Diagnosis
Classification of Pleural Effusions
Accurate classification of pleural effusions using a combination of imaging and laboratory findings is essential, guiding management of both the effusion and the causative agent. Pleural effusions form when the rate of pleural fluid formation exceeds the ability of the lymphatics in the parietal pleura to reabsorb the fluid. Under normal physiologic conditions, fluid enters the pleural space via capillaries in the parietal pleura; however, it can also originate from interstitial spaces in the lung, visceral pleura, or tiny holes in the diaphragm under pathologic conditions. The rate of reabsorption in humans has been estimated at 0.36 mL/kg/h per hemithorax, or in more practical terms, 470 mL per hemithorax per day in a 70-kg patient. 1 On a standard chest X-ray, effusions of as little as 50 mL can be detected on lateral films and 200 mL on standard PA view ( Fig. 1 ).
Fig. 1.
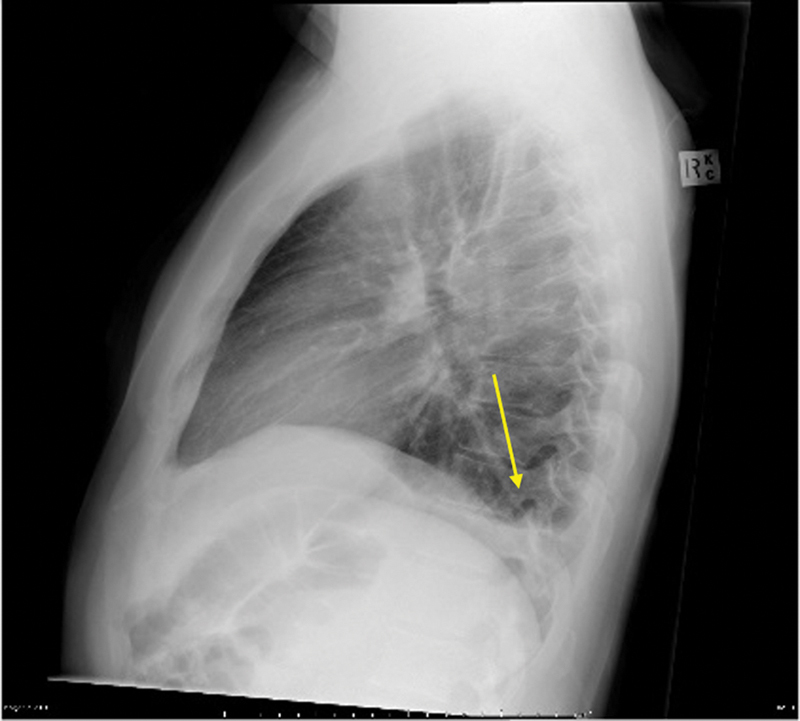
Lateral chest radiograph demonstrates a small pleural effusion collecting in the posterior sulcus ( arrow ).
Traditionally, the first step in the diagnosis of pleural effusions has been to determine whether it is transudative or exudative. A transudative effusion occurs due to alteration of systemic factors which influence the formation and absorption of pleural fluid and an exudative effusion occurs when local factors are altered. Application of lights criteria serves as a good initial step, identifying the transudative effusions which can be managed by treating the systemic factors. Further characterization of the more common exudative effusions is described later.
Parapneumonic Effusions
Parapneumonic effusions occur as a result of bacterial pneumonia, lung abscess, or bronchiectasis. In the initial exudative stage (0–2 weeks), interstitial fluid accumulates around the infection site and crosses the visceral pleura into the pleural space. This fluid is typically sterile and will have a normal pH and glucose with a high protein content. In the subsequent fibropurulent stage (2–4 weeks), bacterial invasion of the pleural space accelerates the immune response causing further migration of neutrophils and activation of the coagulation cascade, often with formation of fibrin barriers which result in loculated pockets. In the final organizing stage (4–6 weeks), fibroblastic response results in creation of a thick pleural peel which resists respiratory motion and negates the efficacy of percutaneous drainage. 2
In 2000, the American College of Chest Physicians (ACCP) developed a categorization system for parapneumonic pleural effusions based on imaging findings and pleural fluid analysis. 3
Category 1 —Any minimal free-flowing effusion with an unknown pleural fluid analysis. A minimal effusion is defined as less than 10 mm on lateral decubitus radiograph. With the increased reliance on CT imaging and infrequent use of lateral decubitus radiographs, fluid separating the lung from the chest wall by less than 10 mm can also serve to define a category 1 effusion. The risk of poor outcome in these scenarios is described as very low and no drainage is indicated. 4 Of note, if a thoracentesis was performed in this case and positive cultures/Gram stain or glucose less than 60 was found on pleural fluid analysis, findings are likely to be false positive. Consideration should then be given for repeat thoracentesis should the effusion enlarge or if the patient's clinical condition deteriorates.
Category 2 —A small to moderate free-flowing effusion with negative culture/Gram stain and pH greater than or equal to 7.2, regardless of prior antibiotic use. Small to moderate is defined as pleural fluid separating the lung from the chest wall by greater than 10 mm but occupying less than one-half of the hemithorax. The risk of poor outcome is low and drainage is not considered necessary. However, should the patient's condition deteriorate, consideration should be given to repeat thoracentesis and drainage.
Category 3 —Any large free-flowing effusion occupying more than one-half of the hemithorax, any effusion with loculations or thickened parietal pleura, and a small or larger (>10 mm of fluid separating the chest wall) effusion with thickened parietal pleura. Risk of poor outcome is considered moderate. Of note, larger pleural effusions are more difficult to drain, probably due to the increased likelihood of loculations. 5 And although perhaps intuitive, the presence of loculations portends a worse prognosis. 6 Category 3 effusions warrant drainage.
Category 4 —Empyema. The term “empyema” is reserved for frank purulent effusions. Empyema is associated with a high risk of poor outcome and drainage is indicated. When assessing imaging of patients with parapneumonic effusions, it is important to note that thickening of the parietal pleura on contrast-enhanced CT is suggesting of empyema. 7
Malignant Pleural Effusions
A malignant pleural effusion results from direct invasion of the pleura by cancer cells of primary or secondary tumors. Tumor-induced angiogenesis subsequently increases vascular permeability and vascular leakage of fluid resulting in pleural effusion. On CT, the most reliable features for distinguishing benign versus malignant pleural disease are the presence of a pleural rind (defined as circumferential involvement of the hemithorax, including the mediastinum), nodular pleural thickening, pleural thickening greater than 1 cm, and mediastinal pleural involvement, with pleural calcifications indicating a benign process. 8 FDG-PET/CT has also shown promise in distinguishing benign from malignant pleural disease. In a study of 79 patients with exudative pleurisy, SUV values were significantly higher in all malignant pleural diseases, with a cutoff value of 2.2 SUVbw (SUV normalized for body weight) resulting in an accuracy of 82.3% for the diagnosis of malignant pleural effusion. 9
Ultimately, pleural fluid analysis is required to confirm the diagnosis of malignant pleural effusion. Chemical analysis (total protein, glucose, albumin, lactate dehydrogenase, pH) can be helpful in identifying if the fluid is an exudate, as almost all malignant pleural effusions are exudates; however, several other entities manifest with exudative effusions including bacterial pneumonia, viral process, and pancreatitis, to name a few. Fluid cytology yields a cytopathologic diagnosis conclusive for cancer in 90.5% of cases with the additional advantage of distinguishing the carcinomatous type. 10 If cytology is negative and there is continued high clinical suspicion for malignant pleural effusion, a pleural biopsy can be obtained. Image-guided percutaneous approaches have an overall sensitivity of 87.5% (95% when pleural thickening is > 1 cm) and thoracoscopic biopsies have a sensitivity of 94.1%. 11
Hemothorax
Hemothorax is the presence of blood in the pleural space and is defined as pleural fluid hematocrit of at least 50% of the peripheral blood hematocrit. Often seen in the setting of trauma, hemothorax can also occur following thoracic interventional procedures such as lung tumor ablation. 12 Iatrogenic hemothorax is typically the result of damage to an intercostal or chest wall artery.
Chylothorax
Chylothorax is the presence of intestinal lymph (chyle) within the pleural space and results from leakage of the lymphatic vessels, most commonly the thoracic duct. 1 The etiology of chylothorax generally divided into traumatic and nontraumatic etiologies with iatrogenic injury accounting for 80% of traumatic causes. Nontraumatic etiologies include malignancy, infection, systemic diseases such as lupus erythematosus, and congenital disorders of the lymphatic system. 13
The thoracic duct transports chyle from the intestinal system and flow rate ranges from 10 to 100 mL/kg of body weight per day, or on average 2.5 L per day. 14 The thoracic duct transports 70 to 80% of ingested fat in addition to fat-soluble vitamins and proteins. Unsurprisingly, the clinical findings of thoracic duct rupture include metabolic, nutritional, and immunologic deficiencies such as malnutrition and muscle wasting.
Aspirate of a chylothorax has traditionally been described as milky and odorless; however, not all effusions with these characteristics are necessarily chylous, and other etiologies such as empyema and pseudochylothorax need to be excluded with laboratory analysis. Pseudochylothorax is also known as cholesterol pleural effusion and can occur in longstanding pleural effusions, thought to result from the destruction of inflammatory cells. 14 Diagnosis of pseudochylothorax can be confirmed by the presence of cholesterol crystals in the aspirate.
The most widely used criteria for assessing the presence of chyle were initially published by Staats et al 15 where the diagnosis of chylothorax can be confirmed with fluid triglyceride levels greater than 110 mg/dL and triglyceride levels less than 50 mg/dL unlikely for chyle. The use of lipoprotein electrophoresis has been described in the diagnosis of chylothorax; however, it remains too costly and labor intensive for routine use and should only be considered in cases where triglyceride levels are equivocal (between 50 and 110 mg/dL) or where clinical suspicion of chylothorax is high. 16
Intervention
Parapneumonic Effusions
A tremendous body of literature exists regarding the management of pleural effusions with varying recommendations, particularly with regard to management of parapneumonic effusions. There is continued debate as to which patients would benefit from early decortication versus chest tube drainage in the setting of parapneumonic effusion. 1 Per ACCP guidelines, fibrinolytics, video-assisted thoracoscopic surgery (VATS), and surgery are acceptable approaches for managing patients with category 3 and category 4 parapneumonic effusions (level C evidence). 3
Surgical options include VATS and open thoracotomy. Surgical interventions offer the advantages of direct visual inspection of the pleural space, tissue sampling, evacuation of necrotic material (debridement), and allowing the trapped lung to expand by peeling the organized cortex from the visceral pleura (decortication). 1 In cases of early-stage empyema, chest tube drainage, antibiotics, and fibrinolytics are indicated. 1 In the later stages of empyema, when loculations and a pleural peel have formed, surgical intervention is indicated. The decision of VATS versus open thoracotomy depends on the degree of organization and fibrosis, patient clinical status, and operator preference. Current surgical literature comparing VATS versus open debridement suggests comparable postoperative outcomes between the two, with lower postoperative complications in patients undergoing VATS. 17
Malignant Pleural Effusions
In patients with potentially treatable metastatic lung carcinoma such as lymphoma, breast carcinoma, or small cell lung carcinoma, chemotherapy is the preferred initial treatment. 18 Local treatment is reserved for cases where systemic chemotherapy fails to control the effusion. The purpose of local therapy is to aid in reexpansion of the lung and provide relief of symptoms by evacuating pleural fluid and preventing its reaccumulation. Initial treatment of a malignant pleural effusion is a therapeutic thoracentesis, which determines effects of drainage on dyspnea and helps determine rate of reaccumulation. In cases where thoracentesis does not improve dyspnea, additional etiologies including thromboembolism, atelectasis, and lymphangitic cancer need to be considered. 19 Although thoracentesis usually improves acute symptoms, the fluid can rapidly reaccumulate and frequent repeat thoracenteses leads to increased risk of infections, loculations, and adhesions.
Chemical pleurodesis involves instillation of a chemical agent into the pleural space via an intrapleural catheter or thoracoscopic approach, resulting in inflammation and fibrin deposition, leading to adhesion between the pleural layers. The procedure is reserved for patients who have recurrent symptomatic pleural effusions which have previously responded to thoracentesis, life expectancy longer than 3 months, and an effusion not responsive to chemotherapy. 18 19 Of note, the only symptom that will be relieved by pleurodesis is dyspnea. When selecting patients for pleurodesis, it is important to prove that the lung is reexpandable, which can be established by performing a therapeutic thoracentesis. Multiple different pleural agents can be used for the procedure including talc, bleomycin, and doxycycline, with little consensus on the safest and most effective agent despite numerous clinical trials. 19 20
In patients where chemical pleurodesis is contraindicated or not desired, an indwelling pleural catheter has shown comparable efficacy in the treatment of recurrent malignant pleural effusions when compared with doxycycline pleurodesis 21 and reduced total hospitalization days when compared with talc pleurodesis. 22 Now routinely used in the United States and increasingly elsewhere, an indwelling pleural catheter is “tunneled” under the skin and into the pleural space with a subcutaneous cuff allowing the catheter to remain in place for extended periods of time. 23
Chylothorax
Regardless of etiology of chylothorax, initial management is typically conservative and involves drainage of the pleural cavity, reduction of chyle flow, nutritional support, and prevention of metabolic complications. 14 Due to the composition of chyle, nutrient loss and dehydration can occur rapidly, and aggressive oral or parenteral nutritional support is recommended. Pleural cavity drainage via tube thoracostomy serves the dual purpose of aiding in lung reexpansion and allowing for the quantification of output. Reduction of chyle flow through conservative means is accomplished by eliminating all dietary fat except for medium chain triglycerides.
When conservative measures fail, surgical and nonsurgical lymphatic interventions can be considered. Thoracic duct embolization has become a viable alternative to surgery, as it is both minimally invasive and allows for identification of the location of the chyle leak. 13 In cases of lymphatic conduction disorders or where thoracic lymphatic masses result in leakage of chyle into the pleural space, direct percutaneous obliteration of the masses or aberrant channels have been effective treatments.
Hemothorax
The goal of chest tube placement in hemothorax is to both quantify the volume of blood lost and assist in evacuating blood products from the pleural space. Chest tube placement may also assist in tamponading the bleeding source by closer apposition of the parietal and visceral pleura. 12 Traditionally, large bore chest tubes were placed in patients with hemothorax; however, a 2009 study demonstrated no significant difference in complication rate and efficacy when placing a small bore thoracostomy tube (8–16 Fr) versus a large bore thoracostomy tube (32–40 Fr). 24
Techniques of Chest Tube Placement
Techniques of chest tube insertion include blunt dissection, the Seldinger guidewire technique, and the trocar technique. 25 In the blunt dissection technique, a large incision is made parallel to the rib and the subcutaneous tissues are dissected away, often followed with digital palpation of the pleural space before a large bore chest tube is inserted. This offers the advantage of palpating the pleural space, with the disadvantages of a large incision, bigger scar, and more painful insertion for the patient.
With the trocar technique, a sharp tipped trocar is loaded into a chest tube which is then inserted through a skin incision into the pleural space. The trocar is then withdrawn with the catheter left in the pleural space. This technique has a high risk of complications due to the large size of the trocar/catheter combination inserted into the chest.
The Seldinger technique can be used for both large- and small-bore chest tubes and involves advancement of a needle into the pleural space, followed by a guidewire, dilators, and then a chest tube ( Table 1 ). The advantages of the Seldinger technique are less pain, a smaller incision, and a smaller scar; however, this technique results in limited ability to direct the chest tube and does not allow for digital examination of the pleural space.
Table 1. Steps for placing a chest tube using the Seldinger technique.
| 1. Inject local anesthesia (1–2% lidocaine) into the soft tissues |
| 2. A small incision is made in the skin to facilitate catheter entry |
| 3. An 18-guage trocar needle is introduced through the incision into the pleural space |
| 4. The inner stylet is removed and position is confirmed by aspiration of pleural fluid |
| 5. A 0.038-in stiff guidewire is advanced through the trocar into the pleural space and the trocar is removed |
| 6. Serial dilation is performed to the required catheter size |
| 7. The drain is advanced with a stiffener over the guidewire. As soon as the catheter enters the pleural space, the stiffener is disconnected and held in place as the catheter is advanced into the pleural space |
| 8. When the catheter is in satisfactory position, the stiffener and guidewire are removed leaving the catheter in place |
| 9. The pigtail is locked and secured externally with a suture |
| 10. Up to 1 L is aspirated to avoid reexpansion of pulmonary edema |
Chest Tube Management
Following thoracostomy tube placement, the catheter has traditionally been placed on a water seal suction system followed by a trial period of 2 to 10 days, depending on operator preference. 2 Of note, in patients with chylothorax gravity, drainage is recommended as suction may worsen the leak. If thoracostomy tube drainage is unsuccessful, then the patient is taken for surgical drainage. Factors leading to failure of surgical thoracostomy tubes includes adhesions forming locules of fluid away from the drain, unsatisfactory positioning of the drain, fibrinous debris or septations occluding the tube, or formation of a bronchopleural fistula.
Image-guided placement of chest tubes allows for placement of the drainage catheter into specific locations within the pleural effusion or within specific locules, facilitating drainage of complex pleural effusions where nonguided placement may preclude adequate placement of the catheter. In serous collections, a 10- to 12-Fr catheter provides adequate drainage, with larger 24- to 28-Fr chest tubes required for thick collections with particulate debris. 26 Even when proper positioning is obtained and aggressive catheter management is performed, chest tube drainage may ultimately fail due to repeated occlusion by particulate debris and formation of loculations outside the area of the drain. Flushing the tube with 30 mL of sterile saline every 6 to 8 hours is recommended to prevent tube blockage. 27
Fibrinolytics and DNase
While commonly referred to as “fibrinolytics,” the majority of the agents used are technically thrombolytic drugs and it is worth clarifying the mechanism of action. Thrombolytic drugs work by activating plasminogen to form plasmin, an enzyme which breaks apart fibrin molecules and dissolves clots. The key difference between tissue plasminogen activator (tPA) and streptokinase is that tPA is more selective for fibrin-bound plasminogen, whereas streptokinase binds to any circulating or fibrin-bound plasminogen to form plasmin. The goal of all intracavitary fibrinolytic therapy is to liquidate congealed pleural fluid and dissolve fibrous septations, allowing for reexpansion of the underlying lung and evacuation of fluid through small bore thoracostomy tubes.
Numerous small, open, and randomized controlled studies have been performed over the last several decades which have suggested benefits in surrogate outcomes such as increased chest tube output and improved radiologic change when using intracavitary fibrinolytic therapy. 28 29 30 31 32 33 However, a large multicenter double-blind trial (MIST1) released in 2005 comparing intrapleural streptokinase versus placebo in complicated parapneumonic effusions failed to demonstrate improvement in mortality, rate of surgery, or length of hospital stay in the treatment arm. 34 A subsequent meta-analysis in 2008 concluded that there was potential benefit with fibrinolytics in loculated/septated pleural effusions, but data were incomplete and no significant benefits were shown in the subgroup of high-quality trials. 35
Deoxyribonuclease (DNase) catalyzes cleavage of phosphodiester linkages in the DNA backbone resulting in the degradation of DNA. Crude bacterial extracts of DNase and streptokinase from streptococci were used in facilitating catheter drainage of loculated pleural effusions as early as 1949 36 ; however, the impure extracts resulted in allergic reactions. In subsequent decades, commercial extracts became available followed by recombinant human DNase. Due to the high DNA content of empyema, a study in 2000 demonstrated significant reduction in pus viscosity when using DNase compared with streptokinase. 37 The MIST2 trial, published in 2011, is the largest randomized controlled trial to date comparing the use of tPA and DNase. The MIST2 trial demonstrated improved fluid drainage and reduced frequency of surgical referrals and duration of hospital stay when using a combination of DNase and tPA with no benefits when using either agent alone.
Commonly used fibrinolytic dosages are provided in Table 2 . 38 A recent article suggests the need for tailoring dosing and frequency of fibrinolytic therapy as the content and volume of empyema varies significantly for each individual case. This may in part explain the variable results in the decades of previous trials attempting to establish the efficacy of fibrinolytics. Suggested dosing of combination tPA and DNase is provided in Table 3 . 39
Table 2. Commonly used dosages of intrapleural fibrinolytics.
| Thrombolytic | Dose | Instillation | Duration |
|---|---|---|---|
| Streptokinase | 250,000 IU | 100–200 mL saline | Daily up to 7 d |
| Urokinase | 100,000 IU | 100 mL saline | Daily up to 3 d |
| tPA | 10–25 mg | 100 mL saline | Twice daily up to 3 d |
Abbreviation: tPA, tissue plasminogen activator.
Source: Parapneumonic pleural effusion and empyema. Respiration 2008. 38
Table 3. Suggested dosages of combination tPA and DNase.
| Agent | Dose | Frequency | Duration |
|---|---|---|---|
| tPA | 10 mg | Twice daily | 3 d |
| DNase | 5 mg | Twice daily | 3 d |
Abbreviation: tPA, tissue plasminogen activator.
Source: Intrapleural use of tissue plasminogen activator and DNase in pleural infection. The New England Journal of Medicine 2011. 40
References
- 1.Perikleous P, Rathinam S, Waller D A. VATS and open chest surgery in diagnosis and treatment of benign pleural diseases. J Vis Surg. 2017;3:84. doi: 10.21037/jovs.2017.05.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moulton J S. Image-guided management of complicated pleural fluid collections. Radiol Clin North Am. 2000;38(02):345–374. doi: 10.1016/s0033-8389(05)70167-6. [DOI] [PubMed] [Google Scholar]
- 3.Colice G L, Curtis A, Deslauriers J et al. Medical and surgical treatment of parapneumonic effusions: an evidence-based guideline. Chest. 2000;118(04):1158–1171. doi: 10.1378/chest.118.4.1158. [DOI] [PubMed] [Google Scholar]
- 4.Light R W, Girard W M, Jenkinson S G, George R B. Parapneumonic effusions. Am J Med. 1980;69(04):507–512. doi: 10.1016/0002-9343(80)90460-x. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson A D, Prescott R J, Selkon J B, Watson D, Swinburn C R. The clinical course and management of thoracic empyema. QJM. 1996;89(04):285–289. doi: 10.1093/qjmed/89.4.285. [DOI] [PubMed] [Google Scholar]
- 6.Himelman R B, Callen P W. The prognostic value of loculations in parapneumonic pleural effusions. Chest. 1986;90(06):852–856. doi: 10.1378/chest.90.6.852. [DOI] [PubMed] [Google Scholar]
- 7.Waite R J, Carbonneau R J, Balikian J P, Umali C B, Pezzella A T, Nash G. Parietal pleural changes in empyema: appearances at CT. Radiology. 1990;175(01):145–150. doi: 10.1148/radiology.175.1.2315473. [DOI] [PubMed] [Google Scholar]
- 8.Leung A N, Müller N L, Miller R R. CT in differential diagnosis of diffuse pleural disease. AJR Am J Roentgenol. 1990;154(03):487–492. doi: 10.2214/ajr.154.3.2106209. [DOI] [PubMed] [Google Scholar]
- 9.Duysinx B C, Larock M P, Nguyen D et al. 18F-FDG PET imaging in assessing exudative pleural effusions. Nucl Med Commun. 2006;27(12):971–976. doi: 10.1097/01.mnm.0000243366.96012.c0. [DOI] [PubMed] [Google Scholar]
- 10.Johnston W W. The malignant pleural effusion. A review of cytopathologic diagnoses of 584 specimens from 472 consecutive patients. Cancer. 1985;56(04):905–909. doi: 10.1002/1097-0142(19850815)56:4<905::aid-cncr2820560435>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 11.Koegelenberg C F, Diacon A H. Image-guided pleural biopsy. Curr Opin Pulm Med. 2013;19(04):368–373. doi: 10.1097/MCP.0b013e32835f4c23. [DOI] [PubMed] [Google Scholar]
- 12.Robert Sheu Y, Hong K. Percutaneous lung tumor ablation. Tech Vasc Interv Radiol. 2013;16(04):239–252. doi: 10.1053/j.tvir.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Nadolski G. Nontraumatic chylothorax: diagnostic algorithm and treatment options. Tech Vasc Interv Radiol. 2016;19(04):286–290. doi: 10.1053/j.tvir.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Pillay T G, Singh B. A review of traumatic chylothorax. Injury. 2016;47(03):545–550. doi: 10.1016/j.injury.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 15.Staats B A, Ellefson R D. The Lipoprotein analysis of chylous and non-chylous pleural effusions. Mayo Clin Proc. 1980;55:700–704. [PubMed] [Google Scholar]
- 16.Gibbons S M, Ahmed F.Chylothorax diagnosis: can the clinical chemistry laboratory do more? Ann Clin Biochem 201552(Pt 1):173–176. [DOI] [PubMed] [Google Scholar]
- 17.Tong B C, Hanna J, Toloza E M et al. Outcomes of video-assisted thoracoscopic decortication. Ann Thorac Surg. 2010;89(01):220–225. doi: 10.1016/j.athoracsur.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 18.Braun M A. Interventions in the pleural space. J Vasc Interv Radiol. 1997;8(01):154–160. [Google Scholar]
- 19.Lombardi G, Zustovich F, Nicoletto M O, Donach M, Artioli G, Pastorelli D. Diagnosis and treatment of malignant pleural effusion: a systematic literature review and new approaches. Am J Clin Oncol. 2010;33(04):420–423. doi: 10.1097/COC.0b013e3181aacbbf. [DOI] [PubMed] [Google Scholar]
- 20.Clive A O, Jones H E, Bhatnagar R, Preston N J, Maskell N. Interventions for the management of malignant pleural effusions: a network meta-analysis. Cochrane Database Syst Rev. 2016;(05):CD010529. doi: 10.1002/14651858.CD010529.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Putnam J B, Jr, Light R W, Rodriguez R M et al. A randomized comparison of indwelling pleural catheter and doxycycline pleurodesis in the management of malignant pleural effusions. Cancer. 1999;86(10):1992–1999. [PubMed] [Google Scholar]
- 22.Thomas R, Fysh E TH, Smith N A et al. Effect of an indwelling pleural catheter vs talc pleurodesis on hospitalization days in patients with malignant pleural effusion: the AMPLE randomized clinical trial. JAMA. 2017;318(19):1903–1912. doi: 10.1001/jama.2017.17426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McElnay P J, Lim E. Modern techniques to insert chest drains. Thorac Surg Clin. 2017;27(01):29–34. doi: 10.1016/j.thorsurg.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Rivera L, O'Reilly E B, Sise M J et al. Small catheter tube thoracostomy: effective in managing chest trauma in stable patients. J Trauma. 2009;66(02):393–399. doi: 10.1097/TA.0b013e318173f81e. [DOI] [PubMed] [Google Scholar]
- 25.Mahmood K, Wahidi M M. Straightening out chest tubes: what size, what type, and when. Clin Chest Med. 2013;34(01):63–71. doi: 10.1016/j.ccm.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Klein J S, Schultz S, Heffner J E. Interventional radiology of the chest: image-guided percutaneous drainage of pleural effusions, lung abscess, and pneumothorax. AJR Am J Roentgenol. 1995;164(03):581–588. doi: 10.2214/ajr.164.3.7863875. [DOI] [PubMed] [Google Scholar]
- 27.Yarmus L, Feller-Kopman D. Pneumothorax in the critically ill patient. Chest. 2012;141(04):1098–1105. doi: 10.1378/chest.11-1691. [DOI] [PubMed] [Google Scholar]
- 28.Diacon A H, Theron J, Schuurmans M M, Van de Wal B W, Bolliger C T. Intrapleural streptokinase for empyema and complicated parapneumonic effusions. Am J Respir Crit Care Med. 2004;170(01):49–53. doi: 10.1164/rccm.200312-1740OC. [DOI] [PubMed] [Google Scholar]
- 29.Bouros D, Schiza S, Tzanakis N, Chalkiadakis G, Drositis J, Siafakas N. Intrapleural urokinase versus normal saline in the treatment of complicated parapneumonic effusions and empyema. A randomized, double-blind study. Am J Respir Crit Care Med. 1999;159(01):37–42. doi: 10.1164/ajrccm.159.1.9803094. [DOI] [PubMed] [Google Scholar]
- 30.Davies R J, Traill Z C, Gleeson F V. Randomised controlled trial of intrapleural streptokinase in community acquired pleural infection. Thorax. 1997;52(05):416–421. doi: 10.1136/thx.52.5.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jerjes-Sánchez C, Ramirez-Rivera A, Elizalde J J et al. Intrapleural fibrinolysis with streptokinase as an adjunctive treatment in hemothorax and empyema: a multicenter trial. Chest. 1996;109(06):1514–1519. doi: 10.1378/chest.109.6.1514. [DOI] [PubMed] [Google Scholar]
- 32.Moulton J S, Benkert R E, Weisiger K H, Chambers J A. Treatment of complicated pleural fluid collections with image-guided drainage and intracavitary urokinase. Chest. 1995;108(05):1252–1259. doi: 10.1378/chest.108.5.1252. [DOI] [PubMed] [Google Scholar]
- 33.Fraedrich G, Hofmann D, Effenhauser P, Jander R. Instillation of fibrinolytic enzymes in the treatment of pleural empyema. Thorac Cardiovasc Surg. 1982;30(01):36–38. doi: 10.1055/s-2007-1022203. [DOI] [PubMed] [Google Scholar]
- 34.Maskell N A, Davies C W, Nunn A J et al. U.K. Controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med. 2005;352(09):865–874. doi: 10.1056/NEJMoa042473. [DOI] [PubMed] [Google Scholar]
- 35.Cameron R, Davies H R. Intra-pleural fibrinolytic therapy versus conservative management in the treatment of adult parapneumonic effusions and empyema. Cochrane Database Syst Rev. 2008;(02):CD002312. doi: 10.1002/14651858.CD002312.pub3. [DOI] [PubMed] [Google Scholar]
- 36.Tillett W S, Sherry S. The effect in patients of streptococcal fibrinolysin (streptokinase) and streptococcal desoxyribonuclease on fibrinous, purulent, and sanguinous pleural exudations. J Clin Invest. 1949;28(01):173–190. doi: 10.1172/JCI102046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simpson G, Roomes D, Heron M. Effects of streptokinase and deoxyribonuclease on viscosity of human surgical and empyema pus. Chest. 2000;117(06):1728–1733. doi: 10.1378/chest.117.6.1728. [DOI] [PubMed] [Google Scholar]
- 38.Koegelenberg C F, Diacon A H, Bolliger C T. Parapneumonic pleural effusion and empyema. Respiration. 2008;75(03):241–250. doi: 10.1159/000117172. [DOI] [PubMed] [Google Scholar]
- 39.Idell S, Florova G, Shetty S et al. Precision-guided, personalized intrapleural fibrinolytic therapy for empyema and complicated parapneumonic pleural effusions: the case for the fibrinolytic potential. Clin Pulm Med. 2017;24(04):163–169. doi: 10.1097/CPM.0000000000000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rahman N M, Maskell N A, West A et al. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. The New England Journal of Medicine. 2011;365(06):518–526. doi: 10.1056/NEJMoa1012740. [DOI] [PubMed] [Google Scholar]


