Florence Niedergang introduces work by Turan et al. demonstrating that herpes virus egress from dendritic cells is differentially regulated by kinesin-dependent positioning of lysosomes and autophagic degradation of nuclear lamins.
Abstract
Herpes simplex viruses bud into the nuclear membrane of infected cells. Turan et al. (2019. J. Cell Biol. https://doi.org/10.1083/jcb.201801151) demonstrate that mature dendritic cells control the peripheral location of lysosomes, reducing autophagic degradation of lamins and inhibiting viral release.
Viruses have unexpected ways to divert cellular machineries. Herpes simplex virus type 1, or human herpesvirus 1 (HSV-1), represents common and contagious viruses that cause sores around the mouth. Around two-thirds of the global population is infected. The virus persists in neuronal cells, where it remains hidden from the immune system. It has been reported to have a rapid lytic cycle. As for other Alphaherpesvirinae, an important step in viral production is the budding of the viral capsid in the inner nuclear membrane, before the crossing of the outer nuclear membrane and the release in the cytosol. It was determined that the nuclear lamina plays a crucial role in preventing viral egress (1), and its reorganization appears to be a conserved mechanism, triggered by herpesviruses to promote nuclear egress. In this issue, Turan et al. show that this reorganization is differentially regulated in immature and mature dendritic cells, potentially explaining the disparity in their permissiveness for infection (2).
Dendritic cells are sentinel cells, constantly sampling their environment for antigens and solutes (3). They link innate and adaptive immune responses by capturing, processing, and presenting antigens to lymphocytes. They come in two flavors, immature and mature dendritic cells. Immature dendritic cells have a wide range of innate receptors enabling the recognition of pathogens and a high internalization capacity. Dendritic cells undergo a differentiation program, or maturation, while migrating from the periphery to the draining lymphoid organs or tissues where they present antigens. Mature dendritic cells express molecules that expose antigens to, interact with, and stimulate lymphocytes. Drastic reorganization of intracellular structures and surface extensions accompany this differentiation program.
While the replication of HSV-1 has previously been considered inefficient in mature dendritic cells, in this issue, Turan et al. (2) have demonstrated that the virus is produced intracellularly, without release from mature dendritic cells. The authors compared monocyte-derived immature and mature dendritic cells and provide microscopic evidence that the nuclear capsid egress is blocked in mature dendritic cells. Strikingly, the level of lamin A/C proteins was reduced only in immature dendritic cells upon viral infection, not in mature dendritic cells. This decrease was further shown to occur at the protein level by an autophagy-related process. Thus, HSV utilizes autophagy to facilitate the nuclear egress of progeny capsids and, therefore, viral release and spread.
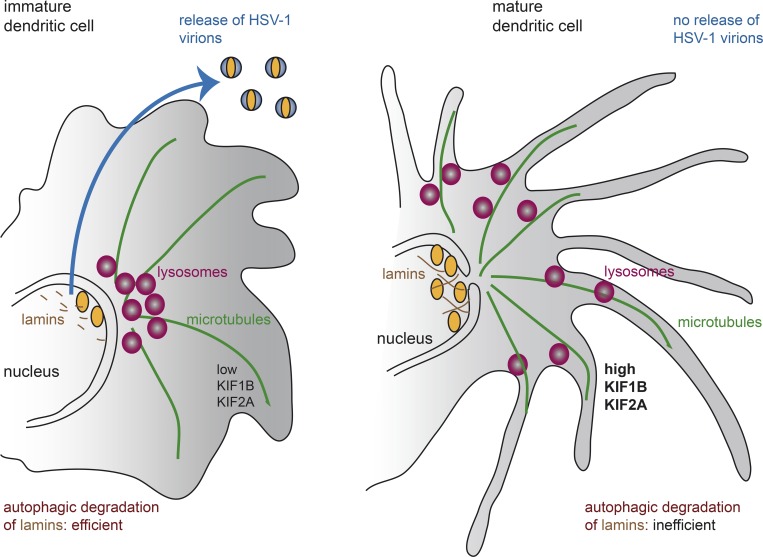
Autophagy is a cellular degradation pathway to digest and recycle intracellular proteins and organelles via the lysosomal pathway (4). In Turan et al., the authors further show that lysosomal positioning by two kinesin family members, KIF1B and KIF2A, and an ARF-like GTPase 8 A/B, contribute to inefficient autophagy in mature dendritic cells. This contrasts with immature dendritic cells, where lower expression of these kinesins results in perinuclear position of lysosomes and more efficient autophagic degradation of lamins (Fig. 1). The authors provide compelling evidence for the roles of these kinesins in controlling autophagy and, thus, viral egress. Lysosomal positioning was described previously to play a crucial role in the autophagic pathway in relation to the activation of mTORC1 signaling and in response to a variety of stressors (5).
Figure 1.
Lysosome positioning regulates herpesvirus egress from the nucleus and viral release in dendritic cells. Immature dendritic cells (left) have low levels of expression of the KIF1B and KIF2A kinesins. Lysosomes accumulate in the center of the cell. Autophagy induction following herpesvirus infection leads to efficient degradation of the nuclear intermediate filaments lamins and efficient viral egress. In mature dendritic cells (right), the high kinesins activity maintains a peripheral location of the lysosomes, which prevents efficient degradation of lamins and therefore limits viral egress.
More than 60 years after Christian de Duve described and named them, lysosomes remain fascinating organelles. Lysosomal biogenesis and positioning is tightly regulated in cells. Advanced imaging techniques now allow for high temporal and spatial analysis of organelle dynamics. Lysosomes were recently revealed to form clusters that are positioned within cells via interactions with the actin and microtubule cytoskeleton and with other organelles such as the ER (6). The position of lysosomes is essential for efficient fusion of lysosomes with various endosomes, including phagosomes, both in normal and pathological conditions. Besides their role in genetic lysosomal storage diseases, lysosomes have to be considered as central players in many infectious or metabolic disorders (7, 8).
The study by Turan et al. raises questions on how nuclear lamins are degraded by an autophagic pathway: Where and how are the lamins recognized by the macroautophagic machinery and how is the phagophore formed? What is the source of membrane used for that? What is the time and space regulation between the autophagic degradation of lamins and the nuclear envelope-dependent autophagy induced in some cell types (9)?
How lysosomal positioning affects the fate of the virus in subsequent steps of the viral cycle in dendritic cells is also of interest, in particular, for the subsequent step generating the secondary envelope that occurs in the cytoplasm. Despite the fact that autophagy induction occurs both in immature and mature dendritic cells, the elevated KIF1B and KIF2A levels are key determinants for the inhibition of autophagosome–lysosome fusion and the impairment of viral capsids nuclear egress. It is worth noting, however, that the level of expression of KIF1B and KIF2A in dendritic cells might affect not only the location of lysosomes, but of other organelles as well. How more global differences in the intracellular trafficking in immature versus mature dendritic cells affect viral production is yet another question.
Finally, many questions remain concerning the role of dendritic cells as antigen-presenting cells that are central to the orchestration of immune responses. What is the fate of immature dendritic cells once they internalize the virus? Among the range of innate receptors enabling the recognition of pathogens, many activate dendritic cells through signaling pathways that elicit their maturation. Mature cells were prepared with a specific cocktail of cytokines by the authors in their study. Would it change the results if the maturation protocol was different? The authors infect immature and mature dendritic cells as two distinct cell types generated in vitro, but the immature dendritic cells are very plastic and often evolve quickly into mature cells. What are the functional consequences of the infection of immature and mature dendritic cells with HSV-1, in particular for antigen presentation and immune escape mechanisms? How is the antigen presentation machinery of dendritic cells affected by viral production? How many of the subversions of dendritic cell functions that have already been reported (10) can be related to the kinesin-dependent pathways described in this paper? Although many of these questions remain to be answered, the study by Turan et al. represents an important step forward in the effort to better understand how viruses interact with important immune cells that are meant to drive their elimination, but are also prominent targets for hijacking by the viruses.
Acknowledgments
I thank Drs. Anna Mularski and Fatah Ouaaz for critical reading of the manuscript.
F. Niedergang is funded by Centre National de la Recherche Scientifique, Institut National de la Santé et de la Recherche Médicale, and Université Paris Descartes, and grants from Agence Nationale de Recherches sur le Sida et les Hépatites (ANRS AO2012-2) and Fondation pour la Recherche Médicale (FRM DEQ20130326518).
The author declares no competing financial interests.
References
- 1.Mou F., et al. . 2008. J. Virol. 82:8094–8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turan A., et al. J. Cell Biol. 2019 doi: 10.1083/jcb.201801151. [DOI] [Google Scholar]
- 3.Merad M., et al. . 2013. Annu. Rev. Immunol. 31:563–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao Y.G., and Zhang H. J. Cell Biol. 2019 doi: 10.1083/jcb.201810099. [DOI] [Google Scholar]
- 5.Korolchuk V.I., et al. . 2011. Nat. Cell Biol. 13:453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ba Q., et al. . 2018. Cell Reports. 23:3591–3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim C.Y., and Zoncu R.. 2016. J. Cell Biol. 214:653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawrence R.E., and Zoncu R. Nat. Cell Biol. 2019 doi: 10.1038/s41556-018-0244-7. [DOI] [PubMed] [Google Scholar]
- 9.Radtke K., et al. . 2013. J. Virol. 87:3990–3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grosche L., et al. . 2017. Front. Microbiol. 8:2149. [DOI] [PMC free article] [PubMed] [Google Scholar]