Autonomic Function. J. Neurosci 33, 10503–10511. 10.1523/JNEUROSCI.1103-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol 57, 289–300. [Google Scholar]
- Ben-Shaanan TL, Azulay-Debby H, Dubovik T, Starosvetsky E, Korin B, Schiller M, Green NL, Admon Y, Hakim F, Shen-Orr SS, Rolls A, 2016. Activation of the reward system boosts innate and adaptive immunity. Nat. Med 22, 940–944. 10.1038/nm.4133 [DOI] [PubMed] [Google Scholar]
- Berntson GG, Sarter M, Cacioppo JT, 2003. Ascending visceral regulation of cortical affective information processing . Eur. J. Neurosci 18, 2103–2109. 10.1046/j.1460-9568.2003.02967.x [DOI] [PubMed] [Google Scholar]
- Blatteis CM, Bealer SL, Hunter WS, Llanos-Q J, Ahokas RA, Mashburn TA, 1983. Suppression of fever after lesions of the anteroventral third ventricle in guinea pigs. Brain Res. Bull 11, 519–526. [DOI] [PubMed] [Google Scholar]
- Bluthé RM, Walter V, Parnet P, Layé S, Lestage J, Verrier D, Poole S, Stenning BE, Kelley KW, Dantzer R, 1994. Lipopolysaccharide induces sickness behaviour in rats by a vagal mediated mechanism. C. R. Acad. Sci III 317, 499– 503. [PubMed] [Google Scholar]
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ, 2000. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405, 458–462. 10.1038/35013070 [DOI] [PubMed] [Google Scholar]
- Brett M, Christoff K, Cusack R, Lancaster J, 2001. Using the Talairach atlas with the MNI template. NeuroImage 13, S85. [Google Scholar]
- *.Brydon L, Harrison NA, Walker C, Steptoe A, Critchley HD, 2008. Peripheral Inflammation is Associated with Altered Substantia Nigra Activity and Psychomotor Slowing in Humans. Biol. Psychiatry 63, 1022–1029. 10.1016/j.biopsych.2007.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhle JT, Kober H, Ochsner KN, Mende-Siedlecki P, Weber J, Hughes B, Kross E, Atlas LY, McRae K, Wager TD, 2012. Common representation of pain and negative emotion in the midbrain periaqueductal gray. Soc. Cogn. Affect. Neurosci 10.1093/scan/nss038 [DOI] [PMC free article] [PubMed]
- Button KS, Ioannidis JPA, Mokrysz C, Nosek BA, Flint J, Robinson ESJ, Munafò MR, 2013. Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci 14, 365–376. 10.1038/nrn3475 [DOI] [PubMed] [Google Scholar]
- Cameron OG, 2009. Visceral brain–body information transfer. NeuroImage, Brain Body Medicine 47, 787–794. 10.1016/j.neuroimage.2009.05.010 [DOI] [PubMed] [Google Scholar]
- Capuron L, Miller AH, 2011. Immune system to brain signaling: Neuropsychopharmacological implications. Pharmacol. Ther 130, 226–238. 10.1016/j.pharmthera.2011.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Capuron L, Pagnoni G, Demetrashvili M, Woolwine BJ, Nemeroff CB, Berns GS, Miller AH, 2005. Anterior Cingulate Activation and Error Processing During Interferon-Alpha Treatment. Biol. Psychiatry 58, 190–196. 10.1016/j.biopsych.2005.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Capuron L, Pagnoni G, Demetrashvili MF, Lawson DH, Fornwalt FB, Woolwine B, Berns GS, Nemeroff CB, Miller AH, 2007. Basal ganglia hypermetabolism and symptoms of fatigue during interferon-alpha therapy. Neuropsychopharmacology 32, 2384–2392. 10.1038/sj.npp.1301362 [DOI] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivey JR, Crowe RJ, Votaw JR, Goodman MM, Miller AH, 2012. Dopaminergic Mechanisms of Reduced Basal Ganglia Responses to Hedonic Reward During Interferon Alfa Administration. Arch. Gen. Psychiatry 69, 1044–1053. 10.1001/archgenpsychiatry.2011.2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cechetto DF, Saper CB, 1987. Evidence for a viscerotopic sensory representation in the cortex and thalamus in the rat. J. Comp. Neurol 262, 27–45. 10.1002/cne.902620104 [DOI] [PubMed] [Google Scholar]
- Costafreda SG, 2009. Pooling fMRI data: meta-analysis, mega-analysis and multi- center studies. Front. Neuroinformatics 3 10.3389/neuro.11.033.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD, 2009. How do you feel — now? The anterior insula and human awareness. Nat. Rev. Neurosci 10, 59–70. 10.1038/nrn2555 [DOI] [PubMed] [Google Scholar]
- Craig AD, 2003. Interoception: the sense of the physiological condition of the body. Curr. Opin. Neurobiol 13, 500–505. 10.1016/S0959-4388(03)00090-4 [DOI] [PubMed] [Google Scholar]
- Craig AD, 2002. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci 3, 655–666. 10.1038/nrn894 [DOI] [PubMed] [Google Scholar]
- Cremers HR, Wager TD, Yarkoni T, 2017. The relation between statistical power and inference in fMRI. PLOS ONE 12, e0184923 10.1371/journal.pone.0184923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Harrison NA, 2013. Visceral Influences on Brain and Behavior. Neuron 77, 624–638. 10.1016/j.neuron.2013.02.008 [DOI] [PubMed] [Google Scholar]
- Dantzer R, Konsman JP, Bluthé RM, Kelley KW, 2000. Neural and humoral pathways of communication from the immune system to the brain: parallel or convergent? Auton. Neurosci. Basic Clin 85, 60–65. 10.1016/S1566-0702(00)00220-4 [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW, 2008. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci 9, 46–56. 10.1038/nrn2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dev SI, Moore RC, Soontornniyomkij B, Achim CL, Jeste DV, Eyler LT, 2017. Peripheral inflammation related to lower fMRI activation during a working memory task and resting functional connectivity among older adults: a preliminary study. Int. J. Geriatr. Psychiatry 32, 341–349. 10.1002/gps.4482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N, 2009. A probabilistic MR atlas of the human cerebellum. NeuroImage 46, 39–46. 10.1016/j.neuroimage.2009.01.045 [DOI] [PubMed] [Google Scholar]
- Dipasquale O, Cooper EA, Tibble J, Voon V, Baglio F, Baselli G, Cercignani M, Harrison NA, 2015. Interferon-α acutely impairs whole-brain functional connectivity network architecture - A preliminary study. Brain. Behav. Immun 10.1016/j.bbi.2015.12.011 [DOI] [PMC free article] [PubMed]
- Dosenbach NUF, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RAT, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE, 2007. Distinct brain networks for adaptive and stable task control in humans. Proc. Natl. Acad. Sci 104, 11073–11078. 10.1073/pnas.0704320104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell NG, Cooper EA, Tibble J, Voon V, Critchley HD, Cercignani M, Harrison NA, 2016. Acute Changes in Striatal Microstructure Predict the Development of Interferon-Alpha Induced Fatigue. Biol. Psychiatry 79, 320– 328. 10.1016/j.biopsych.2015.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Levinthal DJ, Strick PL, 2016. Motor, cognitive, and affective areas of the cerebral cortex influence the adrenal medulla. Proc. Natl. Acad. Sci 113, 9922–9927. 10.1073/pnas.1605044113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbarth H, Chang LJ, Wager TD, 2016. Multivariate Brain Prediction of Heart Rate and Skin Conductance Responses to Social Threat. J. Neurosci 36, 11987–11998. 10.1523/JNEUROSCI.3672-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR, 2010. Inflammation-Induced Anhedonia: Endotoxin Reduces Ventral Striatum Responses to Reward. Biol. Psychiatry 68, 748–754. 10.1016/j.biopsych.2010.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Cole SW, 2012. Social neuroscience and health: neurophysiological mechanisms linking social ties with physical health. Nat. Neurosci 15, 669– 674. 10.1038/nn.3086 [DOI] [PubMed] [Google Scholar]
- *.Eisenberger NI, Inagaki TK, Rameson LT, Mashal NM, Irwin MR, 2009. An fMRI study of cytokine-induced depressed mood and social pain: The role of sex differences. NeuroImage 47, 881–890. 10.1016/j.neuroimage.2009.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ek M, Kurosawa M, Lundeberg T, Ericsson A, 1998. Activation of vagal afferents after intravenous injection of interleukin-1beta: role of endogenous prostaglandins. J. Neurosci. Off. J. Soc. Neurosci 18, 9471–9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H, 2016. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci 113, 7900–7905. 10.1073/pnas.1602413113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD, 2007. Functional Neuroimaging of Anxiety: A Meta-Analysis of Emotional Processing in PTSD, Social Anxiety Disorder, and Specific Phobia. Am. J. Psychiatry 164, 1476–1488. 10.1176/appi.ajp.2007.07030504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X, Miller AH, 2016. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol. Psychiatry 21, 1358–1365. https://doi.org/10.1038/mp . 10.1038/mp2015.168. 2015.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Treadway MT, 2017. Inflammation Effects on Motivation and Motor Activity: Role of Dopamine. Neuropsychopharmacology 42, 216–241. 10.1038/npp.2016.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filiano AJ, Xu Y, Tustison NJ, Marsh RL, Baker W, Smirnov I, Overall CC, Gadani SP, Turner SD, Weng Z, Peerzade SN, Chen H, Lee KS, Scott MM, Beenhakker MP, Litvak V, Kipnis J, 2016. Unexpected role of interferon-γ in regulating neuronal connectivity and social behaviour. Nature advance online publication [DOI] [PMC free article] [PubMed]
- Frenois F, Moreau M, O’Connor J, Lawson M, Micon C, Lestage J, Kelley KW, Dantzer R, Castanon N, 2007. Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive- like behavior. Psychoneuroendocrinology 32, 516–531. 10.1016/j.psyneuen.2007.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Imaizumi M, Zoghbi SS, Fujimura Y, Farris AG, Suhara T, Hong J, Pike VW, Innis RB, 2008. Kinetic analysis in healthy humans of a novel positron emission tomography radioligand to image the peripheral benzodiazepine receptor, a potential biomarker for inflammation. NeuroImage 40, 43–52. 10.1016/j.neuroimage.2007.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbott PLA, Warner TA, Jays PRL, Salway P, Busby SJ, 2005. Prefrontal cortex in the rat: Projections to subcortical autonomic, motor, and limbic centers. J. Comp. Neurol 492, 145–177. 10.1002/cne.20738 [DOI] [PubMed] [Google Scholar]
- Gaykema RPA, Goehler LE, 2011. Ascending caudal medullary catecholamine pathways drive sickness-induced deficits in exploratory behavior: brain substrates for fatigue? Brain. Behav. Immun 25, 443–460. 10.1016/j.bbi.2010.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Marsland AL, Kuan DC-H, Schirda BL, Jennings JR, Sheu LK, Hariri AR, Gross JJ, Manuck SB, 2014. An Inflammatory Pathway Links Atherosclerotic Cardiovascular Disease Risk to Neural Activity Evoked by the Cognitive Regulation of Emotion. Biol. Psychiatry 10.1016/j.biopsych.2013.10.012 [DOI] [PMC free article] [PubMed]
- Gianaros PJ, Marsland AL, Sheu LK, Erickson KI, Verstynen TD, 2012a. Inflammatory Pathways Link Socioeconomic Inequalities to White Matter Architecture. Cereb. Cortex bhs191 10.1093/cercor/bhs191 [DOI] [PMC free article] [PubMed]
- Gianaros PJ, Onyewuenyi IC, Sheu LK, Christie IC, Critchley HD, 2012b. Brain systems for baroreflex suppression during stress in humans. Hum. Brain Mapp 33, 1700–1716. 10.1002/hbm.21315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Sheu LK, 2009. A review of neuroimaging studies of stressor-evoked blood pressure reactivity: Emerging evidence for a brain-body pathway to coronary heart disease risk. NeuroImage 47, 922–936. 10.1016/j.neuroimage.2009.04.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Sheu LK, Uyar F, Koushik J, Jennings JR, Wager TD, Singh A, Verstynen TD, 2017. A Brain Phenotype for Stressor‐ Evoked Blood Pressure Reactivity. J. Am. Heart Assoc 6, e006053 10.1161/JAHA.117.006053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Wager TD, 2015. Brain-Body Pathways Linking Psychological Stress and Physical Health. Curr. Dir. Psychol. Sci 24, 313–321. 10.1177/0963721415581476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons JD, 1993. Nonparametric measures of association Sage. [Google Scholar]
- Ginty AT, Kraynak TE, Fisher JP, Gianaros PJ, 2017. Cardiovascular and autonomic reactivity to psychological stress: Neurophysiological substrates and links to cardiovascular disease. Auton. Neurosci., Imaging and the Autonomic Nervous System 207, 2–9. 10.1016/j.autneu.2017.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH, 2010. Mechanisms Underlying Inflammation in Neurodegeneration. Cell 140, 918–934. 10.1016/j.cell.2010.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goehler LE, Gaykema RPA, Hansen MK, Anderson K, Maier SF, Watkins LR, 2000. Vagal immune-to-brain communication: a visceral chemosensory pathway. Auton. Neurosci 85, 49–59. 10.1016/S1566-0702(00)00219-8 [DOI] [PubMed] [Google Scholar]
- Gorgolewski KJ, Varoquaux G, Rivera G, Schwarz Y, Ghosh SS, Maumet C, Sochat VV, Nichols TE, Poldrack RA, Poline J-B, Yarkoni T, Margulies DS, 2015. NeuroVault.org: a web-based repository for collecting and sharing unthresholded statistical maps of the human brain. Front. Neuroinformatics 9 10.3389/fninf.2015.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V, 2004. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc. Natl. Acad. Sci. U. S. A 101, 4637–4642. 10.1073/pnas.0308627101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewold NA, Opmeer EM, de Jonge P, Aleman A, Costafreda SG, 2013. Emotional valence modulates brain functional abnormalities in depression: Evidence from a meta-analysis of fMRI studies. Neurosci. Biobehav. Rev 37, 152–163. 10.1016/j.neubiorev.2012.11.015 [DOI] [PubMed] [Google Scholar]
- Haas HS, Schauenstein K, 1997. Neuroimmunomodulation via limbic structures— the neuroanatomy of psychoimmunology. Prog. Neurobiol 51, 195–222. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Farmer M, Fogelman P, Gotlib IH, 2015. Depressive Rumination, the Default-Mode Network, and the Dark Matter of Clinical Neuroscience. Biol. Psychiatry, Depression 78, 224–230. 10.1016/j.biopsych.2015.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannestad J, 2013. Neuroimaging and Clinical Studies on Brain–Immune Interactions, in: Cui C, Grandison L, Noronha A (Eds.), Neural-Immune Interactions in Brain Function and Alcohol Related Disorders Springer US, Boston, MA, pp. 95–132. [Google Scholar]
- Hannestad J, Gallezot J-D, Schafbauer T, Lim K, Kloczynski T, Morris ED, Carson RE, Ding Y-S, Cosgrove KP, 2012a. Endotoxin-induced systemic inflammation activates microglia: [11C]PBR28 positron emission tomography in nonhuman primates. NeuroImage 63, 232–239. 10.1016/j.neuroimage.2012.06.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Hannestad J, Subramanyam K, DellaGioia N, Planeta-Wilson B, Weinzimmer D, Pittman B, Carson RE, 2012b. Glucose Metabolism in the Insula and Cingulate Is Affected by Systemic Inflammation in Humans. J. Nucl. Med 53, 601–607. 10.2967/jnumed.111.097014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E, Raison CL, Miller AH, 2012. Psychoneuroimmunology Meets Neuropsychopharmacology: Translational Implications of the Impact of Inflammation on Behavior. Neuropsychopharmacology 37, 137–162. 10.1038/npp.2011.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E, Woolwine BJ, Chen X, Pace TW, Parekh S, Spivey JR, Hu XP, Miller AH, 2014. IFN-Alpha-Induced Cortical and Subcortical Glutamate Changes Assessed by Magnetic Resonance Spectroscopy. Neuropsychopharmacology 39, 1777–1785. 10.1038/npp.2014.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, 2017. Brain Structures Implicated in Inflammation-Associated Depression. Curr. Top. Behav. Neurosci 31, 221–248. 10.1007/7854_2016_30 [DOI] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD, 2009a. Inflammation Causes Mood Changes Through Alterations in Subgenual Cingulate Activity and Mesolimbic Connectivity. Biol. Psychiatry 66, 407–414. 10.1016/j.biopsych.2009.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Dolan RJ, Critchley HD, 2009b. Neural Origins of Human Sickness in Interoceptive Responses to Inflammation. Biol. Psychiatry 66, 415–422. 10.1016/j.biopsych.2009.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Harrison NA, Cercignani M, Voon V, Critchley HD, 2015a. Effects of Inflammation on Hippocampus and Substantia Nigra Responses to Novelty in Healthy Human Participants. Neuropsychopharmacology 40, 831–838. 10.1038/npp.2014.222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Cooper E, Dowell NG, Keramida G, Voon V, Critchley HD, Cercignani M, 2015b. Quantitative Magnetization Transfer Imaging as a Biomarker for Effects of Systemic Inflammation on the Brain. Biol. Psychiatry 78, 49–57. 10.1016/j.biopsych.2014.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Harrison NA, Cooper E, Voon V, Miles K, Critchley HD, 2013. Central autonomic network mediates cardiovascular responses to acute inflammation: Relevance to increased cardiovascular risk in depression? Brain. Behav. Immun 31, 189–196. 10.1016/j.bbi.2013.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Harrison NA, Doeller CF, Voon V, Burgess N, Critchley HD, 2014. Peripheral Inflammation Acutely Impairs Human Spatial Memory via Actions on Medial Temporal Lobe Glucose Metabolism. Biol. Psychiatry 76, 585–593. 10.1016/j.biopsych.2014.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart BL, 1988. Biological basis of the behavior of sick animals. Neurosci. Biobehav. Rev 12, 123–137. [DOI] [PubMed] [Google Scholar]
- Heimer L, Van Hoesen GW, 2006. The limbic lobe and its output channels: Implications for emotional functions and adaptive behavior. Neurosci. Biobehav. Rev., The Limbic Brain: Structure and Function 30, 126–147. 10.1016/j.neubiorev.2005.06.006 [DOI] [PubMed] [Google Scholar]
- Heppner FL, Ransohoff RM, Becher B, 2015. Immune attack: the role of inflammation in Alzheimer disease. Nat. Rev. Neurosci 16, 358–372. 10.1038/nrn3880 [DOI] [PubMed] [Google Scholar]
- Hermans EJ, Marle H.J.F. van, Ossewaarde L, Henckens MJAG, Qin S, Kesteren M.T.R. van, Schoots VC, Cousijn H, Rijpkema M, Oostenveld R, Fernández G, 2011. Stress-Related Noradrenergic Activity Prompts Large-Scale Neural Network Reconfiguration. Science 334, 1151–1153. 10.1126/science.1209603 [DOI] [PubMed] [Google Scholar]
- Hikosaka O, 2010. The habenula: from stress evasion to value-based decision- making. Nat. Rev. Neurosci 11, 503–513. 10.1038/nrn2866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingorani AD, Cross J, Kharbanda RK, Mullen MJ, Bhagat K, Taylor M, Donald AE, Palacios M, Griffin GE, Deanfield JE, MacAllister RJ, Vallance P, 2000. Acute Systemic Inflammation Impairs Endothelium-Dependent Dilatation in Humans. Circulation 102, 994–999. 10.1161/01.CIR.102.9.994 [DOI] [PubMed] [Google Scholar]
- Hostinar CE, Nusslock R, Miller GE, 2017. Future Directions in the Study of Early- Life Stress and Physical and Emotional Health: Implications of the Neuroimmune Network Hypothesis. J. Clin. Child Adolesc. Psychol 1–15. 10.1080/15374416.2016.1266647 [DOI] [PMC free article] [PubMed]
- Hotamisligil GS, 2006. Inflammation and metabolic disorders. Nature 444, 860–867. 10.1038/nature05485 [DOI] [PubMed] [Google Scholar]
- Inagaki TK, Muscatell KA, Irwin MR, Cole SW, Eisenberger NI, 2012. Inflammation Selectively Enhances Amygdala Activity to Socially Threatening Images. Neuroimage 59, 3222–3226. 10.1016/j.neuroimage.2011.10.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki TK, Muscatell KA, Irwin MR, Moieni M, Dutcher JM, Jevtic I, Breen EC, Eisenberger NI, 2015. The role of the ventral striatum in inflammatory- induced approach toward support figures. Brain. Behav. Immun 44, 247–252. 10.1016/j.bbi.2014.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H, Behrens TEJ, Sillery E, Ciccarelli O, Thompson AJ, Smith SM, Matthews PM, 2005. Functional–Anatomical Validation and Individual Variation of Diffusion Tractography-based Segmentation of the Human Thalamus. Cereb. Cortex 15, 31–39. 10.1093/cercor/bhh105 [DOI] [PubMed] [Google Scholar]
- Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, Fleshner M, 2005. Adrenergic receptors mediate stress-induced elevations in extracellular Hsp72. J. Appl. Physiol. Bethesda Md 1985. 99, 1789–1795. 10.1152/japplphysiol.00390.2005 [DOI] [PubMed] [Google Scholar]
- Juengling FD, Ebert D, Gut O, Engelbrecht MA, Rasenack J, Nitzsche EU, Bauer J, Lieb K, 2000. Prefrontal cortical hypometabolism during low-dose interferon alpha treatment. Psychopharmacology (Berl.) 152, 383–389. 10.1007/s002130000549 [DOI] [PubMed] [Google Scholar]
- Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA, 2015. Large-Scale Network Dysfunction in Major Depressive Disorder: A Meta-analysis of Resting-State Functional Connectivity. JAMA Psychiatry 72, 603–611. 10.1001/jamapsychiatry.2015.0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanegawa N, Collste K, Forsberg A, Schain M, Arakawa R, Jucaite A, Lekander M, Olgart Höglund C, Kosek E, Lampa J, Halldin C, Farde L, Varrone A, Cervenka S, 2016. In vivo evidence of a functional association between immune cells in blood and brain in healthy human subjects. Brain. Behav. Immun 54, 149–157. 10.1016/j.bbi.2016.01.019 [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R, 2003. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc. Natl. Acad. Sci. U. S. A 100, 9090–9095. 10.1073/pnas.1531903100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner IR, Zhang J, Touroutoglou A, Chanes L, Xia C, Simmons WK, Quigley KS, Dickerson BC, Barrett LF, 2017. Evidence for a large-scale brain system supporting allostasis and interoception in humans. Nat. Hum. Behav 1, 0069 10.1038/s41562-017-0069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD, 2008. Functional grouping and cortical–subcortical interactions in emotion: A meta- analysis of neuroimaging studies. NeuroImage 42, 998–1031. 10.1016/j.neuroimage.2008.03.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Wager TD, 2010. Meta-analysis of neuroimaging data. Wiley Interdiscip. Rev. Cogn. Sci 1, 293–300. 10.1002/wcs.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisel T, Frank MG, Licht T, Reshef R, Ben-Menachem-Zidon O, Baratta MV, Maier SF, Yirmiya R, 2013. Dynamic microglial alterations underlie stress- sinduced depressive-like behavior and suppressed neurogenesis. Mol. Psychiatry 10.1038/mp.2013.155 [DOI] [PubMed]
- Krushel LA, van Der Kooy D, 1988. Visceral cortex: Integration of the mucosal senses with limbic information in the rat agranular insular cortex. J. Comp. Neurol 270, 39–54. 10.1002/cne.902700105 [DOI] [PubMed] [Google Scholar]
- *.Kullmann JS, Grigoleit J-S, Lichte P, Kobbe P, Rosenberger C, Banner C, Wolf OT, Engler H, Oberbeck R, Elsenbruch S, Bingel U, Forsting M, Gizewski ER, Schedlowski M, 2013. Neural response to emotional stimuli during experimental human endotoxemia. Hum. Brain Mapp 34, 2217–2227. 10.1002/hbm.22063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Kullmann JS, Grigoleit J-S, Wolf OT, Engler H, Oberbeck R, Elsenbruch S, Forsting M, Schedlowski M, Gizewski ER, 2014. Experimental human endotoxemia enhances brain activity during social cognition. Soc. Cogn. Affect. Neurosci 9, 786–793. 10.1093/scan/nst049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekander M, Karshikoff B, Johansson E, Soop A, Fransson P, Lundström JN, Andreasson A, Ingvar M, Petrovic P, Axelsson J, Nilsonne G, 2015. Intrinsic functional connectivity of insular cortex and symptoms of sickness during acute experimental inflammation. Brain. Behav. Immun 10.1016/j.bbi.2015.12.018 [DOI] [PubMed]
- Libby P, Ridker PM, Maseri A, 2002. Inflammation and Atherosclerosis. Circulation 105, 1135–1143. 10.1161/hc0902.104353 [DOI] [PubMed] [Google Scholar]
- Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF, 2012. The brain basis of emotion: A meta-analytic review. Behav. Brain Sci 35, 121–143. 10.1017/S0140525X11000446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist D, Wolkowitz OM, Mellon S, Yehuda R, Flory JD, Henn-Haase C, Bierer LM, Abu-Amara D, Coy M, Neylan TC, Makotkine I, Reus VI, Yan X, Taylor NM, Marmar CR, Dhabhar FS, 2014. Proinflammatory milieu in combat-related PTSD is independent of depression and early life stress. Brain. Behav. Immun 42, 81–88. 10.1016/j.bbi.2014.06.003 [DOI] [PubMed] [Google Scholar]
- Linnman C, Moulton EA, Barmettler G, Becerra L, Borsook D, 2012. Neuroimaging of the periaqueductal gray: State of the field. NeuroImage 60, 505–522. 10.1016/j.neuroimage.2011.11.095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestroni GJM, Mazzola P, 2003. Langerhans cells beta 2-adrenoceptors: role in migration, cytokine production, Th priming and contact hypersensitivity. J. Neuroimmunol 144, 91–99. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Gianaros PJ, Abramowitch SM, Manuck SB, Hariri AR, 2008. Interleukin-6 Covaries Inversely with Hippocampal Grey Matter Volume in Middle-Aged Adults. Biol. Psychiatry 64, 484–490. 10.1016/j.biopsych.2008.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland AL, Gianaros PJ, Kuan DC-H, Sheu LK, Krajina K, Manuck SB, 2015. Brain morphology links systemic inflammation to cognitive function in midlife adults. Brain. Behav. Immun 48, 195–204. 10.1016/j.bbi.2015.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland AL, Kuan DC-H, Sheu LK, Krajina K, Kraynak TE, Manuck SB, Gianaros PJ, 2017a. Systemic inflammation and resting state connectivity of the default mode network. Brain. Behav. Immun 62, 162–170. 10.1016/j.bbi.2017.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland AL, Walsh C, Lockwood K, John-Henderson NA, 2017b. The effects of acute psychological stress on circulating and stimulated inflammatory markers: A systematic review and meta-analysis. Brain. Behav. Immun 64, 208–219. 10.1016/j.bbi.2017.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Matsunaga M, Isowa T, Kimura K, Miyakoshi M, Kanayama N, Murakami H, Sato S, Konagaya T, Nogimori T, Fukuyama S, Shinoda J, Yamada J, Ohira H, 2008. Associations among central nervous, endocrine, and immune activities when positive emotions are elicited by looking at a favorite person. Brain. Behav. Immun 22, 408–417. 10.1016/j.bbi.2007.09.008 [DOI] [PubMed] [Google Scholar]
- McDade TW, Hawkley LC, Cacioppo JT, 2006. Psychosocial and behavioral predictors of inflammation in middle-aged and older adults: the Chicago health, aging, and social relations study. Psychosom. Med 68, 376–381. [DOI] [PubMed] [Google Scholar]
- Ménard C, Pfau ML, Hodes GE, Kana V, Wang VX, Bouchard S, Takahashi A, Flanigan ME, Aleyasin H, LeClair KB, Janssen WG, Labonté B, Parise EM, Lorsch ZS, Golden SA, Heshmati M, Tamminga C, Turecki G, Campbell M, Fayad ZA, Tang CY, Merad M, Russo SJ, 2017a. Social stress induces neurovascular pathology promoting depression. Nat. Neurosci 1 10.1038/s41593-017-0010-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménard C, Pfau ML, Hodes GE, Russo SJ, 2017b. Immune and Neuroendocrine Mechanisms of Stress Vulnerability and Resilience. Neuropsychopharmacology 42, 62–80. 10.1038/npp.2016.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Haroon E, Felger JC, 2017. Therapeutic Implications of Brain–Immune Interactions: Treatment in Translation. Neuropsychopharmacology 42, 334– 359. 10.1038/npp.2016.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Haroon E, Raison CL, Felger JC, 2013. Cytokine Targets in the Brain: Impact on Neurotransmitters and Neurocircuits. Depress. Anxiety 30, 297–306. 10.1002/da.22084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med 3, e123–e130. [PMC free article] [PubMed] [Google Scholar]
- Müller VI, Cieslik EC, Laird AR, Fox PT, Radua J, Mataix-Cols D, Tench CR, Yarkoni T, Nichols TE, Turkeltaub PE, Wager TD, Eickhoff SB, in press Ten simple rules for neuroimaging meta-analysis. Neurosci. Biobehav. Rev 10.1016/j.neubiorev.2017.11.012 [DOI] [PMC free article] [PubMed]
- *.Muscatell KA, Dedovic K, Slavich GM, Jarcho MR, Breen EC, Bower JE, Irwin MR, Eisenberger NI, 2015. Greater amygdala activity and dorsomedial prefrontal-amygdala coupling are associated with enhanced inflammatory responses to stress. Brain. Behav. Immun 43, 46–53. 10.1016/j.bbi.2014.06.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell KA, Eisenberger NI, Dutcher JM, Cole SW, Bower JE, 2016a. Links between inflammation, amygdala reactivity, and social support in breast cancer survivors. Brain. Behav. Immun 53, 34–38. 10.1016/j.bbi.2015.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell KA, Moieni M, Inagaki TK, Dutcher JM, Jevtic I, Breen EC, Irwin MR, Eisenberger NI, 2016b. Exposure to an inflammatory challenge enhances neural sensitivity to negative and positive social feedback. Brain. Behav. Immun 57, 21–29. 10.1016/j.bbi.2016.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers B, Scheimann JR, Franco-Villanueva A, Herman JP, 2016. Ascending mechanisms of stress integration: Implications for brainstem regulation of neuroendocrine and behavioral stress responses. Neurosci. Biobehav. Rev 10.1016/j.neubiorev.2016.05.011 [DOI] [PMC free article] [PubMed]
- Napadow V, Dhond R, Conti G, Makris N, Brown EN, Barbieri R, 2008. Brain correlates of autonomic modulation: Combining heart rate variability with fMRI. NeuroImage 42, 169–177. 10.1016/j.neuroimage.2008.04.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C, Ding A, 2010. Nonresolving inflammation. Cell 140, 871–882. 10.1016/j.cell.2010.02.029 [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline J-B, 2005. Valid conjunction inference with the minimum statistic. NeuroImage 25, 653–660. 10.1016/j.neuroimage.2004.12.005 [DOI] [PubMed] [Google Scholar]
- Nusslock R, Miller GE, 2016. Early-Life Adversity and Physical and Emotional Health Across the Lifespan: A Neuroimmune Network Hypothesis. Biol. Psychiatry, Inflammation and Immune Mechanisms in Neuropsychiatry 80, 23–32. 10.1016/j.biopsych.2015.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.O’Connor M-F, Irwin MR, Wellisch DK, 2009. When grief heats up: Pro- inflammatory cytokines predict regional brain activation. NeuroImage 47, 891– 896. 10.1016/j.neuroimage.2009.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Ohira H, Fukuyama S, Kimura K, Nomura M, Isowa T, Ichikawa N, Matsunaga M, Shinoda J, Yamada J, 2009. Regulation of natural killer cell redistribution by prefrontal cortex during stochastic learning. NeuroImage 47, 897–907. 10.1016/j.neuroimage.2009.04.088 [DOI] [PubMed] [Google Scholar]
- *.Ohira H, Isowa T, Nomura M, Ichikawa N, Kimura K, Miyakoshi M, Iidaka T, Fukuyama S, Nakajima T, Yamada J, 2008. Imaging brain and immune association accompanying cognitive appraisal of an acute stressor. NeuroImage 39, 500–514. 10.1016/j.neuroimage.2007.08.017 [DOI] [PubMed] [Google Scholar]
- *.Ohira H, Matsunaga M, Osumi T, Fukuyama S, Shinoda J, Yamada J, Gidron Y, 2013. Vagal nerve activity as a moderator of brain–immune relationships. J. Neuroimmunol 260, 28–36. 10.1016/j.jneuroim.2013.04.011 [DOI] [PubMed] [Google Scholar]
- Ongür D, Price JL, 2000. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb. Cortex N. Y. N 1991 10, 206–219. [DOI] [PubMed] [Google Scholar]
- Oppenheimer S, Cechetto D, 2016. The Insular Cortex and the Regulation of Cardiac Function. Compr. Physiol 6, 1081–1133. 10.1002/cphy.c140076 [DOI] [PubMed] [Google Scholar]
- Owen DRJ, Gunn RN, Rabiner EA, Bennacef I, Fujita M, Kreisl WC, Innis RB, Pike VW, Reynolds R, Matthews PM, Parker CA, 2011. Mixed-affinity binding in humans with 18-kDa translocator protein ligands. J. Nucl. Med. Off. Publ. Soc. Nucl. Med 52, 24–32. 10.2967/jnumed.110.079459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli WM, O’Reilly RC, Yarkoni T, Wager TD, 2016. Regional specialization within the human striatum for diverse psychological functions. Proc. Natl. Acad. Sci 113, 1907–1912. 10.1073/pnas.1507610113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov VA, Tracey KJ, 2017. Neural regulation of immunity: molecular mechanisms and clinical translation. Nat. Neurosci 20, 156–166. 10.1038/nn.4477 [DOI] [PubMed] [Google Scholar]
- Pfau ML, Russo SJ, 2015. Peripheral and central mechanisms of stress resilience. Neurobiol. Stress, Stress Resilience 1, 66–79. 10.1016/j.ynstr.2014.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Pomykala KL, Ganz PA, Bower JE, Kwan L, Castellon SA, Mallam S, Cheng I, Ahn R, Breen EC, Irwin MR, Silverman DHS, 2013. The association between pro-inflammatory cytokines, regional cerebral metabolism, and cognitive complaints following adjuvant chemotherapy for breast cancer. Brain Imaging Behav 7, 511–523. 10.1007/s11682-013-9243-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather AA, Carroll JE, Fury JM, McDade KK, Ross D, Marsland AL, 2009. Gender differences in stimulated cytokine production following acute psychological stress. Brain. Behav. Immun., Special Issue on Gender, Ethnicity, and Immunity 23, 622–628. 10.1016/j.bbi.2008.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz M, Priller J, 2017. The role of peripheral immune cells in the CNS in steady state and disease. Nat. Neurosci 20, 136–144. 10.1038/nn.4475 [DOI] [PubMed] [Google Scholar]
- Quan N, Banks WA, 2007. Brain-immune communication pathways. Brain. Behav. Immun 21, 727–735. 10.1016/j.bbi.2007.05.005 [DOI] [PubMed] [Google Scholar]
- Raison CL, Miller AH, 2011. Is Depression an Inflammatory Disorder? Curr. Psychiatry Rep 13, 467–475. 10.1007/s11920-011-0232-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmächer T, 2001. Cytokine-associated emotional and cognitive disturbances in humans. Arch. Gen. Psychiatry 58, 445–452. [DOI] [PubMed] [Google Scholar]
- Rinaman L, 2007. Visceral sensory inputs to the endocrine hypothalamus. Front. Neuroendocrinol 28, 50–60. 10.1016/j.yfrne.2007.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Rosenkranz MA, Busse WW, Sheridan JF, Crisafi GM, Davidson RJ, 2012. Are there neurophenotypes for asthma? Functional brain imaging of the interaction between emotion and inflammation in asthma. PloS One 7, e40921 10.1371/journal.pone.0040921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Rosenkranz MA, Esnault S, Christian BT, Crisafi G, Gresham LK, Higgins AT, Moore MN, Moore SM, Weng HY, Salk RH, Busse WW, Davidson RJ, 2016. Mind-body interactions in the regulation of airway inflammation in asthma: A PET study of acute and chronic stress. Brain. Behav. Immun 58, 18– 30. 10.1016/j.bbi.2016.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz Vargas E, Sörös P, Shoemaker JK, Hachinski V, 2016. Human cerebral circuitry related to cardiac control: A neuroimaging meta-analysis. Ann. Neurol 79, 709–716. 10.1002/ana.24642 [DOI] [PubMed] [Google Scholar]
- Salimi-Khorshidi G, Smith SM, Keltner JR, Wager TD, Nichols TE, 2009. Meta- analysis of neuroimaging data: a comparison of image-based and coordinate- based pooling of studies. NeuroImage 45, 810–823. 10.1016/j.neuroimage.2008.12.039 [DOI] [PubMed] [Google Scholar]
- Sandiego CM, Gallezot J-D, Pittman B, Nabulsi N, Lim K, Lin S-F, Matuskey D, Lee J-Y, O’Connor KC, Huang Y, Carson RE, Hannestad J, Cosgrove KP, 2015. Imaging robust microglial activation after lipopolysaccharide administration in humans with PET. Proc. Natl. Acad. Sci 112, 12468–12473. 10.1073/pnas.1511003112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, 2002. The central autonomic nervous system: Conscious visceral perception and autonomic pattern generation. Annu. Rev. Neurosci 25, 433– 69. [DOI] [PubMed] [Google Scholar]
- Satpute AB, Wager TD, Cohen-Adad J, Bianciardi M, Choi J-K, Buhle JT, Wald LL, Barrett LF, 2013. Identification of discrete functional subregions of the human periaqueductal gray. Proc. Natl. Acad. Sci 110, 17101–17106. 10.1073/pnas.1306095110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedlowski M, Engler H, Grigoleit J-S, 2014. Endotoxin-induced experimental systemic inflammation in humans: A model to disentangle immune-to-brain communication. Brain. Behav. Immun 35, 1–8. 10.1016/j.bbi.2013.09.015 [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD, 2007. Dissociable Intrinsic Connectivity Networks for Salience Processing and Executive Control. J. Neurosci 27, 2349–2356. 10.1523/JNEUROSCI.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerdahl AR, Mezue M, Okell TW, Farrar JT, Tracey I, 2015. The dorsal posterior insula subserves a fundamental role in human pain. Nat. Neurosci advance online publication. 10.1038/nn.3969 [DOI] [PMC free article] [PubMed]
- Shattuck DW, Mirza M, Adisetiyo V, Hojatkashani C, Salamon G, Narr KL, Poldrack RA, Bilder RM, Toga AW, 2008. Construction of a 3D probabilistic atlas of human cortical structures. NeuroImage 39, 1064–1080. 10.1016/j.neuroimage.2007.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard RN, 1980. Multidimensional scaling, tree-fitting, and clustering. Science 210, 390–398. 10.1126/science.210.4468.390 [DOI] [PubMed] [Google Scholar]
- *.Slavich GM, Way BM, Eisenberger NI, Taylor SE, 2010. Neural sensitivity to social rejection is associated with inflammatory responses to social stress. Proc. Natl. Acad. Sci 107, 14817–14822. 10.1073/pnas.1009164107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg EM, 2006. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat. Rev. Immunol 6, 318–328. 10.1038/nri1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone EA, Lehmann ML, Lin Y, Quartermain D, 2006. Depressive Behavior in Mice Due to Immune Stimulation is Accompanied by Reduced Neural Activity in Brain Regions Involved in Positively Motivated Behavior. Biol. Psychiatry 60, 803–811. 10.1016/j.biopsych.2006.04.020 [DOI] [PubMed] [Google Scholar]
- Swartz JR, Prather AA, Hariri AR, 2017. Threat-related amygdala activity is associated with peripheral CRP concentrations in men but not women. Psychoneuroendocrinology 78, 93–96. 10.1016/j.psyneuen.2017.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Tashiro M, Itoh M, Kubota K, Kumano H, Masud MM, Moser E, Arai H, Sasaki H, 2001. Relationship between trait anxiety, brain activity and natural killer cell activity in cancer patients: a preliminary PET study. Psychooncology 10, 541–546. 10.1002/pon.548 [DOI] [PubMed] [Google Scholar]
- Thayer JF, Åhs F, Fredrikson M, Sollers III JJ, Wager TD, 2012. A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev 36, 747– 756. 10.1016/j.neubiorev.2011.11.009 [DOI] [PubMed] [Google Scholar]
- Thayer JF, Sternberg EM, 2010. Neural aspects of immunomodulation: Focus on the vagus nerve. Brain. Behav. Immun 24, 1223–1228. 10.1016/j.bbi.2010.07.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey KJ, 2009. Reflex control of immunity. Nat. Rev. Immunol 9, 418–428. 10.1038/nri2566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaziri ND, Rodríguez-Iturbe B, 2006. Mechanisms of Disease: oxidative stress and inflammation in the pathogenesis of hypertension. Nat. Rev. Nephrol 2, 582–593. 10.1038/ncpneph0283 [DOI] [PubMed] [Google Scholar]
- Verstynen TD, Weinstein A, Erickson KI, Sheu LK, Marsland AL, Gianaros PJ, 2013. Competing physiological pathways link individual differences in weight and abdominal adiposity to white matter microstructure. NeuroImage 79, 129– 137. 10.1016/j.neuroimage.2013.04.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP, 2004. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 51, 32–58. 10.1002/syn.10279 [DOI] [PubMed] [Google Scholar]
- Wager TD, Atlas LY, Lindquist MA, Roy M, Woo C-W, Kross E, 2013. An fMRI- Based Neurologic Signature of Physical Pain. N. Engl. J. Med 368, 1388–1397. 10.1056/NEJMoa1204471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Lindquist M, Kaplan L, 2007. Meta-analysis of functional neuroimaging data: current and future directions. Soc. Cogn. Affect. Neurosci 2, 150–158. 10.1093/scan/nsm015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Lindquist MA, Nichols TE, Kober H, Van Snellenberg JX, 2009a. Evaluating the consistency and specificity of neuroimaging data using meta- analysis. NeuroImage, Mathematics in Brain Imaging 45, S210–S221. 10.1016/j.neuroimage.2008.10.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, van Ast VA, Hughes BL, Davidson ML, Lindquist MA, Ochsner KN, 2009b. Brain mediators of cardiovascular responses to social threat, Part II: Prefrontal-subcortical pathways and relationship with anxiety. NeuroImage, Brain Body Medicine 47, 836–851. 10.1016/j.neuroimage.2009.05.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Waugh CE, Lindquist M, Noll DC, Fredrickson BL, Taylor SF, 2009c. Brain mediators of cardiovascular responses to social threat: Part I: Reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. NeuroImage, Brain Body Medicine 47, 821–835. 10.1016/j.neuroimage.2009.05.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Maier SF, 2000. The Pain of Being Sick: Implications of Immune-to- Brain Communication for Understanding Pain. Annu. Rev. Psychol 51, 29–57. 10.1146/annurev.psych.51.1.29 [DOI] [PubMed] [Google Scholar]
- Weaver JD, Huang M-H, Albert M, Harris T, Rowe JW, Seeman TE, 2002. Interleukin-6 and risk of cognitive decline MacArthur Studies of Successful Aging. Neurology 59, 371–378. 10.1212/WNL.59.3.371 [DOI] [PubMed] [Google Scholar]
- Wik G, Lekander M, Fredrikson M, 1998. Human Brain–Immune Relationships: A PET Study. Brain. Behav. Immun 12, 242–246. 10.1006/brbi.1998.0529 [DOI] [PubMed] [Google Scholar]
- Wohleb ES, Franklin T, Iwata M, Duman RS, 2016. Integrating neuroimmune systems in the neurobiology of depression. Nat. Rev. Neurosci 17, 497–511. 10.1038/nrn.2016.69 [DOI] [PubMed] [Google Scholar]
- Wrona D, 2006. Neural–immune interactions: An integrative view of the bidirectional relationship between the brain and immune systems. J. Neuroimmunol 172, 38–58. 10.1016/j.jneuroim.2005.10.017 [DOI] [PubMed] [Google Scholar]
- Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zöllei L, Polimeni JR, Fischl B, Liu H, Buckner RL, 2011. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol 106, 1125–1165. 10.1152/jn.00338.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R, Goshen I, 2011. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain. Behav. Immun 25, 181–213. 10.1016/j.bbi.2010.10.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


