Abstract
Purpose
The adult mammalian retina is typically incapable of regeneration when damaged by disease or trauma. Restoration of function would require generation of new adult neurons, something that until recently, mammals were thought to be incapable of doing. However, previous studies from this laboratory have shown that the α7 nicotinic acetylcholine receptor (α7 nAChR) agonist, PNU-282987, induces cell cycle reentry of Müller glia and generation of mature retinal neurons in adult rats, in the absence of detectible injury. This study analyzes how PNU-282987 treatment in RPE leads to robust BrdU incorporation in Müller glia in adult mice and leads to generation of Müller-derived retinal progenitors and neuronal differentiation.
Methods
Retinal BrdU incorporation was examined after eye drop application of PNU-282987 in adult wild-type and transgenic mice that contain tamoxifen-inducible tdTomato Müller glia, or after intraocular injection of conditioned medium from PNU-282987–treated cultured RPE cells.
Results
PNU-282987 induced robust incorporation of BrdU in all layers of the adult mouse retina. The α7 nAChR agonist was found to stimulate cell cycle reentry of Müller glia and their generation of new retinal progenitors indirectly, via the RPE, in an α7 nAChR-dependent fashion.
Conclusions
The results from this study point to RPE as a contributor to Müller glial neurogenic responses. The manipulation of the RPE to stimulate retinal neurogenesis offers a new direction for developing novel and potentially transformative treatments to reverse the loss of neurons associated with neurodegenerative disease, traumatic injury, or aging.
Keywords: adult mammalian neurogenesis, alpha7 nicotinic ACh receptor, PNU-282987, Müller glia
Blindness associated with retinal degeneration caused by diseases, such as glaucoma, retinitis pigmentosa, macular degeneration, diabetic retinopathy, age, or trauma, can lead to a significant decrease in the quality of life of affected individuals, and is a major burden on health care systems and families.1,2 Moreover, these diseases have no cure. Currently available treatments cannot restore neurons that have already been lost to disease.3,4 The need for new interventions to restore vision has stimulated significant interest in identifying strategies for regenerating retinal neurons.5,6
In some nonmammalian vertebrates, such as teleost fish (e.g., zebrafish) and, to a lesser extent, post-hatch chicks, retinal regeneration occurs regularly and predictably in response to retinal damage.7–10 In these animals, the retinal damage stimulates interkinetic nuclear migration of Müller glia, and their reentry into the cell cycle to produce retinal progenitor cells (RPCs).11,12 Injury-induced RPCs ultimately differentiate into all types of retinal neurons and thereby restore visual function.9,13,14 In fish and birds, retinal injury and the resulting death of retinal neurons is required for robust cell cycle reentry by Müller glia and the subsequent neuronal regeneration.
However, until recently, adult mammals were thought to be incapable of generating new retinal neurons. In mice, retinal neurogenesis is complete by postnatal day 11, with no evidence for subsequent production of new neurons.15 In the mammalian retina, the primary response to retinal injury is gliosis, which is characterized by glial hypertrophy, limited proliferation, and formation of a glial scar.16 Nevertheless, isolated mammalian Müller glia proliferate,17,18 and given the appropriate signals, can generate various types of retinal neurons in vitro.18–20 These findings have given impetus to research and identify mechanisms that can switch the injury response of Müller glia away from gliosis, and toward proliferation, neurogenesis, and retinal regeneration. In rodents, a subpopulation of Müller glia can be driven toward a neurogenic response by exogenous factors, similar to those implicated in retinal regeneration in lower vertebrates. These include epidermal growth factor,10 sonic hedgehog,21 and forced overexpression of acetyl-CoA synthetase long-chain family member 1 in the Müller glia.22 Nevertheless, none of these factors stimulate proliferative or neurogenic responses of Müller glia in the absence or loss of retinal neurons.
Previous work from our laboratory demonstrated that in adult rats, the alpha7 nicotinic acetylcholine receptor (α7 nAChR) agonist, PNU-282987, is sufficient to induce de novo neurogenesis, even in the absence of detectible retinal injury or neuronal death.23,24 In rats, topical application of PNU-282987–containing eye drops resulted in cell cycle reentry of vimentin-positive Müller glia, as well as the production of nestin-positive RPCs, as demonstrated by bromodeoxyuridine (BrdU) and proliferating cell nuclear antigen (PCNA) labeling. Importantly, PNU-282987 effects were dose-dependent and, when followed over time, resulted in persistent BrdU labeling in cells within all retinal layers, and a dramatic accumulation of BrdU-labeled retinal ganglion cells (RGCs).24 These data support the hypothesis that the adult mammalian retina is capable of regeneration given appropriate conditions. Here, we extend these studies to mice to take advantage of transgenic manipulations and examine the contributions of Müller glia and the role of RPE in the proliferative response induced by PNU-282987.
Methods
Animals
Wild-type and transgenic 129/SVJ mice were used in this study. Transgenic mice were obtained as a generous gift from Deborah Otteson, PhD (University of Houston College of Optometry) and bred in the animal facility at Western Michigan University according to breeding procedures listed below. Wild-type 129/SVJ mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA). Animals were housed in a normal experimental room and exposed to a 12-hour light/dark cycle with free access to food and water. An equal number of males and females were used for each experiment in this study. No sex difference was observed in any experiment. Animals used in this study were between 3 and 4 months of age and weighed between 202 and 258 mg. All research adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Cell Culture
Rat RPE cells (RPE-J; 4–6 passages), were grown in Dulbecco's modified Eagle's medium containing 8% fetal bovine serum, 1% L-Glutamine, and 1% penicillin-streptomycin, using standard cell culture techniques. Unless specified, cell culture reagents were obtained from Thermo Fisher (Waltham, MA, USA). For PNU-282987 and methyllycaconitine (MLA) treatments (Sigma-Aldrich, St. Louis, MO, USA), RPE-J cells were grown to 70% to 80% confluence at 33°C and treated with normal culture media containing PNU-282987 (100 nM) for 24 hours, or MLA (1 μM) for 8 hours, followed by 100 nM PNU-282987 for 24 hours. Subsequently, cells were washed three times with PBS to remove any trace of added compounds, fresh media was added, and cells were incubated for an additional 72 hours. Conditioned media was collected at multiple time points following the thorough rinsing. Treatment of RPE-J cells with 100 nM PNU-282987 for 24 hours was used in this study as a result of dose and time-dependent studies in which concentrations between 1 and 1000 nM were applied to cultured cells for various amounts of time (Linn CL, et al. IOVS 2018;59:ARVO E-Abstract B0174). The 100 nM PNU-282987 applied to cells for 24 hours produced the maximal proliferative response in animals injected with treated RPE-J supernatant and was therefore used for all culture experiments described in this study. Similarly, previous dose- and time-dependent studies using MLA to block the effect of PNU-282987 demonstrated that incubation of cultured RPE-J cells with 1 μM MLA for 8 hours produced the maximal inhibition of the effect of PNU-282987 (Linn CL, et al. IOVS 2018;59:ARVO E-Abstract B0174).
Eye Drop Treatments, RPE Supernatant Injection and Retina Preparation
Both eyes of each experimental animal were treated once daily with eye drops containing PBS, 1 mg/mL BrdU, and 1 mM PNU-282987. All animals received this treatment for a maximum of 2 weeks. Other animals also received eye drops containing 1 mM MLA 1 hour before PNU-282987 and BrdU eye drop treatment. Details of the eye drop treatment are described in Linn et al.25 Eye drop treatments never caused irritation to the animals or inflammation to the eyes. At specific times following the start of treatment, mice were euthanized by carbon dioxide asphyxiation. Eyes were removed, and retinas were excised, flat-mounted, and fixed in 4% paraformaldehyde overnight at 4°C. Retinal sections (50 μm) were obtained from flat-mounted tissue.
In some experiments, conditioned media from PNU-282987– or MLA+PNU-282987–treated RPE cells was collected as described above, and 1 μL of media was injected into the vitreal chamber of mice; 1 μL is the standard volume injected into the vitreal chamber of adult mice, as the total vitreous volume is relatively small.26–28 Other eyes received an injection of control RPE media (untreated), an injection of a saline vehicle, or an injection of RPE media obtained immediately after PNU-282987–treated RPE cells were thoroughly washed.
Transgenic Constructs, Breeding, and Genotyping
Mice of the 129Svj strain carrying the RlbpCre-ER and Rosa-tdTomatofloxstopflox transgenes were used to label Müller glia in some experiments. RlbpCre-ER mice carry a tamoxifen-inducible, Cre-transgene under the Rlbp promoter that drives Cre recombinase expression specifically in Müller glia in the retina.29 Following Cre-excision of the stop codon in the transgene, the Rosa-tdTomato Cre-reporter is expressed. To generate experimental mice, male animals heterozygous for RlbpCre-ER, and homozygous or heterozygous for the tdTomato reporter, were crossed with female animals that lacked the RlbpCre-ER transgene (homozygous wild-type) and were homozygous for the tdTomato reporter. Pups were genotyped by PCR analysis of deoxyribonucleic acid obtained from tail biopsies.
Antibody Labeling
Following fixation, whole-mounted retinas were labeled with various combinations of primary antibodies: sheep anti-BrdU (7.5 μL/mL, Abcam [Cambridge, UK] ab1893; research resource identifier [RRID]: AB_302659); chicken anti-PAX6 (2 μL/mL Developmental Studies Hybridoma Bank [University of Iowa, Iowa City, IA, USA]; RRID: AB_528427), and rabbit anti-RFP/tdTomato (5 μL/mL Rockland [Limerick, PA, USA] 600-401-379; RRID: AB_2209751) or rabbit anti-cone arrestin (5 μL/mL Sigma-Aldrich; RRID: AB_15282). For BrdU staining, antigen retrieval was done as in Webster et al.24 Retinas were blocked in PBS containing 1% Triton X-100 and 1% bovine serum. Retinas were incubated in primary antibodies overnight at room temperature in PBS containing 1% bovine serum and 1% goat serum, rinsed in PBS, and incubated overnight with appropriate Alexa Fluor conjugated secondary antibodies (1:300; Life Technologies, Carlsbad, CA, USA) diluted in PBS without serum.
Cell Counting and Normalization
Fixed immunostained retinas were counterstained with 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI; Sigma-Aldrich), sectioned at 50 μm, and imaged using a Nikon (Tokyo, Japan) C2+ scanning laser confocal microscope. DAPI-stained nuclei were counted from four 200 μm2 section images of the inner nuclear layer (INL) from each retinal section and averaged. DAPI-stained cells that colocalized with anti-BrdU were counted and normalized as a mean percentage of the total cells. Percentage of BrdU+ cells in the INL were obtained from five different animals. Normalization of BrdU+ cells was required in these studies due to variation in cell counts obtained in each retinal section. Variations in retinal counts were due to normal variations found between using different animals and due to small changes in the position of the retina when obtaining retinal sections.
Statistical Analysis
Statistical analysis used the Kruskal-Wallis nonparametric ANOVA, with post hoc multiple comparisons (Dunn's test), with P < 0.001 was considered statistically different using GraphPad software (La Jolla, CA, USA).
Results
BrdU+ Cells in the Adult Mouse Retina
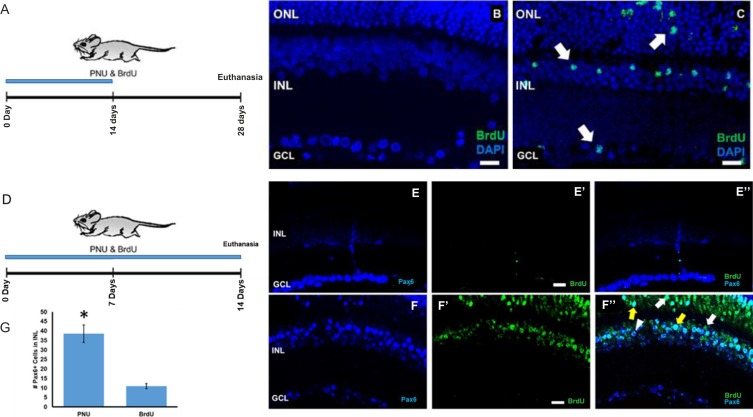
Previous studies from this laboratory using adult Long Evans rats demonstrated that the α7 nAChR agonist, PNU-282987, induces cell cycle reentry of cells located in the INL, resulting in an increase in the number of BrdU+ cells throughout the retina, and new RGCs in the ganglion cell layer (GCL).24 In the present studies, using BrdU incorporation as a marker for entry into S phase, we observed a similar outcome in the retinas of adult mice. In adult mice, no BrdU-labeled cells were detected in the retina following 2 weeks of topical BrdU treatment (Fig. 1B). In contrast, adult mice treated with eye drops containing 1 mM PNU-282987 and 1 mg/mL BrdU for 2 weeks and euthanized after 4 weeks (Fig. 1A) displayed BrdU-labeled cells in all retinal lamina (Fig. 1C).
Figure 1.
PNU-282987 treatment induces BrdU+ and Pax6+ labeling. Confocal images of adult mouse retinas following 2 weeks of topical BrdU only (B) or with PNU-282987 plus BrdU (C). Treatment schemes shown in (A) and (D). No BrdU+ cells are detected in retinas that received only BrdU (B), whereas the PNU-282987/BrdU-treated eye contains BrdU+ cells (green) located in all retinal lamina (arrows in C). In (E, E'), antibodies against Pax6 and BrdU were applied to control untreated retinas. Antibodies against PAX6-labeled mature RGCs in the GCL and amacrine cells of the INL (blue) in BrdU-only treated retinas in control untreated mice (E). No evidence of BrdU+ cells were seen in control animals (E', E”). When the retina was treated with PNU-282987/BrdU, however, there was a significant increase of Pax6+ cells in the INL (F) and evidence of BrdU+ cells (F'). Multiple double-labeled PAX6+/BrdU+ cells in the ONL of PNU-282987–treated retinas (yellow arrows in F”). Arrowhead represents a PAX6+ cell and the white arrow points to a BrdU+ cell. The average number of PAX6+ cells in a 200-μm2 section of the INL treated with PNU-282987/BrdU or just BrdU are shown in (C). *Represents a significant difference from retinas treated with BrdU only. n = 6 mice for each experimental condition. Scale bar: 30 μm.
BrdU and PAX6 Colocalization
PAX6, a transcription factor required for normal retinal development, is expressed in retinal progenitors during embryonic development as well as in RGCs and amacrine cells after differentiation in the mature retina.30–33 In the retinas of control adult mice treated with BrdU for only 2 weeks, PAX6 immunoreactivity in the nuclei of differentiated RGCs and cells in the INL is seen (Fig. 1E). However, BrdU-labeled cells were not observed in eyes that received drops containing BrdU alone (Figs. 1E', 1E” merged image). In contrast, in the retinas of mice treated with both PNU-282987 and BrdU, there was more robust immunostaining of PAX6 in the INL (Fig. 1F) and BrdU+ cells were present in the INL and outer nuclear layer (ONL) (Fig. 1F'). A few BrdU+ cells colabeled for PAX6 (Fig. 1F”, yellow arrows). The bar graph in Figure 1G compares the average numbers of PAX6-positive cells counted from averaged 200 μm2 sections treated with BrdU only or with PNU-282987 and BrdU. There were significantly more PAX6-positive cells in the PNU-282987–treated retinas in the INL (Fig. 1G). In retinas treated with PNU-282987, there was an average of 38.5 ± 9.14 PAX6-positive INL cells per 200 μm2 section, whereas there was an average of only 11.0 ± 2.70 PAX6-positive cells in control retinas. The INL demonstrated the greatest change in the percentage of PAX6-positive cells after treatment with PNU-282987.
A Transgenic Mouse Line
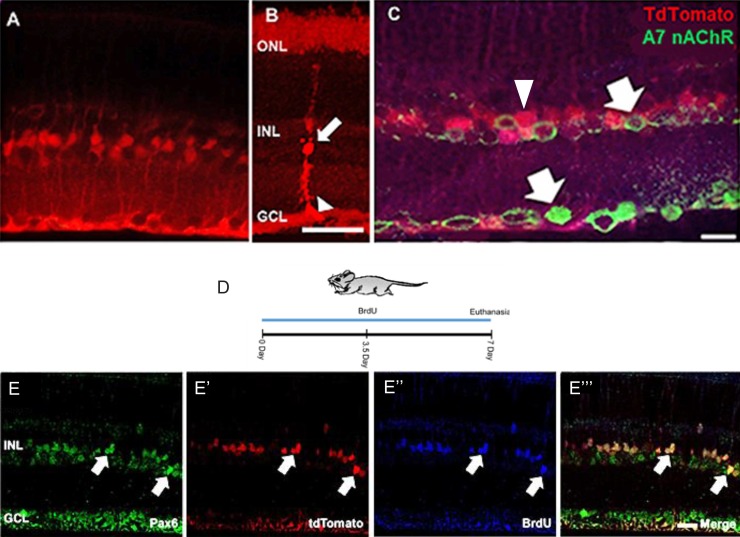
To determine if Müller glia were the source of BrdU+ cells, a transgenic mouse model that carries a tamoxifen-inducible Cre recombinase driven by the Müller glia-specific Rlbp promotor (RlbpCre-ER) and a floxed tdTomato (Rosa-tdTomatofloxstopflox) reporter was used to label Müller glia in vivo.29 In mice 3 to 4 months old, heterozygous for RlbpCre-ER/+ and homozygous for Rosa-tdTomatofloxstopflox, five daily intraperitoneal injections of tamoxifen treatment resulted in expression of tdTomato in Müller glia within the retina (Fig. 2A). The tdTomato reporter was visible in the Müller glial cell bodies within the INL (Fig. 2B, arrow), and their inner, radial processes, which extend through the GCL to form end feet at the inner limiting membrane of the retina (Fig. 2B, arrowhead). The outer radial processes of the Müller glia were faintly labeled by the tdTomato reporter. Thus, tdTomato expression in Müller glia in these mice can serve as a lineage tracer. Unfixed tissue shows clear processes extending from the INL (Figs. 2A, 2B). If Z-stacks of these fresh preparations are taken and the images condensed into one image, even further detail can be observed (Fig. 2B). However, fixation of the tissue diminishes native tdTomato fluorescence and in some experiments, visualization of the tdTomato-positive Müller glia was done using an antibody against tdTomato (Fig. 2C, arrowhead). Interestingly, Müller glia do not express α7 nAChRs, the target of PNU-282987 (Fig. 2C, arrows).34–36 In Figure 2C, α7 nAChRs are found in cells of the INL and GCL, but do not colocalize with the antibody against tdTomato.
Figure 2.
Tamoxifen induction of RlbpERCre labels Müller glia and RPCs with Rosa-tdTomato reporter in adult mouse retina. Confocal image showing tdTomato expression in the retina of a 12-week-old mouse collected 5 weeks after tamoxifen induction of CreER (five consecutive daily intraperitoneal injections of 50 μL of 4-hydroxy tamoxifen at 5 mg/mL). tdTomato-positive cells, shown in a fresh unfixed section (A) in an unfixed z-stack flattened section (B) or in a section fixed and antibody stained (C). Fresh preparations have the typical morphology of Müller glia, with cell bodies and nuclei in the INL (arrow in B) and radial processes ending in characteristic end feet (arrowhead in B). Radial processes also extend through the ONL but are more faintly labeled. tdTomato fluoresce detected with anti-tdTomato antibody staining (C). Müller glia lack the α7 nAChR as shown in (C) (arrows indicate α7 nAChR and arrowheads indicate Müller glia). The treatment scheme for confocal images seen in (E–E''') is shown in (D). Following 2 weeks of topical PNU-282987+BrdU, tdTomato-labeled cells in the INL (arrows) were triple-labeled for (E) PAX6, (E') tdTomato, and BrdU (E”). (E''') shows the three channels merged. tdTomato expression was demonstrated in PAX6+/BrdU+ cells. Scale bar: 50 μm for (A–C) and 20 μm for (E–E'''). n = 6 mice for each experimental condition.
To determine if BrdU+ cells triggered by application of PNU-282987 arise from Müller glia, expression of tdTomato in Müller glia was induced in adult RlbpCre-ER; RosatdTomato mice, followed by application of eye drops containing PNU-282987 and BrdU for 7 days (Fig. 2D). Retinal sections were triple-labeled with antibodies against PAX6 (Fig. 2E), tdTomato (Fig. 2E') and BrdU (Fig. 2E”). At the end of treatment, tdTomato-positive cells (red) were present in the INL (Fig. 2E'). Many tdTomato-positive cells also labeled for BrdU (Fig. 2E”) and PAX6 (Fig. 2E). The merged triple-labeled cells are shown in Figure 2E'''.
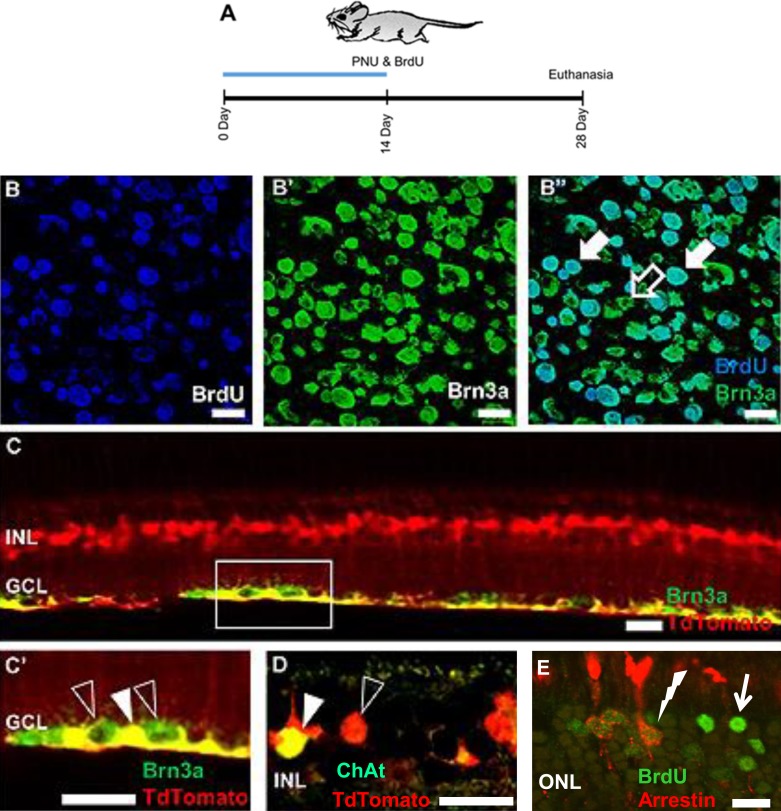
In previous studies from this laboratory, adult rodent RGCs were shown to be BrdU+ following treatment with PNU-282987.24 To determine if new Müller glia-derived RPCs generated RGCs and other adult neurons, the transgenic mouse line containing tdTomato-labeled Müller glia was used to identify cells derived from Müller glia that had differentiated as RGCs (Brn3a) or the starburst amacrine cells (ChAT) (Fig. 3). New RGCs were observed in flat-mounted retinas following 2 weeks of PNU-282987 and BrdU eye drops when euthanized 4 weeks after the start of treatment (Figs. 3B–B”). When the PNU-282987–treated retina was sectioned, RGCs robustly colabeled with tdTomato and Brn3a (Figs. 3C, 3C'). Starburst amacrine cells also colabeled with antibodies against tdTomato and choline acetyltransferase (ChAT) (Fig. 3D). Last, BrdU+ photoreceptors cells were colabeled with antibodies against arrestin in the ONL (Fig. 3E).
Figure 3.
Genesis of tdTomato-positive adult retinal neurons following PNU-282987 treatment. Confocal images from adult transgenic mice that were treated with PNU-282987 for 2 weeks and euthanized 4 weeks after the start of treatment (A). RGCs (B–B” and C–C') and starburst amacrine cells (D) showed colabeling for the Müller glia marker tdTomato. In addition, cone photoreceptors double-labeled with antibodies against BrdU and arrestin (E). The open arrow in (B” ) indicates a Brn3a+, BrdU− RGC, whereas closed arrows show cells in the GCL double-labeled with antibodies against both Brn3a+ and BrdU. The open arrowhead in (C') shows Brn3a+ RGCs, whereas the closed arrowhead illustrates a double-labeled Brn3a+, tdTomato+ RGC. The open arrowhead in (D) illustrates cell bodies of Müller glia in the INL (red), whereas the closed arrowhead indicates a ChAT+, tdTomato+ starburst amacrine cell. In (E), the solid arrow points to a BrdU+ cell in the ONL, whereas the lightning bolt illustrates a double-labeled cell in the ONL labeled with antibodies against arrestin and BrdU. Scale bars: 20 μm. n = 6 mice for each experimental condition.
MLA Inhibition of Cell Cycle Reentry
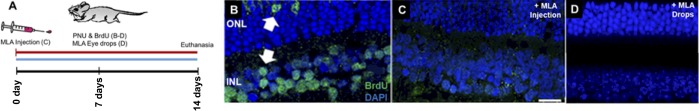
PNU-282987 is a potential and specific agonist of α7 nAChRs. Inhibition of the receptor using the α7 nAChR-specific antagonist, MLA, was expected to reduce the number of BrdU+ cells generated by PNU-282987 treatment in mice. MLA was introduced in adult mice intravitreally using 1 μL of 1 mM MLA an hour before PNU-282987+BrdU eye drop treatment, or as eye drops 1 hour before topical application of PNU-282987+BrdU (Fig. 4A). Eyes treated with PNU-282987+BrdU showed robust staining for BrdU in many retinal cells (Fig. 4B); however, retinas from eyes that received intravitreal injections of MLA before PNU-282987+BrdU consistently contained significantly fewer BrdU+ cells (Fig. 4C). Chronic exposure to topical MLA was also able to inhibit PNU-282987–mediated BrdU incorporation when MLA was applied as an eye drop 1 hour before the eye drop containing PNU-282987 and BrdU (Fig. 4D). This supports the hypothesis that the effects of PNU-282987 on Müller glia proliferation are mediated through α7 nAChRs. However, the absence of α7 nAChRs on Müller glia is consistent with an indirect effect of PNU-282987; likely mediated through other cells in the eyes that express α7 nAChRs. In the retina, RGCs, some cone bipolar cells, and glycinergic amacrine cells express α7 nAChRs,36–38 and we have reported α7 nAChR immunostained cells in the GCL and INL of the mouse retina (Fig. 2). However, retinal neurons cannot be responsible for proliferation of Müller glia in response to the α7 nAChR agonist PNU-282987, as ACh release studies in the vertebrate retina demonstrate tonic release of ACh from starburst amacrine cells that is increased in response to light.39,40 ACh released from starburst amacrine cells onto RGCs, bipolar cells, and other amacrine cells fail to induce neurogenesis under physiological conditions39,40; however, RPE cells also express α7 nAChRs in the eye.36–38 Therefore, the RPE was investigated to determine if it could mediate the effects of PNU-282987.
Figure 4.
MLA Inhibition of α7 nAChRs blocks BrdU incorporation in Müller glia. Treatment scheme shown in (A). Representative confocal images of retinas from mice treated for 2 weeks with (B) PNU-282987+BrdU showing typical pattern of BrdU incorporation in nuclei. (C, D) Retinas from eyes pretreated with MLA before PNU-282987+BrdU contain no BrdU-labeled cells. Scale bar: 50 μm. n = 6 mice for each experimental condition.
PNU-282987–Conditioned Cell Culture Supernatant From RPE Cells Induces Cell Cycle Reentry
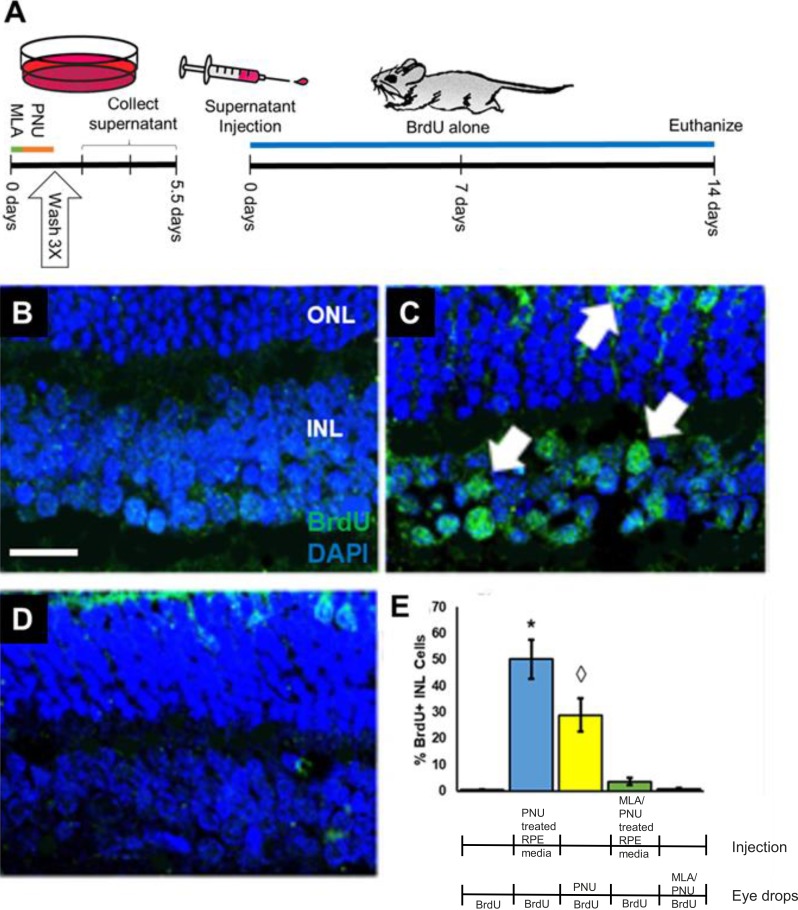
The RPE-J cell line was cultured to 80% confluency and remained untreated, or cells were treated for 24 hours with 100 nM PNU-282987 (PNU-282987–conditioned media) or with 1 μM MLA for 8 hours followed by 100 nM PNU-282987 for 24 hours (MLA+PNU-282987–conditioned media). After treatments, RPE cells were thoroughly washed three times to remove any remaining residual agents and were cultured for various amounts of time (0–72 hours). Modified culture media was collected after various amounts of time, and then injected into the vitreal chamber of adult mice (Fig. 5A). BrdU eye drops were then subsequently added daily to the injected animals for 2 weeks. In eyes injected with control untreated media collected from cultured RPE, no BrdU+ cells were seen in the INL after 2 weeks of BrdU treatment (Fig. 5B). Likewise, no BrdU+ cells were seen if PNU-282987–treated cells were washed thoroughly, and media was injected into mice from cells allowed to culture for 0 to 24 hours after washing out PNU-282987 treatment. However, eyes that were injected with PNU-282987–conditioned media, collected 72 hours after removal of the PNU-282987, induced many BrdU+ cells in adult mice in the INL, and in the ONL (Fig. 5C, arrows). BrdU+ cells were significantly reduced if cultured RPE cells were treated with MLA before PNU-282987 treatment (Fig. 5D).
Figure 5.
Conditioned media from RPE cells induces BrdU incorporation when injected into adult mice eyes. Treatment scheme shown in (A). (B) No BrdU labeling was detected in the INL of retinas from eyes receiving intravitreal injection of conditioned media obtained within 1 hour after washing away PNU-282987 from treated RPE-J cells. (C) Robust BrdU incorporation in the nuclei of cells in the INL and ONL (arrows) was present in retinas from eyes receiving intravitreal injection of 72-hour conditioned media from PNU-282987–treated RPE-J cells. (D) Few BrdU-labeled nuclei are present in the retinas of eyes injected with 72-hour conditioned media from RPE-J cells treated with the α7AChR inhibitor, MLA, and PNU-282987. (E) Quantification of BrdU labeling in retinas treated with indicated combinations of PNU-282987 (PNU) and MLA, applied either intraocularly as conditioned media or topically as eye drops. For intraocular injections, conditioned media from PNU-282987, or conditioned medium from MLA/PNU-282987–treated RPE-J cells, was injected intraocularly one time into 3-month-old mice. BrdU drops were then applied for an additional 2 weeks before mice were euthanized. For topical treatments, animals received a continuous application of 1 mM MLA in the same eye drops containing PNU-282987 and BrdU before being euthanized. The star and diamond represent significant differences from all other categories. Scale bar: 50 μm. n = 6 mice for each experimental condition.
These qualitative observations were confirmed and quantified by cell counts (Fig. 5E). Injections of PNU-282987–conditioned media collected from treated RPE resulted in BrdU incorporation in an average of 50.13% ± 7.43% of the INL cells, which represents a significant increase from BrdU+ cells induced with PNU-282987 eye drops. However, both treatment paradigms were significantly reduced under MLA treatment (Fig. 5E).
Discussion
Previous studies from our laboratory showed that topical treatment of the α7 nAChR agonist, PNU-282987, to the eyes of adult rats stimulate Müller glia to reenter the cell cycle and generate new retinal neurons in the absence of retinal damage.24 Here, we support and extend those findings, demonstrating for the first time that PNU-282987 induces production of RPCs and differentiated neurons from Müller glia in the adult murine retina. Intriguingly, our data show that PNU-282987 does not act directly on Müller glia, but instead, functions indirectly through activation of α7 nAChRs on the RPE. The generation of retinal neurons from Müller glia in the rodent is not entirely unexpected. It is clear that Müller glia are the cellular source for regeneration of retinal neurons in other vertebrate systems.11,12,14,41 There is growing evidence that similar, although less robust regenerative responses, can be induced in the mammalian retina through a variety of interventions. Others have shown that treatment with growth factors in vitro can cause Müller glia dedifferentiation and cell cycle reentry.19,20,42–44 However, these factors alone are insufficient to induce robust RPC genesis in vivo,45–48 and the numbers of neurons generated is modest, particularly when compared with the robust retinal neurogenesis induced by PNU-282987 in vivo.
The neurogenic responses of Müller glia in fish, birds, and mammals reported to date are contingent on retinal damage.8,41,49 In fish, damage to the retina has been shown to elicit robust, spontaneous regeneration, but several studies showed that this occurred only when the outer retina had also sustained injury. Retinal regeneration in fish can be stimulated by the neurotoxin, ouabain, which poisons the Na/K ATPase pumps and, at appropriate doses, kills large numbers of retinal neurons.50,51 The RPE also contains Na/K ATPase pumps on the apical and basolateral membranes.52 Doses of ouabain sufficient to damage the outer retina could also affect the RPE, increasing permeability, as previously demonstrated by reductions in transepithelial resistance following ouabain inhibition of Na/K-ATPase.53 Likewise, surgical excision of full-thickness retinal tissue in fish elicits regeneration of all retinal layers, but also results in traumatic injury to the RPE.54 It is tempting to speculate that in these injury paradigms, the damage to the RPE could permit endogenous retinal acetylcholine to reach the RPE, triggering release of factors that promote the regenerative response of the Müller glia.
However, in this study, retinal damage does not appear to be a prerequisite for PNU-282987–induced neurogenesis. The overall histology of the retina was well preserved following topical PNU-282987 treatment in both mice (this study) and rats,24 and no active caspase-3–positive cells were detected in the rat retina following 2 weeks of topical PNU-282987.24 We cannot exclude the possibility that some of the BrdU labeling results in unscheduled DNA synthesis triggered by DNA damage. However, our previous studies in rats showed changes in the number and laminar position of the BrdU-labeled cells over time, consistent with Müller glia in the INL reentering the cell cycle to produce migrating RPCs. In addition, we previously showed that PNU-282987 increased the number of RGCs in the rat retina. In addition, the Müller glia lineage marker, tdTomato, was detected in presumptive RPCs (PAX6+) and in BRN3a-positive RGCs and ChAT-positive amacrine cells following PNU-282987 treatment. Finally, there is no evidence in the literature that activation of α7 nAChRs causes DNA damage or stimulates DNA repair and we have seen no increase of caspase activation after eye drop treatments. Indeed, others have shown that activation of α7 nAChRs reduces oxidative stress,55 suggesting that this drug is more likely to reduce DNA damage. Thus, current evidence favors reentry into the cell cycle as the basis of the BrdU incorporation stimulated by the α7 nAChR agonist, PNU-282987. Another potential concern involves BrdU incorporation as a response to PNU-282987 and not actual cell proliferation. However, adult cells in the rodent INL have also stained with other proliferating markers besides BrdU after eye drop treatment with PNU-282987. For instance, cells in the adult rodent retina immunostained with antibodies against PCNA after PNU-282987 treatment.24
The RPE is a novel candidate for producing signals capable of activating the regenerative responses of Müller glia. Robust BrdU labeling of retinal tissue resulted after intravitreal injection of culture media obtained from PNU-282987–treated RPE cells. It is suspected that a signaling molecule (or molecules) is released from the RPE following PNU-282987 treatment that induces cell cycle reentry in Müller glia and the subsequent production of retinal neurons in adult mammals. The RPE is critical to normal retinal homeostasis and can produce a variety of growth factors.7,56 In some vertebrate models, such as newt and embryonic chicks, the RPE is capable of transdifferentiation into a variety of retinal neuron types.7,57 This is not the case in retinal regeneration in fish,58 or mice, although developmental reprogramming of RPE into retina occurs in animals with mutations in Mitf.59 There is evidence that transdifferentiation of RPE contributes to fibrosis, but not neurogenesis, in proliferative vitreoretinopathy.60 We observed no evidence for RPE transdifferentiation in our studies. In previous time-dependent studies, BrdU+ cells first emerge in the INL, where the Müller glia nuclei are located. In the adult mouse retina, BrdU+ cells emerge in the INL as early as 3 days following PNU-282987 treatment. At later time periods, BrdU+ cells are found in the ONL and in the GCL.
Previously published data from this laboratory showed that intravitreal injections or topical application (eye drops) of the α7 nAChR agonist, PNU-282987, prevented the loss of RGCs normally associated with induced ocular hypertension, a model of experimental glaucoma in adult rats.23,61,62 This effect on RGC survival was blocked if the α7 nAChR antagonist, MLA, was injected before application of PNU-28298723,61 and suggested a novel neuroprotective role for α7 nAChRs in the retina. In addition, a presynaptic mechanism for acetylcholine neuroprotection in the retina at the starburst amacrine cell to RGC synapse has been described by Zhou et al.62 How then could activation of α7 nAChRs in one set of studies be interpreted as neuroprotection, whereas results presented here provide evidence of neurogenesis?
It is clear that acetylcholine release from starburst amacrine cells onto α7 nAChRs in the retina does not normally induce neurogenesis in adult mammals. At the starburst amacrine cell–RGC synapse, acetylcholine released from starburst amacrine cells in response to light has been proposed to have a neuroprotective function, as previously described in several studies now.23,61,62 However, in this study, RPE cells were artificially stimulated with an exogenous α7 nAChR agonist and likely cause the release of a factor(s) that stimulate Müller glial cell proliferation. In vivo, PNU-282987 may be absorbed through the sclera at the back of the eye or enter the choroid circulation to reach the RPE cells.23 We also showed that PNU-282987 can act directly on cultured RPE cells to trigger release of the proliferation-inducing factor(s). It is unlikely that stimulation of the α7 nChRs on RPE cells from endogenous retinal sources would occur under normal physiological conditions. Thus, α7 nAChRs–induced neurogenesis is likely to be triggered by pharmacological stimulation of the RPE cells, whereas neuroprotection is more likely mediated directly through retinal neurons under physiological conditions.
The results from this study could ultimately lead to significant changes for those living with neurodegenerative retinal diseases, or for those experiencing loss of vision due to traumatic injury or age. If topical agents can be applied to regenerate neurons, vision loss may be reversed and significantly increase the quality of life for many visually impaired people. Additional studies are currently under way to determine the functionality and connectivity of newly generated neurons, as well as to determine the identity of signaling factors released from the RPE that are capable of inducing this unique response.
Acknowledgments
Supported by National Institutes of Health National Eye Institute Grant EY027970 (CLL) and a Faculty Research and Creative Activities Award (FRACAA) from Western Michigan University (CLL).
Disclosure: M.K. Webster, None; B.J. Barnett, None; M.L. Stanchfield, None; J.R. Paris, None; S.E. Webster, None; C.A. Cooley-Themm, None; E.M. Levine, None; D.C. Otteson, None; C.L. Linn, None
References
- 1.Kirtland KA, Saaddine JB, Geiss LS, Thompson TJ, Cotch MF, Lee PP. Geographic disparity of severe vision loss – United States, 2009-2013. MMWR Morb Mortal Wkly Rep. 2015;64:513–517. [PMC free article] [PubMed] [Google Scholar]
- 2.Rossetti L, Digiuni M, Montesano G, et al. Blindness and glaucoma: a multicenter data review from 7 academic eye clinics. PLoS One. 2015;24(10):e0136632. doi: 10.1371/journal.pone.0136632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beidoe G, Mousa SA. Current primary open-angle glaucoma treatments and future directions. Clin Ophthalmol. 2012;6:1699–1707. doi: 10.2147/OPTH.S32933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bucolo C, Platania CB, Reibaldi M, et al. Controversies in glaucoma: current medical treatment and drug development. Curr Pharm Des. 2015;21:4673–4681. doi: 10.2174/1381612821666150909095553. [DOI] [PubMed] [Google Scholar]
- 5.Gamm DM, Wong R. Report on the National Eye Institute audacious goals initiative: photoreceptor regeneration and integration workshop. Trans Vis Sci Tech. 2015;4(6):2. doi: 10.1167/tvst.4.6.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vetter ML, Hitchcock PF. Report on the National Eye Institute audacious goals initiative: replacement of retinal ganglion cells from endogenous cell sources. Trans Vis Sci Tech. 2017;6(2):5. doi: 10.1167/tvst.6.2.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiba C. The retinal pigment epithelium: an important player of retinal disorders and regeneration. Exp Eye Res. 2014;123:107–114. doi: 10.1016/j.exer.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Gorsuch RA, Hyde DR. Regulation of Muller glial dependent neuronal regeneration in the damaged adult zebrafish retina. Exp Eye Res. 2014;123:131–140. doi: 10.1016/j.exer.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyake A, Araki M. Retinal stem/progenitor cells in the ciliary marginal zone complete retinal regeneration: a study of retinal regeneration in a novel animal model. Dev Neurobiol. 2014;74:739–756. doi: 10.1002/dneu.22169. [DOI] [PubMed] [Google Scholar]
- 10.Todd L, Fischer AJ. Hedgehog signaling stimulates the formation of proliferating Muller glia-derived progenitor cells in the chick retina. Development. 2015;142:2610–2622. doi: 10.1242/dev.121616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lenkowski JR, Raymond PA. Muller glia: stem cells for generation and regeneration of retinal neurons in teleost fish. Prog Retin Eye Res. 2014;40:94–123. doi: 10.1016/j.preteyeres.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lahne M, Hyde DR. Interkinetic nuclear migration in the regenerating retina. Adv Exp Med Biol. 2016;854:587–593. doi: 10.1007/978-3-319-17121-0_78. [DOI] [PubMed] [Google Scholar]
- 13.Luz-Madrigal A, Grajales-Esquivel E, McCorkle A, et al. Reprogramming of the chick retinal pigmented epithelium after retinal injury. BMC Biol. 2014;12:28. doi: 10.1186/1741-7007-12-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wan J, Goldman D. Retina regeneration in zebrafish. Curr Opin Genet Dev. 2016;40:41–47. doi: 10.1016/j.gde.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dhomen NS, Balaggan KS, Pearson RA, et al. Absence of chx10 causes neural progenitors to persist in the adult retina. Invest Ophthalmol Vis Sci. 2006;47:386–396. doi: 10.1167/iovs.05-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bringmann A, Wiedemann P. Müller glial cells in retinal disease. Ophthalmologica. 2012;227:1–19. doi: 10.1159/000328979. [DOI] [PubMed] [Google Scholar]
- 17.Hicks D, Courtois Y. The growth and behaviour of rat retinal Müller cells in vitro. 1. An improved method for isolation and culture. Exp Eye Res. 1990;51:119–129. doi: 10.1016/0014-4835(90)90063-z. [DOI] [PubMed] [Google Scholar]
- 18.Phillips MJ, Otteson DC. Differential expression of neuronal genes in Müller glia in two- and three-dimensional cultures. Invest Ophthalmol Vis Sci. 2011;52:1439–1449. doi: 10.1167/iovs.10-6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singhal S, Bhatia B, Jayaram H, et al. Human Muller glia with stem cell characteristics differentiate into retinal ganglion cell (RGC) precursors in vitro and partially restore RGC function in vivo following transplantation. Stem Cells Transl Med. 2012;1:188–199. doi: 10.5966/sctm.2011-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao C, Tao Z, Xue L, et al. Lin28b stimulates the reprogramming of rat Muller glia to retinal progenitors. Exp Cell Res. 2017;352:164–174. doi: 10.1016/j.yexcr.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Wan J, Ramachandran R, Goldman D. HB-EGF is necessary and sufficient for Muller glia dedifferentiation and retina regeneration. Dev Cell. 2012;22:334–347. doi: 10.1016/j.devcel.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ueki Y, Wilken MS, Cox KE, Chipman LB, Bermingham-McDonogh O, Reh TA. A transient wave of BMP signaling in the retina is necessary for Muller glial differentiation. Development. 2015;142:533–543. doi: 10.1242/dev.118745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mata D, Linn DM, Linn CL. Retinal ganglion cell neuroprotection induced by activation of alpha7 nicotinic acetylcholine receptors. Neuropharmacology. 2015;99:337–346. doi: 10.1016/j.neuropharm.2015.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Webster MK, Cooley-Themm CA, Barnett JD, et al. Evidence of BrdU positive retinal neurons after application of an Alpha7 nicotinic acetylcholine agonist. Neuroscience. 2017;346:437–446. doi: 10.1016/j.neuroscience.2017.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linn CL, Webster SE, Webster MK. Eye drops for delivery of bioactive compounds and BrdU to stimulate proliferation and label mitotically active cells in the adult rodent retina. Bio-Protocols. 2018;8:e3076. doi: 10.21769/BioProtoc.3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hombrebueno JR, Luo C, Guo L, Chen M, Xu H. Intravitreal injection of normal saline induces retinal degeneration in the C57BL/6J mouse. Trans Vis Sci Tech. 2014;3(2):3. doi: 10.1167/tvst.3.2.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mizutani T, Fowler BJ, Kim Y, et al. Nucleoside reverse transcriptase inhibitors suppress laser-induced choroidal neovascularization in mice. Invest Ophthalmol Vis Sci. 2015;56:7122–7129. doi: 10.1167/iovs.15-17440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nickells RW, Schmitt HM, Maes ME, Schlamp CL. AAV2-mediated transduction of the mouse retina after optic nerve injury. Invest Ophthalmol Vis Sci. 2017;58:6091–6104. doi: 10.1167/iovs.17-22634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vazquez-Chona FR, Clark AM, Levine EM. Rlbp1 promoter drives robust Muller glial GFP expression in transgenic mice. Invest Ophthalmol Vis Sci. 2009;50:3996–4003. doi: 10.1167/iovs.08-3189. [DOI] [PubMed] [Google Scholar]
- 30.Quinn JC, West JD, Hill RE. Multiple functions for Pax6 in mouse eye and nasal development. Gene Dev. 1996;10:435–446. doi: 10.1101/gad.10.4.435. [DOI] [PubMed] [Google Scholar]
- 31.de Melo J, Qiu X, Du G, Cristante L, Eisenstat DD. Dlx1, Dlx2, Pax6, Brn3b, and Chx10 homeobox gene expression defines the retinal ganglion and inner nuclear layers of the developing and adult mouse retina. J Comp Neurol. 2003;461:187–204. doi: 10.1002/cne.10674. [DOI] [PubMed] [Google Scholar]
- 32.Joly S, Pernet V, Samardzija M, Grimm C. Pax6-positive Muller glia cells express cell cycle markers but do not proliferate after photoreceptor injury in the mouse retina. Glia. 2011;59:1033–1046. doi: 10.1002/glia.21174. [DOI] [PubMed] [Google Scholar]
- 33.Shaham O, Menuchin Y, Farhy C, Ashery-Padan R. Pax6: a multi-level regulator of ocular development. Prog Retin Eye Res. 2012;31:351–376. doi: 10.1016/j.preteyeres.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 34.Keyser KT, MacNeil MA, Dmitrieva N, Wang F, Masland RH, Lindstrom JM. Amacrine, ganglion, and displaced amacrine cells in the rabbit retina express nicotinic acetylcholine receptors. Vis Neurosci. 2000;17:743–752. doi: 10.1017/s095252380017508x. [DOI] [PubMed] [Google Scholar]
- 35.Strang CE, Andison ME, Amthor FR, Keyser KT. Rabbit retinal ganglion cells express functional alpha7 nicotinic acetylcholine receptors. Am J Physiol Cell Physiol. 2005;289:C644–C655. doi: 10.1152/ajpcell.00633.2004. [DOI] [PubMed] [Google Scholar]
- 36.Dmitrieva NA, Strang CE, Keyser KT. Expression of alpha 7 nicotinic acetylcholine receptors by bipolar, amacrine, and ganglion cells of the rabbit retina. J Histochem Cytochem. 2007;55:461–476. doi: 10.1369/jhc.6A7116.2006. [DOI] [PubMed] [Google Scholar]
- 37.Maneu V, Gerona G, Fernandez L, Cuenca N, Lax P. Evidence of alpha 7 nicotinic acetylcholine receptor expression in retinal pigment epithelial cells. Vis Neurosci. 2010;27:139–147. doi: 10.1017/S0952523810000246. [DOI] [PubMed] [Google Scholar]
- 38.Matsumoto H, Shibasaki K, Uchigashima M, et al. Localization of acetylcholine-related molecules in the retina: implication of the communication from photoreceptor to retinal pigment epithelium. PLoS One. 2012;7:e42841. doi: 10.1371/journal.pone.0042841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Linn DM, Massey SC. Acetylcholine release from the rabbit retina mediated by NMDA receptors. J Neurosci. 1991;11:123–133. doi: 10.1523/JNEUROSCI.11-01-00123.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Firth S, Li W, Massey SC, Marshak DW. AMPA receptors mediate ACh release from starburst amacrine cells in the rabbit retina. J Comp Neur. 2003;466:80–90. doi: 10.1002/cne.10880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gallina D, Todd L, Fischer AJ. A comparative analysis of Muller glia-mediated regeneration in the vertebrate retina. Exp Eye Res. 2014;123:121–130. doi: 10.1016/j.exer.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jian Q, Tao Z, Li Y, Yin ZQ. Acute retinal injury and the relationship between nerve growth factor, Notch1 transcription and short-lived dedifferentiation transient changes of mammalian Muller cells. Vision Res. 2015;110:107–117. doi: 10.1016/j.visres.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 43.Song WT, Zhang XY, Xia XB. Atoh7 promotes the differentiation of Muller cells-derived retinal stem cells into retinal ganglion cells in a rat model of glaucoma. Exp Biol Med. 2017;240:682–690. doi: 10.1177/1535370214560965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yao K, Qiu S, Tian L, et al. Wnt regulates proliferation and neurogenic potential of Muller glial cells via a Lin28/let-7 miRNA-dependent pathway in adult mammalian retinas. Cell Rep. 2016;17:165–178. doi: 10.1016/j.celrep.2016.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang HM, Hung KH, Hsu CC, Lin TC, Chen SY. Using induced pluripotent stem cell-derived conditional medium to attenuate the light-induced photodamaged retina of rats. J Chin Med Assoc. 2015;78:169–176. doi: 10.1016/j.jcma.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 46.Koss MJ, Falabella P, Stefanini FR, et al. Subretinal implantation of a monolayer of human embryonic stem cell-derived retinal pigment epithelium: a feasibility and safety study in Yucatan minipigs. Graefes Arch Clin Exp Ophthalmol. 2016;254:1553–1565. doi: 10.1007/s00417-016-3386-y. [DOI] [PubMed] [Google Scholar]
- 47.Thomas BB, Zhu D, Zhang L, et al. Survival and functionality of hESC-derived retinal pigment epithelium cells cultured as a monolayer on polymer substrates transplanted in RCS rats. Invest Ophthalmol Vis Sci. 2016;57:2877–2887. doi: 10.1167/iovs.16-19238. [DOI] [PubMed] [Google Scholar]
- 48.Mandai M, Fujii M, Hashiguchi T, et al. iPSC-derived retina transplants improve vision in rd1 end-stage retinal-degeneration mice. Stem Cell Rep. 2017;8:69–83. doi: 10.1016/j.stemcr.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Todd L, Palazzo I, Squires N, Mendonca N, Fischer AJ. BMP- and TGFβ-signaling regulate the formation of Muller glia-derived progenitor cells in the avian retina. Glia. 2017;65:1640–1655. doi: 10.1002/glia.23185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maier W, Wolburg H. Regeneration of the goldfish retina after exposure to different doses of ouabain. Cell Tissue Res. 1979;202:99–118. doi: 10.1007/BF00239223. [DOI] [PubMed] [Google Scholar]
- 51.Raymond PA, Reifler MJ, Rivlin PK. Regeneration of goldfish retina: rod precursors are a likely source of regenerated cells. J Neurobiol. 1988;19:431–463. doi: 10.1002/neu.480190504. [DOI] [PubMed] [Google Scholar]
- 52.Burke JM, McKay BS. In vitro aging of bovine and human retinal pigment epithelium: number and activity of the Na/K ATPase pump. Exp Eye Res. 1993;57:51–57. doi: 10.1006/exer.1993.1098. [DOI] [PubMed] [Google Scholar]
- 53.Rajasekaran SA, Hu J, Gopal J, et al. Na, K-ATPase inhibition alters tight junction structure and permeability in human retinal pigment epithelial cells. Am J Physiol Cell Physiol. 2003;284:C1497–C1507. doi: 10.1152/ajpcell.00355.2002. [DOI] [PubMed] [Google Scholar]
- 54.Hitchcock PF, Macdonald RE, VanDeRyt JT, Wilson SW. Antibodies against Pax6 immunostain amacrine and ganglion cells and neuronal progenitors, but not rod precursors, in the normal and regenerating retina of the goldfish. J Neurobiol. 1996;29:399–413. doi: 10.1002/(SICI)1097-4695(199603)29:3<399::AID-NEU10>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 55.Navarro E, Buendia I, Parada E, et al. Alpha7 nicotinic receptor activation protects against oxidative stress via heme-oxygenase I induction. Biochem Pharmacol. 2015;97:473–481. doi: 10.1016/j.bcp.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 56.Fuhrmann S, Zou C, Levine EM. Retinal pigment epithelium development, plasticity, and tissue homeostasis. Exp Eye Res. 2014;123:141–150. doi: 10.1016/j.exer.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coulombre JL, Coulombre AJ. Regeneration of neural retina from the pigmented epithelium in the chick embryo. Dev Biol. 1965;12:79–92. doi: 10.1016/0012-1606(65)90022-9. [DOI] [PubMed] [Google Scholar]
- 58.Knight JK, Raymond PA. Retinal pigmented epithelium does not transdifferentiate in adult goldfish. J Neurobiol. 1995;27:447–456. doi: 10.1002/neu.480270402. [DOI] [PubMed] [Google Scholar]
- 59.Mochii M, Ono T, Matsubara Y, Eguchi G. Spontaneous transdifferentiation of quail pigmented epithelial cell is accompanied by a mutation in the Mitf gene. Dev Biol. 1998;196:145–159. doi: 10.1006/dbio.1998.8864. [DOI] [PubMed] [Google Scholar]
- 60.Dvashi Z, Goldberg M, Adir O, Shapira M, Pollack A. TGF-beta1 induced transdifferentiation of rpe cells is mediated by TAK1. PLoS One. 2015;10:e0122229. doi: 10.1371/journal.pone.0122229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iwamoto K, Birkholz P, Schipper A, Mata D, Linn DM, Linn CL. A nicotinic acetylcholine receptor agonist prevents loss of retinal ganglion cells in a glaucoma model. Invest Ophthalmol Vis Sci. 2014;55:1078–1087. doi: 10.1167/iovs.13-12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou X, Cheng Y, Zhang R, et al. Alpha7 nicotinic acetylcholine receptor agonist promotes retinal ganglion cell function via modulating GABAergic presynaptic activity in a chronic glaucomatous model. Sci Rep. 2017;7:1734. doi: 10.1038/s41598-017-02092-6. [DOI] [PMC free article] [PubMed] [Google Scholar]