Summary
Neutrophils constitute the first line of cellular defense against invading microorganisms and modulate the subsequent innate and adaptive immune responses. In order to execute a rapid and precise response to infections, neutrophils rely on preformed effector molecules stored in a variety of intracellular granules. Neutrophil granules contain microbicidal factors, the membrane-bound components of the respiratory burst oxidase, membrane-bound adhesion molecules, and receptors that facilitate the execution of all neutrophil functions including adhesion, transmigration, phagocytosis, degranulation, and neutrophil extracellular trap formation. The rapid mobilization of intracellular organelles is regulated by vesicular trafficking mechanisms controlled by effector molecules that include small GTPases and their interacting proteins. In this review, we focus on recent discoveries of mechanistic processes that are at center stage of the regulation of neutrophil function, highlighting the discrete and selective pathways controlled by trafficking modulators. In particular, we describe novel pathways controlled by the Rab27a effectors JFC1 and Munc13-4 in the regulation of degranulation, reactive oxygen species and neutrophil extracellular trap production, and endolysosomal signaling. Finally, we discuss the importance of understanding these molecular mechanisms in order to design novel approaches to modulate neutrophil-mediated inflammatory processes in a targeted fashion.
Keywords: inflammation, neutrophil function, Rab GTPase, vesicular trafficking
1 | REGULATION OF NEUTROPHIL FUNCTION BY MEDIATORS OF VESICULAR TRAFFICKING
Neutrophils constitute the first line of cellular defense against invading pathogens and modulate subsequent innate and adaptive immune responses.1 As fast acting effector cells, neutrophils are rapidly recruited to the site of infection to exert their antimicrobial function. To enable this response to external stimuli, neutrophils contain preformed effector molecules stored in a variety of intracellular granules. 2,3 The various subsets of neutrophil granules contain microbicidal substances, the membrane-associated components of the respiratory burst oxidase, membrane-bound adhesion molecules such as integrins that mediate neutrophil-endothelial adhesion and transmigration, extracellular matrix proteins, and soluble mediators of inflammation. 4,5 In response to an infectious stimulus or to endogenous proinflammatory mediators, activated neutrophils adhere to the activated endothelium, migrate to the site of infection, and ultimately eliminate the infectious agent by the release of microbicidal substances, phagocytosis, and the generation of reactive oxygen species (ROS).6–8 Neutrophils also form neutrophil extracellular traps (NETs) that help trap bacteria and contain infections6–8 (Fig. 1). Most of these functions depend on the mobilization of cytoplasmic granule subsets at the right place and time. Tight regulation of granule dynamics is important for two reasons: first, to ensure that the contents of different granules are released in a timely manner that correlates with the functions that their cargoes play in the sequential steps of neutrophil response to infections (Fig. 1). Second, to ensure that the toxic granule contents are only released in a targeted manner upon reaching the site of infection so as to avoid damage to the host tissues.
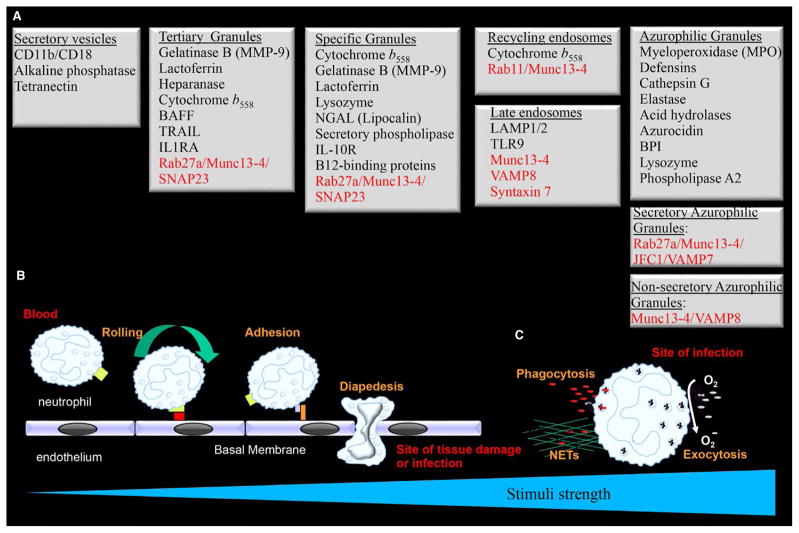
FIGURE 1.
The hierarchy of neutrophil granule exocytosis. (A) Neutrophils contain various subsets of cytoplasmic granules that are classified based on their content as follows: secretory vesicles, tertiary or gelatinase granules, specific or secondary granules, and azurophilic or primary granules. They also contain at least two sets of additional organelles with secretory capacity: late endosomes and recycling endosomes. Membrane and cargo proteins are indicated in black, while secretory effectors are in red. Notice that Rab27a confers a subpopulation of azurophilic granules the category of secretory organelle. (B) The granules are exocytosed in a hierarchical order with the secretory vesicles being the most readily exocytosed, followed by the tertiary and specific granules. The hierarchy of exocytosis of these granules also correlates with the functions exhibited by their cargo proteins. (C) The azurophilic granules, with the most toxic cargoes, are ideally exocytosed in response to the strongest stimuli found at the site of infection where the concentration of pathogens is the highest
The subcellular secretory granules of mature human neutrophils are classified into four subsets based on their cargoes and on how readily they can be mobilized: azurophilic granules (primary granules), specific granules (secondary granules), gelatinase granules (tertiary granules), and secretory vesicles.9 At least two other types of mobilizable organelles have been identified in neutrophils: multivesicular bodies/late endosomes (LE) and recycling endosomes (Fig. 1). LEs are enriched in LAMP1 and LAMP2 (lysosome-associated membrane proteins), but not LAMP3 (CD63), which localizes at azurophilic granules instead,10–13 while recycling endosomes carry the marker Rab11.14
2 | VESICULAR TRAFFICKING MECHANISMS REGULATING NEUTROPHIL FUNCTIONS
Vesicular trafficking is of central importance in maintaining the functional organization of eukaryotic cells. Vesicular transport is responsible for the movement of molecules between different compartments of the cells, nutrient transport, organelle fusion and fission, receptor recycling, secretory protein compartmentalization, and secretion. Thus, trafficking is essential to maintain cellular homeostasis and for the regulation of cell-specific functions.15,16 Neutrophils are unique in having a variety of vesicles containing proteins that need to be mobilized differentially to exert their anti-microbial functions in a timely manner. Tight regulation of granule-specific trafficking, tethering, docking, and fusion is required for this to be achieved. The mobilizable organelles undergo highly dynamic processes that include seemingly random vesicular movements that increase the likelihood of important molecular interactions between the vesicle trafficking machinery and motor proteins and between fusible vesicles.17 Trafficking also facilitates movement through cortical actin followed by tethering, reversible docking, and fusion with the plasma membrane or with membranes of other vesicles. The directionality and efficiency of vesicle delivery are also determined by the actin and microtubule networks, which facilitate local and long range movements, respectively.18 Vesicular trafficking in eukaryotic cells including neutrophils is regulated by members of the Rab protein family, which are Ras-like monomeric GTPases with over 60 known Rabs identified in humans.19–22 Rab-GTPases are central to the control of membrane identity, sorting, and fusion and act by recruiting various effector molecules, tethering factors, and SNAREs. The Rabs function as molecular switches that cycle between a GDP-bound ‘off’ form and a GTP-bound ‘on’ state, which causes a conformational change allowing for recruitment of specific effectors.19 Rab effectors are the ultimate mediators of Rab protein functions.23 The Rab effectors facilitate migration of the vesicles through the cytoskeleton and subsequent tethering. Fusion ensues once the vesicle and the target membrane are jux-taposed upon tethering. Downstream specificity at the stage of vesicle fusion is conferred by a set of proteins collectively referred to as SNAREs (soluble NSF-attachment protein [SNAP] receptors; NSF is N-ethylmaleimide sensitive factor).24 Proteins of the VAMP (v-SNARE) and syntaxin (t-SNARE) family that are present on the transport vesicles and the target membrane, respectively, are responsible for the specificity of the membrane fusion reactions (for review 25).
In order for neutrophils to upregulate adhesion molecules at the plasma membrane and to deliver cargo proteins in a timely, spatial and sequential manner, the diverse secretory compartments in neutrophils are associated with and regulated by different trafficking machineries. The specific secretory machinery of a given neutrophil vesicle defines the fate of that particular organelle beyond and independently of its cargo content. Thus, two granules with identical cargo would become functionally different based on, for example, the Rab-GTPases that are associated with their membranes. Based on this concept, the categorization of neutrophil organelles should be expanded to classify them according to their trafficking machinery, which ultimately defines the function of the granules (Fig. 1). For example, azurophilic granules have been found to be heterogeneous not only based on physical properties26 but also on their ability to engage either in exocytosis or phagosomal maturation, properties specified by the presence or absence of Rab27a in the granule membrane, respectively,27,28 and thus, we propose the classification as secretory and non-secretory azurophilic granules based on this property (Fig. 1). Thus far, neutrophils have been shown to express Rab3a, Rab4, Rab5a, Rab11, Rab27a, and Rab27b, and many other Rab-GTPases have been identified by mass spectrometry analyses of neutrophil granules.4,5,14,27,29,30 Although various Rab-GTPases have been identified in neutrophils, functional characterization in these cells has been performed for only a few of them. Rab5a was shown to be in the cytosol and on the membrane of azurophilic granules in human neutrophils. Upon neutrophil stimulation with the protein kinase C activator, PMA (phorbol 12-myristate 13-acetate), the membrane-associated fraction of Rab5a increases with a concomitant decrease in the cytosolic fraction.31 Rab5a was found to regulate phagolysosome fusion in human neutrophils.32 Rab11 proteins have been implicated in a variety of cellular trafficking pathways, such as recycling endosome trafficking, transport of cargo from sorting endosomes, and the trans-Golgi network to the endosomal recycling compartment and the regulation of trafficking during cytokinesis.33 A recent study has identified the importance of Rab11 in neutrophil functions, in particular, in the regulation of extracellular ROS production.14 The Rab-GTPase, Rab27a, is associated with a genetic disorder leading to disease in humans. Genetic defects in the Rab27a gene cause a rare defect in patients known as Griscelli syndrome type 2 (GS2), which is characterized by pigmentary disorder associated with melanosome transport and severe immunodeficiency associated with defects in T cell, neutrophil, and natural killer cell function.34–38
All of the functions of neutrophils rely on the correct trafficking of their different vesicular compartments and the delivery of their cargo to acceptor membranes or compartments. In this review, we will discuss how various Rab family members along with their effectors regulate trafficking of different granules subsets and consequently modulate neutrophil functions. We will also discuss the emergence of trafficking regulators as potential targets for the control of neutrophil-mediated inflammation.
3 | REGULATION OF NEUTROPHIL EXOCYTOSIS: RAB27A AND ITS EFFECTORS JFC1/SLP1 AND MUNC13-4 AT CENTER STAGE
The different exocytic responses of neutrophil granules correlate with the functions their cargoes play in the sequential steps that take place during the neutrophil response to infections. Thus, secretory vesicles, which express molecules involved in the initial neutrophil response, are mobilized in response to relatively weak stimulation as compared to the stronger stimuli needed to mobilize gelatinase and specific granules. Ideally, the azurophilic granules that contain the most toxic cargoes are mobilized at the site of infection, where the concentration of pathogens and pathogen-associated molecular patterns (PAMPs) is the highest. The secretory vesicles contain molecules required for neutrophil adhesion (such as β2-integrins) 39,40 and receptors,41 which are required early on in the neutrophil innate response and therefore are mobilized by nanomolar concentrations of the stimuli.42 The mobilization of secretory vesicles transforms neutrophils from an alert circulating cell to a cell capable of transmigrating to the site of infection. The subsequent mobilization of the gelatinase granules is thought to be important for migration through the basement membrane due to the secretion of gelatinase B, a metalloproteinase capable of degrading type IV collagen.43 The specific granules contain lactoferrin, NGAL, and components of the NADPH oxidase. The azurophilic granules contain cell-lysing peptides, lysosomal hydrolases, and LAMP3 (CD63) and are classified as lysosome-related organelles11,44 though they lack the traditional LAMPs (LAMP1 and LAMP2) that are characteristic of LEs and lysosomes.10,11,45 Azurophilic granules also contain microbicidal proteins, such as bactericidal permeability-increasing protein, elastase, defensins, and myeloperoxidase (MPO). Using gold-conjugated bovine serum albumin, Berger et al.10 demonstrated the presence of LEs/multivesicular bodies in neutrophils. This was followed by another study that describes MVBs and multilaminar components in neutrophils and the presence of LAMP1 and LAMP2 as specific LE markers.11,45 Finally, a recent study has identified recycling endosomes characterized by the presence of Rab11a to be involved in the regulation of extracellular ROS production in neutrophils.14
The timing and specificity of the mobilization of the different granules is regulated by proteins of the Rab-GTPase family. A role for Rab27a in trafficking and exocytosis was first described in melanosomes and cytotoxic T lymphocytes.46,47 This was based on observations made in Rab27a-deficient mice, characterized by impaired lytic granule exocytosis, leading to the inability of cytotoxic T lymphocytes to kill infected cells and by the hypopigmentation caused by deficient melanosome transport.47–49 Similarly, patients with genetic defects in the rab27a gene (type 2 Griscelli syndrome, GS) develop an immunodeficiency disorder characterized by malfunctioning of cytotoxic T lymphocytes and by impaired neutrophil and natural killer cell function. 35,36,50 An isoform of Rab27a, named Rab27b, regulates dense granule secretion from platelets51 and compensates for abnormal platelet function in the absence of Rab27a in the ashen (Rab27a−/−) mice.52 Several Rab27 effector proteins have been identified, including synaptotagmin-like proteins Slp1 (also known as JFC1), Slp2, Slp3, Slp4 Slac2-a/b, and Slp5.53–56 Munc13-4 was also identified as a Rab27a-GTPase binding effector protein in neutrophils, CTLs, and platelets.57–59 Rab27a effectors have been extensively reviewed in these references.54,60
3.1 | Rab27a and the regulation of neutrophil function
The small GTPase Rab27a was first demonstrated to play a central role in neutrophil exocytosis by Munafo et al.27 In that study, the secretion of MPO from azurophilic granules was shown to be severely impaired in neutrophils from Rab27a-deficient mice as well as in neutrophilic differentiated promyelocytic cells that were downregulated for the expression of the GTPase Rab27a. Using subcellular localization analysis by fractionation and immunofluorescence analysis by confocal microscopy, the authors showed that Rab27a is present in a small population of MPO-containing azurophilic granules. Only 20% of the total azurophilic granules are exocytosable,61 and secretion of MPO was completely impaired in the absence of Rab27a,27 suggesting the presence of an exocytosable subpopulation of azurophilic granules regulated by Rab27a, while the rest of the population is non-releasable and lacks the necessary secretory machinery.27,30 The role of Rab27a in azurophilic granule exocytosis was further demonstrated in vivo using two different models of Rab27a deficiency (Concrete and ashen).27,62 Along with Rab27a, neutrophils express another isoform, Rab27b, which shares 72% homology with Rab27a at the amino acid level. Rab27b deficiency impairs azurophilic granule release in neutrophils albeit to a lesser extent than that seen in Rab27a deficiency.30 Thus, while azurophilic granule secretion was abolished in the absence of Rab27a, it was only partially impaired in the absence of Rab27b.30 Furthermore, immunofluorescence analysis showed that Rab27b primarily localizes on peripherally distributed structures and has poor spatial localization at azurophilic granules in unstimulated cells, whereas Rab27a shows better colocalization with azurophilic granules and is present in granules that are homogeneously distributed in neutrophils. These data suggested that Rab27b plays a downstream role in azurophilic granule secretion in relationship to Rab27a function.30 Rab27a deficiency leads to the upregulation of Rab27b, suggesting that the expression of the two isoforms is linked at the transcriptional or translational level.30 Despite the increase in expression of Rab27b in Rab27a knockout neutrophils, the azurophilic granule exocytosis defect is not restored, suggesting that the two isoforms regulate independent steps in neutrophil exocytosis.30 Almost all MPO-containing granules that localize close to the plasma membrane contain Rab27a which is in contrast with the observation that only a subpopulation of the azurophilic granule containing MPO also express Rab27a. These data reiterate the fact that only those azurophilic granules containing Rab27a are able to engage in exocytosis and hence move closer to the plasma membrane. Other GTPases, including Rac2, were also shown to regulate azurophilic granule exocytosis.63,64 Rac2 has been proposed to regulate actin remodeling to facilitate secretion.64 Whether Rab27a and Rac2 cooperate to regulate exocytosis of azurophilic granules is currently unknown. Rab27a and Rac2 were shown not to be involved in the exocytosis of CD11b expressing secretory vesicles.30,63
3.2 | Rab27a effectors and the regulation of neutrophil function
The function of Rab27a in neutrophils is coordinated by two specific effectors: synaptotagmin-like protein 1 (Slp1/JFC1) and Munc13-4 (Figs 2 and 3).59 The Rab27a effector JFC1, widely expressed in tissues with a secretory function, was originally isolated from leukocytes as a binding partner of the NADPH oxidase subunit p67phox.65 JFC1, a 562 amino acid protein, contains an amino-terminal Rab-binding domain and tandem C2-domains in its carboxy-terminus. The C2A domain of JFC1 binds 3′-phosphoinositides and helps localize the protein to the plasma membrane.66 Owing to its ability to bind to Rab27a through its amino-terminal domain and to plasma membrane–associated phosphoinositides through its C2A domain, JFC1 has been postulated to regulate vesicular tethering or docking (Fig. 2A).66 In granulocytes, endogenous JFC1 colocalizes with Rab27a at the azurophilic granule membrane.27
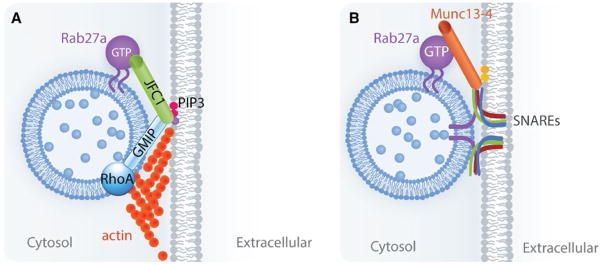
FIGURE 2.
The sequential steps and molecules involved in the exocytosis of azurophilic granules. (A) The Rab27a effector JFC1 associates with the secretory granules by binding to Rab27a through its Rab-binding domain.59 JFC1 interaction with the RhoA-GAP GMIP induces the inhibition of RhoA, facilitating actin depolymerization around the secretory vesicle, thus allowing the vesicles to access the exocytic active zone.80 JFC1 is further capable of causing loose tethering by binding to PIP3 on the plasma membrane through its C2A domain.66 (B) Once the vesicles are in the exocytic active zone, the Rab27a effector Munc13-4 facilitates docking and subsequent SNARE-mediated fusion of the vesicle membrane with the plasma membrane28,69,76
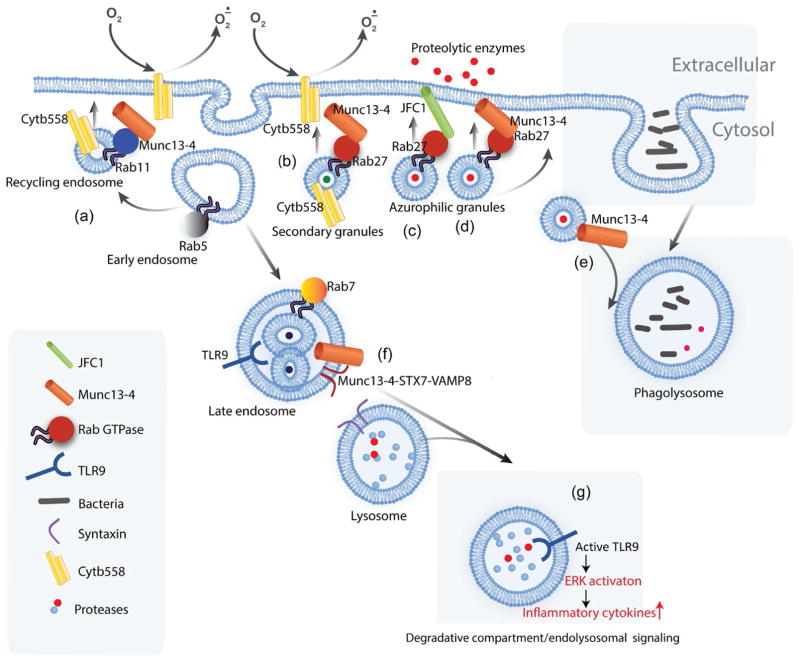
FIGURE 3.
The trafficking machinery determines the fate and function of the vesicles. Neutrophils contain different types of granules and vesicles that not only differ in their cargo content but also in the trafficking molecules associated with them. Recycling endosomes contain Rab11 which along with its effector Munc13-4 regulates the translocation of the flavocytochrome b558 (Cytb558) to the plasma membrane and extracellular ROS production (a).14 Specific and gelatinase granule exocytosis is regulated by Rab27a along with its effector Munc13-4. This process regulates extracellular ROS production independently of Rab11 (b).14,30 Secretory azurophilic granules contain Rab27a which recruits its effectors JFC1 (c) 59,80 and Munc13-4 (d),59,76 sequentially, to regulate exocytosis (see also Fig. 2). Further classification of azurophilic granules arises based on the trafficking machinery associated with them. While secretory azurophilic granules containing Rab27a are able to engage in exocytosis, azurophilic granules lacking Rab27a can fuse with the phagosome in a Rab27a-independent but Munc13-4-dependent manner (e).28 The late endosomal compartment contains a tripartite complex of Munc13-4 Syntaxin 7 and VAMP8 that regulates the fusion of the late endosome with the lysosome (f).45 This fusion event subsequently regulates endosomal signaling pathways such as TLR9 signaling which relies on the proteolytic cleavage of the TLR9 receptor in the endolysosome to initiate downstream signaling events including Erk activation (g)45
In the study by Munafo et al.,27 the authors show that Rab27a and JFC1 colocalize with azurophilic granule markers and that interfering with the Rab27a secretion machinery using the plasma membrane-binding domain of JFC1 impairs MPO secretion. In a following study by Brzezinska et al.,59 siRNA-mediated downregulation of JFC1 was also shown to impair azurophilic granule exocytosis in HL-60 promyelocytic cells. Similar studies using siRNA-mediated downregulation of JFC1 in human neutrophils showed impaired secretion of MPO upon fMLF (N-Formylmethionyl-leucyl-phenylalanine) stimulation, while the response to LPS priming prior to fMLF stimulation was defective only in Rab27a-downregulated cells but not in JFC1-downregulated cells.59 Rab27a but not JFC1 also colocalizes with MMP-9 present in gelatinase granules in human neutrophils, and consequently, interfering with Rab27a but not JFC1 by means of inhibitory antibodies impaired MMP-9 secretion in human neutrophils upon fMLF stimulation, suggesting that Rab27a regulates MMP-9 secretion through interaction with another effector.59 However, recent studies performed with JFC1-KO mice suggest that gelatinase granule exocytosis is also regulated by JFC1 (Ramadass and Catz, unpublished).
Munc13-4 is a 120-kDa protein which is highly expressed in hematopoietic cells and also in lungs, kidneys, and other organs with secretory functions.57,67 Munc13-4 contains two Munc-homology domains (MHD) and two C2 domains. The non-canonical Rab27a-binding motif in the N-terminus of Munc13-4 requires amino acids 240–543 containing the C-terminal end of the C2A domain, and the residues 280FQLIHK285 are particularly important for binding.68 The Munc13-4 C2 domains specifically interact with calcium and regulate exocytosis in a calcium-and SNARE-dependent manner.69 In addition, Munc13-4 is able to bind to phospholipids through its C2 domains70 (Fig. 2B). Munc13-4 was found to regulate exocytosis in many cellular systems, including neutrophils,28,59,70 natural killer cells,71 cytotoxic T lymphocytes,72 mast cells,57 and platelets.73 Mutations in munc13-4 gene are causative of familial hemophagocytic lymphohistiocytosis type 3, an immunodefective disease with impaired lymphocyte cytotoxicity (FHL3).72,74
Munc13-4 was first shown to be a Rab27a effector in platelets58 and CTLs.57 Studies in human neutrophils have shown that Munc13-4 colocalizes with both MPO (azurophilic) and MMP-9 (gelatinase) positive vesicles.59 siRNA downregulation of Munc13-4 impairs MMP-9 and MPO exocytosis, suggesting that Munc13-4 operates together with Rab27a to regulate gelatinase granule exocytosis.59 Using neutrophils from the Munc13-4 knockout mice (Jinx), the authors confirmed the impairment of azurophilic granule exocytosis in the absence of Munc13-4 upon heat-killed Listeria monocytogenes stimulation. 59 Furthermore, IL10-receptor and LAMP2 containing vesicle mobilization was impaired in the absence of Munc13-4, describing further roles for Munc13-4 in multivesicular bodies and specific granule exocytosis.59 Similar to that observed in Rab27a deficiency, CD11b mobilization is unaffected in Munc13-4 deficiency,30,59 thus excluding a role for Munc13-4 in exocytosis of the readily mobilizable pool of secretory vesicles. A recent work by Zhang et al.75 identified CCM3 and STK24 as two Munc13-4-interacting proteins that inhibit both azurophilic granule and tertiary granule secretion, thus supporting previous findings that Munc13-4 is a central regulator of exocytosis of these granule populations.
Although the roles of Rab27a and its effectors in exocytosis have been extensively examined, studies in static systems preclude an understanding of the specific steps in the exocytosis process that are regulated by the different molecules. In the study published by Johnson et al.,76 the authors utilized novel mice models expressing EGFP-Rab27a under the endogenous Rab27a promoter and expressing or lacking Munc13-4 to study vesicular dynamics in real time using live-TIRF (total internal reflection fluorescence) microscopy. This approach was utilized to help understand the roles for Rab27a and Munc13-4 in the dynamic processes that occur at the plasma membrane before vesicle fusion. It was observed that LPS stimulation causes a dramatic reduction in the speed and displacement of Rab27a-expressing vesicles, a mechanism impaired in the absence of Munc13-4.76 These motion-restricted Rab27a-positive vesicles were still able to visit areas in close proximity to their docking site identifying a role for Munc13-4 in tethering and loose docking of the vesicles upon LPS priming. This constitutes the first evidence that Munc13-4 directly controls the fate of exocytosable, Rab27a-positive vesicles in neutrophils, and demonstrates that Munc13-4 is essential for Rab27a-positive vesicle docking in these cells.
Since deficiencies in both Rab27a effectors, Munc13-4 and JFC1, impair azurophilic granule exocytosis, these two molecules must be involved in the regulation of exocytosis in conjunction with Rab27a. Secretion of the cytoplasmic granules involves traversing the actin cytoskeleton, followed by vesicle tethering, docking, and fusion to the plasma membrane. Several reports have suggested both inhibitory and facilitatory roles for the actin cytoskeleton in exocytosis.77,78 In neutrophils, it has been proposed that the actin cytoskeleton limits the access of granules to the plasma membrane.79 To further elucidate the specific roles played by the Rab27a effector JFC1 in vesicular trafficking, Johnson et al.80 performed a mass spectrometric analysis to identify endogenous JFC1-binding partners that might work downstream of the Rab27a–JFC1 complex. The RhoA GTPase-activating protein (RhoA-GAP) GMIP (Gem-interacting protein) was identified as a JFC1-binding partner, and the binding was mapped to the C2B domain of JFC1.80 GMIP carries a Rho-GAP activity that acts specifically on RhoA, but not on Rac or Cdc42, and mediates an inhibitory effect on actin polymerization through the GMIP-RhoA-GAP domain.81 Downregulation of GMIP in HL60 cells increased RhoA activity and actin polymerization in these cells. Live-cell experiments further showed that GMIP downregulation significantly decreased the number of fast-moving vesicles while increasing the number of vesicles with slow or no motility.80 A role for GMIP in actin-remodeling mediated exocytosis was further supported by the observations that downregulation of GMIP decreases MPO secretion while increasing actin polymerization. As a consequence, GMIP downregulated cells show impaired vesicular movement, increased actin polymerization, and enhanced vesicular trapping in the actin network, which is relieved upon depolymerization of the cytoskeleton.80 Further attributing the role of GMIP in exocytosis regulation to its RhoA-GAP activity, an inhibitor of RhoA signaling was shown to enhance azurophilic granule exocytosis.80 Importantly, azurophilic granules were shown to be trapped in the cortical actin in JFC1-KO neutrophils, which correlated with an increase in RhoA activity at the secretory granules as measured using a specific RhoA biosensor, by FRET, in primary neutrophils.80 In JFC1-KO neutrophils, granules get trapped in and are unable to traverse cortical actin; therefore, exocytosis is impeded. Contrarily, in wildtype neutrophils, JFC1-positive granules are surrounded by a halo of depolymerized actin induced by GMIP-mediated RhoA inactivation, allowing azurophilic granules to traffic through actin and to engage in exocytosis.80 These results ascribe a novel role for JFC1 in preventing actin polymerization around the secretory granules, thus facilitating the movement of the vesicles toward the exocytic active zone (Fig. 2A).
Altogether, these findings elucidate a sequential role for the Rab27a effectors in the exocytosis of neutrophil granules. While JFC1 facilitates actin depolymerization around the granules via its interaction with the RhoA-GAP GMIP to allow for the movement of vesicles to the exocytic active zone, Munc13-4 plays a role as a tethering factor allowing loose docking and thus increasing the probability of fusion events at the plasma membrane (Fig. 2).
3.3 | Regulation of secretory vesicle exocytosis
In comparison to primary, secondary, and tertiary granules, the secretory machinery that regulates exocytosis of secretory vesicles is less understood. Secretory vesicles are thought to be formed through endocytic pathways82 though recent works suggest that the involvement of endocytosis in vesicle formation may not be as predominant as previously thought.41 Although there is evidence that secretory vesicles are readily releasable and exocytose in response to relatively weak stimuli, the mechanisms underlying this process is far from clear. For example, secretory vesicle exocytosis would require that these vesicles overcome the barrier presented by cortical actin in a much more efficient and rapid manner than other neutrophil secretory organelles. Although the mechanisms and regulatory factors involved in this process are currently unknown, several potential candidates have been identified in recent studies by Uriarte et al.41 and Rørvig et al.5 by performing mass spectrometry analysis of isolated vesicles. These studies have identified various vesicular trafficking proteins with documented roles in exocytosis in other cells and are, therefore, potential candidates to regulate exocytosis of secretory vesicles in neutrophils. Some of these factors are discussed in further detail below.
3.3.1 | Rab3a
Expressed in secretory vesicles,29 this protein is the first small GTPase known to regulate exocytosis in neuroendocrine cells and has been linked to several secretory pathways.83 Involvement of Rab3a in vesicle priming during exocytosis by interaction with Munc13-1 and Munc18-1 has been demonstrated in PC12 cells.84 Rab3a also regulates acrosomal exocytosis 85 and is involved in calcium-dependent secretion in other systems,83 highlighting this protein as a potential candidate capable of regulating secretory vesicle exocytosis in neutrophils.
3.3.2 | Rab11a
This small GTPase has been recently identified in neutrophils as a mediator of the plasma membrane upregulation of the membrane-associated subunit of the oxidase.14 Rab11a vesicles constitute a different population from Rab27a-positive granules.14 However, whether all secretory vesicles are Rab11a-positive or all Rab11a-containing vesicles constitute secretory vesicles is currently unknown. Similar to that found in neutrophils, Rab11a, although generally associated with recycling endosomes, has been linked to exocytosis in several processes. This includes its role in bladder umbrella cells, for the exocytosis of discoidal/fusiform-shaped vesicles, a mechanism regulated by Rab8, Rab11, and Myo5B.86 Similarly, Rab11 regulates the secretory process necessary for apical membrane amplification in Drosophila photoreceptors,87 calcium-dependent exocytosis in neuronal cells,88 and cAMP-potentiated insulin secretion in pancreatic beta cell lines.89
3.3.3 | Rab14
The small GTPase Rab14 has been shown to be involved in endocytic90 rather than exocytic pathways, and it is, therefore, likely to participate in secretory vesicle maturation. However, some secretory mechanisms seem to utilize Rab14. For example, Rab14 regulates apical targeting in polarized epithelial cells, by controlling the delivery of cargo to the apical domain of epithelial cells.91
3.3.4 | Rab15
This small GTPase has recently been implicated in the regulation of Weibel-Palade bodies’ exocytosis,92 where it is proposed to interact with the secretory factor Munc13-4 to regulate secretion of von Willebrand factor. It is present in neutrophil secretory vesicles, but its function is unknown.
3.3.5 | Rab35
This small GTPase has been associated with several steps of vesicular trafficking. Both associations with endocytic pathways and exocytosis have been shown for Rab35, suggesting that this GTPase is a good candidate to regulate both maturation and exocytosis of secretory vesicles, an organelle of partial endocytic origin that engages in secretion. Rab35 has been shown to upregulate T-cell receptor expression at the immunological synapse in Jurkat cells93 and to regulate exosome secretion from glial cells.94 Furthermore, an association between Rab35 function and both actin remodeling and cell protrusion formation through interaction with Rho GTPases has been established,95 further pointing toward a possible regulation of the actin network by this GTPase during secretory vesicle exocytosis. Its function in secretory vesicle exocytosis remains uncharacterized.
Other molecules identified in neutrophil secretory vesicles with potential importance in the regulation of the secretory process are EXOC5 (Sec10), a member of the exocyst, a complex speculated to tether secretory vesicles to the plasma membrane,96–98 Exophilin 5 (Slac-2b), a Rab27b effector known to regulate exosome secretion,99 and SNAREs, such as VAMP2,100 VAMP3, and VAMP8,5,41 which are capable of mediating fusion to the plasma membrane.
4 | LATE ENDOSOMAL MATURATION AND SIGNALING: A PROCESS REGULATED BY MUNC13-4 THROUGH INTERACTION WITH SNARE PROTEINS
Another feature of regulation and specificity of the process of vesicle exocytosis occurs at the level of membrane fusion. This step is regulated by a family of proteins called the SNAREs (soluble NSF-attachment protein receptors where NSF stands for N-ethyl-maleimide-sensitive factor protein), which are highly conserved, and reside on transport vesicles (v-SNAREs) or target membranes (t-SNAREs). All SNAREs contain a characteristic coiled-coil SNARE motif about 70 amino acids long. The interaction of cognate v- and t-SNAREs leads to the formation of a trans-SNARE complex where four SNARE motifs assemble as a twisted parallel four-helix bundle to bring the opposing membranes together and that eventually drives membrane fusion.24 The docking and fusion of vesicles with the plasma membrane is mediated by the specific interaction of vesicle proteins, v-SNAREs, including synaptobrevins/vesicle-associated membrane proteins (VAMPs), with target plasma membrane-located proteins, t-SNAREs, including syntaxins, synaptosome-associated protein (SNAP)-25, and SNAP-23.
Various SNARE proteins have been identified in neutrophils, and they seem to have a compartment-specific distribution. SNAP23 is expressed in human neutrophils, and its expression in HL-60 cells increases upon myeloid differentiation to neutrophilic cells.101 SNAP-25 was also found to be present in neutrophil cytoplasmic granules, specifically in the peroxidase-negative tertiary and gelatinase granules. 102 The expression of multiple syntaxins (syntaxin 1A, 3, 4, 5, 6, 7, 9, 11, and 16) and VAMPs (VAMP1, 2, 7, and 8) completes the large spectrum of SNARE expression in neutrophils, thus demonstrating the importance of molecular diversity for the precise regulation of the control of exocytosis in these cells.45,103,104 This is exemplified by recent findings that have characterized the use of SNARE-specific interfering peptides to modulate the process of exocytosis. In particular, using a cell-permeable peptide that inhibits SNAP23 function and, therefore, prevents exocytosis of gelatinase and specific but not azurophilic granules, Uriarte et al.105 demonstrated that neutrophil granule exocytosis contributes to phagocytosis-induced respiratory burst. Similar approaches have been used by others to demonstrate the roles of discrete SNAREs in the regulation of exocytosis of neutrophil subsets, extensively described in reference.106
Interestingly, recent studies have highlighted functions for SNAREs in neutrophils that go beyond their role in the regulation of exocytosis. In this regard, particular attention has been paid to syntaxin 7 and VAMP8,45 two SNAREs known to regulate the endocytic pathway in other cells.107 In HL60 cells, while undifferentiated promyelocytes do not express syntaxin 7, its expression is induced upon neutrophilic differentiation, suggesting that it plays a specialized role in the mature cell.103 Syntaxin 7 is also expressed in human neutrophils with a major location originally attributed to azurophilic granules and a secondary but extensive localization at other compartments.104 VAMP8 was also found to localize on azurophilic granules in neutrophils although it was found not to be involved in secretion.108 In a recent report, He et al.45 utilized immunofluorescence analysis of endogenous proteins to demonstrate high colocalization of both syntaxin 7 and VAMP8 with Munc13-4 at LEs in primary neutrophils. Thus, He et al.45 have identified a novel mechanism of late endosomal maturation in neutrophils that involves the molecular interaction between the SNAREs syntaxin 7 and VAMP8 and the tethering factor Munc13-4. Munc13-4 binding to syntaxin 7 was significantly increased by calcium, highlighting a role for Munc13-4 as a calcium sensor in the process of late endosomal maturation. Colocalization of Munc13-4 and syntaxin 7 at LEs was demonstrated by super-resolution and live-cell microscopy.45 Further supporting a role for Munc13-4 in late endosomal maturation, the authors showed that syntaxin 7, VAMP8, and Munc13-4 form a tripartite molecular entity suggesting that Munc13-4 is able to associate with the complex formed by these SNAREs. Direct evidence that Munc13-4 regulates late endosomal maturation arises from evidence showing that Munc13-4-deficient cells have increased numbers of significantly enlarged LEs, a phenotype that was mimicked by the fusion inhibitor chloroquine in wildtype cells and rescued by expression of wildtype Munc13-4 but not by a syntaxin 7-binding-deficient mutant.45
LEs control various cellular processes, such as signaling, protein degradation, receptor maturation, and phagosomal maturation.109,110 Their function as endocytic substrate processing and degradation compartments requires their fusion with lysosomes.109 Calcium is known to have an important regulatory function in homotypic and heterotypic fusion of LEs and lysosomes.111 The fact that the enlarged late endosomal phenotype seen in Munc13-4-KO cells can be rescued by expressing wildtype Munc13-4 protein but not the syntaxin 7-and Ca2+-binding-deficient mutant of Munc13-445 emphasizes the importance of the Ca2+-binding properties of Munc13-4 in late endosomal maturation. These findings by He et al.,45 showing that Munc13-4 regulates calcium association with endosomal SNAREs and modulates endosomal maturation, have postulated Munc13-4 as a calcium sensor for endosomal maturation in neutrophils.
LE function requires the delivery of degradative enzymes into the LE lumen through heterotypic fusion with lysosomes.109 In innate immune cells including neutrophils, this process facilitates the partial degradation and maturation of nucleic acid-sensing receptors including toll-like receptors 7 and 9 (TLR7 and TLR9). Thus, endosome maturation is a requirement for endosomal-initiated signaling in response to several stimuli including unmethylated bacterial DNA, a ligand of the pattern recognition receptor TLR9.112 Using endocytic substrates that become fluorescent upon digestion, it was shown that delivery of degradative enzymes into the LE is retarded in the absence of Munc13-4 in neutrophils.45 This was further demonstrated by immunofluorescence analysis of endogenous proteins showing decreased fusion of cathepsin-bearing lysosomes with late endosomal TLR9 in Munc13-4 deficient neutrophils.45 These fusion defects have deleterious functional consequences as neutrophils lacking Munc13-4 show significant impairment in the signaling pathway triggered by unmethylated DNA, manifested as a net decrease in the Erk phosphorylation response.45 This correlated with an impaired response of Munc13-4-deficient neutrophils to nucleic acid-sensing stimulation, characterized by decreased upregulation of adhesion proteins in response to CpG.45 The specificity of this defect for endosomal signaling was further supported by the observation that the upregulation of the same adhesion protein was not altered in Munc13-4-KO neutrophils when stimulated by plasma membrane receptor ligands.45 Altogether, these findings identify Munc13-4 as a novel calcium sensor for endosomal maturation, nucleic acid sensing, and associated functions in neutrophils (Fig. 3). The data also highlight that although Munc13-4 seems to regulate multiple independent docking and fusion mechanisms in neutrophils, the specificity of these processes is defined by the protein–protein interactions that are specific for each individual trafficking pathway (Fig. 3).
5 | THE ROLE OF VESICULAR TRAFFICKING REGULATORY PROTEINS IN THE REGULATION OF THE NADPH OXIDASE IN NEUTROPHILS
ROS are at the core of neutrophil-mediated innate immunity. In neutrophils, ROS production relies on the NADPH oxidase, a multi-subunit enzymatic complex responsible for the monoelectronic reduction of oxygen to produce superoxide anion (O2−).113 Patients with chronic granulomatous disease, whose NADPH oxidase is inactive, suffer recurrent bacterial and fungal infections, thus highlighting the importance of the oxidase in the neutrophil-mediated defense against microorganisms.114 The NADPH oxidase consists of the cytosolic factors p47phox, p67phox, and p40phox, the membrane-associated cytochrome b558 (composed of p22phox and gp91phox) and the accessory proteins Rac2 and Rap1a. The oxidase is activated in a process that involves the mobilization of the membrane-associated cytochrome b558 from tertiary and specific granules to the plasma membrane or the phagosome, in response to stimulation by soluble or particulate PAMPs, respectively.115,116 A role for Rab27a GTPase in neutrophil ROS generation was first shown by Johnson et al.30 using neutrophils from Rab27a-deficient mice in response to the chemotactic factor fMLF. Impaired extracellular ROS generation was observed in Rab27a-deficient cells using cell-impermeant isoluminol in the presence of sodium azide to inhibit endogenous MPO activity.30 The absence of Rab27a expression significantly impaired NADPH oxidase activity; however, a significant oxidative response was still detected in the absence of Rab27a, supporting the idea that Rab27a-dependent and Rab27a-independent mechanisms cooperate to assemble the NADPH oxidase at the plasma membrane.30 Interestingly, Rab27b-deficient neutrophils also showed decreased NADPH oxidase activity, suggesting that these GTPases have independent roles in the regulation of ROS in neutrophils.30 Although Rab27a/b double-knockout neutrophils showed impaired oxidative response to the chemotactic peptide, a significant response was still detected in the absence of both GTPases,30 supporting the idea that Rab27-dependent and Rab27-independent mechanisms collaborate to assemble the NADPH oxidase at the plasma membrane. Contrary to that observed in dendritic cells,117 intraphagosomal ROS production was unaffected in Rab27-deficient neutrophils.28 Thus, substantial intracellular ROS production was observed in the absence of both the Rab27a and Rab27b GTPases,30 suggesting that cytochrome b558-containing granules likely utilize different trafficking molecules to assemble at the phagosome, an observation that was confirmed in phagocytosis studies using Rab27a-KO neutrophils and live Pseudomonas aeruginosa.28
Since exocytosis is important for NADPH oxidase activation and Munc13-4 regulates exocytosis in neutrophils through a Rab27a-dependent mechanism,59 a possible role for Munc13-4 in the regulation of ROS production was also studied. The role of Munc13-4 in ROS production was analyzed by Monfregola et al. using neutrophils from Munc13-4-KO mice.28 Using total internal reflection fluorescence microscopy, the authors analyzed the localization of flavocytochrome b558-expressing granules in relationship with the plasma membrane in Munc13-4-KO cells. The study shows that the translocation of the p22phox-expressing organelles to the exocytic active zone in response to physiological stimuli is impaired in Munc13-4-KO neutrophils. 28 This phenomenon is explained by a defect in the trafficking of gelatinase and specific granules, which contain more than 85% of the intracellular flavocytochrome b558, toward the plasma membrane. To investigate a possible role for Munc13-4 in the regulation of flavocytochrome b558 integration into the plasma membrane, the authors performed immunofluorescence analysis of wildtype and Munc13-4-KO neutrophils in which the plasma membrane was fluorescently labeled with specific dyes. Using transversal confocal microscopy analysis of fMLF-stimulated neutrophils, they showed that upon stimulation, p22phox-positive granules translocate to and integrate into the plasma membrane in wildtype cells, as visualized by the colocalization of the flavocytochrome-subunit p22phox with the fluorescent plasma membrane. 28 In contrast, although p22phox-expressing vesicles appeared to translocate to areas near the plasma membrane after stimulation in Munc13-4-KO neutrophils, these organelles were unable to integrate into the plasmalemma, suggesting that Munc13-4 is necessary for the final fusion step and the subsequent translocation of cytochrome b558 from the granule to the plasma membrane,28 a crucial step for extracellular ROS production.
Accordingly, Munc13-4-deficient neutrophils show impaired extracellular ROS production upon fMLF stimulation both under basal conditions or primed by pre-exposure to the TLR4-ligand lipopolysaccharide. Intracellular ROS generation upon treatment with live serum-opsonized Pseudomonas aeruginosa was also severely impaired in Munc13-4 deficiency, contrary to the normal intracellular ROS generation observed in Rab27a deficiency.28 While the phagocytosis of bacteria and incorporation of p22phox into the phagosome membrane were unaffected, Munc13-4 deficient cells showed defective phagosome maturation as shown by the failure to incorporate MPO-containing azurophilic granules or LAMP1-containing LEs to the phagosomes.28 Interestingly, intraphagosomal ROS production and phagosomal maturation were unaffected in Rab27a-KO neutrophils, reiterating that although Munc13-4 is a well-established Rab27a effector, it has various Rab27a-independent functions as well (Fig. 3) (the possible role of Rab27a and Munc13-4 in phagosomal maturation was reviewed before and is beyond the scope of this work118).
6 | MUNC13-4 CONTROLS RAB11-POSITIVE VESICLE TETHERING AND EXTRACELLULAR ROS PRODUCTION
Further clarification on the regulation of flavocytochrome b558 trafficking to the plasma membrane in neutrophils comes from a recent work that demonstrates that two small GTPases with secretory functions, Rab11 and Rab27a, localize at discrete but different subcellular compartments in neutrophils, both of them containing subsets of the flavocytochrome b558.14 The mobilization of Rab11-positive recycling endosomes to the plasma membrane contributes to the upregulation of flavocytochrome b558 in a Rab27a-independent manner (Fig. 3).14 This was supported by evidence showing lack of inter-organelle fusion and permanence of Rab11 and Rab27a as independent entities even after cellular stimulation. Since both GTPases bind to and are regulated by Munc13-4, and both, together with Munc13-4 regulate the oxidase activity, the authors proposed that neutrophils have evolved to develop alternative yet complementary mechanisms to elicit the oxidase activation, a function which is of utmost importance in the neutrophil immune response and in the mechanism of innate immunity regulation.14 The importance of Rab11 in the regulation of neutrophil function goes hand-in-hand with previous data that highlight the fundamental role that Rab11 plays in the regulation of vesicular trafficking processes associated with the innate immune response. This includes the regulation of ligand-initiated signaling through the control of receptor recycling,119 the activation of distant regulatory pathways via the secretion of exosomes120 and the control of inflammatory responses through the regulation of endosomal receptor trafficking and sorting.121
TIRFM analyses performed in Munc13-4-deficient neutrophils expressing the small GTPase Rab11 demonstrate that Munc13-4 regulates the trafficking of Rab11-positive organelles and mediates their engagement in exocytosis by inducing tethering or docking at the exocytic active zone.14 The regulation of Rab11 dynamics by endogenous Munc13-4 was further analyzed by live-cell microscopy. Rab11 was found to localize at small, fast-moving vesicles in neutrophils.14 The authors showed that a large number of slow-moving Rab11-positive vesicles were detected in close proximity to the plasma membrane in wildtype cells as compared with Munc13-4-KO neutrophils and quantitative vesicular dynamic analysis demonstrated that in the absence of Munc13-4, Rab11-positive vesicles move at a higher speed in the exocytic active zone further suggesting that Munc13-4 regulates the docking of Rab11-positive vesicles in neutrophils.14 The spatiotemporal colocalization of Munc13-4 and Rab11 was demonstrated in dynamic studies of vesicular trafficking and direct interaction between Rab11 and Munc13-4 was characterized by fluorometric and biochemical methods.14 The interaction between Rab11 and Munc13-4 was further supported by quantitative super-resolution microscopy data showing that endogenous single-molecules of Rab11 and Munc13-4 localize at distances that are compatible with in vivo protein–protein interaction.14 Furthermore, the number of Rab11-positive vesicles in the exocytic active zone upon stimulation with PMA was significantly decreased in the absence of Munc13-4,14 thus directly demonstrating a role for Munc13-4 in Rab11-positive vesicle tethering in neutrophils. Interestingly, Munc13-4 competes with Rip11, another Rab11 effector, for Rab11 binding.14 Rip11 is known to regulate exocytosis through mechanisms involving the motor protein Myo5.87 Thus, Rip11 and Munc13-4 could play sequential roles in Rab11 vesicle transport, with Rip11 controlling actin-mediated trafficking to the plasma membrane and Munc13-4 regulating tethering and docking to the plasma membrane.
Altogether, these data highlight the emergence of Munc13-4 as a novel Rab11 effector. The data also help explain why interference with Rab27a or Rab11 is accompanied by partial decrease in plasma membrane NADPH oxidase activation while deficiency of Munc13-4 shows a much stronger impairment (Fig. 3).
7 | DEGRANULATION TRAFFICKING REGULATORS ARE DISPENSABLE FOR THE FORMATION OF NEUTROPHIL EXTRACELLULAR TRAPS
Neutrophil extracellular trap generation is another mechanism neutrophils use to contain infections by pathogenic bacteria. The NETs are made of processed chromatin bound to cytoplasmic and granular proteins.122 In the study by Munafo et al.,123 neutrophils deficient in Rab27a showed normal NET generation. This differs from the data presented in another study, where using HL-60 cells, Rab27a downregulation is suggested to prevent NET formation.124 Many different reasons may justify these differences. First, while primary neutrophils are excellent NET forming cells, HL-60 cells are poor NET producers. Second, in HL-60 cells, NET formation was extrapolated from measurements of nuclear shape changes visualized with DNA dyes. While these changes may be associated with chromatin decondensation in these cells, formal quantification of extracellular DNA fibers in HL-60 cells expressing or lacking Rab27a has not been performed.124
Surprisingly, NET production in Munc13-4-deficient neutrophils was observed to be twofold higher than that observed in wildtype cells.28 In addition, the NETs produced by Munc13-4-KO cells also showed azurophilic granule puncta in close proximity to trapped bacteria. Impaired killing of extracellular bacteria was observed in Munc13-4-KO neutrophils as compared with wildtype cells when treated with DNase I after the incubation of neutrophils with bacteria, suggesting that the bacteria were trapped but not efficiently killed in Munc13-4-KO cells.28 Thus, although NETs are produced by both Rab27a-and Munc13-4-KO neutrophils, NET-dependent killing was impaired in Munc13-4-deficient cells probably due to defective ROS production and decreased release of secretory proteins. Of note, NET production in Munc13-4-KO cells was independent of ROS,28 a process that is frequently highlighted as an essential signaling pathway that precedes NET formation.125,126 Finally, since exocytosis of azurophilic granules is impaired in both Rab27a and Munc13-4-KO neutrophils, the observations that both deficient cell types produce NETs decorated by azurophilic granule proteins, rule out a possible role for exocytosis in NET formation, and suggest that granular proteins associate with NETs before the DNA is released from the cells.
8 | CHEMOTAXIS IS MODULATED BY VESICULAR TRAFFICKING REGULATORY PROTEINS
A crucial first step in neutrophil defense is their ability to sense a gradient of chemoattractants and chemokines to migrate to the site of infection. Neutrophil migration is a multistep process where the cells first exhibit a polarized phenotype followed by a cycle of cell attachment at the leading edge and detachment at the lagging edge (uropod).127 The β2-integrin LFA1 mediates adhesion followed by CD11b/CD18 (Mac-1)-mediated intraluminal crawling.128 Many cycles of adhesion and de-adhesion are required for neutrophils to migrate to their destination. Shedding of the integrin molecules by cleavage is an important mechanism for neutrophil detachment. The serine proteases, elastase, cathepsin G, and proteinase-3, stored in neutrophil granules, cleave the extracellular domain of CD11b during neutrophil detachment to allow subsequent migration.129 Thus, proper trafficking of vesicular compartments in neutrophils is important for migration. In agreement with this, migration of Rab27a-KO neutrophils was found to be defective both in vitro and in vivo when treated with the neutrophil chemoattractant protein MIP-2.130 Impaired neutrophil migration was also reported in neutrophils from a few patients with Griscelli syndrome, where the neutrophils showed defective migration compared with neutrophils from healthy donors.37,131 In vitro, the Rab27a-KO neutrophils exhibit a defect in uropod retraction, as a consequence of a defect in serine protease release in the absence of Rab27a.132 Similarly, Rab27b-KO neutrophils show impaired neutrophil migration in vitro to MIP-2 and LTB4 and in an in vivo model of lung injury with intranasally delivered MIP-2.130 The defect observed in Rab27b knockout mice130 could possibly be due to impaired primary granule exocytosis observed in these neutrophils.30
Using a RNA-interference screen, a study identified various members of the synaptotagmin family as important molecular players in chemoattractant-mediated guided migration.133 Synaptotagmin 7 (Syt7) and synaptotagmin-like 5 positively regulate T-cell chemotaxis, while synaptotagmin 2 was found to be a negative regulator of chemotaxis. 133 Syt7-deficient neutrophils showed defective migration in terms of velocity and distance migrated and also directionality of cell migration.133 This observation was extended in vivo in a model of gout, where neutrophil recruitment to the air pouch was impaired in Syt7-deficient mice. Since Syt7 is a known positive regulator of lysosome exocytosis,134 lysotracker staining of vesicles during migration of T cells was used to visualize the role of Syt7 in vesicle localization in migrating cells.133 Migrating Syt7−/− T cells showed an increased accumulation of lysotracker red in their uropods, consistent with a reduction in vesicle fusion. The Syt7−/− T cells also showed a defect in releasing their uropods from the substrate, causing a tethered tail phenotype.133 Cell adhesion was also increased in the Syt7−/− T cells, suggesting a possible defect in uropod retraction owing to defective vesicle fusion.133 Although the role for Rab effectors in the regulation of chemotaxis has not been elucidated so far, recent data from our laboratory suggest that JFC1 is an important regulator in this process (Ramadass and Catz, unpublished).
9 | VESICULAR TRAFFICKING REGULATORY PROTEINS AND SYSTEMIC INFLAMMATION
During infections, the exocytosis of specific and azurophilic granules, which contain the most toxic cargoes of all the neutrophil granules, takes place at the infection site where high concentrations of PAMPs are found. However, under pathological conditions, neutrophils may encounter a variety of stimuli capable of inducing dysregulated exocytosis in circulation, a process that is highly deleterious to the host. High levels of neutrophil proteases and pro-oxidative factors are present in plasma in many clinical and experimental conditions associated with systemic inflammation. For example, increased plasma levels of neutrophil secretory proteins are a hallmark of endotoxemia and sepsis and are also observed in sterile disorders including trauma, leading to systemic inflammatory response syndrome (SIRS). In particular, neutrophil-derived MPO, a NO oxidase that interferes with endothelial function,135 and the serine proteinase elastase 136 are both involved in the pathogenesis of several inflammatory processes, including physical trauma, atherosclerosis, arthritis, endotoxemia, and sepsis. Although interference with neutrophil function is, in theory, a plausible mechanism to decrease inflammation and to attenuate inflammatory syndromes, the approach should be considered only while taking into account the state of infection. For example, depletion of neutrophils in humans has been proposed as a possible therapy after initial trials provided statistically significant beneficial outcomes in SIRS and sepsis.137,138 However, similar approach should be taken with care, as the timing of intervention is proposed to have a direct effect on outcome. This is supported by another work, where using experimental models of sepsis, the authors show that neutrophil depletion, if performed too early, correlates with negative outcomes in sepsis as these cells are necessary for the initial control of infection.139 Based on these works, we propose that interfering with neutrophil secretion while maintaining other neutrophil-dependent innate immune defensive mechanisms intact would constitute a more balanced approach to control neutrophil-associated inflammatory processes. In this way, approaches designed to inhibit exocytosis without interfering with phagocytosis, the most powerful antibacterial mechanism displayed by neutrophils, would be, in our opinion, of great benefit. This is supported by works performed in experimental models of acute lung injury, where the authors demonstrate that the use of neutrophil exocytosis inhibitory peptides in models of shock and sepsis decreased extrapulmonary acute lung injury.140 It is also supported by work from our group showing that small-molecule inhibitors of neutrophil exocytosis are beneficial in models of systemic inflammation (Johnson and Catz, submitted).
It has now become evident that approaches targeting the interaction between Rab27a and its effector JFC1 to decrease neutrophil secretion-associated inflammation are potentially effective (Johnson and Catz, submitted). Supporting this, previous studies demonstrate that Rab27a-deficient mice are resistant to lipopolysaccharide (LPS)-induced death in a model of systemic inflammation.62 In addition, JFC1-deficient mice were also found to be resistant to endotoxic shock further highlighting the Rab27a–JFC1 pair as a putative targetable protein–protein interaction to prevent inflammation. Since Rab27a-KO mice are characterized by significantly decreased tumor necrosis factor alpha (TNF-α) in circulation compared with wildtype mice when challenged with endotoxin,62 interference with Rab27a function in inflammation may have beneficial effects that go beyond those achieved by decreasing neutrophil exocytosis. Further supporting a role for Rab27a in inflammation, decreased neutrophil migration into lungs was demonstrated in a Rab27a-deficient mouse model of lung inflammation induced by local MIP-2 administration.132 The defect was associated with the proposed regulation by Rab27a of protease secretion to facilitate neutrophil uropod detachment during chemotaxis. 132 In another study, Rab27a deficiency was associated with decreased neutrophil infiltration into the liver,62 a process explained by the reduced expression of the hyaluronan receptor CD44, which is necessary for liver invasion,141,142 in neutrophils from Rab27a-KO mice. Liver sequestration of neutrophils during endotoxemia requires Rab27a but not Munc13-4.62 Thus, these studies further support the idea that interference with Rab27a would be beneficial in systemic or localized inflammation triggered by PAMPs or endogenous proinflammatory mediators.
Decreased tissue infiltration by neutrophils correlated with reduced manifestation of neutropenia in endotoxin-challenged Rab27a-KO mice.62 Importantly, despite showing a net increase in neutrophil number, Rab27a-KO mice showed marked decrease in the levels of azurophilic granule secretory proteins in circulation and decreased death, further supporting the idea that reduced systemic neutrophil exocytosis is associated with increased survival.62 Highlighting the importance of neutrophil secretory proteins in the development of systemic inflammation associated with infections, Brovkovych et al.143 showed that MPO-KO mice have increased survival to Escherichia coli induced sepsis, suggesting that low plasma concentrations of neutrophil secretory proteins is beneficial for the outcome of systemic inflammation during endotoxemia and sepsis. Decreased plasma levels of azurophilic granule markers and a correlation with increased survival to LPS-induced systemic inflammation was also observed in Munc13-4-deficient mice, further supporting the idea that interference with these secretory proteins is potentially beneficial in the context of sepsis.62
A role for neutrophils in tumor progression has emerged recently with studies showing that the presence of neutrophils in tumor (referred to as tumor-associated neutrophils) correlates with poor prognosis.144–150 In particular, the neutrophil secretory proteins MMP-9 (from gelatinase granules) and MPO (from azurophilic granules) have been largely associated with neovascularization, angiogenesis, and tumor progression in cancers.151–153 Furthermore, granulocyte macrophage colony-stimulating factor (GM-CSF), a proinflammatory cytokine that is a priming factor for neutrophil functions, is secreted by tumor cells and is known to play a role in inflammation and immune suppression in the tumor microenvironment.154–159 A significant increase in GM-CSF levels is also found to be associated with many inflammatory disorders, and accordingly, targeting GM-CSF by using inhibitory antibodies or by depletion has been shown to suppress pathological processes in a wide range of inflammatory and autoimmune diseases such as arthritis, experimental autoimmune encephalomyelitis, asthma, and lung inflammation and psoriasis.160–162 Given the associated roles of GM-CSF and neutrophil secretory proteins in inflammation and cancer progression, our recent finding that Rab27a is the molecular player that regulates GM-CSF priming-mediated secretion of MMP-9 and MPO from neutrophils is highly relevant (M. Ramadass and S.D. Catz, submitted). Furthermore, in addition to its role in systemic inflammation, a role for Rab27a in tumor progression has recently become apparent.163–166 Studies have shown that disrupting Rab27a expression reduces exosome release, tumor growth, and metastasis in melanoma and enhanced expression of Rab27a is associated with breast and pancreatic cancer progression; differential expression of Rab27a and Rab27b correlates with clinical outcome in hepatocellular carcinoma.163–166 In this context, the finding that Rab27a regulates GM-CSF-mediated release of protumorigenic cargo is highly relevant. Altogether, these data highlight important roles for neutrophil trafficking mechanisms in systemic inflammation, sepsis, and cancer.
10 | FUTURE DIRECTIONS AND NEW PERSPECTIVES
Since the identification, isolation, and functional characterization of the secretory organelles of neutrophils, several advances have been made to help understand their differential response to stimuli and involvement in phagosomal maturation. However, although many important trafficking and docking effectors have been characterized, several questions remain unanswered. One such question concerns the exocytic mechanism of secretory vesicles and, in particular, how these rapidly mobilizable vesicles elude cortical actin more efficiently than other neutrophil granules. Another important question relates to the differential characteristics of the secretory machineries of tertiary (gelatinase) and secondary (specific) granules. Perhaps, this is more difficult to address as most of our knowledge of the different characteristics of these two secretory organelles comes from studies in human neutrophils, which are difficult to manipulate. The technical difficulties to achieve granule separation using blood from other species, most notably mice, are based not only on the small volume of the starting material but also on the different density patterns that mouse neutrophil granules present as compared to human cells. The utilization of novel technologies including advanced flow cytometry techniques for the detection of small particles167 in conjunction with the use of mass spectrometry approaches and the development of new and specific antibodies for immune-electron transmission microscopy could help circumvent some of these difficulties in granule characterization. New mobilizable organelles including recycling and LEs carrying the NADPH oxidase and cathepsin G, respectively, two important cargoes involved in the defense against pathogens, have been recently identified in neutrophils.14,45 Further characterization of the interplay between these organelles and neutrophil granules will be important to better understand how neutrophils orchestrate their response to different pathogens. Furthermore, conventional lysosome function and endosomal maturation have been identified as important mechanisms for neutrophil mediating signaling,45 and therefore, future elucidation of the mechanisms involving these trafficking organelles will be essential for a better understanding of how neutrophils combat infections and induce inflammation. Finally, the challenge will be to use this knowledge to design targeted therapies to disrupt neutrophil exocytosis-dependent inflammation without affecting other important neutrophil innate immune responses.
11 | CONCLUDING REMARKS
Elucidating vesicular trafficking mechanisms is an essential step toward a better understanding of the processes that regulate neutrophil functions. Recent discoveries have highlighted the differential roles played by Rab-GTPases and their effectors in the control of the molecular processes that modulate these functions. The identification of specific proteins and interactions that control exocytosis, but are dispensable for other important innate immune responses of neutrophils including phagocytosis and NET formation, will likely lead to the discovery of new targeted approaches for the treatment of inflammatory processes in which neutrophil secretory proteins are involved without interfering with the neutrophil innate immune response.
Acknowledgments
This work was supported by the U.S. Public Health Service grants HL088256 and GM105894 to S. D. Catz and by an American Heart Association Fellowship to Mahalakshmi Ramadass. The authors thank Dr. Jennifer L. Johnson for editorial corrections and suggestions.
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest with the contents of this article.
References
- 1.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 2.Faurschou M, Borregaard N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 2003;5:1317–1327. doi: 10.1016/j.micinf.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Borregaard N, Sørensen OE, Theilgaard-Mönch K. Neutrophil granules: a library of innate immunity proteins. Trends Immunol. 2007;28:340–345. doi: 10.1016/j.it.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Lominadze G, Powell DW, Luerman GC, Link AJ, Ward RA, McLeish KR. Proteomic analysis of human neutrophil granules. Mol Cell Proteomics. 2005;4:1503–1521. doi: 10.1074/mcp.M500143-MCP200. [DOI] [PubMed] [Google Scholar]
- 5.Rørvig S, Østergaard O, Heegaard NHH, Borregaard N. Proteome profiling of human neutrophil granule subsets, secretory vesicles, and cell membrane: correlation with transcriptome profiling of neutrophil precursors. J Leukoc Biol. 2013;94:711–721. doi: 10.1189/jlb.1212619. [DOI] [PubMed] [Google Scholar]
- 6.Falloon J, Gallin JI. Neutrophil granules in health and disease. J Allergy Clin Immunol. 1986;77:653–662. doi: 10.1016/0091-6749(86)90404-5. [DOI] [PubMed] [Google Scholar]
- 7.Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30:459–489. doi: 10.1146/annurev-immunol-020711-074942. [DOI] [PubMed] [Google Scholar]
- 8.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 9.Borregaard N, Cowland JB. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 1997;89:3503–3521. [PubMed] [Google Scholar]
- 10.Berger M, Wetzler E, August JT, Tartakoff AM. Internalization of type 1 complement receptors and de novo multivesicular body formation during chemoattractant-induced endocytosis in human neutrophils. J Clin Invest. 1994;94:1113–1125. doi: 10.1172/JCI117426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cieutat AM, Lobel P, August JT, et al. Azurophilic granules of human neutrophilic leukocytes are deficient in lysosome-associated membrane proteins but retain the mannose 6-phosphate recognition marker. Blood. 1998;91:1044–1058. [PubMed] [Google Scholar]
- 12.Dahlgren C, Carlsson SR, Karlsson A, Lundqvist H, Sjölin C. The lysosomal membrane glycoproteins Lamp-1 and Lamp-2 are present in mobilizable organelles, but are absent from the azurophil granules of human neutrophils. Biochem J. 1995;311(Pt 2):667–674. doi: 10.1042/bj3110667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuijpers TW, Tool AT, van der Schoot CE, et al. Membrane surface antigen expression on neutrophils: a reappraisal of the use of surface markers for neutrophil activation. Blood. 1991;78:1105–1111. [PubMed] [Google Scholar]
- 14.Johnson JL, He J, Ramadass M, et al. Munc13-4 is a Rab11-binding protein that regulates Rab11-positive vesicle trafficking and docking at the plasma membrane. J Biol Chem. 2016;291:3423–3438. doi: 10.1074/jbc.M115.705871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lazar T, Götte M, Gallwitz D. Vesicular transport: how many Ypt/Rab-GTPases make a eukaryotic cell? Trends Biochem Sci. 1997;22:468–472. doi: 10.1016/s0968-0004(97)01150-x. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz AL. Cell biology of intracellular protein trafficking. Annu Rev Immunol. 1990;8:195–229. doi: 10.1146/annurev.iy.08.040190.001211. [DOI] [PubMed] [Google Scholar]
- 17.Degtyar VE, Allersma MW, Axelrod D, Holz RW. Increased motion and travel, rather than stable docking, characterize the last moments before secretory granule fusion. Proc Natl Acad Sci USA. 2007;104:15929–15934. doi: 10.1073/pnas.0705406104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langford GM. Actin-and microtubule-dependent organelle motors: interrelationships between the two motility systems. Curr Opin Cell Biol. 1995;7:82–88. doi: 10.1016/0955-0674(95)80048-4. [DOI] [PubMed] [Google Scholar]
- 19.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 20.Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev. 2011;91:119–149. doi: 10.1152/physrev.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schimmöller F, Simon I, Pfeffer SR. Rab GTPases, directors of vesicle docking. J Biol Chem. 1998;273:22161–22164. doi: 10.1074/jbc.273.35.22161. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz SL, Cao C, Pylypenko O, Rak A, Wandinger-Ness A. Rab GTPases at a glance. J Cell Sci. 2007;120:3905–3910. doi: 10.1242/jcs.015909. [DOI] [PubMed] [Google Scholar]
- 23.Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci USA. 2006;103:11821–11827. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen YA, Scheller RH. SNARE-mediated membrane fusion. Nat Rev Mol Cell Biol. 2001;2:98–106. doi: 10.1038/35052017. [DOI] [PubMed] [Google Scholar]
- 25.Ungar D, Hughson FM. SNARE protein structure and function. Annu Rev Cell Dev Biol. 2003;19:493–517. doi: 10.1146/annurev.cellbio.19.110701.155609. [DOI] [PubMed] [Google Scholar]
- 26.Oren A, Taylor JM. The subcellular localization of defensins and myeloperoxidase in human neutrophils: immunocytochemical evidence for azurophil granule heterogeneity. J Lab Clin Med. 1995;125:340–347. [PubMed] [Google Scholar]
- 27.Munafo DB, Johnson JL, Ellis BA, Rutschmann S, Beutler B, Catz SD. Rab27a is a key component of the secretory machinery of azurophilic granules in granulocytes. Biochem J. 2007;402:229–239. doi: 10.1042/BJ20060950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monfregola J, Johnson JL, Meijler MM, Napolitano G, Catz SD. MUNC13-4 protein regulates the oxidative response and is essential for phagosomal maturation and bacterial killing in neutrophils. J Biol Chem. 2012;287:44603–44618. doi: 10.1074/jbc.M112.414029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaudhuri S, Kumar A, Berger M. Association of ARF and Rabs with complement receptor type-1 storage vesicles in human neutrophils. J Leukoc Biol. 2001;70:669–676. [PubMed] [Google Scholar]
- 30.Johnson JL, Brzezinska AA, Tolmachova T, et al. Rab27a and Rab27b regulate neutrophil azurophilic granule exocytosis and NADPH oxidase activity by independent mechanisms. Traffic. 2010;11:533–547. doi: 10.1111/j.1600-0854.2009.01029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vita F, Soranzo MR, Borelli V, Bertoncin P, Zabucchi G. Subcellular localization of the small GTPase Rab5a in resting and stimulated human neutrophils. Exp Cell Res. 1996;227:367–373. doi: 10.1006/excr.1996.0286. [DOI] [PubMed] [Google Scholar]
- 32.Perskvist N, Roberg K, Kulyté A, Stendahl O. Rab5a GTPase regulates fusion between pathogen-containing phagosomes and cytoplasmic organelles in human neutrophils. J Cell Sci. 2002;115:1321–1330. doi: 10.1242/jcs.115.6.1321. [DOI] [PubMed] [Google Scholar]
- 33.Kelly EE, Horgan CP, McCaffrey MW. Rab11 proteins in health and disease. Biochem Soc Trans. 2012;40:1360–1367. doi: 10.1042/BST20120157. [DOI] [PubMed] [Google Scholar]
- 34.Griscelli C, Durandy A, Guy-Grand D, Daguillard F, Herzog C, Prunieras M. A syndrome associating partial albinism and immunodeficiency. Am J Med. 1978;65:691–702. doi: 10.1016/0002-9343(78)90858-6. [DOI] [PubMed] [Google Scholar]
- 35.Ménasché G, Pastural E, Feldmann J, et al. Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nat Genet. 2000;25:173–176. doi: 10.1038/76024. [DOI] [PubMed] [Google Scholar]
- 36.Meeths M, Bryceson YT, Rudd E, et al. Clinical presentation of Griscelli syndrome type 2 and spectrum of RAB27A mutations. Pediatr Blood Cancer. 2010;54:563–572. doi: 10.1002/pbc.22357. [DOI] [PubMed] [Google Scholar]
- 37.Klein C, Philippe N, Le Deist F, et al. Partial albinism with immunodeficiency (Griscelli syndrome) J Pediatr. 1994;125:886–895. doi: 10.1016/s0022-3476(05)82003-7. [DOI] [PubMed] [Google Scholar]
- 38.Harfi HA, Brismar J, Hainau B, Sabbah R. Partial albinism, immunodeficiency, and progressive white matter disease: a new primary immunodeficiency. Allergy Proc. 1992;13:321–328. doi: 10.2500/108854192778816933. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt S, Moser M, Sperandio M. The molecular basis of leukocyte recruitment and its deficiencies. Mol Immunol. 2013;55:49–58. doi: 10.1016/j.molimm.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 40.Ley K. Molecular mechanisms of leukocyte recruitment in the inflammatory process. Cardiovasc Res. 1996;32:733–742. [PubMed] [Google Scholar]
- 41.Uriarte SM, Powell DW, Luerman GC, et al. Comparison of proteins expressed on secretory vesicle membranes and plasma membranes of human neutrophils. J Immunol. 2008;180:5575–5581. doi: 10.4049/jimmunol.180.8.5575. [DOI] [PubMed] [Google Scholar]
- 42.Borregaard N, Christensen L, Bejerrum OW, Birgens HS, Clemmensen I. Identification of a highly mobilizable subset of human neutrophil intracellular vesicles that contains tetranectin and latent alkaline phosphatase. J Clin Invest. 1990;85:408–416. doi: 10.1172/JCI114453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delclaux C, Delacourt C, D’Ortho MP, Boyer V, Lafuma C, Harf A. Role of gelatinase B and elastase in human polymorphonuclear neutrophil migration across basement membrane. Am J Respir Cell Mol Biol. 1996;14:288–295. doi: 10.1165/ajrcmb.14.3.8845180. [DOI] [PubMed] [Google Scholar]
- 44.Bretz U, Baggiolini M. Biochemical and morphological characterization of azurophil and specific granules of human neutrophilic polymorphonuclear leukocytes. J Cell Biol. 1974;63:251–269. doi: 10.1083/jcb.63.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He J, Johnson JL, Monfregola J, et al. Munc13-4 interacts with syntaxin 7 and regulates late endosomal maturation, endosomal signaling, and TLR9-initiated cellular responses. Mol Biol Cell. 2016;27:572–587. doi: 10.1091/mbc.E15-05-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hume AN, Collinson LM, Rapak A, Gomes AQ, Hopkins CR, Seabra MC. Rab27a regulates the peripheral distribution of melanosomes in melanocytes. J Cell Biol. 2001;152:795–808. doi: 10.1083/jcb.152.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stinchcombe JC, Barral DC, Mules EH, et al. Rab27a is required for regulated secretion in cytotoxic T lymphocytes. J Cell Biol. 2001;152:825–834. doi: 10.1083/jcb.152.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haddad EK, Wu X, Hammer JA, Henkart PA. Defective granule exocytosis in Rab27a-deficient lymphocytes from Ashen mice. J Cell Biol. 2001;152:835–842. doi: 10.1083/jcb.152.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilson SM, Yip R, Swing DA, et al. A mutation in Rab27a causes the vesicle transport defects observed in ashen mice. Proc Natl Acad Sci USA. 2000;97:7933–7938. doi: 10.1073/pnas.140212797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salazar-Cabrera AN, Matos-Martínez M, Sánchez-Villegas MC, et al. The Griscelli-Prunieras syndrome: a case report. Bol Med Hosp Infant Mex. 1993;50:503–507. [PubMed] [Google Scholar]
- 51.Tolmachova T, Abrink M, Futter CE, Authi KS, Seabra MC. Rab27b regulates number and secretion of platelet dense granules. Proc Natl Acad Sci USA. 2007;104:5872–5877. doi: 10.1073/pnas.0609879104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barral DC, Ramalho JSS, Anders R, et al. Functional redundancy of Rab27 proteins and the pathogenesis of Griscelli syndrome. J Clin Invset. 2002;110:247–257. doi: 10.1172/JCI15058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuroda TS, Fukuda M, Ariga H, Mikoshiba K. The Slp homology domain of synaptotagmin-like proteins 1-4 and Slac2 functions as a novel Rab27A binding domain. J Biol Chem. 2002;277:9212–9218. doi: 10.1074/jbc.M112414200. [DOI] [PubMed] [Google Scholar]
- 54.Fukuda M. Rab27 effectors, pleiotropic regulators in secretory pathways. Traffic. 2013;14:949–963. doi: 10.1111/tra.12083. [DOI] [PubMed] [Google Scholar]
- 55.Fukuda M. Regulation of secretory vesicle traffic by Rab small GTPases. Cell Mol Life Sci. 2008;65:2801–2813. doi: 10.1007/s00018-008-8351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuroda TS, Fukuda M, Ariga H, Mikoshiba K. Synaptotagmin-like protein 5: a novel Rab27A effector with C-terminal tandem C2 domains. Biochem Biophys Res Commun. 2002;293:899–906. doi: 10.1016/S0006-291X(02)00320-0. [DOI] [PubMed] [Google Scholar]
- 57.Neeft M, Wieffer M, de Jong AS, et al. Munc13-4 is an effector of rab27a and controls secretion of lysosomes in hematopoietic cells. Mol Biol Cell. 2005;16:731–741. doi: 10.1091/mbc.E04-10-0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shirakawa R, Higashi T, Tabuchi A, et al. Munc13-4 is a GTP-Rab27-binding protein regulating dense core granule secretion in platelets. J Biol Chem. 2004;279:10730–10737. doi: 10.1074/jbc.M309426200. [DOI] [PubMed] [Google Scholar]
- 59.Brzezinska AA, Johnson JL, Munafo DB, et al. The Rab27a effectors JFC1/Slp1 and Munc13-4 regulate exocytosis of neutrophil granules. Traffic. 2008;9:2151–2164. doi: 10.1111/j.1600-0854.2008.00838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fukuda M. Versatile role of Rab27 in membrane trafficking: focus on the Rab27 effector families. J Biochem. 2005;137:9–16. doi: 10.1093/jb/mvi002. [DOI] [PubMed] [Google Scholar]
- 61.Sengeløv H, Kjeldsen L, Borregaard N. Control of exocytosis in early neutrophil activation. J Immunol. 1993;150:1535–1543. [PubMed] [Google Scholar]
- 62.Johnson JL, Hong H, Monfregola J, Catz SD. Increased survival and reduced neutrophil infiltration of the liver in Rab27a-but not Munc13-4-deficient mice in lipopolysaccharide-induced systemic inflammation. Infect Immun. 2011;79:3607–3618. doi: 10.1128/IAI.05043-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abdel-Latif D, Steward M, Macdonald DL, Francis GA, Dinauer MC, Lacy P. Rac2 is critical for neutrophil primary granule exocytosis. Blood. 2004;104:832–839. doi: 10.1182/blood-2003-07-2624. [DOI] [PubMed] [Google Scholar]
- 64.Mitchell T, Lo A, Logan MR, Lacy P, Eitzen G. Primary granule exocytosis in human neutrophils is regulated by Rac-dependent actin remodeling. Am J Physiol Cell Physiol. 2008;295:65. doi: 10.1152/ajpcell.00239.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McAdara Berkowitz JK, Catz SD, Johnson JL, Ruedi JM, Thon V, Babior BM. JFC1, a novel tandem C2 domain-containing protein associated with the leukocyte NADPH oxidase. J Biol Chem. 2001;276:18855–18862. doi: 10.1074/jbc.M011167200. [DOI] [PubMed] [Google Scholar]
- 66.Catz SD, Johnson JL, Babior BM. The C2A domain of JFC1 binds to 3′-phosphorylated phosphoinositides and directs plasma membrane association in living cells. Proc Natl Acad Sci USA. 2002;99:11652–11657. doi: 10.1073/pnas.172382799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koch H, Hofmann K, Brose N. Definition of Munc13-homology-domains and characterization of a novel ubiquitously expressed Munc13 isoform. Biochem J. 2000;349:247–253. doi: 10.1042/0264-6021:3490247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elstak ED, Neeft M, Nehme NT, et al. The munc13-4-rab27 complex is specifically required for tethering secretory lysosomes at the plasma membrane. Blood. 2011;118:1570–1578. doi: 10.1182/blood-2011-02-339523. [DOI] [PubMed] [Google Scholar]
- 69.Boswell KL, James DJ, Esquibel JM, et al. Munc13-4 reconstitutes calcium-dependent SNARE-mediated membrane fusion. J Cell Biol. 2012;197:301–312. doi: 10.1083/jcb.201109132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pivot-Pajot C, Varoqueaux F, de Saint Basile G, Bourgoin SG. Munc13-4 regulates granule secretion in human neutrophils. J Immunol. 2008;180:6786–6797. doi: 10.4049/jimmunol.180.10.6786. [DOI] [PubMed] [Google Scholar]
- 71.Wood SM, Meeths M, Chiang SCC, et al. Different NK cell-activating receptors preferentially recruit Rab27a or Munc13-4 to perforin-containing granules for cytotoxicity. Blood. 2009;114:4117–4127. doi: 10.1182/blood-2009-06-225359. [DOI] [PubMed] [Google Scholar]
- 72.Feldmann J, Callebaut I, Raposo G, et al. Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3) Cell. 2003;115:461–473. doi: 10.1016/s0092-8674(03)00855-9. [DOI] [PubMed] [Google Scholar]
- 73.Ren Q, Wimmer C, Chicka MC, et al. Munc13-4 is a limiting factor in the pathway required for platelet granule release and hemostasis. Blood. 2010;116:869–877. doi: 10.1182/blood-2010-02-270934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ishii E, Ueda I, Shirakawa R, et al. Genetic subtypes of familial hemophagocytic lymphohistiocytosis: correlations with clinical features and cytotoxic T lymphocyte/natural killer cell functions. Blood. 2005;105:3442–3448. doi: 10.1182/blood-2004-08-3296. [DOI] [PubMed] [Google Scholar]
- 75.Zhang Y, Tang W, Zhang H, et al. A network of interactions enables CCM3 and STK24 to coordinate UNC13D-driven vesicle exocytosis in neutrophils. Dev Cell. 2013;27:215–226. doi: 10.1016/j.devcel.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Johnson JL, Hong H, Monfregola J, Kiosses WB, Catz SD. Munc13-4 restricts motility of Rab27a-expressing vesicles to facilitate lipopolysaccharide-induced priming of exocytosis in neutrophils. J Biol Chem. 2011;286:5647–5656. doi: 10.1074/jbc.M110.184762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Muallem S, Kwiatkowska K, Xu X, Yin HL. Actin filament disassembly is a sufficient final trigger for exocytosis in nonexcitable cells. J Cell Biol. 1995;128:589–598. doi: 10.1083/jcb.128.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lang T, Wacker I, Wunderlich I, et al. Role of actin cortex in the sub-plasmalemmal transport of secretory granules in PC-12 cells. Biophys J. 2000;78:2863–2877. doi: 10.1016/S0006-3495(00)76828-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jog NR, Rane MJ, Lominadze G, Luerman GC, Ward RA, McLeish KR. The actin cytoskeleton regulates exocytosis of all neutrophil granule subsets. Am J Physiol Cell Physiol. 2007;292:700. doi: 10.1152/ajpcell.00384.2006. [DOI] [PubMed] [Google Scholar]
- 80.Johnson JL, Monfregola J, Napolitano G, Kiosses WB, Catz SD. Vesicular trafficking through cortical actin during exocytosis is regulated by the Rab27a effector JFC1/Slp1 and the RhoA-GTPase-activating protein Gem-interacting protein. Mol Biol Cell. 2012;23:1902–1916. doi: 10.1091/mbc.E11-12-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aresta S, de Tand-Heim M-FF, Béranger F, de Gunzburg j. A novel Rho GTPase-activating-protein interacts with Gem, a member of the Ras superfamily of GTPases. Biochem J. 2002;367:57–65. doi: 10.1042/BJ20020829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Borregaard N, Lollike K, Kjeldsen L, et al. Human neutrophil granules and secretory vesicles. Eur J Haematol. 1993;51:187–198. doi: 10.1111/j.1600-0609.1993.tb00629.x. [DOI] [PubMed] [Google Scholar]
- 83.Johannes L, Lledo PM, Roa M, Vincent JD, Henry JP, Darchen F. The GTPase Rab3a negatively controls calcium-dependent exocytosis in neuroendocrine cells. EMBO J. 1994;13:2029–2037. doi: 10.1002/j.1460-2075.1994.tb06476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huang C-CC, Yang D-MM, Lin C-CC, Kao L-SS. Involvement of Rab3A in vesicle priming during exocytosis: interaction with Munc13-1 and Munc18-1. Traffic. 2011;12:1356–1370. doi: 10.1111/j.1600-0854.2011.01237.x. [DOI] [PubMed] [Google Scholar]
- 85.Yunes R, Tomes C, Michaut M, et al. Rab3A and calmodulin regulate acrosomal exocytosis by mechanisms that do not require a direct interaction. FEBS Lett. 2002;525:126–130. doi: 10.1016/s0014-5793(02)03102-2. [DOI] [PubMed] [Google Scholar]
- 86.Khandelwal P, Prakasam HS, Clayton DR, et al. A Rab11a-Rab8a-Myo5B network promotes stretch-regulated exocytosis in bladder umbrella cells. Mol Biol Cell. 2013;24:1007–1019. doi: 10.1091/mbc.E12-08-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li BX, Satoh AK, Ready DF. Myosin V, Rab11, and dRip11 direct apical secretion and cellular morphogenesis in developing Drosophila photoreceptors. J Cell Biol. 2007;177:659–669. doi: 10.1083/jcb.200610157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Khvotchev MV, Ren M, Takamori S, Jahn R, Südhof TC. Divergent functions of neuronal Rab11b in Ca2+-regulated versus constitutive exocytosis. J Neurosci. 2003;23:10531–10539. doi: 10.1523/JNEUROSCI.23-33-10531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sugawara K, Shibasaki T, Mizoguchi A, Saito T, Seino S. Rab11 and its effector Rip11 participate in regulation of insulin granule exocytosis. Genes Cells. 2009;14:445–456. doi: 10.1111/j.1365-2443.2009.01285.x. [DOI] [PubMed] [Google Scholar]
- 90.Reed SE, Hodgson LR, Song S, et al. A role for Rab14 in the endocytic trafficking of GLUT4 in 3T3-L1 adipocytes. J Cell Sci. 2013;126:1931–1941. doi: 10.1242/jcs.104307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kitt KN, Hernández-Deviez D, Ballantyne SD, Spiliotis ET, Casanova JE, Wilson JM. Rab14 regulates apical targeting in polarized epithelial cells. Traffic. 2008;9:1218–1231. doi: 10.1111/j.1600-0854.2008.00752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zografou S, Basagiannis D, Papafotika A, et al. A complete Rab screening reveals novel insights in Weibel-Palade body exocytosis. J Cell Sci. 2012;125:4780–4790. doi: 10.1242/jcs.104174. [DOI] [PubMed] [Google Scholar]
- 93.Patino-Lopez G, Dong X, Ben-Aissa K, et al. Rab35 and its GAP EPI64C in T cells regulate receptor recycling and immunological synapse formation. J Biol Chem. 2008;283:18323–18330. doi: 10.1074/jbc.M800056200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hsu C, Morohashi Y, Yoshimura S-I, et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol. 2010;189:223–232. doi: 10.1083/jcb.200911018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang J, Fonovic M, Suyama K, Bogyo M, Scott MP. Rab35 controls actin bundling by recruiting fascin as an effector protein. Science. 2009;325:1250–1254. doi: 10.1126/science.1174921. [DOI] [PubMed] [Google Scholar]
- 96.Pfeffer SR. Transport-vesicle targeting: tethers before SNAREs. Nat Cell Biol. 1999;1:22. doi: 10.1038/8967. [DOI] [PubMed] [Google Scholar]
- 97.Guo W, Sacher M, Barrowman J, Ferro-Novick S, Novick P. Protein complexes in transport vesicle targeting. Trends Cell Biol. 2000;10:251–255. doi: 10.1016/s0962-8924(00)01754-2. [DOI] [PubMed] [Google Scholar]
- 98.Whyte JR, Munro S. Vesicle tethering complexes in membrane traffic. J Cell Sci. 2002;115:2627–2637. doi: 10.1242/jcs.115.13.2627. [DOI] [PubMed] [Google Scholar]
- 99.Ostrowski M, Carmo NB, Krumeich S, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12:19. doi: 10.1038/ncb2000. [DOI] [PubMed] [Google Scholar]
- 100.Brumell JH, Volchuk A, Sengelov H, et al. Subcellular distribution of docking/fusion proteins in neutrophils, secretory cells with multiple exocytic compartments. J Immunol. 1995;155:5750–5759. [PubMed] [Google Scholar]
- 101.Mollinedo F, Lazo PA. Identification of two isoforms of the vesicle-membrane fusion protein SNAP-23 in human neutrophils and HL-60 cells. Biochem Biophys Res Commun. 1997;231:808–812. doi: 10.1006/bbrc.1997.6196. [DOI] [PubMed] [Google Scholar]
- 102.Nabokina S, Egea G, Blasi J, Mollinedo F. Intracellular location of SNAP-25 in human neutrophils. Biochem Biophys Res Commun. 1997;239:592–597. doi: 10.1006/bbrc.1997.7515. [DOI] [PubMed] [Google Scholar]
- 103.Martín-Martín B, Nabokina SM, Lazo PA, Mollinedo F. Co-expression of several human syntaxin genes in neutrophils and differentiating HL-60 cells: variant isoforms and detection of syntaxin 1. J Leukoc Biol. 1999;65:397–406. doi: 10.1002/jlb.65.3.397. [DOI] [PubMed] [Google Scholar]
- 104.Xie L-x, Calafat J, Janssen H, de la Iglesia-Vicente J, Mollinedo F. Intra-cellular location of syntaxin 7 in human neutrophils. Immunol Lett. 2010;129:72–77. doi: 10.1016/j.imlet.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 105.Uriarte SM, Rane MJ, Luerman GC, et al. Granule exocytosis contributes to priming and activation of the human neutrophil respiratory burst. J Immunol. 2011;187:391–400. doi: 10.4049/jimmunol.1003112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mollinedo F, Calafat J, Janssen H, et al. Combinatorial SNARE complexes modulate the secretion of cytoplasmic granules in human neutrophils. J Immunol. 2006;177:2831–2841. doi: 10.4049/jimmunol.177.5.2831. [DOI] [PubMed] [Google Scholar]
- 107.Mullock BM, Smith CW, Ihrke G, et al. Syntaxin 7 is localized to late endosome compartments, associates with Vamp 8, and is required for late endosome-lysosome fusion. Mol Biol Cell. 2000;11:3137–3153. doi: 10.1091/mbc.11.9.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Logan MR, Lacy P, Odemuyiwa SO, et al. A critical role for vesicle-associated membrane protein-7 in exocytosis from human eosinophils and neutrophils. Allergy. 2006;61:777–784. doi: 10.1111/j.1398-9995.2006.01089.x. [DOI] [PubMed] [Google Scholar]
- 109.Luzio PJ, Gray SR, Bright NA. Endosome–lysosome fusion. Biochem Soc Trans. 2010;38:1413–1416. doi: 10.1042/BST0381413. [DOI] [PubMed] [Google Scholar]
- 110.Huotari J, Helenius A. Endosome maturation. EMBO J. 2011;30:3481–3500. doi: 10.1038/emboj.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pryor PR, Mullock BM, Bright NA, Gray SR, Luzio JP. The role of intraorganellar Ca(2+) in late endosome-lysosome heterotypic fusion and in the reformation of lysosomes from hybrid organelles. J Cell Biol. 2000;149:1053–1062. doi: 10.1083/jcb.149.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Häcker H, Mischak H, Miethke T, et al. CpG-DNA-specific activation of antigen-presenting cells requires stress kinase activity and is preceded by non-specific endocytosis and endosomal maturation. EMBO J. 1998;17:6230–6240. doi: 10.1093/emboj/17.21.6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Babior BM. The respiratory burst oxidase. Curr Opin Hematol. 1995;2:55–60. doi: 10.1097/00062752-199502010-00008. [DOI] [PubMed] [Google Scholar]
- 114.Babior BM. The respiratory burst oxidase and the molecular basis of chronic granulomatous disease. Am J Hematol. 1991;37:263–266. doi: 10.1002/ajh.2830370410. [DOI] [PubMed] [Google Scholar]
- 115.Clark RA. Activation of the neutrophil respiratory burst oxidase. J Infect Dis. 1999;179(Suppl 2):17. doi: 10.1086/513849. [DOI] [PubMed] [Google Scholar]
- 116.Li XJ, Tian W, Stull ND, Grinstein S, Atkinson S, Dinauer MC. A fluorescently tagged C-terminal fragment of p47phox detects NADPH oxidase dynamics during phagocytosis. Mol Biol Cell. 2009;20:1520–1532. doi: 10.1091/mbc.E08-06-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jancic C, Savina A, Wasmeier C, et al. Rab27a regulates phagosomal pH and NADPH oxidase recruitment to dendritic cell phagosomes. Nat Cell Biol. 2007;9:367–378. doi: 10.1038/ncb1552. [DOI] [PubMed] [Google Scholar]
- 118.Catz SD. The role of Rab27a in the regulation of neutrophil function. Cell Microbiol. 2014;16:1301–1310. doi: 10.1111/cmi.12328. [DOI] [PubMed] [Google Scholar]
- 119.Fan G-HH, Lapierre LA, Goldenring JR, Sai J, Richmond A. Rab11-family interacting protein 2 and myosin Vb are required for CXCR2 recycling and receptor-mediated chemotaxis. Mol Biol Cell. 2004;15:2456–2469. doi: 10.1091/mbc.E03-09-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Savina A, Fader CM, Damiani MTT, Colombo MII. Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic. 2005;6:131–143. doi: 10.1111/j.1600-0854.2004.00257.x. [DOI] [PubMed] [Google Scholar]
- 121.Yu S, Nie Y, Knowles B, et al. TLR sorting by Rab11 endosomes maintains intestinal epithelial-microbial homeostasis. EMBO J. 2014;33:1882–1895. doi: 10.15252/embj.201487888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 123.Munafo DB, Johnson JL, Brzezinska AA, Ellis BA, Wood MR, Catz SD. DNase I inhibits a late phase of reactive oxygen species production in neutrophils. J Innate Immun. 2009;1:527–542. doi: 10.1159/000235860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kawakami T, He J, Morita H, et al. Rab27a is essential for the formation of neutrophil extracellular traps (NETs) in neutrophil-like differentiated HL60 cells. PLoS ONE. 2014;9:e84704. doi: 10.1371/journal.pone.0084704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Remijsen Q, Vanden Berghe T, Wirawan E, et al. Neutrophil extracellular trap cell death requires both autophagy and superoxide generation. Cell Res. 2011;21:290–304. doi: 10.1038/cr.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fuchs TA, Abed U, Goosmann C, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sánchez-Madrid F, Serrador JM. Bringing up the rear: defining the roles of the uropod. Nat Rev Mol Cell Biol. 2009;10:353–359. doi: 10.1038/nrm2680. [DOI] [PubMed] [Google Scholar]
- 128.Phillipson M, Heit B, Colarusso P, Liu L, Ballantyne CM, Kubes P. Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. J Exp Med. 2006;203:2569–2575. doi: 10.1084/jem.20060925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zen K, Guo Y-LL, Li L-MM, Bian Z, Zhang C-YY, Liu Y. Cleavage of the CD11b extracellular domain by the leukocyte serprocidins is critical for neutrophil detachment during chemotaxis. Blood. 2011;117:4885–4894. doi: 10.1182/blood-2010-05-287722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Singh RK, Furze RC, Birrell MA, Rankin SM, Hume AN, Seabra MC. A role for Rab27 in neutrophil chemotaxis and lung recruitment. BMC Cell Biol. 2014;15:39. doi: 10.1186/s12860-014-0039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kurugöl Z, Ozkinay F, Vardar F, et al. Griscelli syndrome: report of a case and review of the literature. Pediatr Int. 2001;43:298–301. doi: 10.1046/j.1442-200x.2001.01383.x. [DOI] [PubMed] [Google Scholar]
- 132.Singh RK, Liao W, Tracey-White D, et al. Rab27a-mediated protease release regulates neutrophil recruitment by allowing uropod detachment. J Cell Sci. 2012;125:1652–1656. doi: 10.1242/jcs.100438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Colvin RA, Means TK, Diefenbach TJ, et al. Synaptotagmin-mediated vesicle fusion regulates cell migration. Nat Immunol. 2010;11:495–502. doi: 10.1038/ni.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Martinez I, Chakrabarti S, Hellevik T, Morehead J, Fowler K, Andrews NW. Synaptotagmin VII regulates Ca(2+)-dependent exocytosis of lysosomes in fibroblasts. J Cell Biol. 2000;148:1141–1149. doi: 10.1083/jcb.148.6.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Eiserich JP, Baldus S, Brennan M-LL, et al. Myeloperoxidase, a leukocyte-derived vascular NO oxidase. Science. 2002;296:2391–2394. doi: 10.1126/science.1106830. [DOI] [PubMed] [Google Scholar]
- 136.Massberg S, Grahl L, von Bruehl M-LL, et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med. 2010;16:887–896. doi: 10.1038/nm.2184. [DOI] [PubMed] [Google Scholar]
- 137.Lewis SM, Khan N, Beale R, Treacher DF, Brown KA. Depletion of blood neutrophils from patients with sepsis: treatment for the future? Int Immunopharmacol. 2013;17:1226–1232. doi: 10.1016/j.intimp.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 138.Treacher DF, Sabbato M, Brown KA, Gant V. The effects of leucodepletion in patients who develop the systemic inflammatory response syndrome following cardiopulmonary bypass. Perfusion. 2001;16(Suppl):67–73. doi: 10.1177/026765910101600i110. [DOI] [PubMed] [Google Scholar]
- 139.Hoesel LM, Neff TA, Neff SB, et al. Harmful and protective roles of neutrophils in sepsis. Shock. 2005;24:40–47. doi: 10.1097/01.shk.0000170353.80318.d5. [DOI] [PubMed] [Google Scholar]
- 140.Uriarte SM, Rane MJ, Merchant ML, et al. Inhibition of neutrophil exocytosis ameliorates acute lung injury in rats. Shock. 2013;39:286–292. doi: 10.1097/SHK.0b013e318282c9a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Khan AI, Kerfoot SM, Heit B, et al. Role of CD44 and hyaluronan in neutrophil recruitment. J Immunol. 2004;173:7594–7601. doi: 10.4049/jimmunol.173.12.7594. [DOI] [PubMed] [Google Scholar]
- 142.McDonald B, McAvoy EF, Lam F, et al. Interaction of CD44 and hyaluronan is the dominant mechanism for neutrophil sequestration in inflamed liver sinusoids. J Exp Med. 2008;205:915–927. doi: 10.1084/jem.20071765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Brovkovych V, Gao X-PP, Ong E, et al. Augmented inducible nitric oxide synthase expression and increased NO production reduce sepsis-induced lung injury and mortality in myeloperoxidase-null mice. Am J Physiol Lung Cell Mol Physiol. 2008;295:103. doi: 10.1152/ajplung.00450.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rao H-LL, Chen J-WW, Li M, et al. Increased intratumoral neutrophil in colorectal carcinomas correlates closely with malignant phenotype and predicts patients’ adverse prognosis. PLoS ONE. 2012;7:e30806. doi: 10.1371/journal.pone.0030806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li Y-WW, Qiu S-JJ, Fan J, et al. Intratumoral neutrophils: a poor prognostic factor for hepatocellular carcinoma following resection. J Hepatol. 2011;54:497–505. doi: 10.1016/j.jhep.2010.07.044. [DOI] [PubMed] [Google Scholar]
- 146.Jensen HK, Donskov F, Marcussen N, Nordsmark M, Lundbeck F, von der Maase H. Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. J Clin Oncol. 2009;27:4709–4717. doi: 10.1200/JCO.2008.18.9498. [DOI] [PubMed] [Google Scholar]
- 147.Shen M, Hu P, Donskov F, Wang G, Liu Q, Du J. Tumor-associated neutrophils as a new prognostic factor in cancer: a systematic review and meta-analysis. PLoS ONE. 2014;9:e98259. doi: 10.1371/journal.pone.0098259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fioretti F, Fradelizi D, Stoppacciaro A, et al. Reduced tumorigenicity and augmented leukocyte infiltration after monocyte chemotactic protein-3 (MCP-3) gene transfer: perivascular accumulation of dendritic cells in peritumoral tissue and neutrophil recruitment within the tumor. J Immunol. 1998;161:342–346. [PubMed] [Google Scholar]
- 149.Hirose K, Hakozaki M, Nyunoya Y, et al. Chemokine gene transfection into tumour cells reduced tumorigenicity in nude mice in association with neutrophilic infiltration. Br J Cancer. 1995;72:708–714. doi: 10.1038/bjc.1995.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lee LF, Hellendall RP, Wang Y, et al. IL-8 reduced tumorigenicity of human ovarian cancer in vivo due to neutrophil infiltration. J Immunol. 2000;164:2769–2775. doi: 10.4049/jimmunol.164.5.2769. [DOI] [PubMed] [Google Scholar]
- 151.Bekes EM, Schweighofer B, Kupriyanova TA, et al. Tumor-recruited neutrophils and neutrophil TIMP-free MMP-9 regulate coordinately the levels of tumor angiogenesis and efficiency of malignant cell intravasation. Am J Pathol. 2011;179:1455–1470. doi: 10.1016/j.ajpath.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Deryugina EI, Zajac E, Juncker-Jensen A, Kupriyanova TA, Welter L, Quigley JP. Tissue-infiltrating neutrophils constitute the major in vivo source of angiogenesis-inducing MMP-9 in the tumor microenvironment. Neoplasia. 2014;16:771–788. doi: 10.1016/j.neo.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rymaszewski AL, Tate E, Yimbesalu JP, et al. The role of neutrophil myeloperoxidase in models of lung tumor development. Cancers. 2014;6:1111–1127. doi: 10.3390/cancers6021111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dolcetti L, Peranzoni E, Ugel S, et al. Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur J Immunol. 2010;40:22–35. doi: 10.1002/eji.200939903. [DOI] [PubMed] [Google Scholar]
- 155.Queen MM, Ryan RE, Holzer RG, Keller-Peck CR, Jorcyk CL. Breast cancer cells stimulate neutrophils to produce oncostatin M: potential implications for tumor progression. Cancer Res. 2005;65:8896–8904. doi: 10.1158/0008-5472.CAN-05-1734. [DOI] [PubMed] [Google Scholar]
- 156.Park B, Zhang H, Zeng Q, et al. NF-κB in breast cancer cells promotes osteolytic bone metastasis by inducing osteoclastogenesis via GM-CSF. Nat Med. 2006;13:62–69. doi: 10.1038/nm1519. [DOI] [PubMed] [Google Scholar]
- 157.Wang Y, Han G, Wang K, et al. Tumor-derived GM-CSF promotes inflammatory colon carcinogenesis via stimulating epithelial release of VEGF. Cancer Res. 2014;74:716–726. doi: 10.1158/0008-5472.CAN-13-1459. [DOI] [PubMed] [Google Scholar]
- 158.Bayne LJ, Beatty GL, Jhala N, et al. Tumor-Derived Granulocyte-Macrophage Colony-Stimulating Factor Regulates Myeloid Inflammation and T Cell Immunity in Pancreatic Cancer. Cancer Cell. 2012;21:822–835. doi: 10.1016/j.ccr.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pylayeva-Gupta Y, Lee K, Hajdu CH, Miller G, Bar-Sagi D. Oncogenic kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell. 2012;21:836–847. doi: 10.1016/j.ccr.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wicks IP, Roberts AW. Targeting GM-CSF in inflammatory diseases. Nat Rev Rheumatol. 2016;12:37–48. doi: 10.1038/nrrheum.2015.161. [DOI] [PubMed] [Google Scholar]
- 161.van Nieuwenhuijze A, Koenders M, Roeleveld D, Sleeman MA, van den Berg W, Wicks IP. GM-CSF as a therapeutic target in inflammatory diseases. Mol Immunol. 2013;56:675–682. doi: 10.1016/j.molimm.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 162.Hamilton JA. GM-CSF in inflammation and autoimmunity. Trends Immunol. 2002;23:403–408. doi: 10.1016/s1471-4906(02)02260-3. [DOI] [PubMed] [Google Scholar]
- 163.Wang Q, Ni Q, Wang X, Zhu H, Wang Z, Huang J. High expression of RAB27A and TP53 in pancreatic cancer predicts poor survival. Med Oncol. 2015;32:372. doi: 10.1007/s12032-014-0372-2. [DOI] [PubMed] [Google Scholar]
- 164.Wang J-SS, Wang F-BB, Zhang Q-GG, Shen Z-ZZ, Shao Z-MM. Enhanced expression of Rab27A gene by breast cancer cells promoting invasiveness and the metastasis potential by secretion of insulin-like growth factor-II. Mol Cancer Res. 2008;6:372–382. doi: 10.1158/1541-7786.MCR-07-0162. [DOI] [PubMed] [Google Scholar]
- 165.Dong W-WW, Mou Q, Chen J, Cui J-TT, Li W-MM, Xiao W-HH. Differential expression of Rab27A/B correlates with clinical outcome in hepatocellular carcinoma. World J Gastroenterol. 2012;18:1806–1813. doi: 10.3748/wjg.v18.i15.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Peinado H, Alečković M, Lavotshkin S, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gaudin R, Barteneva NS. Sorting of small infectious virus particles by flow virometry reveals distinct infectivity profiles. Nat Commun. 2015;6:6022. doi: 10.1038/ncomms7022. [DOI] [PMC free article] [PubMed] [Google Scholar]