ABSTRACT
The global obesity epidemic continues its relentless advance, currently affecting >2 billion people. This paper explores alternative ways to assess the potential disease impact of the epidemic, which is currently based almost exclusively on body mass index (BMI) data. It also argues in favor of concerted efforts to modify the built ecosystem that is driving the obesity epidemic. Most of the epidemiologic data on obesity are based on BMI (in kg/m2) and use the ranges of 18.5–24.9 for normality, 25–29.9 for overweight, and ≥30 for obesity. But the gap between the median of the “normal” BMI distribution (∼22) and the current population BMI of, for example, the United States (27.7) has become so wide that it is unlikely that we will be able to close that gap in the near future. Furthermore, the correlation between BMI and disease risk is not linear. Over 60% of the global disease burden of obesity affects individuals with a BMI ≥30, who comprise only ∼10% of the global population of overweight/obese persons. Furthermore, BMI accounts for only ∼17% of the risk of insulin resistance and subsequent type 2 diabetes in the BMI ≥25 population. Epigenetics, specifically DNA methylation, appears to play a far more important role than BMI in determining the risk of obesity's comorbidities, such as diabetes. Similarly, socioeconomic status carries a higher risk than BMI level for the development of obesity-related noncommunicable diseases. Finally, the built environment that sustains our species’ lifestyle is a major driver of the obesity epidemic. Modifying that ecosystem will require no less than a social movement, one able to promote and sustain the necessary coordinated action of virtually all sectors of society.
Keywords: epidemiology, international health, obesity, obesity epidemiology, public health
Introduction
Recent statistics indicate that overweight/obesity continues its relentless global rise, with the number of people with excess body weight reaching >2 billion, ∼30% of the world population. The Global Burden of Disease Group reported in 2017 that “since 1980, the prevalence of obesity has doubled in more than 70 countries and has continuously increased in most other countries” (1). In children, UNICEF in 2017 concluded that “there has been no progress to stem the rate of overweight in more than 15 years” (2). Experts are not optimistic on the prospects of reducing the obesity epidemic. On the contrary, leading scientists concluded that “if post-2000 trends continue, the probability of meeting the global obesity target is virtually zero” (3). This target is to reduce the rate of obesity by half by 2025 (4). Clearly, so far, we seem to be losing the war against obesity.
The aim of this paper is to explore alternative ways to assess the potential disease burden of the obesity epidemic, which is currently based almost exclusively on BMI data. It also offers some estimates on the likelihood that humans will return to a “pre-epidemic” BMI level. Finally, it discusses global obesity as a component of the overall human ecosystem.
When Did This Epidemic Start?
The 1998 WHO report on global obesity (5) was arguably the first major public document to use the term “epidemic” referring to obesity. The term has been widely used since, but it is not clear that it fits the characteristics of the obesity “epidemic”. A typical epidemic has an outbreak, a peak, an ebb, and eventually is resolved because every susceptible individual either dies or recovers. As discussed below, rather than starting as an outbreak, obesity (i.e., increasing population BMI) has progressively emerged over the past many decades.
In fact, historical records indicate that humans have been increasing their BMI continuously for the past 300 y. The economist R Fogel tracked the relationship between body size and productivity from the early 1700s (6, 7) through the use of data from healthier populations in economically advanced countries at that time (Scandinavian countries, France, United Kingdom). Figure 1 depicts Fogel's data from 1705 to 1975, plus the most recent data from the United States. As can be seen, in 1705 the average BMI of this select population was below the minimum considered adequate by current WHO standards. Over the past 300 y, BMI increased progressively, as a result of gains in height and weight. BMI values continued to rise until in recent decades they crossed the threshold of “normality”, reaching, in the case of the United States, an average BMI (in kg/m2) of 27.8 in 2014. Thus, it can be argued that rather than an “epidemic”, obesity is the result of humans’ longstanding efforts to protect themselves against famine and to increase body size, a factor that was a critical source of productivity and military domination for centuries. With the emergence of mechanized warfare and industrial automation, body size became less critical as a source of power, but efforts to feed the poor redoubled after World War II, with the creation of the FAO in 1945. Its founding director, John Boyd-Orr (9), a fierce advocate of hunger alleviation, took numerous initiatives to increase the per-capita dietary energy available to poor populations. Those efforts metamorphosed into modern mass production of foods and commercial competition for “stomach share” of consumers. Hunger still certainly exists in many parts of the world, but most experts agree that it is more related to disparities in access to food than to insufficient production at a global level.
FIGURE 1.
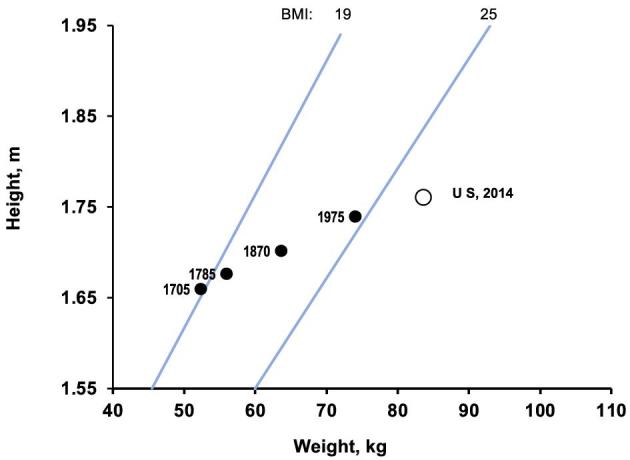
Trends in median BMI, 1705–1975, from select populations from established Western countries (e.g., France, Norway). Data from Fogel and Costa (6). Data point for 2014 from national US survey (8).
Coupled with the continuing gain in body weight, there is some slowing of gain in height, as some population groups begin to approach their full genetic potential for stature. Fogel's last data point, from 1975, is almost BMI = 25, so one could take approximately that year as the beginning of the “epidemic” of excess weight. Certainly, one should not overinterpret Fogel's data on their own, but most other historical views on the topic are consistent in recognizing this long journey of humans from scarcity to mass food production and consumption.
This historical view of the trajectory of today's high BMI is important because it highlights the major social and economic factors that have been sustaining the continuing rise in human BMI. These are the factors that will need to be controlled in order to stop and reverse the continuing rise in obesity prevalence in the global population.
Can the Epidemic Be Reversed?
The ultimate objective in confronting a typical communicable disease epidemic is eradication, i.e., to return the population to the status quo ante, where presumably none or only a nonsignificant fraction of the population was infected. In the case of the obesity epidemic, it would be to return to a pre-epidemic (“normal”) BMI distribution. The median BMI of the current reference distribution (with limits of 18.5–24.99) is ∼22. Thus, in the United States for example, reversing the obesity epidemic would entail moving the median BMI distribution of the population from its current 27.8 to 22. Even allowing for 5–10% of the population with excess BMI, that gap seems substantial. How feasible is it to produce this change? I am not aware of experimental data to answer this question, but a natural quasi-experiment in the country of Cuba offers some clues.
The Cuban Special Period
Cuba is a country of ∼10 million people, with an excellent primary health care system and a centralized social wealth distribution. With the collapse of the Soviet Union in 1990, Cuba lost billions of dollars in Soviet subsidies to its main export, sugar, and its privileged price for oil import. As a consequence, between 1990 and 1995 widespread food shortages affected virtually all the population, leading to a substantial decrease in dietary energy intake (10, 11). In addition, because of the oil shortage, mechanized transportation was virtually halted, replaced by bicycles distributed by the government. People biked or walked to work, and thus physical activity increased dramatically (12). The inevitable consequence was a negative energy balance and weight loss. The uniqueness of this situation was that it affected an otherwise healthy population, where most individuals were affected both by the problem and by the solutions, and that the health care system included a strong data collection and epidemiologic surveillance system.
The population-wide weight loss resulted in a shift to the left of the population BMI distribution. Rates of obesity were reduced by almost 50%. The impact could be likened to enrolling the whole population in a weight loss program, and a very successful one at that. But after 4 y of this Special Period, equivalent to a mandatory weight loss and exercise program, the shift in median BMI was of only 1.5 units (11). For comparison, the shift needed to “eradicate” obesity in the United States would be one of 5.7 points, from 27.7 to 22. It would be fair to say the probability of closing that gap in the near future is remote. Moreover, after the Cuban economy began to recover around 1995, population-wide BMI increased progressively, eventually reaching and even surpassing its pre-crisis level (12).
Thus, at least when judging by the BMI criteria, it is likely that for several more generations, excess body weight will continue to be the norm rather than the exception. We may be able to achieve a modest reduction in the median BMI, in the United States for example, from 27.7 to 25 or 26. It is also possible that proportionally more people in the obese category will be able to reduce their BMI to the overweight category, just as it happened in the Cuban experience. But we will not be able to eradicate this epidemic, i.e., to return to a status quo ante. In this scenario, many individuals will be in the BMI 25–29.9 category. And it is therefore particularly important to understand the disease risk carried by this category.
Excess BMI and Disease
Most of the alarming statistics on global obesity (the “>2 billion people”) include overweight and obese persons, i.e., everyone with a BMI ≥25. But >60% of the disease and mortality burden associated with excess BMI occurs at BMI ≥30, despite this BMI category representing only ∼10% of global excess BMI prevalence (1). In fact, the true disease burden associated with BMIs between 25 and 29.9 is a matter of debate, with some data showing that mortality in this range is not higher than in the normal BMI range (13). These and other data led some researchers to suggest that the BMI point for lowest mortality may have been increasing over time (14), and that perhaps the traditional range of normality of BMI = 18.5–25 should be re-evaluated. Exploring this issue in detail is beyond the focus of this paper; suffice it to say that there are also other data suggesting that the current range for normal BMI is still valid (15–18). But these controversies do have an impact in the form of inconsistent advice given to the 25–29.9 group. For example, the advice of US federal experts is that, unless they have other risk factors, they should “avoid further weight gain rather than lose weight” (19). It appears that this advice is heeded by the US population, because the number of people trying to lose weight continues to decline (20).
The correlation between BMI and obesity-related early metabolic disorders, such as insulin resistance, is also remarkably weak across a wide range of BMIs. A systematic review of studies published from 1994 to 2015 found that BMI explained only 16% of the variance in insulin resistance (21). Other studies show that when insulin resistance is present, it is a much stronger predictor of disease risk than BMI, at virtually any BMI level. In a prospective 5.5-y follow-up, insulin-sensitive obese persons had 40% lower incidence of diabetes than the insulin-resistant obese. Conversely, lean persons with insulin resistance had an 80% higher risk of developing diabetes than insulin-sensitive individuals (22).
The precise mechanisms by which some individuals with excess BMI move to the next step in the causal path, to insulin resistance, dyslipidemia, or high blood pressure, are the focus of great research interest, with genetics as one major focus (23). A recent study used epigenome-wide association to explore the link between BMI and changes in DNA methylation (24). The authors propose that adiposity is a major trigger of DNA methylation, and that this process in turn results in alterations in critical components of lipoprotein metabolism, substrate transport, and inflammation pathways. The study measured DNA methylation scores in >5000 people, sorting them by BMI status into quintiles of methylation score in each BMI category (normal, overweight, obese). The data showed that the main predictor for type 2 diabetes (T2D) was DNA methylation score, and that a lean person in quintile 5 of methylation score had a higher risk of T2D than an obese person in quintile 1 (24). If confirmed by further studies, these findings open the possibility of targeting high-methylators as a high-risk group for developing T2D, independently of BMI status.
Physical activity (PA) is also a powerful modulator of obesity-related cardiovascular disease risk. The Rotterdam study, a 15-y prospective follow-up of >5000 middle-age participants (25), found no difference in risk between obese/overweight individuals who maintained a high level of PA and those of normal BMI. One mechanism by which PA can modulate the effects of obesity may be through its effect on gene expression. A large meta-analysis performed interaction analysis between PA and the fat mass and obesity associated (FTO) gene, a well-known obesity susceptibility gene. The presence of the FTO gene significantly increased the odds of obesity, but this risk was attenuated by almost 30% in physically active individuals (26). Similar gene-modulating effects of PA were reported in a meta-analysis by Graff et al. (27).
Those data underline the fact that there are many individuals with excess BMI who have no higher risk of obesity comorbidities than normal-BMI persons. This occurs not only in the overweight category (BMI: 25–29.9) but also among the obese (BMI ≥30). According to some surveys, mostly in white Western populations, 10–16% of obese persons are metabolically normal, as assessed by fasting plasma glucose and insulin concentrations, 2-h glucose, and HOMA-IR (28). Overall, the prevalence of T2D in individuals with metabolic syndrome was 37%, compared with 4% in those without, irrespective of BMI classification. Alternative approaches to define obesity that do not rely on BMI have been proposed, e.g., “adiposity-based chronic disease” (29), or a more integrative combination of etiologic factors, degree of adiposity, and other coexisting clinical features (30). These approaches may be useful for case management but may not be practical for population-wide screening.
Socioeconomic Status
In assessing the health impact of the global obesity epidemic, another crucial factor is socioeconomic disparity. There is ample quantitative evidence of the extreme level of income inequality in the modern world. According to recent data, <1% of the world population owns >30% of the world's wealth (31). This extreme disparity has widespread effects on health and quality of life. Stringhini et al. (32) recently reported results of a meta-analysis of 48 studies with data on socioeconomic status (SES) and the risk factors included in the WHO 25 × 25 project (PA, smoking, hypertension, high alcohol intake, diabetes, and obesity), and estimated HRs for all-cause and cause-specific mortality. The leading factors were smoking, PA, and SES. The latter was associated with a 2.1-y reduction in life expectancy, compared with 0.7 y for obesity (Table 1). Other cohort studies have shown that adherence to health-promotion interventions tends to be lower in low-SES populations (33), resulting in a phenomenon that Frohlich and Potvin (34) termed “benefit disparity,” where the benefits of an intervention favor the better-off rather than the target, more disadvantaged population. These data underline the importance of SES for any intervention aimed at reducing obesity-related disease risks.
TABLE 1.
Reduction in life expectancy due to selected risk factors for chronic noncommunicable diseases1
| Risk factor | Reduction in life expectancy, y |
|---|---|
| Current smoking | 4.8 |
| Diabetes | 3.9 |
| Physical inactivity | 2.4 |
| Low socioeconomic status | 2.1 |
| Hypertension | 1.6 |
| Obesity | 0.7 |
1Data from Stringhini et al. (32).
Obesity as Part of the Anthropocene
The unofficial term Anthropocene describes the current phase of human evolution in which human activity is the dominant determinant of planetary change (35, 36). For thousands of years, that dominant force of change was nature itself, with all species, including humans and their ancestors, struggling to adapt and survive. Now, Homo sapiens’ footprint is wide and disruptive, and is the main factor responsible for the rapid changes in nature, including deforestation, rising ocean levels, increasing carbon dioxide concentrations, and rising surface temperatures (36). Several components of this expanding human footprint are linked to diet and thus to the global epidemic of excess weight. For example, continuing increase in the size and number of mega-cities will place an increasing number of individuals in an urban living environment characterized by sedentarism and consumption of energy-dense, unhealthy foods (37, 38). According to the last UN Habitat Summit (39), 2 billion more people will live in an urban environment by 2035. Another example of the link between environment and diet and health is deforestation. A prime driver for forest destruction is the use of the land to produce animal feed, particularly soy. The European Union for example, an avid importer of soy from Brazil and Argentina, allocates >80% of this crop to animal feed (40). The demand for animal feeds in turn is driven by the rising demand for animal protein, mostly consumed as low-cost fast foods. In another category, the continuing increase in environmental pollutants, including increasing levels of hormone disruptors, has also been linked to the obesity epidemic (41). It is evident that these factors cannot be confronted nor eradicated without a concerted effort from the various disciplines working on improving human health and the environment.
Confronting the Obesity Epidemic
One of the key difficulties in confronting the obesity epidemic at this point is that many of the crucial risk factors cannot be changed only by individual or group efforts, instead requiring national and even global concerted action (e.g., food production and marketing, urbanization, economic disparity, etc.). Until these powerful forces begin to show positive changes, there will be at best only modest progress in reducing global obesity. One is reminded of another effort, decades ago, the one to reduce population growth in poor countries. A variety of programs attempted to reduce fertility in the poorest communities, with little success. Only the eventual reduction in poverty and in child mortality led to a dramatic fall in fertility.
What to do in the short term? There are a number of initiatives around the world that show that there are still many opportunities to implement obesity prevention programs. Here are a few key points.
Childhood obesity: few, if any of the considerations discussed above that are related to the use of BMI to predict disease risk apply to childhood obesity. First, the BMI cutoffs for children and adolescents are not primarily risk-based, but reference-based. BMI cutoffs for overweight/obesity are selected by consensus from reference charts for normal growth. These charts are prescriptive for 0- to 5-y-old children (42) or descriptive for 5–20 y of age, and use a composite of older data; hence, obesity prevalence in the reference population was lower than it is today (42, 43). The key risk factor for childhood obesity is obesity itself: having excess BMI during the first 10 y of life carries more risk of becoming an obese adult than having one or both parents obese (44). Overweight adolescents also carry a higher risk of long-term morbidity and mortality (45). Thus, preventing obesity during childhood is the best way to reduce adult obesity and its comorbidities.
Furthermore, many of the prevention interventions aimed at reducing obesity during childhood are also beneficial for child health in general: exclusive breastfeeding, adequate weaning foods, healthy dietary practices, and introducing early on the practice of an active lifestyle.
Regarding adult obesity, we need to go beyond BMI, and target those at risk of noncommunicable diseases, regardless of BMI. The majority of individuals with BMI between 25 and 30 and with no additional risk factors do not have higher risk of cardiovascular disease or diabetes than lean persons. The data discussed above suggest that even persons with a BMI ≥30 without insulin resistance may not have additional risk. Thus, it is critical to develop practical means to identify those truly at risk and formulate preventive interventions. Unfortunately, there are still no easy and inexpensive tests for insulin resistance, or degree of DNA methylation, for example. But the pace of progress in science and technology suggests that those goals may not be far ahead.
In terms of research needs, almost all the data discussed here were obtained from white populations from developed countries. Thus, another important task will be to obtain information and understand the causative path of obesity in low-income populations. There is ample evidence that nutritional injury early in life increases the risk of some noncommunicable diseases in adulthood, but it is not clear whether or how some of the epigenetic changes discussed here operate in those populations. Similarly, research on intervention approaches has been largely confined to developed countries. A report by the WHO (46) compiled 261 obesity prevention interventions, of which only 13 were in developing countries.
On a more general level, it seems clear that most of the elements sustaining the current obesogenic environment are the result of major choices made by humans: our built environment, transportation, agricultural policy, global food commerce, etc. The adverse trends in many of these factors can only be reversed by a sustained social movement encompassing not only those of us working in the field of nutrition, but all other disciplines seeking to change the global conditions of the Anthropocene: environmentalists, urban planners, social activists, political leaders, and innovators in industry and technology. The social movement against nuclear weapons may be a good example of this kind of coalition. It involved not only nuclear scientists, but also legal scholars, pacifists, social activists, politicians, journalists, etc. That coalition has secured the pledge of >50 countries so far to never possess nuclear weapons and was awarded the Nobel Peace Prize in 2016. Creating such a movement may take a while, but it will certainly never happen if we don't start. In the broader area of diet and health, which has a strong overlap with obesity, there are a number of initiatives, involving governments, civil leaders, nongovernmental organizations, and the private sector, that are targeting the obesogenic ecosystem: urban planning to favor active outdoor lifestyles, promotion of public transportation, improving availability of local fresh produce, protecting children from unhealthy foods, etc. (47). These initiatives are critical not only for obesity prevention but for reducing chronic disease risk in general, and ultimately for improving quality of life.
Acknowledgments
The sole author had responsibility for all parts of the manuscript. The author acknowledges the support received over the years for obesity research from the National Institutes of Health (National Heart, Lung and Blood Institute), the World Health Organization/Pan American Health Organization, and the Center for a Livable Future at the Johns Hopkins Bloomberg School of Public Health.
Notes
Published in a supplement to Advances in Nutrition. Presented at the International Union of Nutritional Sciences (IUNS) 21st International Congress of Nutrition (ICN) held in Buenos Aires, Argentina, October 15-20, 2017. The International Union of Nutritional Sciences (IUNS) thanks Mead Johnson Nutrition and Herbalife Nutrition for generously providing grants to support the publication and distribution of the present supplement from the 21st International Union of Nutritional Sciences. The contents of this supplement are solely the responsibility of the authors and do not necessarily represent official views of the IUNS. The supplement coordinators were Angel Gil and Alfredo Martinez. The supplement coordinators had no conflicts of interest to disclose.
Author disclosures: BC, no conflicts of interest.Publication costs for this supplement were defrayed in part by the payment of page charges. This publication must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors or the publisher, Editor, or Editorial Board of Advances in Nutrition.
Abbreviations used: PA, physical activity; SES, socioeconomic status; T2D, type 2 diabetes.
References
- 1. GBD 2015 Obesity Collaborators Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 2017;377(1):13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. UNICEF, WHO , The World Bank Group Joint child malnutrition estimates—levels and trends (2017 edition). [Internet] [cited 2017 Dec 16]. Available from: http://www.who.int/nutgrowthdb/estimates2016/en/. [Google Scholar]
- 3. NCD Risk Factor Collaboration Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016;387(10026):1377–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization Global action plan for the prevention and control of noncommunicable diseases 2013–2020. Geneva, Switzerland: WHO; 2013. [Google Scholar]
- 5. World Health Organization Obesity—preventing and managing the global epidemic. Report of the WHO Consultation of Obesity Geneva, Switzerland: WHO; 1998. [PubMed] [Google Scholar]
- 6. Fogel RW, Costa DL. A theory of technophysio evolution, with some implications for forecasting population, health care costs, and pension costs. Demography 1997;34(1):49–66. [PubMed] [Google Scholar]
- 7. Fogel RW, Grotte N. Major findings from the changing body: health, nutrition, and human development in the Western world since 1700. J Econ Asymmetries 2011;8(2):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Centers for Disease Control Anthropometric reference data for children and adults: United States, 2011–2014. Vital and Health Statistics, Series 3, Number 39 Washington (DC): US Department of Health and Human Services; 2016. [Google Scholar]
- 9. Boyd-Orr J. The food problem. Scientific American 1950;183:5. [Google Scholar]
- 10. Hedges TR 3rd, Hirano M, Tucker K, Caballero B. Epidemic optic and peripheral neuropathy in Cuba: a unique geopolitical public health problem. Surv Ophthalmol 1997;41(4):341–53. [DOI] [PubMed] [Google Scholar]
- 11. Franco M, Ordunez P, Caballero B, Tapia Granados JA, Lazo M, Bernal JL, Guallar E, Cooper RS. Impact of energy intake, physical activity, and population-wide weight loss on cardiovascular disease and diabetes mortality in Cuba, 1980–2005. Am J Epidemiol 2007;166(12):1374–80. [DOI] [PubMed] [Google Scholar]
- 12. Franco M, Bilal U, Ordunez P, Benet M, Morejon A, Caballero B, Kennelly JF, Cooper RS. Population-wide weight loss and regain in relation to diabetes burden and cardiovascular mortality in Cuba 1980–2010: repeated cross sectional surveys and ecological comparison of secular trends. BMJ 2013;346:f1515. [DOI] [PubMed] [Google Scholar]
- 13. Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA 2005;293(15):1861–7. [DOI] [PubMed] [Google Scholar]
- 14. Wang Z, Peng Y, Dong B. Is body mass index associated with lowest mortality increasing over time? Int J Obes (Lond) 2017;41(8):1171–5. [DOI] [PubMed] [Google Scholar]
- 15. Global BMI Mortality Collaboration Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 2016;388(10046):776–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Read SH, Lewis SC, Halbesma N, Wild SH. Measuring the association between body mass index and all-cause mortality in the presence of missing data: analyses from the Scottish National Diabetes Register. Am J Epidemiol 2017;185(8):641–9. [DOI] [PubMed] [Google Scholar]
- 17. Yu E, Ley SH, Manson JE, Willett W, Satija A, Hu FB, Stokes A. Weight history and all-cause and cause-specific mortality in three prospective cohort studies. Ann Intern Med 2017;166(9):613–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Howell CR, Fontaine K, Ejima K, Ness KK, Cherrington A, Mehta T. Maximum lifetime body mass index and mortality in Mexican American adults: the National Health and Nutrition Examination Survey III (1988–1994) and NHANES 1999–2010. Prev Chronic Dis 2017;14:E67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. NIH-NHLBI Aim for a healthy weight [Internet]. [cited 2018 Jan 24]. Available from: https://www.nhlbi.nih.gov/health/educational/lose_wt/risk.htm.
- 20. Snook KR, Hansen AR, Duke CH, Finch KC, Hackney AA, Zhang J. Change in percentages of adults with overweight or obesity trying to lose weight, 1988–2014. JAMA 2017;317(9):971–3. [DOI] [PubMed] [Google Scholar]
- 21. Vidwans HB, Watve MG. How much variance in insulin resistance is explained by obesity? J Insul Resist 2017;2(1):5. [Google Scholar]
- 22. Owei I, Umekwe N, Provo C, Wan J, Dagogo-Jack S. Insulin-sensitive and insulin-resistant obese and non-obese phenotypes: role in prediction of incident pre-diabetes in a longitudinal biracial cohort. BMJ Open Diabetes Res Care 2017;5(1):e000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goodarzi MO. Genetics of obesity: what genetic association studies have taught us about the biology of obesity and its complications. Lancet Diabetes Endocrinol 2018;6(3):223–36. [DOI] [PubMed] [Google Scholar]
- 24. Wahl S, Drong A, Lehne B, Loh M, Scott WR, Kunze S, Tsai PC, Ried JS, Zhang W, Yang Y et al. . Epigenome-wide association study of body mass index, and the adverse outcomes of adiposity. Nature 2017;541(7635):81–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koolhaas CM, Dhana K, Schoufour JD, Ikram MA, Kavousi M, Franco OH. Impact of physical activity on the association of overweight and obesity with cardiovascular disease: the Rotterdam Study. Eur J Prev Cardiol 2017;24(9):934–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kilpelainen TO, Qi L, Brage S, Sharp SJ, Sonestedt E, Demerath E, Ahmad T, Mora S, Kaakinen M, Sandholt CH et al. . Physical activity attenuates the influence of FTO variants on obesity risk: a meta-analysis of 218,166 adults and 19,268 children. PLoS Med 2011;8(11):e1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Graff M, Scott RA, Justice AE, Young KL, Feitosa MF, Barata L, Winkler TW, Chu AY, Mahajan A, Hadley D et al. . Genome-wide physical activity interactions in adiposity―a meta-analysis of 200,452 adults. PLoS Genet 2017;13(4):e1006528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pajunen P, Kotronen A, Korpi-Hyovalti E, Keinanen-Kiukaanniemi S, Oksa H, Niskanen L, Saaristo T, Saltevo JT, Sundvall J, Vanhala M et al. . Metabolically healthy and unhealthy obesity phenotypes in the general population: the FIN-D2D Survey. BMC Public Health 2011;11:754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mechanick JI, Hurley DL, Garvey WT. Adiposity-based chronic disease as a new diagnostic term: the American Association of Clinical Endocrinologists and American College of Endocrinology position statement. Endocr Pract 2017;23(3):372–8. [DOI] [PubMed] [Google Scholar]
- 30. Hebebrand J, Holm JC, Woodward E, Baker JL, Blaak E, Durrer Schutz D, Farpour-Lambert NJ, Fruhbeck G, Halford JGC, Lissner L et al. . A proposal of the European Association for the Study of Obesity to improve the ICD-11 diagnostic criteria for obesity based on the three dimensions etiology, degree of adiposity and health risk. Obes Facts 2017;10(4):284–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alvaredo F, Chancel L, Piketty T, Saez E, Zucman G. World inequality report. World Inequality Lab; 2018. [cited 2018 Jul 30]. Available from: http://wir2018.wid.world.
- 32. Stringhini S, Carmeli C, Jokela M, Avendano M, Muennig P, Guida F, Ricceri F, d'Errico A, Barros H, Bochud M et al. . Socioeconomic status and the 25 × 25 risk factors as determinants of premature mortality: a multicohort study and meta-analysis of 1.7 million men and women. Lancet 2017;389(10075):1229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bonaccio M, Di Castelnuovo A, Pounis G, Costanzo S, Persichillo M, Cerletti C, Donati MB, de Gaetano G, Iacoviello L; Moli-sani Study Investigators High adherence to the Mediterranean diet is associated with cardiovascular protection in higher but not in lower socioeconomic groups: prospective findings from the Moli-sani study. Int J Epidemiol 2017;46(5):1478–87. [DOI] [PubMed] [Google Scholar]
- 34. Frohlich KL, Potvin L. Transcending the known in public health practice: the inequality paradox: the population approach and vulnerable populations. Am J Public Health 2008;98(2):216–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wikipedia The Anthropocene [Internet]. [cited 2018 Jan 30]. Available from: https://en.wikipedia.org/wiki/anthropocene.
- 36. The Anthropocene [Internet]. Available from: www.anthropocene.info.
- 37. McMichael AJ. The urban environment and health in a world of increasing globalization: issues for developing countries. Bull World Health Organ 2000;78(9):1117–26. [PMC free article] [PubMed] [Google Scholar]
- 38. Fraser B. Latin America's urbanisation is boosting obesity. Lancet 2005;365(9476):1995–6. [DOI] [PubMed] [Google Scholar]
- 39. UN Habitat UN Habitat III [Internet]. [cited 2017 Dec 27]. Available from: https://unhabitat.org/habitat-iii/.
- 40. Muller A, Bautze L. Agriculture and deforestation. The EU Common Agricultural Policy, soy, and deforestation: proposals for reform. Moreton in Marsh, UK: FERN; 2017. p. 57. [Google Scholar]
- 41. Lind L, Lind PM, Lejonklou MH, Dunder L, Bergman A, Guerrero-Bosagna C, Lampa E, Lee HK, Legler J, Nadal A et al. . Uppsala consensus statement on environmental contaminants and the global obesity epidemic. Environ Health Perspect 2016;124(5):A81–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. World Health Organization WHO Child Growth Standards. Geneva (Switzerland): World Health Organization; 2006. [Google Scholar]
- 43. Centers for Disease Control CDC Growth Charts for the United States. 2000. [cited 2018 Jul 29]. Available from: https://www.cdc.gov/growthcharts/cdc_charts.htm. [Google Scholar]
- 44. Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med 1997;337:869–73. [DOI] [PubMed] [Google Scholar]
- 45. Must A, Jacques PF, Dallal GE, Bajema CJ, Dietz WH. Long-term morbidity and mortality of overweight adolescents. N Engl J Med 1992;327:1350–5. [DOI] [PubMed] [Google Scholar]
- 46. World Health Organization Interventions on diet and physical activity: what works. Geneva: WHO; 2009. p. 42. [PubMed] [Google Scholar]
- 47. Perez-Escamilla R, Lutter CK, Rabadan-Diehl C, Rubinstein A, Calvillo A, Corvalan C, Batis C, Jacoby E, Vorkoper S, Kline L et al. . Prevention of childhood obesity and food policies in Latin America: from research to practice. Obes Rev 2017;18(Suppl 2):28–38. [DOI] [PubMed] [Google Scholar]


