Abstract
Acute limb ischemia is an emergent limb and life-threatening condition with high morbidity and mortality. An understanding of the presentation, clinical evaluation, and initial workup, including noninvasive imaging evaluation, is critical to determine an appropriate management strategy. Modern series have shown endovascular revascularization for acute limb ischemia to be safe and effective with success rates approaching surgical series and with similar, or even decreased, perioperative morbidity and mortality. A thorough understanding of endovascular techniques, associated pharmacology, and perioperative care is paramount to the endovascular management of patients presenting with acute limb ischemia. This article discusses the diagnosis and strategies for endovascular treatment of acute limb ischemia.
Keywords: acute limb ischemia, endovascular, thrombolysis, thrombectomy, interventional radiology
Objectives : Upon completion of this article, the reader will be able to discuss the diagnosis and strategies for endovascular treatment of acute limb ischemia.
Accreditation : This activity has been planned and implemented in accordance with the accreditation requirements and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit : Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit ™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Definition and Incidence of Acute Limb Ischemia
Acute limb ischemia is defined as a sudden decrease in limb perfusion that threatens limb viability and is less than 14 days in duration. 1 2 3 More commonly, acute limb ischemia involves the lower extremities. The annual incidence of acute limb ischemia in the United States is between 1.4 and 2.6 cases per 10,000 patients. 1 2 4 5 Morbidity is high with amputation rates approaching 15% during the acute hospitalization, many above the knee, and mortality rates between 15 and 40%. 1 2 The acuity and severity of the inciting event are due to the lack of opportunity for development of collateral vessels unlike chronic ischemia. 1
Clinical Evaluation and Workup of Acute Limb Ischemia
History
A focused history includes an evaluation of symptoms, prior interventions (both endovascular and surgical), risk factors, and contraindications to thrombolysis. 2 Patients with acute limb ischemia will present with new or worsening claudication or, more commonly, rest pain, numbness, weakness, and change in the color or temperature of the extremity. 1 2 Clinical symptoms may be recalled by employing the “6 Ps”: pain, paresthesia, paralysis, pallor, poikilothermia (cool extremity), and pulselessness. 1
Risk Factors
Relevant risk factors depend on the underlying etiology for acute limb ischemia. The two most common etiologies are thrombosis and embolism. 3 Thrombosis is most often due to prior bypass grafts or atherosclerotic disease in the region of occlusion which is associated with a history of diabetes, smoking, known peripheral arterial disease, myocardial infarction, or cerebrovascular accident. 1 2 Embolism, from a distant source, is most often due to atrial fibrillation, acute myocardial infarction, left ventricular dysfunction, and prosthetic cardiac valves. 1 6 Less common risk factors for acute limb ischemia include systemic thrombophilia, dissection, intimal hyperplasia, vasculitis, and aneurysm thrombosis. 1 3
Physical Examination Findings
The physical examination is critical, as the Rutherford classification that drives treatment decisions is based solely on the clinical examination. 7 A focused physical examination includes evaluation of pulses (tactile and Doppler), sensation to light touch, and muscular strength. 2 Findings of acute limb ischemia include absent pulses, decreased strength, cool skin, and reduced sensation. 1
Classification of Acute Limb Ischemia
Clinical classification of acute limb ischemia was described by Rutherford in 1986 and updated in 1997, and is still the primary means by which clinical prognosis and management is stratified. 1 2 3 6 8 9 10 11 The classification is derived from components of the clinical exam and is detailed in Table 1 . 7 If patients have an audible pulse with Doppler, the limb is classified as viable. If the patient has profound sensory loss and especially muscle weakness, the limb is classified as irreversibly ischemic. If the limb does not fit these criteria, it is considered threatened, which is stratified as marginal or immediate, the determination of which is based on the degree of impairment in sensory and motor function.
Table 1. Rutherford classification of acute limb ischemia 7 .
| Class | Description | Sensory loss | Muscle weakness | Arterial Doppler | Venous Doppler |
|---|---|---|---|---|---|
| I. Viable | Not immediately threatened | None | None | Audible | Audible |
| IIa. Marginally threatened | Salvageable with prompt treatment | None or minimal (toes only) | None | Absent | Audible |
| IIb. Immediately threatened | Salvageable with immediate revascularization | More than toes; rest pain | Mild to moderate | Absent | Audible |
| III. Irreversible | Major tissue loss, inevitable nerve damage | Profound | Profound | Absent | Absent |
Laboratory Evaluation
Laboratory analysis is not necessary for the diagnosis of acute limb ischemia, but is necessary to prepare for definitive management. 2 Basic metabolic panel should be obtained, with attention to renal function to guide the use of intravenous contrast, as well as evidence of hyperkalemia and acidosis, which are complications of limb ischemia. 3 Hemoglobin, hematocrit, and type and screen should be obtained, and are particularly useful if the patient may require open surgical intervention to determine the need for preoperative or intraoperative transfusion. 2 Baseline coagulation studies including partial thromboplastin time, prothrombin time, international normalized ratio, and fibrinogen should be obtained, particularly if the patient is to undergo heparinization or thrombolysis. 2
Contraindications to Thrombolysis
As discussed previously, the focused history should evaluate the patient for contraindications to thrombolysis, as detailed in Table 2 . Absolute contraindications include active bleeding, intracranial hemorrhage, compartment syndrome, and contraindications to anticoagulation or thrombolytic medications. 3
Table 2. Contraindications to thrombolysis per Society of Interventional Radiology 3 .
| Absolute contraindications |
| Active clinically significant bleeding |
| Intracranial hemorrhage |
| Presence/development of compartment syndrome |
| Absolute contraindication to anticoagulation |
| Relative contraindications |
| Bleeding diathesis |
| Disseminated intravascular coagulation |
| Established cerebrovascular accident (within 2 mo) |
| Neurosurgery or intracranial trauma (within 3 mo) |
| Cardiopulmonary resuscitation (within 10 d) |
| Major surgery or trauma (within 10 d) |
| Eye surgery (within 3 mo) |
| Intracranial tumor, vascular malformation, aneurysm, or seizure disorder |
| Uncontrolled hypertension |
| Recent internal hemorrhage or visceral biopsy |
| Recent major gastrointestinal bleed (within 10 d) |
| Serious allergic reaction to thrombolytic agent, anticoagulant, or contrast which cannot be controlled by premedication |
| Severe thrombocytopenia |
| Pregnancy or immediate postpartum state |
| Severe liver dysfunction with associated coagulopathy |
| Bacterial endocarditis |
| Bleeding diathesis |
| Disseminated intravascular coagulation |
| Diabetic hemorrhagic retinopathy |
| Life expectancy less than 1 y |
Noninvasive Imaging Workup (Duplex Ultrasound, Pulse Volume Recordings, Computed Tomography Angiography, Magnetic Resonance Angiography)
Though catheter-based arteriography is often employed in cases of acute limb ischemia due to the ability to provide diagnostic information and treatment in the same session, noninvasive imaging may be considered in patients with viable or marginally threatened limbs (Rutherford classification I or IIa) and in some cases of immediately threatened, limbs (Rutherford classification IIb). 1 If noninvasive imaging is pursued, the preferred study is commonly computed tomography angiography of the abdomen and pelvis with run-offs, as computed tomography angiography may identify the level of occlusion, demonstrate prior surgical or endovascular intervention, and may be obtained expeditiously. 2 Other potentially useful, and often complementary, studies include magnetic resonance angiography (may not be available emergently and may be difficult to obtain a good-quality study depending on the patient's potential level of pain), segmental pressures (which may reveal the level of occlusion), and duplex ultrasound (which may elucidate the site of occlusion). 6 If the clinical examination precludes all of the aforementioned studies, surgical history along with plain radiographs of the abdomen, pelvis, and extremities may identify prior stents or hardware that may influence the feasibility and type of endovascular approach. 2
Endovascular Intervention
See Figs. 1 and 2 . Once acute limb ischemia has been identified, initial management should aim to prevent thrombus propagation with intravenous unfractionated heparin while triaging the patient to undergo conservative management, endovascular revascularization, open surgical revascularization, or hybrid intervention based on clinical severity (i.e., Rutherford classification), anatomy, surgical risk factors, and whether the patient is a candidate for catheter-directed thrombolysis or endovascular thrombectomy. 1 2 3 6 8 9 10 11
Fig. 1.
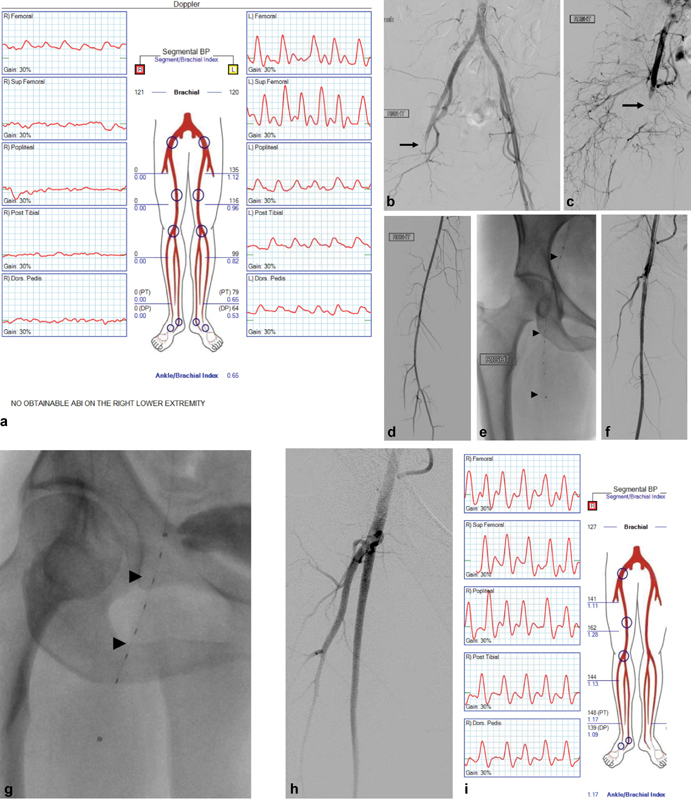
A 65-year-old male presenting with acute-onset right hip and thigh pain with numbness and cold temperature of the right foot starting 16 hours earlier with findings of Rutherford class IIa acute limb ischemia. ( a ) Noninvasive arterial examination confirmed poor perfusion to the right leg with ankle/brachial index 0. ( b–d ) Patient was started on heparin drip and aortography and right lower extremity arteriography confirmed right common femoral embolus. ( e–g ) Catheter-directed thrombolysis was performed, initially into the superficial femoral artery and subsequently into the profundal femoris artery (0.5 mg/hour alteplase over total 12 hours). ( h ) Completion arteriography demonstrated resolution of the embolus with restored in-line flow to the foot confirmed by follow-up physical examination and ( I ) noninvasive arterial examination. Patient was found to have new-onset atrial fibrillation and was continued on anticoagulation. Sup, superficial; Post, posterior; Dors, dorsalis; BP, blood pressure.
Fig. 2.
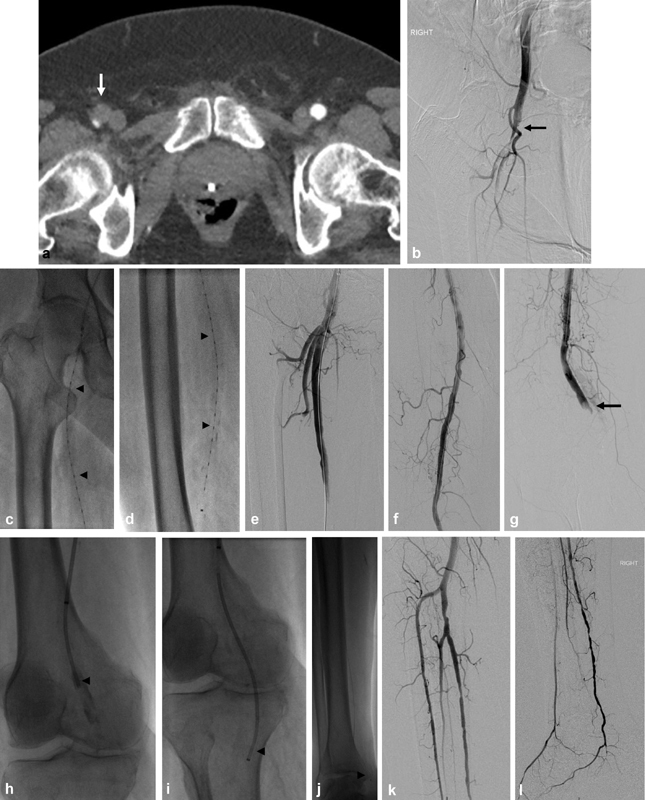
A 79-year-old male with known atrial fibrillation presented with sudden-onset pain and cold temperature of the right leg and foot. His presenting international normalized ratio was 1.4. On physical examination, he was found to have Rutherford class IIb acute limb ischemia. He was started on heparin drip and ( a ) computed tomography angiography confirmed occlusion of the right common and superficial femoral arteries. ( b ) Right lower extremity arteriography confirmed the same with poor distal run-off. ( c and d ) Catheter-directed thrombolysis was initiated from the common femoral through superficial femoral arteries (0.5 mg/hour alteplase for a total of 8 hours). ( e–g ) Follow-up arteriography confirmed revascularization of the common and superficial femoral arteries with residual thromboembolic occlusions of the popliteal artery and infrapopliteal run-off. ( h–j ) Adjunct endovascular thromboembolectomy of the popliteal and infrapopliteal arteries was performed with Penumbra Cat-6 and Cat-3 catheters. ( k and l ) Completion right lower extremity arteriography demonstrated restoration of in-line flow to the right foot. Patient was maintained on therapeutic anticoagulation postoperatively.
Patient Selection and Timing of Intervention
The timing and treatment for acute limb ischemia is, in part, determined by the Rutherford classification ( Table 1 ). In patients presenting with a viable limb (class I), there is time to acquire noninvasive diagnostic imaging, noninvasive vascular studies (e.g., venous and arterial duplex ultrasonography, pulse volume recordings), and laboratory studies to characterize possible underlying chronic vascular disease and determine the need for elective vascular intervention while optimizing modifiable vascular disease and surgical risk factors. 1 2 9 11 12 Patients with Rutherford class IIa limb ischemia require urgent revascularization and are often amenable to endovascular treatment, whereas patients with class IIb ischemia require emergent revascularization and have traditionally been treated with open surgery (e.g., open thromboembolectomy, endarterectomy, bypass surgery, patch angioplasty, or intraoperative thrombolysis), though more recent case series have demonstrated similar revascularization rates with endovascular revascularization with decreased 30-day morbidity and mortality compared with open surgery. 1 2 9 11 12 13 Patients with irreversible limb ischemia (Rutherford class III) may require amputation without attempted revascularization as reperfusion abruptly releases toxic by-products of ischemic tissue (potassium, myoglobin, and reactive oxygen species) into the systemic circulation. 6 14 15 There is a large degree of overlap between endovascular and open indications and based on currently available data, endovascular revascularization is a reasonable option for patients with Rutherford classes I and II acute limb ischemia. 4 13 16 Regarding timing of intervention, patients with class I acute limb ischemia need urgent revascularization (i.e., within 12 hours of presentation) and patients with class II acute limb ischemia need emergent revascularization (i.e., within 2–6 hours of presentation).
Endovascular Revascularization
The goals of endovascular treatment are prompt revascularization of the ischemic lower extremity and management of the underlying thrombogenic lesion. First, arteriographic evaluation of inflow and run-off should be obtained. 1 2 12 17 Distal aortography and aortoiliac arteriography may reveal embolic sources such as aortic aneurysm with associated mural thrombus, dissection, traumatic injury, and other proximal thrombogenic lesions. 12 Additional important arteriographic findings include the site of occlusion, the presence or absence of distal reconstitution, and evidence of collateral vessels. In general, embolic disease should be considered if the occlusion lies at a vessel bifurcation or trifurcation. 12 18 Additionally, if an arterial conduit is present and thrombosed, the offending lesion typically resides at the site of vessel inflow or outflow. 1 12 18 Once the area of occlusion has been identified, crossing the lesion may be attempted. Anticoagulation should be titrated to an activated clotting time of at least 250 seconds. 12 In general, it is recommended to begin probing the lesion with hydrophilic guidewires and progressing to wires with greater penetration power. The acuity of the thrombosis may be estimated by the ease in which a guidewire passes through the occlusion, with fresh thrombus being easily traversable and chronic lesions requiring more advanced wires and techniques. If the thrombus is unable to be crossed, proximal infusion of thrombolytics may be attempted for several hours followed by reattempt at crossing the lesion. 2 9 12
Once the lesion has been crossed, an infusion catheter may be placed across the thrombus with subsequent infusion of a thrombolytic agent. Thrombolytic medications currently available in the United States include alteplase (recombinant tissue plasminogen activator), Retavase (genetically engineered tissue plasminogen activator), and tenecteplase (genetically engineered tissue plasminogen activator). Thrombolytic dosing and duration vary widely in the literature. For example, alteplase infusions ranging from 2.5 to 48 hours have been studied with no consensus on an optimal strategy; however, some important generalizations have been derived. 4 9 Low-dose, long-duration thrombolytic infusions may carry an increased risk of hemorrhagic complications, while high-dose, short-duration thrombolytic infusions may carry an increased risk of distal embolization. 9 In patients with emergent need for revascularization (class IIb), short high-dose infusions are typically recommended. 4 9 Moreover, the method of infusion of the thrombolytic agent may vary, as it may be administered in a continuous, pulse-spray fashion, or initial pulse-spray with subsequent continuous infusion. The theoretical advantage of pulse-spray infusion is more rapid penetration and fragmentation of the thrombus; however, there has yet to be large cohort series or systematic meta-analysis that shows a clinical benefit between infusion methods. 4 9 12
In addition to catheter-directed thrombolysis, endovascular thrombectomy may serve as a complimentary or standalone technique for percutaneous revascularization that ideally speeds revascularization and limits the need for thrombolysis. 19 20 21 22 23 24 25 Endovascular thrombectomy has seen rapid innovation and increase in the number of devices available over the past decades. The devices are often categorized into four groups: mechanical aspiration, rheolytic, ultrasonic, or combination. Drawbacks to some of these devices include increased risk of distal embolization. Embolization risk may be offset with protection devices and is advisable in high-risk patients with single vessel run-off. 2 9 19 22 26
It is important to recall that endovascular thrombolysis or thromboembolectomy, at best, restores the vasculature to its baseline condition and the remaining thrombogenic lesion must be addressed in an endovascular (angioplasty or stent placement), open surgical, or hybrid technique to achieve a durable result.
Pharmacology
All currently used thrombolytic agents cleave plasminogen to plasmin which then degrades fibrin matrices leading to thrombus disorganization and degradation. 9 27 28 An ideal thrombolytic agent would have selective activity for fibrin-bound plasminogen within the target thrombus and thus limit the potential hemorrhagic complications. Multiple generations of thrombolytic agents have been developed with each generation acquiring increased specificity for fibrin-bound plasminogen and less activity against free circulating plasminogen. 9 First-generation thrombolytics (streptokinase, urokinase) are no longer used in acute limb ischemia due to their increased risk of hemorrhagic complications and slower clot clearing times secondary to the lack of specificity for fibrin. 28 Second-generation thrombolytics, of which recombinant tissue plasminogen activator (alteplase) is the most commonly used agent, acts almost exclusively on fibrin-bound plasminogen. 9 11 29 30 Third-generation thrombolytics are slight modifications of the second generation and are engineered for greater thrombus penetration, resistance to endogenous inactivating factors in the plasma (tenecteplase), and less affinity for free circulating plasminogen (reteplase). 31 32 Theoretically, third-generation, compared with second-generation, agents may be more effective with less bleeding complications; however, it is debated whether in vivo pharmacologic differences have any clinical relevance. 28 29
In addition to thrombolytic agents, adjunctive anticoagulation and antiplatelet agents may also have a role in revascularization. Continuous unfractionated heparin infusion during thrombolysis is thought to increase the efficacy of lysis by preventing rethrombosis and is utilized by most interventionalists. 3 9 28 29 33 Small series, however, show no improvement in efficacy with similar complication rates without or with heparinization during thrombolysis. 9 11 Similarly, adjunctive administration of platelet glycoprotein IIb/IIIa antagonists has been proposed to accelerate natural thrombolysis. While conclusive studies have not been conducted for acute limb ischemia, there is some encouraging evidence in lysis for acute ischemic stroke that may be extrapolated.
During catheter-directed thrombolysis, many interventionalists monitor serial plasma fibrinogen levels, and halt or reduce lysis infusion when less than 100 to 150 mg/dL according to recent survey results. 11 Yet, the largest prospective study, the PURPOSE trial that contained 241 patients, found no statistically significant difference in either major or minor bleeding complications with fibrinogen less than 100 mg/dL compared with greater than 100 mg/dL. 34 35 36 37 38 Current Society of Interventional Radiology acute limb ischemia practice guidelines do not recommend nor dissuade fibrinogen monitoring due to the lack of prospective randomized trials. 3 While serial fibrinogen levels may anecdotally aid in reducing bleeding events for some interventionalists, the practice may contribute to premature termination of lysis infusion.
Catheter-Directed Thrombolysis versus Surgery
Five prospective randomized controlled trials in the 1990s including the STILE (Surgery vs. Thrombolysis for Ischemia of the Lower Extremity; specifically, the subgroup with limb ischemia less than 14-days duration), TOPAS (Thrombolysis or Peripheral Arterial Surgery), and Ouriel et al's trials demonstrated at least equal efficacy of catheter-directed thrombolysis with urokinase compared with open thrombectomy. 39 40 41 Cochrane meta-analysis of these studies concluded no difference in limb salvage or mortality at 1, 6, and 12 months. 41 Stroke (1.3%), major bleeding events (8.8%), and distal embolization (12.4%), however, were higher in patients who underwent thrombolysis compared with surgery (0, 3.3, and 0%, respectively). 16 Since these trials, endovascular techniques and thrombolytic agents have shown improved outcomes and contemporary studies further support catheter-directed thrombolysis for patients with mild to moderate acute limb ischemia (classes I–II). Additionally, in the PEARL registry (PEripheral Use of AngioJet Rheolytic Thrombectomy with a variety of catheter Lengths), Leung et al demonstrated favorable results using rheolytic pharmacomechanical thrombectomy with technical success in 83 and in 52% of cases without the use of additional catheter-directed lysis. 21 Newer devices such as suction thrombectomy devices dedicated are also being utilized in acute limb ischemia without and with adjunctive catheter-directed thrombolysis with recent case series by Baumann et al showing favorable safety and technical success using the Penumbra system. 22
Complications
Patients are at greatest risk for hemorrhagic complications during thrombolytic infusion and must be examined in the intensive care unit very closely for neurological or neurovascular changes. New abnormalities identified on neurological exam should prompt immediate cessation of thrombolytics and noncontrast head computed tomography to screen for intracranial hemorrhage. New-onset flank pain, tachycardia, hypotension, or significant reductions in hemoglobin concentration (>1 g/dL) may indicate retroperitoneal hemorrhage, either spontaneous or secondary to catheter manipulation within the aortoiliac vessels. In addition to holding thrombolytic infusion, aortography or computed tomography angiography should be considered depending on the degree of acuity. Patients in extremis may undergo emergent aortography for localization of the bleeding vessel with possible embolization.
Distal embolization may occur during thrombolysis. Typically, emboli will resolve with continued infusion of the thrombolytic agent; however, if resolution does not occur, calcific or atheroembolic plaque should be suspected, and require suction or surgical embolectomy.
Minor bleeding from the arterial access site(s) is the most common complication encountered, and typically resolves with manual compression. 2 12 42 43 Access site bleeding may resolve without further complication or lead to hematoma and pseudoaneurysm formation which may be treated with ultrasound-guided thrombin injection and rarely requires open repair. 44
There are also a set of complications stemming from reperfusion injury. Revascularization of ischemic skeletal muscle leads to washout of high concentrations of potassium, myoglobin, and reactive oxygen species into the systemic circulation with both local and systemic consequences. 15 45 Hyperkalemia destabilizes cardiomyocytes leading to arrhythmias. High concentrations of excreted myoglobin precipitates in the renal tubules causing obstruction and potentially renal failure. 46 47 Local intracellular antioxidant processes are overwhelmed leading to cell death and release of inflammatory cytokines and cell adhesion molecules that propagate an inflammatory milieu causing tissue swelling that may increase compartment pressures compromising the microvasculature leading to compartment syndrome. 15 Management of reperfusion syndrome should include aggressive hydration, potassium temporization, diuresis, and possible dialysis, and consideration of prophylactic fasciotomy if new-onset pain and paresthesias prompt a diagnosis of compartment syndrome.
Contrast-induced nephropathy, or post-contrast acute kidney injury, is debated to result in any clinically significant renal injury. 48 Thus, poor renal function should not hinder the diagnosis and treatment of acute limb ischemia.
Long-Term Management
Following successful endovascular revascularization for acute limb ischemia, long-term management should focus on preservation of native vessel or conduit patency, and optimization of modifiable cardiovascular risk factors (i.e., smoking cessation, diabetes management, statin therapy, counseling on diet, and exercise). 49 Patients may be seen in clinic at 1 week, 1 month, and every 3 months thereafter for follow-up evaluation. An outpatient follow-up visit should include reassessment of symptoms (i.e., 6 “Ps”), vascular ultrasound, arterial brachial indices, and pulse volume recordings, and laboratory studies including complete blood count, electrolyte panel, serum blood urea nitrogen, serum creatinine, and coagulation studies if applicable. Patients who underwent angioplasty or stenting are typically placed on dual-antiplatelet therapy with aspirin (81 or 325 mg) and clopidogrel (75 mg), while patients with underlying thromboembolic disease are typically anticoagulated with warfarin or a novel oral anticoagulant. Some patients may require at least short-term anticoagulation along with antiplatelet monotherapy. There are limited data; however, to direct exact antiplatelet and anticoagulation management. 49 Although the recent COMPASS trial suggests low-dose rivaroxaban plus aspirin is superior to aspirin alone to reduce major adverse cardiovascular and limb events. 50
Conclusion
Acute limb ischemia is a vascular emergency that carries significant risk of limb loss and mortality. Thus, rapid diagnosis is paramount for limb salvage. In addition to starting heparin infusion, triaging the patient to endovascular or surgical management should be based on clinical symptoms using Rutherford classification and noninvasive imaging when available. Recent data support that viable and threatened limb ischemia (Rutherford classes I–IIb) may be safely and effectively treated using endovascular techniques including catheter-directed thrombolysis and/or endovascular thromboembolectomy.
Footnotes
Drs. Hage and McDevitt contributed equally and share first authorship for this manuscript.
References
- 1.Creager M A, Kaufman J A, Conte M S. Clinical practice. Acute limb ischemia. N Engl J Med. 2012;366(23):2198–2206. doi: 10.1056/NEJMcp1006054. [DOI] [PubMed] [Google Scholar]
- 2.Gilliland C, Shah J, Martin J G, Miller M J., Jr Acute limb ischemia. Tech Vasc Interv Radiol. 2017;20(04):274–280. doi: 10.1053/j.tvir.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 3.Patel N H, Krishnamurthy V N, Kim S et al. Quality improvement guidelines for percutaneous management of acute lower-extremity ischemia. J Vasc Interv Radiol. 2013;24(01):3–15. doi: 10.1016/j.jvir.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 4.Lukasiewicz A, Lichota W, Thews M. Outcomes of accelerated catheter-directed thrombolysis in patients with acute arterial thrombosis. Vasc Med. 2016;21(05):453–458. doi: 10.1177/1358863X16635291. [DOI] [PubMed] [Google Scholar]
- 5.Baril D T, Ghosh K, Rosen A B. Trends in the incidence, treatment, and outcomes of acute lower extremity ischemia in the United States Medicare population. J Vasc Surg. 2014;60(03):669–7700. doi: 10.1016/j.jvs.2014.03.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falluji N, Mukherjee D. Critical and acute limb ischemia: an overview. Angiology. 2014;65(02):137–146. doi: 10.1177/0003319712470966. [DOI] [PubMed] [Google Scholar]
- 7.Rutherford R B, Baker J D, Ernst C et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26(03):517–538. doi: 10.1016/s0741-5214(97)70045-4. [DOI] [PubMed] [Google Scholar]
- 8.Morrison H L. Catheter-directed thrombolysis for acute limb ischemia. Semin Intervent Radiol. 2006;23(03):258–269. doi: 10.1055/s-2006-948765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lukasiewicz A. Treatment of acute lower limb ischaemia. Vasa. 2016;45(03):213–221. doi: 10.1024/0301-1526/a000527. [DOI] [PubMed] [Google Scholar]
- 10.Sedghi Y, Collins T J, White C J. Endovascular management of acute limb ischemia. Vasc Med. 2013;18(05):307–313. doi: 10.1177/1358863X13505643. [DOI] [PubMed] [Google Scholar]
- 11.Smeds M R, Sandhu H K, Leake S S, Miller C C, Charlton-Ouw K M. Patterns in the management of acute limb ischemia: a VESS survey. Ann Vasc Surg. 2017;38:164–171. doi: 10.1016/j.avsg.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Valle J A, Waldo S W. Current endovascular management of acute limb ischemia. Interv Cardiol Clin. 2017;6(02):189–196. doi: 10.1016/j.iccl.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Grip O, Wanhainen A, Michaëlsson K, Lindhagen L, Björck M. Open or endovascular revascularization in the treatment of acute lower limb ischaemia. Br J Surg. 2018;105(12):1598–1606. doi: 10.1002/bjs.10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gillani S, Cao J, Suzuki T, Hak D J. The effect of ischemia reperfusion injury on skeletal muscle. Injury. 2012;43(06):670–675. doi: 10.1016/j.injury.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Blaisdell F W.The Pathophysiology of Skeletal Muscle Ischemia and the Reperfusion Syndrome: A Review. 2016Available at:http://dx.doi.org/101177/096721090201000620. Accessed November 15, 2018 [DOI] [PubMed]
- 16.Berridge D C, Kessel D O, Robertson I.Surgery versus thrombolysis for initial management of acute limb ischaemia(Review)Cochrane Database Syst Rev 201306CD002784. [DOI] [PubMed] [Google Scholar]
- 17.Funaki B. Thrombolysis for acute limb-threatening ischemia: a practical approach. Semin Intervent Radiol. 2012;29(03):201–203. doi: 10.1055/s-0032-1326930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McNamara T O, Fischer J R. Thrombolysis of peripheral arterial and graft occlusions: improved results using high-dose urokinase. AJR Am J Roentgenol. 1985;144(04):769–775. doi: 10.2214/ajr.144.4.769. [DOI] [PubMed] [Google Scholar]
- 19.Kwok C HR, Fleming S, Chan K KC et al. Aspiration thrombectomy versus conventional catheter-directed thrombolysis as first-line treatment for noniatrogenic acute lower limb ischemia. J Vasc Interv Radiol. 2018;29(05):607–613. doi: 10.1016/j.jvir.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 20.Heller S, Lubanda J C, Varejka P et al. Percutaneous mechanical thrombectomy using Rotarex® S device in acute limb ischemia in infrainguinal occlusions. BioMed Res Int. 2017;2017:2.362769E6. doi: 10.1155/2017/2362769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leung D A, Blitz L R, Nelson T et al. Rheolytic pharmacomechanical thrombectomy for the management of acute limb ischemia: results from the PEARL registry. J Endovasc Ther. 2015;22(04):546–557. doi: 10.1177/1526602815592849. [DOI] [PubMed] [Google Scholar]
- 22.Baumann F, Sharpe E, III, Peña C, Samuels S, Benenati J F. Technical results of vacuum-assisted thrombectomy for arterial clot removal in patients with acute limb ischemia. J Vasc Interv Radiol. 2016;27(03):330–335. doi: 10.1016/j.jvir.2015.11.061. [DOI] [PubMed] [Google Scholar]
- 23.Davis F M, Albright J, Gallagher K A et al. Early outcomes following endovascular, open surgical, and hybrid revascularization for lower extremity acute limb ischemia. Ann Vasc Surg. 2018;51:106–112. doi: 10.1016/j.avsg.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 24.Nehler M R, Mueller R J, McLafferty R B et al. Outcome of catheter-directed thrombolysis for lower extremity arterial bypass occlusion. J Vasc Surg. 2003;37(01):72–78. doi: 10.1067/mva.2003.42. [DOI] [PubMed] [Google Scholar]
- 25.Raabe R D. Ultrasound-accelerated thrombolysis in arterial and venous peripheral occlusions: fibrinogen level effects. J Vasc Interv Radiol. 2010;21(08):1165–1172. doi: 10.1016/j.jvir.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 26.Saxon R R, Benenati J F, Teigen C, Adams G L, Sewall L E; PRISM Trialists.Utility of a power aspiration-based extraction technique as an initial and secondary approach in the treatment of peripheral arterial thromboembolism: results of the multicenter PRISM trial J Vasc Interv Radiol 2018290192–100. [DOI] [PubMed] [Google Scholar]
- 27.Grip O, Kuoppala M, Acosta S, Wanhainen A, Åkeson J, Björck M. Outcome and complications after intra-arterial thrombolysis for lower limb ischaemia with or without continuous heparin infusion. Br J Surg. 2014;101(09):1105–1112. doi: 10.1002/bjs.9579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Acosta S, Kuoppala M. Update on intra-arterial thrombolysis in patients with lower limb ischemia. J Cardiovasc Surg (Torino) 2015;56(02):317–324. [PubMed] [Google Scholar]
- 29.Robertson I, Kessel D O, Berridge D C. Fibrinolytic agents for peripheral arterial occlusion. Cochrane Database Syst Rev. 2013;(12):CD001099. doi: 10.1002/14651858.CD001099.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Razavi M K, Wong H, Kee S T, Sze D Y, Semba C P, Dake M D. Initial clinical results of tenecteplase (TNK) in catheter-directed thrombolytic therapy. J Endovasc Ther. 2002;9(05):593–598. doi: 10.1177/152660280200900508. [DOI] [PubMed] [Google Scholar]
- 31.Qureshi A I, Pande R U, Kim S H, Hanel R A, Kirmani J F, Yahia A M. Third generation thrombolytics for the treatment of ischemic stroke. Curr Opin Investig Drugs. 2002;3(12):1729–1732. [PubMed] [Google Scholar]
- 32.Verstraete M. Third-generation thrombolytic drugs. Am J Med. 2000;109(01):52–58. doi: 10.1016/s0002-9343(00)00380-6. [DOI] [PubMed] [Google Scholar]
- 33.Theodoridis P G, Davos C H, Dodos I et al. Thrombolysis in acute lower limb ischemia: review of the current literature. Ann Vasc Surg. 2018;52:255–262. doi: 10.1016/j.avsg.2018.02.030. [DOI] [PubMed] [Google Scholar]
- 34.Laroia S, Alexander S, Morales S A, Miller N G, Laroia A. Making the best out of the lab values; correlation of fibrinogen level with rate of thrombus resolution during tissue plasminogen (TPA) therapy. J Vasc Interv Radiol. 2015;26:S159–S160. [Google Scholar]
- 35.Poorthuis M HF, Brand E C, Hazenberg C EVB et al. Plasma fibrinogen level as a potential predictor of hemorrhagic complications after catheter-directed thrombolysis for peripheral arterial occlusions. J Vasc Surg. 2017;65(05):1519–1.527E29. doi: 10.1016/j.jvs.2016.11.025. [DOI] [PubMed] [Google Scholar]
- 36.Lee K, Istl A, Dubois L et al. Fibrinogen level and bleeding risk during catheter-directed thrombolysis using tissue plasminogen activator. Vasc Endovascular Surg. 2015;49(07):175–179. doi: 10.1177/1538574415611234. [DOI] [PubMed] [Google Scholar]
- 37.Skeik N, Gits C C, Ehrenwald E, Cragg A H. Fibrinogen level as a surrogate for the outcome of thrombolytic therapy using tissue plasminogen activator for acute lower extremity intravascular thrombosis. Vasc Endovascular Surg. 2013;47(07):519–523. doi: 10.1177/1538574413497107. [DOI] [PubMed] [Google Scholar]
- 38.Ouriel K, Kandarpa K, Schuerr D M, Hultquist M, Hodkinson G, Wallin B. Prourokinase versus urokinase for recanalization of peripheral occlusions, safety and efficacy: the PURPOSE trial. J Vasc Interv Radiol. 1999;10(08):1083–1091. doi: 10.1016/s1051-0443(99)70196-x. [DOI] [PubMed] [Google Scholar]
- 39.Ouriel K, Veith F J, Sasahara A A; Thrombolysis or Peripheral Arterial Surgery (TOPAS) Investigators.A comparison of recombinant urokinase with vascular surgery as initial treatment for acute arterial occlusion of the legs N Engl J Med 1998338161105–1111. [DOI] [PubMed] [Google Scholar]
- 40.Results of a prospective randomized trial evaluating surgery versus thrombolysis for ischemia of the lower extremity. The STILE trial Ann Surg 199422003251–266., discussion 266–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Darwood R, Berridge D C, Kessel D O, Robertson I, Forster R. Surgery versus thrombolysis for initial management of acute limb ischaemia. Cochrane Database Syst Rev. 2018;8:CD002784. doi: 10.1002/14651858.CD002784.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Byrne R M, Taha A G, Avgerinos E, Marone L K, Makaroun M S, Chaer R A. Contemporary outcomes of endovascular interventions for acute limb ischemia. J Vasc Surg. 2014;59(04):988–995. doi: 10.1016/j.jvs.2013.10.054. [DOI] [PubMed] [Google Scholar]
- 43.Urbak L, de la Motte L, Rørdam P, Siddiqi A, Sillesen H. Catheter-directed thrombolysis in the treatment of acute ischemia in lower extremities is safe and effective, especially with concomitant endovascular treatment. Ann Vasc Dis. 2017;10(02):125–131. doi: 10.3400/avd.oa.16-00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tisi P V, Callam M J. Treatment for femoral pseudoaneurysms. Cochrane Database Syst Rev. 2013;(11):CD004981. doi: 10.1002/14651858.CD004981.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gillani S, Cao J, Suzuki T, Hak D J. The effect of ischemia reperfusion injury on skeletal muscle. Injury. 2012;43(06):670–675. doi: 10.1016/j.injury.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 46.Watson J D, Gifford S M, Clouse W D.Biochemical markers of acute limb ischemia, rhabdomyolysis, and impact on limb salvage Semin Vasc Surg 201427(3-4):176–181. [DOI] [PubMed] [Google Scholar]
- 47.Petejova N, Martinek A. Acute kidney injury due to rhabdomyolysis and renal replacement therapy: a critical review. Crit Care. 2014;18(03):224. doi: 10.1186/cc13897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aycock R D, Westafer L M, Boxen J L, Majlesi N, Schoenfeld E M, Bannuru R R. Acute kidney injury after computed tomography: a meta-analysis. Ann Emerg Med. 2018;71(01):44–530000. doi: 10.1016/j.annemergmed.2017.06.041. [DOI] [PubMed] [Google Scholar]
- 49.Lambert M A, Belch J JF. Medical management of critical limb ischaemia: where do we stand today? J Intern Med. 2013;274(04):295–307. doi: 10.1111/joim.12102. [DOI] [PubMed] [Google Scholar]
- 50.Anand S S, Bosch J, Eikelboom J Wet al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial Lancet 201820;391(10117):219–229. [DOI] [PubMed] [Google Scholar]


