Abstract
Background and objectives
Dorsal root ganglion stimulation is an emerging therapy in the treatment of chronic pain. Compared with traditional spinal cord stimulation, it allows a discretely targeted stimulation profile and may act via differing mechanisms of action. Despite these advantages, little is known about the complications associated with this new modality.
Methods
We queried the MAUDE (Manufacturer and User Facility Device Experience) database for all entries named ‘Dorsal root ganglion stimulator for pain relief’ reported between May 1, 2016 and December 31, 2017. We verified these data through the Office of the Freedom of Information Act at the US Food and Drug Administration. We then eliminated duplicate entries and categorized each complication based on the event description. A secondary analysis was performed to characterize the serious adverse events and the severity of new neurologic symptoms and infections.
Results
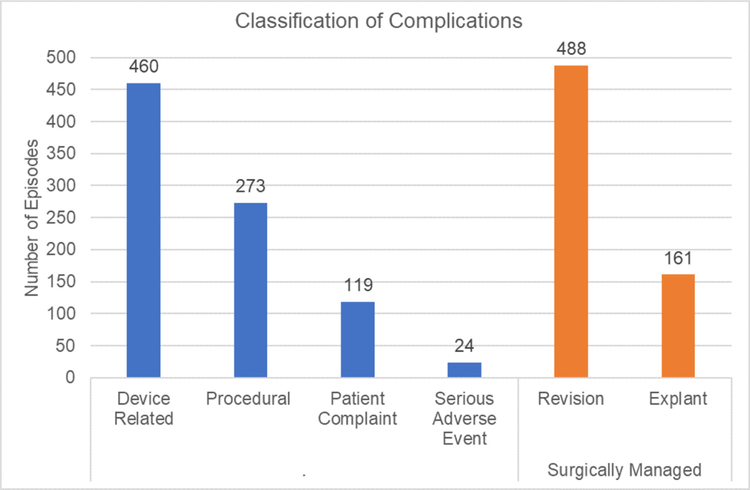
We identified 979 unique episodes following our process of deduplication. Almost half (47%) of entries were categorized as device-related complications, a quarter (28%) as procedural complications, with the remainder as patient complaints (12%), serious adverse events (2.4%), and ‘other’ complications (4.6%). The majority of complications were managed surgically with revision (n = 488; 49.8%) rather than explant (n = 161; 16.4%) events, respectively.
Conclusions
The ‘Dorsal root ganglion stimulator for pain relief’ device has been publicized as a breakthrough in neuromodulation technologies. As with any new technology, we must proceed with caution and reevaluate effectiveness as information becomes available. The MAUDE database has provided safety data unique for this device that will aid in informed consent and further refinement of this innovative therapy.
INTRODUCTION
Dorsal root ganglion (DRG) stimulation is rapidly emerging as a treatment for chronic pain, with an estimated 5000 trials and 3000 permanent implants (J. Sysantos, Abbott, personal communication, December 1, 2017) performed in the USA since approval in February 2016. The earliest report of stimulation directly targeting the DRG appears to have occurred in 1995 for the treatment of lumbar discogenic pain.1 This report and another early case used traditional dorsal column system components, modifying the procedural technique to facilitate placement overlying the DRG.1,2 Limitations of traditional systems for DRG stimulation led to the development of Abbott Laboratories, Austin, Texas, USA proprietary device designed specifically for this purpose. Compared with spinal cord stimulation (SCS) leads, these DRG specific leads contain a smaller cross sectional area, contact size, intercontact spacing, less rigidity and are implanted via a unique contralateral technique.3,4 Although the DRG stimulator received US Food and Drug Administration (FDA) approval only recently, the device has been available in Europe and Australia since receiving regulatory Conformité Européene (CE) Mark approval in late 2011.5,6
Since the device is relatively new, scant data are available regarding complications. Outside of safety reporting in clinical trials, a literature review did not reveal any publications focusing specifically on complications associated with DRG stimulation.7–9
The most comparable safety data comes from conventional SCS, which has a reported incidence of complications ranging from 32% to 43%.10–14 However, stark differences in design and implantation technique limit similarities and possibly generalization of this data to DRG stimulation. It is unknown whether these rates are comparable with DRG stimulation or if there are unique complications specific to this new modality.
The FDA supports the Manufacturer and User Facility Device Experience (MAUDE) database for reporting of medical device-related adverse events.15 This information is available to the public online or with a Freedom of Information Act (FOIA) request directed to the FDA. Device manufacturers, importers and device user facilities are considered mandatory reporters by the FDA and must submit known complications. Device user facilities encompass hospitals, outpatient diagnostic or treatment facilities, nursing homes, and ambulatory surgical centers. Patients, family, and healthcare providers are also able to report events as voluntary reporters; however, this is an uncommon occurrence. As manufacturers often rely on facilities or health-care providers to inform them of adverse events, not all events are captured. Consequently, the FDA acknowledges that the incidence or prevalence of an event should not be derived from this database alone and that these data in and of itself should not be used to compare devices or determine temporal changes in event rates.
In the context of these constraints, the FDA asserts that the purpose of the MAUDE database is ‘…to monitor device performance, detect potential device-related safety issues, and contribute to benefit-risk assessments of these products.’15 We hypothesized that the MAUDE database would provide insight into both unique and commonly reported complications associated with DRG stimulation. This information will aid in risk: benefit assessment prior to implantation, and provide important clues on potential etiologies for postoperative complications.
METHODS
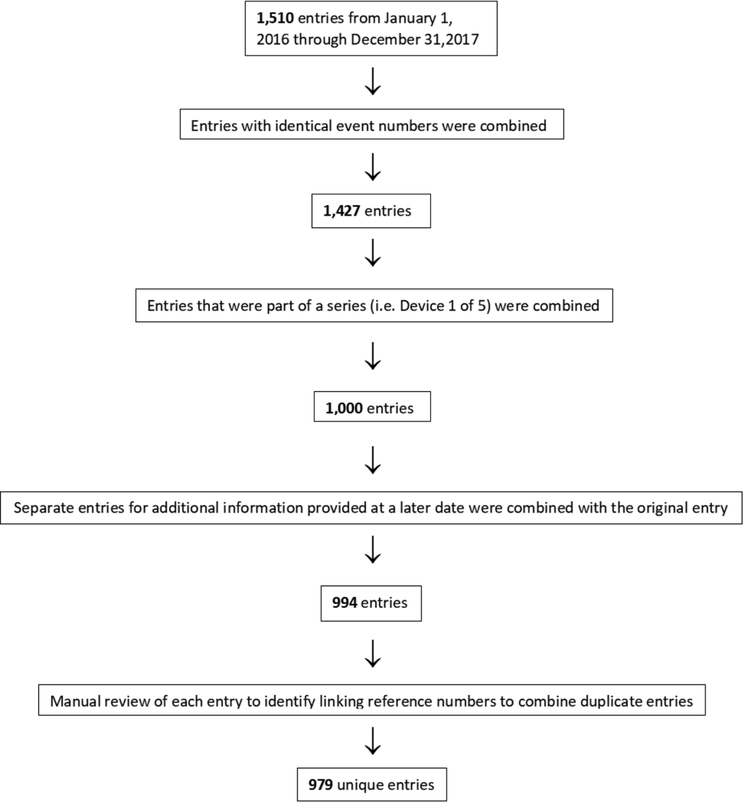
We conducted a retrospective database study of publicly reported safety events for DRG stimulation which included trials and implants. We accessed adverse events reports from the MAUDE database (https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.cfm). The primary exposure was defined as device reports with the product code ‘PMP,’ a term which uniquely identifies devices named ‘Dorsal root ganglion stimulator for pain relief.’ We queried the MAUDE database on January 27, 2018, for all events reported between May 1, 2016, and December 31, 2017. No entries before this date were noted as the device only recently received approval for the US market. These data were verified with identical data received from a Freedom of Information Act Request on January 3, 2018, to the Center for Devices and Radiological Health at the FDA.16 Each entry contained a report number, event date, event type, manufacturer, date received, product code, brand name, and event text.
We examined descriptive statistics using counts and percentages to categorize the complications in tabular form as described below, given inferential statistical methods were inappropriate as the total number of procedures for the search period was unknown. Additionally, the FDA advises that the database should not be used to derive event trends.
For each entry, we noted the report number and event description. Duplicate entries with identical event report numbers and descriptions were removed using a series of steps outlined below. The reporting process often includes each device component as a separate entry even when they are implanted in the same episode. For example, an implant surgery involving four leads and one implantable pulse generator (IPG) could be reported as five separate reports. In order to avoid overcounting complications for the same episode, duplicate reports were combined into a single event. These were identified as devices in a series (ie, device 1 of 5) and through a linking reference number provided in the event description. Because additional information regarding the same event was sometimes entered as a separate entry at a later date, we combined all entries labeled as additional information in the event description with the original event. We then manually reviewed each entry and further eliminated repetitive entries which contained a linking reference number.
Our primary outcome was type of complication. We classified complications based on categories defined by authors ES, MCB, and SPC (table 1). If multiple complications occurred for the same episode, we recorded each as a separate complication. For example, if a migration and lead fracture were both listed for the same report number, we considered these two separate complications as occurring separately for the same episode. If a particular complication was considered serious (eg, prolonged neurological weakness, meningitis) and managed via a separately recorded category (eg, deep infection requiring explant), it was reported in each category (ie, a deep infection resulting in meningitis was an explant, infection, and serious adverse event). If a definite plan for future surgery was stated, this was included in the surgically managed complications; however, if it was written that surgery ‘may’ occur, this was not included as a surgically managed complication. Each entry was manually reviewed and categorized based on the definitions. The major categories were device-related complications, procedural complications, patient complaints, surgically managed complications, serious adverse events, and other.
Table 1.
Definitions and examples of complication categories.
| Device-related complications | |
| Migration | Migration of any lead from the original placement site |
| Erosion | Lead protruding through the skin |
| Lead damage | Breakage, fracture, kink, or other damage |
| Damage to sheath | Breakage, fracture, kink, or other damage |
| Lead connection failure | Loose connection or disconnection |
| Hardware malfunction | Battery failure, inoperable device, or IPG communication error |
| Difficult insertion | Difficulty with lead placement |
| Difficult removal | Difficulty with lead removal |
| Procedural complications | |
| Neurologic symptoms | New or worsened neurologic symptoms which most commonly involve numbness, paresthesias, weakness, or pain not including musculoskeletal back pain at entry points* |
| Dural puncture | Visualized cerebrospinal fluid, suspected dural puncture, postdural puncture symptoms |
| Infection | Infectious symptoms associated with non-prophylactic treatment |
| Hematoma | Abnormal collection of blood |
| Patient complaints | |
| IPG site pain | Abnormal patient-reported discomfort at IPG site |
| Unwanted stimulation | Inappropriate or overstimulation, electrical shock-like sensations, or stimulation in an unwanted area |
| Surgically managed complications | |
| Explant | Removal of all implanted components† |
| Revision | Removal of any implanted component |
| Serious adverse event | Epidural abscess, meningitis, seizure, asystole/cardiac arrest, stroke, death, neuraxial hematoma, admission to long-term rehabilitation, deep venous thrombosis, laminectomy/decompression surgery |
| Other | Events not included in the above definitions |
Not including symptoms which were stimulation related and ceased upon decreasing or eliminating stimulation.
Removal of trials leads was not considered an explant.
IPG, implantable pulse generator.
We identified 16 different types of complications, which included the following categories:
Device related complications—migration, erosion, lead damage, sheath damage, lead connection failure, hardware malfunction, difficult removal, and difficult insertion.
Procedural complications—new neurologic symptoms, dural puncture, infection, and hematoma.
Patient complaints—IPG site pain and unwanted stimulation.
Surgically managed complications—a revision or an explant.
Other—all entries which did not relate to one of the above classifications.
After the initial categorization, we performed a secondary analysis based on a classification to determine the severity of infections and new neurologic symptoms. Infections which were associated with a revision, explant, or serious adverse event were designated as deep infections, while infections not associated with surgery were designated as superficial infections. New neurologic symptoms associated with a revision, explant, or serious adverse event were designated as severe. Symptoms that did not result in surgical intervention or a serious adverse event were designated as self-limited neurologic symptoms. Authors ES and SPC independently allocated the serious adverse events into subcategories of related, possibly related, or unrelated to the device or procedure, with discrepancies resolved via discussion in all cases. The inter-rater reliability and the average of the two ratings are reported.
RESULTS
A total of 1510 entries were found between May 1, 2016 through December 31, 2017. After deduplication, we identified 979 unique episodes (figure 1). Almost half (47.0%) of the entries were categorized as device-related complications, a quarter (24.2%) as procedural complications, with the remainder as patient complaints (12.2%), serious adverse events (2.4%), and ‘other’ complications (4.6%) (figure 2). The majority of complications were managed surgically with revision rather than explant, with 488 (49.8%) and 161 (16.4%) events, respectively (figure 2).
Figure 1.
Flowchart for the filtering process to eliminate duplicate complication entries.
Figure 2.
Major categories for complications. *If multiple complications occurred for the same episode, each was recorded as a separate complication. If a particular complication was considered serious and managed via a separately recorded category, it was reported in both categories.
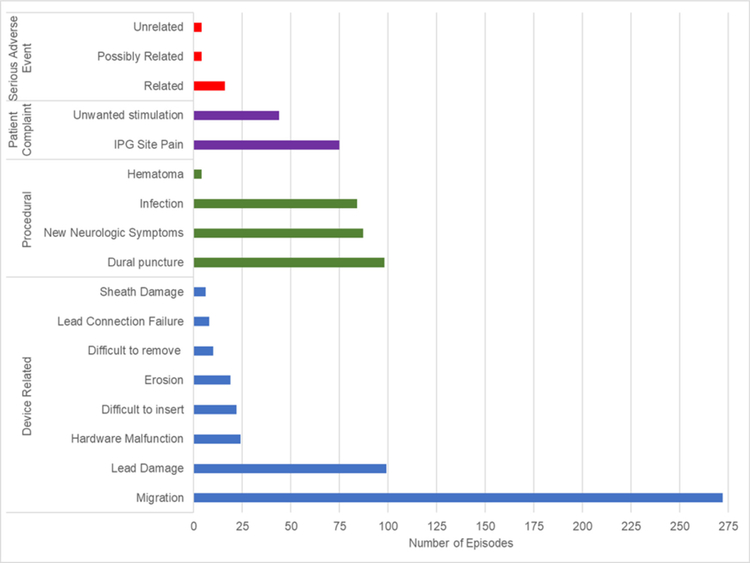
Among device-related complications, migration and lead damage were the most common, being reported 272 and 99 times, respectively (figure 3). There were numerous other types of device related complications including: 6 cases of sheath damage, 19 of erosion, 8 with lead connection failure, 24 with hardware malfunction, 10 complicated by difficult removal, and 22 associated with difficult insertion. Procedural complications included 98 dural punctures, 87 cases with new neurologic symptoms, 84 postoperative infections, and 4 reports of hematoma (figure 3). New or worsening radiculopathy was the most common new neurologic symptom, reported in 79 of 87 cases. Among the 119 patient complaints, 75 related to IPG site pain and the other 44 to unwanted stimulation (figure 3).
Figure 3.
Subcategorization of device-related, procedural, patient complaint, and serious adverse event complication categories. *If multiple complications occurred for the same episode, each was recorded each as a separate complication. If a particular complication was considered serious and managed via a separately recorded category, it was reported in both categories. IPG, implantable pulse generator.
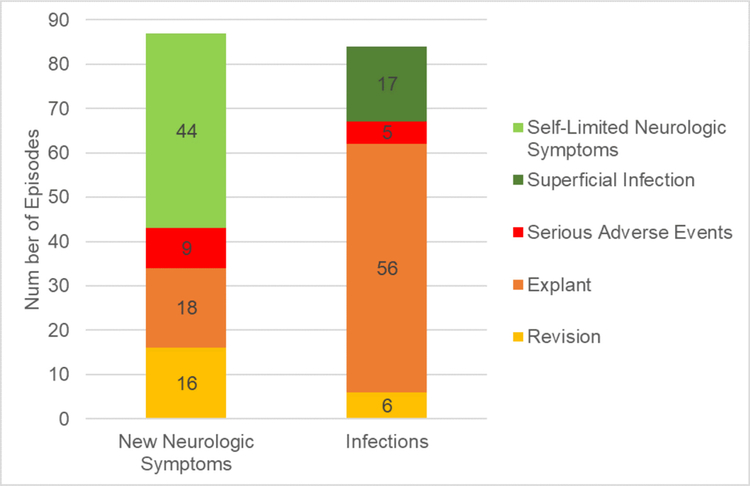
Secondary analysis of the serious adverse events identified 16 related, 4 possibly related, and 4 unrelated complication, with an inter-rater reliability of 90.0% (figure 3). Seventeen infections (20.2%) were characterized as superficial, while 67 infections (79.8%) were characterized as deep. Among the latter, 56 were associated with explant, 6 with revision, and 5 were considered to constitute a serious adverse event (figure 4). In the 87 cases of new or worsening neurological symptoms, 44 (50.6%) were characterized as self-limited, while 43 (49.4%) were characterized as severe. Severe neurological complications were further categorized as serious in 9 individuals and associated in explant or revision in 18 and 16 patients, respectively (figure 4).
Figure 4.
New neurologic symptoms and infections associated with serious adverse events, explant, or revision. Superficial infections and self-limited neurologic symptoms are not included in these events.
DISCUSSION
This survey of the MAUDE database analyzed rates of complication from DRG stimulation and found many complications which appear similar to traditional SCS; however, some complications including dural puncture, neurologic injury, migration, and component damage may be related to features of the device itself.
Similar to traditional SCS, lead migration was the most frequently reported complication.17 DRG lead migration may be a more frequent complication because the traditional method of anchoring SCS lead(s) to the supraspinous ligament or fascia is less commonly used to prevent DRG lead migration.18 Although traditional anchoring and strain relief loops are advised in the manufacturer implantation manual, many providers instead rely solely on strain relief loops, which are created within the epidural space by manipulation of the lead after placement overlying the DRG.3,6,19 A small stab incision is performed with permanent DRG lead placement which may provide less space for the creation of a subcutaneous strain relief loop than is seen with the larger, midline incisions used for traditional SCS.19 It is unknown which anchoring techniques were performed in the entries submitted to the MAUDE database. Future studies comparing anchoring techniques are warranted to shed light on this area (eg, strain relief only as opposed to traditional anchoring). This stab incision, if shallow, may result in superficial lead placement and subsequent erosion through the skin. No recommendations are provided with regard to crossing leads within the epidural space, which is likely to occur with multiple lead placements.19 It is also unknown whether the smaller circumference and flexibility of the DRG lead affects migration.
Although this lead design facilitates placement within the intervertebral foramen and the creation of strain relief loops, it is also likely to increase lead damage. The resultant length of loop formation increases the number of potential damage sites, and the tradeoff for lead flexibility may be altered structural integrity. Lead damage occurred both during insertion and removal. Difficult lead removal often prompted purposeful lead cutting, whole lead retention, or inadvertent lead damage, which resulted in a retained lead segment within the epidural space. Similarly, damage to the sheath used for lead placement sometimes resulted in a retained segment. Difficult lead insertion often led to abandonment of the planned foraminal insertion level or in some cases, the entire procedure. Since these complications are provider dependent and may negatively reflect on practice and experience, they are most likely under-reported.
The reports of new neurologic symptoms and dural puncture may share procedural causality. Although dural puncture is a well-known complication of traditional SCS, new neurologic symptoms are not a prominent classification in studies of traditional SCS-associated complications.10,11,13 The DRG-specific procedural technique typically requires access to the epidural space at a steeper angle than in the conventional SCS antero-grade approach.19 Once access is obtained, a unique semirigid sheath is advanced within the epidural space towards the targeted foramen.3,19 A guidewire may facilitate placement of the sheath in or at the foramen.19 The DRG lead is then advanced through this sheath to its final resting place over the targeted DRG. The epidural needle, sheath, guidewire, or DRG lead can puncture the dura at any point in this process or during removal. Furthermore, it seems plausible that extensive manipulation of these components in order to obtain foraminal access can increase the risk of dural puncture.
New neurologic symptoms from trauma to the DRG may also relate to manipulation of these components at the neural foramen. The fixed space within the neural foramen also makes compressive symptoms from lead placement or trauma more likely than cord compression with traditional SCS leads. Since roughly 50% of these neurologic complications were associated with surgical intervention or a serious adverse event, we recommend that physicians remain vigilant when neurologic symptoms are reported, as they appear less likely to be transient compared with most interventional pain procedures. The physician implant manual explicitly warns physicians with the statement, ‘The patient must be awake and conversant during portions of the procedure to minimize the likelihood of nerve damage.’19 There are also multiple warning labels regarding dural puncture by the lead, guidewire, sheath, and epidural needle. The warning labeling for sedation and dural puncture was not found in the physician implant manuals for traditional SCS systems from several major manufacturers, including the proprietor of DRG stimulation technology, Abbott Laboratories, Austin, Texas, USA but caution against deep sedation is recommended for epidural steroid injections for the same apparent reason (to reduce neurologic complications).20–24
With rates slightly less than that of neurologic symptoms, infection was commonly associated with an explant, revision, or serious adverse event, with only 20.2% deemed as superficial and nonserious. This is likely an overestimation of the true incidence of serious infection, as many physicians choose to immediately explant at even the slightest suspicion of infection as the consequences of deep infections can be catastrophic. It is possible that an increased number of device components required for placement, additional leads (up to 4), or skin erosion over the lead could alter the risk of infection. The dura normally serves as a barrier to the central spread of infection and thus an increased risk of dural puncture is concerning. The operative time for DRG implantation is on average lengthier than for traditional SCS and prolonged operative times have been correlated with increased infection rates in other types of surgery.7,25,26
The patient-reported complaint of IPG site pain is a recognized complication in traditional SCS and this is unlikely to differ with DRG systems because the hardware design and pocket creation are similar. The complications of lead connection failure and hardware malfunction are also probably comparable to traditional SCS for similar reasons. The patient-reported complaint of unwanted stimulation, however, may have a unique etiology with DRG systems. As the lead lies in close proximity to the DRG, lower amplitudes can create overstimulation.27 Although it is possible that increased migration rates could increase the likelihood of stimulation in an unwanted region, we are unable to make any inferences about overall rates of complications based on this database alone.
Overall, most complications of DRG stimulation were associated with surgically managed interventions, which appears similarly when compared with conventional SCS.14 Revisions were reported three times more often than explants, which could suggest that patients had sufficient initial therapeutic effect to justify repeat implantation. In a retrospective review performed in 345 patients who underwent non-DRG SCS, the rates of revision and explanation were equivalent, at 23.9% each.28
Limitations
There are several limitations to this database analysis. Because individual providers are not required to report complications and may be disinclined to do so because of time constraints, lack of knowledge about the process, poor surveillance and concerns about repercussions or embarrassment, databases such as this tend to underestimate incident rates and should not be used to estimate prevalence. This observation notwithstanding, because different sources may report the same complication using different descriptions, despite attempts to screen these out manually, it is impossible to definitively rule out duplicate reporting. For subjective complications, this under-reporting may be even more pronounced. Second, manual review of individual entries is susceptible to human error by both the reviewer and provider. These inaccuracies can result from technical limitations in the online database (ie, inaccurate categorization) and manual downloading of queried entries. Whereas a computerized categorization system would be ideal, the nonstandard reporting process limits the development of algorithms to automatically sort the entries, and the large number of entries should presumably minimize inaccuracies. However, we hope that the large number of entries reviewed minimized overall inaccuracies. Third, the definitions for each complication including serious adverse events are subjective and were determined by consensus among authors. The European Union also employs a reporting system, the Medical Device Vigilance System; however, in this project, we focused on the FDA repository.
Despite our inquiries, we were unable to obtain information from Abbott Laboratories, Austin, Texas, USA regarding the total number of DRG trials and implants performed during the time period of this analysis, as several sources at the company cited concerns regarding implant volumes made available to competitors, and an inability to guarantee elimination of duplicate reporting. Therefore, we cannot calculate the rates of these complications.
CONCLUSION
The DRG has been an attractive target for pharmacologic, surgical, and electrical pain modulation for decades. Exponential technologic advancements in traditional SCS are moving toward precisely treating the region of pain distribution with fewer off-target effects. DRG stimulation is a leap in this direction with the ability to target discrete subdermatomal foci.29,30 Furthermore, DRG stimulation may alleviate chronic pain via different mechanisms of action than traditional SCS.31 The excitement surrounding this new technology should, however, proceed with caution as there are many unknowns. At a time when nonopioid alternatives are urgently needed, the benefits associated with this new modality may outweigh the risks of complications. As the total number of procedures performed during this time period (ie, a denominator) is unknown, a complication incidence could not be estimated. However, extrapolating numbers based on estimates of the number of individuals trained and controversial requirements that each of those individuals vouch to perform at least six implants, it is likely that the risks and complication rate are much lower than those associated with chronic opioid therapy and spine surgery.32 Despite its limitations, the MAUDE database is one resource which can aid in the early detection of safety issues with new technology, though, large multisite studies are necessary to more accurately identify complication rates. Our results should spur future study into modifications of the DRG stimulation device and procedural technique to reduce complications, while still maintaining the advantages of this promising new therapy.
Acknowledgments
Funding ES received support from a Stimulating and Advancing ACCM Research (StAAR) seed grant (E.S.) from the Department of Anesthesiology and Critical Care Medicine at Johns Hopkins University and the National Institute of General Medical Sciences (T32GM075774). MCB received support from the Foundation for Anesthesia Education and Research (FAER). SPC received support from the Centers for Rehabilitation Sciences Research, U.S. Dept. of Defense.
Footnotes
Competing interests SPC has served as a consultant to Abbott in the past 3 years on the topic of radiofrequency ablation. All other authors declare no conflicts of interest.
Patient consent Not required
Provenance and peer review Not commissioned; externally peer reviewed
REFERENCES
- 1.Wright RE, Colliton JW. Neurostimulation of the L2 dorsal root ganglion for intractable disc pain: description of a novel technique. Neurosurgery 1995;36:1101–10.7643988 [Google Scholar]
- 2.Lynch PJ, McJunkin T, Eross E, et al. Case report: successful epiradicular peripheral nerve stimulation of the C2 dorsal root ganglion for postherpetic neuralgia. Neuromodulation 2011;14:58–61. [DOI] [PubMed] [Google Scholar]
- 3.Vancamp T, Levy RM, Peña I, et al. Relevant anatomy, morphology, and implantation techniques of the dorsal root ganglia at the lumbar levels. Neuromodulation 2017;20:690–702. [DOI] [PubMed] [Google Scholar]
- 4.Deer TR, Pope JE. Dorsal root ganglion stimulation approval by Administration: advice on evolving the process. Expert Rev Neurother 2016;16:1123–5. [DOI] [PubMed] [Google Scholar]
- 5.Dorsal root ganglion stimulator for pain relief PMA P150004 approval letter. 2016. Available: https://www.accessdata.fda.gov/cdrh_docs/pdf15/P150004b.pdf [Accessed 17 Dec 2017].
- 6.Harrison C, Epton S, Bojanic S, et al. The efficacy and safety of dorsal root ganglion stimulation as a treatment for neuropathic pain: a literature review. Neuromodulation 2018;21:225–33. [DOI] [PubMed] [Google Scholar]
- 7.Deer TR, Levy RM, Kramer J, et al. Dorsal root ganglion stimulation yielded higher treatment success rate for complex regional pain syndrome and causalgia at 3 and 12 months: a randomized comparative trial. Pain 2017;158:669–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liem L, Russo M, Huygen FJ, et al. One-year outcomes of spinal cord stimulation of the dorsal root ganglion in the treatment of chronic neuropathic pain. Neuromodulation 2015;18:41–9. [DOI] [PubMed] [Google Scholar]
- 9.Deer TR, Grigsby E, Weiner RL, et al. A prospective study of dorsal root ganglion stimulation for the relief of chronic pain. Neuromodulation 2013;16:67–72. [DOI] [PubMed] [Google Scholar]
- 10.Cameron T. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20-year literature review. J Neurosurg 2004;100(3 Suppl):254–67. [DOI] [PubMed] [Google Scholar]
- 11.Turner JA, Loeser JD, Deyo RA, et al. Spinal cord stimulation for patients with failed back surgery syndrome or complex regional pain syndrome: a systematic review of effectiveness and complications. Pain 2004;108:137–47. [DOI] [PubMed] [Google Scholar]
- 12.Taylor RS, Van Buyten JP, Buchser E. Spinal cord stimulation for chronic back and leg pain and failed back surgery syndrome: a systematic review and analysis of prognostic factors. Spine 2005;30:152–60. [DOI] [PubMed] [Google Scholar]
- 13.Kumar K, Wilson JR, Taylor RS, et al. Complications of spinal cord stimulation, suggestions to improve outcome, and financial impact. J Neurosurg Spine 2006;5:191–203. [DOI] [PubMed] [Google Scholar]
- 14.Kumar K, Bishop S. Financial impact of spinal cord stimulation on the healthcare budget: a comparative analysis of costs in Canada and the United States. J Neurosurg Spine 2009;10:564–73. [DOI] [PubMed] [Google Scholar]
- 15.U. S. Food and Drug Administration. Manufacturer and User Facility Device Experience (MAUDE). Available: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.cfm [Accessed 17 Dec 2017].
- 16.U.S. Food and Drug Administration, Center for Devices and Radiological Health. FOIA request 2018–172 2018. [Google Scholar]
- 17.Mironer YE, Brown C, Satterthwaite JR, et al. A new technique of “midline anchoring” in spinal cord stimulation dramatically reduces lead migration. Neuromodulation 2004;7:32–7. [DOI] [PubMed] [Google Scholar]
- 18.Breel J, Zuidema X, Wille F. Long-term outcomes of anchorless lead placement in a dorsal root ganglion (DRG) stimulation cohort. Neuromodulation 2015;18:E227. [Google Scholar]
- 19.St. Jude medical: axium™ neurostimulator system, physician implant manual. Available: https://www.accessdata.fda.gov/cdrh_docs/pdf15/P150004d.pdf [Accessed 23 Dec 2017].
- 20.Boston scientific corporation: precision™ spinal cord stimulation clinician manual. Available: https://www.bostonscientific.com/content/dam/Manuals/us/current-rev-en/91083273-02_Precision_Clinician_Manual_Entrada_2_DFU_en-US_S.pdf [Accessed 24 Dec 2017].
- 21.Physician Implant Manual 11051 Rev A (2015-01-16). 1. Available: https://www.accessdata.fda.gov/cdrh_docs/pdf13/P130022d.pdf [Accessed 24 Dec 2017].
- 22.Vectris® SureScan® MRI 1×8 Subcompact 977A160, 977A175, 977A190, Vectris® SureScan® MRI 1×8 Compact 977A260, 977A275, 977A290 Lead Kit - Implant Manual. 1. Available: http://manuals.medtronic.com/content/dam/emanuals/neuro/CONTRIB_171278.pdf [Accessed 24 Dec 2017].
- 23.Percutaneous Lead Kit Models 3143, 3146, 3149, 3153, 3156, 3159, 3183, 3186, 3189, Clinician’s Manual. 1. Available: https://manuals.sjm.com/ [Accessed 24 Dec 2017].
- 24.Rathmell JP, Benzon HT, Dreyfuss P, et al. Safeguards to prevent neurologic complications after epidural steroid injections: consensus opinions from a multidisciplinary working group and national organizations. Anesthesiology 2015;122:974–84. [DOI] [PubMed] [Google Scholar]
- 25.Peersman G, Laskin R, Davis J, et al. Prolonged operative time correlates with increased infection rate after total knee arthroplasty. Hss J 2006;2:70–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colman M, Wright A, Gruen G, et al. Prolonged operative time increases infection rate in tibial plateau fractures. Injury 2013;44:249–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kent AR, Min X, Hogan QH, et al. Mechanisms of dorsal root ganglion stimulation in pain suppression: a computational modeling analysis. Neuromodulation 2018;21:234–46. [DOI] [PubMed] [Google Scholar]
- 28.Engle MP, Vinh BP, Harun N, et al. Infectious complications related to intrathecal drug delivery system and spinal cord stimulator system implantations at a comprehensive cancer pain center. Pain Physician 2013;16:251–7. [PubMed] [Google Scholar]
- 29.Puigdellívol-Sánchez A, Prats-Galino A, Ruano-Gil D, et al. Sciatic and femoral nerve sensory neurones occupy different regions of the L4 dorsal root ganglion in the adult rat. Neurosci Lett 1998;251:169–72. [DOI] [PubMed] [Google Scholar]
- 30.Liem L, Russo M, Huygen FJ, et al. A multicenter, prospective trial to assess the safety and performance of the spinal modulation dorsal root ganglion neurostimulator system in the treatment of chronic pain. Neuromodulation 2013;16:471–82. [DOI] [PubMed] [Google Scholar]
- 31.Krames ES. The dorsal root ganglion in chronic pain and as a target for neuromodulation: a review. Neuromodulation 2015;18:24–32. [DOI] [PubMed] [Google Scholar]
- 32.Cohen SP, Gallagher RM. Ethical conundrums in pain medicine: the intersection of industry sponsorship, fee-for-service interventions, and access to care. Oxford: Oxford University Press, 2017. [DOI] [PubMed] [Google Scholar]