Abstract
The long-standing challenge in the treatment of prostate cancer is to overcome therapeutic resistance during progression to lethal disease. Aberrant transforming-growth factor-β (TGF-β) signaling accelerates prostate tumor progression in a transgenic mouse model via effects on epithelial-mesenchymal transition (EMT), and neuroendocrine differentiation driving tumor progression to castration-resistant prostate cancer (CRPC). Neuroendocrine prostate cancer (NEPC) is highly aggressive exhibiting reactivation of developmental programs associated with EMT induction and stem cell-like characteristics. The androgen receptor (AR) is a critical driver of tumor progression as well as therapeutic response in patients with metastatic CRPC. The signaling interactions between the TGF-β mechanistic network and AR axis impact the EMT phenotypic conversions, and perturbation of epithelial homeostasis via EMT renders a critical venue for epithelial derived tumors to become invasive, acquire the neuroendocrine phenotype, and rapidly metastasize. Combinations of microtubule targeting taxane chemotherapy and androgen/AR targeting therapies have survival benefits in CRPC patients, but therapeutic resistance invariability develops, leading to mortality. Compelling evidence from our group recently demonstrated that chemotherapy (cabazitaxel, second line taxane chemotherapy), or TGF-β receptor signaling targeted therapy, caused reversion of EMT to mesenchymal-epithelial transition and tumor re-differentiation, in in vitro and in vivo prostate cancer models. In this review, we discuss the functional contribution of EMT dynamic changes to the development of the neuroendocrine phenotype—the newly characterized pathological feature of prostate tumors in the context of the tumor microenvironment-navigated cell lineage changes and the role of this neuroendocrine phenotype in metastatic progression and therapeutic resistance.
Keywords: Neuroendocrine differentiation, Cell polarity, Prostate cancer, Androgen deprivation therapy, Epithelial-mesenchymal transition
1. Introduction
Prostate cancer is the second most common cause of death of men in the United States. In 2018, it is estimated that 164 690 new cases of prostate cancer will be diagnosed [1]. Androgen deprivation therapy (ADT) is the standard first line systemic treatment for men with advanced metastatic prostate cancer. ADT attempts to abrogate critical androgen receptor (AR)-mediated growth but many patients develop castration-resistant prostate cancer (CRPC) [2]. Targeting androgen signaling (anti-androgens) and microtubules (taxane chemotherapy) has survival benefits for patients with metastatic CRPC (mCRPC), but therapeutic resistance develops, resulting in lethal disease [3], [4], [5]. This resistance results from the addiction of CRPC cells to AR signaling and constitutive activation of AR splice variants [6], [7], [8]. A comprehensive epidemiology-based review of the literature by Kirby et al. [9] revealed that 10%–20% of patients with prostate cancer on ADT will develop CRPC within 5 years. Hence, there has been a controversial debate surrounding the efficacy of ADT as an effective therapy for the treatment of advanced disease with persistent AR signaling or activating alterations in the AR signaling axis. The treatment options currently available for patients with advanced cancer is second generation anti-androgens, taxane-based chemotherapy, Radium-223 and Sipuleucel-T [10]. Our team first provided ground-breaking evidence (confirmed by other investigators) that taxane chemotherapy, in addition to targeting microtubule integrity and stabilization, exerts antitumor effects by impairing AR transport along the microtubules, resulting in AR cytoplasmic sequestration and inhibition of AR activity in clinical prostate cancer [11], [12], [13], [14].
A rapidly growing body of work has demonstrated that terminally differentiated cells can be reprogrammed into acquiring pluripotent stem cell characteristics, by expressing the relevant transcription factors towards transdifferentiation, epithelial–mesenchymal transition (EMT) and mesenchymal–epithelial transition (MET) [15], [16], [17]. The cellular process of transdifferentiation is regulated by close associations between EMT and the neuroendocrine phenotype [18]. Neuroendocrine phenotype characteristics of prostate tumors have recently been identified as an active dynamic component of prostate cancer progression [16], [17], [18]. Neuroendocrine tumors have been pathologically well characterized in human clinical disease, primarily as these neuroendocrine prostate cancers (NEPCs) can arise either de novo or as an adaptive response to ADT [16]. In normal cells, the neuroendocrine phenotype may play a role in regulating growth and differentiation of epithelia. However, the neuroendocrine phenotype presents itself in cancer as more aggressive pathological feature, indicating poor clinical outcomes relative to primary neuroendocrine cancers from other organ systems [19]. Although only about 2% of organ-confined prostate cancer are classified as having NEPC, the features associated with the neuroendocrine phenotype are presented in at least 10% of patients [20], [21]. However, due to a general lack of biopsy diagnoses for advanced disease, this may underrepresent the frequency of NEPC. The National Comprehensive Cancer Network (NCCN) guidelines outline critical distinctions associated with NEPC [22]. These prostate cancer patients have poorly differentiated tumors (Gleason score 9–10) and respond poorly to ADT or targeted anti-androgen therapy. The disease is associated with low serum prostate-specific antigen (PSA) and progression to visceral metastasis, in contrast to bony metastases clinically exhibited by traditional prostatic adenocarcinoma. Histological (non-acinar) variants of prostatic carcinoma account for about 5%–10% of carcinomas originating in the prostate, including basal cell carcinoma, and neuroendocrine tumors [23]. Neuroendocrine cells from human NEPC specimens are characteristically small, round epithelial cells that contain irregular nuclei. Prostate tumors from the transgenic mouse model of prostate adenocarcinoma (TRAMP) mice develop neuroendocrine type adenocarcinoma with local invasion at 20 weeks of age and with progression to advanced disease (at 36 weeks) exhibit pathologically aggressive neuroendocrine tumors [24] (Fig. 1). NEPC is a lethal, AR-negative variant that most commonly arises from treated adenocarcinoma of the prostate. NEPC does not respond to AR-directed therapy and has only transient response to chemotherapy, with most patients dying within 12 months. It is estimated that up to 30% of late-stage prostate cancers harbor a predominance of neuroendocrine differentiation. This review delves into the neuroendocrine phenotype to determine its contribution to the process of trans-differentiation during tumor progression to advanced disease. Furthermore, we discuss the current understanding of the functional exchange between the EMT phenotypic landscape and the neuroendocrine phenotype in the context of the prostate tumor microenvironment and the implications on therapeutic resistance in clinical disease.
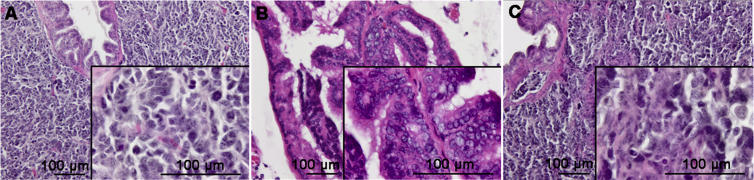
Figure 1.
Histopathological characteristics of neuroendocrine prostate cancer. (A) A representative H–E section of a human prostatectomy specimen from a 57-year-old male with PSA 3.4 ng/mL diagnosed with NEPC. Neuroendocrine cells are pathologically small, round epithelial cells that contain irregular nuclei and reduced cytoplasmic granularity; (B) and (C) Images of prostate tumor sections from the TRAMP mouse model tumors at 20 weeks and 34 weeks respectively exhibiting a characteristic progressive spectrum of neuroendocrine differentiation. PSA, prostate-specific antigen; NEPC, neuroendocrine prostate cancer; TRAMP, transgenic mouse of adenocarcinoma of the prostate.
2. EMT defines the prostate tumor landscape
The heterogeneity and plasticity that characterize prostate tumors suggest that regulatory phenotypic changes in individual cells imposed by the microenvironment may contribute to metastasis and therapeutic resistance [23], [24]. Coordinated molecular and genetic events generating such phenotypic alterations in the tumor landscape are associated with the acquisition of mesenchymal traits by epithelial cells and EMT induction facilitating tumor progression and development of therapeutic resistance [24]. The process of EMT in the normal prostate gland plays a significant role in embryonic development and tissue regeneration during wound healing [25]. However, the process has different implications in oncogenesis due to fundamental changes in the microenvironment. Transforming growth factor-β (TGF-β) is a primary inducer of EMT [13]. TGF-β signaling controls prostate growth by inhibiting proliferation, inducing apoptosis, and promoting migration and invasion through two transmembrane serine/threonine kinases, type I and type II receptors (TβRI and TβRII) [26]. By the stimulation of these receptors, the intracellular effectors Smad2/3 form a complex with Smad4 allowing its nuclear translocation and transcriptional activation of target genes in response to TGF-β [27]. In the early stages of tumorigenesis TGF-β has tumor-suppressor function via apoptosis induction, but it promotes tumor migration and invasion in late stages towards metastasis via effects on the actin cytoskeleton [26], [28]. The biological and clinical impact of the dual functions of TGF-β as a cytokine with apoptosis-inducing, as well as EMT-promoting and invasive properties on human cancer development and metastatic progression has been intimately linked with several malignancies, including urothelial carcinoma [29], [30], breast cancer [31] and prostate cancer [26].
The TGF-β signaling and the AR axis work in mechanistic conjunction to facilitate prostate tumor progression to metastasis. Indeed, there is compelling evidence to suggest that the phenotypic EMT landscape in prostate cancer is partially regulated by androgen signaling [15], [32]. In clinical disease, EMT induction and acquisition of mesenchymal features has been associated with high Gleason grade, biological recurrence and visceral metastasis [16]. Consequentially intricate understanding of the intercellular interactions, cell-extracellular matrix interactions, and complex intracellular signaling programming EMT and its reversal to MET will be imperative for the development of targeted therapies against advanced mCRPC. Cadherins are crucial to the process of EMT because the change in the balancing dynamic between E-cadherin and N-cadherin presence confers either suppressed or expressed invasive properties. E-cadherin, an epithelial marker, is an important contributor to cell–cell communication and functional exchange between cells. E-cadherin depletion and EMT have been shown to confer anoikis resistance (cell death resulting from insufficient cellular interactions) [33]. N-cadherin expression promotes invasive and migratory capacities [34]. E-cadherin/N-cadherin behavior and EMT/MET cycling is controlled by multiple TGF-β regulated genes: TWIST, ZEB1, Slug, and Snail [15]. The genes act as repressors of E-cadherin. TWIST promotes the expression of the prominent pro-angiogenic factor, vascular endothelial growth factor (VEGF) [30]. VEGF is the instrumental paracrine factor in angiogenesis and has been implicated in tumor growth and invasion [35]. ZEB1 is a zinc-finger protein transcription factor that is linked to the TCF8 gene. With increased expression of the TCF8 gene regulated by the AR, there is increased expression of the ZEB1 protein [36]. Ultimately, the downregulation of AR enhances ZEB1 expression, inducing EMT. Snail and Slug are examples of other zinc-finger transcription factors linked with TGF-β and consequently EMT. Snail and Slug possess unique mechanisms in determining EMT outcomes. Snail binds to E-box sequences within the promoter region of E-cadherin genes to repress their expression, resulting in upregulation of mesenchymal protein expression [37], [38]. Slug expression is under transcriptional regulation by androgens and its complex formation with AR results in the repression of E-cadherin [39]. Upregulation of Slug has been characteristically demonstrated in both mouse-derived models, prostate tumors, and human prostate cancer cell lines [40]. With the identification of these significant EMT effectors, targeted therapies can be developed to impair or reverse the molecular and phenotypic traits of tumor aggressiveness and overcome therapeutic resistance — A quite challenging task. Interestingly, evidences from in vitro and in vivo models of pre-clinical and clinical prostate cancer, support that a lineage switch from epithelial cells to neuroendocrine cells compromises the AR-dependency of prostate tumors, enabling them to bypass the AR signaling axis targeting [41], [42], [43].
3. Neuroendocrine transdifferentiation (NEtD) and the tumor microenvironment
Next-generation sequencing studies have identified a few genetic differences between prostate adenocarcinoma and NEPC, mainly related to the functional involvement of oncogenes and tumor suppressor genes [17], [44]. Furthermore, contextual to the tumor microenvironment signaling exchanges between tumor epithelial cells, tumor-associated fibroblasts and endothelial cells can mediate EMT outcomes [28]. As a cellular process, EMT involves trans-differentiation of epithelial-based tumors to a mesenchymal phenotype with an associated increase in invasive and migratory capacities. One must also consider the evidence suggesting that ADT may initiate EMT through downregulation of epithelial-based markers among prostate tumor cell populations [45]. The biological consequences of ADT affect not only the tumor cells themselves but the entire microenvironment, with significant impact on the EMT phenotypic dynamic and neuroendocrine-directed tumor landscape within the microenvironment (and independently of AR axis), towards local invasion and distant metastasis [15], [20], [46].
Complex paracrine signaling and extracellular interactions within the tumor microenvironment demonstrate attractive targeting value for the development of novel effective targeted therapies [47]. Normal paracrine interactions between prostatic stromal and epithelial cells control prostatic growth and exocrine function. In prostate cancer, stromal cells behave aberrantly and demonstrate increased extracellular matrix remodeling and angiogenesis [47]. The changes made in the stromal cells resemble that of wound healing, like EMT in its non-cancerous progression. There are three main tissue/organs that interact with prostate cancer: The prostate gland, lymph node, and bone. In the prostate gland, stromal cells are characterized by distinct alterations, including increased extracellular matrix (ECM) remodeling, increased protease activity, increased angiogenesis, and an infiltration of inflammatory cells [47]. Prostate cancer cells promote stromal reactivity and tumor proliferation in the context of the tumor microenvironment (Fig. 1). In the lymph node, there is loss in cell adhesion resulting in increased migratory capacity. In metastatic lesions, however, there is a complex interplay between the EMT and MET phenotypes as cells become a cohesive lesion in distant sites. The bone is identified as the primary metastatic site for prostate tumors. A study surveying 1 589 patients with prostate cancer found that 35% developed metastases and 90% of those metastases were in the bone [48]; with the osteoblastic characteristics promoting the production of unstructured bones in metastatic disease. Work by Kobayashi et al. [49] identified a protein linking dormancy to metastasis during prostate cancer progression. Bone morphogenic protein 7 (BMP7) is secreted from bone and subsequently allows prostate cancer stem-like cells to arise, conferring metastasis, while providing a promising therapeutic target for metastatic prostate cancer to the bone.
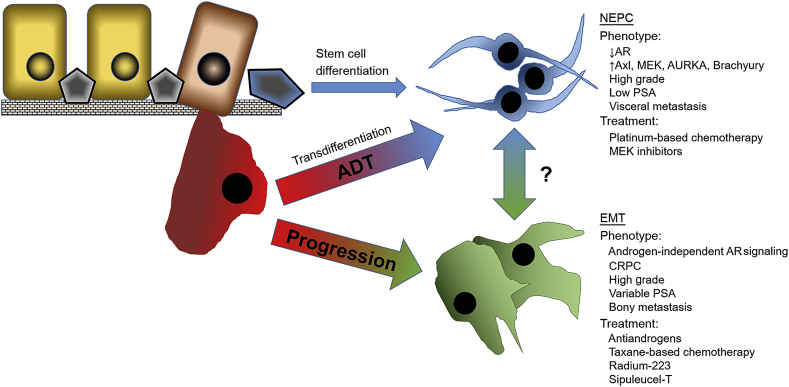
There has been a relatively recent recognition of clinical implications of neuroendocrine prostate cancer and its diagnosis and treatment, but there are conflicting opinions on the etiology of the pathological process leading to neuroendocrine tumors. While there is strong evidence to suggest the neuroendocrine phenotype transdifferentiates from pre-existing adenocarcinoma cells [50], other investigators argue that the cancer stem cells give rise to the neuroendocrine differentiated tumor cells [51]. Moreover the widely-accepted high intra-tumoral heterogeneity confers serious challenges in the effective chemotherapeutic targeting of prostate tumors [52]. Our current understanding of the histopathological progression of human prostate cancer guiding clinical decision-making stems from the Gleason grading system, with neuroendocrine tumors almost universally exhibiting high grade, poorly differentiated pathology [53]. The process of neuroendocrine differentiation during prostate cancer progression to advanced disease emerges is a critical event in facilitating therapeutic resistance and emergence of metastasis. As illustrated in Fig. 2, prostate tumor epithelial cells can follow three diverse routes as they progress towards advanced and therapeutically resistant prostate cancer. Although still a point of controversy, the recognition that tumor progression involves the differentiation of cancer stem cells with the outcome being NEPC, is intriguing [51]. A more attractive route is when tumor cells detach from the ECM and undergo NEtD, consequential to ADT treatment, the molecular changes lead to phenotypic changes. Cells transdifferentiate into the neuroendocrine phenotype (Fig. 1). The phenotype is associated with low AR activity, high levels of cellular plasticity drivers (Axl, MEK, AURKA, Brachyury), high grade, low PSA, and possible treatments include platinum-based chemotherapy and MEK inhibitors. Parallel to the NEPC development, many prostate tumors progress by following an EMT-navigated route that characteristically result in metastatic spread. The invasive phenotype developed is androgen independent, high grade, therapeutically resistant (CRPC), variable PSA, with high expression of plasticity drivers that can lead to bony metastasis (Fig. 2). Considering the clinical evidence that the cancer-specific survival is less than 1 year for patients with NEPC, precise histopathological distinction for timely clinical diagnosis is imperative to eradicate lethal disease. The prevalence of NEtD is increased in response to ADT and disease progression [15], [16]. Androgens can suppress the neuroendocrine phenotype. When the AR is inhibited, the phenotype emerges and is manifested by aggressive features and clinical progression. Prostate cancer cells with neuroendocrine phenotype are characterized by elongated and spindle-like morphology. NEtD is also associated with tumors that are aggressive and resistant to therapy [12]. One mechanism for this resistance is emergence of apoptosis resistance. Furthermore, secretory products from NEPC cells (neuropeptides and growth factors) are thought to have paracrine effects on surrounding prostate cancer cells by decreasing rates of apoptosis [53].
Figure 2.
Progression of primary prostate adenocarcinoma to NEPC and EMT-navigated metastatic prostate cancer follows diverse differentiation spectra. Normal epithelial cells may undergo EMT via loss of basoapical polarity and creating a more irregularly shaped, mesenchymal phenotype. Tumor epithelial cells may, upon detaching from the ECM, also transdifferentiate to NEPC as a cellular response to ADT. Another pathway to the neuroendocrine phenotype is differentiation of cell with stem cell-like properties. In addition to the separate lineages, a potential connection and spectrum of differentiation between the two phenotypes within the microenvironment may have significant functional consequences on prostate tumor progression. ADT, androgen deprivation therapy; ECM, extracellular matrix; EMT, epithelial–mesenchymal transition; NEPC, neuroendocrine-prostate cancer; PSA, prostate-specific antigen; AR, androgen receptor; MEK, mitogen-activated protein kinase; AURKA, aurora kinase A; CRPC, castration-resistant prostate cancer.
In addition to its role as a critical EMT regulator acting as a transcriptional repressor of E-cadherin, Snail can also induce NEtD [12]. Snail decreases cell adhesion by decreasing E-cadherin and other epithelial markers thus inducing EMT. Evidence derived from this study indicated the first functional connection between the process of EMT and the NEtD phenotype, determined by a common mechanism [12]. One essential component to EMT and NEtD activation is cell plasticity, driven predominantly by genetic and epigenetic changes impacting cellular identity and cellular response to signaling within the microenvironment. Drivers controlling changes in cell plasticity and lineage identity include transcription factors Brachyury, Axl, MEK, and Aurora kinase A [15], as well as SOX2 [43]. In recent studies, genes RB1 and Trp53 have been identified as plasticity suppressors [42]. Suppression of RB1 and Trp53 (tumor suppressors) is associated with overexpression of SOX2 [42], [43], implicating an inverse relationship between these factors in terms of their value as promising therapeutic targets to combat EMT and NEtD-related tumor progression and therapeutic resistance. A compelling characteristic of the RB1 and Trp53 genes is they are commonly suppressed in advanced prostate cancer pathological variants, such as NEPC, suggesting also a relation to response to antiandrogens and therapeutic resistance [42], [44]. Indeed, a strong indication of lineage plasticity contributing to therapeutic resistance, stems from clinical evidence that 20%–25% of patients with metastatic CRPC treated with antiandrogens, relapse with tumor cells harboring neuroendocrine features [46].
An intriguing potential mechanism driving prostate tumor therapeutic resistance to ADT towards a CRPC phenotype is the selection of NEtD cells surviving low androgen environments and enriching a selective proliferation within the primary tumor. Mechanistically AR signaling inhibits the neuroendocrine phenotype [55]. Neuroendocrine prostate tumor cells downregulate AR expression and thrive in restricted androgen environment, consequential to ADT (Fig. 2). Assessment of mechanisms underlying CRPC in the absence of AR in AR null and neuroendocrine null cells revealed that in the absence of AR ligands, AR signaling is active in a ligand-independent manner [56]. Since ADT promotes the development of NEPC, its incidence may be more frequently detected in response to second generation antiandrogens (abiraterone and enzalutamide). Thus, chemotherapeutic strategies must anticipate the AR-independent signaling exhibited by neuroendocrine tumors to be clinically efficacious.
4. Neuroendocrine phenotype in metastasis and therapeutic resistance
The phenotypes of both EMT and NEtD, no longer considered rare biological entities, can functionally contribute to prostate cancer progression and emergence of therapeutically-resistant metastatic disease. In terms of EMT, extensive cellular plasticity conferring dramatic polarity manipulations, via AR-dependent or independent signaling mechanisms is seemingly the “smoking gun” behind prostate cancer progression to metastasis [39], [45], [57]. The predominant increase occurs in the expression of N-cadherin—a cell adhesion molecule that, when lost, promotes greater plasticity and migratory capacity. In addition to N-cadherin, there are other markers implicated in metastasis with a close functional association with the EMT and NEtD phenotypes. MEK inhibitors have been evaluated in the treatment of tumors with NEtD induced by previous therapy. Moreover, additional evidence suggests that the MEK pathway downregulates AR mRNA levels in multiple prostate cancer cell lines [41]. Other markers involved in metastasis include ZEB1, mediated by insulin-like growth factor-1 (IGF-1) [58]; β-catenin, downregulation of which is associated with tumorigenesis [59]; and vimentin [60]. Each of these markers serves as promising candidate for therapeutic targeting in advanced metastatic disease, in addition to having an attractive biomarker value. Significantly enough, the functional involvement of all the markers contributes to the overall cancer progression through EMT by increasing polarity and invasive properties. Understanding the relationships between the marker expression/localization and the EMT to MET interconversion dynamic will facilitate identification of new targets for therapeutic development in the treatment of advanced prostate cancer.
Therapeutic resistance to ADT is virtually inevitable but occurs after a unique timeline of therapy for each prostate cancer patient after biochemical recurrence or diagnosis of advanced disease. The treatment options for patients with CRPC, are antiandrogens, targeting the androgen/AR signaling, and taxane chemotherapy, targeting the microtubule organization/stabilization, but therapeutic resistance ultimately develops resulting in lethal disease [61]. Growing evidence has established that docetaxel (first line chemotherapy) inhibits AR nuclear localization in androgen-sensitive prostate tumors, while in CRPC AR splice variants remain capable of nuclear trafficking contributing to taxane therapeutic resistance [14], [32]. Targeting the AR can confer cross-resistance between the antiandrogen enzalutamide and docetaxel, but not cabazitaxel (second line chemotherapy) in CRPC [62]. Moreover, recent work from this laboratory demonstrated that cabazitaxel led to reversion of EMT to MET, kinesin-mediated multi-nucleation, and glandular re-differentiation while retaining nuclear AR in in vivo pre-clinical models of advanced prostate cancer [63]. An appealing target here is Bcl-2 a regulator of apoptosis that enables prostate cancer cells to survive under low androgen environments and overexpression of which is associated with clinical progression to metastasis [64]. Taxane-based chemotherapeutic agents induce post-translational modification in Bcl-2 protein through phosphorylation to impact apoptosis outcomes [31], causing stabilization of the microtubules [64], and impairing AR translocation to the nucleus [11] towards a therapeutic response in prostate cancer. The mechanistic link between Bcl-2 with treatment resistance to taxane chemotherapy among patients with advanced prostate cancer calls for novel therapeutic exploitations of combination strategies to target this apoptosis suppressor.
Ongoing efforts in therapeutic targeting of CRPC led to several promising FDA-approved therapies, including second-generation antiandrogens, abiraterone (CYP inhibitor), and enzalutamide (AR signaling inhibitor), cabazitaxel (second line taxane chemotherapy), and Radium-223 (radionucleotide) (EAU Guidelines) [65]. Recent observations from Phase III randomized controlled trials indicate that abiraterone has efficacy in men at high risk for advanced or metastatic prostate cancer naïve to hormone therapy. The recently completed LATITUDE trial demonstrated that abiraterone with prednisone decreased the risk of death by 38% compared with ADT, and the combination nearly doubled median progression-free survival in patients with recurrent disease (33 months vs. 15 months respectively) [66]. In the TROPIC, a phase III randomized trial of prostate cancer patients with clinical progression on docetaxel for metastatic disease, patients were randomized to mitoxantrone and prednisone vs. cabazitaxel and prednisone. Patients in the cabazitaxel group showed 2.4-month overall survival and 1.4-month progression free survival benefits [67]. Experimental evidence from our group implicates new efficacy of a combination approach of taxane chemotherapy and novel antiandrogens targeting the N-terminal domain of the AR via dictating EMT to MET interconversion [68].
Platinum-based chemotherapy has proven efficacy in the treatment of neuroendocrine tumors. Humeniuk et al. [69] recently demonstrated that 63% of patients with NEPC as opposed to 29% of patients with prostate adenocarcinoma, partially responded to platinum chemotherapy—suggesting the NEPC sensitivity to the platinum-based agents which is clinically consistent with primary neuroendocrine tumors from other organ systems. In the pursuit of a complete characterization of the neuroendocrine phenotype as a driver of progression to advanced prostate cancer, two ground-breaking studies by Beltran and her investigative team [44], have provided fundamental knowledge and enhance our current understanding of the significance of neuroendocrine tumors determining clinically aggressive, therapeutically resistant prostate cancer. The initial molecular characterization of the NEPC phenotype was achieved by performing clinical diagnostics using deep next-generation sequencing towards assessments and subsequent mechanism-driven efforts enhanced our understanding of its impact on tumor cell polarity and contribution to disease progression under ADT [42], [46]. Moreover, while recognizing the vast heterogeneity that characterizes advanced disease in clinical presentation, additional evidence has identified translationally significant characterizations of the phenotype using circulating tumor cells (CTC) [17]. These pioneering studies established that NEPC cells are smaller in pathological size than the “classic” CRPC cells harboring reduced AR expression, opening the way to exploitation of the neuroendocrine phenotype at the pathological, molecular and therapeutic level towards new diagnostic tests and improving clinical outcomes of patients with advanced disease.
5. Conclusion
In 2018, the challenge remains to apply targeted therapies either as in combination approaches or in sequence to achieve clinically meaningful outcomes in prostate cancer patients with advanced disease. The molecular mechanisms underlying the role of NEtD in prostate cancer progression are still not clearly defined. Gaining further mechanistic insights into the cellular mechanisms driving phenotypic transition to the neuroendocrine phenotype would be critical in enhancing our understanding of the acquisition of this “pathologically exotic” feature of prostate tumors. Further characterization of the underlying drivers of transdifferentiation in response to conventional therapies will generate valuable new platforms for therapeutic manipulation throughout the spectrum of disease progression. Recognizing the contribution of the neuroendocrine phenotype to inherent therapeutic resistance in prostate cancer begs the question: how can such resistance be overcome at the molecular and clinical setting of advanced disease? Neuroendocrine tumors of the prostate may provide a unique phenotypic landscape enabling functional connections to EMT with consequences on the therapeutic response in patients with advanced disease. Exploitation of treatment therapies in combination and/or sequencing strategies to facilitate the interconversion of EMT to MET in a temporal “switch” has promising novel utility by conventional therapies. Molecular subtyping of individual of prostate tumors from patients throughout the disease process may tailor treatment strategies targeting the dynamic interconversion rate of EMT to MET towards a spectrum or NEPC differentiation and the degree of intratumoral heterogeneity characterizing these advanced tumors. This will be further facilitated by platforms enabling high throughput screening of biomarkers for neuroendocrine phenotype, disease progression, and therapeutic resistance. Considering the increased recognition of the importance of the neuroendocrine phenotype in advanced prostate cancer [15], [54], [70], the ongoing efforts of molecular exploitation of the landscape of individual tumors using advanced technology, would empower significant molecular correlations between EMT and the neuroendocrine phenotype to impact clinical disease.
Author contributions
Study design: Natasha Kyprianou, Haley Dicken, Patrick Hensley.
Data analysis: Haley Dicken, Patrick Hensley.
Drafting of manuscript: Haley Dicken, Patrick Hensley.
Critical revision of the manuscript: Natasha Kyprianou.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
This work is supported by a Schwab Foundation Grant and the James F. Hardymon Endowment in Urologic Research at the University of Kentucky (NK, PJH), and the University of Kentucky Summer Undergraduate Research Experience in Environmental Health Sciences (SURES) program (HD).
Footnotes
Peer review under responsibility of Second Military Medical University.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics. CA Cancer J Clin. 2018;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Varenhorst E., Klaff R., Berglund A., Heldlund P.O., Sandblom G. Predictors of early androgen deprivation treatment failure in prostate cancer with bone metastases. Cancer Med. 2015:409–414. doi: 10.1002/cam4.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petrylak D.P., Tangen C.M., Hussain M.H., Lara P.N., Jones J.A., Taplin M.E. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 4.Fizazi K., Scher H.I., Molin A., Logothetis C.J., Chi K., Jones R.J. Abiraterone acetate for treatment of metastatic castration resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomized, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13:983–992. doi: 10.1016/S1470-2045(12)70379-0. [DOI] [PubMed] [Google Scholar]
- 5.Beer T.M., Armstrong A.J., Rathkopf D.E., Loriot Y., Sternberg C.N., Higano C.S. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–433. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen C.D., Welsbie D.S., Tran C., Baek S.H., Chen R., Vessella R. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 7.Kahn B., Collazo J., Kyprianou N. Androgen receptor as a driver of therapeutic resistance in advanced prostate cancer. Int J Biol Sci. 2014;10:588–595. doi: 10.7150/ijbs.8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antonarakis E.S., Lu C., Wang H., Luber B., Nakazawa M., Roeser J.C. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028–1038. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirby M., Hirst C., Crawford E.D. Characterising the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract. 2011;65:1180–1192. doi: 10.1111/j.1742-1241.2011.02799.x. [DOI] [PubMed] [Google Scholar]
- 10.Snoeks L.L., Ogilvie A.C., van Haarst E.P., Siegert C.E. New treatment options for patients with metastatic prostate cancer. Neth J Med. 2013;71:290–294. [PubMed] [Google Scholar]
- 11.Zhu M., Horbinski C.M., Garzotto M., Qian D., Beer T., Kyprianou N. Tubulin-targeting chemotherapy impairs androgen receptor activity in prostate cancer. Cancer Res. 2010;70:7992–8002. doi: 10.1158/0008-5472.CAN-10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKeithen C., Graham T., Chung L., Odero-Marah V. Snail transcription factor regulates neuroendocrine differentiation in LNCaP prostate cancer cells. Prostate. 2010;70:982–992. doi: 10.1002/pros.21132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y., Zhou B. Epithelial-mesenchymal transition in breast cancer progression and metastasis. Chin J Cancer. 2011;30:603–611. doi: 10.5732/cjc.011.10226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darshan M.S., Loftus M.S., Thadani-Mulero M., Levy B.P., Escuin D., Zhou X.K. Taxane-induced blockade to nuclear accumulation of the androgen receptor predicts clinical responses in metastatic prostate cancer. Cancer Res. 2011;71:6019–6029. doi: 10.1158/0008-5472.CAN-11-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nouri M., Ratther E., Stylianou N., Nelson C., Hollier B., Williams E. Androgen-targeted therapy-induced epithelial mesenchymal plasticity and neuroendocrine transdifferentiation in prostate cancer: an opportunity for intervention. Front Oncol. 2014;370:1–6. doi: 10.3389/fonc.2014.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parimi V., Goyal R., Poropatich K., Yang X. Neuroendocrine differentiation in prostate cancer: a review. Am J Clin Exp Urol. 2014;2:273–285. [PMC free article] [PubMed] [Google Scholar]
- 17.Beltran H., Jendrisak A., Landers M., Mosquera J.M., Kossai M., Louw J. The initial detection and partial characterization of circulating tumor cells in neuroendocrine prostate cancer. Clin Cancer Res. 2016;22:1510–1519. doi: 10.1158/1078-0432.CCR-15-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vlachostergios P.J., Papandreou C.N. Targeting neuroendocrine prostate cancer: molecular and clinical perspectives. Front Oncol. 2015;5:6. doi: 10.3389/fonc.2015.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hauso O., Gustafsson B.I., Kidd M., Waldum H.L., Drozdov I., Chan A.K. Neuroendocrine tumor epidemiology. Cancer. 2008;113:2655–2664. doi: 10.1002/cncr.23883. [DOI] [PubMed] [Google Scholar]
- 20.Hirano D., Okada Y., Minei S., Takimoto Y., Nemoto N. Neuroendocrine differentiation in hormone refractory prostate cancer following androgen deprivation therapy. Eur Urol. 2004;45:586–592. doi: 10.1016/j.eururo.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 21.diSant'Agnese P. Neuroendocrine differentiation in prostatic carcinomas. Cancer. 1995;75:1850–1859. [Google Scholar]
- 22.National Comprehensive Cancer Network . 2016. Prostate cancer. Version 1.2016.http://nccn.org [Google Scholar]
- 23.Humphrey P.A. Histological variants of prostate carcinoma and their significance. Histopathology. 2012;60:59–74. doi: 10.1111/j.1365-2559.2011.04039.x. [DOI] [PubMed] [Google Scholar]
- 24.Pu H., Collazo J., Jones E., Gayheart D., Sakamoto S., Vogt A. Dysfunctional TGF-β receptor II accelerates prostate tumorigenesis in the TRAMP mouse model. Cancer Res. 2009;69:7366–7374. doi: 10.1158/0008-5472.CAN-09-0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heerboth S., Housman G., Leary M., Longacre M., Byler S., Lapinska K. EMT and tumor metastasis. Clin Transl Med. 2015;4:1. doi: 10.1186/s40169-015-0048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu B., Kyprianou N. Transforming growth factor-β and cancer. In: Alison M.R., editor. The cancer textbook. 2007. pp. 257–271. [Google Scholar]
- 27.Wendt M.K., Allington T.M., Schiemann W.P. Mechanisms of the epithelial-mesenchymal transition by TGF-beta. Future Oncol. 2009;5:1145–1168. doi: 10.2217/fon.09.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collazo J., Zhu B., Larkin S., Pu H., Koochekpour S., Martin S.K. Cofilin regulates cellular invasion responses to TGF-β towards prostate cancer metastasis. Cancer Res. 2014;74:2362–2373. doi: 10.1158/0008-5472.CAN-13-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hensley P., Zetter D., Horbinski C., Strup S., Kyprianou N. Association of epithelial-mesenchymal transition and nuclear cofilin with advanced urothelial cancer. Hum Pathol. 2016;57:68–77. doi: 10.1016/j.humpath.2016.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wallerand H., Robert G., Pasticier G., Ravaud A., Ballanger P., Reiter R.E. The epithelial-mesenchymal transition-inducing factor TWIST is an attractive target in advanced and/or metastatic bladder and prostate cancers. Urol Oncol. 2010;28:473–479. doi: 10.1016/j.urolonc.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 31.Mohile S.G., Petrylak D.P. Taxane-based chemotherapy for prostate cancer. Prostate Cancer. 2007:445–462. [Google Scholar]
- 32.Zhu M.L., Kyprianou N. Role of androgens and the androgen receptor in epithelial–mesenchymal transition and invasion of prostate cancer cells. FASEB J. 2010;24:769–777. doi: 10.1096/fj.09-136994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar S., Park S.H., Cieply B., Schupp J., Killiam E., Zhang F. A pathway for the control of anoikis sensitivity by E-cadherin and epithelial-to-mesenchymal transition. Mol Cell Biol. 2011;31:4036–4051. doi: 10.1128/MCB.01342-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Derycke L.D., Bracke M.E. N-cadherin in the spotlight of cell–cell adhesion, differentiation, embryogenesis, invasion and signaling. Int J Dev Biol. 2004;48:463–476. doi: 10.1387/ijdb.041793ld. [DOI] [PubMed] [Google Scholar]
- 35.Niu G., Chen X. Vascular endothelial growth factor as an anti-angiogenic target for cancer therapy. Curr Drug Targets. 2010;11:1000–1017. doi: 10.2174/138945010791591395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anose B., Sanders M. Androgen receptor regulates transcription of the ZEB1 transcription factor. Int J Endocrinol. 2011;2011:903918. doi: 10.1155/2011/903918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Acloque H., Adams M.S., Fishwick K., Bronner-Fraser M., Nieto M.A. Epithelial mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Investig. 2009;119:1438–1449. doi: 10.1172/JCI38019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baritaki S., Chapman A., Yeung K., Spandidos D.A., Palladino M., Bonavida B. Inhibition of epithelial to mesenchymal transition in metastatic prostate cancer cells by the novel proteasome inhibitor, NPI-0052: pivotal roles of Snail repression and RKIP induction. Oncogene. 2009;28:3573–3585. doi: 10.1038/onc.2009.214. [DOI] [PubMed] [Google Scholar]
- 39.Wu K., Gore C., Yang L., Fazli L., Gleave M., Pong R.C. Slug, a unique androgen-regulated transcription factor, coordinates androgen receptor to facilitate castration resistance in prostate cancer. Mol Endocrinol. 2012;26:1496–1507. doi: 10.1210/me.2011-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu J., Uygur B., Zhang Z., Shao L., Romero D., Vary C. Slug inhibits proliferation of human prostate cancer cells via downregulation of cyclin D1 expression. Prostate. 2010;70:1768–1777. doi: 10.1002/pros.21213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hong S.K., Kim J.H., Lin M.F., Park J.I. The Raf/MEK/extracellular signal-regulated kinase 1/2 pathway can mediate growth inhibitory and differentiation signaling via androgen receptor downregulation in prostate cancer cells. Exp Cell Res. 2011;317:2671–2682. doi: 10.1016/j.yexcr.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ku S.Y., Rosario S., Wang Y., Mu P., Seshadri M., Goodrich Z.W. RB1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science. 2017;355:78–83. doi: 10.1126/science.aah4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mu P., Zhang Z., Benelli M., Karthaus W.R., Prandi D., Hoover E. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science. 2017;355:84–88. doi: 10.1126/science.aah4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beltran H., Yelensky R., Frampton G.M., Park K., Downing S.R., MacDonald T.Y. Targeted next-generation sequencing of advanced prostate cancer identifies potential therapeutic targets and disease heterogeneity. Eur Urol. 2013;63:920–926. doi: 10.1016/j.eururo.2012.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matuszak E.A., Kyprianou N. Androgen regulation of epithelial–mesenchymal transition in prostate tumorigenesis. Expert Rev Endocrinol Metab. 2011;6:469–482. doi: 10.1586/eem.11.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bishop J.L., Thaper D., Vahid S., Davies A., Ketola K., Kuruma H. The master neural transcription factor BRN2 is an androgen receptor suppressed driver of neuroendocrine differentiation in prostate cancer. Cancer Discov. 2017;7:54–71. doi: 10.1158/2159-8290.CD-15-1263. [DOI] [PubMed] [Google Scholar]
- 47.Corn P.G. The tumor microenvironment in prostate cancer: elucidating molecular pathways for therapy development. Cancer Manag Res. 2012;4:183–193. doi: 10.2147/CMAR.S32839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bubendorf L., Schlöpfer A., Wagner U., Sauter G., Moch H., Willi N. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31:578–583. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 49.Kobayashi A., Okuda H., Xing F., Pandey P.R., Watabe M., Hirota S. Bone morphogenetic protein 7 in dormancy and metastasis of prostate cancer stem-like cells in bone. J Exp Med. 2011;208:2641–2655. doi: 10.1084/jem.20110840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yuan T., Veeramani S., Lin M. Neuroendocrine-like prostate cancer cells: neuroendocrine transdifferentiation of prostate adenocarcinoma cells. Endocr Relat Cancer. 2007;14:531–547. doi: 10.1677/ERC-07-0061. [DOI] [PubMed] [Google Scholar]
- 51.Chen X., Rycaj K., Liu X., Tang D.G. New insights into prostate cancer stem cells. Cell Cycle. 2013;12:579–586. doi: 10.4161/cc.23721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wyatt A.W., Mo F., Wang K., McConeghy B., Brahmbratt S., Jong L. Heterogeneity in the inter-tumor transcriptome of high rick prostate cancer. Genome Biol. 2014;15:426. doi: 10.1186/s13059-014-0426-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Terry S., Beltran H. The many faces of neuroendocrine differentiation in prostate cancer progression. Front Oncol. 2014;4:60. doi: 10.3389/fonc.2014.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beltran H., Prandi D., Mosquera J.M., Benelli M., Puca L., Cyrta J. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat Med. 2016;22:298–305. doi: 10.1038/nm.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun Y., Niu J., Huang J. Neuroendocrine differentiation in prostate cancer. Am J Transl Res. 2009;1:148–162. [PMC free article] [PubMed] [Google Scholar]
- 56.Bluemn E., Coleman I., Lucas J., Vessella R.L., Morrissey C., Nelson P.S. Androgen receptor pathway-independent prostate cancer is sustained through FGF signaling. Cancer Cell. 2017;32:474–489. doi: 10.1016/j.ccell.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grant C.M., Kyprianou N. Epithelial mesenchymal transition (EMT) in prostate growth and tumor progression. Transl Androl Urol. 2013;2:202–211. doi: 10.3978/j.issn.2223-4683.2013.09.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Graham T.R., Zhau H.E., Odero-Marah V.A., Osunkoya A.O., Kimbro K.S., Tighiouart M. Insulin-like growth factor-i-dependent up-regulation of ZEB1 drives epithelial-to-mesenchymal transition in human prostate cancer cells. Cancer Res. 2008;68:2479–2488. doi: 10.1158/0008-5472.CAN-07-2559. [DOI] [PubMed] [Google Scholar]
- 59.Sadot E., Geiger B., Oren M., Ben-Ze'ev A. Down-regulation of beta-catenin by activated p53. Mol Cell Biol. 2001;21:6768–6781. doi: 10.1128/MCB.21.20.6768-6781.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Satelli A., Li S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol Life Sci. 2011;68:3033–3046. doi: 10.1007/s00018-011-0735-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sartor O., Gillessen S. Treatment sequencing in metastatic castrate-resistant prostate cancer. Asian J Androl. 2014;16:426–431. doi: 10.4103/1008-682X.126378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Soest R.J., de Morrée E.S., Kweldam C.F., de Ridder C.M.A., Wiemer E.A.C., Mathijssen R.H.J. Targeting the androgen receptor confers in vivo cross-resistance between enzalutamide and docetaxel, but not cabazitaxel, in castration-resistant prostate cancer. Eur Urol. 2015;67:981–985. doi: 10.1016/j.eururo.2014.11.033. [DOI] [PubMed] [Google Scholar]
- 63.Martin S.K., Pu H., Penticuff J., Cao Z., Horbinski C., Kyprianou N. Multinucleation and mesenchymal-to-epithelial transition alleviate resistance to combined cabazitaxel and antiandrogen therapy in advanced prostate cancer. Cancer Res. 2016;76:912–926. doi: 10.1158/0008-5472.CAN-15-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yoshino T., Shiina H., Urakami S., Kikuno N., Yoneda T., Shigeno K. Bcl-2 expression as a predictive marker of hormone-refractory prostate cancer treated with taxane-based chemotherapy. Clin Cancer Res. 2006;12:6116–6124. doi: 10.1158/1078-0432.CCR-06-0147. [DOI] [PubMed] [Google Scholar]
- 65.Mottet N., Bellmunt J., Bolla M., Briers E., Cumberbatch M.G., De Santis M. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71:618–629. doi: 10.1016/j.eururo.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 66.Fizazi K., Tran N., Fein L., Matsubara N., Rodriguez-Antolin A., Alekseev B.Y. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377:352–360. doi: 10.1056/NEJMoa1704174. [DOI] [PubMed] [Google Scholar]
- 67.de Bono J.S., Oudard S., Ozguroglu M., Hansen S., Machiels J.P., Kocak I., TROPIC Investigators Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet Oncol. 2010;376:1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 68.Martin S.K., Banuelos C.A., Sadar M.D., Kyprianou N. N-terminal targeting of androgen receptor variant enhances response of castration resistant prostate cancer to taxane chemotherapy. Mol Oncol. 2015;9:628–639. doi: 10.1016/j.molonc.2014.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Humeniuk S., Gupta R.T., Healy P., McNamara M., Ramalingam S., Harrison M. Platinum sensitivity in metastatic prostate cancer: does histology matter? Prostate Cancer Prostatic Dis. 2018;21:92–99. doi: 10.1038/s41391-017-0017-6. [DOI] [PubMed] [Google Scholar]
- 70.Vashchenko N., Abrahsmsson P. Neuroendocrine differentiation in prostate cancer: implications for new treatment modalities. Eur Urol. 2004;47:147–155. doi: 10.1016/j.eururo.2004.09.007. [DOI] [PubMed] [Google Scholar]