Abstract
Various studies have indicated that the thalamus is involved in controlling both cortico-cortical information flow and cortical communication with the rest of the brain. Detailed anatomy and functional connectivity patterns of the thalamocortical system are essential to understanding the cortical organization and pathophysiology of a wide range of thalamus-related neurological and neuropsychiatric diseases. The current study used resting-state fMRI to investigate the topography of the human thalamocortical system from a functional perspective. The thalamus-related cortical networks were identified by performing independent component analysis on voxel-based thalamic functional connectivity maps across a large group of subjects. The resulting functional brain networks were very similar to well-established resting-state network maps. Using these brain network components in a spatial regression model with each thalamic voxel’s functional connectivity map, we localized the thalamic subdivisions related to each brain network. For instance, the medial dorsal nucleus was shown to be associated with the default mode, the bilateral executive; the medial visual networks, and the pulvinar nucleus was involved in both the dorsal attention and the visual networks. These results revealed that a single nucleus may have functional connections with multiple cortical regions or even multiple functional networks, which may be s potentially related to the function of mediation or modulation of multiple cortical networks. This observed organization of thalamocortical system provided a reference for studying the functions of thalamic sub-regions. The importance of intrinsic connectivity-based mapping of the thalamocortical relationship is discussed, as well as the applicability of the approach for future studies.
Keywords: BOLD, fMRI, thalamus, resting-state
Introduction
The thalamus plays an important role in a diverse array of functions, such as sensory relay, motor control, attention and awareness, which are supported by widely distributed thalamocortical connections. The thalamus can be divided into nine major nuclei, which can be further subdivided into a total of more than 30 nuclei and sub-nuclei (Sherman and Guillery 2013; Morel 1997). The topography of anatomical projections from major thalamic nuclei to main cortical regions has been studied in detail by animal studies and post-mortem human studies (Jones et al. 2007; Llinás and Paré 1991; Nieuwenhuys et al. 2007), and the thalamocortical pathways between first-order relay nuclei and sensorimotor, visual and auditory cortices are well known. However, the vast majority of thalamic sub-regions are formed by higher order relay nuclei, which not only have reciprocal projections with distinct cortical regions but also have nonreciprocal projections from many cortical areas that are not innervated by those nuclei or belong to another circuit system (Guillery et al. 2002; McFarland et al. 2002; Parent et al. 1995; Sherman and Guillery et al. 2002). Moreover, Jones (1998; 2001; 2009) has suggested that within the thalamus, besides the core parvalbumin-immunoreactive neurons which project to the cortex in an area-specific manner, there is a matrix of calbindin-immunoreactive neurons that project diffusely to cortical areas. While the general understanding of structural topography of the thalamocortical relations has not altered significantly in recent years, the relatively simple portrait of information flow between the thalamus and cortical regions has become more complex. On the other hand, thalamic dysfunction has been shown in several psychotic disorders, including major depression (Greicius et al. 2007), Parkinson’s disease (Fasano et al. 2012), and schizophrenia (Andreasen et al. 1994; Corradi-Dell’Acqua et al. 2012; Popken et al. 2000). Therefore, mapping the functional topography of human thalamocortical relationships is important to understand thalamic functions and underlying mechanism of these diseases associated with the thalamus.
Recent advances in neuroimaging techniques have enabled researchers to study human thalamocortical relationships in vivo using diffusion tensor imaging (DTI) (Behrens et al. 2003; Draganski et al. 2008; Traynor et al. 2010; O’Muircheartaigh et al. 2011) and functional MRI (fMRI) (Zhang et al. 2008; Zhang et al. 2010, Kim et al. 2013). Based on the functional connectivity and anatomical connections of the brain, these previous studies have investigated the basic topography of thalamocortical system among distinct thalamic sub-regions and large cortical regions. Studies have also confirmed that the thalamocortical connections observed in humans are similar to those measures of non-human primates (Barbas et al. 1987; Van Essen et al. 1986).
These studies have illustrated the utility of neuroimaging techniques to map thalamocortical relationships in vivo; however, the typically coarse cortical divisions (e.g., the prefrontal lobe, parietal lobe, occipital lobe) of these studies may neglect detailed mapping within each lobe and simplify the underlying cortical organization of the thalamocortical relationships. In a recent study of more detailed thalamocortical relationships, Kim and colleagues performed two-level independent component analysis (ICA) on resting-state fMRI data to parcellate the thalamus into distinct sub-regions and demonstrated that these sub-regions have significant temporal correlations with specific and individual cortical regions (Kim et al. 2013). However, this and previous studies have generally focused on individual anatomical cortical lobes or regions, while the functional organization of the cortex is known to be organized as a set of networks of spatially distinct regions that are not necessarily bounded by lobes (Beckmann et al. 2005; Cordes et al. 2000, 2002; Yeo et al. 2011). Due to the broad functional connections between the thalamus and cortex, it is possible that thalamic nuclei would be expected to not only connect to individual cortical lobes, but also to connect to a set of spatially distinct cortical regions that support similar functions. In order to examine this, we investigated the cortical organization that responds to the thalamus based on the functional connections between the thalamus and cortex, hypothesizing that cortical regions that respond to the thalamus are organized as brain networks.
While the thalamic subdivisions have been examined with increasing specificity, the aforementioned methods have typically ignored the possible existence of functional overlaps within sub-nuclei, instead assigning singular associations to each in a “winner take all” strategy. However, an individual thalamic nucleus may have connections to multiple cortical systems. For example, the medial pulvinar is strongly interconnected with the temporal and prefrontal cortices (Nieuwenhuys et al. 2007; Romanski et al. 1997), and the ventral anterior thalamic nucleus links to the prefrontal and premotor cortices (Zikopoulos and Barbas 2006). In this study, we utilize a methodology specifically designed to be able to both subdivide the thalamus on a fine (voxel-wise) resolution and to determine whether individual sub-region possess multiple cortical associations.
Our goal in this study is to use resting-state fMRI to explore the thalamocortical relationships from a functional perspective. First, whole brain functional connectivity maps are calculated for each voxel within the thalamus. Then, data-driven ICA is performed in order to generate thalamocortical connectivity maps that identify independent patterns of the thalamocortical connections. It is first hypothesized that the pattern of thalamocortical connectivity will be similar to that of the cortical networks typically identified in resting-state fMRI studies. Finally, a spatial regression framework back projects each brain network onto the thalamus, identifying functional subdivisions of the thalamus while also providing the ability to test the hypothesis that individual nuclei have associations with multiple cortical networks.
Materials and Methods
Data Acquisition
Resting-state fMRI and anatomical images were downloaded from the 1000 Functional Connectomes Project (http://fcon_1000.projects.nitrc.org/) (Biswal et al. 2010). The set consisted of 198 subjects (75 males, 123 females), with ages between 18 to 30 years. The fMRI images were collected using a 3T scanner, with TR of 3 seconds, 47 slices, 3mm isotropic voxel size and 119 time points. The first 5 time points were removed, leaving 114 time points for each subject. Each subject’s T1-weighted anatomical scan had been acquired using a magnetization-prepared rapid-acquisition gradient echo (MPRAGE) sequence (192 slices with a 144*192 matrix; voxel size 1.20×1.00×1.33mm3).
Data preprocessing
All fMRI data were preprocessed by using the SPM8 software package (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) and AFNI (http://afni.nimh.nih.gov/afni/), as well as in-house MATLAB (MathWorks) programs. The fMRI datasets were realigned to the first image to correct for head motion and linearly co-registered to each subject’s T1-weighted image. The 6 motion parameters obtained from the rigid body registration and the Euclidean norm of all motion derivatives were regressed out from time series of all the voxels. Each structural image was segmented into grey matter (GM), white matter (WM) and cerebrospinal fluid (CSF). The functional images were then transformed to the standard Montreal Neurological Institute (MNI) template in 3×3×3 mm3 by using the Diffeomorphic Anatomical Registration Through Exponentiated Lie algebra (DARTEL, Ashburner et al. 2007) toolbox. CSF and WM masks were defined by thresholding individual tissue probability maps at 0.95. The first 5 principal components from each of the CSF and WM masks were regressed out from time series of every voxel. No physiologic (e.g., breathing, heartrate, etc.) or global mean signals were regressed (Chang et al. 2013; Saad et al. 2012). Finally, a band pass filter ranging between 0.01–0.1 Hz was applied to each time series. No spatial smoothing was performed.
The thalamus mask
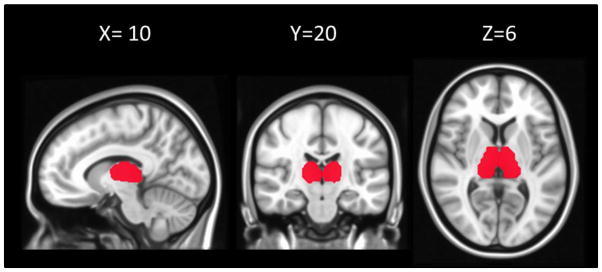
We combined regions 10 (left thalamus) and 49 (right thalamus) from the Harvard-Oxford cortical and subcortical structural atlases (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases) in order to define a mask for the thalamus. This mask was then downsampled into matching 3×3×3 mm3 in MNI space. 679 voxels were included in the final thalamus mask (Figure 1).
Figure 1.
The thalamus mask (679 voxels) defined using the Harvard-Oxford cortical and subcortical structural atlases and overlaid on a MNI template. The slice number of x, y, and z are given in MNI coordinates.
Identifying thalamic connectivity patterns
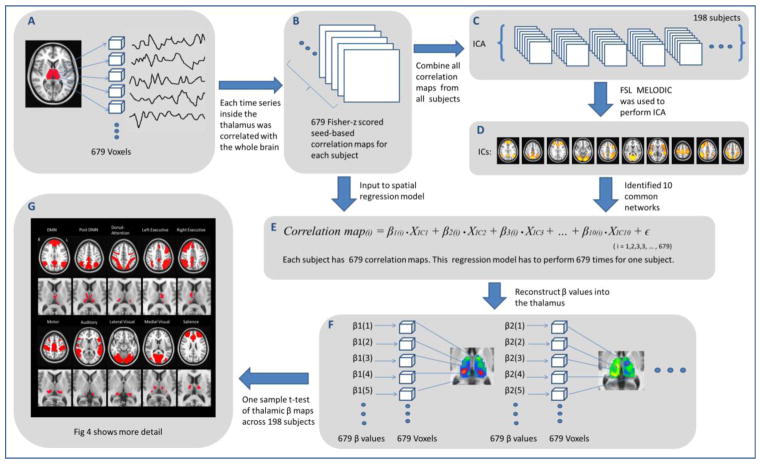
The sequence of steps used to identify the thalamic functional connectivity pattern is presented schematically in Fig 2. First, we calculated the functional connectivity of each voxel in the thalamus to all the voxels across the whole brain using Pearson’s correlation coefficient, resulting in 679 seed-based correlation maps for each subject (Fig 2A, Fig 2B). All correlation maps were Fisher-transformed to Z-scores.
Figure 2.
Schematic flow of thalamocortical functional connectivity analysis. Step A: time series from the thalamus mask (679 voxels in total) were extracted. Step B: each time series from each voxel of the thalamus was correlated to the rest of the whole brain. Step C: ICA was performed on functional connectivity maps. Step D: 10 well-established brain networks were identified (NB: in this panel thresholding is only for visualization; IC maps were not thresholded at this stage of analysis) Step E: the spatial regression model was used to calculate the thalamic β maps. Step F: thalamic β maps were constructed for every network. Step G: one sample t-test was performed across every thalamic β map of each subject.
To identify spatial patterns underlying these thalamic connectivity maps, spatial ICA (Beckmann et al. 2005; Biswal et al. 2010; Greicius et al. 2004) was performed on this set of seed-based correlation maps (Fig 2C). Z-score maps from all subjects were concatenated into a single 4D dataset; since a single volume had 61×73×61 voxels, and there were 679 volumes for each of the 198 subjects, the shape of the final 4D dataset was 61×73×61×134442 voxels. Then FSL’s MELODIC (Multivariate Exploratory Linear Optimized Decomposition into Independent Components) (Beckmann 2005) was used to decompose this 4D data into 20 spatially independent components (ICs), representing large-scale patterns of functional connectivity of the thalamus to the whole brain (Biswal et al. 2010; Damoiseaux et al. 2006; Smith et al. 2009). From this set of ICs, we were able to identify 10 known brain networks for further analysis (Fig 2D).
From brain networks to the thalamus
For each subject, in order to measure the functional connectivity between each thalamic voxel and brain network, we then measured the contributions of each IC network with the individual thalamic connectivity maps (Fig 2E). To do this, the thalamic correlation maps and IC maps were transformed into vectors, and linear regression was used to quantify network relationships per voxel. For a given voxel in the thalamus, a linear regression was used to measure the contribution from each IC (i.e., each was used in vectorised form as a predictor in the model) to the voxel’s seed-based correlation map (i.e., each was used in vectorised form as the dependent variable in the model). For each subject and each voxel in the thalamus, the linear regression model for deriving these contributions was given as follows:
where y(i) is the thalamic correlation map of the ith voxel, VICj is the jth IC map; βj(i) is a matrix of values quantifying the relative contribution of each brain network to every thalamic correlation map; and ε is a vector of the residuals of the model. The regression analysis was performed for all the 679 voxels in the thalamus, and 10 β values were obtained for each voxel. In order to find significant clusters in the thalamus for each network IC, a voxel-wise, one sample t-test was performed on every network’s thalamic β map across subjects using the FSL randomise (Fig 2F), using threshold-free cluster enhancement (TFCE) to threshold the final statistical maps at a family wise error (FWE) corrected value of p<0.05 (Smith et al. 2009).
Identification of the thalamic nuclei
The thalamus atlas by Morel and Krauth (Morel 1997; Krauth 2010) was used to identify the thalamic nuclei. This atlas was primarily constructed based on the postmortem examination of nine stereotactic cuts from five healthy human brains, and built in MNI space including 40 small thalamic nuclei. In addition to visual inspection of the thalamic nuclei locations, we first downsampled the atlas from 1mm to 3mm voxel size (matching the fMRI resolution) and then directly measured the overlap between each nucleus in the atlas and thalamus-corresponding subregion. The correspondence table is provided in the supplementary material.
Results
Group level spatial ICA of thalamic correlation maps
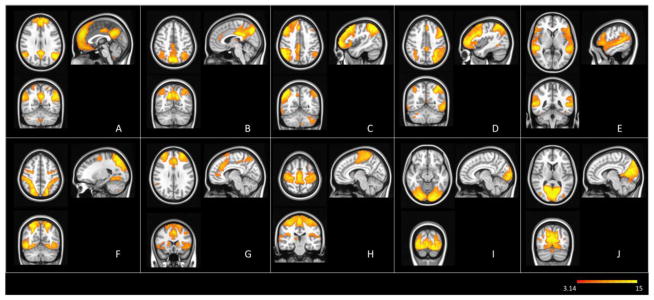
Spatial ICA on thalamic correlation maps across subjects was implemented to produce 20 ICs, of which 10 were visually identified as being quite similar to the standard networks that have generally been observed in resting-state fMRI studies (Beckman et al. 2005; Biswal et al. 1995, 2010). These networks corresponded to: the default mode (DMN), the posterior DMN, left and right executive, auditory, dorsal attention, motor, salience, lateral visual, and medial visual networks (Fig. 3). The peak coordinates of clusters of these networks are listed in supplementary Table S1. The maps of the remaining 10 ICs are also illustrated in supplementary Fig S1. Additionally, several subcortical components were observed (see the supplementary materials), and while these may have functional interactions with the thalamus, they were beyond the scope of the current study, which focused on cortical connections.
Figure 3. Thalamus-related cortical networks from group level ICA.
Networks are thresholded at p<0.05 and rendered on MNI template. Identified brain networks include: (A) the default mode (DMN); (B) the posterior DMN; (C) the left executive; (D) the right executive; (E) the auditory; (F) the dorsal-attention; (G) the salience; (H) the sensorimotor; (I) the lateral visual; and (J) the medial visual networks.
Thalamic mapping of corresponding brain networks
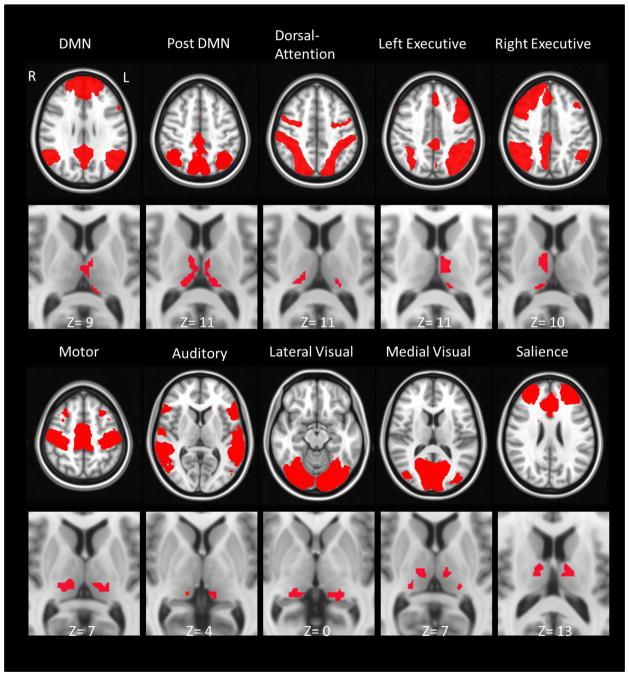
Fig. 4 shows thalamic sub-regions associated with each of the ten final networks. The DMN-related sub-regions encompass the left anterior nucleus (ventral portion), intralamininar nucleius, left ventral lateral nuclei (VL), as well as the mediodorsal nucleus. The thalamic subregion related to the posterior DMN encompasses a large portion of the thalamus, including the intralaminar nucleus, the medial dorsal nucleus, the VL nucleus, and the pulvinar. The dorsal attention network corresponds to the posterior part of the thalamus, mainly the pulvinar, and lateral posterior nucleus. The left and the right executive networks show lateralized and predominantly symmetric patterns, covering the major parts of the mediodorsal nucleus and the pulvinar (medial portion), VL nucleus. The motor network appears to be associated with the ventral posterior lateral nucleus and covers a small portion of the medial dorsal nucleus. Small sub-regions associated with the auditory network are located at the medial pulvinar nucleus around the medial geniculate nucleus. Both the lateral and the medial visual networks are mainly associated with the pulvinar, but only the medial visual network showed relation to the medial portion of the thalamus, such as the intralaminar, mediodorsal and ventral posterior nucleus. The thalamic sub-regions corresponding to the salience network are found mostly at the VL nucleus, also small portion at the anterior part of the thalamus, including the anterior nuclei and the ventral anterior (VA)..
Figure 4.
Thalamic sub-regions that correspond to different brain networks. All those subregions of the thalamus are threshold at FWE corrected p< 0.05.
Overlap between brain network corresponding regions inside the thalamus
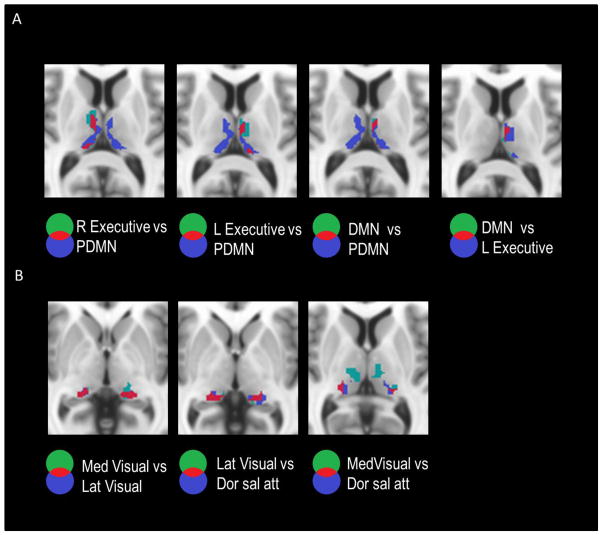
Most of the thalamic sub-divisions corresponding to different networks are discernable from each other (Fig 4). However, spatial overlaps are apparent among some of these sub-divisions. Overlaps among the thalamic regions associated with the DMN and the executive network ICs are shown in Fig 5A. The overlaps of thalamic regions that correspond to the DMN and right executive network were localized in the left thalamus, namely in the medial dorsal nucleus and anterior nucleus, as well as in the pulvinar nucleus. The overlaps of thalamic regions corresponding to the DMN and the left executive network revealed similar patterns but in the right thalamus. In addition, bilateral overlaps were also observed in the pulvinar nucleus that corresponded to the lateral visual, medial visual and attention networks (Fig 5B).
Figure 5.
Overlaps of thalamic regions that corresponding to different networks. In each labelled pair, the overlapping thalamic voxels of the first (green) and second (blue) nuclei are highlighted in red for the following associated networks. Panel A shows the overlaps of thalamus regions that were associated with the DMN and the bilateral executive networks. Panel B demonstrates the overlaps of thalamic regions that were associated with the lateral and medial visual networks and the dorsal attention network.
Discussion
This study utilized resting-state fMRI to investigate the connectivity of the thalamocortical system. The current study demonstrate for the first time that cortical regions that show functional connectivity with the thalamus were organized as networks which were spatially similar to resting state networks. We then identified sub-regions of the thalamus that corresponded to those different brain networks, as well as functional overlaps between those sub-regions. The spatial patterns of thalamic subdivisions observed in the present study are predominantly consistent with a previous cytoarchitectonic study and fMRI studies (Kim et al. 2013; Zhang et al. 2008, 2010).
Due to the heterogeneous variation of the size and location of thalamic nuclei, as well as the necessary spatial resolution constraint of the whole brain fMRI data, it is difficult to precisely identify each thalamic nucleus via the standardized template. Moreover, the thalamus atlas used here as a reference was constructed from histological data (Morel et al. 1997) and then reconstructed in the MNI grid (Kruth et al. 2010). Therefore, a certain inherent mismatch can be expected in the overlap maps. To minimize resulting errors, we mainly focused on the largest of the 40 nuclei present in the Morel atlas, such as the mediodorsal and pulvinar nuclei, without differentiating finer sub-divisions of these nuclei (which would likely be considered ‘sub-resolution’ in this study). However, the detailed nuclei identifications have been included in the supplementary material (Table S3–S6).
Thalamocortical relationships
One critical step in studying thalamocortical relationships is to properly define corresponding cortical target regions, so that the thalamocortical association can be mapped accordingly. Previous studies have investigated relations to individual cortical lobes and regions. In the current study, instead of predefining cortical locations, we started with observing the functional connectivity patterns of thalamocortical system. We performed spatial ICA on a series of functional correlation maps derived using each thalamic voxel as a seed and identified the spatially independent patterns of the thalamocortical connectivity maps. Interestingly, the observed independent cortical networks closely resembled resting-state networks (Biswal et al. 2010; Cole et al. 2010). This result suggested that the thalamus has distributed cortical functional connections across all the distinct networks and stresses the importance of defining cortical target regions based on data driven thalamic interactions. However, because these cortical regions within a network generally have high functional connectivity, it would be possible that only some of these regions within a network are directly connected to the thalamus sub-regions, and the functional connectivity between other regions and the thalamus are mediated by that single region in the network. This question may be resolved by using other imaging methods (e.g. DTI) to examine the white matter connections between certain cortical regions and the thalamus.
Specific networks
By applying a spatial regression model, thalamic sub-regions that were associated with different networks were identified. The thalamic sub-regions associated with different networks agree with previous studies, especially for uni-modal networks such as the sensorimotor, visual and auditory networks. It is commonly acknowledged that the ventral posterior nuclei are involved in sensorimotor functions (Diamond et al. 1992; Jones et al. 2007; Koralek et al. 1988). The thalamic sub-regions of the motor network roughly matched with this description (Fig 4F). Auditory processing usually involves the medial geniculate body (MGN) (Winer et al. 1992), which can be approximately observed in the thalamic sub-regions corresponding to the auditory network (Fig 4G). The lateral geniculate nucleus (LGN), the lateral posterior nucleus and inferior pulvinar nucleus have been shown to be largely connected to the visual cortex (Adams et al. 2000; Li et al. 2012; Sabatinelli et al. 2011; Zou et al. 2009). These nuclei generally agree with the thalamic sub-regions that correspond to the medial and lateral visual networks (Fig 4H and 4I). It should be noted that, considering the limited spatial resolutions of fMRI images, the results in Fig 4 cannot be expected to match the precision of previous histology studies. Based on the imaging protocols, we can only approximately locate the major thalamic nuclei within the MNI template.
ICA identified three independent components that are associated with visual and attention processing: the medial visual, the lateral visual and the dorsal attention components. Thalamic sub-regions related with these three networks showed overlaps as well as disparities. All three networks revealed associated thalamic sub-regions in the posterior thalamus, mainly in the pulvinar nucleus. Considering that the pulvinar nucleus is an important structure in the visual path and highly involved in attention orienting and selection (LaBerge and Buchsbaum 1990; LaBerge et al. 1995; Roux et al. 2013; Saalmann et al. 2012), the observed spatial associations indicate that the pulvinar might play a critical role in mediating or modulating communications among lower visual, higher visual and attention networks. There are several thalamic sub-region including the intralaminar, MD and ventral lateral nucleus that only appears in the medial visual network (mainly V1), but not in the lateral visual network (mainly V2 and V4). The VL is the only nucleus just appears in the medial visual network, but not in the lateral visual network, nor in the dorsal attention network. The MD has a reciprocal and direct connection with the prefrontal cortex and cingulate cortex (Eckert et al. 2012; Klein et al. 2010); the intralaminar nuclei has a wide projection to stritum and several cortical regions including visual cortex, auditory cortex, anterior cingulate gyrus (Kaufman et al. 1984, Jones et al. 1974); The VL projects to the primary motor area and afferent from cerebellar nuclei (Asanuma et al. 1983), but there is no established anatomical connection between these nuclei and the visual cortex. It is possible that some other regions mediate the functional correlation between these nuclei and the medial visual network. Some evidence suggests that the MD is activated in processing information of spatial location and controlling saccade direction (Watanabe et al. 2004), which indicates that the MD nucleus is involved in processing integral information from sensory and motor system. This hypothesis is in line with the current results of the functional relationships between the MD and the medial visual network. The connection between the intralaminar nucleus, VL and the visual cortex has also been reported (Miller et al. 1979; Kaufman 1985). Noticeably, the multisensory integration theory (Ghazanfar et al. 1996) might provide a reasonable explanation, but our results are far from been able to support any of those theories.
The default mode network has higher metabolic rates during the unconstrained mode of brain functioning (Raichle et al. 2001) but is deactivated during task condition (Binder et al. 2012). It is associated with consciousness, memory retrieval, self-reflection, as well as various psychiatric and neuro-degenerative diseases (Anticevic et al. 2012; Buckner et al. 2005; Greicius et al. 2004; Zhang et al. 2009). The current analysis identified two DMN components (the DMN and PDMN), and their corresponding thalamic sub-divisions. Although these two default mode networks are spatially adjacent or even overlap, several recent studies (Buckner et al. 2008; Stevens et al. 2009; Yang et al. 2013; Zuo et al. 2010) have indicated the potential differences between the DMN and PDMN, especially in terms of development (Power et al. 2010) and aging (Andrews-Hanna et al. 2007; Yang et al. 2014). The functional thalamocortical analysis here revealed both overlaps and differentiated patterns of two networks’ corresponding thalamic subregions. The DMN-associated regions mainly encompassed the anterior portion of the thalamus, such as anterior nucleus and the medial dorsal (MD), while those of the posterior DMN covered the whole MD and a large portion of the pulvinar nucleus (Fig 4B). Previous studies have shown that, the anterior thalamic nucleus projects to the posterior cingulate cortex (Vogt et al. 1979; Kim et al. 2013), the MD projects to prefrontal lobe (Ray and Price et al. 1993; Klein et al. 2010; Eckert et al. 2012; Leonard et al. 1969; Krettek et al. 1977; Goldman et al. 1985; Ray et al. 1993; Klein et al. 2010), and the pulvinar nucleus has widely distributed projections to the parietal, the temporal and the prefrontal lobes (Asanuma et al. 1985; Behrens et al. 2003; Grieve et al. 2000; Mufson et al. 1984; Romanski et al. 1997). Therefore, the functional interactions among these thalamic nuclei and default networks agree with the known underlying anatomical connections. The main thalamic differentiation between those two networks appears to be that the DMN preferentially target the rostral part of the MD and midline nucleus, while the PDMN encompasses nearly the entire MD. Additionally, the DMN covers very small portion of the pulvinar nucleus near the midline, and the pDMN covers a large portion of medial the pulvinar nucleus.
In fact, each sub-division of the MD shows a distinct connection to the prefrontal cortex: the medial MD to the lateral orbitofrontal cortex; the caudodorsal MD to cingulate cortex; and the lateral MD to lateral prefrontal cortex (Ray et al. 1993; Barbas et al. 2000; Klein et al. 2010; Mitchell et al. 2007, 2013). Since the DMN and PDMN encompass at different regions of the prefrontal lobe, it is possible that those differences observed here are due to the different projections from MD to the prefrontal lobe. Additionally, those sub-regions corresponding to two default networks also present different patterns inside the thalamus. The DMN is left lateralized, while the PDMN is bilateralized. Several previous studies mentioned the lateralized thalamic function and lateralized structural connection between the thalamus and cortical regions (Alkonyi et al. 2010; De Witte et al. 2011; Exner et al. 2001; Marchetti et al. 2005; Johnson et al. 2000; Oke et al. 1978), and functional lateralization of DMN (Liu et al. 2009). However, there has been no general consensus on the mechanisms for the lateralization of the thalamic sub-regions of DMN, a topic which requires further investigation.
The bilateral executive networks, which encompass mainly the dorsolateral prefrontal and inferior parietal cortices, have been identified in various task-based neuroimaging studies (Coull et al. 1996; Dormal et al. 2013). Based on thalamocortical connectivity, the lateralized frontoparietal network had a corresponding lateralized projection in the thalamus (Fig 4), mainly the MD and the pulvinar nucleus. This localization agrees with previous anatomical studies in monkeys (Selemon et al. 1988). Both of the frontal and parietal cortex project to the MD nucleus and lateral dorsal nuclei. The parietal projection occupied the lateral part of the medial pulvinar (Fig. 4). In addition, sub-regions associated with DMN and bilateral executive networks are highly overlapped in the MD and pulvinar (Fig 5). In fact, the competitive interaction between the DMN (without the differentiated posterior region) and the executive network has been frequently observed (Chen et al. 2013; Fox et al. 2007; Hellyer et al. 2014; Sridharan et al. 2008). However the underlying mechanism that regulates these two networks is still unclear. Based the observed thalamocortical functional connectivity, it is possible that the same neural ensembles within thalamic nucleus interact with both the DMN and executive network. Therefore, these thalamic sub-regions might play a role in mediating or modulating these two major cortical networks. By using specific models such as the physiophysiological interaction (Friston et al. 1997) and the dynamic causal modeling (Friston et al. 2003), future studies might provide more detailed functional relationships among the thalamus, DMN and executive networks.
The salience network, first defined by Seeley and colleagues, mainly covers the bilateral anterior insula, inferior frontal and the anterior cingulate cortices (Seeley et al. 2007). The current analysis identified two corresponding clusters in the anterior portion of the thalamus, including Anterior nucleus, VA, VL, and intralaminar nucleus, which were in line with previous anatomic studies using nonhuman animals, in which, for example, the anterior cingulate cortex projects to MD, VA/VL and intralaminar nuclei (Harber and McFarland 2001; Jeon et al. 2014; Kievit and Kuypers et al. 1975; Xiao and Barbas, 2004, 2009; Zikopoulos and Barbas, 2007). The anterior insula is known to be anatomically connected to the ventromedial posterior nucleus and the intralaminar nuclei (Guldin and Markowitsch 1983). However, the locations of thalamic subdivisions associated with the salience and executive networks in the current study differ from what has been shown in Fig 5 of Seeley et al. (2007). The current results indicated that the thalamic subdivisions of the salience network are functionally connected to the anterior portion of the thalamus and those of the executive networks are in the medial potion, while Seeley et al. (2007) illustrated that the salience-related thalamic regions are more posterior than those of the executive network. Nevertheless, recent studies using high-field fMRI have also shown that the VA and intralaminar nuclei were involved in directing attention toward upcoming stimuli, and that the MD was more associated with the content of the stimuli rather than specific stimuli intensity (Metzger et al. 2010; Walter et al. 2008). These differences may reflect the heterogeneous nature of thalamic connections observed using different experimental paradigms, as well as simply the relatively low spatial resolution of FMRI.. Follow-up investigations with high resolution functional images (such as, Metzger et al. 2013) may provide a more detailed spatial mapping. In addition, other studies have indicated that the thalamus is only involved in the functioning of the salience network when attention resources explicitly are required (Engstrom et al. 2013). Although the precise role that the thalamus plays with regard to the salience network is uncertain, the present study reinforces the evidence of the presence of functional thalamocortical interaction. The disparities of the attributed thalamic functional activity among all of these studies may be due to the divergent functional configuration between task co-activation and resting state network (Boly et al. 2012; Bullmore and Sporns et al. 2012; Di et al. 2013).
Methodology Concerns
The current method provides a novel approach to assessing functional interaction between a particular brain region and the whole brain. By applying this method to study the thalamus, we demonstrated that cortical regions that interacted with the thalamus were organized as spatially distinct networks, in particular as commonly identified (resting state) functional networks. This widespread functional connectivity may result from the fact that the thalamus has widely spread structural connectivity with cortical regions. It is likely that the current method can be applied to other brain regions, e.g., the basal ganglia, the hippocampus, and the visual cortex. However, whether their connectivity maps could show network-like patterns still needs further verification.
The current analysis adopted spatial ICA to unravel thalamocortical connectivity patterns, which introduced some practical problem of ICA. An existing, main concern of ICA is the dependency of results on the selection of the number of ICs that are specified. Since we are interested in major brain networks that are generally analyzed in literatures (Biswal et al. 2010; Cole et al. 2010), a subset of the 20 computed components was extracted for further analysis, namely those which were visually identifiable as networks that have been shown in previous studies. Even so, ICs are known to show degrees of variability as the dimensionality of the decomposition changes, which would subtly alter the overlap pattern of the thalamic subdivisions at small scale. However, it should be noted that the spatial regression model was implemented to reduce the spurious effects of the low magnitude spatial fluctuation. While, some recent studies have suggested that extracting larger number of ICs to present finer network structure (Kim et al. 2013; Smith et al. 2009). It is also possible that increasing model order might also result in less reliable ICs and a decrease in ICA repeatability (Abou-Elseoud et al. 2010).
Previous studies have shown that reconfigurations of functional connectivity between task and rest conditions (Boly et al. 2012; Bullmore and Sporns 2012; Di et al. 2013; Gili et al. 2013). Since this current study is mainly based on the resting-state fMRI, it is unclear whether the thalamocortical interactions remain the same in the task conditions. Therefore, by combining resting-state and task-based fMRI, we might be able to provide a better understanding of the thalamocortical system.
Conclusion
In summary, by using a data driven approach, thalamic nuclei were shown to be functionally associated with networks of cortical regions, which were observed to correspond to known brain networks. Additionally, individual thalamic nuclei were observed to be associated with multiple networks, as hypothesized. This study demonstrated a fine functional topography of the thalamocortical system, provided a functional reference for thalamus studies, and bolstered the idea that the higher-order nuclei, such as VA, MD, pulvinar nucleus might be connected to several functional networks and are involved in multiple functions.
Supplementary Material
Acknowledgments
This research was supported by NIH 5R01NS049176 (BBB).
References
- Abou-Elseoud A, Starck T, Remes J, Nikkinen J, Tervonen O, Kiviniemi V. The effect of model order selection in group PICA. Hum Brain Mapp. 2010;31:1207–1216. doi: 10.1002/hbm.20929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams MM, Hof PR, Gattass R, Webster MJ, Ungerleider LG. Visual cortical projections and chemoarchitecture of macaque monkey pulvinar. J Comp Neurol. 2000;419:377–393. doi: 10.1002/(sici)1096-9861(20000410)419:3<377::aid-cne9>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Alkonyi B, Juhasz C, Muzik O, Behen ME, Jeong JW, Chugani HT. Thalamocortical connectivity in healthy children: asymmetries and robust developmental changes between ages 8 and 17 years. AJNR Am J Neuroradiol. 2011;32:962–969. doi: 10.3174/ajnr.A2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Arndt S, Swayze V, Cizadlo T, Flaum M, O’Leary D, Ehrhardt JC, Yuh WT. Thalamic abnormalities in schizophrenia visualized through magnetic resonance image averaging. Science. 1994;266:294–298. doi: 10.1126/science.7939669. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Cole MW, Murray JD, Corlett PR, Wang XJ, Krystal JH. The role of default network deactivation in cognition and disease. Trends in cognitive sciences. 2012;16:584–592. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanuma C, Andersen RA, Cowan WM. The thalamic relations of the caudal inferior parietal lobule and the lateral prefrontal cortex in monkeys: divergent cortical projections from cell clusters in the medial pulvinar nucleus. J Comp Neurol. 1985;241:357–381. doi: 10.1002/cne.902410309. [DOI] [PubMed] [Google Scholar]
- Asanuma C, Thach WT, Jones EG. Cytoarchitectonic delineation of the ventral lateral thalamic region in the monkey. Brain Res. 1983;286:219–235. doi: 10.1016/0165-0173(83)90014-0. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Barbas H. Connections underlying the synthesis of cognition, memory, and emotion in primate prefrontal cortices. Brain Res Bull. 2000;52:319–330. doi: 10.1016/s0361-9230(99)00245-2. [DOI] [PubMed] [Google Scholar]
- Barbas H, Pandya DN. Architecture and frontal cortical connections of the premotor cortex (area 6) in the rhesus monkey. J Comp Neurol. 1987;256:211–228. doi: 10.1002/cne.902560203. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TEJ, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CAM, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6:750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- Binder JR. Task-induced deactivation and the “resting” state. Neuroimage. 2012;62:1086–1091. doi: 10.1016/j.neuroimage.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo X-N, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski A-M, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kotter R, Li S-J, Lin C-P, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SARB, Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng G-J, Veijola J, Villringer A, Walter M, Wang L, Weng X-C, Whitfield-Gabrieli S, Williamson P, Windischberger C, Zang Y-F, Zhang H-Y, Castellanos FX, Milham MP. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boly M, Perlbarg V, Marrelec G, Schabus M, Laureys S, Doyon J, Pelegrini-Issac M, Maquet P, Benali H. Hierarchical clustering of brain activity during human nonrapid eye movement sleep. Proc Natl Acad Sci U S A. 2012;109:5856–5861. doi: 10.1073/pnas.1111133109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. The economy of brain network organization. Nat Rev Neurosci. 2012;13:336–349. doi: 10.1038/nrn3214. [DOI] [PubMed] [Google Scholar]
- Chang C, Metzger CD, Glover GH, Duyn JH, Heinze HJ, Walter M. Association between heart rate variability and fluctuations in resting-state functional connectivity. Neuroimage. 2013;68:93–104. doi: 10.1016/j.neuroimage.2012.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AC, Oathes DJ, Chang C, Bradley T, Zhou Z-W, Williams LM, Glover GH, Deisseroth K, Etkin A. Causal interactions between fronto-parietal central executive and default-mode networks in humans. Proc Natl Acad Sci U S A. 2013;110:19944–19949. doi: 10.1073/pnas.1311772110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi EY, Yeo BTT, Buckner RL. The organization of the human striatum estimated by intrinsic functional connectivity. J Neurophysiol. 2012;108:2242–2263. doi: 10.1152/jn.00270.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DM, Smith SM, Beckmann CF. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Frontiers in systems neuroscience. 2010;4:8. doi: 10.3389/fnsys.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes D, Haughton V, Carew JD, Arfanakis K, Maravilla K. Hierarchical clustering to measure connectivity in fMRI resting-state data. Magn Reson Imaging. 2002;20:305–317. doi: 10.1016/s0730-725x(02)00503-9. [DOI] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Wendt GJ, Turski PA, Moritz CH, Quigley MA, Meyerand ME. Mapping functionally related regions of brain with functional connectivity MR imaging. AJNR Am J Neuroradiol. 2000;21:1636–1644. [PMC free article] [PubMed] [Google Scholar]
- Corradi-Dell’Acqua C, Tomelleri L, Bellani M, Rambaldelli G, Cerini R, Pozzi-Mucelli R, Balestrieri M, Tansella M, Brambilla P. Thalamic-insular dysconnectivity in schizophrenia: evidence from structural equation modeling. Hum Brain Mapp. 2012;33:740–752. doi: 10.1002/hbm.21246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JT, Frith CD, Frackowiak RS, Grasby PM. A fronto-parietal network for rapid visual information processing: a PET study of sustained attention and working memory. Neuropsychologia. 1996;34:1085–1095. doi: 10.1016/0028-3932(96)00029-2. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SARB, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca M, Smith S, De Stefano N, Federico A, Matthews PM. Blood oxygenation level dependent contrast resting state networks are relevant to functional activity in the neocortical sensorimotor system. Exp Brain Res. 2005;167:587–594. doi: 10.1007/s00221-005-0059-1. [DOI] [PubMed] [Google Scholar]
- De Witte L, Brouns R, Kavadias D, Engelborghs S, De Deyn PP, Marien P. Cognitive, affective and behavioural disturbances following vascular thalamic lesions: a review. Cortex. 2011;47:273–319. doi: 10.1016/j.cortex.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Di X, Gohel S, Kim EH, Biswal BB. Task vs. rest-different network configurations between the coactivation and the resting-state brain networks. Front Hum Neurosci. 2013;7:493. doi: 10.3389/fnhum.2013.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond ME, Armstrong-James M, Ebner FF. Somatic sensory responses in the rostral sector of the posterior group (POm) and in the ventral posterior medial nucleus (VPM) of the rat thalamus. J Comp Neurol. 1992;318:462–476. doi: 10.1002/cne.903180410. [DOI] [PubMed] [Google Scholar]
- Dormal V, Dormal G, Joassin F, Pesenti M. A common right fronto-parietal network for numerosity and duration processing: an fMRI study. Hum Brain Mapp. 2012;33:1490–1501. doi: 10.1002/hbm.21300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B, Kherif F, Kloppel S, Cook PA, Alexander DC, Parker GJM, Deichmann R, Ashburner J, Frackowiak RSJ. Evidence for segregated and integrative connectivity patterns in the human Basal Ganglia. J Neurosci. 2008;28:7143–7152. doi: 10.1523/JNEUROSCI.1486-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert U, Metzger CD, Buchmann JE, Kaufmann J, Osoba A, Li M, Safron A, Liao W, Steiner J, Bogerts B, Walter M. Preferential networks of the mediodorsal nucleus and centromedian-parafascicular complex of the thalamus--a DTI tractography study. Hum Brain Mapp. 2012;33:2627–2637. doi: 10.1002/hbm.21389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engstrom M, Landtblom AM, Karlsson T. Brain and effort: brain activation and effort-related working memory in healthy participants and patients with working memory deficits. Front Hum Neurosci. 2013;7:140. doi: 10.3389/fnhum.2013.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exner C, Weniger G, Irle E. Implicit and explicit memory after focal thalamic lesions. Neurology. 2001;57:2054–2063. doi: 10.1212/wnl.57.11.2054. [DOI] [PubMed] [Google Scholar]
- Fair DA, Bathula D, Mills KL, Dias TG, Blythe MS, Zhang D, Snyder AZ, Raichle ME, Stevens AA, Nigg JT, Nagel BJ. Maturing thalamocortical functional connectivity across development. Frontiers in systems neuroscience. 2010;4:10. doi: 10.3389/fnsys.2010.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano A, Daniele A, Albanese A. Treatment of motor and non-motor features of Parkinson’s disease with deep brain stimulation. Lancet neurology. 2012;11:429–442. doi: 10.1016/S1474-4422(12)70049-2. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003;19:1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Ghazanfar AA, Schroeder CE. Is neocortex essentially multisensory? Trends in cognitive sciences. 2006;10:278–285. doi: 10.1016/j.tics.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Gili T, Saxena N, Diukova A, Murphy K, Hall JE, Wise RG. The thalamus and brainstem act as key hubs in alterations of human brain network connectivity induced by mild propofol sedation. J Neurosci. 2013;33:4024–4031. doi: 10.1523/JNEUROSCI.3480-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Porrino LJ. The primate mediodorsal (MD) nucleus and its projection to the frontal lobe. J Comp Neurol. 1985;242:535–560. doi: 10.1002/cne.902420406. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve KL, Acuna C, Cudeiro J. The primate pulvinar nuclei: vision and action. Trends Neurosci. 2000;23:35–39. doi: 10.1016/s0166-2236(99)01482-4. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Sherman SM. Thalamic relay functions and their role in corticocortical communication: generalizations from the visual system. Neuron. 2002;33:163–175. doi: 10.1016/s0896-6273(01)00582-7. [DOI] [PubMed] [Google Scholar]
- Guldin WO, Markowitsch HJ. Cortical and thalamic afferent connections of the insular and adjacent cortex of the rat. J Comp Neurol. 1983;215:135–153. doi: 10.1002/cne.902150203. [DOI] [PubMed] [Google Scholar]
- Haber S, McFarland NR. The place of the thalamus in frontal cortical-basal ganglia circuits. The Neuroscientist: a review journal bringing neurobiology, neurology and psychiatry. 2001;7:315–324. doi: 10.1177/107385840100700408. [DOI] [PubMed] [Google Scholar]
- Hellyer PJ, Shanahan M, Scott G, Wise RJS, Sharp DJ, Leech R. The control of global brain dynamics: opposing actions of frontoparietal control and default mode networks on attention. J Neurosci. 2014;34:451–461. doi: 10.1523/JNEUROSCI.1853-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood DC, Raza AS, Kay KY, Sandler SF, Xin D, Ritch R, Liebmann JM. A comparison of retinal nerve fiber layer (RNFL) thickness obtained with frequency and time domain optical coherence tomography (OCT) Optics express. 2009;17:3997–4003. doi: 10.1364/oe.17.003997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon HA, Anwander A, Friederici AD. Functional network mirrored in the prefrontal cortex, caudate nucleus, and thalamus: high-resolution functional imaging and structural connectivity. J Neurosci. 2014;34:9202–9212. doi: 10.1523/JNEUROSCI.0228-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H, Behrens TEJ, Robson MD, Drobnjak I, Rushworth MFS, Brady JM, Smith SM, Higham DJ, Matthews PM. Changes in connectivity profiles define functionally distinct regions in human medial frontal cortex. Proc Natl Acad Sci U S A. 2004;101:13335–13340. doi: 10.1073/pnas.0403743101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MD, Ojemann GA. The role of the human thalamus in language and memory: evidence from electrophysiological studies. Brain and cognition. 2000;42:218–230. doi: 10.1006/brcg.1999.1101. [DOI] [PubMed] [Google Scholar]
- Jones EG. Synchrony in the interconnected circuitry of the thalamus and cerebral cortex. Ann N Y Acad Sci. 2009;1157:10–23. doi: 10.1111/j.1749-6632.2009.04534.x. [DOI] [PubMed] [Google Scholar]
- Jones EG. The thalamic matrix and thalamocortical synchrony. Trends Neurosci. 2001;24:595–601. doi: 10.1016/s0166-2236(00)01922-6. [DOI] [PubMed] [Google Scholar]
- Jones EG. The thalamus. Vol. 1. Cambridge university press; 2007. [Google Scholar]
- Jones EG. Viewpoint: the core and matrix of thalamic organization. Neuroscience. 1998;85:331–345. doi: 10.1016/s0306-4522(97)00581-2. [DOI] [PubMed] [Google Scholar]
- Jones EG, Leavitt RY. Retrograde axonal transport and the demonstration of non-specific projections to the cerebral cortex and striatum from thalamic intralaminar nuclei in the rat, cat and monkey. J Comp Neurol. 1974;154:349–377. doi: 10.1002/cne.901540402. [DOI] [PubMed] [Google Scholar]
- Kaufman EF, Rosenquist AC. Efferent projections of the thalamic intralaminar nuclei in the cat. Brain Res. 1985;335:257–279. doi: 10.1016/0006-8993(85)90478-0. [DOI] [PubMed] [Google Scholar]
- Kievit J, Kuypers HG. Subcortical afferents to the frontal lobe in the rhesus monkey studied by means of retrograde horseradish peroxidase transport. Brain Res. 1975;85:261–266. doi: 10.1016/0006-8993(75)90079-7. [DOI] [PubMed] [Google Scholar]
- Kim D-J, Park B, Park H-J. Functional connectivity-based identification of subdivisions of the basal ganglia and thalamus using multilevel independent component analysis of resting state fMRI. Hum Brain Mapp. 2013;34:1371–1385. doi: 10.1002/hbm.21517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein JC, Rushworth MFS, Behrens TEJ, Mackay CE, de Crespigny AJ, D’Arceuil H, Johansen-Berg H. Topography of connections between human prefrontal cortex and mediodorsal thalamus studied with diffusion tractography. Neuroimage. 2010;51:555–564. doi: 10.1016/j.neuroimage.2010.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koralek KA, Jensen KF, Killackey HP. Evidence for two complementary patterns of thalamic input to the rat somatosensory cortex. Brain Res. 1988;463:346–351. doi: 10.1016/0006-8993(88)90408-8. [DOI] [PubMed] [Google Scholar]
- Krauth A, Blanc R, Poveda A, Jeanmonod D, Morel A, Szekely G. A mean three-dimensional atlas of the human thalamus: generation from multiple histological data. Neuroimage. 2010;49:2053–2062. doi: 10.1016/j.neuroimage.2009.10.042. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. The cortical projections of the mediodorsal nucleus and adjacent thalamic nuclei in the rat. J Comp Neurol. 1977;171:157–191. doi: 10.1002/cne.901710204. [DOI] [PubMed] [Google Scholar]
- LaBerge D. Attentional processing: The brain’s art of mindfulness. Vol. 2. Harvard University Press; 1995. [Google Scholar]
- LaBerge D, Buchsbaum MS. Positron emission tomographic measurements of pulvinar activity during an attention task. J Neurosci. 1990;10:613–619. doi: 10.1523/JNEUROSCI.10-02-00613.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard CM. The prefrontal cortex of the rat. I. Cortical projection of the mediodorsal nucleus. II. Efferent connections. Brain Res. 1969;12:321–343. doi: 10.1016/0006-8993(69)90003-1. [DOI] [PubMed] [Google Scholar]
- Li C, Chen K, Han H, Chui D, Wu J. An FMRI study of the neural systems involved in visually cued auditory top-down spatial and temporal attention. PLoS One. 2012;7 doi: 10.1371/journal.pone.0049948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Stufflebeam SM, Sepulcre J, Hedden T, Buckner RL. Evidence from intrinsic activity that asymmetry of the human brain is controlled by multiple factors. Proc Natl Acad Sci U S A. 2009;106:20499–20503. doi: 10.1073/pnas.0908073106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas RR, Pare D. Of dreaming and wakefulness. Neuroscience. 1991;44:521–535. doi: 10.1016/0306-4522(91)90075-y. [DOI] [PubMed] [Google Scholar]
- Long B, Liang S, Xin D, Yang Y, Xiang J. Synthesis, characterization and in vitro antiproliferative activities of new 13-cis-retinoyl ferrocene derivatives. European journal of medicinal chemistry. 2009;44:2572–2576. doi: 10.1016/j.ejmech.2009.01.029. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Dzemidzic M, Lurito JT, Mathews VP, Phillips MD. Correlations in low-frequency BOLD fluctuations reflect cortico-cortical connections. Neuroimage. 2000;12:582–587. doi: 10.1006/nimg.2000.0654. [DOI] [PubMed] [Google Scholar]
- Marchetti C, Carey D, Della Sala S. Crossed right hemisphere syndrome following left thalamic stroke. J Neurol. 2005;252:403–411. doi: 10.1007/s00415-005-0656-8. [DOI] [PubMed] [Google Scholar]
- Metzger CD, Eckert U, Steiner J, Sartorius A, Buchmann JE, Stadler J, Tempelmann C, Speck O, Bogerts B, Abler B, Walter M. High field FMRI reveals thalamocortical integration of segregated cognitive and emotional processing in mediodorsal and intralaminar thalamic nuclei. Frontiers in neuroanatomy. 2010;4:138. doi: 10.3389/fnana.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger CD, van der Werf YD, Walter M. Functional mapping of thalamic nuclei and their integration into cortico-striatal-thalamo-cortical loops via ultra-high resolution imaging-from animal anatomy to in vivo imaging in humans. Front Neurosci. 2013;7:24–24. doi: 10.3389/fnins.2013.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JW, Benevento LA. Demonstration of a direct projection from the intralaminar central lateral nucleus to the primary visual cortex. Neuroscience letters. 1979;14:229–234. doi: 10.1016/0304-3940(79)96153-6. [DOI] [PubMed] [Google Scholar]
- Mitchell AS, Browning PG, Baxter MG. Neurotoxic lesions of the medial mediodorsal nucleus of the thalamus disrupt reinforcer devaluation effects in rhesus monkeys. J Neurosci. 2007;27:11289–11295. doi: 10.1523/JNEUROSCI.1914-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AS, Chakraborty S. What does the mediodorsal thalamus do? Frontiers in systems neuroscience. 2013;7:37. doi: 10.3389/fnsys.2013.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel A, Magnin M, Jeanmonod D. Multiarchitectonic and stereotactic atlas of the human thalamus. J Comp Neurol. 1997;387:588–630. doi: 10.1002/(sici)1096-9861(19971103)387:4<588::aid-cne8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Mesulam MM. Thalamic connections of the insula in the rhesus monkey and comments on the paralimbic connectivity of the medial pulvinar nucleus. J Comp Neurol. 1984;227:109–120. doi: 10.1002/cne.902270112. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R, Voogd Jan, VanHuijzen Christiaan. The Human Central Nervous System: A Synopsis and Atlas. 2007 [Google Scholar]
- O’Muircheartaigh J, Vollmar C, Traynor C, Barker GJ, Kumari V, Symms MR, Thompson P, Duncan JS, Koepp MJ, Richardson MP. Clustering probabilistic tractograms using independent component analysis applied to the thalamus. Neuroimage. 2011;54:2020–2032. doi: 10.1016/j.neuroimage.2010.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oke A, Keller R, Mefford I, Adams RN. Lateralization of norepinephrine in human thalamus. Science. 1978;200:1411–1413. doi: 10.1126/science.663623. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain research Brain research reviews. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- Popken GJ, Bunney WE, Potkin SG, Jones EG. Subnucleus-specific loss of neurons in medial thalamus of schizophrenics. Proc Natl Acad Sci U S A. 2000;97:9276–9280. doi: 10.1073/pnas.150243397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Fair DA, Schlaggar BL, Petersen SE. The development of human functional brain networks. Neuron. 2010;67:735–748. doi: 10.1016/j.neuron.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray JP, Price JL. The organization of projections from the mediodorsal nucleus of the thalamus to orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol. 1993;337:1–31. doi: 10.1002/cne.903370102. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Giguere M, Bates JF, Goldman-Rakic PS. Topographic organization of medial pulvinar connections with the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1997a;379:313–332. [PubMed] [Google Scholar]
- Romanski LM, Giguere M, Bates JF, Goldman-Rakic PS. Topographic organization of medial pulvinar connections with the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1997b;379:313–332. [PubMed] [Google Scholar]
- Roux F, Wibral M, Singer W, Aru J, Uhlhaas PJ. The phase of thalamic alpha activity modulates cortical gamma-band activity: evidence from resting-state MEG recordings. J Neurosci. 2013;33:17827–17835. doi: 10.1523/JNEUROSCI.5778-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, Cox RW. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain connectivity. 2012;2:25–32. doi: 10.1089/brain.2012.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalmann YB, Pinsk MA, Wang L, Li X, Kastner S. The pulvinar regulates information transmission between cortical areas based on attention demands. Science. 2012;337:753–756. doi: 10.1126/science.1223082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatinelli D, Fortune EE, Li Q, Siddiqui A, Krafft C, Oliver WT, Beck S, Jeffries J. Emotional perception: meta-analyses of face and natural scene processing. Neuroimage. 2011;54:2524–2533. doi: 10.1016/j.neuroimage.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: evidence for a distributed neural network subserving spatially guided behavior. J Neurosci. 1988;8:4049–4068. doi: 10.1523/JNEUROSCI.08-11-04049.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thalamus Sherman SM. Scholarpedia 1.9. 2006 [Google Scholar]
- Sherman SM, Guillery Rainer W. Functional Connections of Cortical Areas: A New View from the Thalamus. MIT Press; 2013. [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MC, Pearlson GD, Calhoun VD. Changes in the interaction of resting-state neural networks from adolescence to adulthood. Hum Brain Mapp. 2009;30:2356–2366. doi: 10.1002/hbm.20673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theyel BB, Llano DA, Sherman SM. The corticothalamocortical circuit drives higher-order cortex in the mouse. Nat Neurosci. 2010;13:84–88. doi: 10.1038/nn.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynor C, Heckemann RA, Hammers A, O’Muircheartaigh J, Crum WR, Barker GJ, Richardson MP. Reproducibility of thalamic segmentation based on probabilistic tractography. Neuroimage. 2010;52:69–85. doi: 10.1016/j.neuroimage.2010.04.024. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Newsome WT, Maunsell JH, Bixby JL. The projections from striate cortex (V1) to areas V2 and V3 in the macaque monkey: asymmetries, areal boundaries, and patchy connections. J Comp Neurol. 1986;244:451–480. doi: 10.1002/cne.902440405. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN, Rosene DL. Cingulate cortex of the rhesus monkey: I. Cytoarchitecture and thalamic afferents. J Comp Neurol. 1987;262:256–270. doi: 10.1002/cne.902620207. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Rosene DL, Pandya DN. Thalamic and cortical afferents differentiate anterior from posterior cingulate cortex in the monkey. Science. 1979;204:205–207. doi: 10.1126/science.107587. [DOI] [PubMed] [Google Scholar]
- Walter M, Bermpohl F, Mouras H, Schiltz K, Tempelmann C, Rotte M, Heinze HJ, Bogerts B, Northoff G. Distinguishing specific sexual and general emotional effects in fMRI-subcortical and cortical arousal during erotic picture viewing. Neuroimage. 2008;40:1482–1494. doi: 10.1016/j.neuroimage.2008.01.040. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Funahashi S. Neuronal activity throughout the primate mediodorsal nucleus of the thalamus during oculomotor delayed-responses. II. Activity encoding visual versus motor signal. J Neurophysiol. 2004;92:1756–1769. doi: 10.1152/jn.00995.2003. [DOI] [PubMed] [Google Scholar]
- Winer JA, Wenstrup JJ, Larue DT. Patterns of GABAergic immunoreactivity define subdivisions of the mustached bat’s medial geniculate body. J Comp Neurol. 1992;319:172–190. doi: 10.1002/cne.903190114. [DOI] [PubMed] [Google Scholar]
- Xiao D, Barbas Helen. Circuits through prefrontal cortex, basal ganglia, and ventral anterior nucleus map pathways beyond motor control. Thalamus & Related Systems. 2004;2:325–343. [Google Scholar]
- Xiao D, Zikopoulos B, Barbas H. Laminar and modular organization of prefrontal projections to multiple thalamic nuclei. Neuroscience. 2009;161:1067–1081. doi: 10.1016/j.neuroscience.2009.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Chang C, Xu T, Jiang L, Handwerker DA, Castellanos FX, Milham MP, Bandettini PA, Zuo X-N. Connectivity trajectory across lifespan differentiates the precuneus from the default network. Neuroimage. 2014;89:45–56. doi: 10.1016/j.neuroimage.2013.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L, Polimeni JR, Fischl B, Liu H, Buckner RL. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang Y-F, He Y, Zhu C-Z, Cao Q-J, Sui M-Q, Liang M, Tian L-X, Jiang T-Z, Wang Y-F. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29:83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Zhang D, Snyder AZ, Fox MD, Sansbury MW, Shimony JS, Raichle ME. Intrinsic functional relations between human cerebral cortex and thalamus. J Neurophysiol. 2008;100:1740–1748. doi: 10.1152/jn.90463.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Snyder AZ, Shimony JS, Fox MD, Raichle ME. Noninvasive functional and structural connectivity mapping of the human thalamocortical system. Cerebral cortex. 2010;20:1187–1194. doi: 10.1093/cercor/bhp182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Schuff N, Du AT, Rosen HJ, Kramer JH, Gorno-Tempini ML, Miller BL, Weiner MW. White matter damage in frontotemporal dementia and Alzheimer’s disease measured by diffusion MRI. Brain: a journal of neurology. 2009;132:2579–2592. doi: 10.1093/brain/awp071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikopoulos B, Barbas H. Prefrontal projections to the thalamic reticular nucleus form a unique circuit for attentional mechanisms. J Neurosci. 2006;26:7348–7361. doi: 10.1523/JNEUROSCI.5511-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Q, Long X, Zuo X, Yan C, Zhu C, Yang Y, Liu D, He Y, Zang Y. Functional connectivity between the thalamus and visual cortex under eyes closed and eyes open conditions: a resting-state fMRI study. Hum Brain Mapp. 2009;30:3066–3078. doi: 10.1002/hbm.20728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X-N, Kelly C, Adelstein JS, Klein DF, Castellanos FX, Milham MP. Reliable intrinsic connectivity networks: test-retest evaluation using ICA and dual regression approach. Neuroimage. 2010;49:2163–2177. doi: 10.1016/j.neuroimage.2009.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.