Abstract
Herbaceous peony (Paeonia lactiflora Pall.) is an excellent ornamental plant, which is usually stressed by summer high temperatures, but little is known about its relevant measures. In this study, the effects of trehalose on alleviating high temperature-induced damage in P. lactiflora were examined. High temperature stress in P. lactiflora increased production of reactive oxygen species (ROS), including superoxide anion free radical (O2·−) and hydrogen peroxide (H2O2), enhanced both malondialdehyde (MDA) content and relative electrical conductivity (REC), decreased superoxide dismutase (SOD) activity, increased catalase (CAT) activity, inhibited photosynthesis, and destroyed cell structure. However, exogenous trehalose effectively alleviated its high temperature-induced damage. Trehalose decreased O2·− and H2O2 accumulation, MDA content, and REC, increased the activities of antioxidant enzymes, enhanced photosynthesis, improved cell structure, and made chloroplasts rounder. Additionally, trehalose induced high temperature-tolerant-related gene expressions to different degrees. These results indicated that trehalose decreased the deleterious effect of high temperature stress on P. lactiflora growth by enhancing antioxidant systems, activating photosynthesis, and protecting cell structure. These findings indicate the potential application of trehalose for managing high temperatures in P. lactiflora cultivation.
Keywords: High temperature, Trehalose, Lipid peroxidation, Antioxidant, Photosynthesis
Introduction
With the constant increase in atmospheric carbon dioxide (CO2) levels, the impact of global climatic changes is exacerbated (Eissa and Zaki 2011; Putten et al. 2002). In addition, by the end of the twenty-first century, a 1.8–5.8 °C temperature increase is predicted globally, an increase that is one type of abiotic stress factor (Goraya et al. 2017). Both temporary and long-term elevated temperature increases can lead to a wide range of responses in plants, including morphological, physiological, biochemical, and molecular changes, and these changes usually have an adverse impact on plant growth and development, eventually reducing plant production (Liu et al. 2013). For example, high temperature reduces vegetative growth and potassium allocation in olive (Olea europaea L.) (Benlloch-González et al. 2016), affects male and female reproduction in plants (Sage et al. 2015), and drastically reduces economic yield in potato (Solanum tuberosum L.) (Rykaczewska 2015). Thus, it is crucial to find some method to improve high temperature stress tolerance in plants.
High temperature stress tolerance can be effectively improved by breeding new plant varieties, improving plant growth environments, and applying exogenous chemicals. Of these, spraying exogenous chemicals is the most simple and convenient method to improve high temperature stress tolerance in plants and is the easily to apply in production. For example, Sang et al. (2017) observed the positive effect of exogenous spermidine in tomato (Lycopersicon esculentum Mill.) seedlings’ response to high temperature stress. Salicylic acid alleviated the high temperature stimulus in Ulva prolifera through partion antioxidant-related proteins upregulations, jasmonate signal pathway enhancements, significant changes in Ca2+-binding proteins and photosynthesis-related proteins, the incensement of antioxidant enzyme activities, and changes in photosynthesis index (Fan et al. 2017). Abscisic acid had a protective role in membrane stability, osmotic adjustment, photosynthesis, and hormonal status of lucerne (Medicago sativa L.) under high temperature stress (An et al. 2014). Trehalose is a nonreducing disaccharide, presents in many organisms, that is composed of two glucose residues bound by an α-α-(1→1) linkage, serving as an energy source and as an osmolyte and/or protein/membrane protectant (López-Gómez and Lluch 2012). In addition, previous studies have shown that trehalose can regulate plant responses to environmental stresses, including salt, copper, and drought. (Ali et al. 2012; Mostofa et al. 2015; Shahbaz et al. 2017). Moreover, several studies found that exogenously supplied trehalose protected plants from high temperature-induced damage (Luo et al. 2008, 2010, 2014; Pang et al. 2017). However, it is still unclear whether such response of trehalose against high temperature stress is universal for ornamental plants.
Herbaceous peony (Paeonia lactiflora Pall.), belonging to the Paeoniaceae family, is a traditionally well-recognized flower with a long cultivation history. P. lactiflora has excellent aesthetic characteristics because of its gorgeous color and attractive pattern, and it has been widely used in gardens. P. lactiflora favors sunny environments while avoiding summer high temperature, often leading to high temperature stress in south China environments (Zhang et al. 2016). In this respect, the effects of high temperature stress on P. lactiflora physiology and biochemistry were investigated, and it was found that high temperature-tolerant P. lactiflora cultivars could effectively reduce stress damage by enhancing antioxidant enzyme activities and heat shock protein gene expression (Wu et al. 2016). Moreover, transcriptome and digital gene expression analysis of P. lactiflora were used to screen for thermo-tolerant-related differentially expressed genes (Hao et al. 2016). Additionally, Zhao et al. (2015) found that shade could ameliorate high temperature-induced growth inhibition in P. lactiflora. Nevertheless, the application of exogenous chemicals to improve high temperature stress tolerance in P. lactiflora has not been studied, to the best of our knowledge. To reduce the adverse effects of high temperature on P. lactiflora, trehalose was selected for application in the present study. The responses of stress physiological indices, antioxidant enzyme activities, photosynthesis, anatomy, and high temperature-tolerant gene expressions were investigated. These findings provide a theoretical basis for further elucidating the regulatory mechanism of trehalose-mediated high temperature stress tolerance in P. lactiflora.
Materials and methods
Plant materials and treatments
The experiments were carried out with 6-year-old potted P. lactiflora cv ‘Da Fugui’, one of the popular P. lactiflora cultivars in China. These plants were divided into two groups, with 24 pots per group. For one group, 30 mmol L−1 trehalose solution was sprayed at 17:00 daily, and the other was treated with distilled water as the control. After a 3-day treatment, four pots were adopted randomly per group as the 0th day, while the others were transferred to an artificial climate chamber with 40 °C and 60% relative humidity during daytime (5:00–19:00, 10,000 lx light intensity) and nighttime (19:00–5:00, 0 lx light intensity) for high temperature stress. In addition, the samples were taken separately on 0, 1, 2, and 3 days after treatment. When taking samples, photosynthetic and chlorophyll fluorescence parameters were first measured, and then leaves were taken for stress physiological indices and protective enzyme activity measurements, anatomy observation, and gene expression analysis. Additionally, all leaves were fully expanded and were taken from the fourth apical node.
Stress physiological index measurement
Superoxide anion free radical (O2·–) accumulation was observed by a reagent kit (Shanghai Haling Biotechnology Co., Ltd., China). The samples were observed at 540 nm excitation wavelength and 590 nm emanation wavelength and imaged with a fluorescent microscope (Axio Imager D2, ZEISS, Germany). The fluorescent signals were gathered using ZEN software (ZEISS, Germany).
Hydrogen peroxide (H2O2) accumulation was firstly observed by diaminobenzidine (DAB) staining (Tian et al. 2013). Briefly, P. lactiflora leaves were immersed in 0.1 mg/mL DAB in 50 mM Tris-acetate buffer, pH 5.0, at 25 °C for 24 h in the dark, and then they were boiled in 95% (v/v) ethanol for 15 min and then photographed using a camera (Canon 50D, Japan). Additionally, H2O2 content was detected according to the guideline of reagent kit (Nanjing Jiancheng Bioengineering Institute, China).
Malondialdehyde (MDA) content was evaluated according to the guideline of reagent kit (Nanjing Jiancheng Bioengineering Institute, China). Relative electrical conductivity (REC) was determined according to the method reported by Yang et al. (1996).
Antioxidant enzyme activity measurement
Firstly, 0.5 g leaf was ground to a fine powder with liquid nitrogen and extracted with ice-cold 50 mM phosphate buffer (pH 7.8). The extracts were centrifuged at 4 °C for 15 min at 10,000×g and then the resulting supernatants, thereafter referred to as crude extracts, were collected and used for enzyme activity assay (Zou 2000). Catalase (CAT, EC 1.11.1.6) and superoxide dismutase (SOD, EC 1.15.1.1) activities were evaluated by reagent kits (Nanjing Jiancheng Bioengineering Institute, China).
Photosynthetic and chlorophyll fluorescence parameter measurement
Photosynthetic parameters were measured with a portable photosynthesis system (Li-Cor LI-6400, USA), and the chlorophyll fluorescence parameters were measured with a chlorophyll fluorescence spectrometer (Heinz Walz GmbH 91090 Effeltrich, Germany).
Anatomy observation
The surfaces of leaves were observed by the environmental scanning electron microscopy (Philips XL-30 ESEM, Holland). The fixed leaves were firstly dehydrated in a gradient ethanol solution and treated with the mixtures of acetone/anhydrous alcohol (1:1, 2:1, 1:0, v/v), acetone/isoamyl acetate (1:1, 1:2, v/v), and pure isoamyl acetate. After drying and spraying gold (EIKO IB-3, Hitachi, Japan), the samples were observed.
Moreover, the anatomical details of leaves were observed by the transmission electron microscope (Tecnai 12, Philips, Holland). The fixed leaves were washed three times with 0.1 mol/L phosphate buffer for 15 min and post-fixed with 1% osmium tetroxide for 4 h at room temperature. After washing three times with 0.1 mol/L phosphate buffer for 15 min each, the leaves were dehydrated using 50, 70, 85, 95, and 100% gradient ethanol for 15 min each. Moreover, they were treated with 100% acetone solution (15 min) and acetone solution containing anhydrous sodium sulfate (15 min), infiltrated in Spurr resin, and then hardened at 70 °C for 24 h. Sections (70 nm thick) were cut with a diamond knife using a Leica EM UC6 ultramicrotome (Leica Co., Austria) and stained with 1% uranyl acetate in 70% methanol and 1% lead citrate before examination. After these, the samples were observed and imaged.
Gene expression analysis
Total RNA was extracted from P. lactiflora leaves using MiniBEST Plant RNA Extraction Kit (TaKaRa, Japan). The cDNA was synthesized from RNA using PrimeScript® RT reagent Kit With gDNA Eraser (TaKaRa, Japan). PCR was performed in a total volume of 25 μL reaction system containing total cDNA 2 μL, 10× PCR buffer 2.5 μL, dNTP mixture (2.5 mM each) 2 μL, TaKaRa Taq™ (5 u/μL) 0.2 μL (TaKaRa, Japan), PCR primers (10 μM) (Table S1) 2.5 μL, and ddH2O 15.8 μL. PCR conditions were 3 min at 94 °C, followed by 35 cycles of 30 s at 94 °C, 30 s at 52 °C, and 30 s at 72 °C, with a final extension at 72 °C for 10 min.
Gene transcript levels were analyzed using qRT-PCR with a BIO-RAD CFX Connect™ Optics Module (Bio-Rad, USA), and all gene-specific primers were shown in Table 1. qRT-PCR was performed using the SYBR® Premix Ex Taq™ (Perfect Real Time) (TaKaRa, Japan) and contained 12.5 μL 2 × SYBR Premix Ex Taq™, 2 μL cDNA solution, 2 μL mix solution of target gene primers, and 8.5 μL ddH2O in a final volume of 25 μL. The amplification was carried out under the following conditions: 95 °C for 30 s, 40 cycles at 95 °C for 5 s, 52 °C for 30 s, and 72 °C for 30 s. Gene relative expression levels of target genes were calculated by the 2−△△Ct comparative threshold cycle (Ct) method (Schmittgen and Livak 2008). The Ct values of the triplicate reactions were gathered using the Bio-Rad CFX Manager V1.6.541.1028 software.
Table 1.
Gene-specific primers used in gene expression analysis
| Gene ID | Gene name | Forward primer sequence (5′-3′) | Reverse primer sequence (5′-3′) |
|---|---|---|---|
| Unigene13462 | HSP70-1 | CCCTCGTAGACCTTGAACTGG | GGCATGGAAGACCAGAAGGTT |
| Unigene17547 | HSP70-2 | GTGGCTGGTCGTATGTGTCA | ACAACGGCCTCAGCTAATCC |
| Unigene14964 | HSP70-3 | TGGCACGAGTGATGGTTGAA | GTTGAGGACCCATTGCGAGA |
| CL5807.Contig2 | HSP40-1 | CATTGCCCTCTTCCCACCAT | TCGCTTATCGGAGACTTGCC |
| CL822.Contig1 | HSP40-2 | GGTGGAGACCCTGAGAAGTT | TTATGACTGGCACCTCCCCC |
| CL7816.Contig2 | HSP20 | TTGAGGAGGGTCGGGTTCTT | CTGAATCGGCGGAGGAACTT |
| Unigene38459 | WRKY-1 | GGTTCACAGCCCTGGAAAAA | ATCACTCTCACACGCTCCTTG |
| CL10073.Contig1 | WRKY-2 | ACCCCAACGATGAATGATGGAT | CCTCAGCACTTCTTTGCACC |
| CL5841.Contig1 | POD | ATCGACATGGACCCCAACAC | AGTGGGCCTGGATCTTAGGT |
The gene ID was from high temperature-induced P. lactiflora transcriptome data (accession number in NCBI: SRS774325, SRS774326, and SRS774327)
Statistical analysis
All experiments described here were repeated three times arranged in a completely randomized design. Primers were designed using Primer program (version 5.00). The results were analyzed for variance using the SAS/STAT statistical analysis package (version 6.12, SAS Institute, Cary, NC, USA).
Results
Effects of trehalose on oxidative stress under high temperature stress
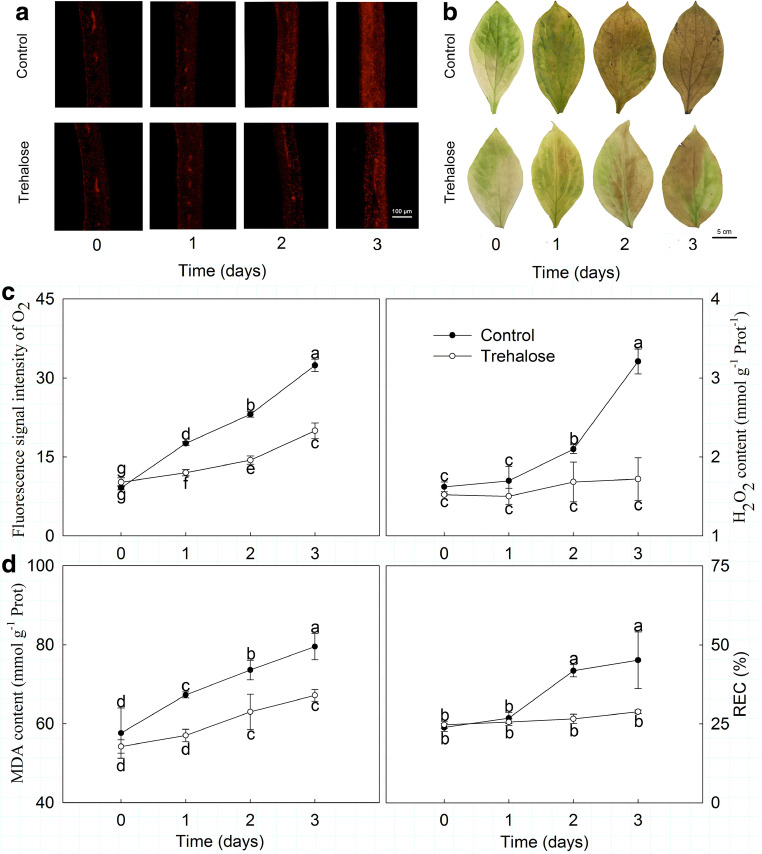
High temperature stress accelerated the production of reactive oxygen species (ROS) in P. lactiflora. O2·− and H2O2 accumulations were first observed using a fluorescence probe and DAB staining, and they all increased due to high temperature stress. However, trehalose pretreatment significantly reduced the high temperature-induced accumulations of O2·− and H2O2; in particular, on day 3, a lighter color and weaker fluorescent signal was observed in trehalose-treated leaves compared with leaves of the control (Fig. 1a, b). When the fluorescent signal intensity of O2·− accumulation and H2O2 content were numerically detected, both exhibited an upward trend in response to high temperature stress, while in trehalose-treated leaves, they were only 53.30 and 46.93% of those of the control on day 3, respectively (Fig. 1c).
Fig. 1.
Effect of trehalose on stress physiological indices of P. lactiflora under high temperature stress. a O2·− accumulation was detected by fluorescence probe. Different colors showed the fluorescent signal of O2·− accumulation. b H2O2 accumulation was detected by DAB staining. c Fluorescent signal intensity of O2·− accumulation and H2O2 content. d MDA content and REC. Values are expressed as the mean ± SE of triplicate assays, and different letters indicate significant differences according to Duncan’s multiple range test (P < 0.05)
Similarly, high temperature stress caused membrane lipid peroxidation injury in P. lactiflora. MDA content and REC increased significantly as high temperature stress developed, and these increases were attenuated by trehalose pretreatment, especially on day 3, when REC was 36.21% lower and MDA content was 15.51% lower in trehalose-treated leaves than in the control leaves (Fig. 1d).
Effects of trehalose on antioxidant enzymes under high temperature stress
Antioxidant enzyme activities, including CAT and SOD, were significantly affected by high temperature stress in P. lactiflora. As shown in Fig. 2, CAT activity presented an upward trend in the control, which increased by 14.37, 53.09, and 77.72% on days 1, 2, and 3, respectively. Moreover, CAT activity in trehalose-treated leaves was always higher than in the control leaves, being on average 1.38 times greater. Conversely, SOD activity showed a downward trend in both the trehalose-treated leaves and the control leaves but was continuously higher in trehalose-treated leaves compared to those of the control. Clearly, their differences gradually narrowed, and the smallest difference was observed on day 3, with 14.33 U g−1 FW.
Fig. 2.
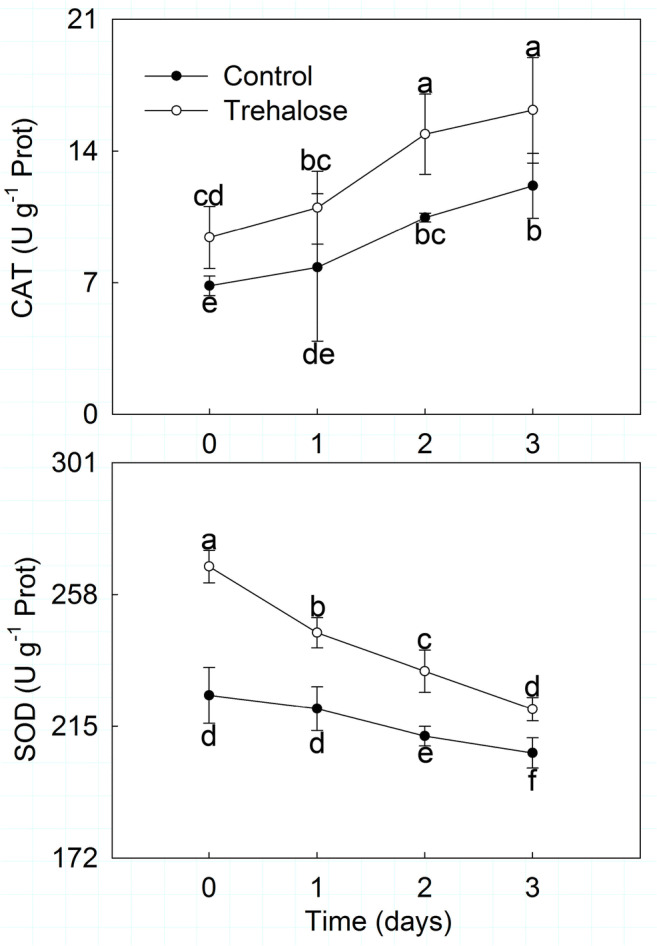
Effect of trehalose on antioxidant enzyme activities of P. lactiflora under high temperature stress. Values are expressed as the mean ± SE of triplicate assays, and different letters indicate significant differences according to Duncan’s multiple range test (P < 0.05)
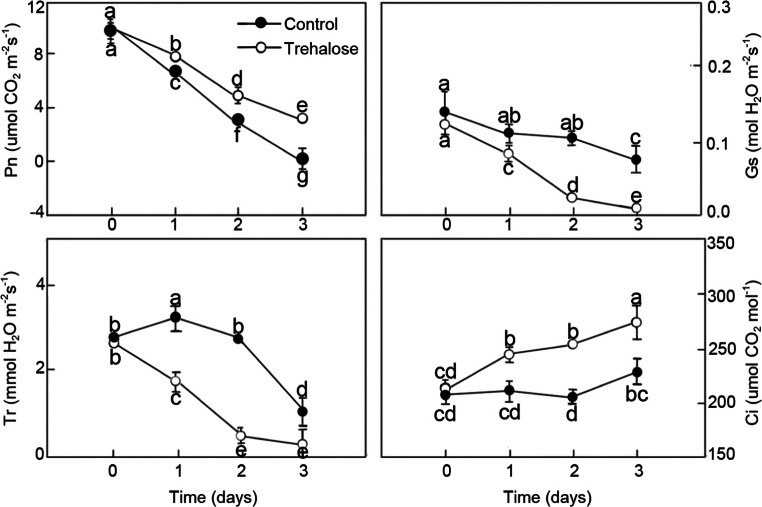
Effects of trehalose on photosynthesis under high temperature stress
High temperature stress affected photosynthetic characteristics in P. lactiflora (Fig. 3). Pn, Gs, and Tr in trehalose-treated leaves and the control basically showed downward trends with the development of high temperature stress, but Ci presented the opposite tendency. Trehalose pretreatment clearly alleviated high temperature-induced reduction in leaf Pn, but in the control leaves, Pn was significantly decreased by 84.59%, while in the trehalose-treated leaves, it decreased by 61.52%. In addition, Ci was enhanced by trehalose pretreatment. However, Gs and Tr in trehalose-treated leaves under high temperature stress were markedly lower than those of the control; Gs and Tr in the control significantly decreased by 40.12 and 92.21%, while trehalose-treated leaves significantly decreased by 50.34 and 85.71%, respectively.
Fig. 3.
Effect of trehalose on photosynthetic characteristics of P. lactiflora under high temperature stress. Values are expressed as the mean ± SE of triplicate assays, and different letters indicate significant differences according to Duncan’s multiple range test (P < 0.05)
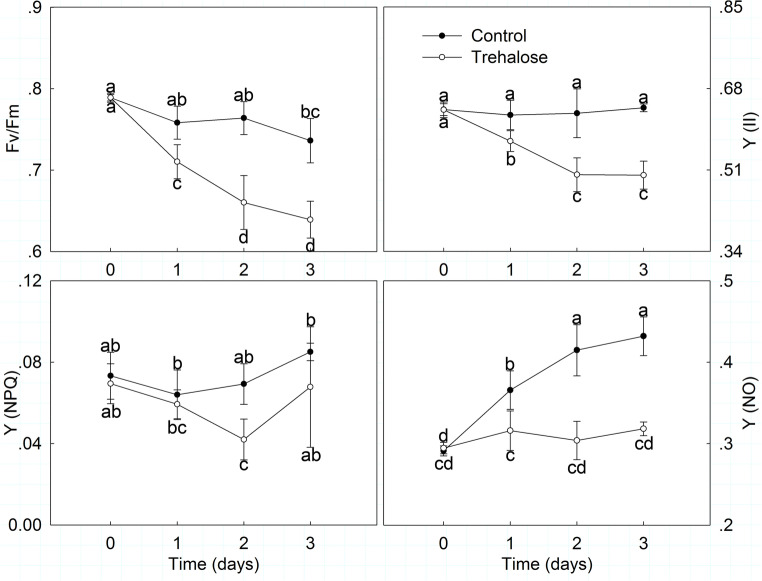
Moreover, P. lactiflora chlorophyll fluorescence parameters were also significantly affected by high temperature stress (Fig. 4). In general, Fv/Fm and Y (II) exhibited downtrends when exposed to high temperature and their values increased in trehalose-treated leaves compared with those of the control. Fv/Fm in trehalose-treated leaves generally decreased and had a slight increase on day 2, which represented the biggest difference from the control. Y (II) presented a slight uptrend in trehalose-treated leaves with a small fluctuation. Conversely, Y (NPQ) and Y (NO) generally presented uptrends when exposed to high temperature and were lower in trehalose-treated leaves than in those of the control. Y (NPQ) first decreased and then increased in trehalose-treated leaves, and the biggest difference was observed on day 2, which was 0.80 times that of the control. Y (NO) increased slowly and had a slight decline in trehalose-treated leaves on day 2; Y (NO) in trehalose-treated leaves and the control increased by 8.03 and 48.30%, respectively.
Fig. 4.
Effect of trehalose on chlorophyll fluorescence parameters of P. lactiflora under high temperature stress. Values are expressed as the mean ± SE of triplicate assays, and different letters indicate significant differences according to Duncan’s multiple range test (P < 0.05)
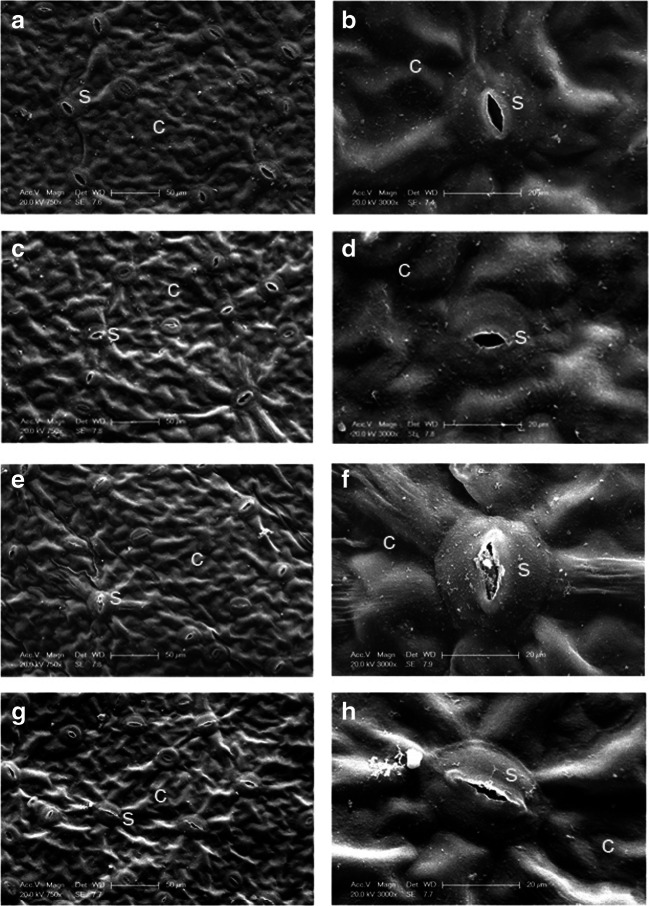
Effects of trehalose on anatomy under high temperature stress
High temperature stress resulted in stomata and mesophyll cell changes. At first, there was no obvious difference between trehalose-treated leaves and those of the control, and most of the stomata remained open on day 0 (Fig. 5a–d). However, in response to high temperature stress, most of the stomata in the control were closed, and only a few opened slightly on day 3. Moreover, some stomata were also blocked by impurities. Conversely, trehalose pretreatment slowed stomatal closure, and most of them remained open (Fig. 5e–h).
Fig. 5.
Effect of trehalose on stomatal characteristics of P. lactiflora under high temperature stress. a Micrograph of control on day 0. b Micrograph of partial enlargement of region in a. c Micrograph of trehalose treatment on day 0. d Micrograph of partial enlargement of region in c. e Micrograph of control on day 3. f Micrograph of partial enlargement of region in e. g Micrograph of trehalose treatment on day 3. g Micrograph of partial enlargement of region in g. C, cuticle; S, stomata
Subsequently, the observation and analysis of mesophyll cell ultrastructures from day 0 to day 3 indicated that the mesophyll cell ultrastructures in trehalose-treated leaves and those of the control were normal and similar to each other on day 0. Chloroplasts were the most prominent cell organelle, and they were arranged close to the cell membrane in greater quantities and contained some bored small lipid spheres (Fig. 6a–d). On day 3, trehalose-treated leaves had more intact chloroplasts than the control did, and the chloroplasts still presented some lipid spheres and starch grains, as previously observed. The membranes of some chloroplasts in the control appeared to be diffuse and not intact, leading to leakage of the grana lamellae (Fig. 6e–h).
Fig. 6.
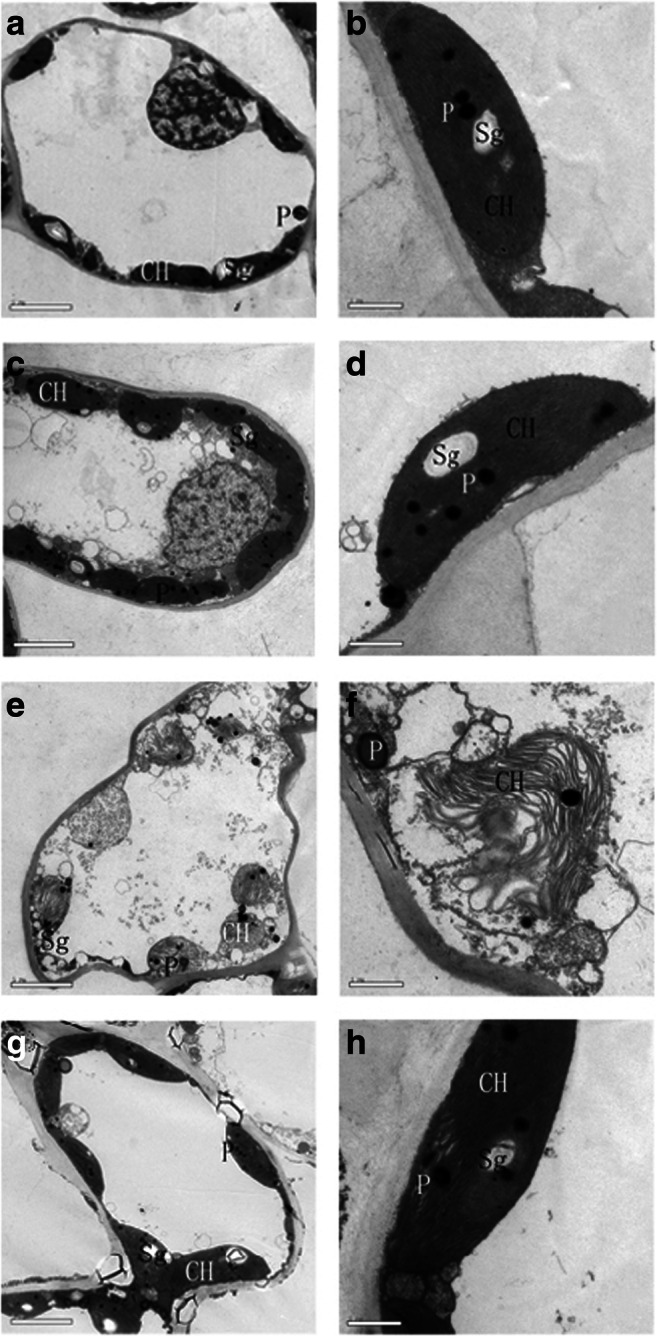
Effect of trehalose on cellular ultrastructure of P. lactiflora under high temperature stress. a Micrograph of control on day 0. b Micrograph of partial enlargement of region in a. c Micrograph of trehalose treatment on day 0. d Micrograph of partial enlargement of region in c. e Micrograph of control on day 3. f Micrograph of partial enlargement of region in e. g Micrograph of trehalose treatment on day 3. h Micrograph of partial enlargement of region in g. CH, chloroplast; P, lipid sphere; Sg, starch grain
Effects of trehalose on high temperature-tolerant gene expression under high temperature stress
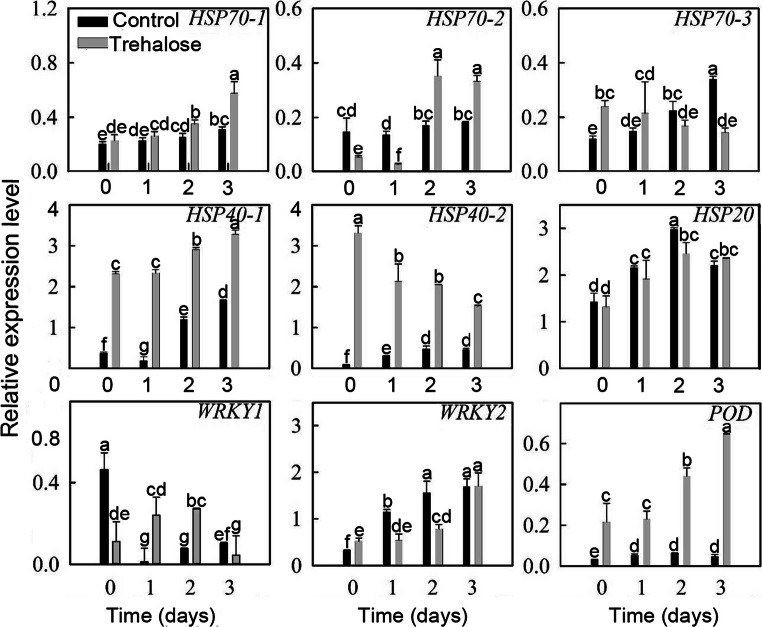
To explore the molecular mechanisms of trehalose conferring high temperature stress tolerance to P. lactiflora, nine high temperature-tolerant genes obtained in a previous study based on transcriptome and digital gene expression analysis (Hao et al. 2016) were used to detect their expression patterns under high temperature stress, and their expression levels responded differently to high temperature stress (Fig. 7). Except for HSP40-2 and WRKY1, the other seven genes showed overall upward trends when exposed to high temperature. Compared with the control, the expression levels of these nine genes in trehalose-treated leaves were all affected to some extent, especially HSP70-1, HSP40-1, HSP40-2, and POD. The expression level of HSP70--1 increased with a slight fluctuation as stress continued until its expression level peaked on day 3, when it was significantly higher in trehalose-treated leaves than in the control. In addition, HSP40-1, HSP40-2, and POD expression levels in trehalose-treated leaves were all significantly higher than those in the control leaves. HSP40-1 expressed similarly in trehalose-treated leaves and the control leaves, generally showing an upward trend with a single drop on day 1. Moreover, the biggest difference in HSP40-1 expression level between trehalose-treated leaves and the control leaves was observed on day 1 and was approximately 12 times higher in trehalose-treated leaves than in the control. With respect to POD expression level, in the control, there was very little change under high temperature, whereas it increased gradually in trehalose-treated leaves, becoming approximately 13.5 times higher than the control. In contrast, HSP40-2 expression level presented a downtrend and had a peak on day 0 which was 41.5 times greater in trehalose-treated leaves than that of the control. Subsequently, the difference of HSP40-2 expression levels between trehalose-treated leaves and the control gradually narrowed. Additionally, the expression levels of the other five genes were different from each other.
Fig. 7.
Effect of trehalose on high temperature-tolerant gene expression of P. lactiflora under high temperature stress. Values are expressed as the mean ± SE of triplicate assays, and different letters indicate significant differences according to Duncan’s multiple range test (P < 0.05)
Discussion
Summer high temperature restricts the normal growth of plants, inducing a series of changes in plant morphology, anatomy, physiology, biochemistry, and phenology (Wahid et al. 2007). Therefore, it is necessary to spray exogenous chemicals to alleviate the damage of high temperature stress to plants. In this study, the effects of trehalose on high temperature stress damage in P. lactiflora were investigated. Trehalose plays a protective role in serving as an osmolyte and/or a protein/membrane (López-Gómez and Lluch 2012). Previous studies revealed that plant abiotic stress tolerance modulation was achieved through engineering the trehalose synthesis pathway or trehalose treatment (Fernandez et al. 2010). In winter wheat (Triticum aestivum L.), exogenous application of trehalose protected thylakoid membranes from high temperature-induced damage (Luo et al. 2010). In addition, our results clearly suggested that exogenous supply of trehalose could alleviate adverse effects of high temperature on P. lactiflora growth, which was consistent with previous studies (Luo et al. 2008, 2010, 2014).
In plants, the initial nonspecific step of the stress response is ROS generation, including O2·− and H2O2, which not only damages proteins but also causes lipid peroxidation in cell membranes (Berlett and Stadtman 1997; Foyer et al. 1997; Halliwell and Gutteridge 1984). Under high temperature, O2·− generating rate and H2O2 content in T. aestivum leaves were significantly enhanced compared with those of the control (Luo et al. 2010), and this result was also observed in P. lactiflora, which suggested that high temperature disrupted the balance between the generation of and the scavenging of ROS, leading to oxidative stress. As a form of high temperature stress, oxidative stress also has an adverse impact on plant growth and development. Moreover, excessive ROS accumulation caused membrane lipid peroxidation injury in P. lactiflora, and MDA content and REC significantly increased with the development of high temperature stress, perhaps because of the decrease in antioxidant enzyme activities. When trehalose treatment was applied, these increases in ROS accumulation, MDA content, and REC were attenuated in P. lactiflora, consistent with a study of T. aestivum (Luo et al. 2010), which suggested that trehalose increased antioxidant enzyme activities of P. lactiflora under high temperature stress. The main antioxidant enzymes eliminating ROS all contain SOD, CAT, peroxidase (POD), and ascorbate peroxidase (APX). SOD is the first line of defense in eliminating ROS in plants and is central to the antioxidant enzyme system, catalyzing the disproportionation reaction of O2·− to generate O2 and H2O2 (Giannopolitis and Ries 1977), and CAT can catalyze H2O2 to generate O2 and H2O (Asada 1999). In this study, CAT activity in P. lactiflora presented an uptrend under high temperature stress, whereas SOD activity showed a downtrend, which was also consistent with the study in T. aestivum (Wang et al. 2016). This result was mainly because ROS elimination consumed a large quantity of SOD under high temperature stress, causing the generation of a large amount of H2O2, which needed more CAT to eliminate it. In trehalose-treated P. lactiflora, CAT and SOD activities were all enhanced, which reduced ROS accumulation and membrane lipid peroxidation injury. This result was also found in T. aestivum (Wang et al. 2016). Moreover, trehalose has the ability to scavenge free radicals such as H2O2 (Benaroudj et al. 2001), another reason for reduced ROS accumulation and membrane lipid peroxidation injury.
Leaves are vital organs of plants, and leaf senescence is a typical representation of high temperature damage, evidenced by low photosynthesis, collapse of cellular structure, chlorophyll degradation, and membrane lipid peroxidation (Yang and Guo 2014). Photosynthesis is one of the most sensitive physiological processes affected by high temperature, including stomatal and nonstomatal limitation. Stomatal limitation emphasizes a decrease in photosynthesis that is mainly due to reduced stomatal conductance caused by high temperature stress and an insufficient supply of CO2. Nonstomatal limitation emphasizes high temperature inhibition of key photosynthetic enzyme activities, gas absorption and diffusion of mesophyll cells, and CO2 dissolution and fixation, which results in an overall photosynthetic decline (Chen et al. 2014). In addition, Wu et al. (2001) suggested that slight high temperature stress led to a decline in photosynthesis through stomatal limitation, whereas severe high temperature stress reduced photosynthesis through nonstomatal limitation. In this study, Pn, Gs, and Tr all decreased under high temperature stress, but Ci increased, suggesting that P. lactiflora photosynthetic decline was caused by nonstomatal limitation; this was consistent with the results obtained in cucumber (Cucumis sativus L.) seedlings during late stages of high temperature stress (Sun et al. 2017). In addition, when P. lactiflora was treated with trehalose, Pn and Ci increased, but Gs and Tr decreased, consistent with results obtained in tomato (L. esculentum Mill.) seedlings treated with 0.5% trehalose and under high temperature stress (Pang et al. 2017). This result might be because leaf surfaces formed a film of trehalose, which closed stomata, reducing the excessive moisture evaporation and maintaining the moisture balance in P. lactiflora, which was related to the water absorption and retaining properties of trehalose (Brett et al. 2015). Moreover, higher temperature weaken efficient use of light energy by plants, and when the light energy absorbed is greater than the light energy photosynthesized and is beyond the plant’s protection ability, numerous ROS will be produced resulting in plant damage, consistent with the results of our study. Photosynthetic inhibition caused by high temperature stress has an important relationship with photosystem II (PS II). When P. lactiflora was stressed by high temperature, Fv/Fm and Y (II) presented upward trends, while Y (NPQ) and Y (NO) showed the opposite tendency, suggesting PS II was damaged, consistent with our previous study (Hao et al. 2017). In terms of trehalose treatment, Fv/Fm and Y (II) increased while Y (NPQ) and Y (NO) decreased, which was consistent with the study in L. esculentum (Pang et al. 2017), suggesting that trehalose protected the photosynthetic organ not by increasing heat dissipation but by maintaining photosynthetic organs to improve the activity of the photosystem. In addition, this speculation was validated by the anatomy changes observed in this study; trehalose-treated leaves had more intact chloroplasts than the control did, and the membranes of some chloroplasts in the control appeared to be diffuse and not intact, leading to leakage of the grana lamellae. Additionally, in P. lactiflora, thermo-tolerant related differently expressed genes were screened by transcriptome and digital gene expression analysis, and 161 genes were obtained (Hao et al. 2016). Of them, the expression levels of nine genes were determined in this study. Except for WRKY2 and HSP20, the expression of the other genes was differentially induced by trehalose, especially HSP40-1, HSP40-2, and POD, and thus these genes could protect plants from high temperature stress (Chakraborty et al. 2018; Rehman et al. 2017).
In conclusion, the present study has demonstrated that trehalose conferred high temperature stress tolerance to P. lactiflora. Under high temperature stress, trehalose improved redox homeostasis by inducing the activities of antioxidant enzymes. In addition, trehalose increased photosynthesis by protecting cell structure. Increased photosynthesis alleviated disruption of cellular redox homeostasis, while improved redox homeostasis contributed to keeping higher photosynthesis, forming a virtuous cycle (Fig. 8). These results unravel the critical role of trehalose in high temperature stress mitigation and suggest that trehalose could be applied to aid in the management of high temperature in P. lactiflora cultivation.
Fig. 8.
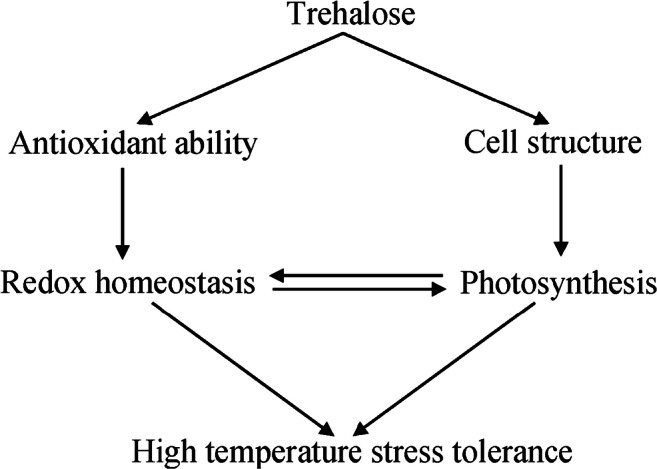
A model showing potential mechanisms of trehalose-induced alleviation of high temperature stress in P. lactiflora
Funding
This work was supported by the Natural Science Foundation of China (31872141), the Young Talent Support Project of Jiangsu Provincial Association for Science and Technology, the Building Project of Combined and Major Innovation Carrier of Jiangsu Province (BM2016008), Agriculture Three New Project of Jiangsu Province (SXGC[2017]297), the Program of Key Members of Yangzhou University Outstanding Young Teacher, and the Priority Academic Program Development from Jiangsu Government.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ali Q, Ashraf M, Anwar F, Al-Qurainy F. Trehalose-induced changes in seed oil composition and antioxidant potential of maize grown under drought stress. J Am Oil Chem Soc. 2012;89:1485–1493. [Google Scholar]
- An Y, Zhou P, Liang JF. Effects of exogenous application of abscisic acid on membrane stability, osmotic adjustment, photosynthesis and hormonal status of two lucerne (Medicago sativa L.) genotypes under high temperature stress and drought stress. Crop Pasture Sci. 2014;65:274–286. doi: 10.1071/CP13162. [DOI] [Google Scholar]
- Asada K. The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Biol. 1999;50:601–639. doi: 10.1146/annurev.arplant.50.1.601. [DOI] [PubMed] [Google Scholar]
- Benaroudj N, Lee DH, Goldberg AL. Trehalose accumulation during cellular stress protects cells and cellular proteins from damage by oxygen radicals. J Biol Chem. 2001;276:24261–24267. doi: 10.1074/jbc.M101487200. [DOI] [PubMed] [Google Scholar]
- Benlloch-González M, Quintero JM, Suárez MP, Sánchez-Lucas R, Fernández-Escobar R, Benlloch M. Effect of moderate high temperature on the vegetative growth and potassium allocation in olive plants. J Plant Physiol. 2016;207:22–29. doi: 10.1016/j.jplph.2016.10.001. [DOI] [PubMed] [Google Scholar]
- Berlett BS, Stadtman ER. Protein oxidation in aging, disease and oxidative stress. J Biol Chem. 1997;272:20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- Brett W, Isaac N, Lalehvash M, Long H, Dickman MB, Zhang X, Mundree S. Trehalose accumulation triggers autophagy during plant desiccation. PLoS Genet. 2015;11:e1005705. doi: 10.1371/journal.pgen.1005705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty K, Bishi SK, Singh AL, Zala PV, Mahatma MK, Kalariya K, Jat R. Rapid induction of small heat shock proteins improves physiological adaptation to high temperature stress in peanut. J Agron Crop Sci. 2018;204:285–297. doi: 10.1111/jac.12260. [DOI] [Google Scholar]
- Chen WL, Yang WJ, Lo HF, Yeh DM. Physiology, anatomy, and cell membrane thermostability selection of leafy radish (Raphanus sativus var. oleiformis Pers.) with different tolerance under heat stress. Sci Hortic. 2014;179:367–375. doi: 10.1016/j.scienta.2014.10.003. [DOI] [Google Scholar]
- Eissa AE, Zaki MM. The impact of global climatic changes on the aquatic environment. Procedia Environ Sci. 2011;4:251–259. doi: 10.1016/j.proenv.2011.03.030. [DOI] [Google Scholar]
- Fan M, Sun X, Xu N, Liao Z, Li Y, Wang J, Fan Y, Cui D, Li P, Miao Z. Integration of deep transcriptome and proteome analyses of salicylic acid regulation high temperature stress in Ulva prolifera. Sci Rep. 2017;7:11052. doi: 10.1038/s41598-017-11449-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez O, Béthencourt L, Quero A, Sangwan RS, Clément C. Trehalose and plant stress responses: friend or foe? Trends Plant Sci. 2010;15:409–417. doi: 10.1016/j.tplants.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Lopez-Delgado H, Dat JF, Scott IM. Hydrogen peroxide and glutathione-associated mechanisms of acclamatory stress tolerance and signaling. Physiol Plant. 1997;100:241–254. doi: 10.1111/j.1399-3054.1997.tb04780.x. [DOI] [Google Scholar]
- Giannopolitis CN, Ries SK. Superoxide dismutase. I. Occurrence in higher plants. Plant Physiol. 1977;59:309–314. doi: 10.1104/pp.59.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goraya GK, Kaur B, Asthir B, Bala S, Kaur G, Farooq M. Rapid injuries of high temperature in plants. J Plant Biol. 2017;60:298–305. doi: 10.1007/s12374-016-0365-0. [DOI] [Google Scholar]
- Halliwell B, Gutteridge JM. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984;219:1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao ZJ, Wei MR, Gong SJ, Zhao D, Tao J. Transcriptome and digital gene expression analysis of herbaceous peony (Paeonia lactiflora Pall.) to screen thermo-tolerant related differently expressed genes. Genes Genom. 2016;38:1201–1215. doi: 10.1007/s13258-016-0465-8. [DOI] [Google Scholar]
- Hao ZJ, Zhou CH, Liu D, Wei MR, Tao J. Effects of high temperature stress on photosynthesis, chlorophyll fluorescence and ultrastructure of herbaceous peony (Paeonia lactiflora Pall.) Mol Plant Breed. 2017;15:2359–2367. [Google Scholar]
- Liu H, Shen G, Fang X, Fu Q, Huang K, Chen Y, Yu H, Zhao Y, Zhang L, Jin L, Ruan S. Heat stress-induced response of the proteomes of leaves from Salvia splendens Vista and King. Proteome Sci. 2013;11:25. doi: 10.1186/1477-5956-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Gómez M, Lluch C. Trehalose and abiotic stress tolerance. In: Ahmad P, Prasad M, editors. Abiotic stress responses in plants. New York: Springer; 2012. [Google Scholar]
- Luo Y, Li WM, Wang W. Trehalose: protector of antioxidant enzymes or reactive oxygen species scavenger under heat stress? Environ Exp Bot. 2008;63:378–384. doi: 10.1016/j.envexpbot.2007.11.016. [DOI] [Google Scholar]
- Luo Y, Li F, Wang GP, Yang XH, Wang W. Exogenously-supplied trehalose protects thylakoid membranes of winter wheat from heat-induced damage. Biol Plant. 2010;54:495–501. doi: 10.1007/s10535-010-0087-y. [DOI] [Google Scholar]
- Luo Y, Gao YM, Wang W, Zou CJ. Application of trehalose ameliorates heat stress and promotes recovery of winter wheat seedlings. Biol Plant. 2014;58:395–398. doi: 10.1007/s10535-014-0397-6. [DOI] [Google Scholar]
- Mostofa MG, Hossain MA, Fujita M, Tran LS. Physiological and biochemical mechanisms associated with trehalose-induced copper-stress tolerance in rice. Sci Rep. 2015;5:11433. doi: 10.1038/srep11433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang CP, Ye L, Ma J, Lu T, Yang ZY, Qi MF. Regulation function of trehalose on tomato seedling leaf photosynthesis under high temperature. Jiangsu Agric Sci. 2017;45:143–146. [Google Scholar]
- Putten M, Schneider S, Root T (2002) Wildlife responses to climate change: north American case studies. Island Press
- Rehman RNU, You Y, Zhang L, Goudia BD, Khan AR, Li P, Ma F. High temperature induced anthocyanin inhibition and active degradation in Malus profusion. Front Plant Sci. 2017;8:1401. doi: 10.3389/fpls.2017.01401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rykaczewska K. The effect of high temperature occurring in subsequent stages of plant development on potato yield and tuber physiological defects. Am J Potato Res. 2015;92:339–349. doi: 10.1007/s12230-015-9436-x. [DOI] [Google Scholar]
- Sage TL, Bagha S, Lundsgaard-Nielsen V, Branch HA, Sultmanis S, Sage RF. The effect of high temperature stress on male and female reproduction in plants. Field Crops Res. 2015;182:30–42. doi: 10.1016/j.fcr.2015.06.011. [DOI] [Google Scholar]
- Sang Q, Shan X, An Y, Shu S, Sun J, Guo S. Proteomic analysis reveals the positive effect of exogenous spermidine in tomato seedlings’ response to high-temperature stress. Front Plant Sci. 2017;8:120. doi: 10.3389/fpls.2017.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;36:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Shahbaz M, Abid A, Masood A, Waraich EA. Foliar-applied trehalose modulates growth, mineral nutrition, photosynthetic ability and oxidative defense system of rice (Oryza Sativa L.) under saline stress. J Plant Nutr. 2017;40:584–599. doi: 10.1080/01904167.2016.1263319. [DOI] [Google Scholar]
- Sun SN, Wang Q, Sun CC, Liu FJ, Bi HG, Ai XZ. Response and adaptation of photosynthesis of cucumber seedlings to high temperature stress. Chinese J Applied Ecol. 2017;28:1603–1610. doi: 10.13287/j.1001-9332.201705.009. [DOI] [PubMed] [Google Scholar]
- Tian F, Gong J, Zhang J, Zhang M, Wang G, Li A, Wang W. Enhanced stability of thylakoid membrane proteins and antioxidant competence contribute to drought stress resistance in the tasg1 wheat stay-green mutant. J Exp Bot. 2013;64:1509–1520. doi: 10.1093/jxb/ert004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahid A, Gelani S, Ashraf M (2007) Heat tolerance in plants: An overview. Environ Exp Bot 61: 199–223
- Wang D, Luo Y, Gao YM, Zhao YY, Zou CJ. Effects of exogenous trehalose on the membrane lipid peroxidation in wheat seedlings under heat stress. J Triticeae Crops. 2016;36:925–932. [Google Scholar]
- Wu HY, Shou SY, Zhu ZJ, Yang X. Effects of high temperature stress on photosynthesis and chlorophyll fluorescence in sweet pepper (Capsicum fructescens L.) Acta Horticulturae Sinica. 2001;28:517–521. [Google Scholar]
- Wu YQ, Zhao DQ, Han CX, Tao J. Biochemical and molecular responses of herbaceous peony to high temperature stress. Can J Plant Sci. 2016;96:474–484. doi: 10.1139/cjps-2015-0255. [DOI] [Google Scholar]
- Yang XF, Guo FQ. Research advances in mechanisms of plant leaf senescence under heat stress. Plant Physiol J. 2014;50:1285–1292. [Google Scholar]
- Yang GP, Rhodes D, Joly RJ. Effects of high temperature on membrane stability and chlorophyll fluorescence in glycinebetaine-deficient and glycinebetaine-containing maize lines. Funct Plant Biol. 1996;23:437–443. doi: 10.1071/PP9960437. [DOI] [Google Scholar]
- Zhang JP, Li DQ, Li K, Xia YP. Reconsideration for the southward plantation of Paeonia lactiflora. Chinese Landscape Architecture. 2016;32:91–95. [Google Scholar]
- Zhao DQ, Han CG, Zhou CH, Tao J. Shade ameliorates high temperature-induced inhibition of growth in herbaceous peony (Paeonia lactiflora) Int J Agric Biol. 2015;17:911–919. doi: 10.17957/IJAB/15.0004. [DOI] [Google Scholar]
- Zou Q. Plant physiology experimental guidance. Beijing: China Agricultural Press; 2000. [Google Scholar]