Abstract
The clinical and biochemical findings in three patients with glutaric aciduria Type 1 (GAT1) are presented. They had a normal postnatal period of three to 14 months. They developed sudden and severe encephalopathy following an infection or trauma (patient 3) that gradually progressed to severe dystonia, choreoathetosis, spastic quadriplegia and mental retardation. Neuroradiologic studies of the brain revealed white matter disease and frontotemporal lobe hypoplasia. The urine findings by gas chromatography/mass spectrometry (GC)/(MS) were characteristic of GAT1. Since GAT1 is an organic acidemia without intermittent acidotic attacks, but primarily manifests with progressive encephalopathy, it is important to recognize the potential of its existence among handicapped children in chronic care facilities. The good clinical response in two of the patients urges early diagnosis in subsequent newborn siblings of the families with the disease. The diagnosis of three patients in less than two years indicates the need for neonatal screening for the recognition of this disease, among other treatable metabolic diseases, in Saudi Arabia.
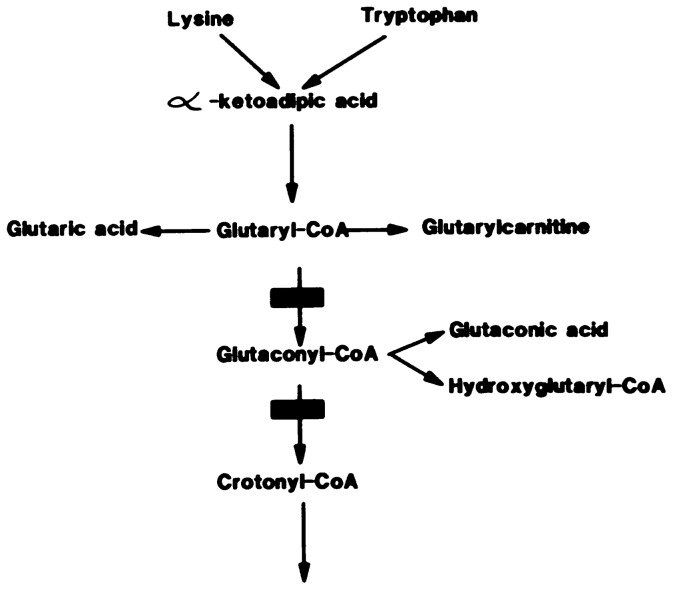
Glutaric aciduria Type 1 (GAT1) is an autosomal recessive disorder caused by a defect of glutaryl CoA dehydrogenase, an enzyme shared by the degradative pathways of L-lysine, L-hydroxylysine, and L-tryptophan (Figure 1).1 It was first described in two siblings with progressive encephalopathy starting at six months and characterized by opisthotonus, dystonia and athetotic posturing.2 Its onset usually is between one and eight months of age.3 It has a characteristic pattern of organic acids in the urine,4 but causes acidosis and hypoglycemia only occasionally.5–7 Since the neurological findings are variable, even within a family,3 and acidosis is usually absent, the diagnosis can be overlooked for many years. In fact, a survey conducted in a handicapped children's facility in Copenhagen, Denmark revealed the presence of five patients with GAT1 among the children older than four years with dystonic cerebral paralysis (CP) who never showed symptoms of an organic acidemia.8
FIGURE 1.
Disturbed metabolic pathway.
The patients in this report were initially considered to have postmeningitic or post-traumatic encephalopathy. The finding that eventually led to the suspicion of GAT1 and the subsequent investigations in the initial patient was the progressive frontotemporal lobe hypoplasia, or open opercular sign, detected on the computed tomography (CT) scan of the brain by the radiologist who recommended the investigations.9 These patients are, to our knowledge, the first cases of GAT1 from the Kingdom of Saudi Arabia. The variable neurologic manifestations of this disease must be appreciated, and the children in chronic care facilities with progressive dystonic or choreoathetotic CP should be routinely screened for GAT1 by gas chromatographic mass spectrometric (GC)/(MS),8 a technique available in the Kingdom. Although no definite treatment is available, the experience of other researchers, as well as the present data, indicate that the neurologic symptoms of the disease and its progression may be alleviated to a certain extent by drugs available.3,10–12 It is therefore important to study all future newborns of families with GAT1 in order to intervene before a devastating encephalopathy appears.
Methods and Patients
Methods
The gas chromatographic mass spectrometric (GC)/(MS) analysis of urine and its interpretation has been described.10 The techniques for CT and magnetic resonance imaging (MRI) scans of the brain have been reported.13 The developmental age was assessed by a qualified child psychologist using the Bayley Scales of Infant Development.
Patients
Patient 1
The patient was a seven-month-old male referred for the evaluation of delayed development, hypotonia and reversed diurnal rhythm of sleep. He was the product of a para 1, gravida 1 mother, and of a first cousin marriage. The delivery was in the hospital. He was normal at birth and achieved expected milestones until three months of age, at which time he was admitted to the referring hospital following 20 days of fever, irritability, vomiting, and two days of excessive crying and refusal to eat. Possible seizures were described. At that time, he was observed to have mild left facial weakness, poor head control, but no meningeal signs nor abnormal movements. A lumbar puncture (LP) was normal; CT scan of the brain revealed enlarged sylvian fissures. He was placed on clonazepam but failed to improve, refusing to feed, with increased irritability and reversed sleep cycle during a one month stay at the referring hospital. After discharge, the frequency of the generalized seizures increased with rolling of the eyes upwards. His motor and intellectual functions began to regress and he was referred for diagnosis. Physical examination at the time of initial encounter showed an acutely ill infant fussing continuously. His weight was in the 3rd, height in the 50th and head circumference in the 25th percentile. He showed severe head lag, hypotonia, and did not follow light. He had no dystonia; he had normal tendon reflexes and other systems were normal. He was considered to have CP as a result of meningitis, although the cerebrospinal fluid (CSF) at the referring hospital showed no evidence of meningitis. Normal laboratory studies included urine and blood chemistries, lactase, blood gases, ammonia, TORCH titers, and metabolic screen. The abnormal findings were: CSF protein 630 mg/L, 1.8 to 2 times the normal level of blood leucine, isoleucine, and valine, borderline increased urine branched chain amino acids and twice the normal blood level of creatine kinase. The electromyogram (EMG) and nerve conduction studies revealed normal peripheral nerve conduction time and normal muscle action potential, supporting the clinical impression of central hypotonia. The electroencephalogram (EEG) showed slow background and high frequency fast activity and paroxysms of high voltage delta waves arising from the left posterior region. A CT scan of the brain showed dilatation of all ventricles and bilateral fluid collection in. the temporal areas extending into the sylvian fissures. Density of the white matter in the frontal and parietal areas was diminished.
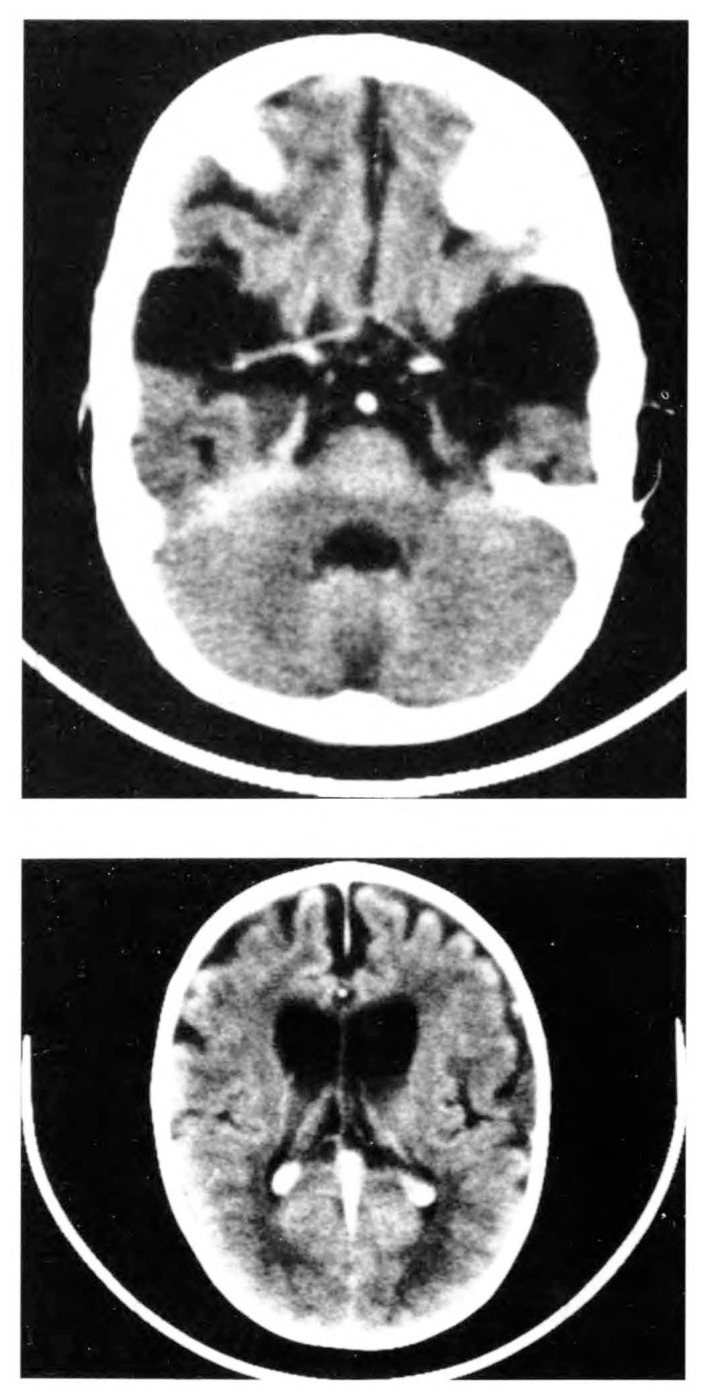
The patient was discharged on phenobarbital and clonazepam but his neurologic condition continued to deteriorate. On repeat admission three months later, the neurologic findings suggested mixed motor double hemiplegia with severe spasticity of the lower limbs and scissoring. He was assessed to have a developmental age of two months at the age of 10 months. A repeat physical examination at 15 months showed his head circumference to be in the 70th percentile and height in the 25th percentile. He showed severe head lag, dystonic posturing, scissoring of the legs, and poor visual follow-up. He was seen in the Inborn Errors of Metabolism Service at 20 months for the evaluation of frontotemporal hypoplasia or open opercular sign. A muscle biopsy showed normal histology. The activities of lysosomal enzymes and aspartoacylase in fibroblast cultures were normal. An MRI brain scan was obtained which indicated increasing frontotemporal hypoplasia and white matter changes suggestive of delayed myelination or demyelination (Figure 2). The radiologic diagnosis was possible GAT1. For this reason, a urine sample was obtained for GC/MS studies, which confirmed the diagnosis (Table 1).
FIGURE 2.
Neuroradiological findings in patient 1: (A) Post contrast CT - large bilateral anterior temporal CSF collections in association with temporal hypoplasia. (B) Post contrast CT - dilated frontal horns of lateral ventricles with widened cortical sulci consistent with cerebral underdevelopment; possible white matter changes in parieto-occipital areas.
Table 1.
Diagnostic organic acids encountered in the urine of patients with glutaric aciduria Type 1.*
| (mmol/mol creatinine) | |||||
|---|---|---|---|---|---|
|
| |||||
| Range (in normal) | Range in glutaric aciduria Type 1 | Patients | |||
| (1) | (2) | (3) | |||
| Glutaric acid | <2 | 500–12000 | 2685 | 376 | 6607 |
| 3-hydroxyglutaric acid | 0–3 | 60–3000 | 211 | 219 | 843 |
| Glutaconic acid | < 2 | 0–360 | 22 | 46 | 53 |
He was placed on riboflavin (100 mg/kg/d), L-carnitine (200 mg/kg/d), baclofen (10 mg b.i.d.), and a diet restricted in lysine and tryptophan (GAT1 diet). His further neurologic deterioration stopped and according to the parents, he started to stand with help and to utter a few words six months later. At present, 12 months later, his neurologic findings remain without further deterioration and he has many words.
Patient 2
A 20-month-old female was referred with a diagnosis of postencephalitic CP. She had achieved normal developmental milestones until the age of 14 months, at which time she developed fever and gastroenteritis, followed by repeated grand mal seizures. Seizure control required five days of intensive care admission. After that episode, she showed generalized central hypotonia, lost her ability to sit, walk, swallow and speak. Since a CT scan of the brain revealed severe white matter disease, she was referred for metabolic and infectious disease evaluation. The parents were first cousins and had five other normal children; no similar disease was known in the family. At the time of referral, the patient showed dystonic posturing, choreoathetosis, and spastic quadriplegia. The deep tendon reflexes were hyperactive.
An MRI brain scan showed mainly frontal white matter disease, increased cerebellar foliation, enlarged ventricles, moderately widened sulci, and the characteristic frontotemporal lobe atrophy or wide operculum sign (images not shown). In view of the frontotemporal lobe atrophy, urine was obtained for GC/MS that revealed the compounds characteristic of GAT1 (Table 1).
The patient was placed on riboflavin (100 mg/kg/d), L-carnitine (200 mg/kd/d), baclofen (10 mg b.i.d.), and GAT1 diet for the past 16 months. At the time of this report, her neurologic findings have deteriorated to severe spastic quadriplegia and severe dystonia.
Patient 3
This male was first seen at the age of 12 months. He had developed normally until seven months, at which time he received a trauma to the head, following which he developed fever, vomiting and diarrhea. The following day he developed episodes of arching his back with crying, and finally, focal seizures involving the left side of the body and the face and he was admitted to the local hospital. An encephalitis was suspected and the CSF revealed 500 mg of protein/L. A CT scan of the brain obtained at the local hospital revealed diffuse brain atrophy and particularly prominent frontotemporal lobe atrophy. He lost his milestones, developed hypotonia, and was referred for detailed studies.
When the patient was first seen, his height and weight were below the 5th percentile and his head was relatively macrocephalic, in the 75th percentile. He was an alert infant but showed dystonic posturing of the hands and opisthotonus. He had spastic quadriplegia and positive Babinski sign. An MRI scan of the brain revealed frontotemporal lobe hypoplasia and he was referred for admission. At the time of admission six months later, his neurologic status remained the same, except he showed choreic grimacing. The laboratory workup revealed compensated metabolic acidosis, a base excess of −7.5 and −6.8 on two occasions; and mild hyperammonemia of 101 and 102 μmol on two occasions. The EEG was found to be slow for age with an asymmetric tracing on the right. The MRI brain scan showed severe atrophy, particularly in the frontotemporal region, enlarged ventricles and para-ventricular white matter disease (images not shown).
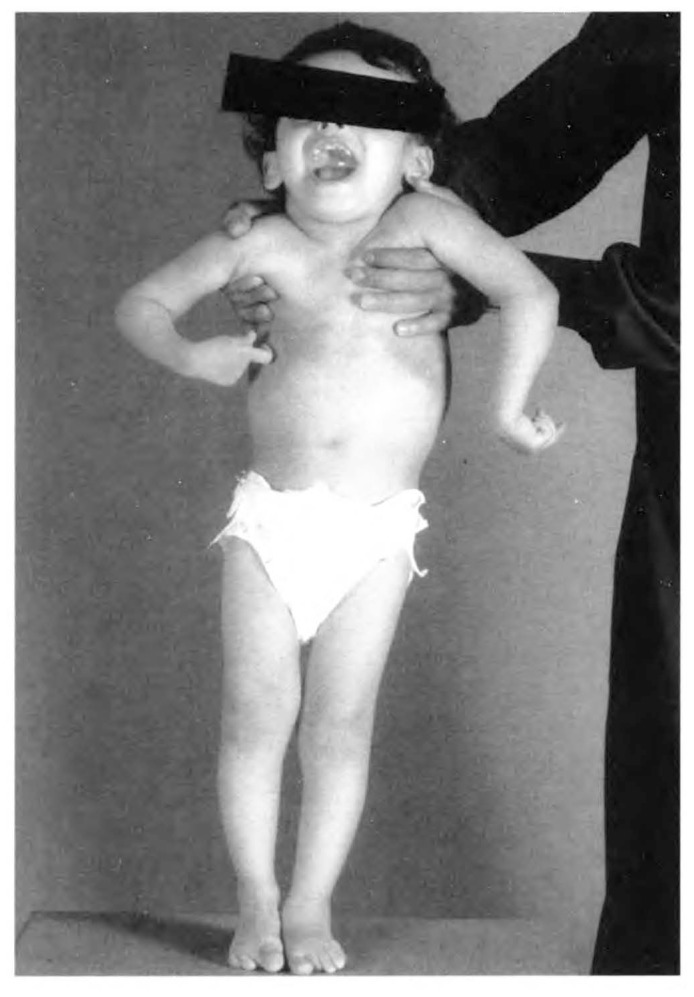
The patient was lost to follow-up and was next seen two years later. He now showed severe dystonic posturing (Figure 3). While his height and weight were below the 5th percentile, the head was macrocephalic and was in the 90th percentile. The eye grounds did not reveal optic atrophy. His muscle tone and deep tendon reflexes were all increased. The plasma aminogram revealed more than 100% increased glutamine, arginine, valine, methionine, phenylalanine and leucine. Blood gases, ammonia and pyruvate levels were normal and lactase was 2.6 mmol, normal upper limits being 2.0 mmol. Visual evoked and brainstem auditory evoked potentials and EEG were normal. A urine GC/MS was then obtained, which showed the characteristic pattern of GAT1 (Table 1).
FIGURE 3.
Patient appearance: dystonic posture of extremities and the choreic grimace.
The patient was placed on riboflavin (100 mg/kg/d), L-carnitine (200 mg/kg/d), baclofen (10 mg b.i.d.) and GAT1 diet. He started to utter a few words within one month; within three months his irritability disappeared and his dystonia had improved. Fifteen months later his neurologic status has improved to a point where he can stand with support and pronounce many words.
Discussion
The reported patients were diagnosed as cases of progressive encephalopathy following an episode of suspected meningitis or trauma. The symptoms progressed to severe spastic quadriplegia within one year. Retrospectively, the cases showed many of the reported clinical features of GAT1.
Over 40 patients with GAT1 have been reported. The pertinent clinical symptoms of reported cases together with the present patients have been summarized in Table 2. The patients in this report developed their first symptoms at the age of three to 14 months. The age of presentation of GAT1 is usually one to 12 months (Table 2). It has been observed in neonates,3,14,15 and patients as old as 36 years.3 One-third of the cases first present after one year (Table 2); and several cases have been detected retrospectively among older patients such as mildly affected relatives of the index patient, or while screening patients in a handicapped children's facility.8
Table 2.
Summary of the symptoms of glutaric aciduria Type 1 in previously published studies.*
| Number reported (39) | Number | Patients | ||
|---|---|---|---|---|
| (1) | (2) | (3) | ||
| Age of onset | ||||
| < 1 mo. | 4 | |||
| < 1 yr. | 19 | + | + | |
| > 1 yr. | 14 | + | ||
| Presenting symptoms | ||||
| Hypotonia | 10 | + | + | + |
| Pyramidal signs | 21 | + | + | + |
| Dystonia or choreoathetosis | 26 | + | + | + |
| Seizures | 13 | Abnormal EEG | + | + |
| Developmental delay | 12 | + | + | + |
| Mental retardation | ||||
| None-mild | 15 | − | − | − |
| Moderate/severe | 23 | + | + | + |
| Macrocephaly | 10 | Relative | − | Relative |
| Preceding infections | 10 | + | + | − |
| Prognosis | ||||
| Handicap | ||||
| Mild | 11 | − | − | − |
| Moderate | 5 | − | − | − |
| Severe | 22 | + | + | + |
| Dead | 6 | − | − | − |
| Blood & CSF chemistries | ||||
| Acidosis | 4 | − | − | +/− |
| Hypoglycemia | 2 | − | − | − |
| Increased amino acids | 19 | + | − | + |
| Elevated CSF protein | 4 | + | − | + |
The most frequent presenting clinical symptoms of GAT1 are dystonia, abnormal movements or choreoathetosis following a period of irritability, refusal to eat, vomiting, or infection (64%) (Table 2). Alternatively, the child may present with pyramidal tract signs in 54% of cases following a similar prodrome (Table 2). Seizures, generalized or myoclonic, have been reported in 33% (Table 2). At the time of initial studies, there might be hypotonia (25%), developmental delay (30%) in younger children, or moderate to severe mental retardation in older children (59%). Undoubtedly the disease is crippling and most patients eventually develop moderate to severe spastic quadriplegia (70%) (Table 2). The disease is compatible with a normal lifestyle in only 30% of the patients. In 30% of the patients, there is only mild mental retardation or normal intelligence despite severe motor handicap (Table 2). The severely affected child will eventually die of complications of a debilitating disease (15%) (Table 2).
The disease is common among consanguineous communities such as the Old Order Amish population in Lancaster, Pennsylvania, USA14 and among Canadian Indians living in Manitoba and Northwest Ontario.3 We have found three Saudi infants with GAT1 among 160 patients with established diagnosis of 20 various types of organic acidemias. The disease may not be common in Saudi Arabia, but patients with pyramidal or extra-pyramidal tract symptoms, in the absence of acidosis and hypoglycemia, are not routinely worked up for organic acidemia. This is particularly true when encephalopathic appearance is preceded by an encephalitic or meningitic picture that is thought to account for the CP as in our patient. The test of urine GC/MS is readily available at our institution and we recommend routine testing of all children with progressive choreoathetotic or dystonic CP.
Laboratory diagnosis of the disease can be achieved by studying the urinary excretion of organic acids,4,6 by measuring the glutaryl CoA dehydrogenase activity in leucocytes, cultured fibroblasts or liver,3,8,16 by loading the patient with L-lysine and studying the increased excretion of glutaric aciduria intermediates,6,12,17 or by administering L-carnitine and detecting the increased excretion of L-glutaryl carnitine.6,16,18 The combined urinary excretion of glutaric acid, 3-hydroxyglutaric acid and glutaconic acid (Table 2) is pathognomonic of GAT1.4,6,19,20 These compounds are derived from glutaryl CoA that accumulates as a result of the deficiency of glutaryl CoA dehydrogenase (Figure 1). In rare instances, the urinary organic acid pattern may be normal,16 and the diagnosis can be reached either by measurement of glutaryl CoA dehydrogenase16 or by increased glutaric acid level in CSF.21 When acidosis occurs, it is usually ketotic20 and rarely lactic.11
The disease is primarily neurotoxic as symptoms indicate (Table 1). Neuroradiological observations further confirm this conclusion (Table 3, Figure 2). It causes severe brain hypoplasia (74%) (Table 3) and ventricular dilatation (33%). The hypoplasia is particularly prominent in frontal and temporal lobes with enlarged sylvian fissures (61%), which has been called the open or wide opercular sign.9,22 Some investigators consider this finding to be due to bilateral arachnoid cysts in the temporal fossa.23 The occurrence of open opercular sign in GAT1 was first described by Amir et al.10 and Yager et al.24 Open opercular sign is nonspecific and can be encountered in a variety of disorders.9,22 The mechanism of open opercular sign is unknown, but it is considered to result from a developmental arrest of neuroblast migration and differentiation in the frontotemporal region in utero between the 28th to 29th week of gestation.
Table 3.
Summary of neuroradiological fundings in previously reported patients with glutaric aciduria Type 1.*
| Number of patients (33) | Number showing the finding | Patients | ||
|---|---|---|---|---|
| (1) | (2) | (3) | ||
| Cerebral atrophy | 24 | + | + | + |
| Ventricular dilatation | 11 | + | + | + |
| Open opercular sign | 20 | + | + | + |
| Demyelination | 11 | + | + | + |
| Caudate lesions | 15 | − | − | + |
| Putamen lesions | 11 | − | − | + |
The pathogenesis of the disease is unknown. It has been suggested, but not yet proven, that the accumulation of glutaryl CoA and glutarate in GABA neurons might inhibit the GABA synthetase activity.11,12,20 Pathological studies revealed decreased GABA content and GABA synthetase (glutamate decarboxylase) activity in affected areas of the brain, suggesting the loss of GABA neurons in the caudate region and the putamen.1,12 This finding correlates with the progressive movement disorder observed in most patients (Table 2). The presence of seizures (33%) and demyelination (32%) in neuroradiological studies suggests additional damage to neurons outside the basal ganglia. The correlation of neuroradiologic and clinical findings has recently been reviewed.25
These findings led to various therapeutic attempts in the past. Riboflavin in very large doses is used to improve the function of the defective enzyme, since glutaryl CoA dehydrogenase is a flavoprotein. In some instances, administration of riboflavin decreased the urinary excretion of glutaric acid, supporting the use of pharmacologic doses of this vitamin in GAT1.11 The Amish type of GAT1, as well as two of our patients, may be riboflavin responsive.3 L-carnitine has been shown to be deficient in GAT1 patients and the administration of L-carnitine increases the excretion of glutaric acid as glutarylcarnitine,6,9,18,20 which supports its use as a nonspecific detoxifying drug. Sodium valproate is an inhibitor of GABA transaminase and baclofen is a structural analogue of GABA. Both compounds increase the function of GABA and both have been used with some success to control the symptoms of GAT1.11,12 Dietary restriction of L-lysine and L-tryptophan, the GAT1 diet, has been advised.6 These observations suggest that therapy should be attempted in all patients with GAT1, using riboflavin, L-carnitine, baclofen and restricting dietary lysine and tryptophan. Valproic acid should be avoided,6 since it requires L-carnitine for its detoxification and may increase the carnitine deficiency.
The detection of GAT1 among Saudi children suggests that any progressive choreoathetotic CP, or a progressive encephalopathy of undetermined etiology but with suggestive clinical or neuroradiological findings, must receive appropriate investigations for this disease. The patient may be responsive to therapy and subsequent newborn siblings with the disease can receive appropriate intervention to prevent, alleviate, or delay the handicap.3,9,11,12 This can be achieved by referring the urine from such children to our service for GC/MS analysis. Alternatively, a dried blood sample on Guthrie test paper can be mailed for diagnosis through Tandem MS at KFSH&RC, Riyadh, Saudi Arabia, a technique recently described for the detection of treatable organic acidemias and aminoacidopathies.26
References
- 1.Goodman SI, Markey SP, Moe PG, et al. Glutaric aciduria: a “new” disorder of amino acid metabolism. Biochem Med. 1975;12:12–21. doi: 10.1016/0006-2944(75)90091-5. [DOI] [PubMed] [Google Scholar]
- 2.Goodman SI, Moe PG, Markey SP. Glutaric aciduria: a “new” inborn error of metabolism (abstract) Am J Hum Genet. 1974;26:36A. [Google Scholar]
- 3.Haworth JC, Booth FA, Chudley AE, et al. Phenotypic variability in glutaric aciduria Type 1: report of fourteen cases in five Canadian Indian kindreds. J Pediatr. 1991;118:52–8. doi: 10.1016/s0022-3476(05)81843-8. [DOI] [PubMed] [Google Scholar]
- 4.Stokke O, Goodman SI, Thompson JA, Miles BS. Glutaric aciduria: presence of glutaconic and beta-hydroxyglutaric acids in urine. Biochem Med. 1975;12:386–91. doi: 10.1016/0006-2944(75)90071-x. [DOI] [PubMed] [Google Scholar]
- 5.Dunger SB, Snodgras GJAI. Glutaric aciduria Type 1 presenting with hypoglycemia. J Inherit Metab Dis. 1984;7:122–4. doi: 10.1007/BF01801769. [DOI] [PubMed] [Google Scholar]
- 6.Ozand PT, Gascon GG. Organic acidurias: a review. Part 1. J Child Neurol. 1991;6:196–219. doi: 10.1177/088307389100600302. [DOI] [PubMed] [Google Scholar]
- 7.Ozand PT, Gascon GG. Organic acidurias: a review. Part 2. J Child Neurol. 1991;6:288–303. doi: 10.1177/088307389100600402. [DOI] [PubMed] [Google Scholar]
- 8.Brandt NJ, Brandt S, Christensen E, et al. Glutaric aciduria in progressive choreoathetosis. Clin Genet. 1978;13:77–80. doi: 10.1111/j.1399-0004.1978.tb04131.x. [DOI] [PubMed] [Google Scholar]
- 9.Amit R, Shapira Y, Berginer J. Significance of the open opercular sign. Ann Neurol. 1990;27:214. doi: 10.1002/ana.410270223. [DOI] [PubMed] [Google Scholar]
- 10.Amir N, El-Peleg O, Shalev RS, Christensen E. Glutaric aciduria Type 1: clinical heterogeneity and neurologic features. Neurology. 1987;37:1654–7. doi: 10.1212/wnl.37.10.1654. [DOI] [PubMed] [Google Scholar]
- 11.Stutchfield P, Edwards MA, Gray RGF, et al. Glutaric aciduria Type 1 misdiagnosed as Leigh's encephalopathy and cerebral palsy. Dev Med Child Neurol. 1985;27:514–21. doi: 10.1111/j.1469-8749.1985.tb04576.x. [DOI] [PubMed] [Google Scholar]
- 12.Leibel RL, Shih VE, Goodman SI, et al. Glutaric acidemia: a metabolic disorder causing progressive choreoathetosis. Neurology. 1980;30:1163–8. doi: 10.1212/wnl.30.11.1163. [DOI] [PubMed] [Google Scholar]
- 13.Brismar J. CT and MRI of the brain in inherited neurometabolic disorders. J Child Neurol. 1992;7(suppl):S112–31. doi: 10.1177/08830738920070011611. [DOI] [PubMed] [Google Scholar]
- 14.Morton H, Bennett M, Nichter C, Kelley RI. Glutaric aciduria type 1 of the Amish (abstract) Am J Hum Genet. 1989;45(suppl):A9. [Google Scholar]
- 15.Floret D, Divry P, Dingeon N, Monnet P. Acide glutarique. Une nouvelle observation. Arch Franc Pediat. 1979;36:462–70. [PubMed] [Google Scholar]
- 16.Bergman I, Finegold D, Gartner JC., Jr Acute profound dystonia in infants with glutaric acidemia. Pediatr. 1989;83:228–34. [PubMed] [Google Scholar]
- 17.Whelan DT, Hill R, Ryan ED, Spate M. L-glutaric acidemia: investigation of a patient and his family. Pediatr. 1979;63:88–93. [PubMed] [Google Scholar]
- 18.Seccombe DW, James L, Booth F. L-carnitine treatment in glutaric aciduria Type 1. Neurol. 1986;36:264–7. doi: 10.1212/wnl.36.2.264. [DOI] [PubMed] [Google Scholar]
- 19.Sweetman L. In: Organic acid analysis Techniques in diagnostic human biochemical genetics: a laboratory manual. Hommes A, editor. New York, USA: Wiley-Liss; 1991. pp. 143–76. [Google Scholar]
- 20.Goodman SI, Norenberg MD, Shikes RH, et al. Glutaric aciduria: biochemical and morphologic considerations. J Pediatr. 1977;90:746–50. doi: 10.1016/s0022-3476(77)81240-7. [DOI] [PubMed] [Google Scholar]
- 21.Campistol J, Ribes A, Alvarez L, et al. Glutaric aciduria Type 1: unusual biochemical presentation. J Pediatr. 1992;121:83–6. doi: 10.1016/s0022-3476(05)82548-x. [DOI] [PubMed] [Google Scholar]
- 22.Tatum WO, Coker SB, Ghobrial M, Abd-Allah S. The open opercular sign: diagnosis and significance. Ann Neurol. 1989;25:196–9. doi: 10.1002/ana.410250216. [DOI] [PubMed] [Google Scholar]
- 23.Hald JK, Nakstadt PH, Skjeldal OH, Stromme P. Bilateral arachnoid cysts of the temporal fossa in four children with glutaric aciduria Type 1. AJNR. 1991;12:407–9. [PMC free article] [PubMed] [Google Scholar]
- 24.Yager JY, McClarty BM, Seshia SS. CT scan findings in an infant with glutaric aciduria Type 1. Dev Med Child Neurol. 1988;30:808–20. doi: 10.1111/j.1469-8749.1988.tb14643.x. [DOI] [PubMed] [Google Scholar]
- 25.Nagasawa H, Yamaguchi S, Suzuki Y, et al. Neuroradiological findings in glutaric aciduria type 1: report of four Japanese patients. Acta Pediatr Jpn. 1992;34:409–15. doi: 10.1111/j.1442-200x.1992.tb00980.x. [DOI] [PubMed] [Google Scholar]
- 26.Millington DS, Terada N, Chace DH, et al. New Developments of Fatty Acid Oxidation. Wiley-Liss Inc; NY, USA: 1992. The role of tandem mass spectrometry in the diagnosis of fatty acid oxidation disorders; pp. 339–54. [PubMed] [Google Scholar]
- 27.Gregersen N, Brandt NJ, Christensen E. Glutaric aciduria: clinical and laboratory findings in two brothers. J Pediatr. 1977;90:740–5. doi: 10.1016/s0022-3476(77)81239-0. [DOI] [PubMed] [Google Scholar]
- 28.Kyllerman M, Stenn G. Intermittently progressive dyskinetic syndrome in glutaric aciduria. Neuropaediatrie. 1977;8:397–404. doi: 10.1055/s-0028-1091535. [DOI] [PubMed] [Google Scholar]
- 29.Goodman SI, Norenberg MD. Glutaric acidemia as a cause of striatal necrosis in childhood. Ann Neurol. 1983;13:582–3. doi: 10.1002/ana.410130525. [DOI] [PubMed] [Google Scholar]