Strange et al. review recent advances in our understanding of the molecular and structural basis of volume-regulated anion channel function within the framework of classical biophysical and physiological studies.
Abstract
The swelling-activated anion channel VRAC has fascinated and frustrated physiologists since it was first described in 1988. Multiple laboratories have defined VRAC’s biophysical properties and have shown that it plays a central role in cell volume regulation and possibly other fundamental physiological processes. However, confusion and intense controversy surrounding the channel’s molecular identity greatly hindered progress in the field for >15 yr. A major breakthrough came in 2014 with the demonstration that VRAC is a heteromeric channel encoded by five members of the Lrrc8 gene family, Lrrc8A–E. A mere 4 yr later, four laboratories described cryo-EM structures of LRRC8A homomeric channels. As the melee of structure/function and physiology studies begins, it is critical that this work be framed by a clear understanding of VRAC biophysics, regulation, and cellular physiology as well as by the field’s past confusion and controversies. That understanding is essential for the design and interpretation of structure/function studies, studies of VRAC physiology, and studies aimed at addressing the vexing problem of how the channel detects cell volume changes. In this review we discuss key aspects of VRAC biophysics, regulation, and function and integrate these into our emerging understanding of LRRC8 protein structure/function.
Introduction
The volume-regulated anion channel (VRAC) is expressed ubiquitously in vertebrate cells where it mediates the swelling-activated efflux of Cl− and organic solutes required for cell volume regulation (Strange et al., 1996; Hoffmann et al., 2009; Jentsch, 2016). Whole-cell patch clamp electrophysiology studies first identified VRAC currents in 1988 in T lymphocytes (Cahalan and Lewis, 1988) and human intestinal epithelial cells (Hazama and Okada, 1988). Several laboratories including our own performed detailed biophysical and cell physiological studies of VRAC throughout the 1990s. During this time, multiple laboratories claimed to have identified the proteins underlying VRAC activity. The most prominent VRAC protein candidates included P-glycoprotein (Gill et al., 1992; Valverde et al., 1992), pICln (Paulmichl et al., 1992; Krapivinsky et al., 1994), and CLC-3 (Duan et al., 1997). Unfortunately, none of these candidates withstood the test of experimental verification by other laboratories (Wine and Luckie, 1996; Strange, 1998; Nilius and Droogmans, 2003).
The extensive confusion and controversy surrounding the molecular identification of VRAC hindered progress in the field for >15 yr. A major breakthrough came in 2014 when two laboratories independently identified the genes encoding VRAC using genome-wide RNA silencing methods and high-throughput fluorescence assays of channel activity (Qiu et al., 2014; Voss et al., 2014). These laboratories demonstrated that VRAC is a heteromeric channel encoded by five members of the Lrrc8 gene family, Lrrc8A–E, a result which has now been confirmed by multiple groups (Hyzinski-García et al., 2014; Yamada et al., 2016; Gradogna et al., 2017). In 2018, four laboratories described cryo-EM structures of LRRC8 channels (Deneka et al., 2018; Kasuya et al., 2018; Kefauver et al., 2018; Kern et al., 2018).
The VRAC field has experienced a stunning rebirth in the last 4 yr. As the melee of structure/function and physiology studies begins, it is critical that this work be framed by a clear understanding of VRAC biophysics, regulation, and cellular physiology. That understanding is essential for the design and interpretation of molecular experiments. This is particularly true for studies aimed at understanding the physiological roles of VRAC and the longstanding and vexing question of how VRAC detects cell volume changes. This paper will review key aspects of VRAC biophysics, regulation, and function and integrate these into our emerging understanding of LRRC8 protein structure/function.
Channel nomenclature
It is important to stress that both vertebrate and invertebrate cells express multiple anion and cation channel types that are activated by cell swelling (Gründer et al., 1992; Niemeyer et al., 2001; Rutledge et al., 2001; Chien and Hartzell, 2007; Toft-Bertelsen et al., 2017) as well as by cell shrinkage (Zhang et al., 2008a; Koch and Korbmacher, 1999; Pan et al., 2011). In other words, there are many volume-regulated channel types. To avoid confusion with other volume-regulated channels, we propose that the defining characteristics of VRAC (VRAC has also been termed VSOAC, Volume-Sensitive organic Osmolyte/Anion Channel; VSOR, Volume-Sensitive Outwardly Rectifying anion channel; and VAAC, Volume-Activated Anion Channel) include activation and inactivation by cell swelling and shrinkage, respectively; modest outward rectification; high selectivity for anions over cations; activation that is modulated by intracellular ionic strength and ATP levels; and mediates volume regulatory organic osmolyte efflux.
The LRRC8A gene has also been termed SWELL1 (Qiu et al., 2014). For consistency and clarity, we use the official gene nomenclature LRRC8A throughout this review.
Lrrc8 genes have not been identified in invertebrates and appear to be vertebrate innovations (Abascal and Zardoya, 2012). Consistent with this, VRAC has to date only been detected in vertebrate cells. Given the abundance of evidence that Lrrc8 genes encode VRAC, we use the terms VRAC and LRRC8 channels interchangeably throughout this review. However, it should be stressed that the five mammalian LRRC8 proteins, LRRC8A–E, appear to assemble in multiple configurations that give rise to VRACs with distinct functional properties. This is discussed in detail below.
Functional characteristics of VRAC currents
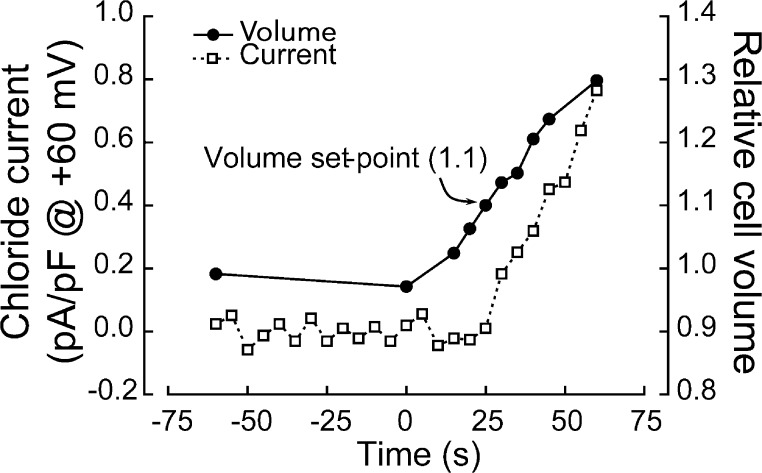
Fig. 1 shows the relationship between VRAC whole-cell current activation and cell swelling. Osmotic swelling was induced by removal of sucrose from the external bath solution. Under these conditions, current activation begins when the cell has swollen 10%, which is defined as the channel volume set point. Current activation will continue until it reaches a stable level, presumably reflecting full activation of all channels in the membrane. Inactivation of the current can be induced by cell shrinkage brought about by raising bath osmolality.
Figure 1.
Relationship between VRAC current activation and cell swelling in a N1E115 neuroblastoma cell. Relative cell volume is measured simultaneously with whole-cell current allowing a direct correlation between VRAC activation and cell swelling, which is the signal that activates the channel. Whole current begins to activate when cell volume has increased 10%, which is defined as the channel volume set point under these physiological conditions. Figure is modified from Bond et al. (1999).
In the whole-cell patch clamp mode, cell swelling is continuous due to intracellular dialysis from the patch pipette and the presence of a constant transmembrane osmotic gradient. Most of our whole-cell electrophysiology studies on VRAC are performed while imaging cells by video-enhanced DIC microscopy (Cannon et al., 1998; Bond et al., 1999). This allows simultaneous quantification of cell volume changes and current activation, which is essential for understanding how VRAC senses cell swelling and shrinkage.
Cell volume can increase manyfold in the whole-cell patch clamp configuration. With very large volume increases, the cell membrane typically dissociates from the underlying cytoplasm. In our studies, we have often seen membrane blebs form during swelling. Blebs are membrane domains that have separated from the cytoskeleton (Ikenouchi and Aoki, 2017). Rapid membrane blebbing is usually associated with a dramatic increase in the rate of VRAC activation (unpublished data) suggesting that physical forces and/or interacting cytoskeletal and other proteins underlying the cell membrane may play a role in regulating VRAC activity.
VRAC is also activated in the absence of cell swelling by large reductions in intracellular ionic strength (Cannon et al., 1998; Nilius et al., 1998; Voets et al., 1999; Sabirov et al., 2000; Best and Brown, 2009), by reactive oxygen species (Shimizu et al., 2004; Liu et al., 2009; Deng et al., 2010), and by intracellular GTP-γ-S (Nilius et al., 1999; Estevez et al., 2001). In endothelial cells (Romanenko et al., 2002) and cardiomyocytes (Browe and Baumgarten, 2003), a VRAC-like current can be activated by shear stress and membrane stretch, respectively. A large body of evidence has demonstrated that VRAC is activated by stimulation of receptors that induce apoptosis and that the channel mediates apoptosis-associated cell shrinkage termed apoptotic volume decease (Okada et al., 2006; Akita and Okada, 2014; Kunzelmann, 2016).
Fully activated VRAC current shows modest outward rectification. Most studies have shown that VRAC has a high selectivity for anions over cations with relative cation permeabilities (Pcation/PCl) of 0.02–0.04 (Kubo and Okada, 1992; Jackson and Strange, 1993; Lewis et al., 1993). However, in skate hepatocytes, Cs+ and Na+ permeabilities relative to Cl− are ∼0.2, and Pcholine/PCl is 0.55. Relative cation permeability increases with decreasing concentrations of CsCl in the patch pipette suggesting that the channel pore contains saturable anion and cation binding sites with different anion and cation affinities (Jackson et al., 1996).
The relative anion permeability of VRAC corresponds to Eisenman’s sequence I (SCN− > I− > NO3− > Br− > Cl− > F−; Strange et al., 1996; Nilius and Droogmans, 2003; Akita and Okada, 2014). VRAC pore diameter has been estimated from electrophysiological measurements to be 1.1–1.3 nm (Nilius and Droogmans, 2003; Ternovsky et al., 2004). No selective inhibitor of VRAC exists, but the channel is inhibited by typical anion transport blockers such as DIDS, SITS, NPPB, carbenoxolone, and DCPIB (Friard et al., 2017). VRAC is also inhibited by lipoxygenase/cytochrome P-450 inhibitors, polyunsaturated fatty acids, phloretin, tamoxifen, and La3+ (Jackson and Strange, 1993; Hand et al., 1997; Akita and Okada, 2014). Several studies have shown that the channel is blocked by extracellular ATP and other nucleotides. ATP block is voltage dependent (Jackson and Strange, 1995a; Tsumura et al., 1996).
VRAC exhibits voltage sensitivity that varies between cell types and physiological conditions. Voltage sensitivity can be negligible, or the channel can show modest to strong inactivation at membrane voltages more positive than +60 mV (Jackson and Strange, 1995a; Voets et al., 1997; Hernández-Carballo et al., 2010). Voltage-dependent inactivation is modulated in part by cytoplasmic and extracellular ions (Voets et al., 1997; Hernández-Carballo et al., 2010).
In addition to inorganic ions, cells also use small, largely uncharged organic solutes termed organic osmolytes for volume regulation (Khan et al., 2010). A number of studies from our laboratory strongly suggested that VRAC is a major pathway for the volume regulatory efflux of these solutes from cells (Jackson and Strange, 1993; Emma et al., 1997; Hand et al., 1997). In the brain, VRAC is thought to be a major pathway for the release of the excitatory amino acids aspartate and glutamate, which may play a role in normal signaling processes as well as excitotoxic injury (Akita and Okada, 2014; Mongin, 2016).
Electrophysiological measurements have shown that VRAC is highly permeable to structurally unrelated organic solutes such as pyruvate, short-chain fatty acids, ketone bodies, amino acids, isethionate, and gluconate (PX/PCl = 0.1–0.4; Jackson and Strange, 1993; Jackson et al., 1996; Manolopoulos et al., 1997). The relative permeability of VRAC to taurine (Ptaurine/PCl), which functions as a major volume regulatory organic osmolyte in diverse cell types (Lambert et al., 2015), is 0.15–0.4 (Jackson and Strange, 1993; Boese et al., 1996; Jackson et al., 1996; Manolopoulos et al., 1997). The uncharged organic osmolytes myo-inositol and sorbitol appear to alter Cl− flux through VRAC, suggesting that they compete with Cl− for binding sites within the channel pore (Jackson and Strange, 1993).
VRAC’s unique single-channel properties
During the early 1990s, several laboratories used stationary noise or variance analysis of whole-cell VRAC current to estimate single-channel conductance (Doroshenko and Neher, 1992; Lewis et al., 1993; Stoddard et al., 1993; Ho et al., 1994; Nilius et al., 1994; Jackson and Strange, 1995b). Noise analysis assumes that graded changes in whole-cell current are due to graded changes in the open probability (Po) of a constant number of independent channels with a fixed unitary conductance (Hille, 2001).
All stationary noises analysis studies, including our own, used cell swelling to activate VRAC. For these studies, cells are swollen continuously while patch clamped in the whole-cell mode. Current records are obtained at various times during the time course of whole-cell current activation. The recording periods are short, typically ∼100 ms. During these brief recordings, whole-cell current magnitude is largely unchanged, and hence, the current is stationary. Noise analysis performed on these current records yields a unitary conductance for VRAC of ∼1 pS at 0 mV (Doroshenko and Neher, 1992; Lewis et al., 1993; Stoddard et al., 1993; Nilius et al., 1994; Jackson and Strange, 1995b). Cahalan and coworkers referred to VRAC as the “mini Cl− channel” and calculated that T-lymphocytes needed to express ∼104 channels to account for the magnitude of whole-cell VRAC currents observed following cell swelling (Lewis et al., 1993).
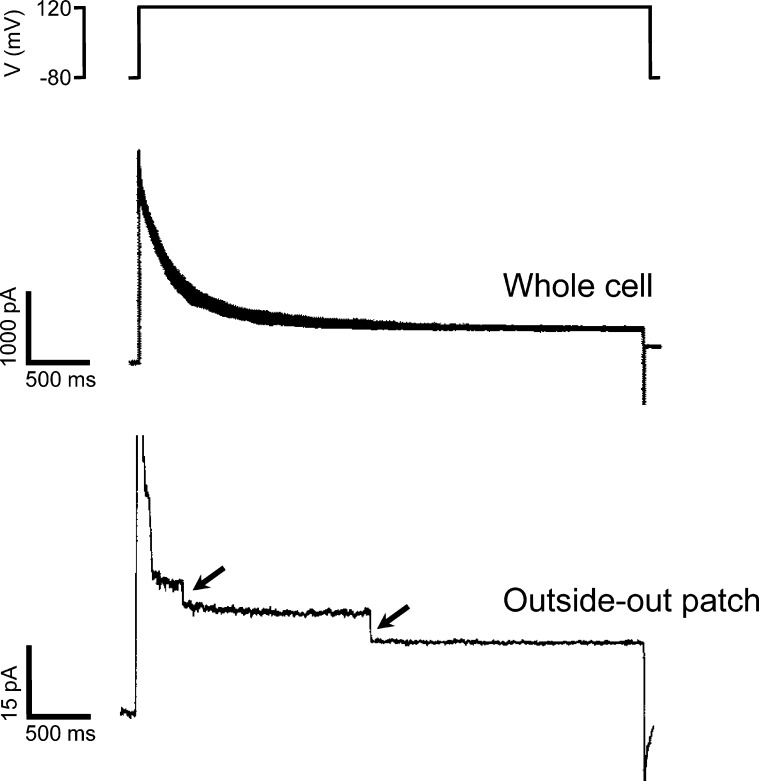
Corroborating unitary conductances of 1 pS or less with single-channel measurements is not feasible because channels with such small conductances are extremely difficult to identify and characterize in isolated membrane patches. To further examine VRAC single-channel properties then, we exploited the channel’s voltage sensitivity. In C6 glioma cells, VRAC whole-cell current is strongly inactivated at positive membrane voltages (Jackson and Strange, 1995a,b). After fully activating VRAC current by cell swelling, whole-cell current was inactivated by stepping membrane voltage to +120 mV. The inactivating current was analyzed by nonstationary stationary noise analysis, which measures current variance continuously during experimentally induced changes in current amplitude (Sigworth, 1980). From these measurements, we estimated a VRAC unitary conductance of 15–20 pS at 0 mV. Single-channel measurements confirmed these estimates (Jackson and Strange, 1995b; Fig. 2).
Figure 2.
Examples of VRAC single-channel currents in a C6 glioma cell. Top panel shows membrane voltage. Middle panel shows voltage-dependent inactivation of whole-cell current induced by stepping membrane voltage from −80 to 120 mV. Bottom panel shows voltage-dependent current inactivation in an outside-out membrane patch stepped from a membrane voltage of −80 to 120 mV. Arrows show closing of single VRAC channels. Figure is modified from Jackson and Strange (1995b).
Why is there such a striking discrepancy between stationary and nonstationary noise analysis estimates of single-channel conductance? Clearly, one or more of the assumptions underlying noise analysis of VRAC current activation during cell swelling are not valid. Further noise analysis studies in C6 glioma cells indicated that in response to cell swelling, VRACs transition in a stepwise fashion from a closed state where Po is effectively 0 to an open state where Po is close to 1. Once in the open state, channels close very infrequently and remain closed for <1 ms (Jackson and Strange, 1995b).
These studies indicate that during swelling-induced activation, VRAC Po is not a graded function of cell volume increase as must be assumed for noise analysis (Hille, 2001). In effect, the number of active channels in the cell membrane increases during cell swelling. It must be stressed, however, that there is no compelling evidence to suggest that VRAC is inserted into the cell membrane in response to volume increases. Instead, VRACs appear to respond in a heterogeneous fashion to cell volume increases.
These unique single-channel activation kinetics have important implications for understanding how VRAC is regulated by cell volume changes. There are two mechanisms that can explain the kinetics of VRAC activation. First, cells may possess multiple types of VRACs with different volume set points. One population of VRACs may require only a minute volume increase to activate, while other populations require much greater degrees of swelling. Given that LRRC8 proteins may assemble in multiple configurations (Voss et al., 2014; Gaitán-Peñas et al., 2016; Syeda et al., 2016; Lutter et al., 2017), it is conceivable that multiple channel types with different volume sensitivities exists. However, homomeric LRRC8 chimeras expressed in Lrrc8−/− cells show normal VRAC activity arguing against this possibility (Yamada and Strange, 2018).
The second, and we believe more likely, explanation is that the primary swelling-induced signal that activates VRAC directly or via other signaling pathways could vary from point to point in the cell during swelling. It seems highly unlikely that a chemical signal such as changes in cytoplasmic ionic strength would vary substantially across the cell. However, changes in mechanical force on the cell membrane induced by swelling are expected to show considerable local variation.
It is well known that the plasma membrane is highly heterogeneous. Membrane microdomains can have widely variable structure, chemical composition, and interactions with cytoplasmic components such as the cytoskeleton (Beedle et al., 2015; Hu et al., 2017; Shi et al., 2018). Local membrane structure and composition in turn impact how microdomains are deformed by mechanical forces such as those associated with cell swelling. If VRAC is activated directly or indirectly by swelling-induced changes in membrane mechanical force, single-channel activation kinetics could be explained readily by channel location. For example, assuming that all other factors such as membrane chemical composition, cytoskeletal interactions, etc., are equal, a greater degree of cell swelling would be required to place a given mechanical force on a membrane invagination compared with a smooth surface membrane microdomain. A channel located in an invagination then would require more cell swelling for activation. We speculate further on the possible regulation of VRAC by mechanical forces in the final section of this review.
Regulation of VRAC by cytoplasmic ionic strength
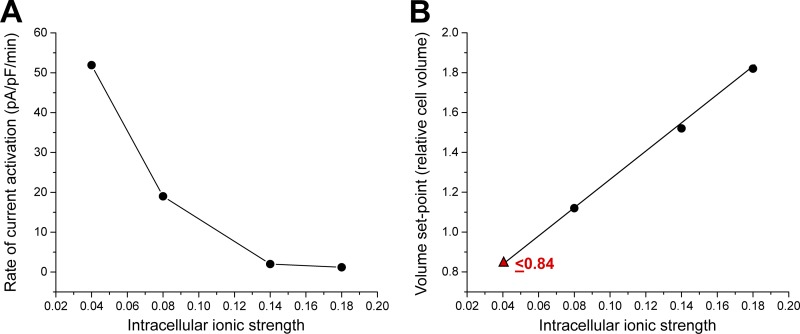
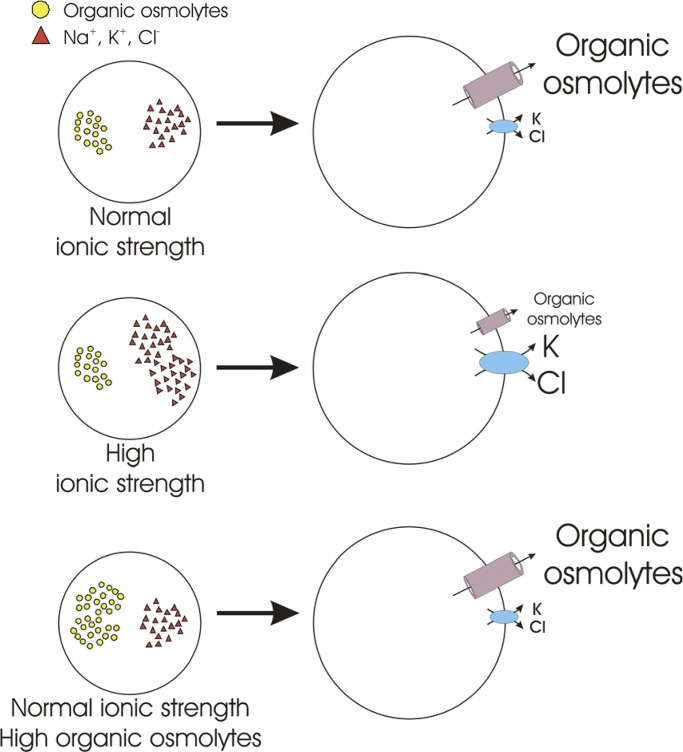
Our laboratory was the first to demonstrate that VRAC is regulated by intracellular ionic composition (Jackson et al., 1996; Emma et al., 1997; Cannon et al., 1998). In early studies on skate hepatocytes (Jackson et al., 1996) we suggested that VRAC is sensitive to intracellular anion composition. However, in a subsequent study in C6 glioma cells, we concluded that VRAC activity is modulated by cytoplasmic ionic strength (Cannon et al., 1998). Intracellular ionic strength alters both the rate of current activation and sensitivity of the current to cell swelling. When intracellular ionic strength is lowered, less cell swelling is required to activate VRAC, and the rate of current activation is increased (Fig. 3, A and B). At very low intracellular ionic strength, VRAC activates spontaneously without swelling and will even activate in shrunken cells (Fig. 3 B; Cannon et al., 1998). We proposed that cytoplasmic ionic strength controls the volume set point of VRAC. Best and Brown (2009) drew similar conclusions from their studies of pancreatic β cells.
Figure 3.
Effect of intracellular ionic strength on VRAC activation in CHO cells. (A) Reduction of intracellular ionic strength increases the rate of swelling-induced current activation. At an ionic strength of 0.04, VRAC activates spontaneously without cell swelling. (B) Reduction of intracellular ionic strength reduces the volume set point of VRAC activation. Set point is defined as the relative cell volume at which VRAC activation is triggered. As ionic strength is lowered, less cell swelling is required to activate the channel. At very low intracellular ionic strength (e.g., 0.04), VRAC activates in shrunken cells. Triangle indicates estimated volume set point obtained from shrunken CHO cells dialyzed with a 0.04 ionic strength pipette solution. Line is a linear regression through the four points. Figure is modified from Cannon et al. (1998).
Shortly after our studies were published, Nilius and coworkers concluded that ionic strength also modulated the volume set point of VRAC in bovine endothelial cells (Nilius et al., 1998). However, in two subsequent studies they concluded that VRAC is not sensitive to cell swelling per se but that it is activated directly by reductions in ionic strength caused by cell swelling (Voets et al., 1999; Sabirov et al., 2000). This conclusion is inconsistent with two experimental observations. First, expanding cell volume by forcing fluid into the cell through the patch pipette robustly activates VRAC (Zhang and Lieberman, 1996; Cannon et al., 1998; Bryan-Sisneros et al., 2000; Poletto Chaves and Varanda, 2008; Best and Brown, 2009). In addition, at least two studies have shown that VRAC can be inactivated by application of negative pressure to the patch pipette after cells have been swollen by exposure to a hypotonic bath solution (Zhang and Lieberman, 1996; Poletto Chaves and Varanda, 2008). Altering cell volume by pressure-induced fluid injection or withdrawal has no effect on cytoplasmic ionic strength.
Second, and very importantly, the reductions in ionic strength required to induce constitutive channel activation in the absence of swelling are substantial. For example, in whole-cell patch clamp recordings on CHO cells, CsCl (the predominant salt in the pipette solution) concentration has to be lowered from 140 to 40 mM to induce VRAC activation in the absence of swelling (Cannon et al., 1998). In bovine endothelial cells, VRAC is activated in the absence of swelling by reduction of intracellular ionic strength from 155 to 95 mM (Nilius et al., 1998). In the whole-cell patch mode, cells are dialyzed continuously by the patch pipette solution. Hence, there should be little to no change in cytoplasmic ionic strength when cells are osmotically swollen. If such changes were occurring, the current reversal potential (Erev) is expected to shift throughout the entire experiment as cytoplasmic Cl− levels are reduced. For example, if the same reduction in cytoplasmic ionic strength that spontaneously activates VRAC in CHO cells (i.e., a reduction from 140 to 40 mM; Cannon et al., 1998) is required to activate the channel during swelling, Erev would shift by more than −30 mV. Shifts in whole-cell current Erev during swelling-induced activation of VRAC have not been reported. Indeed, the reported Erev for VRAC is close to the Nernst potential expected for an anion channel with high selectivity for Cl− over cations (Kubo and Okada, 1992; Jackson and Strange, 1993; Lewis et al., 1993).
Physiological implications of VRAC ionic strength sensitivity
The swelling-activated KCl cotransporter, which mediates volume regulatory efflux of K+ and Cl− from cells, is also sensitive to intracellular ionic strength (Parker et al., 1995). Increases in ionic strength increase the volume sensitivity of the cotransporter. In other words, less swelling is required to activate KCl cotransport when cytoplasmic salt concentration is elevated. Importantly, this effect is opposite to that observed with VRAC where high ionic strength renders the channel less sensitive to cell swelling.
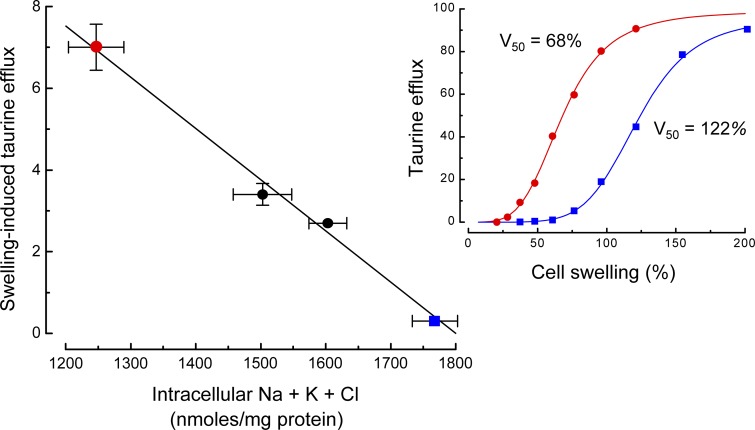
At least two studies have shown in vivo that cytoplasmic inorganic ion levels affect the type of regulatory volume decrease (RVD) mechanism used by cells to restore their volume after swelling. In trout red blood cells (Motais et al., 1991; Guizouarn and Motais, 1999), RVD is mediated by amino acid and KCl efflux. The efflux of amino acids is an inverse function of cytoplasmic ionic strength. At high cytoplasmic ionic strength, little or no amino acid efflux occurs in response to swelling, and volume regulation is instead mediated primarily by coupled KCl efflux. Similarly, swelling-induced efflux of taurine in C6 glioma cells is an inverse function of cytoplasmic inorganic ion levels (Fig. 4, A and B). However, cell volume regulation occurs normally irrespective of cytoplasmic ionic strength indicating that different regulatory mechanisms are active under different physiological conditions (Emma et al., 1997).
Figure 4.
Effect of intracellular inorganic ion levels on swelling-induced 3H-taurine efflux in C6 glioma cells. Left panel shows that swelling-induced taurine efflux is an inverse function of the combined intracellular levels of Na+, K+, and Cl−. Values are means ± standard errors. Inset graph on the right shows the relationship between taurine efflux and cell swelling in cells with low (red circles) versus high (blue squares) intracellular inorganic ion levels. V50 is defined as the amount of cell swelling required for half-maximal taurine efflux. A greater amount of cell swelling is required to activate taurine efflux in cells with high cytoplasmic ionic strength. Figure is based on data from Emma et al. (1997)).
The sensitivity of VRAC and the KCl cotransporter to ionic strength has important physiological implications. Cells regulate not only their volume but also their cytoplasmic ionic composition within a narrow range. Changes in cytoplasmic inorganic ion levels and ionic strength can have profound effects on protein conformation, membrane potential and transport mechanisms, and other critical physiological parameters. The differential sensitivity of VRAC, which plays a critical role in volume regulatory organic solute efflux (Jackson and Strange, 1993; Emma et al., 1997; Hand et al., 1997; Lutter et al., 2017; Schober et al., 2017), and the KCl cotransporter would allow cells to regulate their volume and cytoplasmic ionic strength in a coordinated manner. For example, exposure to a hypotonic extracellular medium reduces intracellular ionic strength. Under these conditions, it would be advantageous for cells to regulate their volume primarily by extrusion of organic solutes via VRAC and/or other organic solute efflux mechanisms in order to maintain inorganic ions at normal levels.
Cell volume increases also occur when there is increased transmembrane influx of salt. This is termed “isotonic” or “isosmotic” swelling because it occurs in the absence of extracellular hypotonicity. Isotonic/isosmotic swelling occurs under both physiological and pathophysiological conditions (Häussinger, 2008; Rungta et al., 2015). Volume regulation mediated primarily by the efflux of organic solutes following isotonic/isosmotic swelling would increase cytoplasmic ionic strength above normal levels. Thus, it would be advantageous for cells to reduce their volume in response to isotonic swelling by extruding inorganic ions via the KCl cotransporter.
Exposure of cells to a hypertonic medium has two effects on cytoplasmic inorganic ion levels. Within seconds to minutes after shrinkage, cells activate regulatory volume increase (RVI) mechanisms that mediate the uptake of NaCl and KCl (Hoffmann et al., 2009; Jentsch, 2016). This acute RVI restores cell volume but also increases cytoplasmic ionic strength. Over a period of hours, the NaCl and KCl are replaced by organic osmolytes, which maintain cell volume while simultaneously restoring inorganic ion concentrations to normal levels.
Cells that have undergone RVI will immediately swell when returned to a normotonic medium and undergo what is referred to as a post-RVI RVD. The use of different solutes for post-RVI RVD again would have distinct advantages for the cell. Once osmolytes have been accumulated, the efflux of these solutes for RVD would maintain cytoplasmic inorganic ion levels. However, if cell swelling occurs while intracellular inorganic ion levels are still elevated, the volume regulatory extrusion of K+ and Cl− via the KCl cotransporter would serve to restore cytoplasmic ionic strength (Fig. 5).
Figure 5.
Regulation of RVD mechanisms by intracellular ionic strength. Large fluctuations in cytoplasmic ionic strength can disrupt protein structure and function and a host of diverse cellular processes. The differential sensitivity of VRAC and the KCl cotransporter to cytoplasmic ionic strength may allow cells to reduce their volume via preferential efflux of organic osmolytes or KCl. For example, under conditions where cell swelling occurs concomitantly with elevated cytoplasmic ionic strength, efflux of KCl would serve to reduce cell volume as well as intracellular inorganic ion levels.
VRAC ATP dependence
Numerous studies demonstrate that swelling-induced activation of VRAC requires the presence of intracellular ATP. In most cases, investigators have shown that ATP can be replaced by nonhydrolyzable analogues indicating that ATP binding to VRAC and/or accessory proteins is required for channel regulation (reviewed by Akita and Okada, 2014). However, some studies have concluded that VRAC ATP dependence is due to regulatory phosphorylation (Meyer and Korbmacher, 1996; Carpenter and Peers, 1997) or that both phosphorylation and ATP binding are required (Bryan-Sisneros et al., 2000).
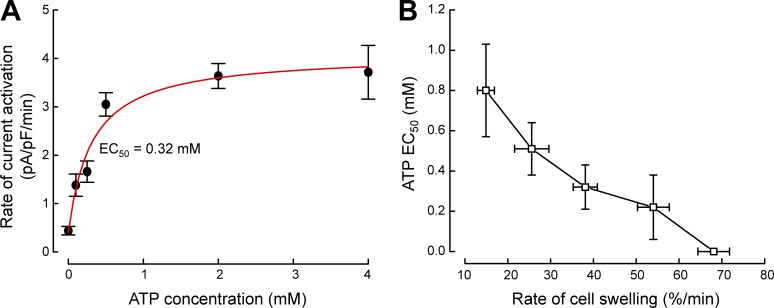
Our studies in N1E115 neuroblastoma (Bond et al., 1999), C6 glioma cells (Jackson et al., 1994), and skate hepatocytes (Jackson et al., 1996) demonstrated that nonhydrolytic ATP binding rather than phosphorylation is required for VRAC activation. We performed detailed studies of VRAC ATP dependence in N1E115 neuroblastoma cells (Bond et al., 1999). The rate of VRAC current activation is a saturable function of intracellular ATP concentration when cells are swollen with transmembrane osmotic gradients of 50–120 mOsm (Fig. 6 A). Current activation is ATP-independent when N1E115 cells are swollen by a 150 mOsm gradient (Fig. 6 B). Volk et al. (1996) made similar observations. VRAC activation requires intracellular ATP when inner medullary collecting duct (IMCD) cells are swollen by a 50 mOsm gradient but not when swelling is induced by an osmotic gradient of 100 mOsm.
Figure 6.
Effect of intracellular ATP on VRAC activation in N1E115 neuroblastoma cells. (A) Rate of current activation is a saturable function of intracellular ATP concentration. Cells were swollen by a 100-mOsm reduction in bath osmolality. Mean rate of cell swelling under these conditions is 38%/min. The half-maximal effective concentration (EC50) of ATP required for current activation is 0.32 mM. (B) Relationship between ATP EC50 and rate of cell swelling. Increased rates of swelling reduce ATP dependence of VRAC activation. Current activation no longer requires intracellular ATP when cells are swollen at rates ≥65%/min. Values are means ± standard errors. Figure is modified from Bond et al. (1999).
The rate of transmembrane water flow and hence cell swelling is a direct function of the osmotic driving force. In N1E115 cells, the half-maximal effective concentration (EC50) of ATP required for current activation is an inverse function of the rate of cell swelling (Bond et al., 1999; Fig. 6 B). At very high rates of swelling, ATP is no longer required. Thus, the rate of cell volume increase modulates the ATP dependence of VRAC.
VRAC mediates the efflux of organic osmolytes (Jackson and Strange, 1993; Emma et al., 1997; Hand et al., 1997), and swelling-induced efflux is ATP dependent (Jackson and Strange, 1993; Ballatori et al., 1995). VRAC is also permeable to important metabolic intermediates such as pyruvate, short-chain fatty acids, ketone bodies, and amino acids (Jackson et al., 1994). These metabolites are major inputs into the ATP generating tricarboxylic acid cycle. Modulation of VRAC ATP dependence by the rate of cell swelling may thus play an important physiological role. Anoxia and ischemia disrupt cellular ATP production and can cause severe cell swelling (Kalogeris et al., 2012). Efflux of metabolites via VRAC in a swollen cell during anoxia/ischemia may further suppress metabolism, which can increase cell swelling and injury. Thus, if swelling is not too severe in a metabolically compromised cell, it may be beneficial to minimize VRAC activation to protect cell metabolism at the expense of volume homeostasis. On the other hand, if swelling is rapid and extreme, volume control may take precedence over energy metabolism as a last-ditch effort to prevent imminent membrane lysis and cell death.
Functional roles of LRRC8 channel subunits
The Lrrc8 gene family comprises five members termed Lrrc8a, Lrrc8b, Lrrc8c, Lrrc8d, and Lrrc8e (Abascal and Zardoya, 2012; Voss et al., 2014). LRRC8A is an essential VRAC subunit and must be coexpressed with at least one other family member to reconstitute normal VRAC activity (Voss et al., 2014; Syeda et al., 2016).
Coexpression of LRRC8A with other LRRC8 proteins gives rise to VRACs with distinct functional properties. LRRC8D increases the permeability of cells to blasticidin, taurine, and platinum-based anticancer drugs (Lee et al., 2014; Planells-Cases et al., 2015). Studies by Schober et al. (2017) and Lutter et al. (2017) suggest that LRRC8A/D heteromers are the major pathway for efflux of uncharged myo-inositol, taurine, and GABA from cells. Gradogna et al. (2017) have shown that LRRC8A/E heteromers are strongly activated by intracellular oxidizing agents, while oxidation inhibits LRRC8A/C and LRRC8A/D heteromers. LRRC8A/E heteromers show very strong inactivation at positive membrane potentials compared with LRRC8A/C and LRRC8A/D (Voss et al., 2014). VRAC subunit composition may also affect single-channel properties (Syeda et al., 2016).
There is little to no VRAC activity in Lrrc8−/− cells heterologously expressing LRRC8A and LRRC8B (Voss et al., 2014). However, small VRAC currents are observed in Lrrc8(C,D,E)−/− HCT116 cells (Voss et al., 2014) but not in HEK293 cells in which the same subunits are knocked out (Lutter et al., 2017). Thus, LRRC8A/B heteromers give rise to VRAC activity that may be dependent on cell type as well as physiological conditions.
Ghosh et al. (2017) recently demonstrated that overexpression or siRNA-induced knockdown of LRRC8B in HEK293 causes modest changes in endoplasmic reticulum (ER) Ca2+ dynamics. The authors conclude that LRRC8B functions as an ER Ca2+ leak channel. However, this conclusion should be viewed cautiously. Indirect effects of LRRC8B overexpression and knockdown on ER functions are possible. More detailed studies are needed before additional channel functions are ascribed to LRRC8 proteins.
The amount of LRRC8A cDNA transfected into cells can have significant effects on VRAC activity. High levels of LRRC8A expression suppress endogenous VRAC activity (Qiu et al., 2014; Voss et al., 2014; Syeda et al., 2016). In Lrrc8−/− cells, decreasing the amount of LRRC8A cDNA transfected relative to other subunits strikingly increases VRAC current amplitude (Yamada et al., 2016; Yamada and Strange, 2018) and can induce VRAC currents that are otherwise undetectable (Yamada and Strange, 2018). This effect does not seem to be simply dependent on the ratio of LRRC8A cDNA transfection relative to other subunits but instead requires a reduction in the amount of transfected LRRC8A cDNA (unpublished data). These findings indicate that high levels of LRRC8A expression may disrupt the proper synthesis and processing of VRAC channels.
LRRC8A appears to traffic to the cell membrane when expressed alone in cells, whereas LRRC8B, LRRC8C, LRRC8D, and LRRC8E expressed alone are retained in the cytoplasm (Voss et al., 2014; Yamada and Strange, 2018). Three studies have shown that LRRC8A homomers may give rise to channel activity (Deneka et al., 2018; Kefauver et al., 2018; Yamada and Strange, 2018). We observed a small outwardly rectifying current that was insensitive to cell swelling in LRRC8A-expressing cells (Yamada and Strange, 2018). A very small (0.2–0.4 pA/pF) swelling-activated current was observed by Kefauver et al. (2018). Deneka et al. (2018) demonstrated that expression of LRRC8A alone induces an outwardly rectifying current that is activated by reducing intracellular ion concentration. Given that high-resolution LRRC8 structures are those of LRRC8A homomeric channels (Deneka et al., 2018; Kasuya et al., 2018; Kefauver et al., 2018; Kern et al., 2018), detailed characterization of currents induced by expressing LRRC8A alone are clearly warranted.
Structure of VRAC/LRRC8 channels
The recently described high-resolution cryo-EM structures of an LRRC8 channel (Deneka et al., 2018; Kasuya et al., 2018; Kefauver et al., 2018; Kern et al., 2018) represent a major breakthrough in the VRAC field. However, a note of caution is warranted. These structures are of LRRC8A homomeric channels. It is unclear whether functional LRRC8A homomers exist in vivo. Furthermore, the channels do not exhibit normal VRAC properties. For example, LRRC8A homomers show little (Kefauver et al., 2018) or no (Yamada and Strange, 2018) sensitivity to cell swelling under normal patch clamp conditions and relatively low sensitivity to reduced intracellular ionic strength (Deneka et al., 2018; unpublished data). Clearly, much additional work is required to fully understand VRAC structure/function relationships. The LRRC8A structures provide a powerful foundation for defining those relationships through hypothesis-driven studies.
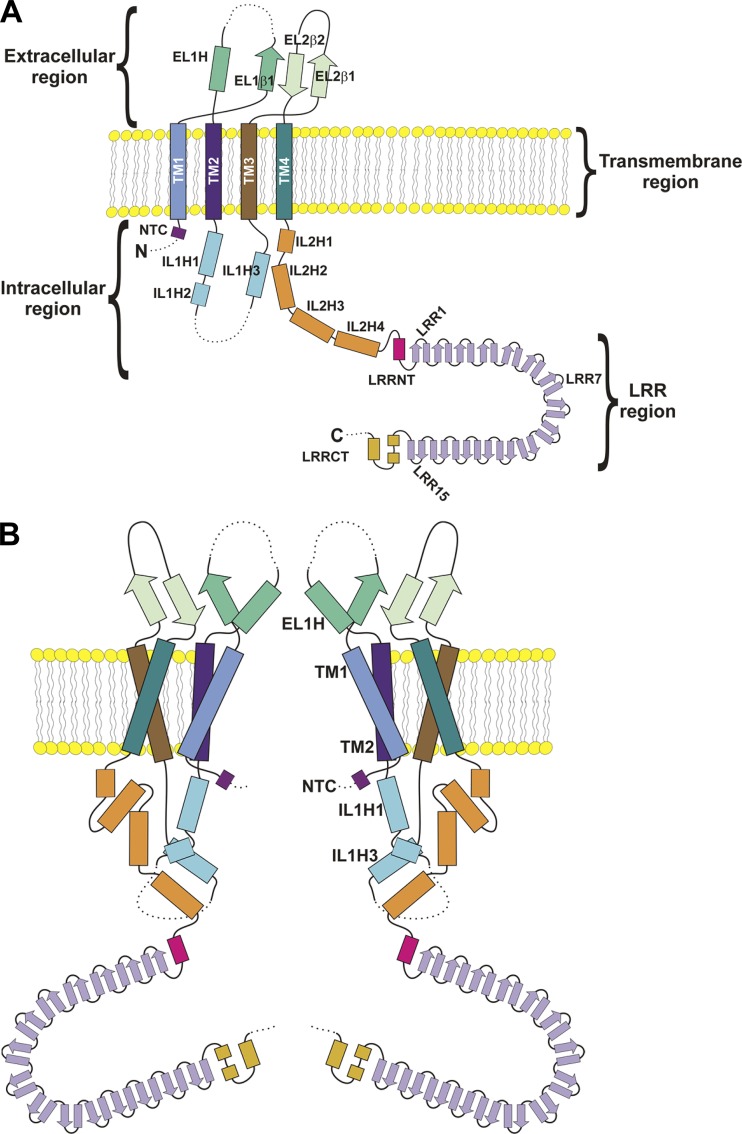
Fig. 7 A shows secondary structural elements identified in the cryo-EM structures of mouse and human LRRC8A (Deneka et al., 2018; Kasuya et al., 2018; Kefauver et al., 2018). Structural elements defined in the three cryo-EM studies are similar with only minor differences that are noted. The terminology we use here is that adopted by Kasuya et al. (2018).
Figure 7.
Two-dimensional structure of the LRRC8A channel. (A) Structural components of the LRRC8A protein. The protein comprises four regions, extracellular, transmembrane, intracellular, and LRR regions. β, β strand; EL, extracellular loop; H, α-helix; IL, intracellular loop; NTC, N-terminal coil; LRRCT, LRR region C terminus; LRRNT, LRR region N terminus; TM, transmembrane. Dashed lines represent protein regions that were not resolved in cryo-EM structures. (B) Subunit structure of the LRRC8A channel. The channel pore is formed primarily from ELH1, TM1 and TM2, NTC, and ILH1 and ILH3.
LRRC8A comprises four regions defined as intracellular (I), transmembrane (TM), extracellular (E), and leucine-rich repeat (LRR). When functioning as a VRAC subunit, LRRC8A N and C termini are located in the cytoplasm. The N terminus is 23 amino acids long and appears to be largely unstructured (Deneka et al., 2018; Kasuya et al., 2018; Kefauver et al., 2018) except for a short N-terminal coil that projects into the channel pore (Kefauver et al., 2018).
The membrane domain consists of four TM helices. Two relatively large extracellular loops, EL1 and EL2, connect TM1 and TM2 and TM3 and TM4, respectively. EL1 contains a β strand (EL1β) followed by an α helix (EL1H) located on the extracellular side of TM2. The region between EL1β and EL1H is disordered and contains a proline-rich domain. EL2 contains two β strands, EL2β1 and EL2β2, that interact with EL1β to form a β sheet.
An intracellular loop (IL1) connects TM2 and TM3. IL1 contains three α-helices, IL1H1–3, and an unstructured region. Kefauver et al. (2018) identified a possible fourth α-helix in IL1. A second intracellular loop, IL2, connects TM4 to the LRR region. IL2 comprises four α-helices (IL2H1–4).
The LRR region comprises an N terminus (LRRNT) containing an α-helix; 15 LRRs, LRR1–15; and a C terminus (LRRCT) containing three α-helices. The 15 LRRs in each LRRC8A subunit form a crescent-shaped structure typical of that observed in many LRR-containing proteins (Bella et al., 2008; Abascal and Zardoya, 2012; Fig. 7, A and B).
LRRC8 proteins are homologous to pannexin and innexin channels and appear to have diverged from this gene family at the origin of the chordates (Abascal and Zardoya, 2012). Based on this homology, Abascal and Zardoya (2012) postulated that LRRC8 proteins form hexameric membrane channels. High-resolution cryo-EM structures demonstrate that LRRC8A homomers have a hexameric structure (Deneka et al., 2018; Kasuya et al., 2018; Kefauver et al., 2018). A lower-resolution cryo-EM structure of LRRC8A/8C demonstrated a hexameric structure for LRRC8 heteromers as well (Deneka et al., 2018).
The structure of the LRRC8A hexamer can be likened to that of an upside-down jellyfish where the transmembrane and extracellular regions form six tentacles, and the jellyfish’s umbrella-shaped bell is formed by the intracellular and LRR regions (Fig. 7 B). The channel length is ∼170 Å. The transmembrane spanning region is 40 Å long, and the extracellular region extends 35 Å above the cell membrane. The intracellular and LRR regions are 35 and 60 Å long, respectively.
The VRAC pore is formed primarily by TM1, TM2, EL1H, IL1H1, IL1H3, and the N terminus (Fig. 7 B). The maximum pore diameter is ∼35 Å at the intracellular side of the TM region. A constriction located at the beginning of the extracellular region narrows the pore to a minimum diameter ∼6–8 Å (Fig. 7 B).
The precise arrangement of the six LRRC8A subunits is somewhat unclear at present. Deneka et al. (2018) and Kefauver et al. (2018) concluded that the extracellular, TM, and intracellular loop regions of the subunits are arranged in a sixfold symmetric fashion (i.e., C6 symmetry). However, the LRR regions exhibit C3 symmetry. The LRR regions dimerize and interact with the neighboring LRR region dimer to form a trimer of dimers structure. This structure contrast with that described by Kasuya et al. (2018) where the entire channel exhibited C3 symmetry.
More recently, Kern et al. (2018) reported a fourth cryo-EM structure of the LRRC8A homomeric channel. They observed C6 symmetry in the extracellular, TM, and intracellular loop regions and an asymmetric and heterogeneous arrangement of the LRR regions. In addition, they observed constricted and expanded structures of the lower portions of the TM regions and IL2. In the expanded state, the intracellular opening of the pore is dilated ∼4 Å. These two structural states may reflect channel gating conformations.
It should be noted that the structures described by Kern et al. (2018) were generated from LRRC8A reconstituted in lipid nanodiscs, which may more closely approximate the cell membrane. In contrast, earlier studies reconstituted LRRC8A in detergent (Deneka et al., 2018; Kasuya et al., 2018; Kefauver et al., 2018). Kern et al. (2018) propose that the arrangement of LRRC8A subunits is modified by the hydrophobic environment in which the protein is embedded.
Kasuya et al. (2018) calculated conservation scores for other LRRC8 family members and mapped the scores onto the LRRC8A structure. They noted that the pore constriction site, the LRR region, and the lipid-facing side of the TM regions showed relatively low conservation. These findings suggest that these regions may confer distinct functional properties on heteromeric VRAC/LRRC8 channels.
LRRC8 structure/function relationships
Identification of the molecular basis of VRAC has opened the door to understanding critical channel properties including regulation by cell swelling and ionic strength, selectivity and permeability, voltage sensitivity, and physiological roles. Qiu et al. (2014) provided the first structure/function insights into VRAC. Using the substituted cysteine accessibility method and electrophysiology, they demonstrated that LRRC8A T44 plays an important role in VRAC ion permeation. T44 is located on the extracellular side of TM1. Mutation of T44 to cysteine strikingly increases inhibition of VRAC by the membrane impermeable, negatively charged sulfhydryl reactive reagent MTSES and increases I− permeability. In contrast, arginine substitution at T44 reduces permeability to I−.
Syeda et al. (2016) demonstrated that the subunit composition of LRRC8 heteromers also affects channel ion selectivity as well as rectification. Furthermore, they showed that mutating T44 to cysteine in LRRC8C, LRRC8D, or LRRC8E subunits increases I− permeability to the same extent as the LRRC8A T44C mutation. Cryo-EM structures confirm that TM1 forms part of the VRAC pore and that T44 projects into the pore on the extracellular side of the cell membrane (Deneka et al., 2018; Kasuya et al., 2018; Kefauver et al., 2018).
α-helix EL1H forms part of the extracellular constriction of the LRRC8A pore (Fig. 7 B; Deneka et al., 2018; Kasuya et al., 2018; Kefauver et al., 2018). The constriction is lined by a ring of positively charged arginine residues, R103, located at the N terminus of ELH1. Mutation of R103 to alanine increases the relative Na+ permeability of LRRC8A(R103A)/8C heteromeric channels (Deneka et al., 2018). Kefauver et al. (2018) observed that replacement of R103 with phenylalanine reduced the Erev of LRRC8A(R103F)/8C heteromeric channel currents suggesting that the mutant channels have increased relative cation permeability. They also observed that voltage-dependent block by extracellular ATP is greatly reduced in LRRC8A(R103F)/8C heteromers (Kefauver et al., 2018).
The cryo-EM structures described by Kern et al. (2018) show LRRC8A homomeric channels blocked by the anion channel inhibitor DCPIB. The negatively charged butanoic acid group of DCPIB appears to interact with the ring of arginine residues at position 103. The remainder of the molecule is extracellular to this region.
Interestingly, R103 is replaced by a leucine residue in LRRC8C and LRRC8E, and a phenylalanine residue in LRRC8D. As suggested by Deneka et al. (2018), the bulky phenylalanine residue in LRRC8D may widen the pore constriction. The widened constriction may allow LRRC8A/D heteromers to transport large organic solutes (Lee et al., 2013, 2014; Planells-Cases et al., 2015; Lutter et al., 2017; Schober et al., 2017).
The first several amino acids of the cytoplasmic LRRC8A N terminus are not resolved in cryo-EM structures and are most likely disordered. However, Kefauver et al. (2018) identified a short coiled region in the N terminus that projects into the intracellular mouth of the LRRC8A homomer pore. Mutation of T5 to cysteine renders LRRC8A(T5C)/8C heteromeric channels sensitive to inhibition by extracellular MTSES. In contrast, MTSES has no effect on wild-type LRRC8A/8C (Qiu et al., 2014) and LRRC8A(T5R)/8C (Kefauver et al., 2018) channels. LRRC8A(T5R)/8C channels also exhibit increased relative I− permeability (Kefauver et al., 2018). Studies by Zhou et al. (2018) demonstrated that mutations in the LRRC8 N terminus alter VRAC voltage sensitivity, ion selectivity, and conductance. Cysteine substitution on the N terminus also rendered the channel sensitive to sulfhydryl reactive reagents. Taken together, the studies of Kefauver et al. (2018) and Zhou et al. (2018) indicate that the LRRC8 N terminus plays an important role in VRAC pore structure/function.
As noted earlier, VRAC is inactivated by positive membrane potentials (Jackson and Strange, 1995a; Voets et al., 1997; Hernández-Carballo et al., 2010). The degree of inactivation varies substantially between cell types. Voss et al. (2014) first demonstrated that VRAC voltage sensitivity is determined in part by the subunit composition of LRRC8. LRRC8A/8E heteromers exhibit the strongest voltage-dependent inactivation compared with LRRC8A/8C and LRRC8A/8D channels.
Ullrich et al. (2016) first suggested that EL1 forms part of the outer channel pore. They demonstrated that charge reversal mutations in LRRC8A K98 and D100 and the equivalent residues in LRRC8C and LRRC8E alter VRAC voltage-dependent gating and I− permeability. These amino acids are located in the C-terminal portion of EL1 (Fig. 7, A and B). They also showed using a chimera approach that swapping the last 33 amino acids of the LRRC8C EL1 with those from LRRC8E is sufficient to induce LRRC8E-like voltage-dependent properties in LRRC8A/8C heteromers and vice versa. α-helix EL1H is located at the C-terminal end of EL1 (Fig. 7 A). K98 and D100 are located immediately upstream of ELH1, which, as noted above, forms part of the extracellular constriction of the VRAC pore (Fig. 7 B; Deneka et al., 2018; Kasuya et al., 2018; Kefauver et al., 2018). Taken together, these studies suggest that ELH1 may play an important role in voltage-dependent channel gating.
The formation of VRACs that exhibit normal volume-dependent regulation requires protein regions of LRRC8A and protein regions from one of the other LRRC8 subunits. We recently used a chimera approach to identify these regions (Yamada and Strange, 2018). Homomeric VRACs exhibiting normal regulation by cell swelling and shrinkage are formed by replacing EL1 of LRRC8A with that of LRRC8C. We also observed normal volume-dependent regulation in VRAC homomers in which IL1 of LRRC8C, LRRC8D, or LRRC8E is replaced with the LRRC8A IL1. Homomeric LRRC8 chimeras exhibit altered anion permeability, rectification, and voltage sensitivity. We suggested that IL1 as well as EL1 contribute to VRAC pore structure and function. Cryo-EM structures show that IL1H1 and IL1H3 are part of the intracellular portion of the VRAC pore (Deneka et al., 2018; Kasuya et al., 2018; Kefauver et al., 2018).
We also observed that a 25–amino acid sequence unique to the LRRC8A IL1 is sufficient to generate homomeric VRACs with normal volume-dependent regulation when it is inserted into the corresponding region of LRRC8C and LRRC8E (Yamada and Strange, 2018).
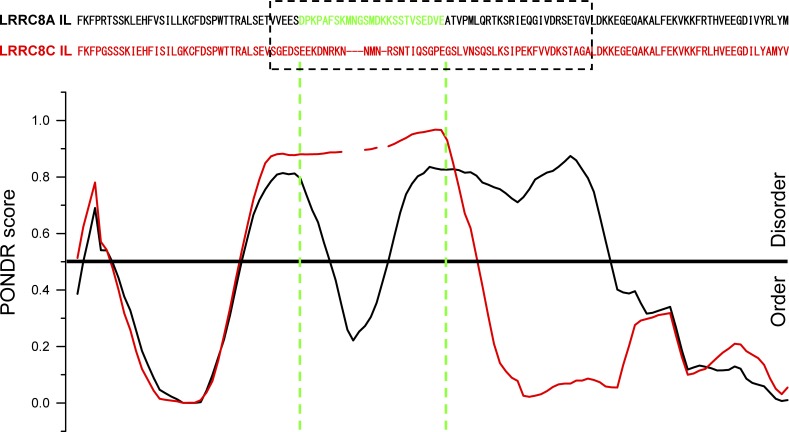
Predictor of Natural Disordered Regions (PONDR) VL-XT analysis revealed interesting differences between IL1 of LRRC8A and the IL1s of LRRC8C, LRRC8D, and LRRC8E. A region separating ILH1 from ILH2 and ILH3, which includes the 25–amino acid sequence unique to LRRC8A, is predicted to be intrinsically disordered (Fig. 8). Consistent with the PONDR prediction, this region is not resolved in cryo-EM structures (Deneka et al., 2018; Kasuya et al., 2018; Kefauver et al., 2018). Interestingly, the PONDR score for the LRRC8A IL1 takes a sharp dip in the region encompassing its unique 25–amino acid sequence (Fig. 8). Such dips in the PONDR score of intrinsically disordered protein regions are thought to predict the existence of molecular recognition elements or features (Oldfield et al., 2005; Mohan et al., 2006; Xue et al., 2010). Molecular recognition elements/molecular recognition features are small stretches of amino acid sequence within intrinsically disordered proteins regions that mediate protein–protein interactions. This unique region of LRRC8A could play a role in the assembly of LRRC8 subunits into VRACs and/or in VRAC regulation by cell volume changes.
Figure 8.
PONDR VL-XT analysis of LRRC8A (black) and LRRC8C (red) intracellular loops (ILs). PONDR scores are aligned with specific amino acid residues, which are shown at the top. Dashed box shows amino acid sequences that diverge significantly in LRRC8A, LRRC8C, LRRC8D, and LRRC8E proteins. Amino acids colored green (D182-E206) in the LRRC8A sequence are the minimal sequence required to give rise to chimeric homomeric LRRC8C-LRRC8A(IL) and LRRC8E-LRRC8A(IL) VRACs with normal swelling-induced activity. Figure is from Yamada and Strange (2018).
Physiological and pathophysiological roles of VRAC and LRRC8 proteins
It is well established that VRAC plays a central role in RVD in many cell types (Strange et al., 1996; Hoffmann et al., 2009; Jentsch, 2016). Consistent with this, several studies have now shown that RVD is disrupted with knockout or knockdown of LRRC8 expression (Qiu et al., 2014; Voss et al., 2014; Formaggio et al., 2018).
VRAC has also been implicated in multiple other physiological processes (reviewed by Stauber, 2015). These early studies relied on the use of relatively nonspecific pharmacological agents to identify possible physiological roles of VRAC. With the molecular identification of the channel it should now be possible to better define its functions. However, it should be stressed that like nonspecific pharmacology, the use of gene modification approaches to assess gene function can be fraught with significant interpretative problems (Schalkwyk et al., 2007; Eisener-Dorman et al., 2009). Thus, a critical assessment of genetic studies is essential to provide clear and definitive understanding of VRAC’s physiological roles. Below we discuss proposed functions of VRAC and LRRC8 proteins that are based on emerging genetic data.
VRAC current amplitude varies during the cell cycle (Shen et al., 2000; Chen et al., 2002), suggesting that the channel may play a role in cell proliferation. Pharmacological inhibition of VRAC slows proliferation of endothelial cells (Maertens et al., 2001; Xue et al., 2018), hepatocytes (Wondergem et al., 2001), glioblastoma cells (Rouzaire-Dubois et al., 2000), and vascular smooth muscle cells (Liang et al., 2014). Knockdown of the essential VRAC subunit LRRC8A was shown recently to inhibit proliferation of glioblastoma cells (Rubino et al., 2018).
As noted earlier, VRAC has been proposed to mediate the cell shrinkage associated with apoptosis (Okada et al., 2006; Akita and Okada, 2014; Kunzelmann, 2016). Recently, Jentsch and colleagues demonstrated that LRRC8A is required for activation of caspase-3 in HCT116 cells by the proapoptosis drugs cisplatin and staurosporine (Planells-Cases et al., 2015).
Our group recently demonstrated that LRRC8A plays a role in zebrafish development (Yamada et al., 2016). Patch clamp experiments showed that zebrafish embryo cells and differentiated adult cells express canonical VRAC currents that are indistinguishable from those of mammalian cells. Morpholino knockdown of LRRC8A expression abolished VRAC currents in embryo cells and caused pericardial edema and defects in trunk elongation and somatogenesis. The precise role of LRRC8A in zebrafish development is unknown. It is possible that VRAC plays a role in cell proliferation and/or apoptosis as discussed above for mammalian cells.
Release of neuroactive amino acids from astrocytes plays an important role in astrocyte–neuron signaling (Akita and Okada, 2014; Mongin, 2016). Uncontrolled release of the excitatory amino acids glutamate and aspartate from astrocytes, which occurs during ischemic brain injury and associated cell swelling, leads to neuronal injury and death (Akita and Okada, 2014; Mongin, 2016). Given the role of VRAC in organic osmolyte efflux, it has been suggested that the channel may be a major pathway for excitatory amino acid release in the brain (Akita and Okada, 2014; Mongin, 2016). Consistent with this idea, intracisternal injection of the VRAC inhibitor DCPIB reduces glutamate release and confers neuroprotection in experimental brain ischemia (Zhang et al., 2008b). Recently, Mongin and colleagues demonstrated that knockdown of LRRC8A (Hyzinski-García et al., 2014) alone or combined knockdown of LRRC8C and LRRC8E (Schober et al., 2017) suppresses swelling-induced glutamate release from cultured astrocytes, suggesting that VRAC may play a key role in excitotoxic brain injury and normal astrocyte–neuron signaling.
Pancreatic β-cells release stored insulin in response to glucose uptake, which increases ATP production resulting in inhibition of KATP channels, membrane depolarization, and activation of voltage-gated Ca2+ channels. Increased Ca2+ influx in turn triggers exocytotic insulin release (Best et al., 2010; Ashcroft and Rorsman, 2013). Best and colleagues have proposed that VRAC may also control β-cell electrical activity and insulin release (Best et al., 2010). Glucose metabolites cause β-cell swelling and subsequent VRAC activation, which in turn depolarizes membrane potential resulting in activation of voltage-gated Ca2+ channels and insulin release (Best et al., 2010).
The findings of recent molecular studies are consistent with the VRAC model proposed by Best et al. (2010). Kang et al. (2018) have shown that loss of the essential VRAC subunit LRRC8A abolishes swelling- and glucose-activated anion current in β-cells and disrupts electrical activity, Ca2+ influx, and insulin secretion evoked by elevated glucose. Mice lacking LRRC8A exhibit reduced glucose-stimulated insulin secretion and glucose tolerance.
Stuhlmann et al. (2018) also observed that ablation of Lrrc8a abolishes swelling- and glucose-activated anion current in β-cells and reduces glucose tolerance in mice. However, the effects of loss of LRRC8A on β-cell electrical activity and Ca2+ influx were delayed rather than nearly abolished as observed by Kang et al. (2018). Consistent with this, Stuhlmann et al. (2018) observed that LRRC8A knockout only reduces the early phase of insulin secretion both in isolated pancreatic islets and in vivo.
The reasons for the differences between the Kang et al. (2018) and Stuhlmann et al. (2018) studies are unclear. Nevertheless, both investigations show that VRAC/LRRC8 channels play a role in controlling β-cell electrical activity and insulin release. While further studies are needed, these findings taken together with those of Best et al. (2010) suggest that disruption of VRAC/LRRC8 channel activity may underlie certain forms of type 2 diabetes and related disorders and that the channel may be a novel pharmacological target for treatment of these diseases.
VRAC may also contribute to maintenance of blood glucose homeostasis through regulation of adipocyte size and associated cell signaling events (Zhang et al., 2017). Adipocytes express robust Lrrc8a-dependent VRAC currents that are larger in adipocytes from obese mice and humans. Knockdown of Lrrc8a expression reduces adipocyte size, lipid content, and glucose uptake and decreases adiposity and impairs glucose tolerance and increases insulin resistance in obese mice.
Glucose uptake and lipid synthesis in adipocytes require signaling through the insulin-phosphoinositol 3-kinase (PI3K)-RAC-α serine/threonine protein kinase 2 (AKT2) pathway (insulin-PI3K-AKT2; Satoh, 2014). LRRC8A knockdown disrupts adipocyte insulin-PI3K-AKT2 signaling (Zhang et al., 2017). Growth factor receptor-bound 2 (GRB2) protein interacts with the insulin receptor and mediates insulin-induced activation of PI3K (Siddle, 2012). GRB2 interacts with caveolion-1 (Cav1), which is enriched in adipocytes and forms insulin signaling microdomains (Ikonen and Vainio, 2005; Pilch and Liu, 2011). Zhang et al. (2017) observed that GRB2 and Cav1 coimmunoprecipitate with LRRC8A and that insulin signaling reduces the GRB2/LRRC8A interaction. They propose that VRAC/LRRC8 channels are activated by adipocyte hypertrophy during periods of high caloric intake. Channel activation in turn increases insulin-PI3K-AKT2 signaling, glucose uptake, lipid synthesis, and further adipocyte expansion. This model is consistent with previous studies showing that LRRC8C (also termed Factor of Adipocyte Differentiation 158) is required for high-fat diet-induced weight gain and insulin resistance (Hayashi et al., 2011).
Germ cell–specific knockout of LRRC8A renders mice infertile due to severe abnormalities in sperm development and function. Developing sperm fail to reduce their cytoplasmic volume, consistent with a role of VRAC activity in this process, and later exhibit disorganized mitochondrial sheaths, malformed flagella, and reduced motility (Lück et al., 2018; see also Bao et al., 2018).
Bao et al. (2018) recently identified an LRRC8A point mutation, R545H, in a patient with a male fertility disorder termed Sertoli cell-only syndrome. Expression in Xenopus oocytes of LRRC8 proteins tagged on their C termini with fluorescent reporters causes constitutive VRAC activation in the absence of swelling (Gaitán-Peñas et al., 2016). Using this assay, Bao et al. (2018) demonstrated that the R545H mutant reduced VRAC current amplitude 25–30% when it was coexpressed with LRRC8C or LRRC8D.
Bao et al. (2018) suggest that the LRRC8A R545H mutation may give rise to infertility. However, such a conclusion should be viewed with caution. First, in our opinion, it is very difficult to interpret heterologous expression studies when expression is performed in cell types such as Xenopus oocytes that express native VRACs. Endogenous VRAC activity and LRRC8 protein expression greatly confound data interpretation. Second, the patient studied is heterozygous for the R545H mutation. Heterozygous Lrrc8a+/− mice have normal fertility (Kumar et al., 2014). Furthermore, VRAC current amplitude is normal when a 1:1 mixture of wild-type and R545H LRRC8A cRNA is coexpressed in Xenopus oocytes with LRRC8C or LRRC8D (Bao et al., 2018) suggesting that the mutation does not disrupt the activity of wild-type VRACs and LRRC8 protein function.
In a genetic screen for suppressors of NF-kB, Lee and colleagues identified LRRC8A and LRRC8D as components of an uptake pathway for the antibiotic drug blasticidin (Lee et al., 2013, 2014). Similarly, LRRC8A and LRRC8D were identified in a screen for loss of sensitivity to the anticancer drug carboplatin (Planells-Cases et al., 2015). Consistent with a role of VRAC activity in drug uptake, cell swelling increased cisplatin accumulation in an LRRC8A-dependent manner. Disruption of LRRC8D expression reduced cisplatin uptake by approximately half despite the presence of robust swelling-activated VRAC currents in LRRC8D-knockout cells. Importantly, interrogation of the Cancer Genome Atlas revealed that patients with low expression of LRRC8D, but not of LRRC8A, had significantly lower survival rates. Taken together, these data suggest a role for the LRRC8D subunit in uptake of platinum-based chemotherapeutics and drug responses in cancer patients. Additional studies are clearly needed to test this idea.
LRRC8A was first identified in a 17-yr-old female patient suffering from a rare immune cell disorder, termed agammaglobulinemia, which is characterized by defective B-cell development (Sawada et al., 2003). A chromosomal translocation in this patient truncates the last 91 amino acids of LRRC8A and adds 35 new amino acids encoded by an intron. Forced expression of LRRC8AΔ91/+35 in bone marrow cells transplanted into irradiated mice causes a dramatic reduction in the number of pre-B cells. Sawada et al. (2003) found that the unaffected LRRC8A allele in the patient was translated as wild-type protein and suggested that LRRC8AΔ91/+35 has a dominant-suppressor effect on B-cell development.
Defining how LRRC8AΔ91/+35 alters immune function requires extensive additional study. If LRRC8AΔ91/+35 does cause agammaglobulinemia, it seems very unlikely that this is due to defective VRAC function. LRRC8AΔ91/+35 does not give rise to VRAC activity when expressed heterologously and, importantly, does not suppress VRAC activity when coexpressed with wild-type LRRC8A (Qiu et al., 2014). Thus, LRRC8AΔ91/+35 does not have a dominant-negative effect on VRAC function. In addition, the absence of VRAC is not tied clearly to immune system dysfunction. Lrrc8a knockout mice exhibit multiple defects including greatly increased prenatal and postnatal mortality (no animals survive beyond 16 wk of age), growth retardation, and multiple severe tissues abnormalities. Lrrc8a−/− animals exhibit only a modest block of B-cell development but have normal B-cell function. They also exhibit severely reduced thymus development, decreased proliferation and increased apoptosis of thymocytes and impaired T-cell function (Kumar et al., 2014).
Studies in the ébouriffé mouse contrast with these findings. ébouriffé mice harbor a spontaneous LRRC8A frameshift mutation that truncates the 15 LRRs of LRRC8A (Fig. 7, A and B). Lrrc8aebo/ebo mice have a phenotype similar to that of Lrrc8a−/− including little or no VRAC activity in their T-cells. However, unlike Lrrc8a−/− animals, Lrrc8aebo/ebo mice have normal T-cell development and function (Platt et al., 2017).
A potentially interesting finding of Kumar et al. (2014) that deserves further study concerns LRRC8A topology. When functioning as a VRAC subunit, LRRC8A N and C termini clearly face the cytoplasm (Qiu et al., 2014; Voss et al., 2014; Fig. 7, A and B). However, fluorescence activated cell sorting studies performed by Kumar et al. (2014) using a polyclonal antibody directed against an epitope tag on the LRRC8A C terminus and a monoclonal antibody against the loop connecting TM2 and TM3 (Fig. 7 A) suggest that a fraction of LRRC8A is present in the plasma membrane with its N and C termini facing the extracellular environment. Kumar et al. (2014) also showed that cross-linking LRRC8A with the TM2/TM3 loop monoclonal antibody activates AKT via PI3K signaling and that LRRC8A coimmunoprecipitates with GRB2, GRB2-associated binding protein 2 (GAB2), and lymphocyte-specific protein tyrosine kinase (LCK). They propose that GRB2 interacts with LRRC8A via a proline-rich region located on the loop connecting TM1 and TM2 (Fig. 7 A) and that the C-terminal LRR region is extracellular. Interaction of the LRR region with a putative LRRC8A ligand activates AKT via PI3K signaling, which in turn regulates T-cell development, survival, and function.
A number of proteins exhibit dual transmembrane orientation such as that suggested for LRRC8A (Rapp et al., 2006; von Heijne, 2006; Fluman et al., 2017). In addition, many if not most LRR-containing membrane proteins are oriented with their LRR regions facing the extracellular environment (Dolan et al., 2007; Abascal and Zardoya, 2012). Clearly, much additional work is needed to determine if LRRC8 proteins exhibit dual topology and carry out functions independent of VRAC.
Conclusions and perspective
Progress in understanding the physiological roles of VRAC and its regulation was stymied for >15 yr by confusion and controversy over the molecular identity of the channel. Within a remarkably short period of time, we have gone from definitive demonstration that VRAC is encoded by Lrrc8 genes (Qiu et al., 2014; Voss et al., 2014) to high-resolution molecular insights into channel structure (Deneka et al., 2018; Kasuya et al., 2018; Kefauver et al., 2018; Kern et al., 2018). There are a myriad of important and fundamental questions that can now be addressed at the molecular level.
From our perspective, there are several pressing problems in the field. What are the physiological and pathophysiological roles of VRAC and LRRC8 proteins that are independent of VRAC function? How is LRRC8 subunit composition of VRAC controlled, and is subunit composition modified to meet physiological demands? How many different types of VRACs exist in vivo? Are VRACs formed only by LRRC8A and one other LRRC8 subunit, or can functional channels comprise three or more different LRRC8 proteins? How are VRACs assembled, and what is the subunit stoichiometry?
High-resolution cryo-EM structures are of the LRRC8A homomer (Deneka et al., 2018; Kasuya et al., 2018; Kefauver et al., 2018; Kern et al., 2018), which does not exhibit normal cell volume–dependent regulation and does not recapitulate all the functional properties of various LRRC8 heteromers. Mutagenesis studies on heteromeric LRRC8 channels are complicated by lack of understanding of subunit assembly and stoichiometry. The LRRC8 chimeric homomers we described recently (Yamada and Strange, 2018) will facilitate more detailed structure/function analysis of LRRC8A/8C, LRRC8A/8D, and LRRC8A/8E heteromeric channels and will be valuable for further cryo-EM studies.
Abascal and Zardoya (2012) have proposed that LRRC8 proteins were formed by the combination of a pannexin channel with an LRR domain. It is possible and perhaps likely that the addition of an LRR domain to the ancestral pannexin conferred volume sensitivity to VRAC/LRRC8 channels. Does the LRRC8 LRR region play other roles as well? LRRs are known to mediate diverse protein–protein inter/actions (Kobe and Kajava, 2001; Abascal and Zardoya, 2012). Like other ion channels (Sheng and Pak, 2000; Kunzelmann and Mehta, 2013; Constantin, 2016), could VRAC also function as a scaffold that coordinates the activity of diverse cellular signaling pathways and functions?
The ATP dependence of VRAC likely plays important physiological roles. How does ATP regulate channel activation? Does ATP bind directly to the channel and/or accessory proteins? LRRC8 proteins do not contain canonical nucleotide binding sites. In addition, reconstituted, purified LRRC8 channels are not sensitive to ATP (Syeda et al., 2016). These observations suggest that interacting proteins mediate ATP-dependent channel regulation.
Finally, an understanding of how VRAC detect volume changes has been a longstanding physiological problem. It has been proposed that a reduction in intracellular ionic strength is the primary signal that activates VRAC during cell swelling (Voets et al., 1999; Sabirov et al., 2000). As we argued above, this hypothesis cannot be reconciled with all experimental observations. We suggest that mechanical force is the signal that activates VRAC, that the LRR region functions as the channel’s mechanosensor, and that the sensitivity of the LRR region to mechanical force is modulated by ionic strength.
Syeda et al. (2016) demonstrated that purified LRRC8 proteins reconstituted into droplet lipid bilayers do not respond to increases in droplet volume brought about by fluid injection. Decreases in ionic strength, though, increase channel Po. These studies suggest that tension within the lipid bilayer does not activate VRAC. However, they do not rule out the possibility that the channel is sensitive to mechanical force applied to the LRR region via interaction with other cellular components such as the cytoskeleton. LRR regions in other proteins have been shown to function as mechanosensors (Ju et al., 2015, 2016).
All four cryo-EM studies noted the existence of several structural arrangements of the LRRC8A channel LRR region (Deneka et al., 2018; Kasuya et al., 2018; Kefauver et al., 2018; Kern et al., 2018). The interface between pairs of LRR region in LRRC8 channels contains numerous charged amino acids. Ionic strength could alter LRR region conformation by altering charge at this interface. Interestingly, Kasuya et al. (2018) noted that the transmembrane region of mouse LRRC8A (Deneka et al., 2018) is more compact than that of the human LRRC8A channel that they described. In their cryo-EM studies, Kasuya et al. (2018) used 150 mM NaCl for preparation of cryogrids. In contrast, the mouse LRRC8A structures were obtained in the presence of 250 mM NaCl (Deneka et al., 2018).
Given these observations, one can envision a model where mechanical force applied to the flexible LRR region alters its conformation (Ju et al., 2015, 2016), which in turn activates that channel. As we have proposed, reductions in cytoplasmic ionic strength reduce the amount of swelling needed for VRAC activation (Jackson et al., 1996; Emma et al., 1997; Cannon et al., 1998). We suggest that the amount of swelling-induced mechanical force required to activate VRAC is reduced by LRR conformational changes caused by lowered ionic strength. At very low cytoplasmic ionic strength, changes in LRR conformation are sufficient to activate VRAC in the absence of swelling as we and others have shown (Cannon et al., 1998; Nilius et al., 1998). This model is illustrated in Fig. 9.
Figure 9.
Cartoon illustrating possible mechanism of VRAC regulation by ionic strength and cell swelling-induced mechanical force. LRR region is shown in orange. Mechanical force is represented by the green springs. Conformation of the LRR region is postulated to be regulated by both cytoplasmic ionic strength and mechanical force. Under conditions of normal ionic strength (top panel), a relatively high degree of swelling is required to induce mechanical force sufficient to alter the conformation of the LRR region and trigger channel activation. Reduced cytoplasmic ionic strength (middle panel) causes a nonactivating conformational change in the LRR region. Less swelling and mechanical force is therefore required to induce further conformational changes that activate VRAC. At very low cytoplasmic ionic strength (bottom panel), conformational changes in the LRR region are sufficient to activate VRAC in the absence of cell swelling.
Our proposed model accounts for activation/inactivation of VRAC by pressure-induced cell volume changes (Zhang and Lieberman, 1996; Cannon et al., 1998; Bryan-Sisneros et al., 2000; Poletto Chaves and Varanda, 2008; Best and Brown, 2009) and activation by mechanical force induced by shear stress and membrane stretch (Romanenko et al., 2002; Browe and Baumgarten, 2003). The model accounts for VRAC activation by GTP-γ-S (Nilius et al., 1999; Estevez et al., 2001), which has well-documented effects on cytoskeletal architecture and mechanical properties (Shumilina et al., 2003; Aldaz et al., 2005; Hawkins et al., 2013). It also accounts for the absence of swelling-induced current Erev changes, which are expected if intracellular ionic strength decreases substantially during swelling in patch clamped cells. Finally, the model accounts for VRAC’s unique swelling-induced single-channel activation kinetics (Jackson and Strange, 1995b).
Clearly, much experimental work needs to be done to test the model shown in Fig. 9 as well as other important hypotheses. After years of confusion and controversy, we finally have the tools and molecular insights needed to carry out those experiments.
Acknowledgments
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases grant R01 DK51610 to K. Strange.
The authors declare no competing financial interests.
Author contributions: All authors contributed to the writing and revision of this paper.
Lesley C. Anson served as editor.
References
- Abascal F., and Zardoya R.. 2012. LRRC8 proteins share a common ancestor with pannexins, and may form hexameric channels involved in cell-cell communication. BioEssays. 34:551–560. 10.1002/bies.201100173 [DOI] [PubMed] [Google Scholar]
- Akita T., and Okada Y.. 2014. Characteristics and roles of the volume-sensitive outwardly rectifying (VSOR) anion channel in the central nervous system. Neuroscience. 275:211–231. 10.1016/j.neuroscience.2014.06.015 [DOI] [PubMed] [Google Scholar]
- Aldaz H., Rice L.M., Stearns T., and Agard D.A.. 2005. Insights into microtubule nucleation from the crystal structure of human γ-tubulin. Nature. 435:523–527. 10.1038/nature03586 [DOI] [PubMed] [Google Scholar]
- Ashcroft F.M., and Rorsman P.. 2013. K(ATP) channels and islet hormone secretion: New insights and controversies. Nat. Rev. Endocrinol. 9:660–669. 10.1038/nrendo.2013.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballatori N., Truong A.T., Jackson P.S., Strange K., and Boyer J.L.. 1995. ATP depletion and inactivation of an ATP-sensitive taurine channel by classic ion channel blockers. Mol. Pharmacol. 48:472–476. [PubMed] [Google Scholar]
- Bao J., Perez C.J., Kim J., Zhang H., Murphy C.J., Hamidi T., Jaubert J., Platt C.D., Chou J., Deng M., et al. . 2018. Deficient LRRC8A-dependent volume-regulated anion channel activity is associated with male infertility in mice. JCI Insight. 3:e99767 10.1172/jci.insight.99767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beedle A.E., Williams A., Relat-Goberna J., and Garcia-Manyes S.. 2015. Mechanobiology—Chemical origin of membrane mechanical resistance and force-dependent signaling. Curr. Opin. Chem. Biol. 29:87–93. 10.1016/j.cbpa.2015.09.019 [DOI] [PubMed] [Google Scholar]
- Bella J., Hindle K.L., McEwan P.A., and Lovell S.C.. 2008. The leucine-rich repeat structure. Cell. Mol. Life Sci. 65:2307–2333. 10.1007/s00018-008-8019-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best L., and Brown P.D.. 2009. Studies of the mechanism of activation of the volume-regulated anion channel in rat pancreatic β-cells. J. Membr. Biol. 230:83–91. 10.1007/s00232-009-9189-x [DOI] [PubMed] [Google Scholar]
- Best L., Brown P.D., Sener A., and Malaisse W.J.. 2010. Electrical activity in pancreatic islet cells: The VRAC hypothesis. Islets. 2:59–64. 10.4161/isl.2.2.11171 [DOI] [PubMed] [Google Scholar]
- Boese S.H., Wehner F., and Kinne R.K.. 1996. Taurine permeation through swelling-activated anion conductance in rat IMCD cells in primary culture. Am. J. Physiol. 271:F498–F507. [DOI] [PubMed] [Google Scholar]
- Bond T., Basavappa S., Christensen M., and Strange K.. 1999. ATP dependence of the ICl,swell channel varies with rate of cell swelling. Evidence for two modes of channel activation. J. Gen. Physiol. 113:441–456. 10.1085/jgp.113.3.441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browe D.M., and Baumgarten C.M.. 2003. Stretch of β 1 integrin activates an outwardly rectifying chloride current via FAK and Src in rabbit ventricular myocytes. J. Gen. Physiol. 122:689–702. 10.1085/jgp.200308899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan-Sisneros A., Sabanov V., Thoroed S.M., and Doroshenko P.. 2000. Dual role of ATP in supporting volume-regulated chloride channels in mouse fibroblasts. Biochim. Biophys. Acta. 1468:63–72. 10.1016/S0005-2736(00)00243-1 [DOI] [PubMed] [Google Scholar]
- Cahalan M.D., and Lewis R.S.. 1988. Role of potassium and chloride channels in volume regulation by T lymphocytes. Soc. Gen. Physiol. Ser. 43:281–301. [PubMed] [Google Scholar]
- Cannon C.L., Basavappa S., and Strange K.. 1998. Intracellular ionic strength regulates the volume sensitivity of a swelling-activated anion channel. Am. J. Physiol. 275:C416–C422. 10.1152/ajpcell.1998.275.2.C416 [DOI] [PubMed] [Google Scholar]
- Carpenter E., and Peers C.. 1997. Swelling- and cAMP-activated Cl- currents in isolated rat carotid body type I cells. J. Physiol. 503:497–511. 10.1111/j.1469-7793.1997.497bg.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Wang L., Zhu L., Nie S., Zhang J., Zhong P., Cai B., Luo H., and Jacob T.J.. 2002. Cell cycle-dependent expression of volume-activated chloride currents in nasopharyngeal carcinoma cells. Am. J. Physiol. Cell Physiol. 283:C1313–C1323. 10.1152/ajpcell.00182.2002 [DOI] [PubMed] [Google Scholar]
- Chien L.T., and Hartzell H.C.. 2007. Drosophila bestrophin-1 chloride current is dually regulated by calcium and cell volume. J. Gen. Physiol. 130:513–524. 10.1085/jgp.200709795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantin B. 2016. Role of scaffolding proteins in the regulation of TRPC-dependent calcium entry. Adv. Exp. Med. Biol. 898:379–403. 10.1007/978-3-319-26974-0_16 [DOI] [PubMed] [Google Scholar]
- Deneka D., Sawicka M., Lam A.K.M., Paulino C., and Dutzler R.. 2018. Structure of a volume-regulated anion channel of the LRRC8 family. Nature. 558:254–259. 10.1038/s41586-018-0134-y [DOI] [PubMed] [Google Scholar]
- Deng W., Baki L., and Baumgarten C.M.. 2010. Endothelin signalling regulates volume-sensitive Cl- current via NADPH oxidase and mitochondrial reactive oxygen species. Cardiovasc. Res. 88:93–100. 10.1093/cvr/cvq125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan J., Walshe K., Alsbury S., Hokamp K., O’Keeffe S., Okafuji T., Miller S.F., Tear G., and Mitchell K.J.. 2007. The extracellular leucine-rich repeat superfamily; a comparative survey and analysis of evolutionary relationships and expression patterns. BMC Genomics. 8:320 10.1186/1471-2164-8-320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doroshenko P., and Neher E.. 1992. Volume-sensitive chloride conductance in bovine chromaffin cell membrane. J. Physiol. 449:197–218. 10.1113/jphysiol.1992.sp019082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan D., Winter C., Cowley S., Hume J.R., and Horowitz B.. 1997. Molecular identification of a volume-regulated chloride channel. Nature. 390:417–421. 10.1038/37151 [DOI] [PubMed] [Google Scholar]
- Eisener-Dorman A.F., Lawrence D.A., and Bolivar V.J.. 2009. Cautionary insights on knockout mouse studies: The gene or not the gene? Brain Behav. Immun. 23:318–324. 10.1016/j.bbi.2008.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emma F., McManus M., and Strange K.. 1997. Intracellular electrolytes regulate the volume set point of the organic osmolyte/anion channel VSOAC. Am. J. Physiol. 272:C1766–C1775. 10.1152/ajpcell.1997.272.6.C1766 [DOI] [PubMed] [Google Scholar]
- Estevez A.Y., Bond T., and Strange K.. 2001. Regulation of I(Cl,swell) in neuroblastoma cells by G protein signaling pathways. Am. J. Physiol. Cell Physiol. 281:C89–C98. 10.1152/ajpcell.2001.281.1.C89 [DOI] [PubMed] [Google Scholar]
- Fluman N., Tobiasson V., and von Heijne G.. 2017. Stable membrane orientations of small dual-topology membrane proteins. Proc. Natl. Acad. Sci. USA. 114:7987–7992. 10.1073/pnas.1706905114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formaggio F., Saracino E., Mola M.G., Rao S.B., Amiry-Moghaddam M., Muccini M., Zamboni R., Nicchia G.P., Caprini M., and Benfenati V.. 2018. LRRC8A is essential for swelling-activated chloride current and for regulatory volume decrease in astrocytes. FASEB J. 33:101–113. [DOI] [PubMed] [Google Scholar]
- Friard J., Tauc M., Cougnon M., Compan V., Duranton C., and Rubera I.. 2017. Comparative effects of chloride channel Inhibitors on LRRC8/VRAC-mediated chloride conductance. Front. Pharmacol. 8:328 10.3389/fphar.2017.00328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaitán-Peñas H., Gradogna A., Laparra-Cuervo L., Solsona C., Fernández-Dueñas V., Barrallo-Gimeno A., Ciruela F., Lakadamyali M., Pusch M., and Estévez R.. 2016. Investigation of LRRC8-mediated volume-regulated anion currents in Xenopus oocytes. Biophys. J. 111:1429–1443. 10.1016/j.bpj.2016.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A., Khandelwal N., Kumar A., and Bera A.K.. 2017. Leucine-rich repeat-containing 8B protein is associated with the endoplasmic reticulum Ca2+ leak in HEK293 cells. J. Cell Sci. 130:3818–3828. 10.1242/jcs.203646 [DOI] [PubMed] [Google Scholar]
- Gill D.R., Hyde S.C., Higgins C.F., Valverde M.A., Mintenig G.M., and Sepúlveda F.V.. 1992. Separation of drug transport and chloride channel functions of the human multidrug resistance P-glycoprotein. Cell. 71:23–32. 10.1016/0092-8674(92)90263-C [DOI] [PubMed] [Google Scholar]
- Gradogna A., Gavazzo P., Boccaccio A., and Pusch M.. 2017. Subunit-dependent oxidative stress sensitivity of LRRC8 volume-regulated anion channels. J. Physiol. 595:6719–6733. 10.1113/JP274795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gründer S., Thiemann A., Pusch M., and Jentsch T.J.. 1992. Regions involved in the opening of CIC-2 chloride channel by voltage and cell volume. Nature. 360:759–762. 10.1038/360759a0 [DOI] [PubMed] [Google Scholar]
- Guizouarn H., and Motais R.. 1999. Swelling activation of transport pathways in erythrocytes: effects of Cl-, ionic strength, and volume changes. Am. J. Physiol. 276:C210–C220. 10.1152/ajpcell.1999.276.1.C210 [DOI] [PubMed] [Google Scholar]
- Hand M., Morrison R., and Strange K.. 1997. Characterization of volume-sensitive organic osmolyte efflux and anion current in Xenopus oocytes. J. Membr. Biol. 157:9–16. 10.1007/s002329900211 [DOI] [PubMed] [Google Scholar]
- Häussinger D. 2008. Osmosensing and osmosignaling in the liver. Wien. Med. Wochenschr. 158:549–552. 10.1007/s10354-008-0593-0 [DOI] [PubMed] [Google Scholar]
- Hawkins T.L., Sept D., Mogessie B., Straube A., and Ross J.L.. 2013. Mechanical properties of doubly stabilized microtubule filaments. Biophys. J. 104:1517–1528. 10.1016/j.bpj.2013.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Nozaki Y., Nishizuka M., Ikawa M., Osada S., and Imagawa M.. 2011. Factor for adipocyte differentiation 158 gene disruption prevents the body weight gain and insulin resistance induced by a high-fat diet. Biol. Pharm. Bull. 34:1257–1263. 10.1248/bpb.34.1257 [DOI] [PubMed] [Google Scholar]
- Hazama A., and Okada Y.. 1988. Ca2+ sensitivity of volume-regulatory K+ and Cl- channels in cultured human epithelial cells. J. Physiol. 402:687–702. 10.1113/jphysiol.1988.sp017229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Carballo C.Y., De Santiago-Castillo J.A., Rosales-Saavedra T., Pérez-Cornejo P., and Arreola J.. 2010. Control of volume-sensitive chloride channel inactivation by the coupled action of intracellular chloride and extracellular protons. Pflugers Arch. 460:633–644. 10.1007/s00424-010-0842-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. 2001. Ionic Channels of Excitable Membranes. Third Edition. Sinauer Associates, Inc., Sunderland, MA. [Google Scholar]
- Ho M.W., Duszyk M., and French A.S.. 1994. Evidence that channels below 1 pS cause the volume-sensitive chloride conductance in T84 cells. Biochim. Biophys. Acta. 1191:151–156. 10.1016/0005-2736(94)90243-7 [DOI] [PubMed] [Google Scholar]
- Hoffmann E.K., Lambert I.H., and Pedersen S.F.. 2009. Physiology of cell volume regulation in vertebrates. Physiol. Rev. 89:193–277. 10.1152/physrev.00037.2007 [DOI] [PubMed] [Google Scholar]
- Hu X., Margadant F.M., Yao M., and Sheetz M.P.. 2017. Molecular stretching modulates mechanosensing pathways. Protein Sci. 26:1337–1351. 10.1002/pro.3188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyzinski-García M.C., Rudkouskaya A., and Mongin A.A.. 2014. LRRC8A protein is indispensable for swelling-activated and ATP-induced release of excitatory amino acids in rat astrocytes. J. Physiol. 592:4855–4862. 10.1113/jphysiol.2014.278887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikenouchi J., and Aoki K.. 2017. Membrane bleb: A seesaw game of two small GTPases. Small GTPases. 8:85–89. 10.1080/21541248.2016.1199266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonen E., and Vainio S.. 2005. Lipid microdomains and insulin resistance: Is there a connection? Sci. STKE. 2005:pe3. [DOI] [PubMed] [Google Scholar]
- Jackson P.S., and Strange K.. 1993. Volume-sensitive anion channels mediate swelling-activated inositol and taurine efflux. Am. J. Physiol. 265:C1489–C1500. [DOI] [PubMed] [Google Scholar]
- Jackson P.S., and Strange K.. 1995a Characterization of the voltage-dependent properties of a volume-sensitive anion conductance. J. Gen. Physiol. 105:661–676. 10.1085/jgp.105.5.661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson P.S., and Strange K.. 1995b Single-channel properties of a volume-sensitive anion conductance. Current activation occurs by abrupt switching of closed channels to an open state. J. Gen. Physiol. 105:643–660. 10.1085/jgp.105.5.643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson P.S., Morrison R., and Strange K.. 1994. The volume-sensitive organic osmolyte-anion channel VSOAC is regulated by nonhydrolytic ATP binding. Am. J. Physiol. 267:C1203–C1209. [DOI] [PubMed] [Google Scholar]
- Jackson P.S., Churchwell K., Ballatori N., Boyer J.L., and Strange K.. 1996. Swelling-activated anion conductance in skate hepatocytes: Regulation by cell Cl- and ATP. Am. J. Physiol. 270:C57–C66. [DOI] [PubMed] [Google Scholar]
- Jentsch T.J. 2016. VRACs and other ion channels and transporters in the regulation of cell volume and beyond. Nat. Rev. Mol. Cell Biol. 17:293–307. 10.1038/nrm.2016.29 [DOI] [PubMed] [Google Scholar]
- Ju L., Lou J., Chen Y., Li Z., and Zhu C.. 2015. Force-induced unfolding of leucine-rich repeats of glycoprotein Ibα strengthens ligand interaction. Biophys. J. 109:1781–1784. 10.1016/j.bpj.2015.08.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju L., Chen Y., Xue L., Du X., and Zhu C.. 2016. Cooperative unfolding of distinctive mechanoreceptor domains transduces force into signals. eLife. 5:e15447 10.7554/eLife.15447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalogeris T., Baines C.P., Krenz M., and Korthuis R.J.. 2012. Cell biology of ischemia/reperfusion injury. Int. Rev. Cell Mol. Biol. 298:229–317. 10.1016/B978-0-12-394309-5.00006-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C., Xie L., Gunasekar S.K., Mishra A., Zhang Y., Pai S., Gao Y., Kumar A., Norris A.W., Stephens S.B., and Sah R.. 2018. SWELL1 is a glucose sensor regulating β-cell excitability and systemic glycaemia. Nat. Commun. 9:367 10.1038/s41467-017-02664-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuya G., Nakane T., Yokoyama T., Jia Y., Inoue M., Watanabe K., Nakamura R., Nishizawa T., Kusakizako T., Tsutsumi A., et al. . 2018. Cryo-EM structures of the human volume-regulated anion channel LRRC8. Nat. Struct. Mol. Biol. 25:797–804. 10.1038/s41594-018-0109-6 [DOI] [PubMed] [Google Scholar]
- Kefauver J.M., Saotome K., Dubin A.E., Pallesen J., Cottrell C.A., Cahalan S.M., Qiu Z., Hong G., Crowley C.S., Whitwam T., et al. . 2018. Structure of the human volume regulated anion channel. eLife. 7:e38461 10.7554/eLife.38461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern D.M., Oh S., Hite R.K., and Brohawn S.G.. 2018. Cryo-EM structures of the DCPIB-inhibited volume-regulated anion channel LRRC8A in lipid nanodiscs. bioRxiv. http://doi.org/ 10.1101/442459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S.H., Ahmad N., Ahmad F., and Kumar R.. 2010. Naturally occurring organic osmolytes: From cell physiology to disease prevention. IUBMB Life. 62:891–895. 10.1002/iub.406 [DOI] [PubMed] [Google Scholar]
- Kobe B., and Kajava A.V.. 2001. The leucine-rich repeat as a protein recognition motif. Curr. Opin. Struct. Biol. 11:725–732. 10.1016/S0959-440X(01)00266-4 [DOI] [PubMed] [Google Scholar]
- Koch J., and Korbmacher C.. 1999. Osmotic shrinkage activates nonselective cation (NSC) channels in various cell types. J. Membr. Biol. 168:131–139. 10.1007/s002329900503 [DOI] [PubMed] [Google Scholar]
- Krapivinsky G.B., Ackerman M.J., Gordon E.A., Krapivinsky L.D., and Clapham D.E.. 1994. Molecular characterization of a swelling-induced chloride conductance regulatory protein, pICln. Cell. 76:439–448. 10.1016/0092-8674(94)90109-0 [DOI] [PubMed] [Google Scholar]
- Kubo M., and Okada Y.. 1992. Volume-regulatory Cl- channel currents in cultured human epithelial cells. J. Physiol. 456:351–371. 10.1113/jphysiol.1992.sp019340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar L., Chou J., Yee C.S., Borzutzky A., Vollmann E.H., von Andrian U.H., Park S.Y., Hollander G., Manis J.P., Poliani P.L., and Geha R.S.. 2014. Leucine-rich repeat containing 8A (LRRC8A) is essential for T lymphocyte development and function. J. Exp. Med. 211:929–942. 10.1084/jem.20131379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzelmann K. 2016. Ion channels in regulated cell death. Cell. Mol. Life Sci. 73:2387–2403. 10.1007/s00018-016-2208-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzelmann K., and Mehta A.. 2013. CFTR: A hub for kinases and crosstalk of cAMP and Ca2+. FEBS J. 280:4417–4429. 10.1111/febs.12457 [DOI] [PubMed] [Google Scholar]
- Lambert I.H., Kristensen D.M., Holm J.B., and Mortensen O.H.. 2015. Physiological role of taurine—From organism to organelle. Acta Physiol. (Oxf.). 213:191–212. 10.1111/apha.12365 [DOI] [PubMed] [Google Scholar]
- Lee C.C., Carette J.E., Brummelkamp T.R., and Ploegh H.L.. 2013. A reporter screen in a human haploid cell line identifies CYLD as a constitutive inhibitor of NF-κB. PLoS One. 8:e70339 10.1371/journal.pone.0070339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.C., Freinkman E., Sabatini D.M., and Ploegh H.L.. 2014. The protein synthesis inhibitor blasticidin s enters mammalian cells via leucine-rich repeat-containing protein 8D. J. Biol. Chem. 289:17124–17131. 10.1074/jbc.M114.571257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R.S., Ross P.E., and Cahalan M.D.. 1993. Chloride channels activated by osmotic stress in T lymphocytes. J. Gen. Physiol. 101:801–826. 10.1085/jgp.101.6.801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W., Huang L., Zhao D., He J.Z., Sharma P., Liu J., Gramolini A.O., Ward M.E., Cho H.C., and Backx P.H.. 2014. Swelling-activated Cl- currents and intracellular CLC-3 are involved in proliferation of human pulmonary artery smooth muscle cells. J. Hypertens. 32:318–330. 10.1097/HJH.0000000000000013 [DOI] [PubMed] [Google Scholar]
- Liu H.T., Akita T., Shimizu T., Sabirov R.Z., and Okada Y.. 2009. Bradykinin-induced astrocyte-neuron signalling: Glutamate release is mediated by ROS-activated volume-sensitive outwardly rectifying anion channels. J. Physiol. 587:2197–2209. 10.1113/jphysiol.2008.165084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lück J.C., Puchkov D., Ullrich F., and Jentsch T.J.. 2018. LRRC8/VRAC anion channels are required for late stages of spermatid development in mice. J. Biol. Chem. 293:11796–11808. 10.1074/jbc.RA118.003853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutter D., Ullrich F., Lueck J.C., Kempa S., and Jentsch T.J.. 2017. Selective transport of neurotransmitters and modulators by distinct volume-regulated LRRC8 anion channels. J. Cell Sci. 130:1122–1133. [DOI] [PubMed] [Google Scholar]
- Maertens C., Droogmans G., Chakraborty P., and Nilius B.. 2001. Inhibition of volume-regulated anion channels in cultured endothelial cells by the anti-oestrogens clomiphene and nafoxidine. Br. J. Pharmacol. 132:135–142. 10.1038/sj.bjp.0703786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolopoulos V.G., Voets T., Declercq P.E., Droogmans G., and Nilius B.. 1997. Swelling-activated efflux of taurine and other organic osmolytes in endothelial cells. Am. J. Physiol. 273:C214–C222. 10.1152/ajpcell.1997.273.1.C214 [DOI] [PubMed] [Google Scholar]
- Meyer K., and Korbmacher C.. 1996. Cell swelling activates ATP-dependent voltage-gated chloride channels in M-1 mouse cortical collecting duct cells. J. Gen. Physiol. 108:177–193. 10.1085/jgp.108.3.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan A., Oldfield C.J., Radivojac P., Vacic V., Cortese M.S., Dunker A.K., and Uversky V.N.. 2006. Analysis of molecular recognition features (MoRFs). J. Mol. Biol. 362:1043–1059. 10.1016/j.jmb.2006.07.087 [DOI] [PubMed] [Google Scholar]
- Mongin A.A. 2016. Volume-regulated anion channel—A frenemy within the brain. Pflugers Arch. 468:421–441. 10.1007/s00424-015-1765-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motais R., Guizouarn H., and Garcia-Romeu F.. 1991. Red cell volume regulation: The pivotal role of ionic strength in controlling swelling-dependent transport systems. Biochim. Biophys. Acta. 1075:169–180. 10.1016/0304-4165(91)90248-F [DOI] [PubMed] [Google Scholar]
- Niemeyer M.I., Cid L.P., Barros L.F., and Sepúlveda F.V.. 2001. Modulation of the two-pore domain acid-sensitive K+ channel TASK-2 (KCNK5) by changes in cell volume. J. Biol. Chem. 276:43166–43174. 10.1074/jbc.M107192200 [DOI] [PubMed] [Google Scholar]
- Nilius B., and Droogmans G.. 2003. Amazing chloride channels: An overview. Acta Physiol. Scand. 177:119–147. 10.1046/j.1365-201X.2003.01060.x [DOI] [PubMed] [Google Scholar]
- Nilius B., Oike M., Zahradnik I., and Droogmans G.. 1994. Activation of a Cl- current by hypotonic volume increase in human endothelial cells. J. Gen. Physiol. 103:787–805. 10.1085/jgp.103.5.787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B., Prenen J., Voets T., Eggermont J., and Droogmans G.. 1998. Activation of volume-regulated chloride currents by reduction of intracellular ionic strength in bovine endothelial cells. J. Physiol. 506:353–361. 10.1111/j.1469-7793.1998.353bw.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B., Voets T., Prenen J., Barth H., Aktories K., Kaibuchi K., Droogmans G., and Eggermont J.. 1999. Role of Rho and Rho kinase in the activation of volume-regulated anion channels in bovine endothelial cells. J. Physiol. 516:67–74. 10.1111/j.1469-7793.1999.067aa.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y., Shimizu T., Maeno E., Tanabe S., Wang X., and Takahashi N.. 2006. Volume-sensitive chloride channels involved in apoptotic volume decrease and cell death. J. Membr. Biol. 209:21–29. 10.1007/s00232-005-0836-6 [DOI] [PubMed] [Google Scholar]
- Oldfield C.J., Cheng Y., Cortese M.S., Romero P., Uversky V.N., and Dunker A.K.. 2005. Coupled folding and binding with alpha-helix-forming molecular recognition elements. Biochemistry. 44:12454–12470. 10.1021/bi050736e [DOI] [PubMed] [Google Scholar]
- Pan Z., Wang Z., Yang H., Zhang F., and Reinach P.S.. 2011. TRPV1 activation is required for hypertonicity-stimulated inflammatory cytokine release in human corneal epithelial cells. Invest. Ophthalmol. Vis. Sci. 52:485–493. 10.1167/iovs.10-5801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker J.C., Dunham P.B., and Minton A.P.. 1995. Effects of ionic strength on the regulation of Na/H exchange and K-Cl cotransport in dog red blood cells. J. Gen. Physiol. 105:677–699. 10.1085/jgp.105.6.677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulmichl M., Li Y., Wickman K., Ackerman M., Peralta E., and Clapham D.. 1992. New mammalian chloride channel identified by expression cloning. Nature. 356:238–241. 10.1038/356238a0 [DOI] [PubMed] [Google Scholar]
- Pilch P.F., and Liu L.. 2011. Fat caves: Caveolae, lipid trafficking and lipid metabolism in adipocytes. Trends Endocrinol. Metab. 22:318–324. 10.1016/j.tem.2011.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planells-Cases R., Lutter D., Guyader C., Gerhards N.M., Ullrich F., Elger D.A., Kucukosmanoglu A., Xu G., Voss F.K., Reincke S.M., et al. . 2015. Subunit composition of VRAC channels determines substrate specificity and cellular resistance to Pt-based anti-cancer drugs. EMBO J. 34:2993–3008. 10.15252/embj.201592409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt C.D., Chou J., Houlihan P., Badran Y.R., Kumar L., Bainter W., Poliani P.L., Perez C.J., Dent S.Y.R., Clapham D.E., et al. . 2017. Leucine-rich repeat containing 8A (LRRC8A)-dependent volume-regulated anion channel activity is dispensable for T-cell development and function. J. Allergy Clin. Immunol. 140:1651–1659.e1. 10.1016/j.jaci.2016.12.974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poletto Chaves L.A., and Varanda W.A.. 2008. Volume-activated chloride channels in mice Leydig cells. Pflugers Arch. 457:493–504. 10.1007/s00424-008-0525-2 [DOI] [PubMed] [Google Scholar]
- Qiu Z., Dubin A.E., Mathur J., Tu B., Reddy K., Miraglia L.J., Reinhardt J., Orth A.P., and Patapoutian A.. 2014. SWELL1, a plasma membrane protein, is an essential component of volume-regulated anion channel. Cell. 157:447–458. 10.1016/j.cell.2014.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp M., Granseth E., Seppälä S., and von Heijne G.. 2006. Identification and evolution of dual-topology membrane proteins. Nat. Struct. Mol. Biol. 13:112–116. 10.1038/nsmb1057 [DOI] [PubMed] [Google Scholar]
- Romanenko V.G., Davies P.F., and Levitan I.. 2002. Dual effect of fluid shear stress on volume-regulated anion current in bovine aortic endothelial cells. Am. J. Physiol. Cell Physiol. 282:C708–C718. 10.1152/ajpcell.00247.2001 [DOI] [PubMed] [Google Scholar]
- Rouzaire-Dubois B., Milandri J.B., Bostel S., and Dubois J.M.. 2000. Control of cell proliferation by cell volume alterations in rat C6 glioma cells. Pflugers Arch. 440:881–888. 10.1007/s004240000371 [DOI] [PubMed] [Google Scholar]
- Rubino S., Bach M.D., Schober A.L., Lambert I.H., and Mongin A.A.. 2018. Downregulation of leucine-rich repeat-containing 8A limits proliferation and increases sensitivity of glioblastoma to temozolomide and carmustine. Front. Oncol. 8:142 10.3389/fonc.2018.00142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rungta R.L., Choi H.B., Tyson J.R., Malik A., Dissing-Olesen L., Lin P.J.C., Cain S.M., Cullis P.R., Snutch T.P., and MacVicar B.A.. 2015. The cellular mechanisms of neuronal swelling underlying cytotoxic edema. Cell. 161:610–621. 10.1016/j.cell.2015.03.029 [DOI] [PubMed] [Google Scholar]
- Rutledge E., Bianchi L., Christensen M., Boehmer C., Morrison R., Broslat A., Beld A.M., George A.L., Greenstein D., and Strange K.. 2001. CLH-3, a ClC-2 anion channel ortholog activated during meiotic maturation in C. elegans oocytes. Curr. Biol. 11:161–170. 10.1016/S0960-9822(01)00051-3 [DOI] [PubMed] [Google Scholar]
- Sabirov R.Z., Prenen J., Tomita T., Droogmans G., and Nilius B.. 2000. Reduction of ionic strength activates single volume-regulated anion channels (VRAC) in endothelial cells. Pflugers Arch. 439:315–320. 10.1007/s004249900186 [DOI] [PubMed] [Google Scholar]
- Satoh T. 2014. Rho GTPases in insulin-stimulated glucose uptake. Small GTPases. 5:e28102 10.4161/sgtp.28102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada A., Takihara Y., Kim J.Y., Matsuda-Hashii Y., Tokimasa S., Fujisaki H., Kubota K., Endo H., Onodera T., Ohta H., et al. . 2003. A congenital mutation of the novel gene LRRC8 causes agammaglobulinemia in humans. J. Clin. Invest. 112:1707–1713. 10.1172/JCI18937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalkwyk L.C., Fernandes C., Nash M.W., Kurrikoff K., Vasar E., and Kõks S.. 2007. Interpretation of knockout experiments: The congenic footprint. Genes Brain Behav. 6:299–303. 10.1111/j.1601-183X.2007.00304.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober A.L., Wilson C.S., and Mongin A.A.. 2017. Molecular composition and heterogeneity of the LRRC8-containing swelling-activated osmolyte channels in primary rat astrocytes. J. Physiol. 595:6939–6951. 10.1113/JP275053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M.R., Droogmans G., Eggermont J., Voets T., Ellory J.C., and Nilius B.. 2000. Differential expression of volume-regulated anion channels during cell cycle progression of human cervical cancer cells. J. Physiol. 529:385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M., and Pak D.T.. 2000. Ligand-gated ion channel interactions with cytoskeletal and signaling proteins. Annu. Rev. Physiol. 62:755–778. 10.1146/annurev.physiol.62.1.755 [DOI] [PubMed] [Google Scholar]
- Shi Y., Cai M., Zhou L., and Wang H.. 2018. The structure and function of cell membranes studied by atomic force microscopy. Semin. Cell Dev. Biol. 73:31–44. 10.1016/j.semcdb.2017.07.012 [DOI] [PubMed] [Google Scholar]
- Shimizu T., Numata T., and Okada Y.. 2004. A role of reactive oxygen species in apoptotic activation of volume-sensitive Cl(-) channel. Proc. Natl. Acad. Sci. USA. 101:6770–6773. 10.1073/pnas.0401604101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumilina E.V., Khaitlina S.Y., Morachevskaya E.A., and Negulyaev Y.A.. 2003. Non-hydrolyzable analog of GTP induces activity of Na+ channels via disassembly of cortical actin cytoskeleton. FEBS Lett. 547:27–31. 10.1016/S0014-5793(03)00663-X [DOI] [PubMed] [Google Scholar]
- Siddle K. 2012. Molecular basis of signaling specificity of insulin and IGF receptors: Neglected corners and recent advances. Front. Endocrinol. 3:34 10.3389/fendo.2012.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth F.J. 1980. The variance of sodium current fluctuations at the node of Ranvier. J. Physiol. 307:97–129. 10.1113/jphysiol.1980.sp013426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauber T. 2015. The volume-regulated anion channel is formed by LRRC8 heteromers—Molecular identification and roles in membrane transport and physiology. Biol. Chem. 396:975–990. 10.1515/hsz-2015-0127 [DOI] [PubMed] [Google Scholar]
- Stoddard J.S., Steinbach J.H., and Simchowitz L.. 1993. Whole cell Cl- currents in human neutrophils induced by cell swelling. Am. J. Physiol. 265:C156–C165. [DOI] [PubMed] [Google Scholar]
- Strange K. 1998. Molecular identity of the outwardly rectifying, swelling-activated anion channel: Time to reevaluate pICln. J. Gen. Physiol. 111:617–622. 10.1085/jgp.111.5.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange K., Emma F., and Jackson P.S.. 1996. Cellular and molecular physiology of volume-sensitive anion channels. Am. J. Physiol. 270:C711–C730. 10.1152/ajpcell.1996.270.3.C711 [DOI] [PubMed] [Google Scholar]
- Stuhlmann T., Planells-Cases R., and Jentsch T.J.. 2018. LRRC8/VRAC anion channels enhance β-cell glucose sensing and insulin secretion. Nat. Commun. 9:1974 10.1038/s41467-018-04353-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syeda R., Qiu Z., Dubin A.E., Murthy S.E., Florendo M.N., Mason D.E., Mathur J., Cahalan S.M., Peters E.C., Montal M., and Patapoutian A.. 2016. LRRC8 proteins form volume-regulated anion channels that sense ionic strength. Cell. 164:499–511. 10.1016/j.cell.2015.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ternovsky V.I., Okada Y., and Sabirov R.Z.. 2004. Sizing the pore of the volume-sensitive anion channel by differential polymer partitioning. FEBS Lett. 576:433–436. 10.1016/j.febslet.2004.09.051 [DOI] [PubMed] [Google Scholar]
- Toft-Bertelsen T.L., Križaj D., and MacAulay N.. 2017. When size matters: Transient receptor potential vanilloid 4 channel as a volume-sensor rather than an osmo-sensor. J. Physiol. 595:3287–3302. 10.1113/JP274135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsumura T., Oiki S., Ueda S., Okuma M., and Okada Y.. 1996. Sensitivity of volume-sensitive Cl- conductance in human epithelial cells to extracellular nucleotides. Am. J. Physiol. 271:C1872–C1878. [DOI] [PubMed] [Google Scholar]
- Ullrich F., Reincke S.M., Voss F.K., Stauber T., and Jentsch T.J.. 2016. Inactivation and anion selectivity of volume-regulated anion channels (VRACs) depend on C-terminal residues of the first extracellular loop. J. Biol. Chem. 291:17040–17048. 10.1074/jbc.M116.739342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde M.A., Díaz M., Sepúlveda F.V., Gill D.R., Hyde S.C., and Higgins C.F.. 1992. Volume-regulated chloride channels associated with the human multidrug-resistance P-glycoprotein. Nature. 355:830–833. 10.1038/355830a0 [DOI] [PubMed] [Google Scholar]
- Voets T., Droogmans G., and Nilius B.. 1997. Modulation of voltage-dependent properties of a swelling-activated Cl- current. J. Gen. Physiol. 110:313–325. 10.1085/jgp.110.3.313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets T., Droogmans G., Raskin G., Eggermont J., and Nilius B.. 1999. Reduced intracellular ionic strength as the initial trigger for activation of endothelial volume-regulated anion channels. Proc. Natl. Acad. Sci. USA. 96:5298–5303. 10.1073/pnas.96.9.5298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk K.A., Zhang C., Husted R.F., and Stokes J.B.. 1996. Cl- current in IMCD cells activated by hypotonicity: Time course, ATP dependence, and inhibitors. Am. J. Physiol. 271:F552–F559. [DOI] [PubMed] [Google Scholar]
- von Heijne G. 2006. Membrane-protein topology. Nat. Rev. Mol. Cell Biol. 7:909–918. 10.1038/nrm2063 [DOI] [PubMed] [Google Scholar]
- Voss F.K., Ullrich F., Münch J., Lazarow K., Lutter D., Mah N., Andrade-Navarro M.A., von Kries J.P., Stauber T., and Jentsch T.J.. 2014. Identification of LRRC8 heteromers as an essential component of the volume-regulated anion channel VRAC. Science. 344:634–638. 10.1126/science.1252826 [DOI] [PubMed] [Google Scholar]
- Wine J.J., and Luckie D.B.. 1996. Cell-volume regulation: P-glycoprotein—A cautionary tale. Curr. Biol. 6:1410–1412. 10.1016/S0960-9822(96)00744-0 [DOI] [PubMed] [Google Scholar]
- Wondergem R., Gong W., Monen S.H., Dooley S.N., Gonce J.L., Conner T.D., Houser M., Ecay T.W., and Ferslew K.E.. 2001. Blocking swelling-activated chloride current inhibits mouse liver cell proliferation. J. Physiol. 532:661–672. 10.1111/j.1469-7793.2001.0661e.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue B., Dunker A.K., and Uversky V.N.. 2010. Retro-MoRFs: Identifying protein binding sites by normal and reverse alignment and intrinsic disorder prediction. Int. J. Mol. Sci. 11:3725–3747. 10.3390/ijms11103725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y., Li H., Zhang Y., Han X., Zhang G., Li W., Zhang H., Lin Y., Chen P., Sun X., et al. . 2018. Natural and synthetic flavonoids, novel blockers of the volume-regulated anion channels, inhibit endothelial cell proliferation. Pflugers Arch. 470:1473–1483. 10.1007/s00424-018-2170-8 [DOI] [PubMed] [Google Scholar]
- Yamada T., and Strange K.. 2018. Intracellular and extracellular loops of LRRC8 are essential for volume-regulated anion channel function. J. Gen. Physiol. 150:1003–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Wondergem R., Morrison R., Yin V.P., and Strange K.. 2016. Leucine-rich repeat containing protein LRRC8A is essential for swelling-activated Cl- currents and embryonic development in zebrafish. Physiol. Rep. 4:e12940 10.14814/phy2.12940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., and Lieberman M.. 1996. Chloride conductance is activated by membrane distention of cultured chick heart cells. Cardiovasc. Res. 32:168–179. 10.1016/S0008-6363(95)00132-8 [DOI] [PubMed] [Google Scholar]
- Zhang X.F., Chen J., Faltynek C.R., Moreland R.B., and Neelands T.R.. 2008a. Transient receptor potential A1 mediates an osmotically activated ion channel. Eur. J. Neurosci. 27:605–611. 10.1111/j.1460-9568.2008.06030.x [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zhang H., Feustel P.J., and Kimelberg H.K.. 2008b. DCPIB, a specific inhibitor of volume regulated anion channels (VRACs), reduces infarct size in MCAo and the release of glutamate in the ischemic cortical penumbra. Exp. Neurol. 210:514–520. 10.1016/j.expneurol.2007.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Xie L., Gunasekar S.K., Tong D., Mishra A., Gibson W.J., Wang C., Fidler T., Marthaler B., Klingelhutz A., et al. . 2017. SWELL1 is a regulator of adipocyte size, insulin signalling and glucose homeostasis. Nat. Cell Biol. 19:504–517. 10.1038/ncb3514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Polovitskaya M.M., and Jentsch T.J.. 2018. LRRC8 N termini influence pore properties and gating of volume-regulated anion channels (VRACs). J. Biol. Chem. 293:13440–13451. 10.1074/jbc.RA118.002853 [DOI] [PMC free article] [PubMed] [Google Scholar]