Abstract
Clinical outcomes in patients with critical limb ischemia (CLI) depend not only on endovascular restoration of macrovascular blood flow but also on aggressive periprocedural wound care. Education about this area of CLI therapy is essential not only to maximize the benefits of endovascular therapy but also to facilitate participation in the multidisciplinary care crucial to attaining limb salvage. In this article, we review the advances in wound care products and therapies that have granted the wound care specialist the ability to heal previously nonhealing wounds. We provide a primer on the basic science behind wound healing and the pathogenesis of ischemic wounds, familiarize the reader with methods of tissue viability assessment, and provide an overview of wound debridement techniques, dressings, hyperbaric therapy, and tissue offloading devices. Lastly, we explore emerging technology on the horizons of wound care.
Keywords: critical limb ischemia, wound care, ulcer, debridement, peripheral arterial disease, interventional radiology
Objectives : Upon completion of this article, the reader will be able to describe the fundamentals of wound care. It is hoped that this knowledge will allow endovascular physicians to provide the best comprehensive care possible to their critical limb ischemia patients, thereby preventing amputation and maximizing potential for limb salvage.
Peripheral vascular disease (PAD) is the third leading cause of cardiovascular morbidity worldwide and affects over 20 million people in the United States. 1 2 3 The most severe form of PAD, critical limb ischemia (CLI), encompasses rest pain, ulceration, frank gangrene, and complications of ischemic infection. CLI is estimated to affect 2 to 3.4 million people in the United States, with numbers expected to skyrocket to over 4 million by the year 2030 given the current aging population and increasing prevalence of risk factors such as diabetes mellitus. 3 The mortality rate associated with a CLI diagnosis exceeds that of most cancers, with most patients dying of cardiovascular disease. CLI patients who have undergone amputation have less than 20% survival at 5 years, compared to 5 year breast and colon cancer survival rates of almost 90 and 65%, respectively. 4 5 The morbidity associated with CLI is equally devastating, with only 45% of patients with CLI alive with both limbs at 1 year after diagnosis. 5
This high rate of lower extremity amputation is associated with a profound economic burden. In general, the economic costs of PAD exceed that of diabetes, coronary artery disease, and all cancers, with 55 to 65% of these costs attributed to CLI care. 3 It is estimated that over $25 billion per year is spent directly on major and minor amputation in CLI patients. 3 Nonhealing wounds themselves account for over $3 billion in healthcare-related costs per year. 6 Preventing amputation and decreasing time to ulcer healing therefore could theoretically result in significantly reduced healthcare expenses.
Recently, great focus has been directed toward improving amputation prevention, especially through improved access to appropriate diagnosis and revascularization. 7 8 However, the restoration of blood flow through revascularization is only one front in the battle to prevent amputation. Wound healing in CLI requires complete optimization of the wound environment. A multidisciplinary team of specialists has been shown to improve wound healing rates. 8 9 10 11 12 13 However, management of CLI wounds is demanding and it is imperative for all physicians treating CLI to be well-versed in wound care. This article will review the pathogenesis of wounds and the physiology of healing in CLI patients. It will also provide the reader with an overview of medical optimization for wound healing and an understanding of debridement, offloading, and hyperbaric therapy. Finally, this article will review new technology and treatments on the horizon of the rapidly changing wound care field. The importance of a team-based, multidisciplinary approach in the care of patients with CLI is a concept that cannot be understated and is further described in another article of this journal.
Patient Empowerment
While lower extremity wound care takes the coordinated care of multiple specialists, the majority of the wound-healing process occurs outside the doctor's office. Wound healing requires great diligence and self-care by patients. Individual empowerment has been shown to enhance motivation and knowledge about health and illness, resulting in increased capacity for self-monitoring of symptoms and care. 9 10 The anticipated result of patient empowerment is to enable autonomous decision making in line with the goals of care.
Patient empowerment should begin at the first patient interaction and must be reinforced by providers throughout subsequent care, in order to maximize wound healing. An ideal model for conceptualizing the framework for patient care is shown in Fig. 1 . Since treatment is often long and complex, it is prone to failure. Therefore, in the first encounter with a peripheral wound patient, the physician must take adequate time to understand the patient's insight into the development of the ulcer. This will help determine the likelihood of patient buy-in and treatment adherence, as well as likelihood of healing. Physicians must also assess expectations and visualize patient willingness to dedicate themselves to the drastic lifestyle changes often necessary to properly heal the wound. Rather than simply stating recommendations for self-care, the physician must perform comprehensive, objective analysis at each visit to determine patient treatment compliance. It is the authors' opinion that this individualized assessment is one of the most essential components of care for every wound.
Fig. 1.
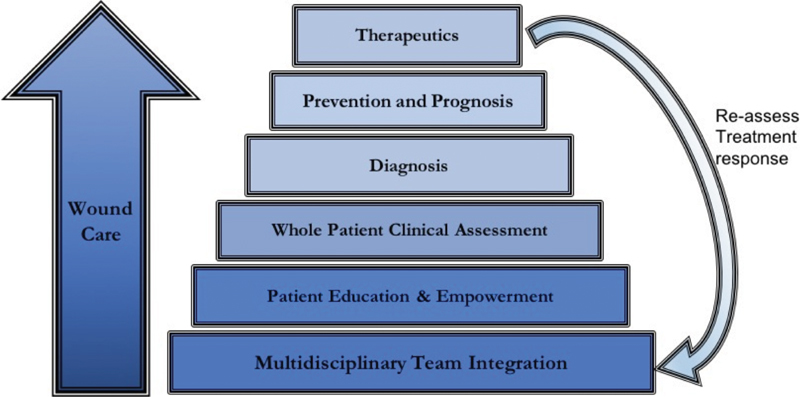
Diagram depicting ideal conceptual framework for addressing each critical limb ischemia patient.
Patients with CLI usually develop their disease through years of unhealthy lifestyle habits such as tobacco use, poor glucose control, obesity, and medication noncompliance. These lifestyle habits can be very difficult to break. One must address each patient individually in attempt to identify who is able follow more aggressive lifestyle changes and those patients more likely to succeed if the modifications are slower. 11 As such, physicians must also be realistic about how many changes can be achieved at a given time. The establishment of reasonable timelines can help the patient maintain and expand progress by reviewing self-monitored goals, targets, and achievements. 12 It has been demonstrated that thoughtful planning of lifestyle changes, endovascular intervention, and wound care increase patient motivation and treatment compliance. 13 14 Through these empowerment methods, the physician–patient interaction is firmly established and a coordinated attack can be launched to prevent amputation.
Visual tools are useful to establish a common understanding about a patient's wound-healing progress. The Society for Vascular Surgery Wound, Ischemia, and foot Infection (WIfI) classification is an algorithm used to stratify amputation risk and determine the need for endovascular or surgical intervention. 15 16 Now available as a smartphone application, patients' risk for amputation can be computed before their eyes, which in the authors experience can dramatically improve a patient's understanding of their condition ( Fig. 2 ).
Fig. 2.

Visual tools, such as the Society for Vascular Surgery Wound, Ischemia, and foot Infection (WIfI) classification smartphone application, shown here in use with a critical limb ischemia patient in clinic (a) and as a screenshot (b) , are very useful for improving a patient's understanding of their condition, the associated risk of amputation, and the potential benefits of revascularization. 15 16
Biology of Wound Healing
A complex, multifactorial wound healing cascade is initiated with the disruption of the normal anatomic structure and function of skin in healthy subjects ( Fig. 3 ).
Fig. 3.

The stages of normal wound healing over time.
Inflammatory Phase: Injury Leads to Hemostasis, Cell Migration, and Phagocytosis
In the first 24 to 48 hours after injury, blood vessels contract and leaked blood coagulates to preserve vascular integrity. 17 Thrombocytes and platelets form a fibrin network, which establishes hemostasis and provides a temporary extracellular matrix (ECM) for cell migration and defense against invading bacteria. 17 This cascade allows fibroblast proliferation and leukocyte response leading to clinical signs of edema and erythema. 17 18 Cytokines are released from immune and inflammatory cells, such as Langerhans cells and neutrophils, leading to focal tissue destruction, nitrous oxide release, and protease activation. 18
By 48 hours, macrophages are recruited to clean the wound. 17 18 19 They augment the inflammatory phase by recruiting additional inflammatory cells to the region and releasing free radicals. 19 Their secretion of prostaglandins is vital, as they act as vasodilators and activators of endothelial cells. 18 As the inner lining of the new vascular bed, endothelial cells release vascular endothelial growth factor, platelet-derived growth factor, and other cytokines that lead to new blood vessel formation and ultimately granulation tissue formation. 20 When macrophages are deficient, wound healing is prevented from progressing to the proliferative phase.
Proliferative Phase: Wound Closure
The proliferative phase of wound healing occurs from 48 hours to 2 weeks after initial injury, occurring via epithelialization and fibrous tissue formation and serving to create the framework for a permanent barrier at the wound site. 17 18 Blood vessel remodeling is the hallmark of the proliferative phase, as new pathways for nutrient delivery and waste removal are crucial in this time of heightened activity. 21 Regenerative cells control this remodeling in three distinct but overlapping processes: angiogenesis—the expansion of capillary vascular networks from preexisting blood vessels; vasculogenesis—the de novo synthesis of new blood vessels from endothelial precursor cells; and arteriogenesis—the remodeling of existing collaterals into functional arteries. 21 These interactions involve innumerable support cells, such as pericytes and circulating progenitor cells, regulating the formation of new blood vessels and granulation tissue. 21 22
At 96 hours, granulation tissue begins to form. 17 18 Fibroblasts recruited to the wound margin create a new collagen ECM in parallel with epithelialization, restoring tensile strength and inciting wound contraction. 18
Remodeling Phase (2–3 Weeks after Injury to 1 Year): Achievement of Tensile Strength
Transforming growth factor β1 (TGF-β1), fibroblast growth factor, and other anti-inflammatory cytokines released by endothelial cells, contribute to collagen maturation by stimulating the formation of cross-linking collagen fibers with a parallel fiber orientation. 18 This delicate collagen synthesis–lysis balance helps prevent hypertrophic scarring. 18 23 Apoptosis and vascular atresia occur over time. Contraction of the new ECM from myofibroblast-derived smooth muscle actin and adhesive glycoproteins results in a healed wound. 18 24
Pathophysiology of Ischemic Wounds
As described in detailed earlier, wound healing involves many chemokines and regulatory factors that function in a precisely orchestrated fashion to ultimately restore wound contraction, function, and strength. However, in the setting of vascular insufficiency, healing is halted due to a combination of lack of oxygen and nutrients and an imbalance of inflammatory factors crucial in the healing cascade. 21 Since little or no vascular supply is available to satisfy the high metabolic needs of the neotissue , blood vessel remodeling fails and fibroblast migration is halted. 25
The reader should recall that in the proliferative phase of wound healing, three distinct but overlapping processes (angiogenesis, vasculogenesis, and arteriogenesis) are necessary for the blood vessel growth and remodeling needed to support epithelialization and fibroplasia. 21 In end-stage CLI patients, these vascular mechanisms are exhausted or absent due to a chronic metabolic attack from increased oxidative stress, chronic inflammation, glucotoxicity, and lipotoxicity. 21
Ischemia normally induces elevation in lactate levels, which in turn stimulates an increase in growth factors in attempt to promote blood vessel formation. 26 The starved tissues of CLI patients, however, are depleted of growth factors, progenitor cells, and regulatory cells needed for basal metabolic activities, let alone the upregulation needed in the hypermetabolic setting of injury. 21 27 This tissue burnout can explain how patients with similar patterns and severity of arterial occlusions can demonstrate drastically different perfusion deficiency-related clinical sequelae.
In addition to tissue burnout, imbalances in specific enzymes and cytokines have been discovered in chronic wounds. The ECM typically promotes cell migration and angiogenesis in normal wound healing. 27 Exudate from chronic wounds contains an imbalance of matrix metalloproteinases (MMPs), endopeptidases responsible for proteolysis of the ECM. 27 Tissue inhibitor of metalloproteinases (TIMPs) and plasma inhibitors control the amount of MMPs produced. Chronic wounds have an excess of MMPs due to decreased TIMPs, which propagates excessive proteolysis, perpetuating a nonhealing chronic wound. 25 27 Furthermore, chronic wounds contain decreased levels of active TGF-β1, a cytokine necessary for angiogenesis, fibroplasia, and collagen synthesis in the proliferative and remodeling phases of wound healing. 25 28
Further complicating wound healing, many patients with CLI also have superimposed venous disease. Venous hypertension itself results in tissue ischemia. 25 29 Histologically, wounds in the setting of venous hypertension contain pericapillary fibrotic cuffs that prevent oxygen and micronutrient delivery from the capillary bed to the dermis, which ultimately induces endothelial damage. 25 Clinically, this manifests as edema and exudate in the bed of granulation tissue. Edema causes dermal thickening, lowering focal tissue perfusion as a result of decreased capillary diffusion. 29 Furthermore, leukocytes trapped in the fibrous cuffs release inflammatory cytokines and reactive oxygen species upon reperfusion, causing cyclic ischemia-reperfusion damage to the tissue. 29
Optimizing the Wound Healing Environment through Medical Management
Up to 25% of patients with CLI will die of cardiovascular causes at 1 year. Therefore much of the currently available data on medical management of CLI is geared toward cardiovascular risk reduction. 5 However, a great deal of research demonstrates that wound healing and amputation prevention rates can also be improved through optimal medical management.
Smoking is the strongest risk factor for development of de novo PAD and additionally promotes progression of disease. 30 Smokers with PAD have reduced patency rates following revascularization and higher amputation rates compared to nonsmokers or prior smokers. 30 31 32 33
The ACCF-AHA guidelines give a class Ia recommendation, regarding smoking cessation stating patients should be asked about tobacco use at every visit. They should be assisted with counseling and develop a plan to quit, including referral to a smoking cessation program and/or the use of adjunctive pharmacotherapy. 34 Smoking cessation should begin as soon as possible and repeated without delay after each failed attempt. Any deferment may result in poor cardiovascular outcomes before any potential revascularization benefit is realized, given the accelerated disease progression inherent in this population. 30 Nicotine replacement therapy, the monocyclic antidepressant bupropion, the nicotine-receptor agonist varenicline, behavioral support therapy, and even simple physician advice have all been found to be effective in increasing smoking cessation rates. 30 35 36 37 38 This can translate to at least partial mitigation of the earlier-mentioned disease states. 30 35 36 37 38
Poor glycemic control in diabetics is another well-known risk factor for the development and progression of PAD: for every 1% rise in HbA1c, there is a 28% increase in risk of PAD. 39 Elevated HbA1c is an independent risk factor for major lower extremity amputation. 40 In another study, for each 1.0% point increase in HbA1c, the daily wound-area healing rate decreased by 0.028 cm 2 /day. 41 As such, control of blood glucose levels is of paramount importance to optimize wound healing.
The benefits of statin medications are well described in the cardiovascular literature. In addition to their lipid-lowering effect, statins are thought to stabilize plaque, reduce inflammation, improve endothelial function, and possibly exert proangiogenic effects. 42 The Reduction of Atherothrombosis for Continued Health (REACH) Registry demonstrated that statin use in 5,861 patients with PAD was associated with an approximately 20% reduction in systemic cardiovascular events. This group also had a corresponding approximately 18% reduction in adverse limb outcomes (worsening symptoms, peripheral revascularization, and amputation). 43 In CLI patients, statins have also demonstrated benefit, with some studies showing improved overall survival, amputation-free survival, and lower rates of systemic vascular events. 44 45 Because of this, the current ACC-AHA guidelines give a class 1a recommendation for the use of high-intensity statin therapy (40–80 mg atorvastatin or 20–40 mg rosuvastatin daily) in patients with PAD/CLI. 46
Cilostazol, a phosphodiesterase-3 inhibitor with vasodilatory and antiplatelet effects, is frequently used in patients with symptomatic claudication. It has also been associated with improvement in amputation-free survival after angioplasty in CLI patients. Cilostazol has also demonstrated potential for improving wound-healing when combined with clopidogrel in patients who are not candidates for revascularization. 47 48
Exercise training is thought to lead to improvements in endothelial function, muscle metabolism, and hemorheology, in addition to inflammation reduction and stimulation of vascular angiogenesis. 49 CLI patients may be at a disadvantage because of tissue loss or immobility. An exercise program may not be feasible until after revascularization has led to healing of all wounds. Still, recent trials suggest that any exercise induces improved oxygen delivery throughout the body. A review of six recent RCTs demonstrated that vigorous upper extremity exercise (arm cranking for 40–70 minutes two to three times per week for 6–24 weeks) results in improvements in mean lower leg peak oxygen consumption, walking distance, claudication distance, and quality-of-life metrics when compared to lower extremity exercise. 50 Given these findings, upper extremity and body exercise may be an alternative method of exercising CLI patients.
Local Wound Care
Wound bed preparation is an approach that seeks to eliminate the numerous factors that impede wound healing. Debridement in some form is often necessary to achieve wound healing. Debridement involves removal of dead, damaged, or infected tissue, resulting in improved healing potential of the remaining healthy tissues. Even nonviable tissue, debris, calluses, or thickened skin surrounding ulcers can result in nonhealing in the periphery. 13 However, when this tissue is removed on a regular basis, it has been shown to speed up wound healing rates and increase the probability of achieving full secondary closure. 13
There are five primary methods of debridement, often referred to as BEAMS ( B iological, E nzymatic, A utolytic, M echanical, S urgical sharp, and conservative sharp debridements; Fig. 4 ). Determining the debridement method is based not only on the wound presentation and evaluation, but also on the patient's history, physical examination, and potential compliance. 51
Fig. 4.

Selective versus nonselective methods of debridement.
Biological Debridement
This debridement technique employs maggots ( Lucilia sericata —green bottle fly) that are grown in a sterile environment and digest dead tissue and pathogens. Maggot debridement has been suggested to work by mechanical and biochemical techniques and is limited to the necrotic wound, sparing healthy tissue. 52 53 The mechanical component of biologic debridement is caused by the mandibles and rough body of the maggot. The biochemical component consists of excretion and secretion of proteolytic enzymes which dissolve the dead and/or infected matrix of the wound bed. 54 Dressings, which can be preassembled or custom made, are used to “confine” sterile maggots to the wound. Unfortunately, despite the efficacy of this debridement technique, many patients are averse to the use of maggots, limiting its use.
Enzymatic Debridement
This technique involves the application of a collagenase ointment that chemically and/or enzymatically degrades only denatured collagen anchored to the wound, leaving functioning structural proteins, crucial for the second phase of the wound healing, unaffected. 55 Collagenase is the only enzymatic debriding ointment available and can be expensive. Enzymatic debridement is commonly used in the long-term care setting because there is less pain than mechanical debridement and it can be applied daily.
Autolytic Debridement
Autolytic debridement is selective, painless, and noninvasive. However, it is slow, contraindicated for infected wounds, and is not suitable when a large amount of necrotic tissue is present. This method entrusts the natural physiologic process (white blood cells, the body's own enzymes, and moisture beneath a dressing during the inflammatory phase of healing) to achieve the ultimate goal of liquefying necrotic tissue. 55 58 All wounds go through autolytic debridement to some extent. Moisture, dressing change frequency, and dressing absorbency are factors that affect the degree of autolytic debridement. Dressing types commonly used are hydrocolloids, hydrogels, and transparent films (semiocclusive and occlusive). 51 55
Mechanical Debridement
Many types of mechanical debridement are available, including the use of wet-to-dry dressings ( Fig. 5 ), scrubbing, wound cleansing, wound irrigation, pulsatile lavage, whirlpool, and use of hydrogen peroxide. It is cost-effective and one of the oldest forms of debridement practiced. 56 57 However, besides being time-consuming and painful, mechanical debridement is nonselective in nature and may also damage healthy tissue, prolonging the inflammatory process and increasing the risk of infection. 56 57
Fig. 5.
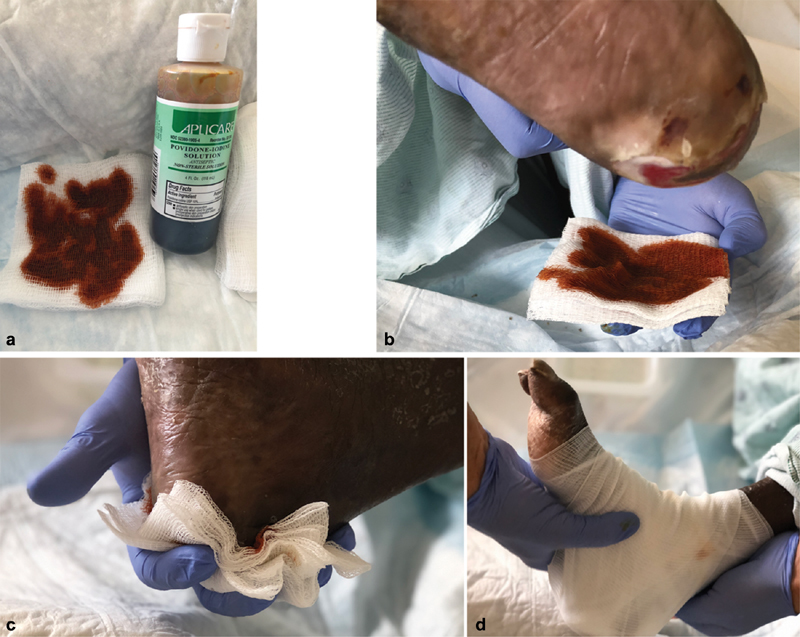
Wet-to-dry dressings are a commonly used form of mechanical debridement. In this patient with an infected heel wound, a povidone-iodine antiseptic solution is used to saturate sterile gauze ( a ). This is then applied to the wound ( b, c ), covered with ample dry gauze, and secured in place ( d ). After approximately 4 to 6 hours, the gauze has dried and become adherent at which point it is removed (not shown), performing nonselective mechanical wound debridement.
Surgical Sharp and Conservative Sharp Debridement
This type of excisional debridement must be performed by a skilled practitioner using surgical instruments for mechanical removal of biofilm and devitalized tissue. Often recommended for necrotic and infected wounds, the extent of the debridement is determined by the amount of devitalized tissue. Surgical debridement is the most aggressive type of debridement and is performed in a surgical operating room, whereas conservative sharp debridement is performed in a clinic setting at the bedside with sterile instruments ( Figs. 6 and 7 ). 51 55
Fig. 6 (a, b).

A patient in clinic undergoes sharp debridement of periulcer callous to promote continued proper healing of the wound.
Fig. 7.

A patient with extensive heel gangrene undergoes surgical debridement in the operating room.
When to Debride: Assessing Microscopic Tissue Viability
Revascularization is often performed to maximize blood flow in the peri-debridement period. Vascular specialists have traditionally relied on angiographic signs of improved perfusion (luminal gain, contrast washout, wound-blush) to assess a successful interventional outcome. 58 However, it has become clear that while technological advances have made it more feasible to obtain a technically successful angiographic result regularly, this macrovascular reconstruction does not always translate to microvascular reperfusion. Reports in the literature demonstrate that up to 10 to 18% of chronic wounds fail to heal even after successful macrovascular reconstruction. 59 60 61 62 Therefore, the assessment of “sufficient tissue perfusion” for wound healing is an area of active investigation and continues to evolve. Two-dimensional perfusion angiography, methylene blue angiography, transcutaneous measurement of oxygen partial pressure (tcPO 2 ), multispectral optoacoustic tomography, hyperspectral tissue oxygenation measurement (HTOM), and near-infrared fluorescence angiography (NIFA) have all demonstrated promise in the assessment of distal perfusion and tissue viability prior to and during debridement. 62 We have found HTOM and NIFA to be particularly effective in our multidisciplinary CLI practice, as they allow real-time, intraoperative assessment of the impact of revascularization on wound perfusion.
HTOM, uses a handheld spectrometer to quantify the absorption of visible light in hemoglobin molecules over a region of interest ( Fig. 8 ). 63 The levels of superficial subcutaneous tissue oxygen saturation, oxyhemoglobin, and deoxyhemoglobin can be determined by differential light absorption and color coded. Scaled composite maps for each of the aforementioned units are constructed, allowing for rapid, real-time pre- and postintervention microcirculatory perfusion analysis. 63 Normative values have been determined and HTOM has demonstrated reliability in determining the severity of PAD compared to standard measures such as transcutaneous oxygen measurement and ankle-brachial index. 64 65 In addition, HTOM has been found to provide a local assessment of microvascular oxygenation status that is predictive of ulcer healing in diabetics, with higher oxyhemoglobin levels in the 85% of diabetic foot ulcers that healed versus the 64% that did not heal. 66
Fig. 8.

HTOM with the HyperView device (HyperMed Imaging) uses a handheld spectrometer (a) to quantify tissue perfusion and viability via the absorption of visible light in hemoglobin molecules over a region of interest. In a region of ulceration (b), the levels of superficial subcutaneous tissue oxyhemoglobin (c) , deoxyhemoglobin (d) , and oxygen saturation (e) can be determined by differential light absorption and are color coded. 63 Images reproduced with permission from HyperMed Imaging, Inc., Memphis, TN.
NIFA makes use of the spectral absorptive properties of FDA-approved water-soluble indocyanine green (ICG) contrast. This allows semiquantitative measurement of skin and subdermal microcirculation. 67 68 First used in 1959 to assess liver function and cardiac output, it has been regularly used for ophthalmological measurement of retinal and choroidal blood flow as well as for flap perfusion analysis in reconstructive plastic surgery. 62 After intravenous injection, ICG binds to plasma proteins and is excited to fluorescence by a near-infrared light source. 67 69 Handheld cameras employing silicon and indium gallium arsenide are then used to measure the emitted light within the optical near-infrared range (700–1,299 nm) from the ICG-bound plasma proteins near the skin surface down to 2 to 4 cm in depth, serving as a surrogate for superficial tissue perfusion. 67 68 69 ICG has been found to reflect statistically significant differences in perfusion in CLI patients before and after revascularization ( Figs. 9 and 10 ).
Fig. 9.

NIFA imaging using ICG in use for intraoperative assessment of tissue viability prior to possible amputation in a patient who underwent successful revascularization after initially presenting with right great hallux (a) and left second digit (b) gangrenous changes. Sterile water and ICG solution (c) are injected intravenously. The NIFA camera and display console are then used to assess viability via the detection of ICG (d). Fluorescence imaging of the gangrenous right hallux demonstrates a concordant paucity of flow ( white arrows ) in the gangrenous region in both anteroposterior (e) and lateral projections (f). Given this lack of viability even after revascularization, decision was made to perform amputation of the right hallux (g, h). Postamputation NIFA imaging of the right foot demonstrates flow to the surgical resection margin in both anteroposterior and lateral projections (i, j). Fluorescence imaging of the gangrenous tip of the left second digit (k) demonstrates focal area of diminished flow ( white arrow ) that is discordant with the amount of gangrene on physical exam, indicating a penumbra of viable tissue. Therefore, surgical debridement of the left second toe was performed rather than amputation.
Fig. 10.

An 80-year-old male with history of smoking, hypertension, hyperlipidemia, and critical limb ischemia gangrenous changes to the right second and third toes (a) with underlying metatarsophalangeal joint abscess. AP angiogram of the right infrageniculate circulation (b) demonstrated ostial occlusion of the right anterior tibial artery * (TASC II type D), subtotal occlusion of the peroneal artery (TASC II type C), and 50% proximal posterior tibial artery stenosis (TASC II type A) with no in-line flow to the foot (not shown). Tibial and pedal plantar loop reconstruction was then performed with complete recanalization of the anterior tibial (AT), tibioperoneal trunk (TP), peroneal (P), dorsalis pedis, and lateral plantar (LP) arteries (c, d) . Second and third digits and metatarsophalangeal joint resection with evacuation of abscess was then performed with apparent adequate bleeding post-revascularization (e) . Fluobeam angiography of the resection site was performed demonstrating unexpected areas of hypoperfusion to the wound site and periphery ( white arrows ; f) . Transmetatarsal amputation was performed with adequate macro/microvasculature for stump healing now demonstrated by NIFA (g). Outpatient follow-up showed complete stump healing and ambulation ( h ).
Quantitative ICG NIFA analysis performed before and after revascularization demonstrates correlation with standard perfusion analyses, such as ABIs, and has been shown to predict revascularization success or failure. 70 71 72 Furthermore, ICG NIFA has been shown to reflect differences in perfusion in patients after revascularization even in situations where other perfusion techniques such as ABI and toe pressure often fail, such as in diffuse medial calcification, making its use evident in the CLI patient. 71 73 74 75
These modalities aid in determining whether our macro-revascularization procedures translate to the microvascular perfusion sufficient to heal wounds and aid in the determination of the appropriateness and extent of debridement.
Osteomyelitis
Osteomyelitis can thwart revascularization efforts, is a leading cause of a nonhealing wound, and carries a high risk of major amputation. 76 77 It can happen as a consequence of a soft-tissue infection that spreads to the bone. Bone involvement should be ruled out in all ulcers, with the highest index of clinical suspicion exercised in diabetic patients, patients with infectious clinical findings, as well as in the settings of chronic nonhealing wounds and ulcer recurrence. 76 77 78 Early revascularization is often necessary to allow adequate blood flow to the infection, improving healing and capability of fighting infection.
Similar to soft-tissue infections, cases of osteomyelitis are typically polymicrobial, depending on the characteristics of the patient, the clinical risk factors, the wounds (extension and depth), and the microenvironment. Because osteomyelitis is often not easily diagnosed clinically in its earliest phases, laboratory, microbiological, and radiological evaluations are often necessary. However, two specific clinical tests, measurement of the width and depth of the foot ulcer and the “probe-to-bone” test have been found to be especially predictive of osteomyelitis and should be performed at initial ulcer evaluation (see Table 1 and Fig. 11 ). 78 79
Table 1. Clinical and laboratory tests for suspected osteomyelitis.
| Test or clinical sign | Details |
|---|---|
| Width and depth of foot ulcer |
• Size > 2 cm
2
: sensitivity of 56%; specificity of 92%
78
• Deep ulcers (>3 mm) are more likely to be associated with an underlying osteomyelitis than superficial ulcers (82 vs. 33%) 78 |
| “Probe-to-bone” test 79 | • Performed by probing ulcer with sterile, blunt probe • Positive if probe reaches bone surface • High specificity and sensitivity when compared to imaging and wound culture |
| Culture/Microbiological evaluation 142 | • Low specificity • Often leads to diagnostic errors because sample not properly obtained or patient already receiving systemic antibiotics |
Fig. 11.

Medial heel ulcer in a critical limb ischemia patient that probes to bone on examination.
Imaging Evaluation
Plain radiography has low sensitivity for detecting acute osteomyelitis. However, when objective radiographic signs are present, the specificity is high. 80
Computed tomography (CT) has superior bony resolution to magnetic resonance imaging (MRI). CT can best demonstrate the classic osseous changes seen in osteomyelitis (cortical destruction, periosteal reactions, and sequestrum formation). 80 MRI has high sensitivity for detection of osteomyelitis and with normal findings virtually excludes the diagnosis. Acute osteomyelitis can be detected as early as 1 to 2 days after initial development when bone marrow edema is visualized. 76 The clinical and biochemical picture must be correlated with the MRI to avoid unnecessary or overly aggressive treatment. 80
Triple-phase bone scan with technetium-99m-labeled MDP (Tc 99m -MDP) has high sensitivity in nonviolated bone. This is lower when bone has been violated (trauma, malignancy, previous surgery). Gallium scans have higher specificity than triple-phase scans, but they take 48 to 72 hours to complete. The combined white cell and marrow scan is the current study of choice for investigating suspected osteomyelitis in violated bone and provides a map of physiological white cell uptake, with any discordance in uptake between the two studies indicating a focus of infection. 80
FDG-PET has the highest sensitivity of all the radionuclide techniques in detection of chronic osteomyelitis as it accumulates in activated macrophages, the predominant cell type found in chronic infection. 81
Bone Biopsy
Although most researchers consider that the histopathological study of bone specimens is the standard for diagnosing osteomyelitis, this method is reserved for cases of diagnostic uncertainty because many clinicians feel that surgically obtaining bone tissue is overly aggressive and puts patients at risk. 82
The Nonhealing Wound
As previously outlined, typical wound healing in healthy populations follows a set order of physiological events. However, patients with CLI generally have at least one factor, often more, that impair wound healing and lead to what are known as chronic, nonhealing wounds. These factors typically include infection, smoking, diabetes and other metabolic and nutritional disorders, vascular disease, immunosuppression, immobilization, and age. The time frame distinguishing acute from chronic wounds can vary based on wound type and is much debated; however, a chronic wound is thought to be any wound that is not healed within 3 months. 83 Due to various factors, chronic wounds tend to stall in one particular phase of the wound healing cascade, most commonly the inflammatory phase. 84 85 In order to properly determine whether a wound is considered chronic and nonhealing, it is important for the wound care physician to monitor the change in size of the wound over time. Many studies have been performed to attempt to correlate the percent reduction in wound size over an acute time period with complete wound healing in the future.
Table 2 highlights the percent reduction in wound surface area over an amount of time as a predictor of wound healing. 86 87 Many studies have shown that if these benchmarks are not attained, complete wound healing is unlikely unless the underlying factors preventing wound healing are better addressed. 86 87 Once a wound has become chronic and nonhealing, it is of vital importance to address the imbalance between certain molecules such as collagen and growth factors which are degraded in chronic wounds by focusing on tissue necrosis, moisture balance, and bacterial burden. 88 89 90
Table 2. Comparative expected size reduction over time as a predictor of complete wound healing.
Chronic wounds may result from the same pathophysiology that created the original wound such as systemic infection or vascular, immune, or nerve insufficiency. However, alternative diagnoses, such as pyoderma gangrenosum ( Fig. 12 ), should always be a consideration in case of a nonhealing ulcerative lesion when all other factors have been optimized ( Fig. 12 ). 91 Given their long-term inflammatory environment, chronic wounds can develop malignant transformation, such as that seen with Marjolin's squamous cell carcinoma. 92 Other classic malignancies included in the differential diagnosis include amelanotic melanoma, aggressive digital adenocarcinoma ( Fig. 13 ), carcinoma cuniculatum, and basal cell carcinoma. 92
Fig. 12.

Pyoderma gangrenosum mimicking a nonhealing wound. (Reprinted with permission from Snyder et al. 91 )
Fig. 13.
Aggressive digital adenocarcinoma mimicking a nonhealed wound. (Reprinted with permission from Vazales et al. 92 )
It has been estimated that up to 10% of all chronic leg wounds may be due to atypical etiologies. 93 Due to this, if a wound has become chronic and unable to reach typical healing benchmarks, it is important to perform multiple punch biopsies from both the wound bed and wound periphery. 93 One study reviewed showed that of 350 ulcer biopsies submitted to the University of Miami Wound Pathology Service between January 2008 and December 2010, 104 (29.7%) were diagnosed as atypical, with PG being the most common seen in 14 cases, followed by malignancy (most common was squamous cell carcinoma), and vasculitis. The least common atypical diagnosis was that of an atypical infection such as Mycobacterium . 93 By monitoring wound size over time, tailoring therapy to clinical changes in wound evolution, and performing biopsies on chronic wounds, the physician increases the chance of stimulating wounds out of their chronic nonhealing state.
Dressings
Throughout history, many different materials have been used to treat wounds and promote healing, including honey, cobwebs, sphagnum moss, and even animal dung. 23 94 The current landscape of wound dressing is equally diverse and a complete discussion is beyond the scope of this review. The current medical literature is unfortunately lacking in rigorous trials comparing efficacy of different dressings. However, as a general rule, wound dressings should be chosen in order to obtain the optimal moist, warm, and clean environment for wound healing. 23
Four basic principles should be remembered when choosing the optimal dressing: dry or desiccated wounds need hydration, excessive exudates need to be absorbed, necrotic tissue should be debrided, and wound infection must be addressed. 23 Three broad categories of dressings exist, each with their own ability to alter the wound environment: autolytic dressings, alginates, and bacteria-reducing dressings. Depending on the characteristics of a wound, different dressings of varying types may need to be applied.
Dressings that facilitate autolytic debridement enhance the patient's own proteolytic enzymes to promote nonvitalized tissue removal. 23 Alginates manipulate the moisture content of the wound bed. 23 The third broad category of wound dressing involves reducing the bacterial load of the wound. 23 All dressing types may be used in conjunction with one another for their additive properties and should be tailored to each individual wound to achieve the optimal wound healing environment. Dressing types may need to be changed intermittently based on clinical appearance of the wound. A variety of commonly used dressing types and their effects are summarized in Table 3 .
Table 3. Dressing types.
| Dressing | Benefit | Negative effects |
|---|---|---|
| Gauze (wet-to-dry dressing) | • Inexpensive • Available • Highly absorbent • Debrides dead tissue |
• Nonselective mechanical debridement • Drying can result in cooling resulting in vasoconstriction • Requires frequent dressing changes • Nonocclusive |
| Impregnated gauze (petroleum, iodine, zinc, etc.) | • Nonadherent • Semiocclusive • Decrease moisture loss Decrease cooling-related vasoconstriction |
• Higher cost • No absorption so not useful in exudative wounds Impregnated compounds can be cytotoxic |
| Hydrogels + hydrocolloids (hydrophilic cross-linked polymers with water base) | • Absorb minimal fluid by swelling + also donate moisture to dry wounds • Facilitate in autolytic debridement • Create moist wound environment |
• Cannot absorb heavy drainage • Maceration of periwound areas can be of concern • Can cause issues in infected wounds |
| Alginates | • Highly absorbent • Hemostatic properties • Nonadherent • Biodegradable • Can prevent microbial contamination |
|
| Antimicrobial dressings: (silver, honey) | • Concept used for thousands of years • Broad-spectrum antimicrobial agents • Reduce inflammation + promotes healing in vitro |
Lack of quality in vivo evidence |
Wet-to-dry dressings, a form of mechanical debridement in themselves, deserve special discussion given their widespread use or overuse ( Fig. 5 ). By applying wet dressings to a wound and allowing them to dry over the course of a few hours, dead tissue adheres to the dressing and is removed during dressing changes. However, this form of nonselective mechanical debridement can also be harmful. Instead of promoting the moist, warm environment necessary for optimal reparative cellular activity and maximal wound healing, the drying process in these dressings results in wound bed desiccation and relative hypothermia. In addition, healthy tissue can also be attached to the gauze in the drying process and is then removed, resulting in bleeding and periwound maceration. 56 57 In addition, gauze fibers left behind in the wound can incite a foreign body inflammatory response. 95 Ultimately, this dressing can impair epithelialization and delay wound healing. Although often considered low cost, the savings are offset by frequent nursing visits, as this type of dressing may need to be changed every 4 to 6 hours to effectively remove all nonviable tissue. 96
Tissue Protection and Offloading
Lower extremity wounds can arise from multiple etiologies. Appropriate diagnosis and management will determine outcome. While purely ischemic wounds will be best treated by revascularization and postinterventional surgical management, neuropathic and neuroischemic wounds will also benefit from aggressive off-loading and biomechanical analysis. 97 98
Patients with diabetes and foot ulcers reflect a special population. Given exceptionally high rates of ulcer-related morbidity and recurrence, caring for diabetic patients with ulcers has been compared to caring for cancer patient. 99 Diabetic patients have higher peak plantar pressures than the general population, with concomitantly higher rates of neuropathy and PAD. 100 101 102 Repetitive pedal stress in the setting of these pathologies can result in osseous and structural abnormalities of the foot, which can be some of the strongest predictors of ulcer development. 102 103 Therefore, the goal in these patients is to shift or redistribute plantar pressures by offloading the foot in order to maintain ulcer remission. 99
Traditional total contact casts (TCCs) are the gold standard for offloading open wounds ( Fig. 14 ). Molded to the shape of the distal phalanges and pedal plantar osseous prominences, they extend proximally beyond the back of the heel and up to the leg. TCCs have been reported to reduce neuropathic site pressure by 84 to 92% and to result in diabetic neuropathic ulcer healing in 6 to 8 weeks. 104 While effective in edema and moderate infection, TCCs are relatively contraindicated in more severe cases of CLI and severe infection, as longer-term device use and haphazard placement can actually worsen disease and allow spread of infection. 104
Fig. 14.

Components of a total contact case.
Removable cast walkers (RCW; Fig. 15 ) are a good alternative to TCCs. They effectively achieve plantar offloading via a rocker bottom sole and also are easily placed and removed, facilitating wound cleaning and inspection. 105 This advantage, however, can also be a weakness: patient adherence to the walker is a major factor in nonhealing ulcers and recurrence, resulting in early failures if not continually worn. 105
Fig. 15.

Removable cast walker ( a ) and removable offloading shoe ( b ).
Custom footwear and orthoses ( Fig. 16 ) have also been promoted in the at-risk population due to their practicality and simultaneous reduction in limitations associated with other offloading devices. When properly fitted, custom footwear and orthoses can effectively alleviate high vertical pressure and shear stress, with one study demonstrating a 30% lower ulcer recurrence in patients who wore diabetic therapeutic shoes in comparison to those who wore normal shoes. 106 107 In patients with preulcerative lesions, silicone orthoses ( Fig. 17 ) have been shown to significantly reduce the incidence of primary ulcer formation. 106 107 Customized shear stress–reducing insoles have been shown to result in significantly reduced ambulatory pedal temperature increases compared with standard insoles. 108 Again, because of the ease of removal, patient adherence with these devices is one of the largest hurdles that must be overcome in dealing with ulcer recurrence.
Fig. 16.

Interior ( a ) and exterior ( b ) of a custom-fitting orthosis.
Fig. 17.

Various offloading shoe inserts.
Comparative evidence of one offloading device over another is weak due to lack of randomized-controlled studies in this area. However, a recent systematic review examined trials evaluating the effectiveness of off-loading practices in diabetic foot wounds, with three categories of interventions identified: removable cast walkers (RCWs), half or heel relief shoes, and therapeutic shoes. Most of the studies compared at least one of these interventions to a TCC, which is considered the gold standard. RCWs were found to be the most effective of the removable devices, followed by half or heel relief shoes, followed by currently available therapeutic shoes. 109
“Smart” technologies and wearable electronics reflect the future of tissue protection and offloading. These devices provide objective data about the patient's daily life and patterns in order to determine causes of treatment success or failure. 110 “SmartSox,” which are sensorized socks made from flexible fiberoptics, are just one example of these wearable electronics. 110 This is especially important in patients with neuropathy, given they lack the ability to sense the effect of physical activity on their feet. Three-dimensional printing has also been used to create personalized exoskeleton suits for paraplegics and may also prove useful in high-risk diabetics with neuropathy to reduce plantar tissue impact and weight-bearing stresses. 111
Hyperbaric Therapy
Hyperbaric oxygen therapy (HBOT) has long been used as an adjunctive therapy for diabetic and other ischemic lower extremity wounds. 112 While a complete discussion of the mechanism of action of HBOT is outside the scope of this review, its premise is based on the gas laws of Boyle, Dalton, and Henry. 113 114 When breathing normobaric air, systemic arterial oxygen tension is around 100 mm Hg and systemic tissue oxygen tension around 55 mm Hg. 115 116 In the extremities, an oxygen tension of greater than 40 mm Hg is needed for sufficient oxygenation and immune response; however, due to impaired macro- and microvascular circulation, diabetic lower extremities often have oxygen tension of less than 20 mm Hg. 115 In HBOT chambers, increasing pressure and oxygen concentrations translate to 10- to 20-fold increases in plasma and therefore tissue oxygen concentrations. For example, at 100% oxygen at 3 atmosphere absolutes (ATA), arterial oxygen tensions increase from 100 to 2,000 mm Hg, and tissue oxygen tensions increase from 55 to around 500 mm Hg. 116 This equates to approximately 20 times the blood oxygen content compared to atmospheric pressure, and can provide maximum support for tissue metabolism without any contribution from hemoglobin-bound oxygen. 114 117
HBOT also has a potent effect on a cellular level. It has been found to mobilize endothelial progenitor cells (EPCs) and other growth factors from bone marrow that eventually will aggregate in the wound. 112 HBOT also has potent antimicrobial effects via the generation of oxygen-free radicals, which oxidize proteins and membrane lipids, damage DNA, and inhibit bacterial metabolic functions, particularly those of anaerobic bacteria. This oxygen-free radical generation also facilitates the oxygen-dependent peroxidase system by which leukocytes kill bacteria. 118 HBOT also improves the oxygen-dependent transport of certain antibiotics across bacterial cell walls. 119
Still, the efficacy of this therapy has been questioned. A recent double-blinded trial randomized 107 diabetic patients with severe foot wounds to receiving either 30 daily sessions of 90 minutes of HBOT (breathing oxygen at 244 kPa) or sham (breathing air at 125 kPa), in addition to comprehensive wound care. 120 At 12 weeks of postrandomization, there was no statistically significant difference in major amputation rates between the two groups. 120
In the “Does Applying More Oxygen (O 2 ) Cure Lower Extremity Sores” (DAMO 2 CLES) trial, 120 patients with diabetic foot wounds were randomized to standard wound care (SC) or standard wound care plus HBOT (SC + HBOT). 121 Unfortunately, this trial was plagued by poor enrollment and high dropout rates, failing to meet its initial calculated required sample size and with only 65% of the HBOT group completing the protocol. 121 122 This resulted in varying results: when examined from a per protocol intention-to-treat analysis, no significant difference was noted in limb salvage rates between the two groups; however, among patients who actually completed the trial, the patients who received HBOT had statistically significant higher rates of limb salvage (SC group 78% vs. SC + HBOT group 95%). 121 122 Despite its criticisms, the DAMO2CLES trial does hint that many CLI patients may be unable to complete a rigorous HBOT regimen necessary to provide substantial benefit. 121 122 In summary, there remains a paucity of well-designed studies in this area, precluding the creation of societal recommendations for or against the use of HBOT in these patients.
Horizons of Wound Care: Current and Future Developments
Intermittent Pneumatic Compression:
Intermittent pneumatic compression (IPC) devices, which provide compression to the foot, calf, and other parts of the leg, have been found to increase arterial blood flow, peak systolic blood flow, as well as pulse volume. 123 Limited prospective data exist to support the widespread use of IPCs in cases of limb salvage and wound healing. 123
Dehydrated Human Amnion/Chorion Membrane
Used in wound healing for over 100 years, human amniotic membrane has been shown to provide a matrix for cellular migration and proliferation, express anti-inflammatory proteins and antibacterial properties, promote scar tissue development, and to be associated with pain reduction at the site of the wound. 124 125 126 The dehydrated amniotic and chorionic tissues retain their molecular composition and laboratory studies have shown that it is capable of inducing angiogenesis due to the presence of multiple proangiogenic factors.
Available therapies of dehydrated human amnion/chorion membrane (dHACM) allografts have been shown in observational studies and randomized-controlled trials to enhance healing of diabetic, venous, and other wounds compared to standard wound care with debridement, moist wound dressing, and compression. 103 127 128 129 130 The cost of the material is still a limitation of its use. In most cases, dHACM use is restricted to cases of poor wound healing after 4 weeks of standard wound care.
Tissue Growth Factors and micro-RNA
Topical application or intra/perilesional injection of growth factors, such as platelet-rich plasma (PRP) and recombinant human epidermal growth factor (rHEGF), after proper debridement has been suggested as another exciting prospect in the field of wound healing. These growth factors are thought to counteract the senescence of fibroblasts and other cells within an ischemic ulcer, stimulating their activation and proliferation. 131 132
Topically applied PRP has been previously used in maxillofacial and plastic surgery to regenerate damaged tissue. 133 It is obtained from autologous blood by a centrifugation process resulting in creation of low-volume plasma gel containing highly concentrated growth factors. 133 134 In addition to being biologically safe, the growth factors found in PRP stimulate production of collagen and ECM; increase the number of undifferentiated mesenchymal cells; and promote angiogenesis, macrophage activation, and mitogenesis. 134 In a study of diabetic patients undergoing transmetatarsal (TMA) amputation with PRP application versus TMA alone, those who had PRP applied for 1 month had a wound healing rate of 96.5% compared to 59.4% in the non-PRP arm. 135
The intra- or perilesional injection of rHEGF has also been shown to improve wound healing rates, with one study demonstrating complete wound healing in 77% of patients at 8 weeks compared to 56% in patients receiving placebo injection. 132 136 In a meta-analysis of RCTs using various growth factors compared to standard of care in patients with diabetic foot ulcers, rHEGF was associated with significantly higher proportion of achieving complete wound healing when compared with standard of care as well as other tissue growth factors. 137
MicroRNAs (miRs) are endogenous, noncoding RNAs of approximately 20 to 22 nucleotides. They regulate gene expression on the posttranscriptional level, with a single miR able to regulate many targets with many downstream effects. 138 Several miRs have been identified with potential downstream effects on angiogenesis induction, skeletal muscle and peripheral nerve regeneration, and atherosclerosis and vascular calcification reduction. While the precise, controlled effects of mIRs have yet to be elucidated, ghrelin, a hormone with pleiotropic effects throughout the body, has demonstrated promise in the upregulation of miRs beneficial in CLI. 139
Currently, the widespread use of tissue growth factors and miR therapy in CLI wound healing is prohibited by their cost and lack of universal approval. Perhaps with the support of larger trials currently underway, this may change in the near future.
Cell Therapy
As described earlier, the mechanisms of vascular growth and repair are often exhausted in end-stage CLI patients. Cell-based therapy seeks to bypass this state through the introduction of progenitor cells with the potential to stimulate blood vessel formation through the release of proangiogenic factors. Cells currently being studied include bone marrow mononuclear cells (BM-MNCs), mesenchymal stem cells (MSCs), adipose-derived stem cells, EPCs, human embryonic stem cells from cord blood, and induced pluripotent stem cells. 2 BM-MNCs and MSCs in particular have shown a great deal of promise in CLI.
BM-MNCs are multipotent stem cells that serve as hematopoietic progenitors. 2 They are obtained by centrifugation of bone marrow aspirates. 2 21 BM-MNCs transplantation via intramuscular or intraarterial injection has been shown to result in improved ABI scores, tissue oxygen pressure (tcPO 2 ), rest pain symptoms, wound healing, and amputation-free survival.
MSCs are also derived from bone marrow but function solely through the release of cytokines which then stimulate endothelial cells to undergo proliferation and differentiation into new blood vessels. They have been described as “biofactories that home to sites of ischemia.” 21 Both allogenic MSCs derived from healthy human donors and those derived from umbilical cord blood have been shown to be safe for patients with CLI. Because they are easy to isolate and modify and their populations can be expanded ex vivo, they have been subject of great interest. MSCs are often introduced therapeutically in conjunction with BM-MNCs, and together have shown improvements in rest pain, ABIs, collateralization, and wound healing rates. 21 140 141
There remain many unanswered questions in regard to the application of cell therapy in CLI: which subgroups of patients may benefit the most from cell therapy, at which time in the course of the disease cell therapy would be most effective, which exact cell populations give the most benefit, and what molecular signals are needed to optimize their function. 21 In addition, the coordination of cells and signals responsible for logical complete vessel regeneration remains a major challenge. The combined delivery of multiple cell lineages embedded within a bioengineered support matrix is a promising method of more closely mimicking the complex orchestra of in vivo blood vessel formation.
Previous Presentations
Parts of the manuscript were previously presented in an education poster entitled “On the Cutting Edge of Wound Care: What Every Interventional Radiologist Needs to Know” at the Society of Interventional Radiology Annual Meeting 2018, Los Angeles, CA.
References
- 1.Fowkes F GR, Rudan D, Rudan Iet al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis Lancet 2013382(9901):1329–1340. [DOI] [PubMed] [Google Scholar]
- 2.Henry J C, Peterson L A, Schlanger R E. Wound healing in peripheral arterial disease: current and future therapy. J Vasc Med Surg. 2014;02(04):157. [Google Scholar]
- 3.Yost M.PAD Costs Economics Amputation Costs Economics, Critical Limb Ischemia, Chronic Venous Disease, Venous Ulcers, Chronic Venus Insufficiency - CLI US Supplement 2016The Sage Group. Available at:http://thesagegroup.us/pages/reports/cli-us-supplement-2016.php. Accessed August 18, 2018
- 4.Noone A, Howlander N, Krapcho M, Miller D.SEER Cancer Statistics Review, 1975–2015National Cancer Institute;2018. Available at:https://seer.cancer.gov/csr/1975_2015/. Accessed August 27, 2018
- 5.Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) - Journal of Vascular Surgery. Available at:https://www.jvascsurg.org/article/S0741-5214(06)02296-8/fulltext. Accessed August 18, 2018 [DOI] [PubMed]
- 6.Menke N B, Ward K R, Witten T M, Bonchev D G, Diegelmann R F. Impaired wound healing. Clin Dermatol. 2007;25(01):19–25. doi: 10.1016/j.clindermatol.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Yost M L. Cost-benefit analysis of critical limb ischemia in the era of the Affordable Care Act: is it fiscally responsible to perform primary amputation as treatment? Endovascular Today. 2014;(05):29–36. [Google Scholar]
- 8.Goodney P P, Travis L L, Nallamothu B K et al. Variation in the use of lower extremity vascular procedures for critical limb ischemia. Circ Cardiovasc Qual Outcomes. 2012;5(01):94–102. doi: 10.1161/CIRCOUTCOMES.111.962233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eurobarometer Qualitative Study on Patient Involvement in Healthcare - European Innovation Partnership - European Commission. Available at:https://ec.europa.eu/eip/ageing/library/eurobarometer-qualitative-study-patient-involvement-healthcare_en. Published May 21,2012. Accessed August 18, 2018
- 10.Corbett L Q, Ennis W J. What do patients want? Patient preference in wound care. Adv Wound Care (New Rochelle) 2014;3(08):537–543. doi: 10.1089/wound.2013.0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Náfrádi L, Nakamoto K, Schulz P J. Is patient empowerment the key to promote adherence? A systematic review of the relationship between self-efficacy, health locus of control and medication adherence. PLoS One. 2017;12(10):e0186458. doi: 10.1371/journal.pone.0186458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koenigsberg M R, Corliss J. Diabetes self-management: facilitating lifestyle change. Am Fam Physician. 2017;96(06):362–370. [PubMed] [Google Scholar]
- 13.Kavitha K V, Tiwari S, Purandare V B, Khedkar S, Bhosale S S, Unnikrishnan A G. Choice of wound care in diabetic foot ulcer: a practical approach. World J Diabetes. 2014;5(04):546–556. doi: 10.4239/wjd.v5.i4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koenigsberg M R, Bartlett D, Cramer J S. Facilitating treatment adherence with lifestyle changes in diabetes. Am Fam Physician. 2004;69(02):309–316. [PubMed] [Google Scholar]
- 15.Mills J L., Sr The application of the Society for Vascular Surgery Wound, Ischemia, and foot Infection (WIfI) classification to stratify amputation risk. J Vasc Surg. 2017;65(03):591–593. doi: 10.1016/j.jvs.2016.12.090. [DOI] [PubMed] [Google Scholar]
- 16.Mills J L, Sr, Conte M S, Armstrong D Get al. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on wound, ischemia, and foot infection (WIfI) J Vasc Surg 20145901220–340., 2 [DOI] [PubMed] [Google Scholar]
- 17.Martin P.Wound healing--aiming for perfect skin regeneration Science 1997276(5309):75–81. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez A de O, Costa T F, Andrade Z A, Medrado A R. Wound healing - a literature review. An Bras Dermatol. 2016;91(05):614–620. doi: 10.1590/abd1806-4841.20164741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tidball J G. Mechanisms of muscle injury, repair, and regeneration. Compr Physiol. 2011;1(04):2029–2062. doi: 10.1002/cphy.c100092. [DOI] [PubMed] [Google Scholar]
- 20.Mendonça R J, Coutinho-Netto J. Cellular aspects of wound healing. An Bras Dermatol. 2009;84(03):257–262. doi: 10.1590/s0365-05962009000300007. [DOI] [PubMed] [Google Scholar]
- 21.Qadura M, Terenzi D C, Verma S, Al-Omran M, Hess D A. Concise review: cell therapy for critical limb ischemia: an integrated review of preclinical and clinical studies. Stem Cells. 2018;36(02):161–171. doi: 10.1002/stem.2751. [DOI] [PubMed] [Google Scholar]
- 22.Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro-oncol. 2005;7(04):452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sood A, Granick M S, Tomaselli N L. Wound dressings and comparative effectiveness data. Adv Wound Care (New Rochelle) 2014;3(08):511–529. doi: 10.1089/wound.2012.0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Midwood K S, Williams L V, Schwarzbauer J E. Tissue repair and the dynamics of the extracellular matrix. Int J Biochem Cell Biol. 2004;36(06):1031–1037. doi: 10.1016/j.biocel.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Stadelmann W K, Digenis A G, Tobin G R.Physiology and healing dynamics of chronic cutaneous wounds Am J Surg 1998176(2A, Suppl):26S–38S. [DOI] [PubMed] [Google Scholar]
- 26.Gould L J, Leong M, Sonstein J, Wilson S. Optimization and validation of an ischemic wound model. Wound Repair Regen. 2005;13(06):576–582. doi: 10.1111/j.1524-475X.2005.00080.x. [DOI] [PubMed] [Google Scholar]
- 27.Medina A, Scott P G, Ghahary A, Tredget E E. Pathophysiology of chronic nonhealing wounds. J Burn Care Rehabil. 2005;26(04):306–319. doi: 10.1097/01.bcr.0000169887.04973.3a. [DOI] [PubMed] [Google Scholar]
- 28.Penn J W, Grobbelaar A O, Rolfe K J. The role of the TGF-β family in wound healing, burns and scarring: a review. Int J Burns Trauma. 2012;2(01):18–28. [PMC free article] [PubMed] [Google Scholar]
- 29.Mustoe T A, O'Shaughnessy K, Kloeters O.Chronic wound pathogenesis and current treatment strategies: a unifying hypothesis Plast Reconstr Surg 2006117(7, Suppl):35S–41S. [DOI] [PubMed] [Google Scholar]
- 30.Hirsch A T, Treat-Jacobson D, Lando H A, Hatsukami D K. The role of tobacco cessation, antiplatelet and lipid-lowering therapies in the treatment of peripheral arterial disease. Vasc Med. 1997;2(03):243–251. doi: 10.1177/1358863X9700200314. [DOI] [PubMed] [Google Scholar]
- 31.Jonason T, Bergström R. Cessation of smoking in patients with intermittent claudication. Effects on the risk of peripheral vascular complications, myocardial infarction and mortality. Acta Med Scand. 1987;221(03):253–260. [PubMed] [Google Scholar]
- 32.Lassila R, Lepäntalo M.Cigarette smoking and the outcome after lower limb arterial surgery Acta Chir Scand 1988154(11-12):635–640. [PubMed] [Google Scholar]
- 33.Faulkner K W, House A K, Castleden W M. The effect of cessation of smoking on the accumulative survival rates of patients with symptomatic peripheral vascular disease. Med J Aust. 1983;1(05):217–219. doi: 10.5694/j.1326-5377.1983.tb99395.x. [DOI] [PubMed] [Google Scholar]
- 34.2011 ACCF/AHA Focused Update of the Guideline for the Management of Patients With Peripheral Artery Disease (Updating the 2005 Guideline)Available at:http://circ.ahajournals.org/content/124/18/2020.full. Accessed January 13, 2016 [DOI] [PubMed]
- 35.Spangler E L, Goodney P P. Smoking cessation strategies in vascular surgery. Semin Vasc Surg. 2015;28(02):80–85. doi: 10.1053/j.semvascsurg.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;5(05):CD009329. doi: 10.1002/14651858.CD009329.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stead L F, Koilpillai P, Lancaster T. Additional behavioural support as an adjunct to pharmacotherapy for smoking cessation. Cochrane Database Syst Rev. 2015;10(10):CD009670. doi: 10.1002/14651858.CD009670.pub3. [DOI] [PubMed] [Google Scholar]
- 38.Rooke T W, Hirsch A T, Misra Set al. 2011 ACCF/AHA Focused Update of the Guideline for the Management of Patients With Peripheral Artery Disease (Updating the 2005 Guideline)Available at:http://circ.ahajournals.org. Published February 5,2016. Accessed February 5, 2016
- 39.Adler A I, Stevens R J, Neil A, Stratton I M, Boulton A JM, Holman R R. UKPDS 59: hyperglycemia and other potentially modifiable risk factors for peripheral vascular disease in type 2 diabetes. Diabetes Care. 2002;25(05):894–899. doi: 10.2337/diacare.25.5.894. [DOI] [PubMed] [Google Scholar]
- 40.Zhou Z-Y, Liu Y-K, Chen H-L, Yang H-L, Liu F. HbA1c and lower extremity amputation risk in patients with diabetes: a meta-analysis. Int J Low Extrem Wounds. 2015;14(02):168–177. doi: 10.1177/1534734615593190. [DOI] [PubMed] [Google Scholar]
- 41.Christman A L, Selvin E, Margolis D J, Lazarus G S, Garza L A. Hemoglobin A1c predicts healing rate in diabetic wounds. J Invest Dermatol. 2011;131(10):2121–2127. doi: 10.1038/jid.2011.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mohler E R, III, Hiatt W R, Creager M A. Cholesterol reduction with atorvastatin improves walking distance in patients with peripheral arterial disease. Circulation. 2003;108(12):1481–1486. doi: 10.1161/01.CIR.0000090686.57897.F5. [DOI] [PubMed] [Google Scholar]
- 43.Kumbhani D J, Steg P G, Cannon C P et al. Statin therapy and long-term adverse limb outcomes in patients with peripheral artery disease: insights from the REACH registry. Eur Heart J. 2014;35(41):2864–2872. doi: 10.1093/eurheartj/ehu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schanzer A, Hevelone N, Owens C D, Beckman J A, Belkin M, Conte M S. Statins are independently associated with reduced mortality in patients undergoing infrainguinal bypass graft surgery for critical limb ischemia. J Vasc Surg. 2008;47(04):774–781. doi: 10.1016/j.jvs.2007.11.056. [DOI] [PubMed] [Google Scholar]
- 45.Westin G G, Armstrong E J, Bang H et al. Association between statin medications and mortality, major adverse cardiovascular event, and amputation-free survival in patients with critical limb ischemia. J Am Coll Cardiol. 2014;63(07):682–690. doi: 10.1016/j.jacc.2013.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stone N J, Robinson J G, Lichtenstein A H et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25) 02:S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 47.Soga Y, Iida O, Hirano K et al. Impact of cilostazol after endovascular treatment for infrainguinal disease in patients with critical limb ischemia. J Vasc Surg. 2011;54(06):1659–1667. doi: 10.1016/j.jvs.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 48.Soga Y, Iida O, Kawasaki D, Hirano K, Yamaoka T, Suzuki K. Impact of cilostazol on angiographic restenosis after balloon angioplasty for infrapopliteal artery disease in patients with critical limb ischemia. Eur J Vasc Endovasc Surg. 2012;44(06):577–581. doi: 10.1016/j.ejvs.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 49.Stewart K J, Hiatt W R, Regensteiner J G, Hirsch A T. Exercise training for claudication. N Engl J Med. 2002;347(24):1941–1951. doi: 10.1056/NEJMra021135. [DOI] [PubMed] [Google Scholar]
- 50.Tompra N, Foster C, Sanchis-Gomar F, de Koning J J, Lucia A, Emanuele E. Upper versus lower limb exercise training in patients with intermittent claudication: a systematic review. Atherosclerosis. 2015;239(02):599–606. doi: 10.1016/j.atherosclerosis.2015.02.038. [DOI] [PubMed] [Google Scholar]
- 51.Wound Debridement Options. The 5 Major Methods. WoundSource. Available at:http://www.woundsource.com/blog/wound-debridement-options-5-major-methods. Published April 19,2018. Accessed August 14, 2018
- 52.Chambers L, Woodrow S, Brown A P et al. Degradation of extracellular matrix components by defined proteinases from the greenbottle larva Lucilia sericata used for the clinical debridement of non-healing wounds. Br J Dermatol. 2003;148(01):14–23. doi: 10.1046/j.1365-2133.2003.04935.x. [DOI] [PubMed] [Google Scholar]
- 53.Mumcuoglu K Y, Miller J, Mumcuoglu M, Friger M, Tarshis M. Destruction of bacteria in the digestive tract of the maggot of Lucilia sericata (Diptera: Calliphoridae) J Med Entomol. 2001;38(02):161–166. doi: 10.1603/0022-2585-38.2.161. [DOI] [PubMed] [Google Scholar]
- 54.Gottrup F, Jørgensen B. Maggot debridement: an alternative method for debridement. Eplasty. 2011;11:e33. [PMC free article] [PubMed] [Google Scholar]
- 55.Advisor W C.Debridement options: BEAMS made easy. Wound Care AdvisorAvailable at:https://woundcareadvisor.com/debridement-options-beams-made-easy_vol2-no/. Published March 25, 2013. Accessed August 17, 2018
- 56.Mechanical Debridement | Wound Debridement Techniques. WoundEducators.com | Online Wound Care Certification Courses. Available at:https://woundeducators.com/wound-debridement-techniques-3-mechanical-debridement/. Published June 16,2012. Accessed August 17, 2018
- 57.Ayello E A, Cuddigan J, Kerstein M D. Skip the knife: debriding wounds without surgery. Nursing. 2002;32(09):58–63, quiz 64. doi: 10.1097/00152193-200209000-00039. [DOI] [PubMed] [Google Scholar]
- 58.Utsunomiya M, Nakamura M, Nakanishi M et al. Impact of wound blush as an angiographic end point of endovascular therapy for patients with critical limb ischemia. J Vasc Surg. 2012;55(01):113–121. doi: 10.1016/j.jvs.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 59.Khan M UN, Lall P, Harris L M, Dryjski M L, Dosluoglu H H.Predictors of limb loss despite a patent endovascular-treated arterial segment J Vasc Surg 200949061440–1445., discussion 1445–1446 [DOI] [PubMed] [Google Scholar]
- 60.Meyer A, Goller K, Horch R E et al. Results of combined vascular reconstruction and free flap transfer for limb salvage in patients with critical limb ischemia. J Vasc Surg. 2015;61(05):1239–1248. doi: 10.1016/j.jvs.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 61.Blevins W A, Jr, Schneider P A. Endovascular management of critical limb ischemia. Eur J Vasc Endovasc Surg. 2010;39(06):756–761. doi: 10.1016/j.ejvs.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 62.Rother U, Lang W. Noninvasive measurements of tissue perfusion in critical limb ischemia. Gefasschirurgie. 2018;23 01:8–12. doi: 10.1007/s00772-018-0368-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.HyperMed Imaging, Inc.Announces CE Mark for New HyperViewTM ProductAvailable at:https://www.businesswire.com/news/home/20180402005574/en/HyperMed-Imaging-Announces-CE-Mark-New-HyperView%E2%84%A2. Published April 2, 2018. Accessed August 21, 2018.
- 64.Neville R, Gupta S. Establishment of normative perfusion values using hyperspectral tissue oxygenation mapping technology. Vascular Disease Management. 2009;6(06):156–161. [Google Scholar]
- 65.Chiang N, Jain J K, Sleigh J, Vasudevan T. Evaluation of hyperspectral imaging technology in patients with peripheral vascular disease. J Vasc Surg. 2017;66(04):1192–1201. doi: 10.1016/j.jvs.2017.02.047. [DOI] [PubMed] [Google Scholar]
- 66.Nouvong A, Hoogwerf B, Mohler E, Davis B, Tajaddini A, Medenilla E. Evaluation of diabetic foot ulcer healing with hyperspectral imaging of oxyhemoglobin and deoxyhemoglobin. Diabetes Care. 2009;32(11):2056–2061. doi: 10.2337/dc08-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smith A M, Mancini M C, Nie S. Bioimaging: second window for in vivo imaging. Nat Nanotechnol. 2009;4(11):710–711. doi: 10.1038/nnano.2009.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marshall M V, Rasmussen J C, Tan I-C et al. Near-infrared fluorescence imaging in humans with indocyanine green: a review and update. Open Surg Oncol J. 2010;2(02):12–25. doi: 10.2174/1876504101002010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bajwa A, Wesolowski R, Patel A et al. Assessment of tissue perfusion in the lower limb: current methods and techniques under development. Circ Cardiovasc Imaging. 2014;7(05):836–843. doi: 10.1161/CIRCIMAGING.114.002123. [DOI] [PubMed] [Google Scholar]
- 70.Braun J D, Trinidad-Hernandez M, Perry D, Armstrong D G, Mills J L., Sr Early quantitative evaluation of indocyanine green angiography in patients with critical limb ischemia. J Vasc Surg. 2013;57(05):1213–1218. doi: 10.1016/j.jvs.2012.10.113. [DOI] [PubMed] [Google Scholar]
- 71.Settembre N, Kauhanen P, Albäck A, Spillerova K, Venermo M. Quality control of the foot revascularization using indocyanine green fluorescence imaging. World J Surg. 2017;41(07):1919–1926. doi: 10.1007/s00268-017-3950-6. [DOI] [PubMed] [Google Scholar]
- 72.Patel H M, Bulsara S S, Banerjee S et al. Indocyanine green angiography to prognosticate healing of foot ulcer in critical limb ischemia: a novel technique. Ann Vasc Surg. 2018;51:86–94. doi: 10.1016/j.avsg.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 73.Igari K, Kudo T, Toyofuku T, Jibiki M, Inoue Y, Kawano T. Quantitative evaluation of the outcomes of revascularization procedures for peripheral arterial disease using indocyanine green angiography. Eur J Vasc Endovasc Surg. 2013;46(04):460–465. doi: 10.1016/j.ejvs.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 74.Igari K, Kudo T, Uchiyama H, Toyofuku T, Inoue Y. Intraarterial injection of indocyanine green for evaluation of peripheral blood circulation in patients with peripheral arterial disease. Ann Vasc Surg. 2014;28(05):1280–1285. doi: 10.1016/j.avsg.2013.12.036. [DOI] [PubMed] [Google Scholar]
- 75.Benitez E, Sumpio B J, Chin J, Sumpio B E. Contemporary assessment of foot perfusion in patients with critical limb ischemia. Semin Vasc Surg. 2014;27(01):3–15. doi: 10.1053/j.semvascsurg.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 76.Eneroth M, Larsson J, Apelqvist J.Deep foot infections in patients with diabetes and foot ulcer: an entity with different characteristics, treatments, and prognosis J Diabetes Complications 199913(5-6):254–263. [DOI] [PubMed] [Google Scholar]
- 77.Hill S L, Holtzman G I, Buse R. The effects of peripheral vascular disease with osteomyelitis in the diabetic foot. Am J Surg. 1999;177(04):282–286. doi: 10.1016/s0002-9610(99)00050-1. [DOI] [PubMed] [Google Scholar]
- 78.Giurato L, Meloni M, Izzo V, Uccioli L. Osteomyelitis in diabetic foot: a comprehensive overview. World J Diabetes. 2017;8(04):135–142. doi: 10.4239/wjd.v8.i4.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grayson M L, Gibbons G W, Balogh K, Levin E, Karchmer A W. Probing to bone in infected pedal ulcers. A clinical sign of underlying osteomyelitis in diabetic patients. JAMA. 1995;273(09):721–723. [PubMed] [Google Scholar]
- 80.Lee Y J, Sadigh S, Mankad K, Kapse N, Rajeswaran G. The imaging of osteomyelitis. Quant Imaging Med Surg. 2016;6(02):184–198. doi: 10.21037/qims.2016.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Palestro C J. FDG-PET in musculoskeletal infections. Semin Nucl Med. 2013;43(05):367–376. doi: 10.1053/j.semnuclmed.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 82.Lavery L A, Armstrong D G, Peters E JG, Lipsky B A. Probe-to-bone test for diagnosing diabetic foot osteomyelitis: reliable or relic? Diabetes Care. 2007;30(02):270–274. doi: 10.2337/dc06-1572. [DOI] [PubMed] [Google Scholar]
- 83.Braddock M. Euroconference on tissue repair and ulcer/wound healing: molecular mechanisms, therapeutic targets and future directions. Expert Opin Investig Drugs. 2005;14(06):743–749. doi: 10.1517/13543784.14.6.743. [DOI] [PubMed] [Google Scholar]
- 84.Snyder R J. Treatment of nonhealing ulcers with allografts. Clin Dermatol. 2005;23(04):388–395. doi: 10.1016/j.clindermatol.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 85.Taylor J E, Laity P R, Hicks J et al. Extent of iron pick-up in deforoxamine-coupled polyurethane materials for therapy of chronic wounds. Biomaterials. 2005;26(30):6024–6033. doi: 10.1016/j.biomaterials.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 86.Sheehan P, Jones P, Caselli A, Giurini J M, Veves A. Percent change in wound area of diabetic foot ulcers over a 4-week period is a robust predictor of complete healing in a 12-week prospective trial. Diabetes Care. 2003;26(06):1879–1882. doi: 10.2337/diacare.26.6.1879. [DOI] [PubMed] [Google Scholar]
- 87.Snyder R J, Cardinal M, Dauphinée D M, Stavosky J. A post-hoc analysis of reduction in diabetic foot ulcer size at 4 weeks as a predictor of healing by 12 weeks. Ostomy Wound Manage. 2010;56(03):44–50. [PubMed] [Google Scholar]
- 88.Gist S, Tio-Matos I, Falzgraf S, Cameron S, Beebe M. Wound care in the geriatric client. Clin Interv Aging. 2009;4:269–287. doi: 10.2147/cia.s4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Edwards J V, Howley P, Cohen I K.In vitro inhibition of human neutrophil elastase by oleic acid albumin formulations from derivatized cotton wound dressings Int J Pharm 2004284(1-2):1–12. [DOI] [PubMed] [Google Scholar]
- 90.Schönfelder U, Abel M, Wiegand C, Klemm D, Elsner P, Hipler U-C. Influence of selected wound dressings on PMN elastase in chronic wound fluid and their antioxidative potential in vitro. Biomaterials. 2005;26(33):6664–6673. doi: 10.1016/j.biomaterials.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 91.Snyder R J, Ead J, Glick B, Cuffy C. Dehydrated human amnion/chorion membrane as adjunctive therapy in the multidisciplinary treatment of pyoderma gangrenosum: a case report. Ostomy Wound Manage. 2015;61(09):40–49. [PubMed] [Google Scholar]
- 92.Vazales R, Constant D, Snyder R J. A rare case of aggressive digital adenocarcinoma of the lower extremity, masquerading as an ulcerative lesion that clinically favored benignancy. Healthcare (Basel) 2014;2(03):315–323. doi: 10.3390/healthcare2030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tang J C, Vivas A, Rey A, Kirsner R S, Romanelli P.Atypical ulcers: wound biopsy results from a university wound pathology service Ostomy Wound Manage 2012580620–22., 24, 26–29 [PubMed] [Google Scholar]
- 94.Forrest R D. Early history of wound treatment. J R Soc Med. 1982;75(03):198–205. doi: 10.1177/014107688207500310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wodash A J. Wet-to-dry dressings do not provide moist wound healing. J Am Coll Clin Wound Spec. 2013;4(03):63–66. doi: 10.1016/j.jccw.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dale B A, Wright D H. Say goodbye to wet-to-dry wound care dressings: changing the culture of wound care management within your agency. Home Healthc Nurse. 2011;29(07):429–440. doi: 10.1097/NHH.0b013e31821b726e. [DOI] [PubMed] [Google Scholar]
- 97.Khan T, Shin L, Woelfel S, Rowe V, Wilson B L, Armstrong D G. Building a scalable diabetic limb preservation program: four steps to success. Diabet Foot Ankle. 2018;9(01):1.452513E6. doi: 10.1080/2000625X.2018.1452513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kinlay S. Management of critical limb ischemia. Circ Cardiovasc Interv. 2016;9(02):e001946. doi: 10.1161/CIRCINTERVENTIONS.115.001946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Boghossian J A, Miller J D, Armstrong D G. Offloading the diabetic foot: toward healing wounds and extending ulcer-free days in remission. Chronic Wound Care Management and Research. 2017;4:83–88. [Google Scholar]
- 100.Fernando M E, Crowther R G, Lazzarini P A et al. Plantar pressures are higher in cases with diabetic foot ulcers compared to controls despite a longer stance phase duration. BMC Endocr Disord. 2016;16(01):51. doi: 10.1186/s12902-016-0131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Crews R.Diabetes: Improving foot care complianceLower Extremity Review Magazine. October 2009. Available:http://lermagazine.com/article/diabetes-improving-foot-care-compliance. Accessed August 20, 2018
- 102.Bus S A, van Deursen R W, Armstrong D G, Lewis J E, Caravaggi C F, Cavanagh P R; International Working Group on the Diabetic Foot.Footwear and offloading interventions to prevent and heal foot ulcers and reduce plantar pressure in patients with diabetes: a systematic review Diabetes Metab Res Rev 2016320199–118. [DOI] [PubMed] [Google Scholar]
- 103.Armstrong D G, Boulton A J. Activity monitors: should we begin dosing activity as we dose a drug? J Am Podiatr Med Assoc. 2001;91(03):152–153. doi: 10.7547/87507315-91-3-152. [DOI] [PubMed] [Google Scholar]
- 104.Cavanagh P R, Bus S A. Off-loading the diabetic foot for ulcer prevention and healing. Plast Reconstr Surg. 2011;127 01:248S–256S. doi: 10.1097/PRS.0b013e3182024864. [DOI] [PubMed] [Google Scholar]
- 105.Brem H, Sheehan P, Boulton A JM.Protocol for treatment of diabetic foot ulcers Am J Surg 2004187(5A):1S–10S. [DOI] [PubMed] [Google Scholar]
- 106.Peters E JG, Armstrong D G, Lavery L A. Risk factors for recurrent diabetic foot ulcers: site matters. Diabetes Care. 2007;30(08):2077–2079. doi: 10.2337/dc07-0445. [DOI] [PubMed] [Google Scholar]
- 107.Uccioli L, Faglia E, Monticone G et al. Manufactured shoes in the prevention of diabetic foot ulcers. Diabetes Care. 1995;18(10):1376–1378. doi: 10.2337/diacare.18.10.1376. [DOI] [PubMed] [Google Scholar]
- 108.Wrobel J S, Ammanath P, Le T et al. A novel shear reduction insole effect on the thermal response to walking stress, balance, and gait. J Diabetes Sci Technol. 2014;8(06):1151–1156. doi: 10.1177/1932296814546528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Healy A, Naemi R, Chockalingam N. The effectiveness of footwear and other removable off-loading devices in the treatment of diabetic foot ulcers: a systematic review. Curr Diabetes Rev. 2014;10(04):215–230. doi: 10.2174/1573399810666140918121438. [DOI] [PubMed] [Google Scholar]
- 110.Najafi B, Mohseni H, Grewal G S, Talal T K, Menzies R A, Armstrong D G. An optical-fiber-based smart textile (Smart Socks) to manage biomechanical risk factors associated with diabetic foot amputation. J Diabetes Sci Technol. 2017;11(04):668–677. doi: 10.1177/1932296817709022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ventola C L. Medical applications for 3D printing: current and projected uses. P&T. 2014;39(10):704–711. [PMC free article] [PubMed] [Google Scholar]
- 112.Sunkari V G, Lind F, Botusan I R et al. Hyperbaric oxygen therapy activates hypoxia-inducible factor 1 (HIF-1), which contributes to improved wound healing in diabetic mice. Wound Repair Regen. 2015;23(01):98–103. doi: 10.1111/wrr.12253. [DOI] [PubMed] [Google Scholar]
- 113.Gill A L, Bell C NA. Hyperbaric oxygen: its uses, mechanisms of action and outcomes. QJM. 2004;97(07):385–395. doi: 10.1093/qjmed/hch074. [DOI] [PubMed] [Google Scholar]
- 114.Gabb G, Robin E D. Hyperbaric oxygen. A therapy in search of diseases. Chest. 1987;92(06):1074–1082. doi: 10.1378/chest.92.6.1074. [DOI] [PubMed] [Google Scholar]
- 115.Bhutani S, Vishwanath G. Hyperbaric oxygen and wound healing. Indian J Plast Surg. 2012;45(02):316–324. doi: 10.4103/0970-0358.101309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tibbles P M, Edelsberg J S. Hyperbaric-oxygen therapy. N Engl J Med. 1996;334(25):1642–1648. doi: 10.1056/NEJM199606203342506. [DOI] [PubMed] [Google Scholar]
- 117.Leach R M, Rees P J, Wilmshurst P.Hyperbaric oxygen therapy BMJ 1998317(7166):1140–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Knighton D R, Halliday B, Hunt T K. Oxygen as an antibiotic. The effect of inspired oxygen on infection. Arch Surg. 1984;119(02):199–204. doi: 10.1001/archsurg.1984.01390140057010. [DOI] [PubMed] [Google Scholar]
- 119.Mader J, Adam K, Couch L.Potentiation of tobramycin by hyperbaric oxygen in experimental Pseudomonas aeruginosa osteomyelitis (Abstract 1331)Presented at the: American Society for Microbiology: the 27th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, DC;1987
- 120.Fedorko L, Bowen J M, Jones W et al. Hyperbaric oxygen therapy does not reduce indications for amputation in patients with diabetes with nonhealing ulcers of the lower limb: a prospective, double-blind, randomized controlled clinical trial. Diabetes Care. 2016;39(03):392–399. doi: 10.2337/dc15-2001. [DOI] [PubMed] [Google Scholar]
- 121.Santema K TB, Stoekenbroek R M, Koelemay M JW et al. Hyperbaric oxygen therapy in the treatment of ischemic lower- extremity ulcers in patients with diabetes: results of the DAMO 2 CLES multicenter randomized clinical trial . Diabetes Care. 2018;41(01):112–119. doi: 10.2337/dc17-0654. [DOI] [PubMed] [Google Scholar]
- 122.Huang E. Comment on Santema et al. Hyperbaric oxygen therapy in the treatment of ischemic lower-extremity ulcers in patients with diabetes: results of the DAMO 2 CLES multicenter randomized clinical trial. diabetes care 2018;41:112-119 . Diabetes Care. 2018;41(04):e61. doi: 10.2337/dc17-2440. [DOI] [PubMed] [Google Scholar]
- 123.Moran P S, Teljeur C, Harrington P, Ryan M. A systematic review of intermittent pneumatic compression for critical limb ischaemia. Vasc Med. 2015;20(01):41–50. doi: 10.1177/1358863X14552096. [DOI] [PubMed] [Google Scholar]
- 124.Fetterolf D E, Snyder R J. Scientific and clinical support for the use of dehydrated amniotic membrane in wound management. Wounds. 2012;24(10):299–307. [PubMed] [Google Scholar]
- 125.Kesting M R, Wolff K-D, Hohlweg-Majert B, Steinstraesser L. The role of allogenic amniotic membrane in burn treatment. J Burn Care Res. 2008;29(06):907–916. doi: 10.1097/BCR.0b013e31818b9e40. [DOI] [PubMed] [Google Scholar]
- 126.Koob T J, Lim J J, Zabek N, Massee M. Cytokines in single layer amnion allografts compared to multilayer amnion/chorion allografts for wound healing. J Biomed Mater Res B Appl Biomater. 2015;103(05):1133–1140. doi: 10.1002/jbm.b.33265. [DOI] [PubMed] [Google Scholar]
- 127.Angiogenic properties of dehydrated human amnion/chorion allografts: therapeutic potential for soft tissue repair and regeneration | Vascular CellAvailable at:https://vascularcell.com/index.php/vc/article/view/10.1186-2045-824X-6-10. Accessed August 28, 2018 [DOI] [PMC free article] [PubMed]
- 128.Serena T E, Carter M J, Le L T, Sabo M J, DiMarco D T; EpiFix VLU Study Group.A multicenter, randomized, controlled clinical trial evaluating the use of dehydrated human amnion/chorion membrane allografts and multilayer compression therapy vs. multilayer compression therapy alone in the treatment of venous leg ulcers Wound Repair Regen 20142206688–693. [DOI] [PubMed] [Google Scholar]
- 129.Zelen C M, Snyder R J, Serena T E, Li W W. The use of human amnion/chorion membrane in the clinical setting for lower extremity repair: a review. Clin Podiatr Med Surg. 2015;32(01):135–146. doi: 10.1016/j.cpm.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 130.Zelen C M, Serena T E, Denoziere G, Fetterolf D E. A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int Wound J. 2013;10(05):502–507. doi: 10.1111/iwj.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Loots M A, Lamme E N, Mekkes J R, Bos J D, Middelkoop E.Cultured fibroblasts from chronic diabetic wounds on the lower extremity (non-insulin-dependent diabetes mellitus) show disturbed proliferation Arch Dermatol Res 1999291(2-3):93–99. [DOI] [PubMed] [Google Scholar]
- 132.Berlanga J, Fernández J I, López E et al. Heberprot-P: a novel product for treating advanced diabetic foot ulcer. MEDICC Rev. 2013;15(01):11–15. doi: 10.37757/MR2013V15.N1.4. [DOI] [PubMed] [Google Scholar]
- 133.Marx R E. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62(04):489–496. doi: 10.1016/j.joms.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 134.Massara M, Barillà D, De Caridi Get al. Application of autologous platelet-rich plasma to enhance wound healing after lower limb revascularization: a case series and literature review Semin Vasc Surg 201528(3-4):195–200. [DOI] [PubMed] [Google Scholar]
- 135.Serra R, Buffone G, Dominijanni A, Molinari V, Montemurro R, de Franciscis S. Application of platelet-rich gel to enhance healing of transmetatarsal amputations in diabetic dysvascular patients. Int Wound J. 2013;10(05):612–615. doi: 10.1111/iwj.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fernández-Montequín J I, Valenzuela-Silva C M, Díaz O G et al. Intra-lesional injections of recombinant human epidermal growth factor promote granulation and healing in advanced diabetic foot ulcers: multicenter, randomised, placebo-controlled, double-blind study. Int Wound J. 2009;6(06):432–443. doi: 10.1111/j.1742-481X.2009.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sridharan K, Sivaramakrishnan G. Growth factors for diabetic foot ulcers: mixed treatment comparison analysis of randomized clinical trials. Br J Clin Pharmacol. 2018;84(03):434–444. doi: 10.1111/bcp.13470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Caporali A, Emanueli C. MicroRNA regulation in angiogenesis. Vascul Pharmacol. 2011;55(04):79–86. doi: 10.1016/j.vph.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 139.Neale J PH, Pearson J T, Katare R, Schwenke D O. Ghrelin, MicroRNAs, and critical limb ischemia: hungering for a novel treatment option. Front Endocrinol (Lausanne) 2017;8:350. doi: 10.3389/fendo.2017.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lu D, Chen B, Liang Z et al. Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: a double-blind, randomized, controlled trial. Diabetes Res Clin Pract. 2011;92(01):26–36. doi: 10.1016/j.diabres.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 141.Gupta P K, Chullikana A, Parakh R et al. A double blind randomized placebo controlled phase I/II study assessing the safety and efficacy of allogeneic bone marrow derived mesenchymal stem cell in critical limb ischemia. J Transl Med. 2013;11:143. doi: 10.1186/1479-5876-11-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Andersen C A, Roukis T S.The diabetic foot Surg Clin North Am 200787051149–1177., x [DOI] [PubMed] [Google Scholar]
- 143.Ramirez A T, Soroff H S, Schwartz M S, Mooty J, Pearson E, Raben M S. Experimental wound healing in man. Surg Gynecol Obstet. 1969;128(02):283–293. [PubMed] [Google Scholar]
- 144.Flanagan M. Improving accuracy of wound measurement in clinical practice. Ostomy Wound Manage. 2003;49(10):28–40. [PubMed] [Google Scholar]
- 145.Günes U Y. A prospective study evaluating the Pressure Ulcer Scale for Healing (PUSH Tool) to assess stage II, stage III, and stage IV pressure ulcers. Ostomy Wound Manage. 2009;55(05):48–52. [PubMed] [Google Scholar]
- 146.Margolis D J, Berlin J A, Strom B L. Which venous leg ulcers will heal with limb compression bandages? Am J Med. 2000;109(01):15–19. doi: 10.1016/s0002-9343(00)00379-x. [DOI] [PubMed] [Google Scholar]


