Leandro and Houten highlight new work from Zhou et al. exploring the disease relevance and toxicity of lysine metabolites in mitochondria.
Abstract
Saccharopine, a nonproteinogenic amino acid originally isolated from the yeast Saccharomyces cerevisiae, is an intermediate in lysine metabolism. In this issue, Zhou et al. (2019. J. Cell Biol. https://doi.org./10.1083/jcb.201807204) show that abnormal accumulation of saccharopine results in defective mitochondrial dynamics and function in worm and mouse models.
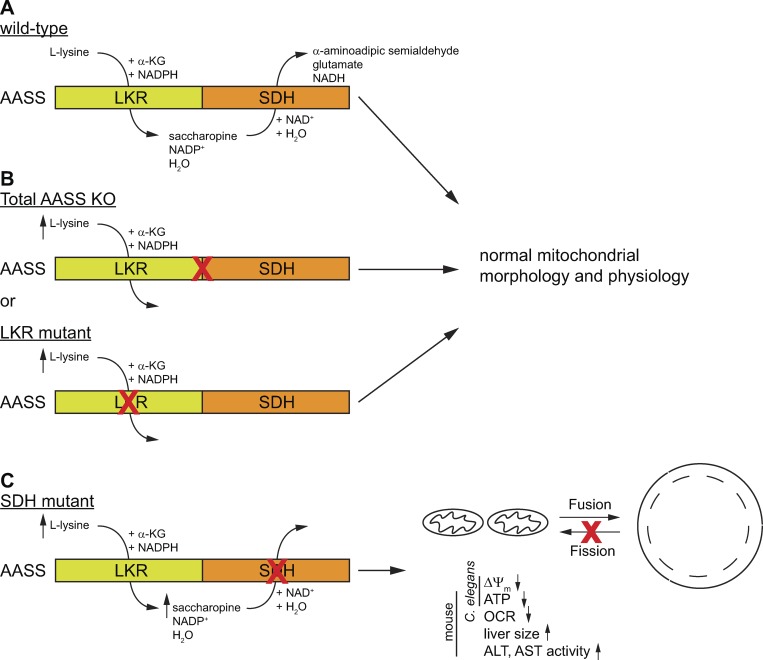
Lysine is an essential amino acid in humans. It is primarily catabolized through the saccharopine pathway in liver mitochondria, leading to the production of acetyl-CoA. The first enzyme of the lysine degradation pathway is α-aminoapidate semialdehyde synthase (AASS). AASS is of particular interest because it is a bifunctional enzyme that essentially catalyzes the ε-deamination of lysine (Fig. 1). In the first step, lysine and α-ketoglutarate (α-KG) are converted into saccharopine by the lysine-ketoglutarate reductase domain (LKR; EC 1.5.1.8). Saccharopine is then oxidized to α-aminoapidate semialdehyde and glutamate by the saccharopine dehydrogenase domain (SDH; EC 1.5.1.9).
Figure 1.
Schematic representation of the consequences of different mutations in AASS on mitochondrial dynamics and function. (A) The wild type AASS consist of two domains. LKR catalyzes the NADPH-dependent formation of saccharopine from lysine and α-KG. SDH catalyzes the NAD-dependent formation of α-aminoapidate semialdehyde from saccharopine. (B) Mutations that produce a total AASS KO or an isolated defect in LKR lead to hyperlysinemia, but without any apparent clinical or mitochondrial consequences. (C) Mutations that cause an isolated defect in SDH lead to hyperlysinemia with saccharopinuria that ultimately produces mitochondrial defects and liver disease in mice. ΔΨm, mitochondrial membrane potential; ALT, plasma alanine aminotransferase; AST, plasma aspartate aminotransferase.
The lysine degradation pathway is also clinically relevant, as several defects in this pathway can cause neurometabolic disorders. For example, the step directly following AASS in the lysine degradation pathway involves the conversion of α-aminoapidate semialdehyde into α-aminoapidate acid by α-aminoapidate semialdehyde dehydrogenase (ALDH7A1/antiquitin). Mutations in ALDH7A1 are a cause of pyridoxine-dependent epilepsy (PDE). In this disease, accumulation of α-aminoapidate semialdehyde, the ALDH7A1 substrate, leads to depletion of pyridoxal phosphate, an essential coenzyme derived from vitamin B6 (1). Importantly, inhibition of the upstream AASS enzyme has been proposed as substrate reduction therapy for the treatment of PDE (2). Therefore understanding of the consequences of AASS inhibition is of the utmost importance.
Human genetics has offered a unique insight into the consequences of AASS inhibition. Mutations in AASS cause hyperlysinemia, a condition characterized by an increased plasma lysine concentration (3). Seminal work by Dancis et al. has established that hyperlysinemia is most likely a benign metabolic variant without clinical significance (4). In very few cases, hyperlysinemia is accompanied by saccharopinuria (sometimes referred to as hyperlysinemia type 2), which indicates that there is also abnormal excretion of saccharopine in urine. While in most hyperlysinemia cases the entire AASS enzyme or the LKR domain is defective, these saccharopinuria cases are characterized by an isolated defect in the SDH domain (Fig. 1). Although most experts in metabolic diseases consider both forms hyperlysinemia biochemical phenotypes of questionable clinical significance (5), an association between neurological disease and a pronounced biochemical abnormality remains a challenging clinical question, especially if there is a paucity in available data.
In this issue, Zhou et al. establish a link between abnormal saccharopine accumulation and mitochondrial dynamics and function (6). By performing a mutagenesis screen in Caenorhabditis elegans expressing mitochondria-targeted GFP in the hypodermis, they observed abnormal enlargement of mitochondria in the presence of mutations of the aass-1 gene, the orthologue of human AASS. Importantly, only mutations in the SDH domain with the consequent increase in saccharopine and lysine levels induced mitochondrial enlargement. High lysine levels due to mutations in the LKR domain did not cause abnormal mitochondrial morphology. Furthermore, inactivation of aass-1 by RNAi did not lead to mitochondrial abnormalities, but instead was able to suppress the mitochondrial enlargement in the SDH mutants. These data convincingly illustrate that the accumulation of saccharopine is most likely responsible for the observed mitochondrial abnormalities.
Mitochondria are dynamic organelles, changing shape by fusion and fission processes. They also establish contact with other organelles such as the ER. Impairment of such processes has been associated with several neurological disorders (7). Zhou et al. (6) demonstrated that saccharopine accumulation results in impairment of fission and ER-dependent mitochondrial tubulation, while ongoing fusion is responsible for mitochondrial enlargement. Saccharopine accumulation also led to mitochondrial damage and functional loss, and ultimately impaired growth in adult C. elegans. Using additional assays including a suppressor screen in C. elegans, Zhou et al. furthermore showed that inactivation of genes controlling the levels of mitochondrial lysine (slc-25A29) and α-KG (slc-25A18.1, gdh-1, and idh-2), the precursors of saccharopine, ameliorate the mitochondrial phenotype of the SDH mutant.
To investigate if saccharopine accumulation also induces mitochondrial dysfunction in mammals, Zhou et al. created two knock-in mouse models. The first model harbors a mutation in the LKR domain, leading to complete AASS deficiency, equivalent to that observed in a previously reported hyperlysinemia case (8). The second model has a mutation in the SDH domain that leads to impaired SDH activity with preserved LKR function. Whereas the LKR mutant had no detectable clinical phenotype, the SDH mutant presented with liver disease, developmental delay, and premature death. This phenotype coincided with mitochondrial defects reminiscent of the mitochondrial phenotype of the C. elegans SDH mutants (6).
How accumulation of saccharopine, an adduct of lysine and α-KG, affects mitochondrial morphology, dynamics, and function remains to be elucidated. It might result from a direct effect of saccharopine on proteins involved in the fusion/fission and tubulation machinery, or indirect via inhibition of a protein or enzyme in another pathway. Notably SDH deficiency could result in changes of the mitochondrial α-KG/glutamate ratio and/or the NADP+/NADPH ratio. However, limiting the mitochondrial production of glutamate and α-KG was shown to rescue the saccharopine-induced phenotype, arguing against a prominent role of these metabolites in the observed phenotypes. Further mechanistic studies of the worm and mouse models generated by Zhou et al. will undoubtedly yield additional insights. Given the association of saccharopinuria with neurological disease, the study of the SDH mutant mice should not only focus on the liver, but also other organs such as the brain.
In summary, Zhou et al. demonstrate that saccharopine is a mitochondrial toxin detrimental to mitochondrial dynamics and function (6). This work establishes that AASS mutations leading to an isolated defect of SDH are a new cause of mitochondrial dysfunction that can result in a potentially harmful condition at least in worms and mice. Therefore hyperlysinemia with saccharopinuria in humans warrants careful observation and potentially a therapeutic intervention. At the same time, this work confirms the notion that hyperlysinemia due to complete AASS or isolated LKR deficiency is a biochemical abnormality without clinical consequences, which further paves the way for the development of LKR inhibition as a potential strategy for the treatment of PDE and saccharopinuria.
Acknowledgments
The work on genetic disorders of the lysine degradation pathway in the Houten laboratory is supported by the National Institutes of Health’s Eunice Kennedy Shriver National Institute of Child Health and Human Development under award numbers R03HD092878 and R21HD088775.
The authors declare no competing financial interests.
References
- 1.Mills P.B., et al. 2006. Nat. Med. 12:307–309. [DOI] [PubMed] [Google Scholar]
- 2.Pena I.A., et al. 2017. Biochim Biophys Acta Mol Basis Dis. 1863:121–128. [DOI] [PubMed] [Google Scholar]
- 3.Sacksteder K.A., et al. 2000. Am. J. Hum. Genet. 66:1736–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dancis J., et al. 1983. Am. J. Hum. Genet. 35:438–442. [PMC free article] [PubMed] [Google Scholar]
- 5.Goodman S.I., and Duran M.. 2014. Biochemical phenotypes of questionable clinical significance. In Physician’s guide to the diagnosis, treatment, and follow-up of inherited metabolic diseases. Springer-Verlag Berlin Heidelberg; 691–705. [Google Scholar]
- 6.Zhou J., et al. 2019. J. Cell Biol. https://doi.org./10.1083/jcb.201807204. [Google Scholar]
- 7.Bertholet A.M., et al. 2016. Neurobiol. Dis. 90:3–19. [DOI] [PubMed] [Google Scholar]
- 8.Houten S.M., et al. 2013. Orphanet J. Rare Dis. 8:57. [DOI] [PMC free article] [PubMed] [Google Scholar]