ABSTRACT
Probiotics are living microorganisms that confer health benefits to the host when administered in adequate amounts; however, dead bacteria and their components can also exhibit probiotic properties. Bifidobacterium and strains of lactic acid bacteria are the most widely used bacteria that exhibit probiotic properties and are included in many functional foods and dietary supplements. Probiotics have been shown to prevent and ameliorate the course of digestive disorders such as acute, nosocomial, and antibiotic-associated diarrhea; allergic disorders such as atopic dermatitis (eczema) and allergic rhinitis in infants; and Clostridium difficile–associated diarrhea and some inflammatory bowel disorders in adults. In addition, probiotics may be of interest as coadjuvants in the treatment of metabolic disorders, including obesity, metabolic syndrome, nonalcoholic fatty liver disease, and type 2 diabetes. However, the mechanisms of action of probiotics, which are diverse, heterogeneous, and strain specific, have received little attention. Thus, the aim of the present work was to review the main mechanisms of action of probiotics, including colonization and normalization of perturbed intestinal microbial communities in children and adults; competitive exclusion of pathogens and bacteriocin production; modulation of fecal enzymatic activities associated with the metabolization of biliary salts and inactivation of carcinogens and other xenobiotics; production of short-chain and branched-chain fatty acids, which, in turn, have wide effects not only in the intestine but also in peripheral tissues via interactions with short-chain fatty acid receptors, modulating mainly tissue insulin sensitivity; cell adhesion and mucin production; modulation of the immune system, which results mainly in the differentiation of T-regulatory cells and upregulation of anti-inflammatory cytokines and growth factors, i.e., interleukin-10 and transforming growth factor; and interaction with the brain-gut axis by regulation of endocrine and neurologic functions. Further research to elucidate the precise molecular mechanisms of action of probiotics is warranted.
Keywords: bifidobacteria, lactic acid bacteria, lactobacilli, mechanism of action, probiotics, volatile fatty acids, brain-gut axis, immune system
Introduction
The term “probiotics” refers to microorganisms that confer health benefits to hosts when administered in adequate amounts (1–3). A true probiotic should preferably be of human origin, safe, and free of vectors that are able to transfer resistance to antibiotics and of pathogenicity or toxicity factors. In addition, a probiotic should have great capacity to survive under intestinal conditions (acidic pH, enzymes, biliary salts, etc.). Moreover, a probiotic should exhibit antagonism against pathogens and stimulation of the immune system and, ultimately, must have demonstrable beneficial effects on the host. Finally, maintenance of the activity, viability, and growth efficacy of the probiotic upon technologic treatment should be demonstrated (4, 5).
Ilya Ilyich Mechnikov (6) performed the first investigations on lactic acid–producing bacteria (LAB) and their health effects in humans, and results from these first investigations suggested that LAB ingestion improved host health. LAB are a heterogeneous group of microorganisms that are often present in the human gut, being introduced via the ingestion of fermented foods, such as yogurt and other fermented milk products, various cheeses, and fermented cured meat by-products. Strains of Bifidobacterium, Enterococcus, Lactobacillus, Saccharomyces boulardii, and Escherichia coli Nissle 1917 are the most widely used probiotic bacteria. However, other strains such as Lactococcus, Leuconostoc, Pediococcus, and Streptococcus are also used as probiotics (7–9).
In 2014, the International Scientific Association for Probiotics and Prebiotics stated that the development of metabolic by-products, dead microorganisms, or other microbe-based nonviable products has potential; however, these do not fall under the probiotic construct (3). Nevertheless, several studies have shown that dead bacteria and bacterial molecular components display probiotic properties (4, 5, 10). Currently, the term “postbiotic” refers to soluble components with biological activity that, could therefore be a safer alternative to the use of whole bacteria (11).
The effects of probiotics on host health have been reported in many articles, reviews, and systematic reviews (12, 13). These studies have documented the role of probiotics in the prevention of health problems, including digestive disorders such as diarrhea caused by infections (4), antibiotic-associated diarrhea (14), irritable bowel syndrome (IBS) (15), Clostridium difficile–associated diarrhea in adults and children (16), inflammatory bowel disease (IBD), only in ulcerative colitis (17), and allergic disorders such as atopic dermatitis (eczema) (18) and allergic rhinitis (19).
Even though many probiotic strains are well documented as safe or denoted “generally recognized as safe,” the European Food Safety Authority (EFSA) and the US FDA do not attribute the ability to prevent or treat diseases to probiotic administration. Probiotics are purchased as dietary supplements in many countries and follow current market policies.
The EFSA has not approved any product with health claims associated with probiotic administration. More than 300 approval requests have been submitted for 200 probiotic strains or combinations of strains, claiming >60 beneficial effects (20). The principal reasons for these approval requests being denied were as follows: insufficient characterization, undefined claims, nonbeneficial claims, lack of relevant human studies, lack of measurable outcomes that reflect direct benefit for humans, and finally, the quality of the presented studies (20). In addition, the FDA might regulate probiotic strains as a dietary supplement, food ingredient, or drug (21). Similarly to the EFSA, the FDA has not approved any probiotics to prevent or treat health problems (22). Both food agencies have emphasized the following notions: each health claim is unique for each probiotic strain; scientific requirements have to be considered in the context of each application; guidelines and past evaluations are valuable sources of information; it is important to understand the rationale behind the principles being applied; no recipe for success can be provided; and finally, researchers and companies need to try, fail, learn, and try again (20, 22). Moreover, many studies of probiotics lack insight into the potential mechanism of action.
However, Health Canada approves a multistrain probiotic [Streptococcus salivarius subsp. thermophilus (SD5207), Bifidobacterium breve (SD5206), Lactobacillus plantarum (SD5209), Lactobacillus paracasei (SD5218), Bifidobacterium animalis subsp. lactis (SD5220, SD5219), Lactobacillus acidophilus (SD5212), and Lactobacillus helveticus (SD5210)] and a single strain of B. animalis spp. lactis LAFTI B94 as natural health products for relief of IBS symptoms, such as abdominal discomfort, gas, and bloating (23).
Currently, it is accepted that gut dysbiosis refers to changes in the quantitative and qualitative composition of microbiota, that these changes may lead to altered host microbial interaction that can contribute to a disease state often with inflammation, and that this is associated with the development of many noncommunicable human diseases, but the mechanisms via which homeostasis is maintained are not yet completely understood (4, 24). Recent investigations have proposed that, during homeostasis, epithelial hypoxia limits oxygen availability in the colon, leading to the maintenance of a balanced microbiota that functions as a microbial organ, producing metabolites that contribute to host nutrition, immune training, and niche security (24, 25).
Probiotics are a current strategy to treat dysbiosis, restoring microbial diversity and altering the perturbed intestinal microbiota with specific mechanisms of action that have not been completely elucidated (26, 27). For this reason, we performed a literature review of the varied mechanisms of action of probiotics to understand the role of various strains in host homeostasis. A comprehensive search of the relevant literature was performed with the use of electronic databases, including MEDLINE (PubMed), EMBASE, and the Cochrane Library. MEDLINE through PubMed was searched for scientific articles in English through the use of the terms “probiotics” combined with “mechanism of action,” “competitive exclusion,” “volatile fatty acids,” “mucin,” “immune system,” and “brain-gut axis.” The following mechanisms have been reviewed: 1) colonization and normalization of perturbed intestinal microbial communities in children and adults; 2) competitive exclusion of pathogens and bacteriocin production; 3) enzymatic activity and production of volatile fatty acids; 4) cell adhesion, cell antagonism, and mucin production; 5) modulation of the immune system; and 6) interaction with the brain-gut axis.
Colonization and Normalization of Perturbed Intestinal Microbial Communities in Children and Adults
Children
Early colonization of the infant gastrointestinal tract is likely to be a key determinant in the establishment of the gut microbiome in later life (28). Assembly of the intestinal microbiota begins before childbirth and continues into childhood. Several factors influence initial intestinal colonization, such as the genetic constitution of the newborn, method of childbirth, use of antibiotics, type of feeding, and whether the mother is under stress or expresses an inflammatory condition (29). Bacteria isolated from the placenta, umbilical cord blood, and meconium (Enterococcus faecium, Propionibacterium acnes, Staphylococcus epidermidis, and Escherichia coli) are among those that might affect colonization (30, 31). However, the bacteria present in the vagina and in human milk seem to be more important for infant gut colonization (32, 33). These bacteria can spread from the digestive tract to extradigestive sites via dendritic cells (DCs), which can penetrate the epithelium and take the bacteria directly from the intestinal lumen. Once inside DCs or macrophages, the bacteria can be transported to other areas by immune cell circulation through the bloodstream (34). Adhesion of bacteria to host surfaces is a crucial aspect of host colonization because it prevents the mechanical clearing of pathogens. In addition to pili, which are polymeric hair-like organelles protruding from the surface of bacteria, and which represent a first class of structures involved in the binding of bacteria to host cells, a wide range of bacterial surface factors with adhesive properties have been described. These adhesins recognize various classes of host molecules including transmembrane proteins such as integrins or cadherins, or components of the extracellular matrix such as collagen, fibronectin, laminin, or elastin (35). Preclinical studies in children that used probiotics found positive results such as normalization of perturbed microbiota composition, intestinal maturation, decreased pathogenic load and infections, and improved immune response; however, only a few of these studies documented specific changes in the composition of the microbiota (13). In clinical studies in children, specific administered probiotic strains have shown promise in attenuation of the severity of different pathologies such as necrotizing enterocolitis (NEC), IBD, nosocomial and antibiotic-associated diarrhea, colic, and allergies (13, 36, 37).
Breastfeeding and formula feeding modify microbial succession in the gut in infants. Although commercially available formulas are supplemented with bacteria considered to be probiotics, little is known about the ability of these bacteria formulas to have a long-term impact on infant gut microbial composition and function (38). Specifically, changes in the composition of the gut microbiota have been observed to be directly correlated with increased concentrations of biomarkers of innate and acquired immunity after the use of a fermented milk product containing heat-killed cells of the probiotic strain L. paracasei CBAL74. Infants fed with this product showed higher amounts of Bacteroides and specific oligotypes of Roseburia, Faecalibacterium, and Blautia, which showed a positive correlation with secretory IgA (sIgA) and fecal defensin concentrations. In addition, an increase in the relative abundance of genes predicted to be involved in butyrate synthesis and higher fecal butyrate amounts associated with the consumption of this product have been described (39). On the other hand, infants exposed to bifidobacteria-supplemented formula showed slight differences, such as decreased occurrence of Bacteroides fragilis and Blautia spp., compared with infants fed a placebo. To confirm colonization of the supplemented bifidobacteria, authors performed strain-specific analysis, detecting Bifidobacterium bifidum, B. breve, and Bifidobacterium longum in month 4. At 2 y of age, the strains were no longer detectable, suggesting that the supplemented bifidobacteria failed to stably colonize the infant gut due to competition within the ecosystem over time. The authors established these time points to study colonization and found that long-term colonization was not shown (38). This lack of probiotic colonization at 24 mo might be a benefit of their use, because the organism can be depleted from the gut through the effects of colonization resistance (40). Moreover, the most significant differences in the composition of the microbiota and in metabolite concentrations have been found between breastfed infants and those fed formula and between infants birthed vaginally and those birthed by cesarean delivery (38).
It has been suggested that pathogenesis in severe NEC must be multifactorial and may involve an overactive response of the immune system, causing an insult that might be ischemic, infectious, related to the introduction of enteric feeds, or a response to the translocation of normal enteric bacteria. Prophylactic treatment with probiotics in premature newborns has been shown to reduce the risk of severe NEC. Probiotic preparations containing Lactobacillus alone or in combination with Bifidobacterium have led to decreased mortality, days of hospitalization, and days after which exclusive enteral nutrition is achieved (41, 42). There are no reports in the literature about mechanisms associated with these positive effects on health; nevertheless, this treatment could help control the outgrowth of pathogenic bacteria due to the immature immune system of premature neonates (42).
With regard to infant colic, there is evidence that the use of Lactobacillus reuteri improves crying spells, but only after 2–3 wk of treatment, even with the natural evolution of this disorder (43–45). Other bacterial strains (bacilli and bifidobacteria) also appear to have some beneficial effects in alleviating the symptoms of infant colic and can lead to changes in the composition of the gut microbiota. Lactobacillus rhamnosus GG consumption resulted in increased abundance of different Bifidobacterium species compared with the effect seen upon consumption of a placebo. In general, Bifidobacterium was associated with differences between infants suffering from colic and healthy controls; infants with colic tended to be less frequently colonized with B. breve than healthy infants at a baseline level and at the end of the study, despite intervention (46).
With respect to probiotics for the prevention of pediatric diarrhea and antibiotic-associated diarrhea, it has been described that probiotics can restore microbial balance and thus inhibit the proliferation of pathogens such as C. difficile, acting as both preventive and treatment; however, most of the studies mainly provided clinical effects and tolerance and safety data but did not provide potential mechanisms of action (16, 45, 47, 48).
The intestinal microbiota has been analyzed to determine the long-term effects of L. rhamnosus GG intake on antibiotic use by preschool children and on antibiotic-associated gastrointestinal complaints. This intervention increased the abundance of Prevotella, Lactococcus, and Ruminococcus and decreased the abundance of Escherichia, appearing to prevent some of the changes in the microbiota associated with penicillin use but not those associated with macrolide use and preventing certain bacterial infections for ≤3 y after the trial (49). Recently, in children with acute watery diarrhea randomly assigned to receive L. acidophilus or placebo, no differences were observed in the daily fecal concentrations of rotavirus and norovirus or in Lactobacillus colonization in both groups (50).
Lactose intolerance usually leads to diarrhea; the effect of L. acidophilus strain LBKV-3 on fecal residual lactase activity in undernourished children <10 y of age was tested. Lactase activity increased over the course of the treatment (51). Some probiotics promote lactose digestion in lactose intolerance through increasing the overall hydrolytic capacity in the small intestine and increasing the colonic fermentation (52), and they can decrease lactose concentration in fermented products, and also increase active lactase enzyme entering the small intestine with the fermented products (53, 54).
The microbiome of lactose-intolerant individuals is represented by Firmicutes, Bacteroidetes, Proteobacteria, Fusobacteria, Tenericutes, Elusimicrobia, Actinobacteria, Synergistetes, Cyanobacteria, and Lentisphaerae (55). There is some evidence suggesting the clinical potential of probiotics against lactose intolerance, and B. animalis has been established among the most well-researched and effective strains (54, 56, 57).
Cow-milk allergy (CMA) is mostly a disease of infancy and early childhood. The majority of affected children have ≥1 symptoms involving ≥1 organ systems, mainly the gastrointestinal tract and/or skin (58). Many infants develop symptoms in ≥2 organ systems. Typical IgE-mediated symptoms include urticaria, angioedema, vomiting, diarrhea, and anaphylaxis. Dermatitis and rhinitis can be IgE and non–IgE mediated. Vomiting, constipation, hemosiderosis, malabsorption, villous atrophy, eosinophilic proctocolitis, enterocolitis, and eosinophilic esophagitis are non–IgE-mediated reactions (59). Samples from subjects with CMA showed that Firmicutes and Clostridia were enriched in the infant gut microbiome of subjects whose milk allergy resolved by age 8 y, whereas Bacteroidetes and Enterobacter were characteristic of subjects whose milk allergy did not resolve by age 8 y (60). Specifically, there seems to be a link between dysbiosis in the composition of the intestinal microbiota and the pathogenesis of CMA. The administration of probiotics such as L. rhamnosus GG in an extensively hydrolyzed formula led to increased tolerance in infants with CMA compared with those treated with hydrolyzed formula alone, which was due in part to changes in the structure of the bacterial community in the intestines of the infants (61).
The use of probiotics in adult diseases
After the administration or consumption of probiotic strains, the process of colonization begins. Few studies have assessed this step, and most have only evaluated major outcomes and drawn associations between those results and microbial administration.
In healthy adults, probiotic administration increases the production of SCFAs (see below), fecal moisture, frequency of defecation, and volume of stools (62). Recorded gastrointestinal symptoms, defecation frequency, and stool consistency were not influenced by L. rhamnosus PRSF-L477, indicating that this bacterium was well tolerated. The detection of L. rhamnosus in the feces of subjects in the probiotic-treated group was an important issue (63). Tolerance to Lactobacillus salivarius CECT5713 was assessed in healthy adults; the strain was tolerated, and no adverse effects were detected, but no attempt was made to evaluate intestinal colonization by this strain (64). However, intestinal persistence was observed in volunteers who received L. rhamnosus CNCM I-4036, as detected through the use of a specific primer for qRT-PCR (65). The effects of probiotics go beyond health status; subjects living with overweight and obesity are good candidates to receive probiotic strains, as individual treatments or as multistrain preparations. De Simone formulation is a multistrain probiotic preparation that has been tested in subjects living with overweight. De Simone formulation administration reduced the concentrations of lipids and inflammatory markers such as high-sensitivity C-reactive protein, enhanced insulin sensitivity, and produced changes in the composition of the gut microbiota (66). In patients living with obesity and hypertension, L. plantarum TENSIA decreased BMI and blood pressure (67).
IBD is a term used to describe a group of systemic pathologies that affect the gastrointestinal tract. In these conditions, the function of the epithelial barrier is affected and is a main factor in the onset of the disease and in further complications (4). Alterations in the gut microbiota might be associated with the initiation and progression of IBD. Probiotic treatment trials in patients living with Crohn disease (CD) showed no remission effect; in contrast, probiotic consumption by patients with ulcerative colitis seems to be more effective in the remission of the pathology, especially upon treatment with De Simone formulation and a combination of Lactobacillus and prebiotics (68).
Butyrate-producing bacterial strains (Butyricicoccus pullicaecorum 25–3T, Butyricicoccus pullicaecorum 1.20, Faecalibacterium prausnitzii, and a mix of Butyricicoccus pullicaecorum 25–3T, Faecalibacterium prausnitzii, Roseburia hominis, Eubacterium hallii, and Anaerostipes caccae) were tested in patients with CD to evaluate mucus stimulation. All the assayed strains exhibited increased butyrate production and improved the integrity of the epithelial barrier (69).
With regard to C. difficile–associated diarrhea, moderate-quality evidence suggests that probiotic administration results in efficient alleviation of this condition (70).
Competitive Exclusion of Pathogens and Bacteriocin Production
Competitive exclusion refers to the situation in which 1 species of bacteria competes for receptor sites in the intestinal tract more vigorously than other species (71). The specific pathways and key regulatory mechanisms underlying these effects of probiotics are largely unknown. Reduction in luminal pH, competition for nutritional sources, and production of bacteriocin or bacteriocin-like substances are among the main proposed mechanisms for competitive exclusion of pathogens (72).
Most studies have focused on the reduction of human pathogens such as Salmonella typhi and E. coli (73). Hence, some probiotic metabolites appear to play a role in the modulation of diverse signaling and metabolic pathways in cells. Indeed, components of the probiotic metabolome (organic acids, bacteriocins, hydrogen peroxide, amines, etc.) have been reported to interact with multiple targets in some metabolic pathways that regulate cellular proliferation, differentiation, apoptosis, inflammation, angiogenesis, and metastasis (74).
Some lactobacilli and bifidobacteria can produce antimicrobial peptides known as bacteriocins, which prevent the proliferation of selected pathogens. The term “colonization resistance” refers to the use of probiotics to prevent or treat enteric pathogens (71). Bacteriocins are small cationic molecules composed of ∼30–60 amino acids. These molecules act at bacterial cytoplasmic membranes and target energized membrane vesicles to disrupt the proton-motive force (75). Bacteriocins are classified into 4 main types based on their primary structures, molecular weights, post-translational modifications, and genetic characteristics (76). In particular, some of these compounds produced by L. plantarum and L. acidophilus have been shown to inhibit the growth of Helicobacter, C. difficile, rotaviruses, and multidrug-resistant Shigella spp. and E. coli in some gastrointestinal conditions (77) and have activity against a number of uropathogens (76).
Enzymatic Activities and Production of Volatile Fatty Acids
Enzymatic activities
The enzymatic activities of probiotics in the gut lumen can play a role in the biological effects of these probiotics. Lactobacilli and bifidobacteria exhibit >20 different enzymatic activities, with β-galactosidase activity being the most typical.
Intestinal bacterial β-glucuronidase hydrolyzes glucuronidated metabolites to their toxic forms in intestines, resulting in intestinal damage. In addition, low β-glucuronidase activity in fecal material has been linked to an increase in the amounts of substances such as carcinogens in the colonic lumen (78). B. longum, when added to the diet, contributes to changes in the intestinal microbiota, lowering the activity of β-glucuronidase, which is associated with the inhibition of aberrant crypt formation and is an early preneoplastic marker of malignant potential in the process of colon carcinogenesis (79). Moreover, in a systematic review of randomized clinical trials (RCTs) testing probiotics, prebiotics, or both (synbiotics) for the treatment of nonalcoholic fatty liver disease (NAFLD) in adult patients, a reduction in liver aminotransferase activity was documented (80).
In an RCT involving 30 healthy adults to evaluate the effects of a fermented product containing 2 probiotic strains (Lactobacillus gasseri CECT5714 and Lactobacillus coryniformis CECT5711), compared with standard yogurt, on host intestinal function, 19 enzymatic activities were detected in the feces of volunteers. The pattern of enzymatic activity exhibited by the control and the probiotic-treated groups was very stable throughout the study. However, the naphthol-AS-BI-phosphohydrolase activity, a typical feature of lactobacilli, was augmented in the feces of the probiotic-treated group. In addition, the leucine arylamidase activity, which is characteristic of probiotic strains, also increased, whereas the β-glucuronidase activity exhibited a decreasing trend (62).
Probiotics interact with bile acids in the gut lumen, modifying bile acid metabolism and in turn influencing cholesterol absorption. Bile salt hydrolase (BSH) is an enzyme produced by bacterial species of several genera associated with the gastrointestinal tract and by most of the known probiotics; this enzyme may participate in the first reaction of the deconjugation of biliary salts (81). Considering these beneficial effects of BSH-containing bacteria, BSH activity has been included in FAO/WHO guidelines for the evaluation of probiotics for food use (1). Enzymatic deconjugation of bile acids by BSH from probiotics has been considered to be one of the main mechanisms of the hypocholesterolemic effect attributed to probiotics (81, 82).
Volatile fatty acids
In an RCT with adult volunteers, the treatment group that received L. gasseri CECT5714 and L. coryniformis CECT5711 exhibited increased production of fecal butyrate compared with a group who received yogurt. Similarly, production of propionic and acetic acid was higher in the probiotic-treated group after 2 wk of treatment. At the end of the washout period, the production of butyrate in the probiotic-treated group was still higher than that in the control group (62, 83).
Another RCT study, conducted to determine the impact after 4 wk of daily consumption of a capsule containing ≥24 × 109 viable L. paracasei DG on the intestinal microbial ecology of healthy volunteers, reported that participants with a butyrate concentration of >100 mmol/kg of wet feces had a butyrate reduction of 49% ± 21% (mean ± SD) and a concomitant decrease in the total abundance of 6 genera of Clostridiales, namely, Faecalibacterium, Blautia, Anaerostipes, Pseudobutyrivibrio, Clostridium, and Butyrivibrio, after the probiotic intervention. However, in subjects with initial butyrate concentrations of <25 mmol/kg of wet feces, the probiotic contributed to a very high increase in butyrate concentrations concomitantly with a ∼55% decrease in Ruminococcus abundance and a 150% increase in an abundantly represented unclassified Bacteroidales genus. Therefore, the authors concluded that the impact of the intake of L. paracasei DG on the microbiota and on SCFAs seems to depend on the initial characteristics of the intestinal microbial ecosystem, and specifically, fecal butyrate content might represent an important biomarker for identifying subjects who may benefit from probiotic treatment (84).
Another RCT study recruited 33 healthy subjects, including young (mean age of 26 y), middle-aged (mean age of 51 y), and elderly (mean age of 76 y) volunteers, who were given a single daily oral dose of L. plantarum Lp-8. The concentrations of both acetate and propionate, but not butyrate, increased significantly and peaked at week 5 in all 3 age groups. After Lp-8 consumption was terminated, the concentrations of both acetate and propionate gradually decreased but remained higher than the baseline concentrations (85). Hence, the production of fecal butyrate by different probiotics appears to strictly depend on the specific bacteria used.
Worthley et al. (86) carried out a 4-wk crossover RCT of resistant starch and Bifidobacterium lactis, either alone or as a combined synbiotic preparation, in 20 human volunteers. This synbiotic supplementation at the doses used induced unique changes in the fecal microbiota but did not significantly alter any other fecal, serum, or epithelial variables: for example, fecal SCFA concentrations were unchanged from the baseline. In contrast, a 4-wk crossover RCT carried out in 43 older volunteers that used a synbiotic comprising the probiotic B. longum and an inulin-based prebiotic reported increased production of acetate, succinate, butyrate, and isobutyrate compared with the placebo-treated group at the end of the treatment. Thus, short-term synbiotic use could be effective in improving the metabolic activity of the colonic bacterial microbiota in older people (83). Osmotic diarrhea and antibiotic-associated diarrhea are significant problems in patients receiving total enteral nutrition, particularly elderly patients, because enteral feeding may change the intestinal microbiota and SCFA composition (87).
The results of an RCT showed that short-term treatment (∼6 d) with the probiotic yeast S. boulardii may decrease the incidence of diarrhea in patients receiving total enteral nutrition. Fecal butyrate concentrations were lower in patients than in controls, and treatment with S. boulardii increased the total fecal SCFA concentrations in the patients. At the end of the treatment with S. boulardii, the patients had higher fecal and total SCFA concentrations, which remained high 9 d after treatment was discontinued. Thus, the increase in fecal SCFA concentrations, particularly butyrate, may contribute to explaining the preventive effects of S. boulardii on total enteral nutrition–induced diarrhea (88).
L. plantarum 299v has been shown to enhance the concentrations of fecal SCFAs in patients with recurrent C. difficile–associated diarrhea, contributing to reducing the adverse effects of antibiotics. In fact, after consumption of metronidazole, a significant decrease in total SCFA concentrations was observed in the placebo-treated group but not in the probiotic-treated group. Moreover, the concentration of fecal butyrate was higher in the Lactobacillus-treated group than in the placebo-treated group. At the end of the study and after cessation of placebo or probiotic treatment, the total SCFA concentrations were restored to the pre–antibiotic-treatment levels in the placebo-treated group (89).
Nagata et al. (90) conducted an RCT in 77 elderly people (mean age of 84 y) at a long-stay health service facility. The study evaluated the effect of the intake of probiotic-fermented milk containing Lactobacillus casei strain Shirota on norovirus gastroenteritis, which occurs in the winter season, during the intake period. While the duration of norovirus-gastroenteritis–related processes decreased, Bifidobacterium and Lactobacillus were found to be the dominant genera; the abundance of Enterobacteriaceae decreased in the fecal samples of the probiotic-treated group; and a significant increase in fecal acetic acid concentration was observed. In a more recent RCT carried out by Nagata et al. (91) with elderly residents who randomly received either L. casei Shirota or a placebo beverage once daily for 6 mo, the counts of C. difficile were significantly lower and the fecal acetic acid concentration and total acidity were significantly higher in the L. casei Shirota–treated group, and these results were associated with significantly lower incidence of fever and improved bowel movements.
Changes in fecal SCFA or branched-chain fatty acid (BCFA) concentrations can partially explain the effect of probiotics and the role of probiotics in the nutritional status of, and risk of diarrhea in, children. L. paracasei Lpc-37 or B. lactis HN019 consumption by 2- to 5-y-old children was found to reduce the risk of diarrhea and was associated with higher concentrations of selected SCFAs and BCFAs in subjects who had experienced diarrhea. The concentrations of SCFAs, namely, acetate, propionate, and butyrate, were found to correlate with each other. Likewise, the concentrations of the BCFAs isobutyrate, 2-methylbutyrate, and isovalerate also correlated with each other. After this intervention, L. paracasei Lpc-37 abundance correlated positively with total Bifidobacterium counts and isovalerate concentrations. B. lactis HN019 counts were found to correlate positively with total bacterial counts and negatively with propionate concentrations (92).
Riezzo et al. (93), in an RCT, evaluated the effects of probiotic-enriched artichokes, compared with ordinary artichokes, on SCFA patterns in constipated subjects. Each patient consumed 180 g of ordinary artichokes/d or artichokes/d enriched with L. paracasei IMPC 2.1 for 15 d. Propionic acid concentrations were higher than baseline in the probiotic-treated group, and this result was associated with a lower constipation score (93).
The addition of prebiotics or probiotics to infant formula to improve the intestinal microbiota of formula-fed infants is a matter of great interest for consumers and stakeholders and for the food industry.
An RCT evaluated the effects of an infant formula containing L. salivarius CECT5713 compared with a control standard formula over 6 mo. Consumption of the probiotic formula led to an increase in the fecal lactobacilli content and the fecal concentration of butyric acid over 6 mo (94).
Abnormal colonization of low-birth-weight infants usually occurs because of a number of reasons, including cesarean delivery, prolonged hospital stay, and immature intestines, and can have adverse effects on health. An RCT evaluated the oral application of a probiotic, usually called B. lactis Bb12 (true name B. animalis), on selected indicators of health status in preterm infants. The fecal pH in the probiotic-treated group was significantly lower than that in the placebo-treated group, and the fecal concentrations of acetate and lactate were 42% and 38% higher in the probiotic-treated group than in the placebo-treated group, respectively (95). The lower fecal pH in feces of infants, as seen in breastfed infants, is associated with a lower incidence of diarrhea. Another RCT compared the effect of 2 prebiotic/probiotic products on the weight gain, stool microbiota, and stool SCFA content of premature infants. Even the bifidobacteria content was higher in the infants who were fed the probiotic formulas, and significant differences in fecal SCFA content were detected between the groups (96).
Based on the roles of probiotics in the modulation of the immune system (see below), various studies have investigated the potential effects of probiotics in the prevention of childhood eczema and allergies. Probiotic supplementation (B. bifidum W23, B. animalis subsp. lactis W52, and Lactococcus lactis W58) resulted in fewer children developing eczema at the age of 3 mo compared with the controls. In addition, the probiotic-treated group exhibited higher concentrations of lactate and SCFAs (acetate, butyrate, propionate, and isobutyrate) and lower concentrations of lactose and succinate than the group who had received the placebo. Moreover, lower concentrations of SCFAs, succinate, phenylalanine, and alanine were detected in fecal samples of the children who later developed eczema, whereas the amounts of glucose, galactose, lactate, and lactose were higher than those in the children who did not develop eczema (78). These results emphasize the roles that SCFAs and other probiotic metabolites may play in the regulation of the immune system.
Extensively hydrolyzed casein formula (eHCF) represents an elective treatment for infants diagnosed with CMA, and supplementation with probiotics, particularly L. rhamnosus GG (eHCF + LGG), seems to accelerate antigenic tolerance (61). Regardless of changes in the gut microbial communities, after treatment with eHCF, most tolerant infants showed a significant increase in fecal butyrate concentrations, which was associated with enrichment of Blautia and Roseburia species (61). Thus, eHCF + LGG treatment seems to promote tolerance in infants with CMA by influencing the bacterial community structure and the capacity to produce SCFAs, mainly butyrate.
Both human and experimental obesity is associated with changes in the intestinal microbiota, as characterized by relatively lower abundances of Firmicutes and higher abundances of Bacteroidetes. In addition, some obesity-associated comorbidities, namely type 2 diabetes (T2D) and NAFLD, also exhibit perturbation of the intestinal microbiota. In these conditions, both probiotics and synbiotics may provide beneficial health effects because these treatments can influence the intestinal microbial ecology and immunity. A recent article by our group reviewed the effects of probiotics and synbiotics on obesity, insulin resistance syndrome, T2D, and NAFLD in a human RCT (97). Selected probiotics and synbiotics exhibited beneficial effects in patients with obesity, mainly affecting the BMI and fat mass. Some probiotics had beneficial effects on insulin resistance syndrome, decreasing the concentrations of some biomarkers of cardiovascular disease. Moreover, selected probiotics improved the carbohydrate metabolism, fasting blood glucose concentrations, insulin sensitivity, and antioxidant status and reduced the metabolic stress in subjects with T2D. Some probiotics and synbiotics also improved the liver and metabolic markers in patients with NAFLD (97).
An RCT has evaluated the effects of L. salivarius Ls-33 on the fecal microbiota of obese adolescents over 12 wk. The ratio of bacteria of the Bacteroides-Prevotella-Porphyromonas group to Firmicutes, including Clostridium cluster XIV, Blautia coccoides, Eubacteria rectale, and R. intestinalis, was significantly increased after Ls-33 administration. However, the abundances of the Lactobacillus group and Bifidobacterium were not significantly altered by the intervention, and similarly, the SCFA concentrations remained unaffected (98).
De Simone formulation is a high-concentration probiotic preparation of 8 live freeze-dried bacterial species that are normal components of the human gastrointestinal microbiota, including 4 strains of lactobacilli (L. casei, L. plantarum, L. acidophilus, and L. delbrueckii subsp. bulgaricus), 3 strains of bifidobacteria (B. longum, B. breve, and B. infantis), and S. salivarius subsp. thermophilus. De Simone formulation treatment induced changes in the NAFLD urinary metabolic phenotype; these changes occurred primarily at the level of host amino-acid metabolism (i.e., valine, tyrosine, 3-amino-isobutyrate, or β-aminoisobutyric acid), nucleic acid degradation (pseudouridine), creatinine metabolism (methylguanidine), and also at the level of gut microbial amino-acid metabolism (i.e., 2-hydroxyisobutyrate from valine degradation). Furthermore, the concentrations of some of these metabolites correlated with clinical primary and secondary trial endpoints after De Simone formulation treatment, particularly alanine aminotransferase and active glucagon-like peptide 1 (99). Thus, in addition to the beneficial effects of some probiotics in lowering the concentrations of liver lipids (100), the induced changes in fecal metabolite concentrations may play an important role in the pathogenesis of NAFLD, and some of these metabolites may be considered as noninvasive effective biomarkers to evaluate the response to treatment (99).
Consistent with in vitro and in vivo data, SCFAs have been reported to have numerous physiologic, biochemical, and molecular effects in many tissues, including intestine, liver, adipose, muscle, and brain tissues (101). It has been proposed that acetate produced by bifidobacteria can improve the intestinal defense mediated by epithelial cells and can protect the host against lethal infection. Thus, genes encoding an ATP-binding-cassette–type carbohydrate transporter present in certain bifidobacteria contribute to protecting mice against death induced by E. coli O157:H7, and this effect can be attributed, at least in part, to increased production of acetate and to translocation of the E. coli O157:H7 (102).
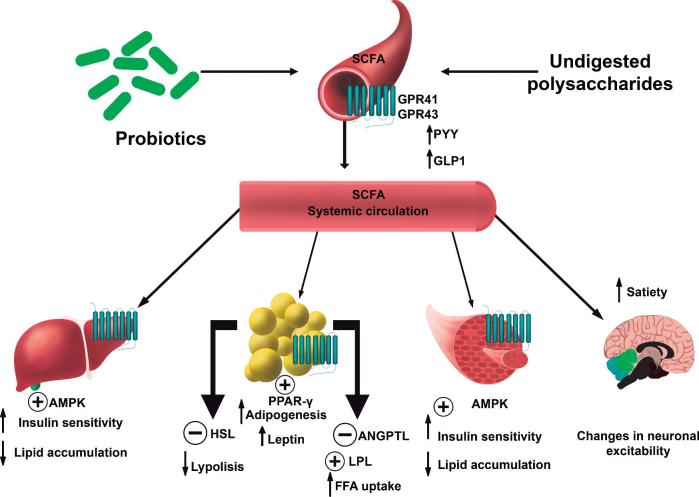
SCFAs are an important source of energy for enterocytes and are key signaling molecules for the maintenance of gut health. In addition, SCFAs can enter the systemic circulation and interact with cell receptors in peripheral tissues. In fact, SCFAs have an important role in the regulation of energy homeostasis and metabolism. Increasing evidence, mainly derived from animal and in vitro studies, has suggested a role for SCFAs in the prevention and treatment of obesity and obesity-related disorders in glucose metabolism and insulin resistance (101). SCFAs can interact with the SCFA receptors G protein–coupled receptor (GPR) 41 and GPR43, leading to an increase in the intestinal secretion of polypeptide YY and glucagon-like peptide 1, respectively, which, in turn, can enhance satiety (101, 103). Moreover, SCFAs might reach the adipose tissue and contribute to decreasing fat accumulation by interacting with GPR43, which would result in decreased lipolysis and inflammation and increased adipogenesis and leptin release. Propionate can increase free fatty acid uptake, possibly by affecting the lipoprotein lipase inhibitor angiopoietin-like 4. Acetate and propionate might also attenuate intracellular lipolysis via decreased hormone-sensitive lipase phosphorylation by interacting with the SCFA receptor GPR43. Similarly, acetate, propionate, and butyrate might increase peroxisome proliferator-activated receptor (PPAR)-γ–mediated adipogenesis, which is possibly regulated by a GPR43-related mechanism. In addition, it has been proposed that acetate, propionate, and butyrate, especially the latter 2, could reduce the secretion of proinflammatory cytokines and chemokines, likely by reducing local macrophage infiltration (101). Furthermore, SCFAs seem to activate AMP kinase in muscles, increasing insulin sensitivity and fatty acid oxidation and decreasing lipid accumulation (101). Figure 1 summarizes the potential biological effects of SCFAs in humans.
FIGURE 1.
Potential biological effects of SCFAs in humans. AMPK, AMP kinase; ANGPTL, angiopoietin-like; GLP1, glucagon-like peptide 1; GPR, G protein–coupled receptor; HSL, hormone-sensitive lipase; LPL, lipoprotein lipase; PPAR-γ, peroxisome proliferator-activated receptor-γ; PYY, polypeptide YY.
Other miscellaneous biological activities of SCFAs might be attributed to probiotics as a result of epigenetic alterations, which may explain the wide range of anticarcinogenic effects attributed to probiotics (104). However, further study is needed in this area, particularly in humans.
Cell Adhesion and Mucin Production
When a microbial strain is indicated to be a probiotic, there are some specific prerequisites that need to be addressed. One of them is adhesion to the intestinal mucosa for colonization and further interaction between the administered probiotic strains and the host (71). This specific interaction is required for the modulation of the antagonism against pathogens and for actions in the immune system (4, 105).
Intestinal epithelial cells secrete mucin to avoid the adhesion of pathogenic bacteria (72). Several Lactobacillus proteins have been shown to promote this adhesion (106), exhibiting surface adhesins that facilitate attachment to the mucous layer (107).
Over the last 30 y, the Caco-2 cell line has been extensively used to determine the adhesion capacity of probiotics in vitro (108). These cells form a homogeneous monolayer that resembles that of mature enterocytes in the small intestines of humans (109) and form crypts, which are typical structures of the epithelial monolayer (7, 110).
L. rhamnosus ATCC 7469 was tested in the presence of an F4-expressing E. coli strain (serotype O149: K91, K88ac) in intestinal porcine epithelial J2 cells. The expression of Toll-like receptor (TLR)-4 and nucleotide-binding oligomerization-domain–containing protein (NOD) 2 (NOD2) was augmented by the presence of E. coli, and these increases were attenuated by L. rhamnosus treatment (111). Pretreatment with L. rhamnosus enhanced Akt phosphorylation and increased zonula occludens-1 and occludin protein expression. The probiotic maintained the epithelial barrier and promoted intestinal epithelial cell activation in response to bacterial infection (111).
In another study, the effects of 3 L. plantarum strains were evaluated on in vivo small intestinal barrier function and gut mucosal gene transcription in human subjects. L. plantarum TIFN101 modulated gene transcription pathways; notably, this probiotic upregulated the matrix metalloproteinase 2, tissue inhibitors of metalloproteinase 1 and 3, and muc2 genes and downregulated genes involved in the tricarboxylic acid cycle II pathway (112).
Modulation of the Immune System
The gut microbiota modulates the immune system via the production of molecules with immunomodulatory and anti-inflammatory functions that are capable of stimulating immune cells. These immunomodulatory effects are due to the interaction of probiotic bacteria with epithelial cells and DCs and with monocytes/macrophages and lymphocytes (113). One of the major mechanisms of action of probiotics is the regulation of host immune response. Thus, the immune system is divided into the innate and adaptive systems. The adaptive immune response depends on B and T lymphocytes, which bind to specific antigens. In contrast, the innate system responds to common structures, called pathogen-associated molecular patterns (PAMPs), shared by a majority of pathogens. The primary response to pathogens is produced by pattern recognition receptors (PRRs), which bind to PAMPs. Consequently, PRRs comprise TLRs, which are transmembrane proteins that are expressed on various immune and nonimmune cells, such as B-cells, natural killer cells, DCs, macrophages, fibroblast cells, epithelial cells, and endothelial cells. Furthermore, PRRs comprise nucleotide-binding oligomerization domains, adhesion molecules, and lectins (114). In addition to TLRs, PRRs include NOD-like intracellular receptors (NODLRs), which guard the cytoplasmic space (115). Other PRRs have also been described, such as C-type lectin receptors, formylated peptide receptors, retinoic acid inducible–like helicases, and intracellular IL-1–converting enzyme protease-activating factor (116). In general, the T cell subset, which is involved in regulating the immune balance, is finely tuned by the host and the microbes with which the host interacts, and disequilibrium between the effector T-helper (Th) cells and regulatory T cells (T-regs) leads to impaired immune response (117). Probiotics help to preserve intestinal homeostasis by modulating the immune response and inducing the development of T-regs (118).
Modulation of sIgA and cytokine production
sIgA is secreted by intestinal B cells and is expressed on the basolateral surface of the intestinal epithelium as an antibody transporter. sIgA facilitates the translocation of IgA dimers to the luminal surfaces of epithelial cells. Several studies have reported that probiotics show potent stimulation of the production of sIgA, thereby enhancing barrier function (119). Regardless, probiotics interact with intestinal and specific immune cells, which results in the production of selected cytokines. Thus, L. salivarius CECT5713 consumption augmented the percentages of NK cells and monocytes as well as the plasmatic concentrations of immunoglobulins M, A, and G and IL-10 in healthy adult volunteers (64). In addition, L. casei Shirota increased the expression of the CD69 activation marker on circulating T cells and NK cells and induced an increase in mucosal salivary IFN-γ, IgA1, and IgA2 concentrations in healthy adults (120). In addition, administration of B. breve CNCM I-4035 resulted in a significant increase in fecal sIgA content; the plasmatic concentrations of IL-4 and IL-10 also increased, whereas the concentrations of IL-12 decreased, in the sera of volunteers treated with this strain. Similar results have been obtained with 2 other probiotic strains, L. rhamnosus and L. casei (65).
Recently, a probiotic strain (E. faecium AL41) was proposed to be effective against Campylobacter jejuni infection in chickens. E. faecium modulates the expression of transforming growth factor (TGF)-β4 but downregulates the relative expression of IL-17 and activates IgA-producing cells in the caeca of chicks infected with C. jejuni (121).
In mice, treatment with L. rhamnosus RC007 for 10 d increased the phagocytic activity of peritoneal macrophages and the number of IgA cells in the lamina propria of the small intestine. Consequently, higher concentrations of monocyte chemoattractant protein 1 (MCP-1), IL-10, and TNF-α were observed, and the ratio of anti- to proinflammatory cytokines (IL-10/TNF-α) in the intestinal fluid increased after L. rhamnosus RC007 treatment (122). Another Lactobacillus strain, L. plantarum 06CC2, is capable of increasing the concentration of IL-12 in co-culture with J774.1 cells, and oral administration induced Th1 cytokine production, activating the Th1 immune response associated with intestinal immunity in normal mice (123). Aktas et al. (124) investigated 7 different L. casei strains for their ability to alter the murine gut microbiota. They observed that L. casei species are capable of modulating the host gut microbiota and the host immune system because there is a relation between the ability of a strain to alter the composition of the gut microbiota, PRR regulation, and antimicrobial peptide regulation (124).
In addition, bifidobacteria strains also modulate the immune system. Accordingly, B. longum subsp. infantis 35624 is a probiotic with immunoregulatory effects, and it has been described that the consumption of B. longum subsp. infantis 35624 resulted in the induction of T-regs and attenuation of nuclear transcription factor κB (NF-κB) activation, preventing the excessive inflammation induced by Salmonella infection in mice. Induction of T-regs by the strain has also been shown in humans, and reduction of systemic proinflammatory biomarkers has been seen in patients with psoriasis, IBS, chronic fatigue syndrome, or ulcerative colitis (125, 126). B. breve C50 releases soluble factors that alleviate the secretion of proinflammatory cytokines by immune cells. Thus, a study carried out by Heuvelin et al. (127) elucidated that B. breve C50 and the soluble factors secreted by this bacterium contribute positively to intestinal homeostasis by attenuating chemokine production, such as CXCL8 secretion by epithelial cells driven by Jun proto-oncogene, AP-1 transcription factor subunit and IκB-α and decreased phosphorylation of p38-MAPK and IκB-α molecules (127).
A probiotic mixture, De Simone formulation, induces NF-κB nuclear translocation in epithelial cells, which is followed by the release of TNF-α, and this effect correlates with reduced epithelial permeability and susceptibility to CD-like ileitis in SAMP1/YitFc mice that spontaneously develop the disease. It has been recently shown that TNF-α can stimulate epithelial cell proliferation, and this stimulation occurs only when TNF-α induces epithelial cell apoptosis in combination with IFN-γ. Hence, it is possible that probiotics can participate in epithelial barrier regeneration by upregulating TNF-α (118, 128). A number of selected species of lactobacilli and bifidobacteria have been demonstrated to be prominent probiotics with anti-inflammatory properties, suppressing proinflammatory responses by increasing the concentrations of IL-10 and Th1-type cytokines. Kwon et al. (129) identified a mixture of probiotics that upregulates CD4+forkhead box P3 (FoxP3)+ T-regs. Thus, administration of the probiotic mixture induced both T cell and B cell hyporesponsiveness and downregulated Th1, Th2, and Th17 cytokines without inducing apoptosis. The probiotic mixture also induced the production of CD4+FoxP3+ T-regs from the CD4+CD25− population and increased the suppressor activity of naturally occurring CD4+CD25+ T-regs. Conversion of T-cells to FoxP3+ T-regs is directly mediated by regulatory DCs that express high levels of IL-10, TGF-β, cyclooxygenase (COX)-2, and indoleamine 2,3-dioxygenase. Administration of the probiotic mixture had therapeutic effects in experimental treatments of IBD, atopic dermatitis, and rheumatoid arthritis. Overall, administration of probiotics that enhance the generation of regulatory DCs and T-regs represents an applicable treatment for inflammatory immune disorders (129).
Interaction of probiotics with TLRs and cell cascade signaling
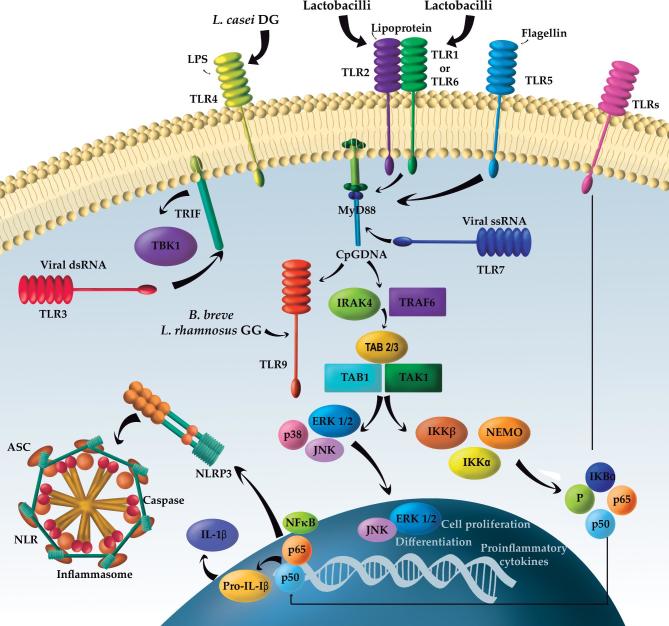
TLRs are a family of evolutionarily conserved PRRs that recognize a wide range of microbial components. In mammals, the TLR family includes 11 proteins (TLR1–TLR11), and the activation of TLRs occurs after the binding of the ligand to extracellular leucine-rich repeats. In humans, TLR1, TLR2, TLR4, TLR5, TLR6, and TLR10 are associated with the outer membrane and primarily respond to bacterial surface–associated PAMPs. TLR3, TLR7, TLR8, and TLR9 are found on the surfaces of endosomes, where they respond primarily to nucleic-acid–based PAMPs from viruses and bacteria. The TLR signaling pathway, with the exception of TLR3, involves the recruitment of myeloid differentiation primary response 88, which activates the MAPK and NF-κB signaling pathways. TLR3 utilizes the adaptor protein TIR-domain-containing adapter-inducing IFN-β, leading to the expression of type 1 IFNs. TLR-mediated signaling has been shown to control DC maturation. TLR9 signaling is essential for the mediation of the anti-inflammatory effect of probiotics (71, 114). Figure 2 summarizes the interaction of probiotics with TLRs.
FIGURE 2.
Main effects of probiotics on the immune system. ASC, apoptosis-associated Speck-like protein containing a CARD; B. breve, Bifidobacterium breve; CpGDNA, Cytosine-phosphate-guanosine DNA; dsRNA, Double strand DNA, ERK, extracellular regulated kinase; IKK, IκB kinase; IRAK4, IL-1 receptor-associated kinase 4; JNK, Jun N-terminal kinase; L. casei, Lactobacillus casei; L. rhamnosus, Lactobacillus rhamnosus; MyD88, myeloid differentiation primary response 88; NEMO, NF-κB essential modulator; NF-κB, nuclear transcription factor; NLR, nucleotide-binding oligomerization domain-like receptors, in short NOD-like receptors; NLRP3, NLR family pyrin domain containing 3; P, Phosphate; ssRNA, TAB1/2/3, TAK binding proteins; TAK1, ubiquitin-dependent kinase of putative mitogen-activated protein kinase (MKK) and IKK; TBK1, serine/threonine-protein kinase 1; TLR, Toll-like receptor; TRAF6, tumor necrosis factor receptor–associated factor 6; TRIF, TIR-domain-containing adapter-inducing interferon-β; Viral ssRNA: Viral single strand DNA.
Probiotics are capable of suppressing intestinal inflammation via the downregulation of TLR expression, secretion of metabolites that may inhibit TNF-α from entering blood mononuclear cells, and inhibition of NF-κB signaling in enterocytes (130). In this sense, signaling by cell wall components of lactobacilli can potentially occur via the binding of TLR2 and TLR6, stimulating cytokine production. In addition, TLR2 recognizes peptidoglycan, which is the main component of gram-positive bacteria, including those of the Lactobacillus genus. L. casei 431 interacts with epithelial cells via TLR2, and the interaction between L. casei and gut-associated immune cells induces an increase in the number of CD-206 and TLR2 receptors (131). Indeed, several strains, such as L. plantarum CCFM634, L. plantarum CCFM734, L. fermentum CCFM381, L. acidophilus CCFM137, and S. thermophilus CCFM218, stimulate TLR2/TLR6, and these interactions between PRRs such as TLRs are strain specific. Thus, TLR2/TLR6 signaling is essential in immune regulatory processes (132). In addition, Shida et al. (133) showed that L. casei induces a high amount of IL-12 production in both wild-type and TLR2-deficient macrophages and that peptidoglycan induces low amounts of IL-12 production in wild-type macrophages and even lower amounts in TLR2-deficient macrophages (133). Moreover, L. rhamnosus GG and L. plantarum BFE 1685 enhance TLR2 activity in human intestinal cells, and L. casei CRL 431 has similar effects in mice infected with Salmonella enterica serovar typhimurium (134, 135). Furthermore, L. plantarum was shown to activate TLR2 signaling, and subsequently, protein kinase C-α and -δ activation has also been implicated in tight-junction modulation and epithelial permeability (136).
With regard to lactobacilli, L. casei DG and its postbiotic modulate the inflammatory/immune response in postinfection IBS in an ex vivo organ culture model. Thus, IL-1α, IL-6, and IL-8 mRNA levels and TLR4 protein expression were significantly higher, whereas IL-10 mRNA levels were lower, in postinfection IBS than in healthy controls in both the ileum and colon. L. casei DG and postbiotic significantly reduced the mRNA levels of the proinflammatory cytokines IL-1α, IL-6, and IL-8 and TLR4, whereas these bacteria increased the mRNA levels of IL-10, in both the ileum and colon after LPS stimulation. Therefore, there was an attenuation of inflammatory mucosal response in an ex vivo organ culture model of postinfection IBS (137).
Bifidobacteria also stimulate TLR2, and specifically, B. breve C50 induces maturation and IL-10 production and prolongs DC survival (138). Similarly, Zeuthen et al. (139) showed that TLR2–/– DCs produce more IL-2 and less IL-10 in response to bifidobacteria, and the authors concluded that the immunoinhibitory effect of bifidobacteria is dependent on TLR2 (71, 139). B. bifidum OLB 6378 stimulates TLR2 expression in the ileal epithelium and enhances COX-2 expression, increasing the production of prostaglandin E2 in rats with NEC. However, the specific mechanism for this phenomenon has not been elucidated (140). In another study, in which dysbiosis was induced in the rat intestine, treatment with a probiotic mixture of 4 strains, namely B. breve DM8310, L. acidophilus DM8302, L. casei DM8121, and S. thermophilus DM8309, ameliorated the injury to the mucosal barrier, reduced the concentrations of proinflammatory factors and cytokines, and reduced neutrophil infiltration. These results are closely associated with the re-establishment of intestinal microbial homeostasis and alteration of the TLR2 and TLR4 signaling pathways (141).
TLR9 is another relevant TLR that is activated by probiotics, and in vivo, TLR9 exhibits anti-inflammatory effects at the epithelial surface. Hence, TLR9 activation induces intracellular signaling pathways via the apical and basolateral surfaces, and TNF-α–induced NF-κB is expressed. Thus, the abilities of different probiotic species to stimulate TLR9 are likely to be different. TLR9 triggers IκBα degradation and NF-κB pathway activation, whereas apical TLR9 induces cytoplasmic accumulation of ubiquitinated IκB and inhibition of NF-κB activation (71, 142).
Different strains such as B. breve, L. rhamnosus, and L. casei induce different amounts of cytokine production in human and mouse primary immune cells. Thus, B. breve induces cytokine production in a TLR9-dependent manner, and the lower inflammatory profile is due to the inhibitory effects of TLR2 (143). In addition, purified genomic DNA from L. plantarum inhibits LPS-induced TNF production and reduces TLR2, TLR4, and TLR9 gene expression in THP-1 cells (144).
A recent study demonstrated that transplantation of the human gut microbiota into pigs via different dosing regimens of L. rhamnosus GG affected intestinal bacterial communities and modulated the responses of the immune signaling pathway to an oral attenuated human rotavirus vaccine. The authors reported that pigs treated with 9 doses, but not those treated with 14 doses, of L. rhamnosus GG exhibited enhanced IL-6, IL-10, TNF-α, and TLR9 mRNA levels and p38 MAPK and extracellular regulated kinase expression in ileal mononuclear cells. Therefore, 9 doses of L. rhamnosus GG were more effective in activating the TLR9 signaling pathway than 14 doses in human-gut-microbiota–containing pigs vaccinated with attenuated human rotavirus vaccine (119).
Our research group has previously reported that L. paracasei CNCM I-4034 and the culture supernatant of L. paracasei CNCM I-4034 modulate Salmonella-induced inflammation in a novel trans-well co-culture of human intestinal-like dendritic and Caco-2 cells. L. paracasei CNCM I-4034 significantly increased the IL-1β, IL-6, IL-8, TGF-β2; regulated upon activation, normal T cell expressed and secreted (RANTES); and IP-10 levels and decreased the IL-12p40, IL-10, TGF-β1, and macrophage inflammatory protein (MIP)-1α levels in DCs through a physical barrier of Caco-2 cells. In contrast, incubation of the co-culture with cell-free culture supernatants increased IL-1β, IL-6, TGF-β2, and IP-10 production only when S. typhi was present. This induction was correlated with an overall decrease in the expression of all TLR genes except TLR9, which was strongly upregulated (145).
With regard to intestinal diseases, L. rhamnosus HN001 has been reported to have beneficial activity for the treatment of inflammatory diseases such as NEC. Hence, the microbial DNA of L. rhamnosus HN001 can activate TLR9, attenuating NEC in vitro, and no evidence of toxicity has been described (146).
With regard to the role of probiotics in reducing allergies, the underlying mechanisms might include shifting the lymphocyte Th1/Th2 balance toward a Th1 response and consequent decreased secretion of Th2 cytokines, such as IL-4, IL-5, and IL-13, as well as decreased IgE concentrations and increased production of C-reactive protein and IgA (56).
Interaction with the brain-gut axis
In social groups, individuals who interacted physically through social grooming harbored more similar communities of gut bacteria to each other (147). This degree of social interaction explained why there was variation in the gut microbiota even after controlling for diet, host genetics, and shared environment. Social transmission of the microbiota may be beneficial for propagating the microbes themselves, and some evidence suggests that a socially transmitted microbiota could confer beneficial effects to the host communities as well (148).
The intestinal microbiota, the brain-gut signaling system, and the interaction of the microbiota with genetic receptors have been shown to be associated with the health of children and with the development of short- and long-term behavior (149). The role of the gut microbiota in health and disease in the first years of life has become very relevant because of evidence that the gut microbiota can influence many aspects of human behavior (150). Preterm infants differ from term infants in that preterm infants are particularly vulnerable to the effects of stress and pain. Stress activates the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system, which increases intestinal permeability and allows bacteria and bacterial antigens to cross the epithelial barrier, activate the mucosal immune response, and alter the composition of the microbiome (151). In addition, oxidative stress in the intestine modulates the process of microbiome establishment in preterm infants (152).
Autism spectrum disorder (ASD) is a severe neurodevelopmental disorder that impairs a child's ability to communicate and interact with others. Children with neurodevelopmental disorders, including ASD, are regularly affected by gastrointestinal problems and dysbiosis of the gut microbiota (153). For example, Hsiao et al. (154) demonstrated that B. fragilis may play a role in the improvement in ASD-associated behaviors (154). Recently published data have linked the incidence of ASD with maternal obesity and diabetes (155, 156). A high-fat maternal diet was administered to mice with the objective of inducing impaired social behavior in the offspring, and subsequently the animals were administered L. reuteri. Administration of L. reuteri failed to mitigate anxiety but was able to restore oxytocin concentrations, the mesolimbic dopamine reward system, and social behaviors in the offspring that were fed a high-fat maternal diet (157).
Dinan et al. (158) demonstrated that stress caused by physical or psychological factors might be directly associated with the imbalance of the microbiota-brain-gut axis. Messaoudi et al. (159) showed that the consumption of L. helveticus R0052 and B. longum R0175 reduced symptoms of depression in healthy human volunteers (160). Recently, changes in brain structure were found to be associated with diet-dependent changes in gut microbiome populations through the use of a machine learning classifier to quantitatively assess the strength of microbiome–brain region associations (161).
In general, the mechanisms underlying the effects of the gut intestinal microbiota on the central nervous system are multifactorial (neural, endocrine, and immunologic), but these effects are believed to principally occur via the generation of bacterial metabolites (161). SCFAs alter neuronal excitability, and gut bacteria manufacture a wide spectrum of neuroactive compounds, including dopamine, γ-aminobutyric acid, histamine, acetylcholine, and tryptophan, which is a precursor in the biosynthesis of serotonin.
Although additional research is needed to test the causality and directionality of the association between the microbiota and social behavior, these initial studies have asked whether microbiota-mediated changes in social behavior affect social transmission of the microbiota and whether these interactions have consequences for both host and microbial fitness.
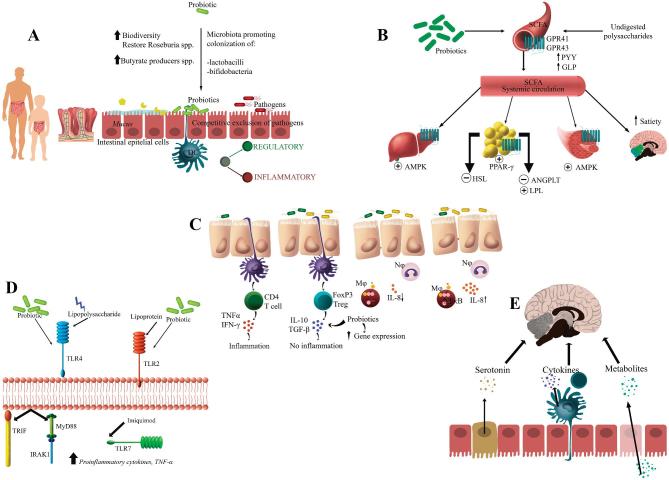
Finally, Figure 3 summarizes the mechanisms of action considered in the present review.
FIGURE 3.
Probiotic mechanisms of action. (A) Colonization and normalization of perturbed intestinal microbial communities in children and adults and competitive exclusion of pathogens and bacteriocin production; (B) enzymatic activity and production of volatile fatty acids; (C) cell adhesion, cell antagonism, and mucin production; (D) modulation of the immune system; and (E) interaction with the brain-gut axis. AMPK, AMP kinase; ANGPTL, angiopoietin-like; DC, dendritic cell; FoxP3, forkhead box P3; GLP, glucagon-like peptide; GPR, G protein–coupled receptor; HSL, hormone-sensitive lipase; IFN, interferon; IRAK1, IL-1 receptor-associated kinase 1; LPL, lipoprotein lipase; MyD88, myeloid differentiation primary response 88; NF-κB, nuclear transcription factor κB; PPAR-γ, peroxisome proliferator-activated receptor-γ; PYY, polypeptide YY; TGF, transforming growth factor; TLR, Toll-like receptor; TRIF, TIR–domain-containing adapter-inducing interferon-β.
Conclusions
Probiotics are safe microorganisms that when administered to human subjects in adequate doses and at appropriate periods confer some beneficial effects to the host. The mechanisms of action of probiotics involve colonization and normalization of perturbed intestinal microbial communities in both children and adults; competitive exclusion of pathogens and bacteriocin production; modulation of enzymatic activities related to metabolization of a number of carcinogens and other toxic substances; and production of volatile fatty acids, namely, SCFAs and BCFAs, which play a role in the maintenance of energy homeostasis and regulation of functionality in peripheral tissues. In addition, probiotics increase intestinal cell adhesion and mucin production and modulate the activity of gut-associated lymphoid tissue and the immune system. Similarly, probiotic metabolites are able to interact with the brain-gut axis and play a role in behavior. All the aforementioned mechanisms of action should encourage investigators, companies, stakeholders, and consumers to learn about the effects of probiotics as a whole and evaluate those strains that show promising results. These steps toward establishing “good science” may result in the approval of health claims in the near future.
Acknowledgments
JP-D, FJR-O, and AG are part of Plan Propio de Investigación 2016, Excellence actions: Units of Excellence; Unit of Excellence on Exercise and Health, University of Granada. The authors’ responsibilities were as follows—all authors: contributed to the design and presentation of the results; read, wrote, discussed, and revised all drafts; and read and approved the final version of the manuscript.
Notes
Published in a supplement to Advances in Nutrition. Presented at the International Union of Nutritional Sciences (IUNS) 21st International Congress of Nutrition (ICN) held in Buenos Aires, Argentina, October 15–20, 2017. The International Union of Nutritional Sciences (IUNS) thanks Mead Johnson Nutrition and Herbalife Nutrition for generously providing grants to support the publication and distribution of the present supplement from the 21st International Union of Nutritional Sciences. The contents of this supplement are solely the responsibility of the authors and do not necessarily represent official views of the IUNS. The supplement coordinators were Angel Gil and Alfredo Martinez. The supplement coordinators had no conflicts of interest to disclose.
Author disclosures: JP-D, FJR-O, MG-C, and AG, no conflicts of interest.Publication costs for this supplement were defrayed in part by the payment of page charges. This publication must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors or the publisher, Editor, or Editorial Board of Advances in Nutrition.
Abbreviations used: ASD, autism spectrum disorder; BCFA, branched-chain fatty acid; BSH, bile salt hydrolase; CD, Crohn disease; CMA, cow-milk allergy; DC, dendritic cell; EFSA, European Food Safety Authority; eHCF, extensively hydrolyzed casein formula; FoxP3, forkhead box P3; GPR, G protein–coupled receptor; IBD, inflammatory bowel disease; IBS, irritable bowel syndrome; LAB, lactic acid–producing bacteria; NAFLD, nonalcoholic fatty liver disease; NEC, necrotizing enterocolitis; NF-κB, nuclear transcription factor κB; PAMP, pathogen-associated molecular pattern; PRR, pattern recognition receptor; RCT, randomized clinical trial; Th, T-helper; TLR, Toll-like receptor; T-reg, regulatory T cell; T2D, type 2 diabetes.
References
- 1. Food and Agriculture Organization/World Health Organization Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Cordoba, Argentina: American Cordoba Park Hotel Rome, Italy: FAO/WHO; 2001. p. 1–2. [Google Scholar]
- 2. Roberfroid MB. Prebiotics and probiotics: are they functional foods? Am J Clin Nutr 2000;71:1682–7. [DOI] [PubMed] [Google Scholar]
- 3. Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 2014;11:506–14. [DOI] [PubMed] [Google Scholar]
- 4. Plaza-Díaz J, Ruiz-Ojeda FJ, Gil-Campos M, Gil A. Immune-mediated mechanisms of action of probiotics and synbiotics in treating pediatric intestinal diseases. Nutrients 2018;10(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Plaza-Díaz J, Robles-Sánchez C, Abadía-Molina F, Sáez-Lara MJ, Vilchez-Padial LM, Gil Á, Gómez-Llorente C, Fontana L. Gene expression profiling in the intestinal mucosa of obese rats administered probiotic bacteria. Sci Data 2017;4:170186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Metchnikoff E. The prolongation of life: optimistic studies. 1st ed Mitchell PC, editor. New York, NY: G.P. Putnam's Sons; 1908. [Google Scholar]
- 7. Fontana L, Bermudez-Brito M, Plaza-Diaz J, Muñoz-Quezada S, Gil A. Sources, isolation, characterisation and evaluation of probiotics. Br J Nutr 2013;109:35–50. [DOI] [PubMed] [Google Scholar]
- 8. Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr 1995;125:1401–12. [DOI] [PubMed] [Google Scholar]
- 9. Ouwehand AC, Salminen S, Isolauri E. Probiotics: an overview of beneficial effects. Antonie Van Leeuwenhoek 2002;82:279–89. [PubMed] [Google Scholar]
- 10. Plaza-Díaz J, Robles-Sánchez C, Abadía-Molina F, Morón-Calvente V, Sáez-Lara MJ, Ruiz-Bravo A, Jiménez-Valera M, Gil Á, Gómez-Llorente C, Fontana L. Adamdec1, Ednrb and Ptgs1/Cox1, inflammation genes upregulated in the intestinal mucosa of obese rats, are downregulated by three probiotic strains. Sci Rep 2017;7:1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tsilingiri K, Barbosa T, Penna G, Caprioli F, Sonzogni A, Viale G, Rescigno M. Probiotic and postbiotic activity in health and disease: comparison on a novel polarised ex-vivo organ culture model. Gut 2012;61:1007–15. [DOI] [PubMed] [Google Scholar]
- 12. Didari T, Solki S, Mozaffari S, Nikfar S, Abdollahi M. A systematic review of the safety of probiotics. Expert Opin Drug Saf 2014;13:227–39. [DOI] [PubMed] [Google Scholar]
- 13. Szajewska H. What are the indications for using probiotics in children? Arch Dis Child 2016;101:398–403. [DOI] [PubMed] [Google Scholar]
- 14. Hempel S, Newberry SJ, Maher AR, Wang Z, Miles JN, Shanman R, Johnsen B, Shekelle PG. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA 2012;307:1959–69. [DOI] [PubMed] [Google Scholar]
- 15. Moayyedi P, Ford AC, Talley NJ, Cremonini F, Foxx-Orenstein AE, Brandt LJ, Quigley EM. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut 2010;59:325–32. [DOI] [PubMed] [Google Scholar]
- 16. Goldenberg JZ, Yap C, Lytvyn L, Lo CK, Beardsley J, Mertz D, Johnston BC. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev 2017;12:CD006095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saez-Lara MJ, Gomez-Llorente C, Plaza-Diaz J, Gil A. The role of probiotic lactic acid bacteria and bifidobacteria in the prevention and treatment of inflammatory bowel disease and other related diseases: a systematic review of randomized human clinical trials. Biomed Res Int 2015:505878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rather IA, Bajpai VK, Kumar S, Lim J, Paek WK, Park YH. Probiotics and atopic dermatitis: an overview. Front Microbiol 2016;7:507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Berings M, Karaaslan C, Altunbulakli C, Gevaert P, Akdis M, Bachert C, Akdis CA. Advances and highlights in allergen immunotherapy: on the way to sustained clinical and immunologic tolerance. J Allergy Clin Immunol 2017;140:1250–67. [DOI] [PubMed] [Google Scholar]
- 20. EFSA Guidance on the scientific requirements for health claims related to the immune system, the gastrointestinal tract and defence against pathogenic microorganisms. EFSA J 2016;14:4369. [Google Scholar]
- 21. Degnan FH. The US Food and Drug Administration and probiotics: regulatory categorization. Clin Infect Dis 2008;46:S133–6. [DOI] [PubMed] [Google Scholar]
- 22. FDA Dietary supplements: New Dietary Ingredient notifications and related issues: guidance for industry, August2016. [cited 2017 Oct 10]. Available from: http://www.fda.gov/downloads/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/UCM515733.pdf.
- 23. Government of Canada Health Canada [Internet]. [cited 2018 Jun 17]. a) Visbiome. Available from: https://health-products.canada.ca/lnhpd-bdpsnh/info.do?licence=80061901 b) Bifidobacterium animalis spp. lactis LAFTI B94 Available from: https://health-products.canada.ca/lnhpd-bdpsnh/info.do?licence=80064384.
- 24. Byndloss MX, Bäumler AJ. The germ-organ theory of non-communicable diseases. Nat Rev Microbiol 2018;16:103–10. [DOI] [PubMed] [Google Scholar]
- 25. Cani PD. Gut microbiota — at the intersection of everything? Nat Rev Gastroenterol Hepatol 2017;14:321–2. [DOI] [PubMed] [Google Scholar]
- 26. Vieira AT, Fukumori C, Ferreira CM. New insights into therapeutic strategies for gut microbiota modulation in inflammatory diseases. Clin Transl Immunology 2016;5:e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mendes MCS, Paulino DS, Brambilla SR, Camargo JA, Persinoti GF, Carvalheira JBC. Microbiota modification by probiotic supplementation reduces colitis associated colon cancer in mice. World J Gastroenterol 2018;24:1995–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang I, Corwin EJ, Brennan PA, Jordan S, Murphy JR, Dunlop A. The infant microbiome: implications for infant health and neurocognitive development. Nurs Res 2016;65:76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hesla HM, Stenius F, Jäderlund L, Nelson R, Engstrand L, Alm J, Dicksved J. Impact of lifestyle on the gut microbiota of healthy infants and their mothers – the ALADDIN birth cohort. FEMS Microbiol Ecol 2014;90:791–801. [DOI] [PubMed] [Google Scholar]
- 30. Jiménez E, Fernández L, Marín ML, Martín R, Odriozola JM, Nueno-Palop C, Narbad A, Olivares M, Xaus J, Rodríguez JM. Isolation of commensal bacteria from umbilical cord blood of healthy neonates born by cesarean section. Curr Microbiol 2005;51:270–4. [DOI] [PubMed] [Google Scholar]
- 31. Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med 2014;6:237ra65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jimenez-Truque N, Tedeschi S, Saye EJ, McKenna BD, Langdon W, Wright JP, Alsentzer A, Arnold S, Saville BR, Wang W et al. Relationship between maternal and neonatal Staphylococcus aureus colonization. Pediatrics 2012;129:e1252–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Murphy K, Curley D, O'Callaghan TF, O'Shea CA, Dempsey EM, O'Toole PW, Ross RP, Ryan CA, Stanton C. The composition of human milk and infant faecal microbiota over the first three months of life: a pilot study. Sci Rep 2017;7:40597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Martín R, Langa S, Reivierngo C, Jiménez E, Marín ML, Olivares M. The comensal microflora of human milk: new perspectives for food bacteriotherapy and probiotics. Trends Food Sci Tech 2004;15:121–7. [Google Scholar]
- 35. Ribet D, Cossart P. How bacterial pathogens colonize their hosts and invade deeper tissues. Microbes Infect 2015;17:173–83. [DOI] [PubMed] [Google Scholar]
- 36. Hashemi A, Villa CR, Comelli EM. Probiotics in early life: a preventative and treatment approach. Food Funct 2016;7:1752–68. [DOI] [PubMed] [Google Scholar]
- 37. Hojsak I, Szajewska H, Canani RB, Guarino A, Indrio F, Kolacek S, Orel R, Shamir R, Vandenplas Y, van Goudoever JB et al. Probiotics for the prevention of nosocomial diarrhea in children. J Pediatr Gastroenterol Nutr 2018;66:3–9. [DOI] [PubMed] [Google Scholar]
- 38. Bazanella M, Maier TV, Clavel T, Lagkouvardos I, Lucio M, Maldonado-Gòmez MX, Autran C, Walter J, Bode L, Schmitt-Kopplin P et al. Randomized controlled trial on the impact of early-life intervention with bifidobacteria on the healthy infant fecal microbiota and metabolome. Am J Clin Nutr 2017;106:1274–86. [DOI] [PubMed] [Google Scholar]
- 39. Berni Canani R, De Filippis F, Nocerino R, Laiola M, Paparo L, Calignano A, De Caro C, Coretti L, Chiariotti L, Gilbert J et al. Specific signatures of the gut microbiota and increased levels of butyrate in children treated with fermented cow's milk containing heat-killed Lactobacillus paracasei CBA L74. Appl Environ Microbiol 2017;83:e01206–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Papadimitriou K, Zoumpopoulou G, Foligné B, Alexandraki V, Kazou M, Pot B, Tsakalidou E. Discovering probiotic microorganisms: in vitro, in vivo, genetic and omics approaches. Front Microbiol 2015;6:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Alfaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev 2014;4:CD005496. [DOI] [PubMed] [Google Scholar]
- 42. Olsen R, Greisen G, Schrøder M, Brok J. Prophylactic probiotics for preterm infants: a systematic review and meta-analysis of observational studies. Neonatology 2016;109:105–12. [DOI] [PubMed] [Google Scholar]
- 43. Xu M, Wang J, Wang N, Sun F, Wang L, Liu XH. The efficacy and safety of the probiotic bacterium Lactobacillus reuteri DSM 17938 for infantile colic: a meta-analysis of randomized controlled trials. PLoS One 2015;10:e0141445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chau K, Lau E, Greenberg S, Jacobson S, Yazdani-Brojeni P, Verma N, Koren G. Probiotics for infantile colic: a randomized, double-blind, placebo-controlled trial investigating Lactobacillus reuteri DSM 17938. J Pediatr 2015;166:74–8. [DOI] [PubMed] [Google Scholar]
- 45. Urbańska M, Szajewska H. The efficacy of Lactobacillus reuteri DSM 17938 in infants and children: a review of the current evidence. Eur J Pediatr 2014;173:1327–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pärtty A, Lehtonen L, Kalliomäki M, Salminen S, Isolauri E. Probiotic Lactobacillus rhamnosus GG therapy and microbiological programming in infantile colic: a randomized, controlled trial. Pediatr Res 2015;78:470–5. [DOI] [PubMed] [Google Scholar]
- 47. Urbańska M, Gieruszczak-Białek D, Szajewska H. Systematic review with meta-analysis: Lactobacillus reuteri DSM 17938 for diarrhoeal diseases in children. Aliment Pharmacol Ther 2016;43:1025–34. [DOI] [PubMed] [Google Scholar]
- 48. Gil-Campos M, López MA, Rodriguez-Benítez MV, Romero J, Roncero I, Linares MD, Maldonado J, López-Huertas E, Berwind R, Ritzenthaler KL et al. Lactobacillus fermentum CECT 5716 is safe and well tolerated in infants of 1–6 months of age: a randomized controlled trial. Pharmacol Res 2012;65:231–8. [DOI] [PubMed] [Google Scholar]
- 49. Korpela K, Salonen A, Virta LJ, Kumpu M, Kekkonen RA, de Vos WM. Lactobacillus rhamnosus GG intake modifies preschool children's intestinal microbiota, alleviates penicillin-associated changes, and reduces antibiotic use. PLoS One 2016;25:e0154012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hong Chau TT, Minh Chau NN, Hoang Le NT, Chung The H, Voong Vinh P, Nguyen To NT, Ngoc NM, Tuan HM, Chau Ngoc TL, Kolader ME et al. A double-blind, randomized, placebo-controlled trial of Lactobacillus acidophilus for the treatment of acute watery diarrhea in Vietnamese children. Pediatr Infect Dis J 2018;37:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hajare ST, Bekele G. Effect of probiotic strain Lactobacillus acidophilus (LBKV-3) on fecal residual lactase activity in undernourished children below 10 years. J Immunoassay Immunochem 2017;38:620–8. [DOI] [PubMed] [Google Scholar]
- 52. Dhama K, Latheef SK, Munjal AK, Khandia R, Samad HA, Iqbal HMN, Joshi SK. Probiotics in curing allergic and inflammatory conditions – research progress and futuristic vision. Recent Pat Inflamm Allergy Drug Discov 2017;10:105–18. [DOI] [PubMed] [Google Scholar]
- 53. He T, Priebe MG, Zhong Y, Huang C, Harmsen HJ, Raangs GC, Antoine JM, Welling GW, Vonk RJ. Effects of yogurt and bifidobacteria supplementation on the colonic microbiota in lactose-intolerant subjects. J Appl Microbiol 2008;104:595–604. [DOI] [PubMed] [Google Scholar]
- 54. Oak SJ, Jha R. The effects of probiotics in lactose intolerance: a systematic review. Crit Rev Food Sci Nutr 2018:1–9. [DOI] [PubMed] [Google Scholar]
- 55. Azcarate-Peril MA, Ritter AJ, Savaiano D, Monteagudo-Mera A, Anderson C, Magness ST, Klaenhammer TR. Impact of short-chain galactooligosaccharides on the gut microbiome of lactose-intolerant individuals. Proc Natl Acad Sci U S A 2017;114:E367–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. West CE, Jenmalm MC, Prescott SL. The gut microbiota and its role in the development of allergic disease: a wider perspective. Clin Exp Allergy 2015;45:43–53. [DOI] [PubMed] [Google Scholar]
- 57. Zhang GQ, Hu HJ, Liu CY, Zhang Q, Shakya S, Li ZY. Probiotics for prevention of atopy and food hypersensitivity in early childhood: a PRISMA-compliant systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2016;95:e2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lifschitz C, Szajewska H. Cow's milk allergy: evidence-based diagnosis and management for the practitioner. Eur J Pediatr 2015;174:141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vandenplas Y. Prevention and management of cow's milk allergy in non-exclusively breastfed infants. Nutrients 2017;9:E731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bunyavanich S, Shen N, Grishin A, Wood R, Burks W, Dawson P, Jones SM, Leung DYM, Sampson H, Sicherer S et al. Early-life gut microbiome composition and milk allergy resolution. J Allergy Clin Immunol 2016;138:1122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Berni Canani R, Sangwan N, Stefka AT, Nocerino R, Paparo L, Aitoro R, Calignano A, Khan AA, Gilbert JA, Nagler CR. Lactobacillus rhamnosus GG-supplemented formula expands butyrate-producing bacterial strains in food allergic infants. ISME J 2016;10:742–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Olivares M, Díaz-Ropero MA, Gómez N, Lara-Villoslada F, Sierra S, Maldonado JA, Martín R, López-Huertas E, Rodríguez JM, Xaus J. Oral administration of two probiotic strains, Lactobacillus gasseri CECT5714 and Lactobacillus coryniformis CECT5711, enhances the intestinal function of healthy adults. Int J Food Microbiol 2006;107:104–11. [DOI] [PubMed] [Google Scholar]
- 63. Wind RD, Tolboom H, Klare I, Huys G, Knol J. Tolerance and safety of the potentially probiotic strain Lactobacillus rhamnosus PRSF-L477: a randomised, double-blind placebo-controlled trial in healthy volunteers. Br J Nutr 2010;104:1806–16. [DOI] [PubMed] [Google Scholar]
- 64. Sierra S, Lara-Villoslada F, Sempere L, Olivares M, Boza J, Xaus J. Intestinal and immunological effects of daily oral administration of Lactobacillus salivarius CECT5713 to healthy adults. Anaerobe 2010;16:195–200. [DOI] [PubMed] [Google Scholar]
- 65. Plaza-Diaz J, Gomez-Llorente C, Campaña-Martin L, Matencio E, Ortuño I, Martínez-Silla R, Gomez-Gallego C, Periago MJ, Ros G, Chenoll E et al. Safety and immunomodulatory effects of three probiotic strains isolated from the feces of breast-fed infants in healthy adults: SETOPROB study. PLoS One 2013;8:e78111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rajkumar H, Mahmood N, Kumar M, Varikuti SR, Challa HR, Myakala SP. Effect of probiotic (VSL#3) and omega-3 on lipid profile, insulin sensitivity, inflammatory markers, and gut colonization in overweight adults: a randomized, controlled trial. Mediators Inflamm 2014:348959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sharafedtinov KK, Plotnikova OA, Alexeeva RI, Sentsova TB, Songisepp E, Stsepetova J, Smidt I, Mikelsaar M. Hypocaloric diet supplemented with probiotic cheese improves body mass index and blood pressure indices of obese hypertensive patients—a randomized double-blind placebo-controlled pilot study. Nutr J 2013;12:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ganji-Arjenaki M, Rafieian-Kopaei M. Probiotics are a good choice in remission of inflammatory bowel diseases: a meta analysis and systematic review. J Cell Physiol 2018;233:2091–103. [DOI] [PubMed] [Google Scholar]
- 69. Geirnaert A, Calatayud M, Grootaert C, Laukens D, Devriese S, Smagghe G, De Vos M, Boon N, Van de Wiele T. Butyrate-producing bacteria supplemented in vitro to Crohn's disease patient microbiota increased butyrate production and enhanced intestinal epithelial barrier integrity. Sci Rep 2017;7:11450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Johnston BC, Ma SS, Goldenberg JZ, Thorlund K, Vandvik PO, Loeb M, Guyatt GH. Probiotics for the prevention of Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Ann Intern Med 2012;157:878–88. [DOI] [PubMed] [Google Scholar]
- 71. Bermudez-Brito M, Plaza-Díaz J, Muñoz-Quezada S, Gómez-Llorente C, Gil A. Probiotic mechanisms of action. Ann Nutr Metab 2012;61:160–74. [DOI] [PubMed] [Google Scholar]
- 72. Collado MC, Gueimonde M, Salminem S. Probiotics in adhesion of pathogens: mechanisms of action. In: Watson RR, Preedy VR, editors. Bioactive foods in promoting health: probiotics and prebiotics. 1st ed. London: Academic Press, Elsevier; 2010. p. 353–70. [Google Scholar]
- 73. Muñoz-Quezada S, Bermudez-Brito M, Chenoll E, Genovés S, Gomez-Llorente C, Plaza-Diaz J, Matencio E, Bernal MJ, Romero F, Ramón D et al. Competitive inhibition of three novel bacteria isolated from faeces of breast milk-fed infants against selected enteropathogens. Br J Nutr 2013;109:S63–9. [DOI] [PubMed] [Google Scholar]
- 74. Kumar M, Nagpal R, Verma V, Kumar A, Kaur N, Hemalatha R, Gautam SK, Singh B. Probiotic metabolites as epigenetic targets in the prevention of colon cancer. Nutr Rev 2013;71:23–34. [DOI] [PubMed] [Google Scholar]
- 75. Umu ÖCO, Rudi K, Diep DB. Modulation of the gut microbiota by prebiotic fibres and bacteriocins. Microb Ecol Health Dis 2017;28:1348886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mokoena MP. Lactic acid bacteria and their bacteriocins: classification, biosynthesis and applications against uropathogens: a mini-review. Molecules 2017;22:1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kumar M, Dhaka P, Vijay D, Vergis J, Mohan V, Kumar A, Kurkure NV, Barbuddhe SB, Malik SVS, Rawool DB. Antimicrobial effects of Lactobacillus plantarum and Lactobacillus acidophilus against multidrug-resistant enteroaggregative Escherichia coli. Int J Antimicrobial Agents 2016;48:265–70. [DOI] [PubMed] [Google Scholar]
- 78. Kim DH, Jin YH. Intestinal bacterial beta-glucuronidase activity of patients with colon cancer. Arch Pharm Res 2001;24:564–7. [DOI] [PubMed] [Google Scholar]
- 79. Kulkarni N, Reddy BS. Inhibitory effect of Bifidobacterium longum cultures on the azoxymethane-induced aberrant crypt foci formation and fecal bacterial β-glucuronidase. Exptal Biol Med 1994;207:278–83. [DOI] [PubMed] [Google Scholar]
- 80. Buss C, Valle-Tovo C, Miozzo S, Alves de Mattos A. Probiotics and synbiotics may improve liver aminotransferases levels in non-alcoholic fatty liver disease patients. Ann Hepatol 2014;13:482–8. [PubMed] [Google Scholar]
- 81. Pavlović N, Stankov K, Mikov M. Probiotics—interactions with bile acids and impact on cholesterol metabolism. Appl Biochem Biotechnol 2012;168:1880–95. [DOI] [PubMed] [Google Scholar]
- 82. Kumar R, Grover S, Batish VK. Hypocholesterolaemic effect of dietary inclusion of two putative probiotic bile salt hydrolase-producing Lactobacillus plantarum strains in Sprague–Dawley rats. Br J Nutr 2011;105:561–73. [DOI] [PubMed] [Google Scholar]
- 83. Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc 2003;62:67–72. [DOI] [PubMed] [Google Scholar]
- 84. Ferrario C, Taverniti V, Milani C, Fiore W, Laureati M, De Noni I, Stuknyte M, Chouaia B, Riso P, Guglielmetti S. Modulation of fecal Clostridiales bacteria and butyrate by probiotic intervention with Lactobacillus paracasei DG varies among healthy adults. J Nutr 2014;144:1787–96. [DOI] [PubMed] [Google Scholar]
- 85. Wang L, Zhang J, Guo Z, Kwok L, Ma C, Zhang W, Lv Q, Huang W, Zhang H. Effect of oral consumption of probiotic Lactobacillus plantarum P-8 on fecal microbiota, SIgA, SCFAs, and TBAs of adults of different ages. Nutrition 2014;30:776–83. [DOI] [PubMed] [Google Scholar]
- 86. Worthley DL, Le Leu RK, Whitehall VL, Conlon M, Christophersen C, Belobrajdic D, Mallitt KA, Hu Y, Irahara N, Ogino S et al. A human, double-blind, placebo-controlled, crossover trial of prebiotic, probiotic, and synbiotic supplementation: effects on luminal, inflammatory, epigenetic, and epithelial biomarkers of colorectal cancer. Am J Clin Nutr 2009;90:578–86. [DOI] [PubMed] [Google Scholar]
- 87. Macfarlane S, Cleary S, Bahrami B, Reynolds N, Macfarlane GT. Synbiotic consumption changes the metabolism and composition of the gut microbiota in older people and modifies inflammatory processes: a randomised, double-blind, placebo-controlled crossover study. Aliment Pharmacol Ther 2013;38:804–16. [DOI] [PubMed] [Google Scholar]
- 88. Schneider SM, Girard-Pipau F, Filippi J, Hebuterne X, Moyse D, Hinojosa GC, Pompei A, Rampal P. Effects of Saccharomyces boulardii on fecal short-chain fatty acids and microflora in patients on long-term total enteral nutrition. World J Gastroenterol 2005;11:6165–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wullt M, Johansson Hagslätt ML, Odenholt I, Berggren A. Lactobacillus plantarum 299v enhances the concentrations of fecal short-chain fatty acids in patients with recurrent Clostridium difficile-associated diarrhea. Dig Dis Sci 2007;52:2082–6. [DOI] [PubMed] [Google Scholar]
- 90. Nagata S, Asahara T, Ohta T, Yamada T, Kondo S, Bian L, Wang C, Yamashiro Y, Nomoto K. Effect of the continuous intake of probiotic-fermented milk containing Lactobacillus casei strain Shirota on fever in a mass outbreak of norovirus gastroenteritis and the faecal microflora in a health service facility for the aged. Br J Nutr 2011;106:549–56. [DOI] [PubMed] [Google Scholar]
- 91. Nagata S, Asahara T, Wang C, Suyama Y, Chonan O, Takano K, Daibou M, Takahashi T, Nomoto K, Yamashiro Y. The effectiveness of Lactobacillus beverages in controlling infections among the residents of an aged care facility: a randomized placebo-controlled double-blind trial. Ann Nutr Metab 2016;68:51–9. [DOI] [PubMed] [Google Scholar]
- 92. Hemalatha R, Ouwehand AC, Saarinen MT, Prasad UV, Swetha K, Bhaskar V. Effect of probiotic supplementation on total lactobacilli, bifidobacteria and short chain fatty acids in 2–5-year-old children. Microb Ecol Health Dis 2017;10:1298340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Riezzo G, Orlando A, D'Attoma B, Guerra V, Valerio F, Lavermicocca P, De Candia S, Russo F. Randomised clinical trial: efficacy of Lactobacillus paracasei-enriched artichokes in the treatment of patients with functional constipation—a double-blind, controlled, crossover study. Aliment Pharmacol Ther 2012;35:441–50. [DOI] [PubMed] [Google Scholar]
- 94. Maldonado J, Lara-Villoslada F, Sierra S, Sempere L, Gómez M, Rodriguez JM, Boza J, Xaus J, Olivares M. Safety and tolerance of the human milk probiotic strain Lactobacillus salivarius CECT5713 in 6-month-old children. Nutrition 2010;26:1082–7. [DOI] [PubMed] [Google Scholar]
- 95. Mohan R, Koebnick C, Schildt J, Mueller M, Radke M, Blaut M. Effects of Bifidobacterium lactis Bb12 supplementation on body weight, fecal pH, acetate, lactate, calprotectin, and IgA in preterm infants. Pediatr Res 2008;64:418–22. [DOI] [PubMed] [Google Scholar]
- 96. Underwood MA, Salzman NH, Bennett SH, Barman M, Mills DA, Marcobal A, Tancredi DJ, Bevins CL, Sherman MP. A randomized placebo-controlled comparison of 2 prebiotic/probiotic combinations in preterm infants: impact on weight gain, intestinal microbiota, and fecal short-chain fatty acids. J Pediatr Gastroenterol Nutr 2009;48:216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Sáez-Lara MJ, Robles-Sanchez C, Ruiz-Ojeda FJ, Plaza-Diaz J, Gil A. Effects of probiotics and synbiotics on obesity, insulin resistance syndrome, type 2 diabetes and non-alcoholic fatty liver disease: a review of human clinical trials. Int J Mol Sci 2016;17:928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Larsen N, Vogensen FK, Gøbel RJ, Michaelsen KF, Forssten SD, Lahtinen SJ, Jakobsen M. Effect of Lactobacillus salivarius Ls-33 on fecal microbiota in obese adolescents. Clin Nutr 2013;32:935–40. [DOI] [PubMed] [Google Scholar]
- 99. Miccheli A, Capuani G, Marini F, Tomassini A, Praticò G, Ceccarelli S, Gnani D, Baviera G, Alisi A, Putignani L et al. Urinary (1)H-NMR-based metabolic profiling of children with NAFLD undergoing VSL#3 treatment. Int J Obes (Lond) 2015;39:1118–25. [DOI] [PubMed] [Google Scholar]
- 100. Plaza-Diaz J, Gomez-Llorente C, Abadia-Molina F, Saez-Lara MJ, Campaña-Martin L, Muñoz-Quezada S, Romero F, Gil A, Fontana L. Effects of Lactobacillus paracasei CNCM I-4034, Bifidobacterium breve CNCM I-4035 and Lactobacillus rhamnosus CNCM I-4036 on hepatic steatosis in Zucker rats. PLoS One 2014;9:e98401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol 2015;11:577–91. [DOI] [PubMed] [Google Scholar]
- 102. Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, Tobe T, Clarke JM, Topping DL, Suzuki T et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011;469:543–7. [DOI] [PubMed] [Google Scholar]
- 103. Hur KY, Lee MS. Gut microbiota and metabolic disorders. Diabetes Metab J 2015;39:198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kim HK, Rutten NB, Besseling-van der Vaart I, Niers LE, Choi YH, Rijkers GT, van Hemert S. Probiotic supplementation influences faecal short chain fatty acids in infants at high risk for eczema. Benef Microbes 2015;m6:783–90. [DOI] [PubMed] [Google Scholar]
- 105. Yadav AK, Tyagi A, Kumar A, Panwar S, Grover S, Saklani AC, Hemalatha R, Batish VK. Adhesion of lactobacilli and their anti-infectivity potential. Crit Rev Food Sci Nutr 2017;57:2042–56. [DOI] [PubMed] [Google Scholar]
- 106. Van Tassell ML, Miller MJ. Lactobacillus adhesion to mucus. Nutrients 2011;3:613–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Buck BL, Altermann E, Svingerud T, Klaenhammer TR. Functional analysis of putative adhesion factors in Lactobacillus acidophilus NCFM. Appl Environ Microbiol 2005;71:8344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Dicks LM, Botes M. Probiotic lactic acid bacteria in the gastro-intestinal tract: health benefits, safety and mode of action. Benef Microbes 2010;1:11–29. [DOI] [PubMed] [Google Scholar]
- 109. Lenaerts K, Bouwman FG, Lamers WH, Renes J, Mariman EC. Comparative proteomic analysis of cell lines and scrapings of the human intestinal epithelium. BMC Genomics 2007;8:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Huang SH, He L, Zhou Y, Wu CH, Jong A. Lactobacillus rhamnosus GG suppresses meningitic E. coli K1 penetration across human intestinal epithelial cells in vitro and protects neonatal rats against experimental hematogenous meningitis. Int J Microbiol 2009:647862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Zhang W, Zhu YH, Yang JC, Yang GY, Zhou D, Wang JF. A selected Lactobacillus rhamnosus strain promotes Egfr-independent Akt activation in an enterotoxigenic Escherichia coli k88-infected IPEC-J2 cell model. PLoS One 2015;10:e0125717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Mujagic Z, de Vos P, Boekschoten MV, Govers C, Pieters HH, de Wit NJ, Bron PA, Masclee AA, Troost FJ. The effects of Lactobacillus plantarum on small intestinal barrier function and mucosal gene transcription; a randomized double-blind placebo controlled trial. Sci Rep 2017;7:40128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. D'Amelio P, Sassi F. Gut microbiota, immune system, and bone. Calcif Tissue Int 2017;102(4):415–25. [DOI] [PubMed] [Google Scholar]
- 114. Gómez-Llorente C, Muñoz S, Gil A. Role of toll-like receptors in the development of immunotolerance mediated by probiotics. Proc Nutr Soc 2010;69:381–9. [DOI] [PubMed] [Google Scholar]
- 115. Claes AK, Zhou JY, Philpott DJ. NOD-like receptors: guardians of intestinal mucosal barriers. Physiology (Bethesda) 2015;30:241–50. [DOI] [PubMed] [Google Scholar]
- 116. Hevia A, Delgado S, Sánchez B, Margolles A. Molecular players involved in the interaction between beneficial bacteria and the immune system. Front Microbiol 2015;6:1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Yousefi M, Movassaghpour AA, Shamsasenjan K, Ghalamfarsa G, Sadreddini S, Jadidi-Niaragh F, Hojjat-Farsangi M. The skewed balance between Tregs and Th17 in chronic lymphocytic leukemia. Future Oncol 2015;11:1567–82. [DOI] [PubMed] [Google Scholar]
- 118. Giorgetti G, Brandimarte G, Fabiocchi F, Ricci S, Flamini P, Sandri G, Trotta MC, Elisei W, Penna A, Lecca PG et al. Interactions between innate immunity, microbiota, and probiotics. J Immunol Res 2015:501361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Wang H, Gao K, Wen K, Allen IC, Li G, Zhang W, Kocher J, Yang X, Giri-Rachman E, Li GH et al. Lactobacillus rhamnosus GG modulates innate signaling pathway and cytokine responses to rotavirus vaccine in intestinal mononuclear cells of gnotobiotic pigs transplanted with human gut microbiota. BMC Microbiol 2016;16(1):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Harbige LS, Pinto E, Allgrove J, Thomas LV. Immune response of healthy adults to the ingested probiotic Lactobacillus casei Shirota. Scand J Immunol 2016;84:353–64. [DOI] [PubMed] [Google Scholar]
- 121. Letnická A, Karaffová V, Levkut M, Revajová V, Herich R. Influence of oral application of Enterococcus faecium AL41 on TGF-β4 and IL-17 expression and immunocompetent cell distribution in chickens challenged with Campylobacter jejuni. Acta Vet Hung 2017;65:317–26. [DOI] [PubMed] [Google Scholar]
- 122. Dogi C, García G, De Moreno de LeBlanc A, Greco C, Cavaglieri L. Lactobacillus rhamnosus RC007 intended for feed additive: immune-stimulatory properties and ameliorating effects on TNBS-induced colitis. Benef Microbes 2016;7:539–47. [DOI] [PubMed] [Google Scholar]
- 123. Takeda S, Kawahara S, Hidaka M, Yoshida H, Watanabe W, Takeshita M, Kikuchi Y, Bumbein D, Muguruma M, Kurokawa M. Effects of oral administration of probiotics from Mongolian dairy products on the Th1 immune response in mice. Biosci Biotechnol Biochem 2013;77:1372–8. [DOI] [PubMed] [Google Scholar]
- 124. Aktas B, De Wolfe TJ, Safdar N, Darien BJ, Steele JL. The impact of Lactobacillus casei on the composition of the cecal microbiota and innate immune system is strain specific. PLoS One 2016;11:e0156374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. O'Mahony C, Scully P, O'Mahony D, Murphy S, O'Brien F, Lyons A, Sherlock G, MacSharry J, Kiely B, Shanahan F et al. Commensal-induced regulatory T cells mediate protection against pathogen-stimulated NF-κB activation. PLoS Pathog 2008;4:e1000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Thomas LV, Suzuki K, Zhao J. Probiotics: a proactive approach to health. A symposium report. Br J Nutr 2015;114:S1–15. [DOI] [PubMed] [Google Scholar]
- 127. Heuvelin E, Lebreton C, Grangette C, Pot B, Cerf-Bensussan N, Heyman M. Mechanisms involved in alleviation of intestinal inflammation by Bifidobacterium breve soluble factors. PLoS One 2009;4:e5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Pagnini C, Saeed R, Bamias G, Arseneau KO, Pizarro TT, Cominelli F. Probiotics promote gut health through stimulation of epithelial innate immunity. Proc Natl Acad Sci U S A 2010;107:454–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Kwon HK, Lee CG, So JS, Chae CS, Hwang JS, Sahoo A, Nam JH, Rhee JH, Hwang KC, Im SH. Generation of regulatory dendritic cells and CD4+Foxp3+ T cells by probiotics administration suppresses immune disorders. Proc Natl Acad Sci U S A 2010;107:2159–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Wells JM. Immunomodulatory mechanisms of lactobacilli. Microb Cell Fact 2011;10:S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Vinderola G, Matar C, Perdigón G. Role of the epithelial cells in the immune effects mediated by gram-positive probiotic bacteria. Involvement of toll-like receptors. Clin Diagn Lab Immunol 2005;12:1075–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ren C, Zhang Q, de Haan BJ, Zhang H, Faas MM, de Vos P. Identification of TLR2/TLR6 signalling lactic acid bacteria for supporting immune regulation. Sci Rep 2016;6:34561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Shida K, Kiyoshima-Shibata J, Nagaoka M, Nanno M. Peptidoglycan from lactobacilli inhibits interleukin-12 production by macrophages induced by Lactobacillus casei through toll-like receptor 2-dependent and independent mechanisms. Inmmunology 2009;128:e858–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Abreu MT, Fukata M, Arditi M. TLR signaling in the gut in health and diseases. J Immunol 2005;174:4453–60. [DOI] [PubMed] [Google Scholar]
- 135. Castillo NA, Perdigón G, De Moreno de Le Blanc A. Oral administration of a probiotic Lactobacillus modulates cytokine production and TLR expression improving the immune response against Salmonella enterica serovar typhimurium infection in mice. BMC Microbiol 2011;11:177–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Karczewski J, Troost FJ, Konings I, Dekker J, Kleerebezem M, Brummer RJ, Wells JM. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am J Physiol Gastrointest Liver Physiol 2010;298:G851–9. [DOI] [PubMed] [Google Scholar]
- 137. Compare D, Rocco A, Coccoli P, Angrisani D, Sgamato C, Iovine B, Salvatore U, Nardone G. Lactobacillus casei DG and its postbiotic reduce the inflammatory mucosal response: an ex-vivo organ culture model of post-infectious irritable bowel syndrome. BMC Gastroenterol 2017;17:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Hoarau C, Martin L, Faugaret D, Baron C, Dauba A, Aubert-Jacquin C, Velge-Roussel F, Lebranchu Y. Supernatant from Bifidobacterium differentially modulates transduction signaling pathways for biological functions of human dendritic cells. PLoS One 2008;3:e2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Zeuthen LH, Fink LN, Frokiaer H. Toll-like receptor 2 and nucleotide-binding oligomerization domain-2 play divergent roles in the recognition of gut derived lactobacilli and bifidobacteria in dendritic cells. Inmmunology 2008;124:489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Kailova L, Mount Patrick SK, Arganbright KM, Halpern M, Kinouchi T, Dvorak B. Bifidobacterium bifidum reduces apoptosis in the intestinal epithelium in necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 2010;299:G1118–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Tang Y, Wu Y, Huang Z, Dong W, Deng Y, Wang F, Li M, Yuan J. Administration of probiotic mixture DM#1 ameliorated 5-fluorouracil-induced intestinal mucositis and dysbiosis in rats. Nutrition 2017;33:96–104. [DOI] [PubMed] [Google Scholar]
- 142. Lee J, Mo JH, Katura K, Alkalay I, Rucker AN, Liu YT, Lee HK, Shen C, Cojocaru G, Shenouda S et al. Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat Cell Biol 2006;8:1327–36. [DOI] [PubMed] [Google Scholar]
- 143. Plantiga TS, van Maren WWC, van Bergenhenegouwen J, Hameetman M, Nierkens S, Jacobs C, de Jong DJ, Joosten LAB, van't Land B, Garssen J et al. Differential toll-like receptor recognition and induction of cytokine profile by Bifidobacterium breve and Lactobacillus strains of probiotics. Clin Vaccine Immunol 2011;18:621–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Kim CH, Kim HG, Kim JY, Kim NR, Jung BJ, Jeong JH, Chung DK. Probiotic genomic DNA reduces the production of pro-inflammatory cytokine tumor necrosis factor-alpha. FEMS Microbiol Lett 2012;328:13–19. [DOI] [PubMed] [Google Scholar]
- 145. Bermudez-Brito M, Muñoz-Quezada S, Gómez-Llorente C, Matencio E, Romero F, Gil A. Lactobacillus paracasei CNCM I-4034 and its culture supernatant modulate Salmonella-induced inflammation in a novel transwell co-culture of human intestinal-like dendritic and Caco-2 cells. BMC Microbiol 2015;15:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Good M, Sodhi CP, Ozolek JA, Buck RH, Goehring KC, Thomas DL, Vikram A, Bibby K, Morowitz MJ, Firek B et al. Lactobacillus rhamnosus HN001 decreases the severity of necrotizing enterocolitis in neonatal mice and preterm piglets: evidence in mice for a role of TLR9. Am J Physiol Gastrointest Liver Physiol 2014;306:G1021–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Tung J, Barreiro LB, Burns MB, Grenier JC, Lynch J, Grieneisen LE, Altmann J, Alberts SC, Blekhman R, Archie EA. Social networks predict gut microbiome composition in wild baboons. ELife 2015;4:e05224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Vuong HE, Yano JM, Fung TC, Hsiao EY. The microbiome and host behavior. Annu Rev Neurosci 2017;40:21–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Tillisch K, Labus J, Kilpatrick L, Jiang Z, Stains J, Ebrat B, Guyonnet D, Legrain-Raspaud S, Trotin B, Naliboff B et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 2013;144:1394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Cong X, Xu W, Romisher R, Poveda S, Forte S, Starkweather A, Henderson WA. Gut microbiome and infant health: brain-gut-microbiota axis and host genetic factors. Yale J Biol Med 2016;89:299–308. [PMC free article] [PubMed] [Google Scholar]
- 151. Bonaz BL, Bernstein CN. Brain-gut interactions in inflammatory bowel disease. Gastroenterology 2013;144:36–49. [DOI] [PubMed] [Google Scholar]
- 152. Arboleya S, Salazar N, Solís G, Fernández N, Hernández-Barranco AM, Cuesta I, Gueimonde M, de los Reyes-Gavilán CG. Assessment of intestinal microbiota modulation ability of Bifidobacterium strains in in vitro fecal batch cultures from preterm neonates. Anaerobe 2013;19:9–16. [DOI] [PubMed] [Google Scholar]
- 153. Yang Y, Tian J, Yang B. Targeting gut microbiome: a novel and potential therapy for autism. Life Sci 2017;194:111–19. [DOI] [PubMed] [Google Scholar]
- 154. Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, Chow J, Reisman SE, Petrosino JF et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013;155:1451–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Li M, Fallin MD, Riley A, Landa R, Walker SO, Silverstein M, Caruso D, Pearson C, Kiang S, Dahm JL et al. The association of maternal obesity and diabetes with autism and other developmental disabilities. Pediatrics 2016;137:e20152206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Nahum Sacks K, Friger M, Shoham-Vardi I, Abokaf H, Spiegel E, Sergienko R, Landau D, Sheiner E. Prenatal exposure to gestational diabetes mellitus as an independent risk factor for long-term neurologic morbidity of the offspring. Am J Obstet Gynecol 2016;214:S48–9. [DOI] [PubMed] [Google Scholar]
- 157. Buffington SA, Di Prisco GV, Auchtung TA, Ajami NJ, Petrosino JF, Costa-Mattioli M. Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell 2016;165:1762–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Dinan TG, Quigley EM, Ahmed SM, Scully P, O'Brien S, O'Mahony L, O'Mahony S, Shanahan F, Keeling PW. Hypothalamic-pituitary-gut axis dysregulation in irritable bowel syndrome: plasma cytokines as a potential biomarker? Gastroenterology 2006;130:304–11. [DOI] [PubMed] [Google Scholar]
- 159. Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A, Bisson JF, Rougeot C, Pichelin M, Cazaubiel M et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr 2011;105:755–64. [DOI] [PubMed] [Google Scholar]
- 160. de la Fuente-Nunez C, Meneguetti BT, Franco OL, Lu TK. Neuromicrobiology: how microbes influence the brain. ACS Chem Neurosci 2018;9(2):141–50. [DOI] [PubMed] [Google Scholar]
- 161. Ong IM, Gonzalez JG, McIlwain SJ, Sawin EA, Schoen AJ, Adluru N, Alexander AL, Yu JJ. Gut microbiome populations are associated with structure-specific changes in white matter architecture. Transl Psychiatry 2018;8:6. [DOI] [PMC free article] [PubMed] [Google Scholar]