Abstract
Peripheral arterial disease (PAD) is a currently underdiagnosed and underrecognized vascular disease afflicting up to 200 million people worldwide, with at least 1 million of those suffering from critical limb ischemia (CLI). The 5-year mortality after major amputation for CLI (70%) is twice the average 5-year cancer mortality in the United States, and as many as 50% of CLI patients proceed directly to amputation without preceding vascular assessment or revascularization. Each year, twice as many breast augmentations are performed as leg revascularizations. Strong evidence in the literature supports markedly improved outcomes when multidisciplinary care teams across specialties are engaged to evaluate, treat, and manage patients with lower extremity wounds. This article assists the vascular specialist in differentiating the three most common lower extremity wound types.
Keywords: peripheral arterial disease, critical limb ischemia, lower extremity wounds, ulcers, interventional radiology
Objectives : Upon completion of this article, the reader will be able to distinguish among the three most common lower extremity wound types to guide successful management and prevent the significant morbidity and mortality associated with amputation.
Accreditation : This activity has been planned and implemented in accordance with the accreditation requirements and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit : Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit ™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In the United States, chronic lower extremity wounds are estimated to affect up to 13% of the population and represent an estimated annual cost to the healthcare system of at least $20 billion. 1 2 3 4 An estimated 65,000 to 75,000 major and 134,000 minor amputations are performed annually for critical limb ischemia (CLI), costing the U.S. healthcare system over $25 billion annually. 5 The estimated in-hospital cost of an amputation is $55,311 and the lifetime cost of amputation is estimated at $509,275. 6 7 Within five years of a major amputation, 70% of CLI patients will die. 8
The increasing prevalence of lower extremity wounds is thought to be due to an aging population coupled with the increasing incidence of (the big four) vascular disease associated risk factors: smoking, diabetes, hypertension, and hyperlipidemia. In addition, it is estimated that 35% of the U.S. population is classified as obese (5% morbidly), with numbers continuing to rise. 9 Obesity is known to contribute to poor wound healing, is implicated in venous ulcer disease, and can limit a patient's ability to engage in physical activity, which has, to date, been considered the gold standard for conservative management of claudication (“the walking cure”).
Unfortunately, half as many leg revascularization procedures are performed each year as breast augmentations, and about the same number as abdominoplasty. 10 As many as 25 to 50% of patients are going straight to amputation, and an alarming proportion of those without prior vascular testing.
Mortality after major amputation is very high: 25 to 40% at 1 year, 35 to 55% at 2 years, and a stunning 60 to 75% mortality at 5 years. 11 Five-year survival rates after amputation are worse than for every type of cancer except pancreatic (9%), hepatobiliary (17%), esophageal (18%), and lung (18%) 12 ( Fig. 1 ). According to the Centers for Disease Control and Prevention (CDC), the average 5-year cancer survival in the United States is 66%, compared with an average 5-year survival of 30% after amputation. 12
Fig. 1.
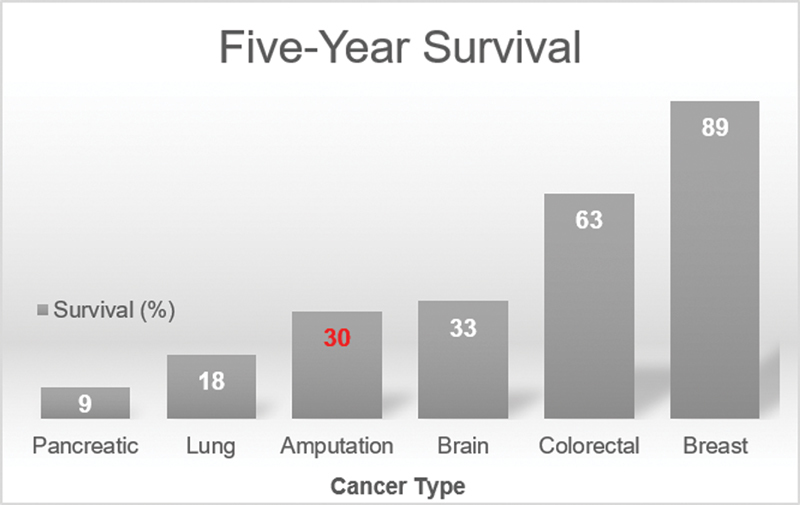
Five-year survival rates for patients with common malignancies, compared with amputation.
Practitioners in all specialties are increasingly more likely to encounter lower extremity wounds, and due to the devastating impact on survival rates and the high cost to the healthcare system, appropriate physical examination, noninvasive imaging evaluation and interpretation, diagnosis, and intervention (or referral) are of paramount importance in reducing the incidence of lower extremity wound–associated morbidity and mortality.
History and Physical Examination of the Lower Extremity Wound
Efficient clinical decision making accomplished by obtaining a thorough history and physical examination cannot be overemphasized. A focused history and physical examination can assist the clinician in distinguishing among the various ulcer types and consequently guide management.
History
Elements of the history that should not be overlooked include medical comorbidities such as diabetes (including HbA1c), hypertension, hyperlipidemia, as well as obesity, rheumatologic/autoimmune disorders (many of which require steroid or immunosuppressive pharmacotherapy), malignancy (which can both cause wounds and impair wound healing), and, of paramount importance to wound healing, nutritional status (without which, no amount of revascularization will suffice to heal a wound).
Critical elements of the social history include smoking, illicit drug use (many illicit drugs can either cause wounds or instigate scratching that can produce wounds [excoriation]), and occupation (someone who spends all day standing at work may have more difficulty healing venous wounds).
With regard to the history of present illness, critical components include the following: how long the ulcer has been present, has it been increasing/decreasing in size, is there associated pain (either in the wound or peri-wound tissues, which can be a sign of infection), any discharge (and if so, what color), has there been any odor, has the patient noticed any skin changes or hair loss, is there associated edema, any thermal disturbances in the extremities, how the leg compares to the other leg with regard to pain/temperature/numbness, how far the patient is able to walk and is there any associated buttock/thigh/calf cramping, are there calf cramps at night, is there pain in the calf or foot at rest, and is there a sensation of heaviness in the lower extremities. Patients with wounds secondary to the ischemia of peripheral arterial disease (PAD) will experience pain relief with dependent position of the limb. These patients may describe, similar to congestive heart failure patients, sleeping in a recliner or, alternatively, waking up at night and dangling their legs over the side of the bed to alleviate a cramp (rest pain). It is also often illuminating to question the patient with regard to any prior ulcers and what was done for them.
Past surgical history should also be noted, as arterial occlusions and lymphedema can be the result of prior surgery, great saphenous vein harvest can uncover deep venous reflux and lower extremity edema, and occluded bypass grafts are important to note when planning intervention or referral, as well as crafting an appropriate physical examination and pharmacotherapeutic regimen. Moreover, a thorough surgical history may further elucidate the natural history of the patient's current presenting condition. For example, a patient with bilateral lower extremity claudication may present for lower extremity revascularization after having already previously undergone lumber spine surgery and bilateral total knee arthroplasties before it was realized that his leg pain was the result of femoropopliteal occlusive disease. A simple pedal pulse exam would have sufficed to raise the possibility of a vascular etiology.
Physical Examination
Physical examination in a patient with a lower extremity wound should begin with a general overall assessment of the patient. Does the patient appear to be in pain? Anxious? Did he or she arrive with a prosthesis or in a wheelchair? Are there any existing amputations? Does the patient smell strongly of cigarettes?
During the examination, the affected extremity should be compared with the contralateral limb. Assessment for varicosities, color, pigmentation, turgor, skin texture, dryness, hair distribution, calluses, surgical scars, and bony deformities should be performed. 13 The temperature and color of both legs and feet should be compared. Any rubor should be noted. Asymmetric edema or atrophy should also be noted, as should sensory loss or motor impairment. If an assessment of either lower extremity reveals distended veins anywhere other than in the foot and ankle (where the finding is normal), venous pathology (venous insufficiency) is implicated. 13
With regard to wound characterization, the anatomic location of the wound should be described and, if an arterial ulcer is suspected, the angiosomal distribution should be noted. There is evidence in the literature that angiosomal directed revascularization (both surgical and endovascular) results in improved wound healing. 14 15 16
Wound size should be measured in centimeters in two orthogonal planes, preferably one of which should be aligned with the patient's head (for consistency), and the depth should also be noted (if any). The presence of any tunneling or undermining should be noted, and the amount of granulation tissue, necrosis, exudate, and presence or absence of rolled edges (epibole). Capillary refill adjacent to the wound should be assessed, although it may remain normal even in PAD due to the presence of collateralization. However, significantly prolonged capillary refill in the tissues adjacent to an ulcer is virtually diagnostic of severe PAD. 13
Elevational pallor should be assessed by raising the affected leg to 60 degrees and observing the color of the soles of the feet. Normally, no color change should be observed. Pallor in a light skinned patient or ashen hue in a dark skinned patient indicates arterial perfusion deficit. Pallor within 25 seconds indicates severe arterial occlusive disease, 25 to 40 seconds indicates moderate arterial occlusive disease, and 40 to 60 seconds is consistent with mild arterial occlusive disease. 13
Dependent rubor is an important sign of PAD. In the dependent position (usually hanging off the edge of the table, but in severe disease, even in the supine position), the foot (and/or lower leg) is erythematous. When raised above the level of the heart, it will normalize in color. This effect, suggestive of severe PAD, is due to the chronic dilatation (and loss of vasoconstriction) of dermal arterioles and capillaries despite increased hydrostatic pressure secondary to chronic ischemia. 17 Notably, this same effect is responsible for reperfusion edema after arterial revascularization.
Differentiating Lower Extremity Wounds
Definitions
An ulcer is a wound with full-thickness depth (epidermis, dermis, and subcutaneous tissues) and slow healing tendency. 2 A chronic leg ulcer is a wound of the lower extremity that has demonstrated no tendency to heal after 3 months of appropriate treatment or is not fully healed at 12 months. 2
Venous Ulcers
Venous ulcers are the most common of all leg ulcers (80–90%). 13 Each year, 2.5 million Americans will develop venous ulcers, affecting patients as young as 20, and costing the U.S. healthcare system over $3 billion annually. 13 Due to the relative paucity of invasive endovascular or surgical options for venous insufficiency (especially deep venous insufficiency) compared with arterial ulcers, the recurrence rate within 5 years is high (40%), resulting in a frustrating wound type to treat for both patient and physician.
Characteristic changes on physical examination in the lower extremities in a patient with venous disease most commonly consist of edema and hyperpigmentation (hemosiderosis), with or without varicosities and telangiectasias. 13 18 The most classic finding associated with venous insufficiency is hyperpigmentation (hemosiderosis) of the lower leg, sometimes extending into the foot. These changes are thought to be the result of venous hypertension and capillary hyperpermeability, with extravasated red blood cells (RBCs) depositing hemosiderin in patients with venous hypertension due to deep or superficial venous reflux 18 ( Fig. 2 ).
Fig. 2.
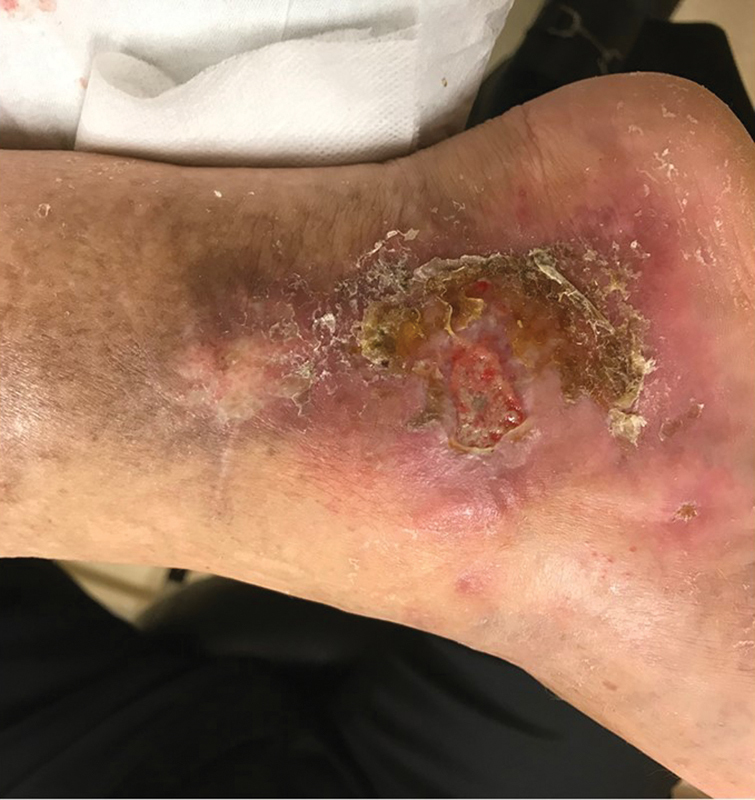
Venous ulcer. Note hemosiderosis, classic location at medial malleolus, crusting, edema, and shallow irregular borders.
Hemosiderosis is thought to play an important role both in ulceration and the development of lipodermatosclerosis, a hardening and fibrosis of the skin indicating long-standing venous insufficiency, thought to be additionally causally related to edema and associated inflammatory change. 13 18 Often, a notable “narrowing” of the affected part of the extremity (typically the “sock” area of the leg) is appreciable due to the fibrotic change, characterized as an inverted champagne bottle appearance, and produces a “woody” hard texture to the skin on palpation. 13
The edema of venous disease can be distinguished from that of lymphedema by its involvement of the lower leg (between the ankle and knee) only, in contrast to the edema of lymphedema, which involves the entire lower extremity. 13
Venous dermatitis is common, consisting of scaling, crusting, weeping, and itching. 13 A lesser known phenomenon in lower extremity venous disease is atrophie blanche, which most commonly occurs near the ankle or foot and consists of thin, smooth, atrophic white plaque with hyperpigmented border, sometimes with peripheral tortuous telangectasias. 13 This is considered a high-risk lesion for impending ulceration, and should not be mistaken for a previously healed ulcer.
A classic venous ulcer is most commonly located in the “sock” (or “gaiter”) region, or medial malleolus (due to greatest hydrostatic pressure), and is typically shallow with irregular borders, moderate to high amount of exudate, and commonly exhibits peri-wound maceration (due to the generally large volume of exudate), as well as a ruddy red wound bed with abundant adherent or loose fibrinous slough. 13
Arterial Ulcers
Arterial ulcers are the second most common wound type (10%). Arterial ulcers are most commonly located at the points farthest from the heart (toes, forefoot) and classically have well-defined margins, a pale or necrotic wound bed, and minimal to no exudate. 13 A characteristic finding in patients with PAD are trophic changes of the bilateral lower extremities, which occur when the growth/development of skin, hair, and nails is compromised secondary to arterial hypoperfusion. 13 These changes characteristically consist of thin shiny epidermis, dry scaly skin, hair loss, and nail thickening. In contradistinction to the patient with venous disease, who tends to have lower legs of generous magnitude and significant discoloration, the classic patient with PAD tends to have thin pale ruborous legs with scaly skin, cold feet, and no (or abnormal) palpable pedal pulses ( Fig. 3 ). Arterial ulcers are less common than venous ulcers, although PAD is estimated to affect one-third of patients older than 65 years, and the prevalence in younger smokers and diabetics is rising. 13 Pure arterial ulcers are the least common of the ulcer types, with mixed arterial/venous and mixed arterial/neuropathic being more common. 13
Fig. 3.
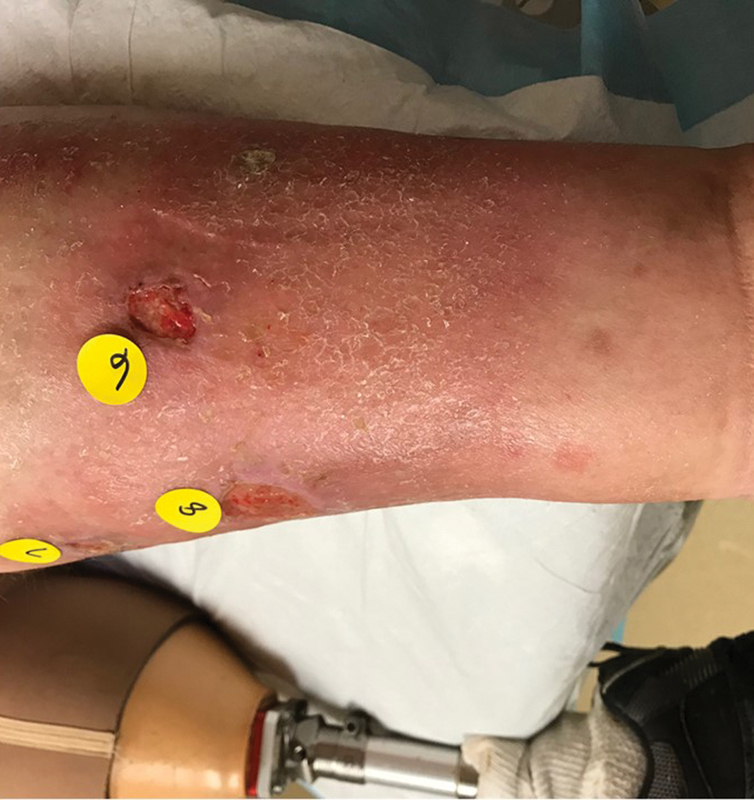
Arterial ulcers. Note the dry scaly skin, hair loss, and rubor, as well as the contralateral below-knee amputation.
When examining a patient with a suspected arterial ulcer, the importance of bilateral pulse examination cannot be overemphasized. Both radial pulses should be checked, as asymmetric radial pulses can confirm a diagnosis of arterial disease. The common femoral artery (CFA), popliteal artery, dorsalis pedis (DP), and posterior tibial (PT) artery pulses should be assessed in the involved extremity, as a nonpalpable pulse at each level can indicate the level of the pathology. For example, in a patient with a nonhealing right great toe ulcer and no palpable right CFA pulse, the presence of inflow disease (suprainguinal, typically iliac occlusion or high-grade stenosis) can guide the plan for revascularization of the inflow prior to addressing any infrainguinal pathology. In a patient with a normal right CFA pulse and no palpable right popliteal pulse, SFA occlusion or high-grade stenosis is implicated. In a patient with normal CFA and popliteal artery pulses and no palpable pedal pulses, below the knee (and sometimes below the ankle) revascularization is indicated. A thorough pulse examination is an important element in diagnosing the level of arterial pathology in a patient with PAD. However, it should be noted that although absent pedal pulses in the vast majority of cases indicate absence of inline flow to the foot, palpable pedal pulses do not exclude PAD and, conversely, approximately 2% of the population has absent DP and PT pulses. 13
Although the manual pulse exam is the least expensive noninvasive exam technique to diagnose the distribution of a patient's PAD, the magnitude of additional clinical information regarding arterial vascular patency, stenosis, or occlusion provided by the vascular Doppler (inexpensive, compared with other noninvasive imaging modalities) should not be underestimated. A nonpalpable DP pulse that is “dopplerable” suggests a modicum of flow, though the expected phasicity of the pulse signal through this artery should correspond to a biphasic or monophasic waveform. For practice, this abnormal pulse signal can be compared with the signal from an artery with a normal pulse on physical exam, which should correspond to a normal audible triphasic waveform pulse signal by vascular Doppler. In a patient with a nonpalpable DP pulse and no audible signal by vascular Doppler, the DP artery can be diagnosed as occluded with a great degree of certainty. Importantly, this technique may also be employed to surveil ongoing patency of arteries in the office post-revascularization, as changes in the vascular Doppler signal phasicity may indicate developing stenosis.
Although the majority of arterial ulcers are due to atherosclerotic disease, less common etiologies that should be considered in unusual cases or presentations include thromboangiitis obliterans (Buerger's disease), sickle cell, and the vasculitides. 13 An abnormal ankle-brachial index is an independent predictor of increased risk of cardiovascular death, and patients with newly diagnosed PAD should be referred to a cardiologist for cardiovascular assessment. 13
Neuropathic Ulcers
Diabetes is the leading cause of nontraumatic lower extremity amputations in the United States. Also known as diabetic ulcers, or diabetic foot ulcers (DFU), it is estimated that 25% of diabetic patients will develop an ulcer in their lifetime, and the risk of contralateral limb amputation within 2 to 3 years of a major amputation is 50 to 84%. 13 19 The 5-year survival of a diabetic after a major amputation is 28%. 13 DFUs account for 20% of hospital admissions in diabetic patients. 20 Lower extremity amputation rates in diabetic patients are 15 times that of nondiabetics, and it is estimated that 50 to 70% of all lower extremity amputations are due to DFU. 20 Every 30 seconds, one leg is amputated due to DFU. 20
Musculoskeletal deformities of the feet are common in diabetics, and result in abnormal foci of increased loading as well as thinning of the fat pads under the metatarsal heads. A diabetic with Charcot deformity is four times more likely to develop a foot ulcer. 13 The typical Charcot foot exhibits a “rocker bottom” deformity and can often be warm to the touch, erythematous, or edematous ( Fig. 4 ). 13 In addition, there is destruction and deformity of the foot and ankle joints. Hammertoes can develop due to the motor neuropathy of diabetes resulting in atrophy of distal motor nerves and atrophy of the small intrinsic muscles of the foot. 13 These changes result in altered foot and ankle biomechanics which, coupled with the inability to recognize ongoing damage due to neuropathy, result in abnormal pressure points and eventual ulceration.
Fig. 4.
Charcot arthropathy. Note the “rocker bottom” foot, hammertoes, and deformity of the ankle joint, all of which produce altered biomechanics leading to ulceration.
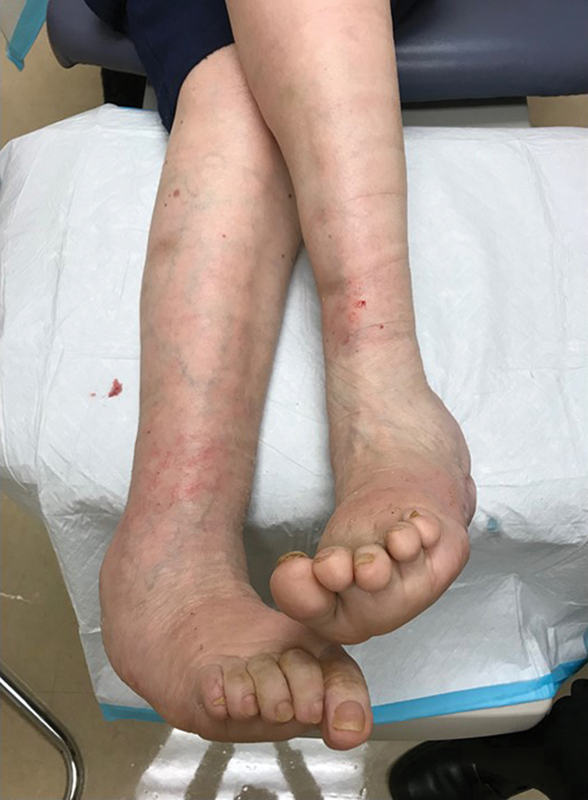
The typical neuropathic ulcer is located on the plantar surface of the foot, although the lateral or dorsal aspects of the foot can also be involved if unrecognized pressure trauma is sustained in these regions ( Fig. 5 ). The general appearance is often of a laceration, puncture, or blister (rounded or oblong). 13 The edges are typically well defined and smooth, and the wound base is usually red but may be pale, pink, or necrotic. 13 The exudate is usually small to moderate, and generally serous or clear. 13 Peri-wound callous buildup is very common in neuropathic (DFU) ulcers. This is due to the biomechanical nature of the formation of these lesions secondary to unrecognized trauma in the neuropathic foot. The callus itself can increase foot pressures by up to 30%, and has been associated with a 77-fold increase in subsequent ulceration of the tissues underlying the callus. 13 In fact, it has been observed that neuropathic ulcers may occur exclusively at callus sites. 13 Hemorrhage into a callus is a strong indicator of underlying ulceration, and sharp debridement of the callus is recommended to assess for underlying ulcer ( Fig. 5 ).
Fig. 5.
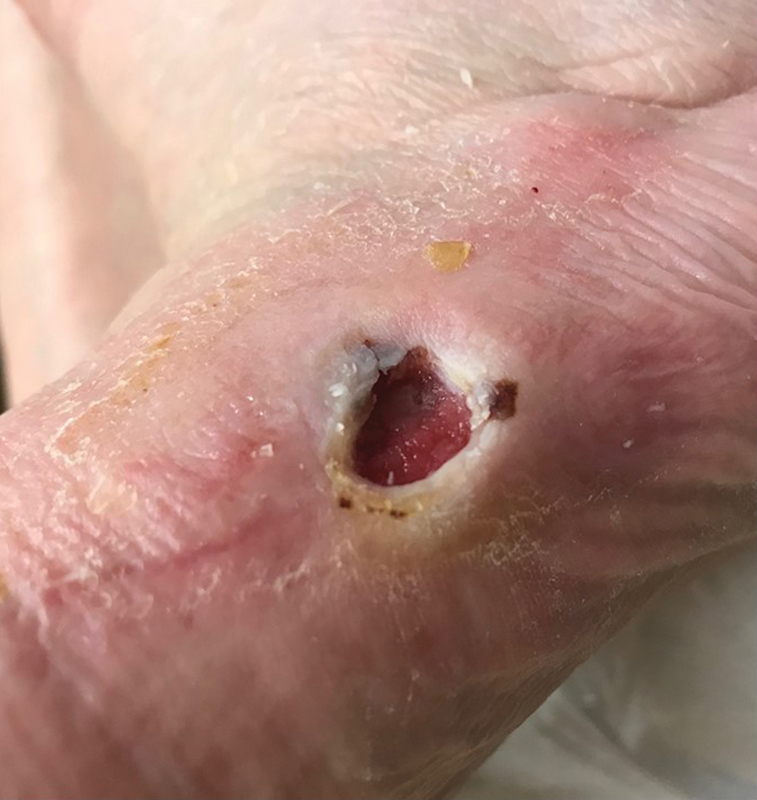
Neuropathic ulcer. Note the peri-wound callus, well-defined edges (“punched-out” appearance), red wound bed, no fibrinous exudate, and the location on the lateral aspect of the left foot of the same patient as in Fig. 4 , an abnormal pressure point due to Charcot deformity.
A summary of wound characteristics by wound type is provided in Table 1 .
Table 1. Summary of wound characteristics.
| Arterial | Venous | Neurotrophic | |
|---|---|---|---|
| Skin changes | Shiny, thin, flaky, hair loss, rubor (pinkish red) | Hyperpigmented (hemosiderosis—purple, dark reddish brown), telangiectasias, thickening (lipodermatosclerosis), peri-wound maceration, scaling/crusting | Callus |
| Location | Foot more often than leg | Lower leg, almost never foot | Almost always plantar foot, sometimes side or dorsum of foot |
| Laterality of leg | Usually lateral | Usually medial | N/A |
| Distribution | Angiosomal | Gaiter | Pressure points |
| Wound edges | Well defined | Irregular, poorly defined | Frequent callous, well defined (“punched-out” appearance) |
| Wound bed | Pale or necrotic | Dark red, fibrinous slough | Typically red |
| Eschar | Common | Never | Sometimes |
| Exudate | Rare | Always | Almost always |
| Odor | If infected (gangrene) | Usually none | Strong if infected |
| Pain (in ulcer) | Uncommon unless infected or acute ischemia | Uncommon unless infected | Never |
| Preceding trauma | Common | Uncommon | Common |
| Edema | No | Yes | Usually no unless mixed, Charcot, or infection |
| Sensation | Normal | Normal | Absent or severely diminished, paresthesia |
| Temperature | Cold | Normal | Usually normal unless mixed arterial |
| Pulses | Abnormal | Normal | Abnormal only if arterial component |
| Delayed capillary refill | Sometimes | Only if arterial component | Only if arterial component |
| Elevational pallor | Present | Absent | Absent (unless arterial component) |
| Dependent rubor | Present | Absent | Absent (unless arterial component) |
| Pain with leg raise | Increased | Decreased | No change |
Noninvasive Imaging Evaluation and Interpretation
According to some estimates, as many as 50% of lower extremity amputations in the United States are a first-line intervention and are performed without prior vascular testing. 21 Unfortunately, in these cases, there is no assurance of healing if vascular perfusion is compromised. Regardless of the wound etiology or amputation status, all wounds require adequate vascular perfusion to heal. The many additional factors that strongly impact wound healing are discussed in other sections. There are many factors to consider when selecting appropriate noninvasive imaging to maximize diagnostic yield, minimize risk to the patient, and mitigate against degradation due to artifact inherent in the imaging modality selected, and these are covered in other sections.
Intervention and Referral
The management of arterial, venous, and neuropathic ulcers is beyond the scope of this article. However, there is strong evidence in the literature supporting markedly improved outcomes with regard to morbidity and mortality as well as reduced healthcare costs when multidisciplinary care teams across specialties are engaged to evaluate, treat, and manage patients with lower extremity wounds.
Acknowledgment
The author wishes to thank David M. Deitz, MD of Vasular Surgery, whose mentorship in wound care made this work possible.
References
- 1.Mani R, Romanelli M, Shukla V. London: Springer London; 2013. Measurements in Wound Healing: Science and Practice. [Google Scholar]
- 2.Agale S V. Chronic leg ulcers: epidemiology, aetiopathogenesis, and management. Ulcers. 2013;2013:1–9. [Google Scholar]
- 3.Rayner R, Carville K, Keaton J, Prentice J, Santamaria N. Leg ulcers: atypical presentations and associated co-morbidities. Wound Pract Res. 2009;17(04):168–185. [Google Scholar]
- 4.Järbrink K, Ni G, Sönnergren H et al. The humanistic and economic burden of chronic wounds: a protocol for a systematic review. Syst Rev. 2017;6(01):15. doi: 10.1186/s13643-016-0400-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yost M L.The Cost of Amputation in 2014. Lecture at 4th Annual Amputation Prevention Symposium. August 14-16, 2014
- 6.Peacock J M, Keo H H, Duval S et al. The incidence and health economic burden of ischemic amputation in Minnesota, 2005-2008. Prev Chronic Dis. 2011;8(06):A141. [PMC free article] [PubMed] [Google Scholar]
- 7.MacKenzie E J, Jones A S, Bosse M J et al. Health-care costs associated with amputation or reconstruction of a limb-threatening injury. J Bone Joint Surg Am. 2007;89(08):1685–1692. doi: 10.2106/JBJS.F.01350. [DOI] [PubMed] [Google Scholar]
- 8.Bell D. Current concepts in vascular assessment of wounds. Pod Today. 2018;31(08):26–32. [Google Scholar]
- 9.Pierpont Y N, Dinh T P, Salas R E et al. Obesity and surgical wound healing: a current review. ISRN Obes. 2014;2014:638936. doi: 10.1155/2014/638936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suckow B.Amputation rate and cost of amputation in the United StatesCurrent Data. Lecture at the Amputation Prevention Symposium 2018. August 8, 2018
- 11.Feinglass J, Pearce W H, Martin G J et al. Postoperative and late survival outcomes after major amputation: findings from the Department of Veterans Affairs National Surgical Quality Improvement Program. Surgery. 2001;130(01):21–29. doi: 10.1067/msy.2001.115359. [DOI] [PubMed] [Google Scholar]
- 12.United States Cancer Statistics.Data Visualizations. Survival: 5-year Relative Survival, All Types of Cancer, Invasive Cancers Only, United StatesAvailable at:https://gis.cdc.gov/Cancer/USCS/DataViz.html. Accessed August 22, 2018
- 13.Bryant R, Nix D. St. Louis, MO: Elsevier; 2016. Acute and Chronic Wounds: Current Management Concepts. 5th ed. [Google Scholar]
- 14.Bunte M C, Shishehbor M H. Angiosome-guided intervention in critical limb ischemia. Interv Cardiol Clin. 2017;6(02):271–277. doi: 10.1016/j.iccl.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 15.McCallum J C, Lane J S., III Angiosome-directed revascularization for critical limb ischemia. Semin Vasc Surg. 2014;27(01):32–37. doi: 10.1053/j.semvascsurg.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Špillerová K, Settembre N, Biancari F, Albäck A, Venermo M. Angiosome targeted PTA is more important in endovascular revascularisation than in surgical revascularisation: analysis of 545 patients with ischaemic tissue lesions. Eur J Vasc Endovasc Surg. 2017;53(04):567–575. doi: 10.1016/j.ejvs.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Uzun G, Mutluoglu M. Images in clinical medicine. Dependent rubor. N Engl J Med. 2011;364(26):e56. doi: 10.1056/NEJMicm1010160. [DOI] [PubMed] [Google Scholar]
- 18.Bergan J J, Schmid-Schönbein G W, Smith P D, Nicolaides A N, Boisseau M R, Eklof B. Chronic venous disease. N Engl J Med. 2006;355(05):488–498. doi: 10.1056/NEJMra055289. [DOI] [PubMed] [Google Scholar]
- 19.Leone S, Pascale R, Vitale M, Esposito S. [Epidemiology of diabetic foot] Infez Med. 2012;20 01:8–13. [PubMed] [Google Scholar]
- 20.Yazdanpanah L, Nasiri M, Adarvishi S. Literature review on the management of diabetic foot ulcer. World J Diabetes. 2015;6(01):37–53. doi: 10.4239/wjd.v6.i1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor G I, Palmer J H. The vascular territories (angiosomes) of the body: experimental study and clinical applications. Br J Plast Surg. 1987;40(02):113–141. doi: 10.1016/0007-1226(87)90185-8. [DOI] [PubMed] [Google Scholar]


